Abstract
We recently showed that B cells reduce CNS inflammation in mice with experimental allergic encephalomyelitis (EAE). Here, we demonstrate that adoptively transferred CD5/CD19+ B cells protect against EAE severity. Furthermore, we show that glatiramer acetate (GA), a therapeutic for relapsing multiple sclerosis treatment, amplifies this effect. Transfer of GA-conditioned B cells leads to increased production of immunoregulatory cytokines and reduced CNS inflammation, as well as decreased expression of the chemokine receptor, CXCR5, and elevated BDNF expression in the CNS. Thus B cells can protect against EAE, and GA augments this effect in maintaining immune homeostasis and controlling EAE disease progression.
Keywords: Glatiramer acetate, Inflammation, B cells, CD19, CD5
1. Introduction
During the inflammatory process in experimental allergic encephalomyelitis (EAE), the mouse model for multiple sclerosis (MS), a T helper 1 (Th1) cell bias is believed to occur in which a variety of cytokines including interferon gamma (IFNγ, interleukin (IL)-12, -23, IL-17, and tumor necrosis factor alpha (TNFα) (Thakker et al., 2007; Begum-Haque et al., 2008) are involved in disease progression, while T helper 2 (Th2) polarization reduces disease activity associated with the secretion of IL-10, IL-4, and transforming growth factor (TGF)-β (Karpus and Swanborg, 1991; Kennedy et al., 1992; Bettelli et al., 1998; Young et al., 2000). The role of B cells in mediating the inflammatory process is less well understood. B cells are not only central to humoral immunity, but also influence immune and inflammatory responses. They regulate CD4+ T cell responses to foreign and self-antigens (Bouaziz et al., 2007; Xiu et al., 2008), function as antigen-presenting cells (Constant et al., 1995; Mann et al., 2007), produce cytokines (Harris et al., 2000; Fillatreau et al., 2002), provide co-stimulatory signals (Linton et al., 2003), and promote naïve CD4+ T cell differentiation into Th1 or Th2 subsets (Harris et al., 2000). B cells also exhibit regulatory activity, as B cell deficient mice develop a severe non-remitting form of EAE (Wolf et al., 1996; Matsushita et al., 2006). Both Phase I and Phase II clinical trials in MS utilizing a chimeric anti-CD20 monoclonal antibody (rituximab) have implicated B cells as a critical component in MS pathogenesis (Bar-Or, 2008; Hauser et al., 2008). The effects of the current FDA approved therapies for MS, interferon beta and glatiramer acetate (GA), on the B cell response are not well understood (Gigli et al., 2007; Wiesemann et al., 2008). Recently published studies suggest that GA can alter B cell responsiveness in MS (Bar-Or et al., 2009).
Most immunological studies focusing on GA implicate T cells as the primary therapeutic target (Arnon and Sela, 2003; Arnon and Aharoni, 2007). GA can amplify Th2 polarization associated with the secretion of IL-4, IL-5, and IL-10 (Aharoni et al., 1997). Moreover, GA-sensitized regulatory CD4+ and CD8+ T cells are induced with increased secretion of anti-inflammatory cytokines (Aharoni et al., 1998). GA can also increase regulatory T cells (Tregs) in EAE mice (Kasper et al., 2007) and enhance differentiation of Tregs in an antigen-independent manner (Weber et al., 2007). Recently, we reported that GA treatment leads to a reduction in IL-17 expression in the CNS and peripheral tissue of mice with EAE (Begum-Haque et al., 2008), and also biases towards anti-inflammatory cytokines production by B cells (Begum-Haque et al., 2010). We describe herein the characteristics associated with B cell regulation in EAE following adoptive transfer of naïve B cells or B cells that had been sensitized with GA. Our findings indicate that transferred B cells can alter EAE disease progression via anti-inflammatory cytokines at the expense of pro-inflammatory cytokines and that GA markedly enhances this effect. Moreover, we show that adoptively transferred B cells resolve EAE by elevating production of brain derived nerve factor (BDNF) and down-regulating the expression of chemokine receptors associated with trafficking of inflammatory cells into the CNS.
2. Materials and methods
2.1. Reagents
GA from batch 28704270 and 147245929 was a generous gift from Teva Pharmaceutical Industries (Petach Tiqva, Israel). Myelin oligodendrocyte glycoprotein (MOG)35-55 peptide was purchased from Peptide International (Louisville, Kentucky, USA), Mycobacterium tuberculosis and Complete Freund’s Adjuvant (CFA) from Sigma-Aldrich (St. Louis, MO, USA), Pertussis Toxin (PT) from Biological Laboratories, Inc (Campbell, CA, USA), and B cell sorting kits from Stem Cell Technologies Inc (Vancouver, BC). Lipopolysaccharides (LPS) were purchased from Sigma-Aldrich, β-actin, primers (STAT6, IL-10, SMAD3, STAT4, CXCR4, CXCR5 and BDNF), and probes were purchased from Invitrogen Life Technologies (Carlsbad, CA, USA). For flow cytometry assays, antibodies against CD19, CD5, CD1d, IL-4, and IL-10 and corresponding isotype controls were obtained from eBiosciences and BioLegend (San Diego, CA, USA).
2.2. Mice and GA conditioning
Female 5–6 weeks old C57BL/6 (Ly5.2, CD45.2, H-2b, abbreviated B6) and B6.SJL-PtprcaPep3b/BoyJ (Ly5.1, CD45.1, H-2b) mice were purchased from Jackson Laboratories (Bar Harbor, Maine), and maintained in pathogen-free conditions. The C57Bl/6 mice had either a CD45.1 or 45.2 polymorphism at the CD45 locus that could be identified by staining with CD45.1 and CD45.2 specific antibodies. For production of GA conditioned CD19+ cells, mice received daily GA (150 μg/mouse) by sub-cutaneous (s.c) injections. Seven days later, spleen and lymph nodes were collected to isolate GA conditioned CD19+ cells. All animal study procedures were approved by the Dartmouth Institutional Animal Care Review Committee.
2.3. Induction of EAE, assessment and GA treatment
EAE was elicited by s.c. immunization (via tail) with 250 μg MOG35-55 emulsified in CFA supplemented with 4 mg/ml of Mycobacterium tuberculosis and injected intraperitoneally on days 0 and 2 with 400 ng PT. Clinical disease usually commences between day 10 and day 12 after immunization. GA treated mice received GA daily (150 μg/mouse by s.c injection) starting at day 0 after MOG injection and continuing until day 23 (experiment’s end). Mice were monitored and scored daily for disease progression.
2.4. Cell sorting, cell tracking, and adoptive transfers
Splenic CD19+ B cells (B6 mice) were purified with magnetic beads (Stem Cell Technologies). The enriched CD19+ cells were sorted using a FACSAria Cell-Sorting System (BD Biosciences) following staining with APC-anti CD19+/FITC-anti CD5 into CD19+CD5−ve and CD19+CD5 +ve cells (Fig. 9, Supplemental data), and were adoptively transferred (day 0) intravenously (i.v) into C57BL/6 mice (CD45.2) prior to being challenged with MOG35-55. Mouse tissues were collected on day 18 to assess expression of cytokines and chemokines by RT-PCR or FACS. All EAE induced mice were examined daily and scored as described previously (Begum-Haque et al., 2008).
