Abstract
Heart failure (HF) is a global phenomenon, and the overall incidence and prevalence of the condition are steadily increasing. Medical therapies have proven efficacious, but only a small number of pharmacological options are in development. When patients cease to respond adequately to optimal medical therapy, cardiac resynchronization therapy has been shown to improve symptoms, reduce hospitalizations, promote reverse remodelling, and decrease mortality. However, challenges remain in identifying the ideal recipients for this therapy. The field of mechanical circulatory support has seen immense growth since the early 2000s, and left ventricular assist devices (LVADs) have transitioned over the past decade from large, pulsatile devices to smaller, more-compact, continuous-flow devices. Infections and haematological issues are still important areas that need to be addressed. Whereas LVADs were once approved only for ‘bridge to transplantation’, these devices are now used as destination therapy for critically ill patients with HF, allowing these individuals to return to the community. A host of novel strategies, including cardiac contractility modulation, implantable haemodynamic-monitoring devices, and phrenic and vagus nerve stimulation, are under investigation and might have an impact on the future care of patients with chronic HF.
Introduction
Heart failure (HF) is a global epidemic,1,2 and the lifetime risk of developing HF is 20%.3 Medical therapy with angiotensin-converting-enzyme inhibitors,4,5 β-blockers,6–9 aldosterone antagonists,10,11 and angiotensin-receptor blockers12,13 has significantly improved morbidity and mortality in patients with HF. Despite these improvements, the rates of hospitalization for HF have improved little, and readmission rates remain high at 23–27% within 30 days.14–16 In addition, the 5-year age-adjusted mortality from HF is 59% and 45% for men and women, respectively.17 Remote monitoring is a promising management strategy for ambulatory patients with HF,18 but many individuals with chronic HF require advanced mechanical therapies just to survive. Nevertheless, causes for optimism exist. Cardiac resynchronization therapy (CRT) has been an important addition to our armamentarium for the treatment of HF, and left ventricular assist devices (LVADs) have quickly revolutionized and improved the care of the sickest patients with HF. In this Review, we discuss the development and latest indications for the use of these devices in the management of patients with advanced, chronic HF.
Cardiac resynchronization therapy
As the inherent limitations of medical therapy and the lack of game-changing options on the horizon became clear, the search began for nonpharmacological methods to treat advanced HF. Astute clinicians discovered that intraventricular dyssynchrony was prominent in left bundle branch block (LBBB), and the abnormal interventricular septal motion in LBBB corresponded to periods of asynchrony in contraction and a reduction in the left ventricular ejection fraction (LVEF).19 Autopsy studies revealed that conduction abnormalities are common in HF and, in one report, >80% of patients with idiopathic dilated cardiomyopathy had electrocardiographic evidence of intraventricular conduction abnormalities.20 Furthermore, intraventricular conduction delay in patients with chronic HF has been shown to be a marker of increased mortality.21 In the early 1990s, these discoveries served as the impetus for French investigators to place pacemaker leads into all four cardiac chambers of a man with severe HF and LBBB, with the goal of restoring the natural mechanical activation sequence.22 Remarkably, his NYHA classification improved from class IV to class II.22 In a small study, multisite biventricular pacing acutely improved haemodynamics in patients with severe HF and marked QRS prolongation.23 This improvement was thought to occur by an increase in left ventricular (LV) filling time, a decrease in septal dyskinesis, and a reduction in mitral regurgitation brought about by resynchronization of ventricular contraction.24,25
Experimentation with biventricular pacemakers began to emerge as a means to restore synchronous left and right ventricular contraction. The additional LV lead was initially placed surgically, but eventually the coronary sinus route was shown to be efficacious and safe and, therefore, is now the standard method of implantation (Figure 1).26 The clinical efficacy and safety of this novel therapy was initially tested in 67 patients with severe HF (NYHA class III) resulting from chronic LV systolic dysfunction.27 Investigators in this study enrolled patients with a QRS interval >150 ms. The mean distance walked, quality of life score, and peak oxygen uptake all significantly improved in the patients with active biventricular pacing.27 These very encouraging results led to the first large, prospective, double-blind study of CRT in patients with moderate-to-severe HF (NYHA class III or IV with a LVEF ≤35%) and a prolonged QRS interval (≥130 ms). The landmark MIRACLE study28 demonstrated that patients who received CRT (with the device set to deliver pacing therapy) experienced significant improvements in the distance walked in 6 min, NYHA functional class, quality of life, time on the treadmill during exercise testing, peak maximal oxygen uptake, and cardiac structure and function, compared with control patients (who received a biventricular pacemaker that was not programmed to deliver pacing therapy) over the course of 6 months.28–30 This study led to the approval of the first CRT device in the USA and laid the groundwork for further investigation into the utility of CRT as adjunctive therapy for advanced systolic HF.
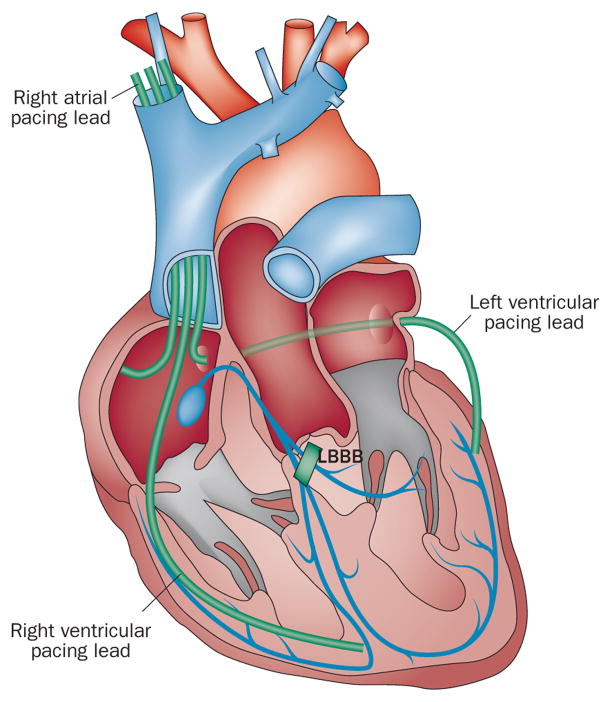
Figure 1.
CRT lead placement. A standard CRT system consists of a right atrial lead, a right ventricular lead (in CRT pacemaker systems) or a right ventricular defibrillation lead (in CRT defibrillator systems), and a left ventricular lead. The left ventricular lead is placed in a tributary of the coronary sinus on the left lateral or posterolateral wall. Abbreviations: CRT, cardiac resynchronization therapy; LBBB, left bundle branch block. Reprinted from Lancet 378 (9792), Holzmeister, J. & Leclercq, C. Implantable cardioverter defibrillators and cardiac resynchronisation therapy, 722–730 © (2011), with permission from Elsevier.
Evolution of CRT: severe HF
The treatment of severe LV dysfunction rapidly gained interest, and novel therapies for advanced HF, such as implantable cardioverter–defibrillators (ICDs), began to be explored. In MADIT II,31 ICDs were shown to reduce mortality in patients with a history of myocardial infarction and severe LV dysfunction. Therefore, MIRACLE ICD32 was designed to test whether combined ICD therapy and CRT was safe in patients with advanced, symptomatic HF. CRT improved the quality of life, exercise capacity, and functional status of patients without being proarrhythmic. However, not until the COMPANION study33 was the combination of an ICD and CRT shown to reduce all-cause mortality compared with medical therapy in patients with severe, symptomatic HF. These finding were instrumental in advancing the role of CRT for the treatment of severe HF, but the results of the CARE-HF trial34 showed definitively that CRT with optimal medical therapy, but without an ICD, was superior to medical therapy alone in decreasing morbidity and mortality (36% reduction in the risk of death with CRT versus medical therapy alone). Additionally, the number needed to treat to reduce the combined end point was six patients, which suggests that CRT is also a cost-effective strategy in the management of HF.34
The culmination of data from the aforementioned studies in severe HF led to the initial criteria for CRT therapy: NYHA class III/IV, a QRS interval ≥130 ms, and a LVEF ≤35% while receiving optimal medical therapy.35 Subsequent studies showed acute and persistent (>6 months) haemodynamic benefits—improved systemic blood pressure, central venous pressure, pulmonary artery pressure, pulmonary capillary wedge pressure, ventricular–arterial coupling, mechanical efficiency, and chronotropic responses—of CRT in severe HF.36,37 Even for gravely ill patients in NYHA class IV, CRT is beneficial and reduces morbidity and mortality if individuals are ambulatory and are not inotrope-dependent.38 Additionally, CRT with or without an ICD in patients with severe, advanced HF was associated with marked reductions in all-cause, cardiac, and HF hospitalization rates compared with optimal pharmacological therapy.39
Despite the use of CRT, mortality in patients in NYHA class IV remains exceptionally high (25% and 38% at 1-year and 2-years, respectively).40 CRT should not be withheld if these individuals are ambulatory, because studies have shown that CRT can decrease LV volumes and improve cardiac function in these patients.40 If the disease is advanced, however, a robust improvement in overall functional status should not be expected.41 A systematic review of 14 randomized trials involving a total of 4,420 patients with a LVEF ≤35% and a QRS duration ≥120 ms, who were in NYHA class III–IV and receiving optimal medical therapy, showed that CRT increased LVEF by 3%, enhanced LV remodelling, quality of life, and exercise capacity, and nearly 60% of patients improved by at least one NYHA class.42 Hospitalizations were decreased by 37%, and all-cause mortality was reduced by 22%.42 These data clearly show that CRT is immensely beneficial in patients in NYHA class III–IV. Subsequently, the search began to determine whether CRT was beneficial in mild-to-moderate HF.
CRT in mild-to-moderate HF
The MIRACLE ICD II study43 was the first trial in which researchers exclusively investigated the role of CRT in patients in NYHA class II. The goal was to examine whether CRT limited disease progression and improved exercise performance in patients with mild HF symptoms. Ultimately, the study revealed that CRT did not alter exercise capacity, but did significantly improve LVEF, LV systolic and diastolic volumes (markers of reverse remodelling), and NYHA class.43 A second study in patients in NYHA class II showed similar findings (significant improvements in LVEF and ventricular volumes), and only 8% of patients had progression of HF symptoms.44 These results led to the large-scale REVERSE study,45 which included patients in NYHA class I (17.5%) or class II (82.5%) with a history of HF symptoms. The primary end point was the HF clinical composite response, which scored patients as ‘improved’, ‘unchanged’, or ‘worsened’, and included ventricular volumes and hospitalizations for HF as secondary end points. This pivotal, double-blinded study showed that CRT (with or without an ICD), in combination with optimal medical therapy, reduced the risk of hospitalization for HF and improved ventricular structure and function, but the improvement in the HF clinical composite score did not reach statistical significance.45
MADIT-CRT46 was the first study in which researchers assessed whether CRT reduced the risk of death or HF events in patients with mild HF. The investigators enrolled patients with ischaemic or nonischaemic cardiomyopathy and NYHA class I (14.5%) or class II (85.5%) symptoms to receive either an ICD, or an ICD with CRT (CRT-D). Importantly, this study was not blinded. No significant difference in the overall risk of death was evident between the two groups, but patients who received CRT had a significant reduction in HF events and experienced an improvement in LVEF and ventricular volumes.46 In the CRT-D group, 17.2% of patients reached the primary end point (death from any cause or a nonfatal HF event) compared with 25.3% of participants in the ICD-only arm. The benefit of CRT-D was completely driven by a 41% reduction in hospitalizations.46 This landmark study in the evolution of CRT led the FDA to expand their criteria and approve CRT with devices made by Boston Scientific for patients with LBBB and who were in NYHA class II or ischaemic class I, with a LVEF <30% and a QRS duration >130 ms.47 In a post hoc analysis of MADIT-CRT,48 the presence of LBBB was a strong factor when determining benefit from CRT with an ICD. Patients in NYHA class II had a greater prevalence of LBBB and QRS duration ≥150 ms than patients in NYHA class I, and a meta-analysis suggested that the benefit of CRT with an ICD is limited to those patients with a QRS duration >150 ms.49
RAFT50 differed from the REVERSE trial and MADIT-CRT in that, initially, patients in NYHA class II or III were included (Table 1). However, after data from the CARE-HF trial showed a clear reduction in mortality for patients in NYHA class III, the protocol was revised to include only patients in NYHA class II. Importantly, this CRT study was the first to show a mortality benefit of CRT-D over ICD alone, particularly in patients in NYHA class II.50 In all previous studies showing a mortality benefit, CRT had been compared with medical therapy. The primary outcome (death from any cause or hospitalization for HF) in RAFT50 occurred in 40% and 33% of the ICD and CRT-D groups, respectively, with a significant delay in time to occurrence of the primary outcome in the CRT-D group. Overall, 23.5% of the patients died. The 5-year actuarial death rate was lower (28.6% versus 34.6%) and the time to death was longer in the patients receiving CRT-D than in those receiving an ICD. On the basis of these results, 14 patients would need to be treated with CRT-D for 5 years to prevent one death.50 Notably, these benefits were at the expense of an increased rate of procedure-related adverse events. Nevertheless, the results from RAFT and the REVERSE trial resulted in the FDA expanding the indication for particular CRT devices to include patients with mildly symptomatic HF (NYHA class II), with a LVEF ≤30%, LBBB, and a QRS duration ≥130 ms.51 Subsequent studies have bolstered the evidence that CRT has a definitive role in patients with mild (NYHA class II) HF.52–58 Because only 17.5% and 14.5% of patients in the REVERSE trial and MADIT-CRT, respectively, were in NYHA class I, CRT is not universally endorsed for this subset of patients, but the FDA has approved CRT for patients with LBBB in ischaemic class I. Further, definitive evidence is needed that individuals with mild HF truly derive a benefit from CRT.
