Abstract
Purpose
The decision to re-induce patients with acute myeloid leukemia (AML) based on results of the day 14 bone marrow (BM) biopsy is variable and lacks evidence based data. The aim of our review was to evaluate the accuracy of a day 14 BM biopsy in determining the need for re-induction chemotherapy.
Methods
Seventy-four patients with newly diagnosed de novo AML treated with induction chemotherapy were retrospectively reviewed for the purpose of evaluating treatment decisions and outcomes based on their day 14 BM biopsy. Response to therapy in this analysis was based on morphology alone.
Results
Of the 74 patients undergoing standard induction, 45 patients (61%) had no evidence of leukemia on their day 14 BM biopsy. Eighteen patients (24%) had definitive residual disease (RD), and 11 patient’s (15%) were classified as indeterminate response (IR). Fifteen patients with RD and one with IR underwent re-induction chemotherapy. However, thirteen patients (3 RD and 10 IR) were observed until count recovery without any re-induction therapy. Eleven of these 13 patients who were observed eventually attained a morphologic complete remission (CR), including two patients with RD.
Conclusions
A day 14 BM biopsy may have suboptimal sensitivity for the detection of residual leukemia. Some patients with an IR on day 14 may not require re-induction chemotherapy, but instead, may benefit from careful observation until count recovery to avoid the mortality and morbidity associated with re-induction chemotherapy.
Keywords: AML, D14 BM biopsy, Re-induction
1. Introduction
Acute myeloid leukemia (AML) is the most common acute leukemia in adults with an incidence of 1.9/100,000 persons [1]. It is an extremely heterogeneous disease characterized by several cytogenetic abnormalities, molecular mutations, and gene overexpressions. Over the past two decades the definition of AML along with its diagnostic and prognostic criteria have undergone tremendous evolution. The traditional therapy for AML off clinical trial is the 7 + 3 regimen: 7 days of cytarabine and 3 days of an anthracycline. This combination leads to complete remission (CR) rates of 55–65% in young AML (<60 years) patients, while their older counterparts do much worse, i.e. CR rates of 25–50%. Initial documentation of an objective response of “no evidence of leukemia” is often based on a day 14 bone marrow (BM) biopsy [2,3]. The timing and interpretation of this biopsy continues to be controversial. Our hypothesis is that a day 14 BM biopsy is not a sensitive test to assess response to induction chemotherapy in AML patients. This analysis aims to analyze the value of a day 14 BM biopsy in clinical decision making for future therapy in AML patients.
2. Methods
2.1. Materials
Patients evaluated between 1995 and 2008, with newly diagnosed de novo AML were identified and screened. Of 100 patients screened, 74 had documented paired (nadir i.e. day 14 and recovery i.e. days 21–42) BM biopsy results in the electronic medical record (Table 1). Many of these patients were seen in consultation after primary treatment – with subsequent pathology review by onsite hematopathology at time of consultation. Patients without pathology review of both nadir and recovery marrows were excluded (n = 26). Documentation of peripheral count recovery varied in detail, and was absent in a considerable number of charts. Patients were classified based on the hematopathologists review of the day 14 BM biopsy as having appropriate (no evidence of leukemia) or suboptimal response (SOR) based on BM cellularity and blast percentage following induction. These biopsy specimens were reviewed by our institution’s hematopathologists for agreement and response criteria were clearly defined (Table 2). All patients underwent an additional BM examination at the time of marrow recovery following initial or subsequent induction chemotherapy between days 28 and 42.
Table 1.
Patient characteristics
| Characteristic | Number |
|---|---|
| Total number | 74 |
| Median age (range) years | 43(18–77) |
| FAB classification | |
| M0 | 5 |
| M1 | 17 |
| M2 | 15 |
| M3 | 0 |
| M4 | 16 |
| M5 | 12 |
| M6 | 3 |
| M7 | 0 |
| Unknown | 6 |
| Cytogenetics | |
| Good-risk | 2 |
| Intermediate risk/normal karyotype | 9/28 |
| Poor-risk | 15 |
| Unknown | 20 |
Table 2.