To assess the recruitment of donor CD19+ B cells to various organs, 10×106 sorted CD19+CD1d+CD5− cells either from untreated or GA conditioned C57Bl/6 (CD45.2) mice (n=10) were injected i.v. into non-irradiated C57BL/6 (CD45.1) recipient mice (n=4) on day 0 prior to challenge with MOG or control PBS. Sixty hr after cell transfer, lymphocytes were isolated from the major immune organs (the peritoneal exudates, cervical lymph nodes, mesenteric lymph nodes, inguinal lymph nodes, spleen, Peyers patches, and bone marrow) in addition to brains of recipient mice and analyzed for the presence of CD45.2+ donor cells.
2.5. Flow cytometry and intracellular cytokine staining
Single cell suspensions from lymph nodes and spleens were prepared and cells were stained with CD5 (clone 53-7.3, FITC) and CD19 (clone 1D3), CD1d antibodies for 40 min at 4 °C. After washing, cells were fixed in 1% paraformaldehyde. Flow cytometric analysis was performed on FACS calliber (Becton Dickinson) running CellQuest software (BD Biosciences). Intracellular staining for IL-4, IL-10 was performed using fluorochrome labeled anti IL-4 and anti IL-10 antibodies (BD Biosciences). All viable lymphocytes were gated on, and analyzed by flow cytometry. Data analysis was performed using FlowJo software (TreeStar, Inc.).
2.6. Collection and processing of brain, spleen and lymph node samples, and RT-PCR
Mice were perfused, tissues collected, and processed for RNA extraction as described previously (Begum-Haque et al., 2008). Brain tissues, spleen and lymph nodes were processed for RNA extraction and stored at −80 °C. mRNA levels for STAT6, IL-10, SMAD3, STAT4, CXCR4, CXCR5 and BDNF were analyzed by real time PCR (RT-PCR) as described elsewhere (Begum-Haque et al., 2008). Expression was normalized to β-actin as described previously (Minns et al., 2006), and was expressed using the ΔCT method, where relative expression=2−(exp−actin) * 1000.
2.7. Histological analysis
Mice were sacrificed 20 days after EAE induction. Spinal cords were removed and fixed in 10% formalin. Paraffin embedded sections (4 μM) were stained with hematoxylin and eosin (H&E) and Luxol fast blue (LFB). Sections were examined with a BX50 Olympus microscope and images captured with Spotinsight.
2.8. Statistical analysis
Disease incidence and severity was analyzed by the Mann-Whitney U test. Analysis of cytokine production, proliferation assays, and real-time expression between the various treatments groups were done using a two-tailed Student’s t-test. Probability values of P<0.05 or less were considered significant. Statistical significance; GA treatment or GA conditioned cell treatment vs EAE or naïve B cell treatment is indicated in Figures by asterisks (*); naïve cell recipient vs EAE by §.
3. Results
3.1. GA treatment can augment the frequency of both IL-4+ and IL-10+ B cells in EAE mice
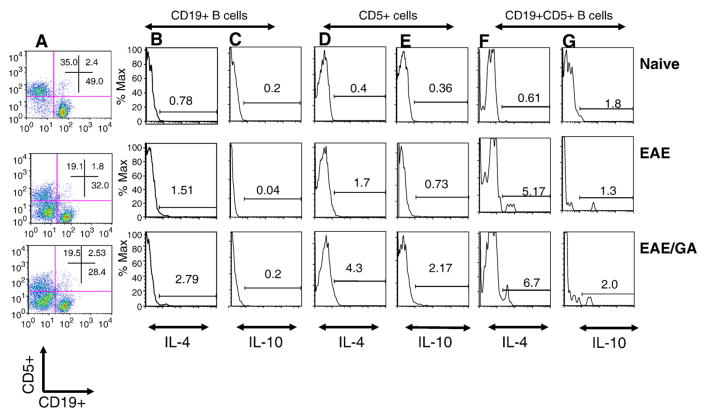
Regulatory IL-10-producing CD1dhiCD5+ B10 cells have been implicated in inhibiting antigen-specific inflammatory reactions (Lund et al., 2005; Yanaba et al., 2008). As we already reported (Begum-Haque et al., 2010), the effects of GA on B cells from mice with EAE (EAE mice) and EAE mice that had been treated with GA (EAE/GA mice) was further determined. Fig. 1A shows the flow (cytometry) distribution of spleen cells expressing CD5 or CD19 or of cells with both markers. Relative to naïve controls, EAE and treatment of EAE with GA resulted in a decline in the percentage of cells that expressed either CD5, CD19, or both markers simultaneously (Fig. 1A). IL-4 secreting CD19+ B cells increased 2-fold in EAE mice (Fig. 1B, middle panel), whereas the expression of IL-4 increased 4-fold in the CD5+ cells population from EAE mice (Fig. 1D, middle panel) relative to naïve animals. Following daily treatment with GA, IL-4+ CD19+ cells increased two-fold, and IL-4+ CD5+ cells increased 2.5 fold (Fig. 1B and D, bottom panels). IL-10 cell frequency dropped 5-fold (P<0.01) in the CD19+ population of EAE mice relative to naïve controls (Fig. 1C, middle panel), whereas GA treatment of EAE restored IL-10+ CD19+ cells to naïve control levels (Fig. 1C, bottom panel). Expression of IL-10 increased 2-fold in CD5 cells from EAE mice relative to naïve controls (Fig. 1E). In response to treatment with GA, IL-10 expression by CD5 cells increased 3-fold more (Fig. 1E, bottom panel). The number of IL-4 expressing CD19+ CD5+ cells increased 1.3-fold in response to GA treatment. These results demonstrate that GA can increase the frequency of both IL-4+ and IL-10+ B cells in EAE mice.
Fig. 1.
Administration of GA to EAE mice increases IL-4 and IL-10 expression by B cells. FACS of spleen and lymph node cells from C57BL/6 mice double-stained with antibodies against CD19+ and CD5+ cells and then intracellular cytokines IL-10 or IL-4. Naïve (n=4): untreated mice; EAE (n=4): EAE induced mice; EAE/GA (n=4): EAE induced mice treated with GA. (A) CD5+ cells (Y-axis) and CD19+ cells (X-axis) from gated lymphocytes; (B–G) Histograms showing the number of cells that express IL-4 (B, D and F) or IL-10 (C, E and G). (B–C) CD19 positive cells that express IL-4 (B) or IL-10 (C). (D–E) CD5 positive cells that express IL-4 (D) or IL-10 (E). (F–G) CD19+ CD5+ cells that express IL-4 (F) or IL-10 (G). Results shown are one representative of 4 independent experiments.