Table 1.
Cardiac resynchronization therapy in patients with mild HF
| Study | Number of patients | NYHA class | LVEF (%) | QRS duration (ms) | Primary end point | Effect on primary end point |
|---|---|---|---|---|---|---|
| REVERSE45 | 610 | I–II | ≤40% | ≥120 | HF clinical composite score | Not significant |
| MADIT-CRT46 | 1,820 | I–II | ≤30% | ≥130 | Death from any cause or a nonfatal HF event | Significant |
| RAFT50 | 1,798 | II–III | ≤30% | ≥130 | Death from any cause or hospitalization for HF | Significant |
Abbreviations: HF, heart failure; LVEF, left ventricular ejection fraction.
QRS duration and morphology with CRT
QRS prolongation on the surface electrocardiogram is used as a surrogate marker of LV dyssynchrony and is the main criterion for determining whether a patient with HF is eligible for CRT. However, the marker might be an imperfect estimate, and many patients with HF could be deprived of beneficial CRT. In a study involving patients with underlying cardiomyopathy, tissue Doppler imaging showed that dyssynchrony was present in >60% of patients with a narrow QRS interval (<120 ms).59 Small, single-centre, prospective studies have suggested that a clinical benefit (improved symptoms and reversed LV remodelling) of CRT exists in patients with a narrow QRS interval.60–62
The RethinQ study63 was the first multicentre, randomized, controlled trial designed to assess the role of CRT in patients with moderate HF and a narrow QRS. Patients enrolled in this study were in NYHA class III, and had a LVEF ≤35% and a QRS duration <130 ms, although they were then stratified into prespecified subgroups of patients with a QRS interval <120 ms or ≥120 ms. In this study, CRT did not improve peak oxygen consumption (the primary end point) in patients with moderate-to-severe HF.63 Data from two subsequent, small, single-centre studies refute these results and suggest that patients with HF and a narrow QRS duration still receive haemodynamic and symptomatic benefit from CRT.64,65 To date, only 244 patients with a QRS interval <120 ms have been enrolled in studies of CRT (Table 2). A benefit seems to exist in this population, but the severely limited number of patients studied precludes definitive recommendations. Ideally, the results of the ongoing EchoCRT trial66 will clarify the role of CRT in these patients. The EchoCRT study will be the largest randomized, controlled trial by far in which the role of CRT will be examined in patients who are in NYHA class III–IV and have a narrow QRS and a LVEF <35%. Morbidity and mortality in patients with echocardiographic signs of ventricular dyssynchrony will be assessed.
Table 2.
CRT in patients with heart failure and a narrow QRS
| Study | Number of patients with narrow QRS (<120 ms) | Average QRS duration (ms) | NYHA class | End point | Outcomes |
|---|---|---|---|---|---|
| Achilli et al. (2003)60 | 14 | 110.0±10.9 | III–IV | Functional capacity and echocardiographic outcomes | Improvements in NYHA class, LVEF, functional capacity, and reverse remodelling |
| Yu et al. (2006)61 | 51 | 103±13 | III–IV | Clinical parameters and echocardiographic outcomes | Improvements in reverse remodelling, mitral regurgitation, NYHA class, exercise capacity, and LVEF |
| Bleeker et al. (2006)62 | 33 | 110±8 | III–IV | Clinical parameters and echocardiographic outcomes | Improvements in 6-min walking distance, quality of life, LVEF, and reverse remodelling |
| RethinQ (2007)63 | 87 | 107±12 | III | Increase of ≥1 ml/kg/min in peak oxygen consumption during cardiopulmonary exercise | Improvement in NYHA class, but not in peak oxygen consumption, quality-of-life scores, 6-min walking distance, or echocardiographic measures |
| Williams et al. (2009)65 | 30 | Not reported (all <120) | III–IV | Short-term haemodynamic improvement in catheterization laboratory | Improvements in cardiac index, left ventricular stroke work, dP/dtmax, with increase in left ventricular filling |
| RESPOND (2011)64 | 29 | 91.5±10.6 | III–IV | Change in 6-min walking distance | Improvement in 6-min walking distance, NYHA class, quality of life, and a composite clinical score |
Abbreviations: CRT, cardiac resynchronization therapy; dP/dtmax, maximum rate of change in left ventricular pressure over time; LVEF, left ventricular ejection fraction.
The data on QRS duration are not conclusive; however, the effect of QRS morphology on CRT outcome is clearer. Patients with LBBB have been shown to benefit more from CRT than those with right bundle branch block—whether from relief of HF symptoms, improved quality of life, slowed HF progression, or reduced risk of ventricular tachyarrhythmias—whereas right bundle branch block has been shown to be a marker of worse outcomes and a poorer response to CRT.48,67–69 The 2011 guidelines from the Heart Failure Society of America state that CRT is recommended for patients in sinus rhythm with a widened QRS interval (≥150 ms) not resulting from right bundle branch block, who have severe LV systolic dysfunction and persistent NYHA functional class II–III symptoms despite optimal medical therapy (Table 3).70
Table 3.
2011 update of CRT guidelines from the Heart Failure Society of America70
| Recommendation | QRS duration (ms) | NYHA class | LVEF | Strength of evidence |
|---|---|---|---|---|
| CRT is recommended for patients in sinus rhythm with a widened QRS interval that is not a result of right bundle branch block, who have severe LV systolic dysfunction and persistent, mild-to-moderate HF despite optimal medical therapy | ≥150 | II–III | ≤35% | A |
| CRT can be considered for ambulatory, severely symptomatic patients with HF and a widened QRS interval and LV systolic dysfunction despite optimal medical therapy | ≥150 | IV | ≤35% | B |
| CRT can be considered for patients with a widened QRS interval and severe LV systolic dysfunction who have persistent, mild-to-severe HF despite optimal medical therapy | ≥120 to <150 | II–IV | ≤35% | B |
| CRT can be considered for patients with atrial fibrillation with a widened QRS interval and severe LV systolic dysfunction who have persistent, mild-to-moderate HF despite optimal medical therapy | ≥120 | II–III | ≤35% | B |
| In patients with a reduced LVEF who require chronic pacing and in whom frequent ventricular pacing is expected, CRT can be considered | No comment | No comment | No comment | C |
Abbreviations: CRT, cardiac resynchronization therapy; HF, heart failure; LV, left ventricular; LVEF, left ventricular ejection fraction. Adapted from J. Card. Fail. 12 (2), Stevenson, W. G. et al. Indications for cardiac resynchronization therapy: 2011 update from the Heart Failure Society of American Guideline Committee, 94–106 © (2012), with permission from Elsevier.
Impact of CRT on mitral regurgitation
Mitral regurgitation can be present in up to 90% of individuals with HF.71 Causes of primary mitral regurgitation include myxomatous degeneration, rheumatic disease, endocarditis, fibroelastic degeneration and, in rare instances, systemic disease processes.72 By contrast, functional mitral regurgitation in HF usually results from abnormal LV geometry because of remodelling, which induces an imbalance between the closing and the tethering forces that act on the mitral valve leaflets.73–75 The results of several studies suggest that CRT has the potential to reduce functional mitral regurgitation in patients with HF.76–79 The mechanism can be either improved papillary muscle synchronization, or an acute increase in the transmitral pressure gradients (mediated by an increased maximal rate of rise in LV systolic pressure caused by improved coordination of LV contraction, which can facilitate effective mitral valve closure).76–79
An observational study showed that an improvement in the grade of mitral regurgitation with CRT did not have an impact on event-free survival.80 A subsequent, single-centre, prospective study showed that CRT can cause an early reversal (within days of initiation) of functional mitral regurgitation, and that the improvement is most profound in patients with moderate-to-severe functional mitral regurgitation at baseline. Additionally, the improvement in severe functional mitral regurgitation was associated with reverse LV remodelling and increased survival.81 These results are the most- convincing to date that CRT has a marked impact on mitral regurgitation and can improve outcomes. The data need to be replicated in a randomized, multicentre trial, but CRT could be considered as a potential treatment option for functional mitral regurgitation.
Molecular benefits of CRT
CRT clearly reduces morbidity and mortality in patients with HF and systolic dysfunction, but additional benefits from this therapy occur at the cellular and molecular levels. Several studies strongly suggest that CRT improves myocardial oxidative metabolism and efficiency, and restores homogeneous myocardial glucose metabolism.82–85 CRT, therefore, is likely to improve ventricular function without increasing global LV oxidative metabolism, thereby improving LV efficiency. Ukkonen and colleagues postulated that successful resynchronization is indicated by increased oxidative metabolism of the interventricular septum relative to the lateral wall, which reflects enhanced work of the septum.82 Additionally, CRT has been shown to restore the balance between collagen type I synthesis and degradation (increased collagen production is a marker of increased fibrosis, which portends worse outcomes). This change, and the reduction in interstitial remodelling with CRT, might determine the overall response to therapy of patients with HF.86–92 Likewise, changes in myocardial gene expression have been described in patients with chronic HF receiving CRT, including an upregulation of β1-adrenoreceptors, myosin-6, and sarcoplasmic/endoplasmic reticulum calcium ATPase 2α, which suggests that CRT induces reverse remodelling at the molecular level.93–95 The benefit of upregulating these contractile proteins can be seen in the anterior wall of the myocardium away from the pacing site of the LV lateral wall, which supports the concept of a global benefit of CRT at the molecular level.96
Optimizing the response to CRT
The main challenge with the implementation of CRT in advanced HF is that 20–30% of patients have either a suboptimal or no response to CRT, despite meeting the clinical indications for therapy.18,28,32,97 Advanced echocardiographic measures have been explored as a possible means of identifying patients who will respond to CRT, and an important study using tissue Doppler imaging described the presence of increased LV dyssynchrony at baseline as a marker of individuals likely to respond to CRT and have a good overall prognosis.98 The main problem with wide-scale implementation of tissue Doppler imaging and echocardiographic techniques to detect dyssynchrony is that many studies show an inability to predict a positive response, and the sensitivity and specificity of the techniques are not strong enough to justify routine clinical use.63,99
Several baseline factors have been identified that might indicate which patients are ‘super responders’ to CRT (defined as an increase in LVEF ≥14.5%). These markers include female sex, no history of myocardial infarction, QRS duration ≥150 ms, presence of LBBB, BMI <30 kg/m2, and low baseline left atrial volume index.100 Interestingly, CRT was associated with greater reductions in death or HF, with consistent echocardiographic evidence of more reverse cardiac remodelling, in women (25% of the study population) than men in MADIT-CRT.101 Some of the benefit might have been driven by the fact that more women than men had LBBB at baseline.