Day 14 bone marrow biopsy classification
| Day 14 response criterion | Bone marrow cellularity | Bone marrow blasts (%) | Other |
|---|---|---|---|
| No evidence of residual disease | Hypocellular | <5 | No auer rods |
| Indeterminate response | Sub-optimal reduction | 5–20 | Blast % by morphology |
| Residual disease | Sub-optimal reduction | >20 | Blast % by morphology |
| Sub-optimal response (SOR) | IR + RD | >5 | Considered a positive screen |
2.2. Definitions for response criteria
The International Working Group (IWG) has published guidelines and definitions of response to therapy, which included early treatment assessment and variations on definitions of complete remission – including definitions of morphologic CR, cytogenetic CR, molecular CR, and partial remission [4]. These latter categories were included for use in clinical trials and it has been stressed that there is no unified agreement on the utility of these seemingly more sensitive measures outside of the research arena. For now, complete remission (CR) is defined by the IWG as a blast percentage of <5% and no auer rods visualized at time of marrow recovery (ANC > 1000/mm3, platelets > 100,000/mm3). Without documentation of peripheral counts at the time of bone marrow recovery, we utilize the term “morphologic leukemia free state”. CR in general cannot be determined based solely on a day 14 BM biopsy given the lack of opportunity for peripheral count recovery at the time of bone marrow nadir.
In our study we have utilized alternative descriptors to define the disease state at the time of bone marrow nadir following induction therapy (Table 2). In particular we have defined sub-optimal response (SOR), as a category that would include both an indeterminate response (IR) and the evidence of definitive residual disease (RD). IR is defined as a >5–20% increase in blasts with some recovering myeloid progenitors in a background marrow yielding a situation where residual disease cannot be definitively ruled out by the reviewing hematopathologist. RD is based on specific morphologic characteristics, including but not limited to a blast percentage of >20% at time of expected BM nadir following induction.
2.3. Statistical methods
The primary aim of our study was to evaluate the accuracy of a day 14 BM biopsy in determining the presence of residual disease in order to clarify its role in treatment decision making at the time of bone marrow nadir following induction therapy for AML. For those patients that were observed without re-induction (n = 58), subgroup analysis allowed for a rudimentary evaluation of the day 14 BM biopsy as a “screening test” for response (Table 3). Patients meeting our definition of an appropriate response with no evidence of residual disease were considered to have a negative screening test on day 14. A recovery BM examination obtained between days 21 and 42 was the confirmatory test for presence or absence of AML. A standard 2 × 2 table was generated, allowing for the calculation of several standard definitions that provide a reasonable platform for evaluation of the day 14 BM biopsy as a screening test. The Fisher’s exact test, which is used in the analysis of contingency tables was employed to look for an association between the nadir (day 14) outcome and the recovery (days 21–42) outcome [5]. Specifically, sensitivity, specificity, positive predictive value, and negative predictive value as well as false positive and negative rates are calculated and presented below for those patients that were observed without re-induction.
Table 3.
Day 14 bone marrow biopsy as a screening test: 2 × 2 table
| Recovery BM: days 21–42 |
||||
|---|---|---|---|---|
| + | − | |||
| Nadir BM | SOR | 2 | 11 | 13 |
| Day 14 | NED | 3 | 42 | 45 |
| 5 | 53 | |||
| Sensitivity | 40% | |||
| Specificity | 79% | |||
| FPR | 21% | |||
| FNR | 60% | |||
| PPV | 15% | |||
| NPV | 93% | |||
3. Results
3.1. Patient demographics