3.2. Adoptive Transfer of CD19+CD5+ Regulatory B cells exposed to GA interferes with EAE disease progression
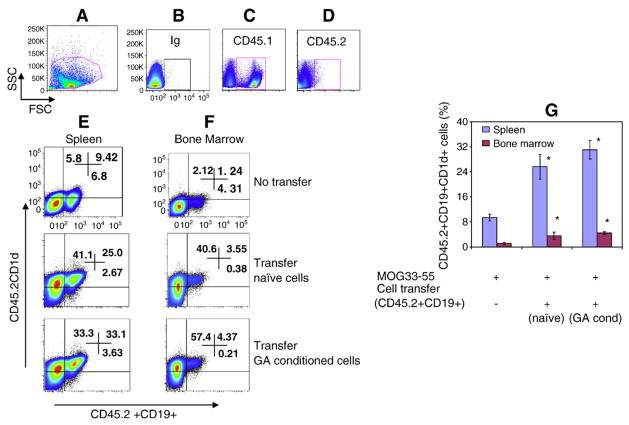
To address whether B cell protection against EAE was transferable between mice, CD19+ B cells were isolated from either naïve animals or animals that were treated daily with GA and then adoptively transferred into C57BL/6, CD45.2+ mice prior to EAE induction. These congenic C57BL/6 mice differ only in the CD45 (leukocyte common antigen) locus, allowing for the discrimination of donor vs. recipient B cells. The distribution of the transferred cells in the recipient animals was assessed sixty hours after cell transfer. Lymphocytes isolated from the major immune organs and brains (Br) were stained for expression of CD45.1, CD45.2, CD19 and CD1d. The distribution of the donor cells was determined by FACS. Fig. 2 shows the comparative levels of viable donor cells in spleen and bone marrow. Regardless of GA treatment, between 25 and 38% of the donor cells were found in the recipient spleen, and less than 5% in the bone marrow. Donor derived CD45.2 cells were distributed in all the major immune organs, with less than 1% of the cells reaching the brain (data not shown).
Fig. 2.
Recruitment of donor B cells to spleen and bone marrow of recipient mice in the presence of EAE. Sixty hours after intravenous injection of 10×106 CD19+ cells, genetically marked with CD45.2, into non-irradiated CD45.1 C57BL/6 mice, spleen and bone marrow of recipient mice were analyzed by FACS for the presence of CD45.2+CD19 cells. Data is presented as the percentage of CD45.2+CD19+ cells. A, representative figure of gating strategy, B, Ig control; C, CD45.1 expression in recipient mouse (Ly5.1, CD45.1, 90%) spleen cells; D, Donor cell (Ly5.2, CD45.2) expression in recipient mouse (Ly5.1, CD45.1, 0.1%) spleen cells; E, recipient mouse spleen cells expressing donor CD45.2+CD19+CD1d+ cells; F, recipient mouse bone marrow cells expressing donor CD45.2+CD19+CD1d+ cells; G, average data (%) from 3 mice/group. Asterisk (*) indicates statistical significance of cell transfer groups vs non-transfer group.
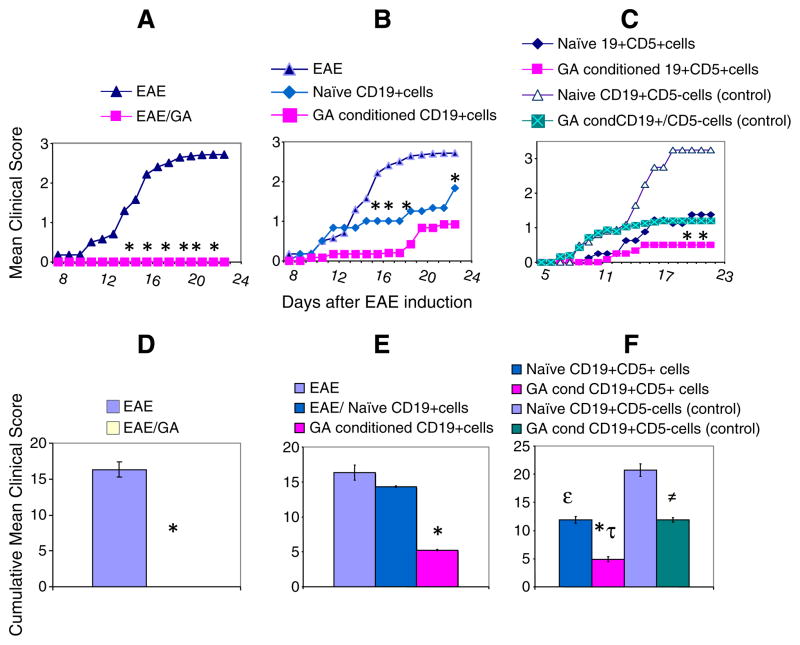
We next examined whether the donor B cells provided protection from EAE clinical symptoms following adoptive cell transfer. Both CD19+ cells (Fig. 3B and E) and CD19+CD5+ cells (Fig. 3C and F), whether isolated from naïve animals or from GA conditioned animals, interfered with EAE disease progression in recipient animals. However, B cells from GA sensitized donor animals with EAE caused a statistically significant (P<0.001) reduction in both the onset and the extent of disease presentation relative to non-treatment (Fig. 3A and D). Transfer of naïve CD19 cells interfered with disease progression as manifested by a limp tail at day 12 post-EAE-induction (Fig. 3B). Adoptive transfer of GA-conditioned CD19 cells delayed presentation of symptoms until day 18, at which time the clinical symptoms were minor (Fig. 3B). Administration of GA-conditioned B cells that co-express CD19 and CD5 resulted in limited disease, manifested by tail limpness at day 14, without further disease progression (P<0.001)(Fig. 3C). In comparison, naïve CD19+CD5+ cell administration reduced disease symptoms only by 23% (Fig. 3D–F). CD19+CD5−ve population, isolated from either naive animals or GA conditioned mice reduced protection. The impact of this was represented by a 57 % reduction in cumulative disease score between the naïve CD19+CD5−ve and GA treated CD19+CD5−ve EAE recipient mice (Fig. 3F). Thus, GA enhances the capacity of CD19+CD5+ B cells to interfere with EAE disease progression upon adoptive transfer. CD5 expressing B cells significantly contribute to the protection, and GA-conditioning of B cells delay disease presentation further.
Fig. 3.
Adoptive transfer of B cells interferes with disease presentation in EAE mice. Clinical scores and cumulative clinical scores of: (A) EAE mice (n=12) that received no treatment (triangles) or were treated with GA (n=12) daily (squares) after MOG immunization; (B) CD19+ cells from naïve (diamonds, n=8) or from GA conditioned mice (squares, n=8) on day 0 before immunization; (C) CD19+CD5+ cells from naïve mice (diamonds, n=8), CD19+CD5− cells from naïve mice (open triangles, n=8); CD19+CD5+ cells from GA conditioned mice (squares, n=8), or CD19+CD5− cells from GA conditioned mice (square X, n=8) administered on day 0 before immunization. Mice were scored according to the following criteria: 0, no disease; 1, decreased tail tone; 2, hind limb weakness or partial paralysis; 3, complete hind limb paralysis; 4, forelimb and hind limb paralysis; 5, moribund state. Significant differences between the means of EAE clinical scores are indicated: *P<0.001 (EAE versus GA treatment); P<0.05 (mice that received CD19+ cells from GA conditioned mice vs. EAE mice); =P<0.01 (EAE mice and mice that received CD19+CD5+ cells from GA conditioned mice); P>0.05 (mice that received CD19+ cells from naive mice vs EAE mice). The lower panels (D–F) depict the cumulative scores of each experimental group: (D) *P<0.0001, EAE-induced GA treated mice vs. EAE-induced mice; (E) *P<0.001, mice that received CD19+ cells (GA conditioned cells vs naïve cells). (F) *P<0.003, mice that received CD19+CD5+ cells (GA conditioned cells vs. naïve cells); ≠P<0.0001, mice that received CD19+CD5− cells (GA conditioned cells vs. naïve cells); *τP<0.004, mice that received GA conditioned CD19+CD5+ cells vs. GA conditioned CD19+CD5− cells; εP<0.0004, mice that received naïve CD19+CD5+ cells vs. CD19+CD5− cells. Cumulative scores were calculated as the sum of all scores from disease onset to day 23 post-induction and divided by the number of mice in each group.