These data increase the complexity of identifying the patients that will benefit most from CRT. The future role of echocardiography with CRT might be to identify the site of latest activation (the LV segment with the greatest delay in mechanical activation) as a possible ‘sweet spot’ for LV lead implantation to improve the overall response to CRT.102–104 Device optimization, which is focused on how the device is set to resynchronize, also remains challenging, and is likely to improve once our ability to identify responders is enhanced. The best approach might include a comprehensive, echocardiography-guided strategy, but time constraints in clinical practice might make such a scheme too challenging to implement.105 An alternative approach might be to develop an optimization clinic with a standardized protocol to treat nonresponders. Every effort should be made to achieve biventricular pacing as close to 100% of the time as possible, which has been shown to reduce mortality.106,107
Left ventricular assist devices
Despite optimal medical therapy and CRT, many patients deteriorate to end-stage refractory HF (defined as symptoms at rest or on minimal exertion, including profound fatigue, inability to perform most activities of daily living, requirement of repeated or prolonged hospitalizations for intensive management, or evidence of refractory cardiogenic shock).108 Cardiac transplantation is the best treatment option for end-stage HF, but a severe shortage of donor organs exists (only 3,742 heart transplantations were reported worldwide in 2009),109 and many patients are poor candidates for transplantation.
Bridge to transplantation
Mechanical circulatory support for ventricular unloading and assistance has been available in various forms since the 1960s, beginning with the extracorporeal left heart bypass pump, which led to the first implantation of a pneumatic total artificial heart in the mid 1980s.110,111 Eventually, interest shifted towards LVADs with an external power source. From 1989 to 1992, single-centre and multicentre trials established that LVADs could serve as a ‘bridge to transplantation’ by showing a 65% rate of survival to transplantation, compared with a 50% rate in patients receiving medical therapy.112,113 In 1994, the FDA approved the use of an LVAD with an external power source as a bridge to transplantation.114
These early devices were implanted through a median sternotomy with an inflow cannula inserted into the LV apex and an outflow tube anastomosed to the ascending aorta. The pneumatically-driven pumping chamber was placed within the abdominal wall (Figure 2a).115 These devices were set to an automatic mode that created pulsatile flow; the device ejected blood when the pump was 90% full, or when it sensed a decreased rate of filling.116 The aortic valve rarely opens when the heart is being supported by an LVAD, and the left ventricle is truly decompressed. A single drive line containing the electrical cable and the atmospheric air vent was tunnelled transcutaneously from the implanted pump to the exterior. These early devices were powered by two rechargeable batteries that provided 4–6 h of power and were usually worn in a shoulder holster, vest, or belt.117 Importantly, improvements to the design of the HeartMate® 1000 IP LVAD (Thoratec Corporation, Pleasanton, CA, USA), such as textured interior surfaces that formed a pseudointima to eliminate direct contact between device surfaces and blood elements, reduced the rate of thromboembolism.118 With advancements in technology, a transition occurred from pneumatically-driven devices to vented electrical LVADs, in particular the HeartMate® VE, which received FDA approval as a bridge to transplantation in 1998.119
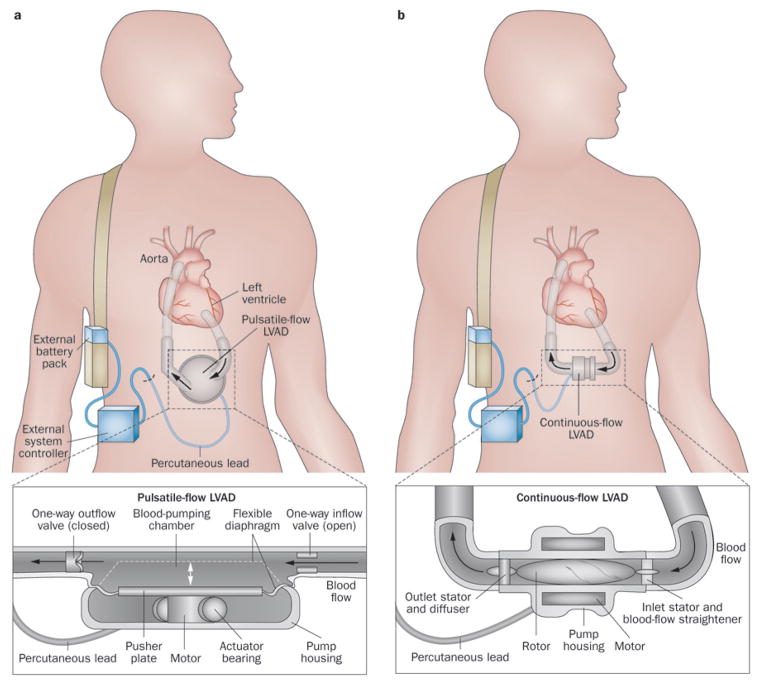
Figure 2.
Designs of LVADs. a | Pulsatile-flow devices use positive displacement pumps to propel blood throughout the body as a healthy ventricle would do. Although pulsatile flow is seemingly more physiological, left ventricular unloading and haemodynamic improvement is comparable to that achieved with continuous-flow pumps. b | Continuous-flow devices use either centrifugal or axial-flow pumps to propel blood continuously throughout the body. These devices are more reliable, have a longer functional life, and operate more quietly than pulsatile devices. Abbreviation: LVAD, left ventricular assist device.
Destination therapy
The use of LVADs in patients deemed unsuitable for transplantation is commonly called ‘destination therapy’. The HeartMate® VE had been approved as suitable for bridge to transplantation, but a randomized, multi-centre trial was conducted to assess the role of LVADs as destination therapy. In May 1998, investigators in REMATCH120 began enrolling extremely morbid patients in NYHA class IV (LVEF <25%, peak oxygen consumption <12 ml/kg/min, or inotrope-dependent) and who were ineligible for cardiac transplantation, to receive a HeartMate® VE LVAD (n = 68) or optimal medical management (n = 61). LVAD placement led to a 48% relative reduction in the risk of death during 30-month follow-up, and a 27% absolute reduction in 1-year mortality.120 Patients with an LVAD also experienced a marked improvement in quality of life; all the individuals with an implanted device who survived to 1 year improved to NYHA class II.120
However, these findings must be put into perspective. These patients were critically ill, and survival was still only 52% and 23% after 1 year and 2 years with LVAD support, respectively.120 Sepsis was the leading cause of death in patients who received an LVAD, most likely because of the external percutaneous drive line; LVAD failure was the second leading cause of death. Furthermore, the neurological event rate with LVAD therapy was not trivial (4.35-fold higher than with medical therapy).120 Nevertheless, REMATCH was a major step forward in the field of mechanical circulatory support, and the FDA approved the HeartMate® VE for destination therapy in 2003.
Unfortunately, long-term follow-up revealed that these devices were not very robust—the 1-year and 2-year freedom from device replacement was 87% and 37%, respectively.121 The HeartMate® XVE model had several enhancements that had the potential to improve device reliability compared with the HeartMate® VE. In particular, the HeartMate® XVE showed a significant reduction in percutaneous lead breaks, and 97% and 82% of patients were free from serious mechanical failures at 6 months and 1 year, respectively—a significant improvement compared with the HeartMate® VE.122 These advances were encouraging, but a need for improved devices for long-term mechanical circulatory support remained.
Continuous-flow LVADs
The concept of continuous-flow LVADs arose from the desire to reduce the size of the pump and to move away from the external venting. Continuous-flow pumps work by an axial mechanism (second-generation pumps with cylindrical rotors and a helical motor, causing the blood to be accelerated and aligned with the rotor’s axis) or a centrifugal mechanism (third-generation pumps whose rotors are shaped to move the blood circumferentially and thereby cause it to move from the centre towards the outer rim of the pump), with the rotor impeller being levitated magnetically in both types of pump (Figure 2b).123 This allowed LVADs to be smaller and simpler because they contained no valves, consumed less power, and had a shaft seal to extend their performance life. Consequently, fewer overall complications were reported than with pulsatile-flow devices.124,125
HeartMate® II axial pump
The HeartMate® II was designed as an axial-flow pump and, after two pivotal trials, was shown to be superior to the pulsatile HeartMate® XVE (significantly improved probability of survival free from stroke and device failure at 2 years; actuarial survival 58% and 24% with the HeartMate® II and the HeartMate® VXE, respectively).126,127 The FDA approved the HeartMate® II for bridge to transplantation in 2008, and for destination therapy in 2010. Consequently, no pulsatile LVADs have been implanted for destination therapy since January 2010.128 The benefits of the HeartMate® II extended up to at least 18 months (72% actuarial survival), and patients had marked improvements in their NYHA functional class and quality of life.129 The incidence of right heart failure with the HeartMate® II was low, and right heart function (in particular the right atrial pressure and right ventricular stroke work index—a surrogate for right ventricular function) might actually improve with this device.130,131 The favourable changes seen in right ventricular function are likely to be the result of the haemodynamic benefits of LV unloading.132 These are all important considerations because right ventricular failure is an important cause of increased morbidity and mortality after LVAD implantation.133
Importantly, both overall quality of life and functional capacity have been shown to improve in patients in NYHA class IV with the HeartMate® II.134 Prospective patient enrolment and data collection in the Interagency Registry For Mechanical Circulatory Support (INTERMACS) began in June 2006, and the Center for Medicaid and Medicare Services mandated that all US hospitals approved for mechanical circulatory support as destination therapy enter patient data into this database.135 The fourth annual report shows that current actuarial survival with continuous-flow pumps exceeds 80% and 70% at 1 year and 2 years, respectively (Table 4).128,136 The report also lists several important risk factors for death after LVAD implantation—advanced age at the time of implantation, large body size, female sex, a high bilirubin level, and elevated right-sided pressures. Notably, patients were at increased risk of death if they presented with critical cardiogenic shock or had a history of CABG surgery, stroke, ascites, pulmonary hypertension, or renal dysfunction (elevated level of blood urea nitrogen, creatinine, or both) at the time of LVAD implantation.128
Table 4.
| Year of implantation | Number of patients | Survival (%)‡ | ||
|---|---|---|---|---|
|
| ||||
| 1 month | 6 months | 12 months | ||
| 2008§ | 458 | 95.8 | 88.3 | 83.4 |
| 2009 | 808 | 93.8 | 87.8 | 82.8 |
| 2010 | 1,445 | 95.1 | 87.0 | 81.4 |
| 2011‖ | 692 | 95.4 | 88.7 | Not available |
With or without a right ventricular assist device.
P = 0.0001.
Two implantations with continuous-flow devices occurred before 2008 (one in 2006 and one in 2007).
Includes January–June 2011.
Abbreviation: LVADs, left ventricular assist devices.