Seventy-four patients were available for retrospective evaluation and their demographics are provided in Table 1. Cytogenetics at time of diagnosis were known for only 73% of patients: 53% had good- or intermediate-risk cytogenetics at time of diagnosis (only 3% patients with t(8:21) or inv(16)). The remaining 47% patients had poor-risk or complex cytogenetics [6]. FAB classification was also documented for most of the patients screened (92%), with the majority of patients nearly equally distributed between M1 (23%), M2 (20%), M4 (22%), and M5 (16%). Data on molecular studies e.g. FLT3-ITD, CEBPA and NPM1 mutation status was not available
3.2. Response
As illustrated in Fig. 1, day 14 BM biopsies of the 74 patients undergoing standard induction chemotherapy revealed that 45 patients (61%) had a HM with less than 5% blasts with no evidence of residual leukemia. Eleven patient’s (15%) BM biopsies were classified as IR. Eighteen patients (24%) had morphologically definitive RD. In all, 29 patients (39%) had a sub-optimal response (SOR) to induction chemotherapy (IR + RD = SOR). The 45 patients with no evidence of residual disease were observed until count recovery as is the usual practice. However, 16 of 29 patients with SOR received re-induction chemotherapy (1 IR and 15 RD) of which 10 patients attained a morphologic leukemia free state (1 IR and 9 RD). The 13 remaining patients (10 IR and 3 RD) were observed without any re-induction therapy, and re-evaluated with a follow-up BM biopsy between days 21 and 42 of initial induction. Interestingly, 11 of the 13 patients in the SOR observation arm had a morphologic leukemia free state on follow-up biopsy, including 2 of 3 patients initially categorized as RD. Using the Fisher’s exact test to look for the association between the nadir and recovery outcomes based on the 2 × 2 table produced a p-value of 0.31, indicating that there is no significant association between the two. Analysis of the paired results from nadir to recovery for the 58 observed patients in this cohort revealed a positive predictive value (PPV) and negative predictive value (NPV) for the day 14 BM biopsy review of 15% (95% CI: 0.02–0.45) and 93% (95% CI: 0.81–0.98) respectively. Sensitivity and specificity were calculated at 40% (95% CI: 0.05–0.85) and 79% (95% CI: 0.66–0.89) respectively, resulting in false positive and false negative rates of 21% (95% CI: 0.11–0.34) and 60% (95% CI: 0.15–0.95) respectively. For our analysis, both IR and RD were considered as a positive test (as this is defined as SOR).
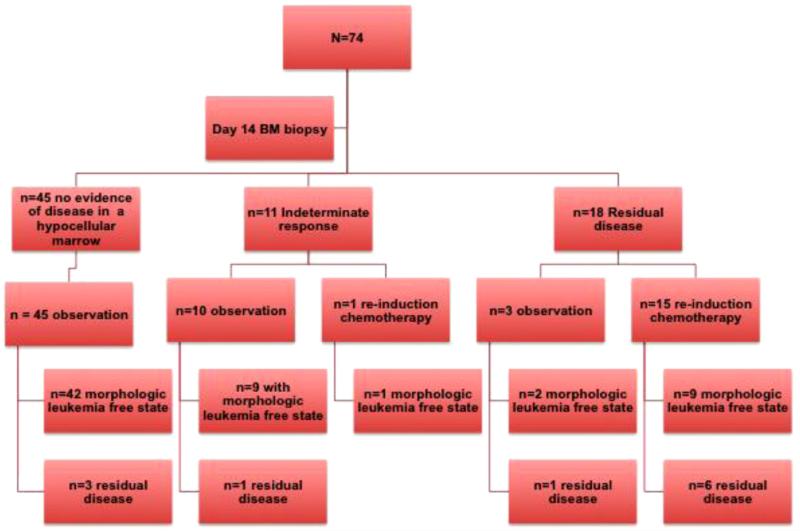
Fig. 1.
Flow diagram representation of outcomes after the day14 BM biopsy. BM: bone marrow. This flow diagram demonstrates that 74 patients with AML underwent induction chemotherapy and underwent a BM examination at nadir i.e. day 14 and a recovery BM i.e. days 21–42. Forty-five patients had no evidence of disease in a hypocellular marrow i.e. leukemia free state. Eleven patients had an Indeterminate response and 18 patients had frank residual leukemia. Of, the 45 patients with initial leukemia free state, 42 continued to be in leukemia free state (morphologic CR) and 3 patients had residual AML when a BM biopsy was done at count recovery i.e. days 21–42. Of the 11 patients with indeterminate response, one patient underwent re-induction chemotherapy and achieved a morphologic CR. However, 10 patients were observed without any more chemotherapy, and of these 9 patients at count recovery had a morphologic CR. Of the 18 patients with frank residual disease, three patients were observed without any more chemotherapy and at count recovery, two of these patients had a morphologic CR. However, 15 patients underwent re-induction chemotherapy and only 9 patients achieved a morphologic CR after re-induction.
4. Discussion
AML is a disease that is difficult to cure and unfortunately continues to have a high rate of relapse and death from progression despite our most aggressive treatment protocols. We have improved our risk stratification systems as our knowledge and understanding of both cytogenetics and molecular testing at the time of diagnosis have matured. We continue, however, to rely mainly on morphologic review techniques of mid-induction (days 14–21) BM biopsies that have historically proven to lack the necessary sensitivity to identify true residual disease that would require early re-induction chemotherapy.