3.3. Reduced inflammation and cell damage is evident in spinal cords of animals following adoptive transfer of B cells
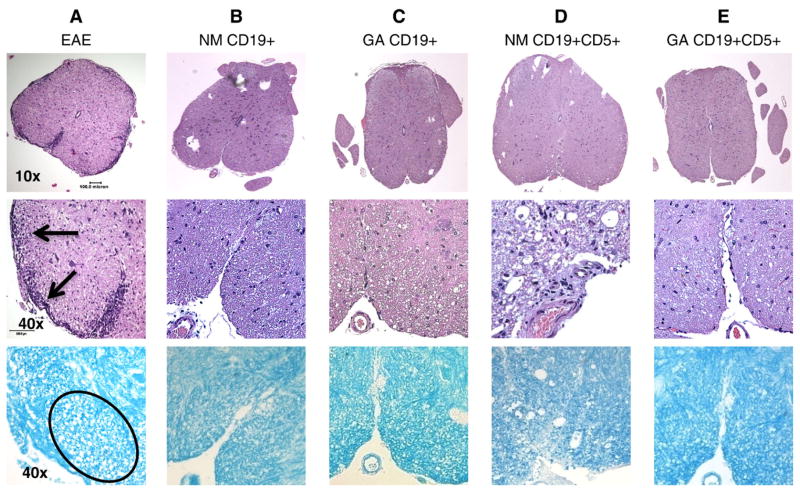
Histological analyses indicated that the number of inflammatory cells in the spinal cords of EAE mice was substantially reduced regardless of the type of B cell transferred. Inflammatory cell numbers decreased when either CD19 positive (Fig. 4B and C, top and bottom panel) or CD5+ CD19 positive (Fig. 4D and E, top and middle panel) cells were transferred. This observation was independent of the origin of the transferred cell, whether from naïve (Fig. 4B and D) or GA conditioned animals (Fig. 4C and E), relative to the untreated EAE animals (Fig. 4A).
Fig. 4.
Minimal inflammation and tissue damage is evident in spinal cords from EAE mice treated with B cells. Representative lumbar spinal cord sections harvested 18 days after MOG immunization and B cell treatment stained with H&E and LFB. Upper panels are 10× magnifications (scale bar: 0.5 mm). Middle and lower panels are 40× magnifications (scale bar: 0.01 mm). Lowest panels are sections stained with LFB to indicate myelin, which appears blue. Spinal cords from: (A) Mouse that developed EAE showing severe infiltration of inflammatory cells and axonal damage; (B) EAE mouse that received CD19+ cells from naïve mice; (C) EAE mice that received CD19+ cells from GA conditioned mice; and (D) EAE mice that received CD19+CD5+ cells from naïve mice. (E) EAE mice that received CD19+CD5+ cells from GA conditioned mice. Similar results were obtained in at least 3 independent experiments.
Of note, GA conditioned cell transfer led to a substantial reduction in cell infiltration and tissue damage (Fig. 4C, E, all 3 panels). Indeed, staining with LFB indicates that the overall integrity of spinal cord myelin was preserved following adoptive cell transfer, despite EAE induction (Fig. 4B–E, bottom panels) relative to untreated mice with EAE (Fig. 4A bottom).
3.4. Adoptive transfer of B cell favors the production of anti-inflammatory cytokines
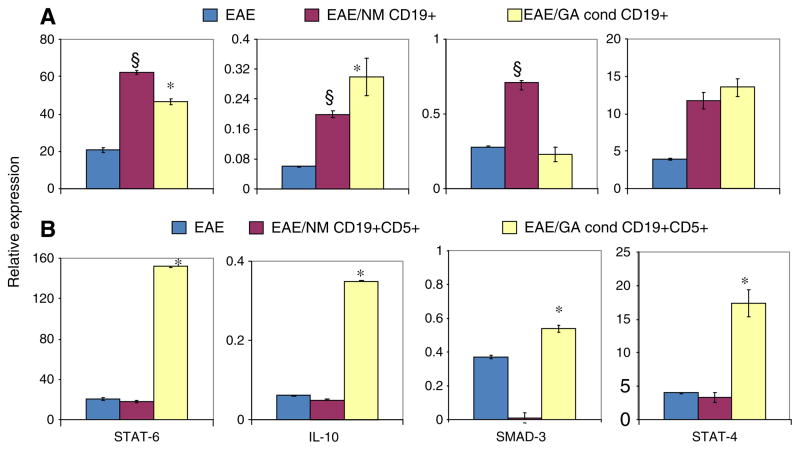
Next we assessed whether the adoptively transferred B cells could impact inflammatory cytokine expression in the recipient EAE mice. The expression of 3 transcription factors associated with immune regulation was measured in spleens of EAE mice that received either CD19+ cells (Fig. 5A) or CD19+CD5+ cells (Fig. 5B) using RT-PCR. STAT-6 is involved in the transduction of Th2 IL-4 and IL-13 intracellular signaling, Smad 3 mediates TGF beta signaling, whereas STAT-4 is involved in Th1 IL-12 expression (Wurster et al., 2000; Wahl, 2007). Following transfer of naïve or GA-conditioned CD19+ cells, STAT-6 levels were several-fold higher than in non-treated animals. However, recipient EAE mice treated with GA conditioned CD5+CD19+ cells had 10-fold higher levels of STAT-6 mRNA, in contrast to animals that received CD5+CD19+ cells from naïve mice that had STAT-6 levels similar to untreated EAE animals. Also consistent with previous studies (Bettelli et al., 1998; Cua et al., 1999; Matsushita et al., 2008), levels of IL-10 were significantly higher following transfer of both CD19 cells and CD19/CD5 positive cells. CD19 cells produced a 3-fold increase, while GA conditioned CD19+ cells produced a 4.5 fold increase relative to controls. IL-10 expression was approximately 10-fold higher following transfer of CD19/CD5+ cells from GA conditioned mice, while transfer of naïve CD19/CD5+ cells had little effect on IL-10 expression in recipient EAE mice. GA conditioned CD19+CD5+ cells and naïve CD19+ cell transfer led to 45–60% (respectively) increased expression of Smad3 in recipient EAE mice relative to untreated EAE mice. The level of STAT-4 was higher in B cell-recipient EAE mice compared with non-treated mice. However, the STAT-4 signal was 6 to 26% (P<0.01) lower than levels of the Th2 signal transducer, STAT-6, suggesting that anti-inflammatory activity is favored in recipient EAE mice. Given the improved clinical presentation of the EAE animals following transfer of B cells relative to the normal progression of EAE in untreated animals, the adoptive transfer of B cells is likely to favor the production of anti-inflammatory cytokines.
Fig. 5.