HeartWare® HVAD centrifugal pump
Another continuous-flow LVAD that is being investigated as a possible option for bridge-to-transplantation and destination therapy is the HeartWare® Ventricular Assist System, which includes a centrifugal pump, the HVAD® (HeartWare, Inc., Miami Lakes, FL, USA; Figure 3). This is an innovative system with a hydro-magnetically levitated rotor that is the only moving part, creating a stable, frictionless impeller system that, unlike the axial pumps, has no mechanical bearings. A short inflow cannula integrated into the pump itself allows intrapericardial placement, which avoids abdominal surgery. The device was initially investigated in a bridge-to-transplantation, multicentre, nonrandomized trial in Europe. A total of 23 patients in NYHA class IV who were taking inotropes received the HVAD®.137 After 1 year, 87% of the patients were still alive. The advanced design of the pump allowed for full cardiac support (estimated mean HVAD® pump flow 6.1 ± 1.1 l/min) with low power consumption by the device (mean power consumption 4.8 ± 1.0 W at a mean rotational speed of 2,741 ± 195 rpm). Infections were the most-common adverse event, but of greater concern was that a thrombus was detected in the pump in six of the first 13 patients to receive the device. Follow-up reports stated that this problem had been resolved.137 After 2-year follow-up in Europe, haemodynamic status, quality of life, and neurocognitive function were improved in the majority of patients with the HVAD®.138
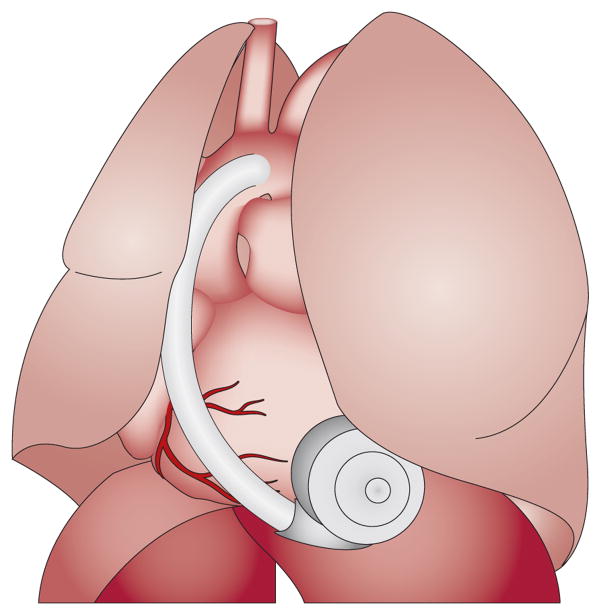
Figure 3.
The positioning of the HVAD® pump (HeartWare, Inc., Miami Lakes, FL, USA) within the pericardial space. Abbreviation: HVAD, HeartWare® Ventricular Assist Device. Reprinted from J. Am. Coll. Cardiol. 57 (12), Strueber, M. et al. Multicenter evaluation of an intrapericardial left ventricular assist system, 1375–1382 © (2011), with permission from Elsevier.
The ADVANCE trial139 was a study conducted in the USA to test the HVAD® as a bridge-to-transplantation device. Patients who received the HVAD® were compared with individuals implanted with commercially available devices (mostly the HeartMate® II). At 1 year, 86% of the 140 enrolled patients were still alive, and the device was shown to be noninferior to established LVADs.139 Bleeding, infections, and perioperative right heart failure were the most-common adverse events, which are typical in patients with all types of LVAD.126,127,129
Haematological issues
Anticoagulation is necessary with all continuous-flow LVADs. Case reports of gastrointestinal bleeding with continuous-flow LVADs have increased awareness of this adverse event.140,141 Subsequent studies strongly indicate that gastrointestinal bleeding is a frequent source of morbidity in patients with the HeartMate® II (incidence 19–40%), but no deaths have been attributed to this adverse effect, and so a negative impact on survival has not been reported.142–145
A possible explanation for the increased incidence of gastrointestinal bleeding is that the HeartMate® II might be associated with impaired platelet aggregation and, therefore, an increased tendency to bleed. Exposure of the blood to high shear stress in the HeartMate® II might cause a qualitative defect in von Willebrand factor, with an increased risk of bleeding even in the 24–48 h postoperative period.146–148 In one study, high-molecular-weight von Willebrand factor multimers were measured in 31 patients with a HeartMate® II and were found to be reduced in all the patients, 58% of whom experienced bleeding.145 The relationship between sheer stress, acquired abnormalities in von Willebrand factor, and arterio–venous malformations has been demonstrated in patients with aortic stenosis, so the same pathological process might occur with the HeartMate® II.149 The bleeding rates might be high with the HeartMate® II from systemic anticoagulation and impaired platelet aggregation, but the thromboembolic rate remains low (2.0–2.5%).150,151
Molecular benefits of LVADs
Patients in NYHA functional class IV have benefited tremendously from LVADs, both as a bridge-to-transplantation and as destination therapy, with reductions in morbidity and mortality, and improved quality of life. Additional benefits at the molecular and cellular levels might accompany LV unloading. Several studies have suggested that LVAD support improves rate-dependent contractility by causing faster decay of the myocyte calcium transient, but this early benefit might not be sustained.152,153 Additionally, LVADs seem to have a favourable impact on the expression and upregulation of genes that improve cardiac function.154–157 Tissue analysis has revealed significant reductions in myocyte size, collagen content, and cardiac tumour necrosis factor with LVAD support.158 From a clinical perspective, LVADs improve end-organ perfusion, and unloading might decrease the number of ICD shocks.159–161
Future directions
CRT and LVADs have ushered in an era of device management of HF. Substantial gains have been made since the introduction of these devices, but further progress is needed in the treatment of HF. In addition to making LVADs smaller,162 other future goals will be to gain an improved understanding of the altered aortic valve biomechanics in patients with an LVAD,163 and to increase the ability to explant the device because several reports describe low rates of device explantation.158,164 Despite the tremendous benefits displayed at the cellular level, explantation of an LVAD has been accomplished in only a small subset of selected patients who are receiving aggressive medical regimens (mostly those with a nonischaemic aetiology or small ventricles before LVAD implantation).165–167
Device-based approaches for the treatment of HF or its comorbidities currently under investigation include cardiac contractility modulation (CCM),168,169 percutaneous ventricular partitioning,170 transvenous phrenic nerve stimulation for central sleep apnoea in HF,171 chronic extra-aortic counterpulsation,172 and a variety of noninvasive and implantable telemonitoring devices.173 Some of these devices will be briefly described, but detailed discussion is beyond the scope of this Review.
Calcium transients and contractions induced by action potentials at 0.5 Hz exhibit phasic and tonic components, known as CCM signals.174 These signals can prolong the action potential and, therefore, increase sarcoplasmic reticulum calcium loading and calcium cycling. Consequently, CCM signal stimulation is a novel mechanism that can be used to enhance myocardial contractility in HF.175 The CCM signal is delivered via a device that resembles a dual-chamber pacemaker (one right atrial and two right ventricular leads are placed transvenously). The device can deliver signals during the absolute refractory period of the cardiac cycle to enhance contractility.176 CCM therapy has been shown to be safe, and a subgroup analysis suggested that patients with a LVEF ≥25% and NYHA class III symptoms might gain the most benefit.168,169
Ventricular partitioning devices divide the dysfunctional and functional portions of the left ventricle in patients with a previous anterior myocardial infarction. The goal is to attenuate or reverse LV remodelling via mechanical reduction, thereby leading to reduced LV volumes and wall stress.170 Preliminary studies suggest that the device is safe and that patients experience an improvement in NYHA class and quality-of-life scores.170
Transvenous phrenic nerve stimulation is being investigated as a possible therapeutic option for patients with HF and concomitant central sleep apnoea. A transvenous lead is placed in the right brachiocephalic vein, left brachiocephalic vein, or left pericardiophrenic vein to stimulate the adjacent phrenic nerve. An implanted pulse generator then provides low-energy nerve stimulation to regulate breathing.171 This therapy has been shown to reduce central sleep apnoea in patients with HF, and is an attractive novel therapy that requires further investigation.
Vagus nerve stimulation is an alternative novel mechanism to treat patients with moderate-to-severe HF. Reduced vagal activity is associated with increased mortality in patients with chronic HF, which makes vagal stimulation an attractive physiological therapy.177 Under anaesthesia, the right vagus is exposed via a surgical incision and an electrode is placed. The implantable vagal neurostimulator system delivers low-current electrical impulses via a pulse stimulation lead. Preliminary studies suggest that implantation is feasible and patients can experience an improvement in their quality of live and overall LV function.178
Conclusions
CRT and LVADs have made a huge impact in the care of patients with chronic HF. Both therapies have decreased morbidity and mortality and, just as importantly, have improved the quality of life for thousands of patients. In our current medical climate, where cost containment and resource utilization are paramount, we must continue to improve upon these therapies to reduce hospitalization and readmission rates in HF. Each therapy also imposes unique challenges that require further research. Optimization of devices and identification of the patients who will benefit most are key areas of current research in CRT. The continued development and miniaturization of devices, and the elimination of external drive lines, are crucial for the long-term durability of LVADs. We are now in an era of mechanical therapy for HF, and the future is promising for all patients affected by this often-devastating condition.
Key points.
Cardiac resynchronization therapy (CRT) has evolved as an effective therapy for many patients with chronic heart failure, especially those with left bundle branch block
CRT device optimization remains challenging, and is an area of intense investigation
Left ventricular assist devices can serve as a bridge to cardiac transplantation or destination therapy for critically ill patients with heart failure, and the use of the latest devices has increased patient survival
Physicians must be aware of various complex issues, including haematological and infectious concerns, when treating patients with chronic heart failure
Several novel, investigational devices for chronic heart failure are on the horizon and hold substantial promise to improve patient outcomes
Review criteria.
The PubMed database was searched for articles using the terms: “chronic heart failure”, “left ventricular assist device”, and “cardiac resynchronization therapy”, combined with “bleeding”, “treatment”, “optimization”, and “management”. We mainly selected full-text, original articles that were written in English and published between 1990 and 2012, and also searched the reference lists of these papers for further leads.
Footnotes
Competing interests W. T. Abraham declares associations with the following companies: Biotronik, Medtronic, and St Jude Medical. See the article online for full details of the relationships. S. A. Smith declares no competing interests.
Author contributions S. A. Smith researched data for the article. Both authors discussed its content, wrote the article, and reviewed and edited the manuscript before submission.