BM evaluation following induction therapy has been adopted and adapted based on clinical experience with pediatric acute lymphoblastic leukemia, and has become an essential part of standard and investigational treatment protocols for AML. BM morphology, specifically as it relates to early blast clearance following induction for AML, has been documented in the literature as an independent predictor of complete remission and overall survival [7,8]. The current International Working Group (IWG) guidelines for clinical response in AML continue to support the use of morphologic review of the BM 7–10 days (i.e. day 14 marrow) following the final dose of induction therapy, as the predominant early indicator of response. With the exception of molecular tests for acute promyelocytic leukemia, flow cytometric analysis, cytogenetic evaluation, and molecular technologies are still considered investigational and currently have no agreed upon role in the determination of CR according to the IWG report. However, the National Comprehensive Cancer Network (NCCN) guidelines do suggest that cytogenetics should be included in the evaluation and determination of a CR at time of marrow recovery following induction if cytogenetic abnormalities existed at the time of diagnosis [9]. Relying solely on morphology and immunohistochemistry places the pathologist, the treating oncologist, and patient at a distinct disadvantage when attempting to identify residual malignant cells in a hypocellular marrow. Specifically, it may be challenging to determine the nature of scattered blasts seen on a nadir BM biopsy. These cells, in fact, may be appropriate and simply represent the normal hematopoietic process in the recovering marrow. Conversely, the blasts may represent a continued population of the leukemic cells that are delayed in response, but may actually be susceptible to the provided therapy, in a slower than average manner. Finally, the blasts could represent a refractory population of cells that will certainly lead to early relapse or induction failure.
Some investigators have suggested that patients who fail to achieve a leukemia free state and subsequent CR with a single cycle of induction therapy should be considered to have refractory AML with anticipated worse outcomes, recommending modifications in both re-induction and post remission treatment strategies [7,8]. Subsequent literature refutes this theory, and instead supports no appreciable difference in outcomes whether one or two cycles of induction therapy are required to achieve a CR, questioning the importance of time to blast clearance and subsequent remission based on current IWG definitions [10]. Many treating physicians are in agreement that attaining a CR is important for long term survival and should be aggressively pursued to optimize clinical outcomes. The question that remains is what are we to do in patients that fail to respond or have a delayed response to induction therapy based on a morphological evaluation of a day 14 BM biopsy? Standard recommendations would support early re-induction based on even low levels of residual blasts (5–20%) as seen on a nadir marrow evaluation [9]. However, early re-induction, especially in elderly AML patients, results in an overall increase in morbidity and mortality from prolonged cytopenias, infectious complications, longer hospital stays, increased transfusion support, as well as resultant poor functional status and reduced quality of life. In contrast to our results, a previous analysis has demonstrated that the day 14 BM biopsy is highly sensitive in predicting CR, but did not predict overall survival [11]. However, that analysis reported sensitivity in respect to achieving a CR, not for the evaluation of residual disease, which is the standard definition of sensitivity as it relates to a screening test. Although our conclusions differ due to this variation in the defined endpoint (CR vs. residual disease), our data are synchronous in respect to outcomes.
For those with definitive RD, the choice for re-induction is often quickly considered and is currently recommended in the guidelines put forth by the NCCN for patients younger than 60 years or those over 60 years with good performance status [9]. These patients, with the appropriate clinical scenario and performance status, most often receive additional cytotoxic chemotherapy at or near the time of the bone marrow nadir, prolonging the period of cytopenias, greatly increasing the risk of bleeding, infections, and other adverse events associated with prolonged hospitalizations. It would be very difficult to defend the alternative – withholding re-induction – based on our limited data set above; however, a pause is certainly appropriate and warranted given that 2 of 3 patients in the observation arm with definitive RD on nadir evaluation attained a CR without additional therapy. It should be noted that these three patients had additional therapy withheld solely due to ongoing infectious complications. Early re-induction for those with IR, however, is not so well supported based on our review. It would seem that these findings reflect the sentiment in the community resulting in a true hesitancy to provide additional therapy at time of count nadir for patients with a borderline marrow. Our data illustrates a positive predictive value of only 15% buttressed by a 21% false positive rate, supporting the instinctual desire to watch and wait for recovery, prior to re-challenging the patient with additional chemotherapy.