Adoptive transfer of B cells increases expression of regulatory molecules in recipient EAE mice. STAT-6, IL-10, SMAD3, and STAT-4 mRNA expression in spleen tissue measured by RT-PCR. (A) Relative mRNA levels in EAE mice that received CD19+ cells from naïve or GA conditioned mice on day 0; (B) Relative mRNA levels in EAE mice that received CD5+CD19+ cells from naïve or GA conditioned mice on day 0. Treatment groups: EAE (EAE induced C57BL/6 mice); EAE/NM CD19+ (EAE induced mice that received CD19+ cells from naïve mice); EAE/GA cond CD19+ (EAE induced mice that received CD19+ cells from GA conditioned mice); EAE/NM CD19+CD5+ (EAE induced mice that received CD19+CD5+ cell from naïve mice); EAE/ GA cond CD19+CD5+; (EAE induced mice that received CD19+CD5+ cells from GA conditioned mice). Expressions of indicated molecules are shown relative to levels seen in naïve mice. Data shown is one experiment that represents results of 3 independent experiments and depict the mean of 6 different mice±SEM. Statistical significance of cell treatments: * (P<0.001), GA conditioned cell recipient mice vs. naïve cell recipient mice or EAE mice; §(P<0,001), Naive cell recipient mice vs. EAE mice.
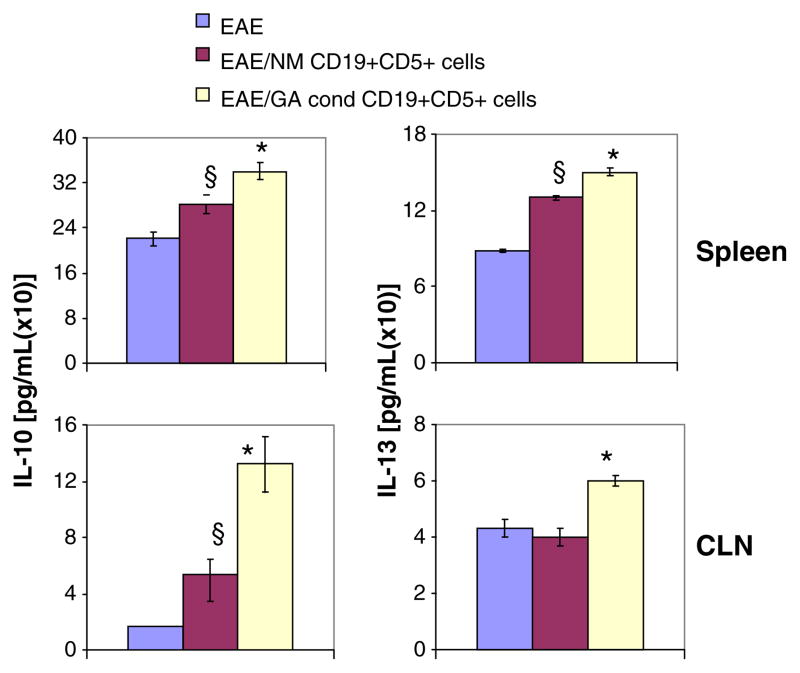
We also determined whether levels of the secreted Th2-type cytokines, IL-10 and IL-13, were affected by adoptive cell transfer. As Fig. 6 shows, IL-10 expression by LPS-stimulated cervical lymph node (CLN) cells was significantly influenced. Transfer of naïve CD19/CD5+ cells induced a 3-fold increase in IL-10 levels secreted by CLN cells from EAE mice, while prior GA exposure led to an 8-fold increase in secreted IL-10 levels relative to untreated EAE mice. In contrast, IL-10 levels secreted by spleen cells were not particularly sensitive to adoptive B cell transfer. Likewise, the effects on IL-13 levels secreted by both spleen and CLN cells were less striking; transferred GA-exposed CD19/CD5+ cells caused a 2 fold increase in IL-13 levels and transferred naïve CD19/CD5+ cells induced a 1.2 fold increase in IL-13 levels secreted by spleen cells.
Fig. 6.
IL-10 and IL-13 production by mice with EAE that received GA-sensitized CD19/CD5+ B cells is increased in a tissue-dependent manner. Total spleen and cervical lymph node (CLN) cells from EAE induced C57BL/6 mice (CD45.2) that were stimulated with LPS (100 ng/ml). Supernatants were collected after 5 hr of culture and IL-10 and IL-13 levels were determined by ELISA. Data are shown as pg/ml and depict the mean of three different experiments±SEM. Asterisks (*P<0.001) indicate statistical significance of GA conditioned CD19+CD5+ cell treatments vs EAE; § (P<0.01), indicates naive cell recipient mice vs. EAE mice. IL-10 and IL-13 levels from naïve mice were below the limit of quantitation. Results are representative of three independent experiments. EAE, EAE induced mice; EAE/NM CD19+CD5+ cells, EAE induced mice that received CD19+CD5+ cells from naïve mice; EAE/GA cond CD19+CD5+ cells, EAE induced mice that received CD19+CD5+ cells from GA conditioned mice.
3.5. Expression of the chemokine receptor, CXCR5, and brain derived neurotrophic factor is modulated to provide neuroprotection following transfer of CD19/CD5+ cells
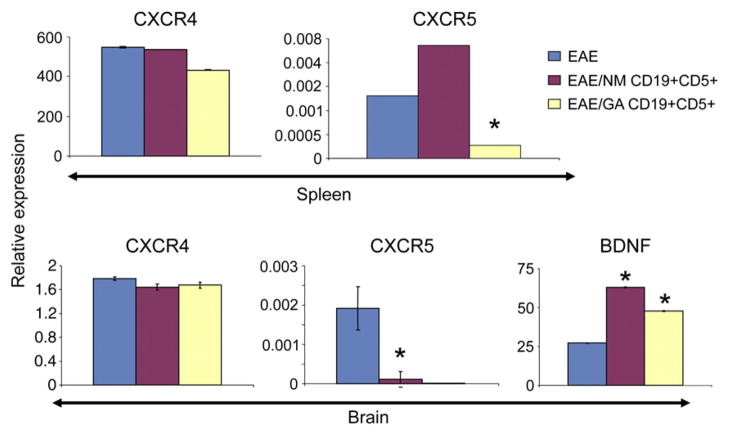
Following adoptive transfer of CD19/CD5+ cells, the CNS perivascular inflammatory foci characteristic of active EAE are not observed (Fig. 4). Since increased expression of chemokines/chemokine receptors is associated with autoimmune diseases (Ishikawa et al., 2001), we hypothesized that the transferred CD19/CD5+ cells might reduce the expression of chemokine receptors involved in leukocyte homing in recipient animals. The expression of the CXC chemokine receptor CXCR 4 and CXCR5 mRNA was compared in EAE mice that received CD19/CD5+ cells either from naïve or GA conditioned mice. In both spleen and brain, the transferred cells had little effect on CXCR4 expression (Fig. 7). In contrast, expression of CXCR5 in animals receiving CD19/CD5+ cells was significantly affected. In the spleen, CXCR5 mRNA levels almost doubled following transfer of CD19/CD5+ cells from naïve mice. There was a striking 5-fold drop in CXCR5 mRNA levels when CD19/CD5+ cells were derived from GA conditioned mice relative to naive CD19/CD5+ cells. The effect of adoptively transferred CD19/CD5+ cells on CXCR5 expression in brain was assessed. CD19/CD5+ cells from naïve animals induced an approx 15-fold drop in brain CXCR5 mRNA levels, whereas prior to GA exposure of the transferred CD19/CD5+ cells derived from GA treated mice resulted in undetectable levels of CXCR5 levels in the CNS.
Fig. 7.