References
- 1.Roger VL, et al. Heart disease and stroke statistics: 2012 update: a report from the American Heart Association. Circulation. 2012;125:e2–e220. doi: 10.1161/CIR.0b013e31823ac046. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Mosterd A, Hoes AW. Clinical epidemiology of heart failure. Heart. 2007;93:1137–1146. doi: 10.1136/hrt.2003.025270. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Lloyd-Jones DM, et al. Lifetime risk for developing congestive heart failure: The Framingham Heart Study. Circulation. 2002;106:3068–3072. doi: 10.1161/01.cir.0000039105.49749.6f. [DOI] [PubMed] [Google Scholar]
- 4.The SOLVD Investigators. Effect of enalapril on mortality and the development of heart failure in asymptomatic patients with reduced left ventricular ejection fractions. N Engl J Med. 1992;327:685–691. doi: 10.1056/NEJM199209033271003. [DOI] [PubMed] [Google Scholar]
- 5.The Heart Outcomes Prevention Evaluation Study Investigators. Effects of an angiotensin-converting-enzyme inhibitor, ramipril, on cardiovascular events in high-risk patients. N Engl J Med. 2000;342:145–153. doi: 10.1056/NEJM200001203420301. [DOI] [PubMed] [Google Scholar]
- 6.CIBIS-II Investigators and Committees. The Cardiac Insufficiency Bisoprolol Study II (CIBIS-II): a randomised trial. Lancet. 1999;353:9–13. [PubMed] [Google Scholar]
- 7.MERIT-HF Study Group. Effect of metoprolol CR/XL in chronic heart failure: Metoprolol CR/XL Randomised Intervention Trial in Congestive Heart Failure (MERIT-HF) Lancet. 1999;353:2001–2007. [PubMed] [Google Scholar]
- 8.Packer M, et al. Effect of carvedilol on survival in severe chronic heart failure. N Engl J Med. 2001;344:1651–1658. doi: 10.1056/NEJM200105313442201. [DOI] [PubMed] [Google Scholar]
- 9.Poole-Wilson P, et al. Comparison of carvedilol and metoprolol on clinical outcomes in patients with chronic heart failure in the Carvedilol Or Metoprolol European Trial (COMET): randomised controlled trial. Lancet. 2003;362:7–13. doi: 10.1016/S0140-6736(03)13800-7. [DOI] [PubMed] [Google Scholar]
- 10.Pitt B, et al. The effect of spironolactone on morbidity and mortality in patients with severe heart failure. N Engl J Med. 1999;341:709–717. doi: 10.1056/NEJM199909023411001. [DOI] [PubMed] [Google Scholar]
- 11.Zannad F, et al. Eplerenone in patients with systolic heart failure and mild symptoms. N Engl J Med. 2011;364:11–21. doi: 10.1056/NEJMoa1009492. [DOI] [PubMed] [Google Scholar]
- 12.Cohn JN, Tognoni G, for the Valsartan Heart Failure Trial Investigators A randomized trial of the angiotensin-receptor blocker valsartan in chronic heart failure. N Engl J Med. 2001;345:1667–1675. doi: 10.1056/NEJMoa010713. [DOI] [PubMed] [Google Scholar]
- 13.McMurray JJV, et al. Effects of candesartan in patients with chronic heart failure and reduced left-ventricular systolic function taking angiotensin-converting-enzyme inhibitors: the CHARM-Added trial. Lancet. 2003;362:767–771. doi: 10.1016/S0140-6736(03)14283-3. [DOI] [PubMed] [Google Scholar]
- 14.Ross JS, et al. Recent national trends in readmission rates after heart failure hospitalization. Circ Heart Fail. 2010;3:97–103. doi: 10.1161/CIRCHEARTFAILURE.109.885210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Chen J, Normand SL, Wang Y, Krumholz HM. National and regional trends in heart failure hospitalization and mortality rates for Medicare beneficiaries, 1998–2008. JAMA. 2011;306:1669–1678. doi: 10.1001/jama.2011.1474. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Jencks SF, Williams MV, Coleman EA. Rehospitalizations among patients in the Medicare fee-for-service program. N Engl J Med. 2009;360:1418–1428. doi: 10.1056/NEJMsa0803563. [DOI] [PubMed] [Google Scholar]
- 17.Levy D, et al. Long-term trends in the incidence of and survival with heart failure. N Engl J Med. 2002;347:1397–1402. doi: 10.1056/NEJMoa020265. [DOI] [PubMed] [Google Scholar]
- 18.Smith SA, Abraham WT. Device therapy in advanced heart failure: what to put in and what to turn off. Remote telemonitoring and implantable hemodynamic devices for advanced heart failure monitoring in the ambulatory setting and the evolving role of cardiac resynchronization therapy. Congest Heart Fail. 2011;17:220–226. doi: 10.1111/j.1751-7133.2011.00238.x. [DOI] [PubMed] [Google Scholar]
- 19.Grines CL, et al. Functional abnormalities in isolated left bundle branch block: the effect of interventricular asynchrony. Circulation. 1989;79:845–853. doi: 10.1161/01.cir.79.4.845. [DOI] [PubMed] [Google Scholar]
- 20.Wilensky RL, et al. Serial electrocardiographic changes in idiopathic dilated cardiomyopathy confirmed at necropsy. Am J Cardiol. 1988;62:276–283. doi: 10.1016/0002-9149(88)90225-1. [DOI] [PubMed] [Google Scholar]
- 21.Shamim W, et al. Intraventricular conduction delay: a prognostic marker in chronic heart failure. Int J Cardiol. 1999;70:171–178. doi: 10.1016/s0167-5273(99)00077-7. [DOI] [PubMed] [Google Scholar]
- 22.Cazeau S, et al. Four chamber pacing in dilated cardiomyopathy. Pacing Clin Electrophysiol. 1994;17:1974–1979. doi: 10.1111/j.1540-8159.1994.tb03783.x. [DOI] [PubMed] [Google Scholar]
- 23.Leclercq C, et al. Acute hemodynamic effects of biventricular DDD pacing in patients with end-stage heart failure. J Am Coll Cardiol. 1998;32:1825–1831. doi: 10.1016/s0735-1097(98)00492-6. [DOI] [PubMed] [Google Scholar]
- 24.Kass DA, et al. Improved left ventricular mechanics from acute VDD pacing in patients with dilated cardiomyopathy and ventricular conduction delay. Circulation. 1999;99:1567–1573. doi: 10.1161/01.cir.99.12.1567. [DOI] [PubMed] [Google Scholar]
- 25.Auricchio A, et al. Effect of pacing chamber and atrioventricular delay on acute systolic function of paced patients with congestive heart failure. Circulation. 1999;99:2993–3001. doi: 10.1161/01.cir.99.23.2993. [DOI] [PubMed] [Google Scholar]
- 26.Daubert JC, et al. Permanent left ventricular pacing with transvenous leads inserted into the coronary veins. Pacing Clin Electrophysiol. 1998;21:239–245. doi: 10.1111/j.1540-8159.1998.tb01096.x. [DOI] [PubMed] [Google Scholar]
- 27.Cazeau S, et al. Effects of multisite biventricular pacing in patients with heart failure and intraventricular conduction delay. N Engl J Med. 2001;344:873–880. doi: 10.1056/NEJM200103223441202. [DOI] [PubMed] [Google Scholar]
- 28.Abraham WT, et al. Cardiac resynchronization in chronic heart failure. N Engl J Med. 2002;346:1845–1853. doi: 10.1056/NEJMoa013168. [DOI] [PubMed] [Google Scholar]
- 29.Yu CM, et al. Left ventricular reverse remodeling but not clinical improvement predicts long-term survival after cardiac resynchronization therapy. Circulation. 2005;112:1580–1586. doi: 10.1161/CIRCULATIONAHA.105.538272. [DOI] [PubMed] [Google Scholar]
- 30.St John Sutton MG, et al. Effect of cardiac resynchronization therapy on left ventricular size and function in chronic heart failure. Circulation. 2003;107:1985–1990. doi: 10.1161/01.CIR.0000065226.24159.E9. [DOI] [PubMed] [Google Scholar]
- 31.Moss AJ, et al. Prophylactic implantation of a defibrillator in patients with myocardial infarction and reduced ejection fraction. N Engl J Med. 2002;346:877–883. doi: 10.1056/NEJMoa013474. [DOI] [PubMed] [Google Scholar]
- 32.Young JB, et al. Combined cardiac resynchronization and implantable cardioversion defibrillation in advanced chronic heart failure: the MIRACLE ICD trial. JAMA. 2003;289:2685–2694. doi: 10.1001/jama.289.20.2685. [DOI] [PubMed] [Google Scholar]
- 33.Bristow MR, et al. Cardiac-resynchronization therapy with or without an implantable defibrillator in advanced chronic heart failure. N Engl J Med. 2004;350:2140–2150. doi: 10.1056/NEJMoa032423. [DOI] [PubMed] [Google Scholar]
- 34.Cleland JG, et al. The effect of cardiac resynchronization on morbidity and mortality in heart failure. N Engl J Med. 2005;352:1539–1549. doi: 10.1056/NEJMoa050496. [DOI] [PubMed] [Google Scholar]
- 35.Vardas PE, et al. Guidelines for cardiac pacing and cardiac resynchronization therapy: the Task Force for Cardiac Pacing and Cardiac Resynchronization Therapy of the European Society of Cardiology: developed in collaboration with the European Heart Rhythm Association. Europace. 2007;9:959–998. doi: 10.1093/europace/eum189. [DOI] [PubMed] [Google Scholar]
- 36.Steendijk P, et al. Hemodynamic effects of long-term cardiac resynchronization therapy: analysis by pressure-volume loops. Circulation. 2006;113:1295–1304. doi: 10.1161/CIRCULATIONAHA.105.540435. [DOI] [PubMed] [Google Scholar]
- 37.Mullens W, et al. Persistent hemodynamic benefits of cardiac resynchronization therapy with disease progression in advanced heart failure. J Am Coll Cardiol. 2009;53:600–607. doi: 10.1016/j.jacc.2008.08.079. [DOI] [PubMed] [Google Scholar]
- 38.Lindenfeld J, et al. Effects of cardiac resynchronization therapy with or without a defibrillator on survival and hospitalizations in patients with New York Heart Association class IV heart failure. Circulation. 2007;115:204–212. doi: 10.1161/CIRCULATIONAHA.106.629261. [DOI] [PubMed] [Google Scholar]
- 39.Anand IS, et al. Cardiac resynchronization therapy reduces the risk of hospitalizations in patients with advanced heart failure: results from the Comparison Of Medical Therapy, Pacing and Defibrillation in Heart Failure (COMPANION) trial. Circulation. 2009;119:969–977. doi: 10.1161/CIRCULATIONAHA.108.793273. [DOI] [PubMed] [Google Scholar]
- 40.van Bommel RJ, et al. Effect of cardiac resynchronization therapy in patients with New York Heart Association functional class IV heart failure. Am J Cardiol. 2010;106:1146–1151. doi: 10.1016/j.amjcard.2010.06.025. [DOI] [PubMed] [Google Scholar]
- 41.Vidal B, et al. Decreased likelihood of response to cardiac resynchronization in patients with severe heart failure. Eur J Heart Fail. 2010;12:283–287. doi: 10.1093/eurjhf/hfq003. [DOI] [PubMed] [Google Scholar]
- 42.McAlister FA, et al. Cardiac resynchronization therapy for patients with left ventricular systolic dysfunction: a systematic review. JAMA. 2007;297:2502–2514. doi: 10.1001/jama.297.22.2502. [DOI] [PubMed] [Google Scholar]
- 43.Abraham WT, et al. Effects of cardiac resynchronization on disease progression in patients with left ventricular systolic dysfunction, an indication for an implantable cardioverter-defibrillator, and mildly symptomatic chronic heart failure. Circulation. 2004;110:2864–2868. doi: 10.1161/01.CIR.0000146336.92331.D1. [DOI] [PubMed] [Google Scholar]
- 44.Bleeker GB, et al. Cardiac resynchronization therapy in patients with systolic left ventricular dysfunction and symptoms of mild heart failure secondary to ischemic or nonischemic cardiomyopathy. Am J Cardiol. 2006;98:230–235. doi: 10.1016/j.amjcard.2006.01.080. [DOI] [PubMed] [Google Scholar]
- 45.Linde C, et al. Randomized trial of cardiac resynchronization in mildly symptomatic heart failure patients and in asymptomatic patients with left ventricular dysfunction and previous heart failure symptoms. J Am Coll Cardiol. 2008;52:1834–1843. doi: 10.1016/j.jacc.2008.08.027. [DOI] [PubMed] [Google Scholar]
- 46.Moss AJ, et al. Cardiac-resynchronization therapy for the prevention of heart-failure events. N Engl J Med. 2009;361:1329–1338. doi: 10.1056/NEJMoa0906431. [DOI] [PubMed] [Google Scholar]
- 47.US Department of Health & Human Sciences: FDA. Summary of safety and effectiveness data (SSED) 2010 [online], http://www.accessdata.fda.gov/cdrh_docs/pdf/P010012S230b.pdf.
- 48.Zareba W, et al. Effectiveness of cardiac resynchronization therapy by QRS morphology in the Multicenter Automatic Defibrillator Implantation Trial—Cardiac Resynchronization Therapy (MADIT-CRT) Circulation. 2011;123:1061–1072. doi: 10.1161/CIRCULATIONAHA.110.960898. [DOI] [PubMed] [Google Scholar]
- 49.Sipahi I, Carrigan TP, Rowland DY, Stambler BS, Fang JC. Impact of QRS duration on clinical event reduction with cardiac resynchronization therapy: meta-analysis of randomized controlled trials. Arch Intern Med. 2011;171:1454–1462. doi: 10.1001/archinternmed.2011.247. [DOI] [PubMed] [Google Scholar]
- 50.Tang AS, et al. Cardiac-resynchronization therapy for mild-to-moderate heart failure. N Engl J Med. 2010;363:2385–2395. doi: 10.1056/NEJMoa1009540. [DOI] [PubMed] [Google Scholar]
- 51.US Department of Health & Human Sciences: FDA. Summary of safety and effectiveness data (SSED) 2012 [online], http://www.accessdata.fda.gov/cdrh_docs/pdf/P010031S232b.pdf.