Thus, although an important tool for the evaluation of AML patients undergoing cytotoxic therapy, a day 14 BM biopsy evaluation after induction chemotherapy should not be the sole marker for treatment modifications, but instead, incorporated into a larger data set to provide a more complete evaluation of a patient’s disease state. Our data suggests that an indeterminate response on BM biopsy at day 14 has a suboptimal sensitivity for the detection of residual disease and those patients do not necessarily require re-induction, and instead may actually benefit from continued observation by avoiding the risks of re-induction and prolonged cytopenias. We make no recommendations in regards to the treatment of patients with definitive residual disease at time of bone marrow nadir based on this limited review; however, a larger prospective study with centralized pathologic evaluation to include flow cytometry, cytogenetics, and molecular studies, with extended clinical endpoints such as relapse free survival and overall survival, is warranted to help better define the utility of nadir bone marrow evaluation with contrast comparison to more sensitive techniques to better define the most appropriate markers of minimal residual disease and poor clinical outcomes early in the treatment process.
References
- [1].Surveillance, Epidemiology, and End Results (SEER) Program Populations (1969–2009) National Cancer Institute . DCCPS, Surveillance Research Program. Cancer Statistics Branch; www.seer.cancer.gov/popdata. released January 2011. [Google Scholar]
- [2].Rao AV, Valk PJ, Metzeler KH, et al. Age-specific differences in oncogenic pathway dysregulation and anthracycline sensitivity in patients with acute myeloid leukemia. J Clin Oncol. 2009;27(33):5580–6. doi: 10.1200/JCO.2009.22.2547. [DOI] [PubMed] [Google Scholar]
- [3].Applebaum FR, Gundacker H, Head DR, et al. Age and acute myeloid leukemia. Blood. 2006;107(9):3481–5. doi: 10.1182/blood-2005-09-3724. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Cheson BD, Bennett JM, Kopecky KJ, et al. Revised recommendations of the International Working Group for Diagnosis, Standardization of Response Criteria, Treatment Outcomes, and Reporting Standards for Therapeutic Trials in Acute Myeloid Leukemia. J Clin Oncol. 2003;21(24):4642–9. doi: 10.1200/JCO.2003.04.036. [DOI] [PubMed] [Google Scholar]
- [5].Agresti A. Categorical data analysis. John Wiley & Sons; New Jersey: 2002. [Google Scholar]
- [6].Byrd JC, Mrozek K, Dodge RK, et al. Pretreatment cytogenetic abnormalities are predictive of induction success, cumulative incidence of relapse, and overall survival in adult patients with de novo acute myeloid leukemia: results from Cancer and Leukemia Group B (CALGB 8461) Blood. 2002;100(13):4325–36. doi: 10.1182/blood-2002-03-0772. [DOI] [PubMed] [Google Scholar]
- [7].Estey EH, Shen Y, Thall PF. Effect of time to complete remission on subsequent survival and disease-free survival time in AML, RAEB-t, and RAEB. Blood. 2000;95(1):72–7. [PubMed] [Google Scholar]
- [8].Kern W, Haferlach T, Schoch C, et al. Early blast clearance by remission induction therapy is a major independent prognostic factor for both achievement of complete and long-term outcome in acute myeloid leukemia: data from the German AML Cooperative Group (AMLCG) 1992 Trial. Blood. 2003;101(1):64–70. doi: 10.1182/blood-2002-02-0532. [DOI] [PubMed] [Google Scholar]
- [9].NCCN Clinical Practice Guidelines in Oncology™ – Acute Myeloid Leukemia Version 2 2011 http://www.nccn.org/professionals/physiciangls/fguidelines.asp#aml.
- [10].Rowe JM, Kim HT, Cassileth PA, et al. Adult patients with acute myeloid leukemia who achieve complete remission after 1 or 2 cycles of induction have a similar prognosis: a report on 1980 patients registered to 6 studies conducted by the Eastern Cooperative Oncology Group. Cancer. 2010;116(21):5012–21. doi: 10.1002/cncr.25263. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [11].Hussein K, Jahagirdar B, Gupta P, et al. Day 14 bone marrow biopsy in predicting complete remission and survival in acute myeloid leukemia. Am J Hematol. 2008;83(6):446–50. doi: 10.1002/ajh.21133. [DOI] [PubMed] [Google Scholar]