Expression of CXCR5 is down-regulated and BDNF mRNA is up-regulated following transfer of GA conditioned CD19/CD5+ B cells. CXCR4, CXCR5, and BDNF mRNA levels from spleen and brain tissues measured by RT-PCR. EAE (EAE induced mice); EAE/NM CD19+CD5+ (EAE mice that received CD19+CD5+ cells from naïve mice), EAE/GA CD19+CD5+ (EAE mice that received CD19+CD5+ cells from GA conditioned mice). Data are presented as relative mRNA expression. Results are representative of four independent experiments and depict the mean of 6 different mice±SEM. Asterisks (*P<0.001) indicate statistical significance of cell treatments vs. non-treatment.
Brain derived neurotrophic factor (BDNF) has been associated with reduction in the severity of EAE (Teitelbaum et al., 2004), thus we determined whether BDNF levels were affected by the adoptively transferred B cells. BDNF levels increased 2.7 and 2.0-fold, respectively, in brains of EAE animals that received naive CD19/CD5+ cells or cells that had been pre-sensitized with GA (Fig. 7). Thus, the transferred B cells influenced the expression of cytokines, chemokines, and nerve growth factors in recipient animals with EAE.
4. Discussion
Our findings demonstrate that a population of immunomodulatory B cells can be identified that play a protective role against the development of EAE and this population can be amplified by prior exposure to GA, an approved immune modulatory drug used in the treatment of human multiple sclerosis. CD19 expressing B cells or B cells that co-express CD19+ and CD5+ cells conferred significant protection against EAE disease that was manifested at the clinical, histological, and molecular levels. Whereas CD19+CD5− cell population negated the transferable protection. B cells modulated the expression of regulatory cytokines and chemokine receptors in recipient animals. This study demonstrates that phenotypically distinct B cell subsets have the capacity to regulate CNS autoimmune disease progression. Adoptive transfer of IL-10-producing regulatory B cells (CD1dhiCD5+) has recently been demonstrated to reduce EAE pathogenesis (Matsushita et al., 2008). Consistent with that report, we observed a moderate increase in the level of CD1d expression by CD19 positive B cells in response to GA. This raises the possibility that this population might be sufficient to have an inhibitory effect on the EAE disease process (Fig. 8, supplemental data). However, further studies are needed to explore this probability.
We found that sixty hours after cell transfer a small percentage of the transferred B cells had crossed the blood-brain barrier, but the majority homed to the spleen and significantly less to the bone marrow (Fig. 2). Thus, although the bulk of adoptively transferred cells potentially modulated immune activity within the spleen, the few that penetrated the CNS were potentially important in modulating the traffic of immune cells into the brain and the cytokines that they express there. Indeed, adoptive transfer experiments have highlighted some important aspects of immune cell compartmentalization and interaction. For instance, Aharoni et al. (2000) previously showed that adoptively transferred labeled GA-specific Th2 cells cross the blood-brain barrier and accumulated in the CNS 7 days after their injection into the periphery. While Jee et al. (2007) found that adoptive transfer of purified Tregs from GA-treated EAE mice was more effective in preventing EAE development than Tregs from untreated EAE controls. When O’Connor et al. (2008) adoptively transferred pure Th1 cells, pure Th17 cells, or a combination of both, they found that Th1 effectors preferentially infiltrated the non-inflamed CNS to initiate inflammation that modified conditions therein, making it both attractive and accessible to Th17 cells. While Mills et al. (2008) reported that adoptive transfer of CD4+ T cells from CD73−/− mice (that lack a 5′-ectonucleotidase that produces extracellular adenosine) into CD73+/+ T cell-deficient mice revealed that free adenosine is required for penetration of the blood-brain barrier. Thus, adoptive transfer experiments facilitate addressing questions that are otherwise relatively inaccessible.
Of note, B cells derived from GA-conditioned animals conferred protection against EAE disease consistent with that afforded by primary administration of GA to EAE mice. This suggests that immunomodulatory B cells are important and perhaps critical factor in controlling both disease induction and progression (Figs. 3 and 4). Increased expression of the cytokines IL-10 and IL-13, and the Smad3 transcription factor that mediates TGF-β, as well as STAT-6 (Figs. 5 and 6) was associated with this protective effect (Young et al., 2000; Ochoa-Reparaz et al., 2008; Sinha et al., 2008). A number of cell compartments including Th2 polarized CD4+ T cells, Foxp3 expressing CD4+ Tregs and type II monocytes have been implicated in EAE disease control (Bettelli et al., 1998; Jee et al., 2007; Weber et al., 2007; Ochoa-Reparaz et al., 2008). In this study, the lower (6%–26%) amount of STAT-4 expression over STAT-6 expression in the spleens of EAE mice that received conditioned B cells suggests that Th2 derived activity is favored over Th1 cell activities following B cell transfer.
The results of our present study indicate that treatment of EAE mice with CD19+ B cells lead to increased expression of BDNF and decreased expression of CXCR5 (Fig. 7). Foxp3 expressing CD4+ Tregs were found to express BDNF (our unpublished observations) indicating that there may be significant cross-talk between the adoptively transferred B cells and the endogenous Tregs in the recipient animals. Indeed, BDNF has been shown to be associated with regeneration and repair of damaged neural tissue (Hellings et al., 2002; Riley et al., 2004). Makar et al. (2009) recently showed that when cells were engineered to generate biologically active BDNF in the brain, inflammation and apoptosis in animals with EAE was reduced significantly.
There is some disagreement as to whether B cells can act as APCs to facilitate early neuroinflammatory disease. Bouaziz et al. (2008) reported that B cell depletion inhibited antigen-specific CD4 (+) T cell expansion. Also, T cell clonal expansion was reduced and the differentiation of T cells, particularly Th2 cells, into cytokine-secreting effector cells, was impaired when the B cell compartment was deficient in MHC class II (Crawford et al., 2006). In contrast, IL-10 production by B cells has been associated with neuroprotection (Fillatreau et al., 2002; Mann et al., 2007; Matsushita et al., 2008). Cua et al. (1999) reported that IL-10 over-expression prevented the development of MOG-induced EAE. Moreover, IL-10−/− knockout mice could not recover from EAE and displayed increased IFN-alpha production (Bettelli et al., 1998). Importantly, this effect was seen when the IL-10 deficiency was restricted to the B cell compartment (Fillatreau et al., 2002). B cells were found to be necessary not only for IL-10 production but also for the timely emergence of Tregs within the CNS (Mann et al., 2007).
Kala et al. (2010) recently reported that B cells from GA treated mice inhibited expansion of autoreactive MOG35-55-specific T cells and reduced expression of the co-stimulatory molecules, CD80 and CD86, on B cells providing further mechanistic insights into how GA limits neuroinflammatory disease progression In contrast to the results presented here, Kala et al. (2010) reported that adoptive transfer of B cells had little effect on EAE disease progression. The B cells used in their study were isolated by depletion of CD43 cells, which removes non-B lymphoid cells but also includes immature B cells, as well as CD5 positive mature B cells. The difference in the isolation method of B cells in our study and that of Kala et al. (2010) perhaps accounts for the observed differences between the two reports.
Our data suggest that regulatory B cells resolve EAE by biasing cytokine expression towards anti-inflammatory cytokines (Figs. 5 and 6). Transferred B cells also enhance production of BDNF in the afflicted brain (Fig. 7 and data not shown), and down-regulate the expression of chemokine receptors that are associated with trafficking of inflammatory cells into the CNS (Fig. 7). Our findings provide a critical insight into how a subset of B cells impacts progression of autoimmune CNS disease by inhibiting inflammatory reactivity. This regulatory effect is amplified by glatiramer acetate, one of the current FDA approved therapies for MS.