- 52.Goldenberg I, et al. Reduction of the risk of recurring heart failure events with cardiac resynchronization therapy. J Am Coll Cardiol. 2011;58:729–737. doi: 10.1016/j.jacc.2011.04.024. [DOI] [PubMed] [Google Scholar]
- 53.Barsheshet A, et al. Response to preventive cardiac resynchronization therapy in patients with ischaemic and nonischaemic cardiomyopathy in MADIT-CRT. Eur Heart J. 2011;32:1622–1630. doi: 10.1093/eurheartj/ehq407. [DOI] [PubMed] [Google Scholar]
- 54.Versteeg H, et al. Effect of cardiac resynchronization therapy-defibrillator implantation on health status in patients with mild versus moderate symptoms of heart failure. Am J Cardiol. 2011;108:1155–1159. doi: 10.1016/j.amjcard.2011.06.019. [DOI] [PubMed] [Google Scholar]
- 55.Adabag S, Roukoz H, Anand IS, Moss AJ. Cardiac resynchronization therapy in patients with minimal heart failure. J Am Coll Cardiol. 2011;58:935–941. doi: 10.1016/j.jacc.2011.05.022. [DOI] [PubMed] [Google Scholar]
- 56.Gold MR, Linde C, Abraham WT, Gardiwal A, Daubert JC. The impact of cardiac resynchronization therapy on the incidence of ventricular arrhythmias in mild heart failure. Heart Rhythm. 2011;8:679–684. doi: 10.1016/j.hrthm.2010.12.031. [DOI] [PubMed] [Google Scholar]
- 57.Al-Majed NS, McAlister FA, Bakal JA, Ezekowitz JA. Meta-analysis: cardiac resynchronization therapy for patients with less symptomatic heart failure. Ann Intern Med. 2011;154:401–412. doi: 10.7326/0003-4819-154-6-201103150-00313. [DOI] [PubMed] [Google Scholar]
- 58.Bank AJ, Rischall A, Gage RM, Burns KV, Kubo SH. Comparison of cardiac resynchronization therapy outcomes in patients with New York Heart Association functional class I/II versus III/IV heart failure. J Card Fail. 2012;18:373–378. doi: 10.1016/j.cardfail.2012.01.015. [DOI] [PubMed] [Google Scholar]
- 59.Perry R, De Pasquale CG, Chew DP, Aylward PE, Joseph MX. QRS duration alone misses cardiac dyssynchrony in a substantial proportion of patients with chronic heart failure. J Am Soc Echocardiogr. 2006;19:1257–1263. doi: 10.1016/j.echo.2006.04.040. [DOI] [PubMed] [Google Scholar]
- 60.Achilli A, et al. Long-term effectiveness of cardiac resynchronization therapy in patients with refractory heart failure and “narrow” QRS. J Am Coll Cardiol. 2003;42:2117–2124. doi: 10.1016/j.jacc.2003.08.024. [DOI] [PubMed] [Google Scholar]
- 61.Yu CM, et al. Benefits of cardiac resynchronization therapy for heart failure patients with narrow QRS complexes and coexisting systolic asynchrony by echocardiography. J Am Coll Cardiol. 2006;48:2251–2257. doi: 10.1016/j.jacc.2006.07.054. [DOI] [PubMed] [Google Scholar]
- 62.Bleeker GB, et al. Cardiac resynchronization therapy in patients with a narrow QRS complex. J Am Coll Cardiol. 2006;48:2243–2250. doi: 10.1016/j.jacc.2006.07.067. [DOI] [PubMed] [Google Scholar]
- 63.Beshai JF, et al. Cardiac-resynchronization therapy in heart failure with narrow QRS complexes. N Engl J Med. 2007;357:2461–2471. doi: 10.1056/NEJMoa0706695. [DOI] [PubMed] [Google Scholar]
- 64.Foley PW, et al. Cardiac resynchronisation therapy in patients with heart failure and a normal QRS duration: the RESPOND study. Heart. 2011;97:1041–1047. doi: 10.1136/hrt.2010.208355. [DOI] [PubMed] [Google Scholar]
- 65.Williams LK, et al. Short-term hemodynamic effects of cardiac resynchronization therapy in patients with heart failure, a narrow QRS duration, and no dyssynchrony. Circulation. 2009;120:1687–1694. doi: 10.1161/CIRCULATIONAHA.108.799395. [DOI] [PubMed] [Google Scholar]
- 66.US National Library of Medicine. ClinicalTrials.gov. 2012 [online], http://clinicaltrials.gov/ct2/results?term=NCT00683696.
- 67.Auricchio A, et al. Clinical efficacy of cardiac resynchronization therapy using left ventricular pacing in heart failure patients stratified by severity of ventricular conduction delay. J Am Coll Cardiol. 2003;42:2109–2116. doi: 10.1016/j.jacc.2003.04.003. [DOI] [PubMed] [Google Scholar]
- 68.Byrne MJ, et al. Diminished left ventricular dyssynchrony and impact of resynchronization in failing hearts with right versus left bundle branch block. J Am Coll Cardiol. 2007;50:1484–1490. doi: 10.1016/j.jacc.2007.07.011. [DOI] [PubMed] [Google Scholar]
- 69.Bilchick KC, Kamath S, DiMarco JP, Stukenborg GJ. Bundle-branch block morphology and other predictors of outcome after cardiac resynchronization therapy in Medicare patients. Circulation. 2010;122:2022–2030. doi: 10.1161/CIRCULATIONAHA.110.956011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Stevenson WG, et al. Indications for cardiac resynchronization therapy: 2011 update from the Heart Failure Society of America Guideline Committee. J Card Fail. 2012;18:94–106. doi: 10.1016/j.cardfail.2011.12.004. [DOI] [PubMed] [Google Scholar]
- 71.Patel JB, et al. Mitral regurgitation in patients with advanced systolic heart failure. J Card Fail. 2004;10:285–291. doi: 10.1016/j.cardfail.2003.12.006. [DOI] [PubMed] [Google Scholar]
- 72.Smith SA, Waggoner AD, de las Fuentes L, Davila-Roman VG. Role of serotoninergic pathways in drug-induced valvular heart disease and diagnostic features by echocardiography. J Am Soc Echocardiogr. 2009;22:883–889. doi: 10.1016/j.echo.2009.05.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.Otsuji Y, et al. Insights from three-dimensional echocardiography into the mechanism of functional mitral regurgitation: direct in vivo demonstration of altered leaflet tethering geometry. Circulation. 1997;96:1999–2008. doi: 10.1161/01.cir.96.6.1999. [DOI] [PubMed] [Google Scholar]
- 74.McKay RG, et al. Left ventricular remodeling after myocardial infarction: a corollary to infarct expansion. Circulation. 1986;74:693–702. doi: 10.1161/01.cir.74.4.693. [DOI] [PubMed] [Google Scholar]
- 75.Yiu SF, Enriquez-Sarano M, Tribouilloy C, Seward JB, Tajik AJ. Determinants of the degree of functional mitral regurgitation in patients with systolic left ventricular dysfunction: a quantitative clinical study. Circulation. 2000;102:1400–1406. doi: 10.1161/01.cir.102.12.1400. [DOI] [PubMed] [Google Scholar]
- 76.Breithardt OA, et al. Acute effects of cardiac resynchronization therapy on functional mitral regurgitation in advanced systolic heart failure. J Am Coll Cardiol. 2003;41:765–770. doi: 10.1016/s0735-1097(02)02937-6. [DOI] [PubMed] [Google Scholar]
- 77.Ypenburg C, et al. Mechanism of improvement in mitral regurgitation after cardiac resynchronization therapy. Eur Heart J. 2008;29:757–765. doi: 10.1093/eurheartj/ehn063. [DOI] [PubMed] [Google Scholar]
- 78.Vinereanu D, et al. Mechanisms of reduction of mitral regurgitation by cardiac resynchronization therapy. J Am Soc Echocardiogr. 2007;20:54–62. doi: 10.1016/j.echo.2006.07.002. [DOI] [PubMed] [Google Scholar]
- 79.Ypenburg C, et al. Acute effects of initiation and withdrawal of cardiac resynchronization therapy on papillary muscle dyssynchrony and mitral regurgitation. J Am Coll Cardiol. 2007;50:2071–2077. doi: 10.1016/j.jacc.2007.08.019. [DOI] [PubMed] [Google Scholar]
- 80.Boriani G, et al. Impact of mitral regurgitation on the outcome of patients treated with CRT-D: data from the InSync ICD Italian Registry. Pacing Clin Electrophysiol. 2012;35:146–154. doi: 10.1111/j.1540-8159.2011.03280.x. [DOI] [PubMed] [Google Scholar]
- 81.Verhaert D, et al. Impact of mitral regurgitation on reverse remodeling and outcome in patients undergoing cardiac resynchronization therapy. Circ Cardiovasc Imaging. 2012;5:21–26. doi: 10.1161/CIRCIMAGING.111.966580. [DOI] [PubMed] [Google Scholar]
- 82.Ukkonen H, et al. Effect of cardiac resynchronization on myocardial efficiency and regional oxidative metabolism. Circulation. 2003;107:28–31. doi: 10.1161/01.cir.0000047068.02226.95. [DOI] [PubMed] [Google Scholar]
- 83.Lindner O, et al. Effect of cardiac resynchronization therapy on global and regional oxygen consumption and myocardial blood flow in patients with non-ischaemic and ischaemic cardiomyopathy. Eur Heart J. 2005;26:70–76. doi: 10.1093/eurheartj/ehi046. [DOI] [PubMed] [Google Scholar]
- 84.Lindner O, et al. Global and regional myocardial oxygen consumption and blood flow in severe cardiomyopathy with left bundle branch block. Eur J Heart Fail. 2005;7:225–230. doi: 10.1016/j.ejheart.2004.07.003. [DOI] [PubMed] [Google Scholar]
- 85.Nowak B, et al. Cardiac resynchronization therapy homogenizes myocardial glucose metabolism and perfusion in dilated cardiomyopathy and left bundle branch block. J Am Coll Cardiol. 2003;41:1523–1528. doi: 10.1016/s0735-1097(03)00257-2. [DOI] [PubMed] [Google Scholar]
- 86.Weber KT, Brilla CG. Pathological hypertrophy and cardiac interstitium: fibrosis and renin–angiotensin–aldosterone system. Circulation. 1991;83:1849–1865. doi: 10.1161/01.cir.83.6.1849. [DOI] [PubMed] [Google Scholar]
- 87.Weber KT, et al. Pathologic hypertrophy with fibrosis: the structural basis for myocardial failure. Blood Press. 1992;1:75–85. doi: 10.3109/08037059209077497. [DOI] [PubMed] [Google Scholar]
- 88.Weber KT, et al. Remodeling and reparation of the cardiovascular system. J Am Coll Cardiol. 1992;20:3–16. doi: 10.1016/0735-1097(92)90130-f. [DOI] [PubMed] [Google Scholar]
- 89.D’Ascia C, Cittadini A, Monti MG, Riccio G, Sacca L. Effects of biventricular pacing on interstitial remodelling, tumor necrosis factor-α expression, and apoptotic death in failing human myocardium. Eur Heart J. 2006;27:201–206. doi: 10.1093/eurheartj/ehi579. [DOI] [PubMed] [Google Scholar]
- 90.Umar S, et al. Myocardial collagen metabolism in failing hearts before and during cardiac resynchronization therapy. Eur J Heart Fail. 2008;10:878–883. doi: 10.1016/j.ejheart.2008.06.019. [DOI] [PubMed] [Google Scholar]
- 91.Garcia-Bolao I, et al. Impact of collagen type I turnover on the long-term response to cardiac resynchronization therapy. Eur Heart J. 2008;29:898–906. doi: 10.1093/eurheartj/ehn098. [DOI] [PubMed] [Google Scholar]
- 92.Orrego CM, et al. Cellular evidence of reverse cardiac remodeling induced by cardiac resynchronization therapy. Congest Heart Fail. 2011;17:140–146. doi: 10.1111/j.1751-7133.2011.00227.x. [DOI] [PubMed] [Google Scholar]
- 93.Iyengar S, et al. Effect of cardiac resynchronization therapy on myocardial gene expression in patients with nonischemic dilated cardiomyopathy. J Card Fail. 2007;13:304–311. doi: 10.1016/j.cardfail.2007.01.005. [DOI] [PubMed] [Google Scholar]