Supplementary Material
Acknowledgments
We thank Dr Jacqueline Channon-Smith (Dartmouth Medical School, Hanover, NH) for helpful discussions, Eric B. York (Dartmouth Hitchcock medical center, Lebanon, NH) for histology slides preparations and Kathy Smith (Dartmouth Medical School, Hanover, NH) for Luminex assay. We also thank Dervla Mellerick, PhD (Science Word Doctor, LLC, Ann Arbor MI) and Pippa Loupe, PhD, (Teva Neuroscience, Kansas City MO) for manuscript assistance. Work was supported by a grant from Teva Pharmaceuticals, LTD, Petah Tiqva, Israel, R01 AI061938 — NIAID and CA1027A1/3 — National Multiple Sclerosis Society. S.B-H. received research funding for this study from Teva Pharmaceuticals, Petah Tiqva, Israel. L.H.K. has received honoraria from Teva Neuroscience, EMD Serono, Bayer Pharmaceuticals, Genzyme, Novartis, Genentech, and Ono Pharmaceuticals.
Abbreviations
- GA
glatiramer acetate
- EAE
experimental allergic encephalomyelitis
- BDNF
brain derived neurotrophic factor
- IL
interleukin
- H&E
hematoxylin and eosin
- PT
Pertussis toxin
- Th1
T helper 1
- Th2
T helper 2
- Tregs
regulatory T cells
Footnotes
Supplementary materials related to this article can be found online at doi:10.1016/j.jneuroim.2010.10.031.
The other authors report no conflicts of interest.
References
- Aharoni R, Teitelbaum D, Leitner O, Meshorer A, Sela M, Arnon R. Specific Th2 cells accumulate in the central nervous system of mice protected against experimental autoimmune encephalomyelitis by copolymer 1. Proc Natl Acad Sci USA. 2000;97:11472–11477. doi: 10.1073/pnas.97.21.11472. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni R, Teitelbaum D, Sela M, Arnon R. Copolymer 1 induces T cells of the T helper type 2 that crossreact with myelin basic protein and suppress experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 1997;94:10821–10826. doi: 10.1073/pnas.94.20.10821. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aharoni R, Teitelbaum D, Sela M, Arnon R. Bystander suppression of experimental autoimmune encephalomyelitis by T cell lines and clones of the Th2 type induced by copolymer 1. J Neuroimmunol. 1998;91:135–146. doi: 10.1016/s0165-5728(98)00166-0. [DOI] [PubMed] [Google Scholar]
- Arnon R, Aharoni R. Neurogenesis and neuroprotection in the CNS—fundamental elements in the effect of Glatiramer acetate on treatment of autoimmune neurological disorders. Mol Neurobiol. 2007;36:245–253. doi: 10.1007/s12035-007-8002-z. [DOI] [PubMed] [Google Scholar]
- Arnon R, Sela M. Immunomodulation by the copolymer glatiramer acetate. J Mol Recognit. 2003;16:412–421. doi: 10.1002/jmr.628. [DOI] [PubMed] [Google Scholar]
- Bar-Or A. The immunology of multiple sclerosis. Semin Neurol. 2008;28:29–45. doi: 10.1055/s-2007-1019124. [DOI] [PubMed] [Google Scholar]
- Bar-Or A, Oger J, Gibbs E, Niino M, Aziz T, Renoux C, Alatab S, Shi FD, Campagnolo D, Jalili F, Rhodes S, Yamashita T, Fan B, Freedman MS, Panitch H, Arnold DL, Vollmer T. Serial combination therapy: is immune modulation in multiple sclerosis enhanced by initial immune suppression? Mult Scler. 2009;15:959–964. doi: 10.1177/1352458509106230. [DOI] [PubMed] [Google Scholar]
- Begum-Haque S, Sharma A, Christy M, Lentini T, Ochoa-Reparaz J, Fayed IF, Mielcarz D, Haque A, Kasper LH. Increased expression of B cell-associated regulatory cytokines by glatiramer acetate in mice with experimental autoimmune encephalomyelitis. J Neuroimmunol. 2010;219:47–53. doi: 10.1016/j.jneuroim.2009.11.016. [DOI] [PubMed] [Google Scholar]
- Begum-Haque S, Sharma A, Kasper IR, Foureau DM, Mielcarz DW, Haque A, Kasper LH. Downregulation of IL-17 and IL-6 in the central nervous system by glatiramer acetate in experimental autoimmune encephalomyelitis. J Neuroimmunol. 2008;204:58–65. doi: 10.1016/j.jneuroim.2008.07.018. [DOI] [PubMed] [Google Scholar]
- Bettelli E, Das MP, Howard ED, Weiner HL, Sobel RA, Kuchroo VK. IL-10 is critical in the regulation of autoimmune encephalomyelitis as demonstrated by studies of IL-10- and IL-4-deficient and transgenic mice. J Immunol. 1998;161:3299–3306. [PubMed] [Google Scholar]
- Bouaziz JD, Yanaba K, Tedder TF. Regulatory B cells as inhibitors of immune responses and inflammation. Immunol Rev. 2008;224:201–214. doi: 10.1111/j.1600-065X.2008.00661.x. [DOI] [PubMed] [Google Scholar]
- Bouaziz JD, Yanaba K, Venturi GM, Wang Y, Tisch RM, Poe JC, Tedder TF. Therapeutic B cell depletion impairs adaptive and autoreactive CD4+ T cell activation in mice. Proc Natl Acad Sci USA. 2007;104:20878–20883. doi: 10.1073/pnas.0709205105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Constant S, Sant’Angelo D, Pasqualini T, Taylor T, Levin D, Flavell R, Bottomly K. Peptide and protein antigens require distinct antigen-presenting cell subsets for the priming of CD4+ T cells. J Immunol. 1995;154:4915–4923. [PubMed] [Google Scholar]
- Crawford A, Macleod M, Schumacher T, Corlett L, Gray D. Primary T cell expansion and differentiation in vivo requires antigen presentation by B cells. J Immunol. 2006;176:3498–3506. doi: 10.4049/jimmunol.176.6.3498. [DOI] [PubMed] [Google Scholar]
- Cua DJ, Groux H, Hinton DR, Stohlman SA, Coffman RL. Transgenic interleukin 10 prevents induction of experimental autoimmune encephalomyelitis. J Exp Med. 1999;189:1005–1010. doi: 10.1084/jem.189.6.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fillatreau S, Sweenie CH, McGeachy MJ, Gray D, Anderton SM. B cells regulate autoimmunity by provision of IL-10. Nat Immunol. 2002;3:944–950. doi: 10.1038/ni833. [DOI] [PubMed] [Google Scholar]
- Gigli G, Caielli S, Cutuli D, Falcone M. Innate immunity modulates autoimmunity: type 1 interferon-beta treatment in multiple sclerosis promotes growth and function of regulatory invariant natural killer T cells through dendritic cell maturation. Immunology. 2007;122:409–417. doi: 10.1111/j.1365-2567.2007.02655.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Harris DP, Haynes L, Sayles PC, Duso DK, Eaton SM, Lepak NM, Johnson LL, Swain SL, Lund FE. Reciprocal regulation of polarized cytokine production by effector B and T cells. Nat Immunol. 2000;1:475–482. doi: 10.1038/82717. [DOI] [PubMed] [Google Scholar]