- 94.Vanderheyden M, et al. Endomyocardial upregulation of β1 adrenoreceptor gene expression and myocardial contractile reserve following cardiac resynchronization therapy. J Card Fail. 2008;14:172–178. doi: 10.1016/j.cardfail.2007.10.016. [DOI] [PubMed] [Google Scholar]
- 95.Vanderheyden M, et al. Myocardial gene expression in heart failure patients treated with cardiac resynchronization therapy responders versus nonresponders. J Am Coll Cardiol. 2008;51:129–136. doi: 10.1016/j.jacc.2007.07.087. [DOI] [PubMed] [Google Scholar]
- 96.Barth AS, et al. Cardiac resynchronization therapy corrects dyssynchrony-induced regional gene expression changes on a genomic level. Circ Cardiovasc Genet. 2009;2:371–378. doi: 10.1161/CIRCGENETICS.108.832345. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.Higgins SL, et al. Cardiac resynchronization therapy for the treatment of heart failure in patients with intraventricular conduction delay and malignant ventricular tachyarrhythmias. J Am Coll Cardiol. 2003;42:1454–1459. doi: 10.1016/s0735-1097(03)01042-8. [DOI] [PubMed] [Google Scholar]
- 98.Bax JJ, et al. Left ventricular dyssynchrony predicts response and prognosis after cardiac resynchronization therapy. J Am Coll Cardiol. 2004;44:1834–1840. doi: 10.1016/j.jacc.2004.08.016. [DOI] [PubMed] [Google Scholar]
- 99.Chung ES, et al. Results of the predictors of response to CRT (PROSPECT) trial. Circulation. 2008;117:2608–2616. doi: 10.1161/CIRCULATIONAHA.107.743120. [DOI] [PubMed] [Google Scholar]
- 100.Hsu JC, et al. Predictors of super-response to cardiac resynchronization therapy and associated improvement in clinical outcome. J Am Coll Cardiol. 2012;59:2366–2373. doi: 10.1016/j.jacc.2012.01.065. [DOI] [PubMed] [Google Scholar]
- 101.Arshad A, et al. Cardiac resynchronization therapy is more effective in women than in men. J Am Coll Cardiol. 2011;57:813–820. doi: 10.1016/j.jacc.2010.06.061. [DOI] [PubMed] [Google Scholar]
- 102.van Bommel RJ, et al. Site of latest activation in patients eligible for cardiac resynchronization therapy: patterns of dyssynchrony among different QRS configurations and impact of heart failure etiology. Am Heart J. 2011;161:1060–1066. doi: 10.1016/j.ahj.2011.03.014. [DOI] [PubMed] [Google Scholar]
- 103.Delgado V, et al. Relative merits of left ventricular dyssynchrony, left ventricular lead position, and myocardial scar to predict long-term survival of ischemic heart failure patients undergoing cardiac resynchronization therapy. Circulation. 2011;123:70–78. doi: 10.1161/CIRCULATIONAHA.110.945345. [DOI] [PubMed] [Google Scholar]
- 104.Singh JP, et al. Left ventricular lead position and clinical outcome in the multicenter automatic defibrillator implantation trial-cardiac resynchronization therapy (MADIT-CRT) trial. Circulation. 2011;123:1159–1166. doi: 10.1161/CIRCULATIONAHA.110.000646. [DOI] [PubMed] [Google Scholar]
- 105.Taha N, et al. Biventricular pacemaker optimization guided by comprehensive echocardiography—preliminary observations regarding the effects on systolic and diastolic ventricular function and third heart sound. J Am Soc Echocardiogr. 2010;23:857–866. doi: 10.1016/j.echo.2010.04.022. [DOI] [PubMed] [Google Scholar]
- 106.Mullens W, et al. Insights from a cardiac resynchronization optimization clinic as part of a heart failure disease management program. J Am Coll Cardiol. 2009;53:765–773. doi: 10.1016/j.jacc.2008.11.024. [DOI] [PubMed] [Google Scholar]
- 107.Hayes DL, et al. Cardiac resynchronization therapy and the relationship of percent biventricular pacing to symptoms and survival. Heart Rhythm. 2011;8:1469–1475. doi: 10.1016/j.hrthm.2011.04.015. [DOI] [PubMed] [Google Scholar]
- 108.Hunt SA, et al. 2009 focused update incorporated into the ACC/AHA 2005 guidelines for the diagnosis and management of heart failure in adults: a report of the American College of Cardiology Foundation/American Heart Association Task Force on Practice Guidelines: developed in collaboration with the International Society for Heart and Lung Transplantation. Circulation. 2009;119:e391–e479. doi: 10.1161/CIRCULATIONAHA.109.192065. [DOI] [PubMed] [Google Scholar]
- 109.Stehlik J, et al. The Registry of the International Society for Heart and Lung Transplantation: twenty-eighth Adult Heart Transplant Report—2011. J Heart Lung Transplant. 2011;30:1078–1094. doi: 10.1016/j.healun.2011.08.003. [DOI] [PubMed] [Google Scholar]
- 110.Dennis C, et al. Clinical use of a cannula for left heart bypass without thoracotomy: experimental protection against fibrillation by left heart bypass. Ann Surg. 1962;156:623–637. doi: 10.1097/00000658-196210000-00009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 111.DeVries WC, et al. Clinical use of the total artificial heart. N Engl J Med. 1984;310:273–278. doi: 10.1056/NEJM198402023100501. [DOI] [PubMed] [Google Scholar]
- 112.Portner PM, et al. Implantable electrical left ventricular assist system: bridge to transplantation and the future. Ann Thorac Surg. 1989;47:142–150. doi: 10.1016/0003-4975(89)90256-7. [DOI] [PubMed] [Google Scholar]
- 113.Frazier OH, et al. Multicenter clinical evaluation of the HeartMate 1000 IP left ventricular assist device. Ann Thorac Surg. 1992;53:1080–1090. doi: 10.1016/0003-4975(92)90393-i. [DOI] [PubMed] [Google Scholar]
- 114.US Department of Health & Human Sciences: FDA. Medical devices: 1994 PMA approvals. 2010 [online], http://www.fda.gov/MedicalDevices/ProductsandMedicalProcedures/DeviceApprovalsandClearances/PMAApprovals/ucm119050.htm.
- 115.Oz MC, Goldstein DJ, Rose EA. Preperitoneal placement of ventricular assist devices: an illustrated stepwise approach. J Card Surg. 1995;10:288–294. doi: 10.1111/j.1540-8191.1995.tb00613.x. [DOI] [PubMed] [Google Scholar]
- 116.McCarthy PM, Sabik JF. Implantable circulatory support devices as a bridge to heart transplantation. Semin Thorac Cardiovasc Surg. 1994;6:174–180. [PubMed] [Google Scholar]
- 117.Goldstein DJ, Oz MC, Rose EA. Implantable left ventricular assist devices. N Engl J Med. 1998;339:1522–1533. doi: 10.1056/NEJM199811193392107. [DOI] [PubMed] [Google Scholar]
- 118.Slater JP, et al. Low thromboembolic risk without anticoagulation using advanced-design left ventricular assist devices. Ann Thorac Surg. 1996;62:1321–1327. doi: 10.1016/0003-4975(96)00750-3. [DOI] [PubMed] [Google Scholar]
- 119.Frazier OH, et al. Multicenter clinical evaluation of the HeartMate vented electric left ventricular assist system in patients awaiting heart transplantation. J Thorac Cardiovasc Surg. 2001;122:1186–1195. doi: 10.1067/mtc.2001.118274. [DOI] [PubMed] [Google Scholar]
- 120.Rose EA, et al. Long-term use of a left ventricular assist device for end-stage heart failure. N Engl J Med. 2001;345:1435–1443. doi: 10.1056/NEJMoa012175. [DOI] [PubMed] [Google Scholar]
- 121.Dembitsky WP, et al. Left ventricular assist device performance with long-term circulatory support: lessons from the REMATCH trial. Ann Thorac Surg. 2004;78:2123–2129. doi: 10.1016/j.athoracsur.2004.02.030. [DOI] [PubMed] [Google Scholar]
- 122.Dowling RD, et al. HeartMate VE LVAS design enhancements and its impact on device reliability. Eur J Cardiothorac Surg. 2004;25:958–963. doi: 10.1016/j.ejcts.2004.03.003. [DOI] [PubMed] [Google Scholar]
- 123.Caccamo M, Eckman P, John R. Current state of ventricular assist devices. Curr Heart Fail Rep. 2011;8:91–98. doi: 10.1007/s11897-011-0050-z. [DOI] [PubMed] [Google Scholar]
- 124.Asama J, Shinshi T, Hoshi H, Takatani S, Shimokohbe A. A compact highly efficient and low hemolytic centrifugal blood pump with a magnetically levitated impeller. Artif Organs. 2006;30:160–167. doi: 10.1111/j.1525-1594.2006.00202.x. [DOI] [PubMed] [Google Scholar]
- 125.Takatani S. Progress of rotary blood pumps: presidential address, International Society for Rotary Blood Pumps 2006, Leuven, Belgium. Artif Organs. 2007;31:329–344. doi: 10.1111/j.1525-1594.2007.00390.x. [DOI] [PubMed] [Google Scholar]
- 126.Miller LW, et al. Use of a continuous-flow device in patients awaiting heart transplantation. N Engl J Med. 2007;357:885–896. doi: 10.1056/NEJMoa067758. [DOI] [PubMed] [Google Scholar]
- 127.Slaughter MS, et al. Advanced heart failure treated with continuous-flow left ventricular assist device. N Engl J Med. 2009;361:2241–2251. doi: 10.1056/NEJMoa0909938. [DOI] [PubMed] [Google Scholar]
- 128.Kirklin JK, et al. The fourth INTERMACS annual report: 4,000 implants and counting. J Heart Lung Transplant. 2012;31:117–126. doi: 10.1016/j.healun.2011.12.001. [DOI] [PubMed] [Google Scholar]
- 129.Pagani FD, et al. Extended mechanical circulatory support with a continuous-flow rotary left ventricular assist device. J Am Coll Cardiol. 2009;54:312–321. doi: 10.1016/j.jacc.2009.03.055. [DOI] [PubMed] [Google Scholar]
- 130.Kormos RL, et al. Right ventricular failure in patients with the HeartMate II continuous-flow left ventricular assist device: incidence, risk factors, and effect on outcomes. J Thorac Cardiovasc Surg. 2010;139:1316–1324. doi: 10.1016/j.jtcvs.2009.11.020. [DOI] [PubMed] [Google Scholar]
- 131.Lee S, et al. Effects of the HeartMate II continuous-flow left ventricular assist device on right ventricular function. J Heart Lung Transplant. 2010;29:209–215. doi: 10.1016/j.healun.2009.11.599. [DOI] [PubMed] [Google Scholar]
- 132.Klotz S, Naka Y, Oz MC, Burkhoff D. Biventricular assist device-induced right ventricular reverse structural and functional remodeling. J Heart Lung Transplant. 2005;24:1195–1201. doi: 10.1016/j.healun.2004.08.005. [DOI] [PubMed] [Google Scholar]
- 133.Morgan JA, John R, Lee BJ, Oz MC, Naka Y. Is severe right ventricular failure in left ventricular assist device recipients a risk factor for unsuccessful bridging to transplant and post-transplant mortality. Ann Thorac Surg. 2004;77:859–863. doi: 10.1016/j.athoracsur.2003.09.048. [DOI] [PubMed] [Google Scholar]
- 134.Rogers JG, et al. Continuous flow left ventricular assist device improves functional capacity and quality of life of advanced heart failure patients. J Am Coll Cardiol. 2010;55:1826–1834. doi: 10.1016/j.jacc.2009.12.052. [DOI] [PubMed] [Google Scholar]
- 135.Kirklin JK, et al. INTERMACS database for durable devices for circulatory support: first annual report. J Heart Lung Transplant. 2008;27:1065–1072. doi: 10.1016/j.healun.2008.07.021. [DOI] [PubMed] [Google Scholar]
- 136.Kirklin JK, et al. Second INTERMACS annual report: more than 1,000 primary left ventricular assist device implants. J Heart Lung Transplant. 2010;29:1–10. doi: 10.1016/j.healun.2009.10.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 137.Wieselthaler GM, et al. Initial clinical experience with a novel left ventricular assist device with a magnetically levitated rotor in a multi-institutional trial. J Heart Lung Transplant. 2010;29:1218–1225. doi: 10.1016/j.healun.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 138.Strueber M, et al. Multicenter evaluation of an intrapericardial left ventricular assist system. J Am Coll Cardiol. 2011;57:1375–1382. doi: 10.1016/j.jacc.2010.10.040. [DOI] [PubMed] [Google Scholar]
- 139.Aaronson KD, et al. Use of an intrapericardial, continuous-flow, centrifugal pump in patients awaiting heart transplantation. Circulation. 2012;125:3191–3200. doi: 10.1161/CIRCULATIONAHA.111.058412. [DOI] [PubMed] [Google Scholar]
- 140.Letsou GV, et al. Gastrointestinal bleeding from arteriovenous malformations in patients supported by the Jarvik 2000 axial-flow left ventricular assist device. J Heart Lung Transplant. 2005;24:105–109. doi: 10.1016/j.healun.2003.10.018. [DOI] [PubMed] [Google Scholar]
- 141.Hetzer R, et al. First experiences with a novel magnetically suspended axial flow left ventricular assist device. Eur J Cardiothorac Surg. 2004;25:964–970. doi: 10.1016/j.ejcts.2004.02.038. [DOI] [PubMed] [Google Scholar]
- 142.Morgan JA, et al. Gastrointestinal bleeding with the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2012;31:715–718. doi: 10.1016/j.healun.2012.02.015. [DOI] [PubMed] [Google Scholar]
- 143.Stern DR, et al. Increased incidence of gastrointestinal bleeding following implantation of the HeartMate II LVAD. J Card Surg. 2010;25:352–356. doi: 10.1111/j.1540-8191.2010.01025.x. [DOI] [PubMed] [Google Scholar]
- 144.Demirozu ZT, et al. Arteriovenous malformation and gastrointestinal bleeding in patients with the HeartMate II left ventricular assist device. J Heart Lung Transplant. 2011;30:849–853. doi: 10.1016/j.healun.2011.03.008. [DOI] [PubMed] [Google Scholar]
- 145.Uriel N, et al. Acquired von Willebrand syndrome after continuous-flow mechanical device support contributes to a high prevalence of bleeding during long-term support and at the time of transplantation. J Am Coll Cardiol. 2010;56:1207–1213. doi: 10.1016/j.jacc.2010.05.016. [DOI] [PubMed] [Google Scholar]
- 146.Tsai HM, Sussman II, Nagel RL. Shear stress enhances the proteolysis of von Willebrand factor in normal plasma. Blood. 1994;83:2171–2179. [PubMed] [Google Scholar]
- 147.Klovaite J, Gustafsson F, Mortensen SA, Sander K, Nielsen LB. Severely impaired von Willebrand factor-dependent platelet aggregation in patients with a continuous-flow left ventricular assist device (HeartMate II) J Am Coll Cardiol. 2009;53:2162–2167. doi: 10.1016/j.jacc.2009.02.048. [DOI] [PubMed] [Google Scholar]
- 148.Heilmann C, et al. Acquired von Willebrand syndrome is an early-onset problem in ventricular assist device patients. Eur J Cardiothorac Surg. 2011;40:1328–1333. doi: 10.1016/j.ejcts.2011.03.021. [DOI] [PubMed] [Google Scholar]
- 149.Vincentelli A, et al. Acquired von Willebrand syndrome in aortic stenosis. N Engl J Med. 2003;349:343–349. doi: 10.1056/NEJMoa022831. [DOI] [PubMed] [Google Scholar]
- 150.John R, et al. Low thromboembolic risk for patients with the HeartMate II left ventricular assist device. J Thorac Cardiovasc Surg. 2008;136:1318–1323. doi: 10.1016/j.jtcvs.2007.12.077. [DOI] [PubMed] [Google Scholar]
- 151.Boyle AJ, et al. Low thromboembolism and pump thrombosis with the HeartMate II left ventricular assist device: analysis of outpatient anti-coagulation. J Heart Lung Transplant. 2009;28:881–887. doi: 10.1016/j.healun.2009.05.018. [DOI] [PubMed] [Google Scholar]
- 152.Chaudhary KW, et al. Altered myocardial Ca2+ cycling after left ventricular assist device support in the failing human heart. J Am Coll Cardiol. 2004;44:837–845. doi: 10.1016/j.jacc.2004.05.049. [DOI] [PubMed] [Google Scholar]
- 153.Ogletree ML, et al. Duration of left ventricular assist device support: effects on abnormal calcium cycling and functional recovery in the failing human heart. J Heart Lung Transplant. 2010;29:554–561. doi: 10.1016/j.healun.2009.10.015. [DOI] [PubMed] [Google Scholar]
- 154.Heerdt PM, et al. Chronic unloading by left ventricular assist device reverses contractile dysfunction and alters gene expression in end-stage heart failure. Circulation. 2000;102:2713–2719. doi: 10.1161/01.cir.102.22.2713. [DOI] [PubMed] [Google Scholar]
- 155.Hall JL, et al. Clinical, molecular, and genomic changes in response to a left ventricular assist device. J Am Coll Cardiol. 2011;57:641–652. doi: 10.1016/j.jacc.2010.11.010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 156.Blaxall BC, Tschannen-Moran B, Milano CA, Koch WJ. Differential gene expression and genomic patient stratification following left ventricular assist device support. J Am Coll Cardiol. 2003;41:1096–1106. doi: 10.1016/s0735-1097(03)00043-3. [DOI] [PubMed] [Google Scholar]
- 157.Ambardekar AV, Buttrick PM. Reverse remodeling with left ventricular assist devices: a review of clinical, cellular, and molecular effects. Circ Heart Fail. 2011;4:224–233. doi: 10.1161/CIRCHEARTFAILURE.110.959684. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 158.Maybaum S, et al. Cardiac improvement during mechanical circulatory support. Circulation. 2007;115:2497–2505. doi: 10.1161/CIRCULATIONAHA.106.633180. [DOI] [PubMed] [Google Scholar]
- 159.Radovancevic B, et al. End-organ function in patients on long-term circulatory support with continuous- or pulsatile-flow assist devices. J Heart Lung Transplant. 2007;26:815–818. doi: 10.1016/j.healun.2007.05.012. [DOI] [PubMed] [Google Scholar]
- 160.Kamdar F, et al. Effects of centrifugal, axial, and pulsatile left ventricular assist device support on end-organ function in heart failure patients. J Heart Lung Transplant. 2009;28:352–359. doi: 10.1016/j.healun.2009.01.005. [DOI] [PubMed] [Google Scholar]
- 161.Refaat MM, et al. Survival benefit of implantable cardioverter-defibrillators in left ventricular assist device-supported heart failure patients. J Card Fail. 2012;18:140–145. doi: 10.1016/j.cardfail.2011.10.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 162.Slaughter MS, et al. HeartWare miniature axial-flow ventricular assist device: design and initial feasibility test. Tex Heart Inst J. 2009;36:12–16. [PMC free article] [PubMed] [Google Scholar]
- 163.John R, Mantz K, Eckman P, Rose A, May-Newman K. Aortic valve pathophysiology during left ventricular assist device support. J Heart Lung Transplant. 2010;29:1321–1329. doi: 10.1016/j.healun.2010.06.006. [DOI] [PubMed] [Google Scholar]
- 164.Mancini DM, et al. Low incidence of myocardial recovery after left ventricular assist device implantation in patients with chronic heart failure. Circulation. 1998;98:2383–2389. doi: 10.1161/01.cir.98.22.2383. [DOI] [PubMed] [Google Scholar]
- 165.Birks EJ, et al. Left ventricular assist device and drug therapy for the reversal of heart failure. N Engl J Med. 2006;355:1873–1884. doi: 10.1056/NEJMoa053063. [DOI] [PubMed] [Google Scholar]
- 166.Birks EJ, et al. Reversal of severe heart failure with a continuous-flow left ventricular assist device and pharmacological therapy: a prospective study. Circulation. 2011;123:381–390. doi: 10.1161/CIRCULATIONAHA.109.933960. [DOI] [PubMed] [Google Scholar]
- 167.Lamarche Y, et al. Successful weaning and explantation of the HeartMate II left ventricular assist device. Can J Cardiol. 2011;27:358–362. doi: 10.1016/j.cjca.2011.01.005. [DOI] [PubMed] [Google Scholar]
- 168.Kadish A, et al. A randomized controlled trial evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure. Am Heart J. 2011;161:329–337.e1–2. doi: 10.1016/j.ahj.2010.10.025. [DOI] [PubMed] [Google Scholar]
- 169.Abraham WT, et al. Subgroup analysis of a randomized controlled trial evaluating the safety and efficacy of cardiac contractility modulation in advanced heart failure. J Card Fail. 2011;17:710–717. doi: 10.1016/j.cardfail.2011.05.006. [DOI] [PubMed] [Google Scholar]
- 170.Mazzaferri EL, Jr, et al. Percutaneous left ventricular partitioning in patients with chronic heart failure and a prior anterior myocardial infarction: results of the PercutAneous Ventricular RestorAtion in Chronic Heart failUre PaTiEnts Trial. Am Heart J. 2012;163:812–820.e1. doi: 10.1016/j.ahj.2012.02.013. [DOI] [PubMed] [Google Scholar]
- 171.Ponikowski P, et al. Transvenous phrenic nerve stimulation for the treatment of central sleep apnoea in heart failure. Eur Heart J. 2012;33:889–894. doi: 10.1093/eurheartj/ehr298. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 172.Hayward CS, et al. Chronic extra-aortic balloon counterpulsation: first-in-human pilot study in end-stage heart failure. J Heart Lung Transplant. 2010;29:1427–1432. doi: 10.1016/j.healun.2010.06.014. [DOI] [PubMed] [Google Scholar]
- 173.Anker SD, Koehler F, Abraham WT. Telemedicine and remote management of patients with heart failure. Lancet. 2011;378:731–739. doi: 10.1016/S0140-6736(11)61229-4. [DOI] [PubMed] [Google Scholar]
- 174.Dipla K, et al. The sarcoplasmic reticulum and the Na+/Ca2+ exchanger both contribute to the Ca2+ transient of failing human ventricular myocytes. Circ Res. 1999;84:435–444. doi: 10.1161/01.res.84.4.435. [DOI] [PubMed] [Google Scholar]
- 175.Burkoff D, et al. Electric currents applied during the refractory period can modulate cardiac contractility in vitro and in vivo. Heart Fail Rev. 2001;6:27–34. doi: 10.1023/a:1009851107189. [DOI] [PubMed] [Google Scholar]
- 176.Pappone C, et al. First human chronic experience with cardiac contractility modulation by nonexcitatory electrical currents for treating systolic heart failure: mid-term safety and efficacy results from a multicenter study. J Cardiovasc Electrophysiol. 2004;15:418–427. doi: 10.1046/j.1540-8167.2004.03580.x. [DOI] [PubMed] [Google Scholar]
- 177.Mortara A, et al. Arterial baroreflex modulation of heart rate in chronic heart failure: clinical and hemodynamic correlates and prognostic implications. Circulation. 1997;96:3450–3458. doi: 10.1161/01.cir.96.10.3450. [DOI] [PubMed] [Google Scholar]
- 178.De Ferrari GM, et al. Chronic vagus nerve stimulation: a new and promising therapeutic approach for chronic heart failure. Eur Heart J. 2011;32:847–855. doi: 10.1093/eurheartj/ehq391. [DOI] [PubMed] [Google Scholar]