- Hauser SL, Waubant E, Arnold DL, Vollmer T, Antel J, Fox RJ, Bar-Or A, Panzara M, Sarkar N, Agarwal S, Langer-Gould A, Smith CH. B-cell depletion with rituximab in relapsing–remitting multiple sclerosis. N Engl J Med. 2008;358:676–688. doi: 10.1056/NEJMoa0706383. [DOI] [PubMed] [Google Scholar]
- Hellings N, Raus J, Stinissen P. Insights into the immunopathogenesis of multiple sclerosis. Immunol Res. 2002;25:27–51. doi: 10.1385/IR:25:1:27. [DOI] [PubMed] [Google Scholar]
- Ishikawa S, Sato T, Abe M, Nagai S, Onai N, Yoneyama H, Zhang Y, Suzuki T, Hashimoto S, Shirai T, Lipp M, Matsushima K. Aberrant high expression of B lymphocyte chemokine (BLC/CXCL13) by C11b+CD11c+ dendritic cells in murine lupus and preferential chemotaxis of B1 cells towards BLC. J Exp Med. 2001;193:1393–1402. doi: 10.1084/jem.193.12.1393. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jee Y, Piao WH, Liu R, Bai XF, Rhodes S, Rodebaugh R, Campagnolo DI, Shi FD, Vollmer TL. CD4(+)CD25(+) regulatory T cells contribute to the therapeutic effects of glatiramer acetate in experimental autoimmune encephalomyelitis. Clin Immunol. 2007;125:34–42. doi: 10.1016/j.clim.2007.05.020. [DOI] [PubMed] [Google Scholar]
- Kala M, Rhodes SN, Piao WH, Shi FD, Campagnolo DI, Vollmer TL. B cells from glatiramer acetate-treated mice suppress experimental autoimmune encephalomyelitis. Exp Neurol. 2010;221:136–145. doi: 10.1016/j.expneurol.2009.10.015. [DOI] [PubMed] [Google Scholar]
- Karpus WJ, Swanborg RH. CD4+ suppressor cells inhibit the function of effector cells of experimental autoimmune encephalomyelitis through a mechanism involving transforming growth factor-beta. J Immunol. 1991;146:1163–1168. [PubMed] [Google Scholar]
- Kasper LH, Haque A, Haque S. Regulatory mechanisms of the immune system in multiple sclerosis. Tregulatory cells: turned on to turn off. J Neurol. 2007;245S:10–14. [Google Scholar]
- Kennedy MK, Torrance DS, Picha KS, Mohler KM. Analysis of cytokine mRNA expression in the central nervous system of mice with experimental autoimmune encephalomyelitis reveals that IL-10 mRNA expression correlates with recovery. J Immunol. 1992;149:2496–2505. [PubMed] [Google Scholar]
- Linton PJ, Bautista B, Biederman E, Bradley ES, Harbertson J, Kondrack RM, Padrick RC, Bradley LM. Costimulation via OX40L expressed by B cells is sufficient to determine the extent of primary CD4 cell expansion and Th2 cytokine secretion in vivo. J Exp Med. 2003;197:875–883. doi: 10.1084/jem.20021290. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lund FE, Garvy BA, Randall TD, Harris DP. Regulatory roles for cytokine-producing B cells in infection and autoimmune disease. Curr Dir Autoimmun. 2005;8:25–54. doi: 10.1159/000082086. [DOI] [PubMed] [Google Scholar]
- Makar TK, Bever CT, Singh IS, Royal W, Sahu SN, Sura TP, Sultana S, Sura KT, Patel N, Dhib-Jalbut S, Trisler D. Brain-derived neurotrophic factor gene delivery in an animal model of multiple sclerosis using bone marrow stem cells as a vehicle. J Neuroimmunol. 2009;210:40–51. doi: 10.1016/j.jneuroim.2009.02.017. [DOI] [PubMed] [Google Scholar]
- Mann MK, Maresz K, Shriver LP, Tan Y, Dittel BN. B cell regulation of CD4+CD25+ Tregulatory cells and IL-10 via B7 is essential for recovery from experimental autoimmune encephalomyelitis. J Immunol. 2007;178:3447–3456. doi: 10.4049/jimmunol.178.6.3447. [DOI] [PubMed] [Google Scholar]
- Matsushita T, Fujimoto M, Hasegawa M, Komura K, Takehara K, Tedder TF, Sato S. Inhibitory role of CD19 in the progression of experimental autoimmune encephalomyelitis by regulating cytokine response. Am J Pathol. 2006;168:812–821. doi: 10.2353/ajpath.2006.050923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsushita T, Yanaba K, Bouaziz JD, Fujimoto M, Tedder TF. Regulatory B cells inhibit EAE initiation in mice while other B cells promote disease progression. J Clin Invest. 2008;118:3420–3430. doi: 10.1172/JCI36030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mills JH, Thompson LF, Mueller C, Waickman AT, Jalkanen S, Niemela J, Airas L, Bynoe MS. CD73 is required for efficient entry of lymphocytes into the central nervous system during experimental autoimmune encephalomyelitis. Proc Natl Acad Sci USA. 2008;105:9325–9330. doi: 10.1073/pnas.0711175105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Minns LA, Menard LC, Foureau DM, Darche S, Ronet C, Mielcarz DW, Buzoni-Gatel D, Kasper LH. TLR9 is required for the gut-associated lymphoid tissue response following oral infection of Toxoplasma gondii. J Immunol. 2006;176:7589–7597. doi: 10.4049/jimmunol.176.12.7589. [DOI] [PubMed] [Google Scholar]
- O’Connor RA, Prendergast CT, Sabatos CA, Lau CW, Leech MD, Wraith DC, Anderton SM. Cutting edge: Th1 cells facilitate the entry of Th17 cells to the central nervous system during experimental autoimmune encephalomyelitis. J Immunol. 2008;181:3750–3754. doi: 10.4049/jimmunol.181.6.3750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochoa-Reparaz J, Rynda A, Ascon MA, Yang X, Kochetkova I, Riccardi C, Callis G, Trunkle T, Pascual DW. IL-13 production by regulatory T cells protects against experimental autoimmune encephalomyelitis independently of autoantigen. J Immunol. 2008;181:954–968. doi: 10.4049/jimmunol.181.2.954. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Riley CP, Cope TC, Buck CR. CNS neurotrophins are biologically active and expressed by multiple cell types. J Mol Histol. 2004;35:771–783. doi: 10.1007/s10735-004-0778-9. [DOI] [PubMed] [Google Scholar]
- Sinha S, Kaler LJ, Proctor TM, Teuscher C, Vandenbark AA, Offner H. IL-13-mediated gender difference in susceptibility to autoimmune encephalomyelitis. J Immunol. 2008;180:2679–2685. doi: 10.4049/jimmunol.180.4.2679. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Teitelbaum D, Aharoni R, Klinger E, Kreitman R, Raymond E, Malley A, Shofti R, Sela M, Arnon R. Oral glatiramer acetate in experimental autoimmune encephalomyelitis: clinical and immunological studies. Ann NY Acad Sci. 2004;1029:239–249. doi: 10.1196/annals.1309.055. [DOI] [PubMed] [Google Scholar]
- Thakker P, Leach MW, Kuang W, Benoit SE, Leonard JP, Marusic S. IL-23 is critical in the induction but not in the effector phase of experimental autoimmune encephalomyelitis. J Immunol. 2007;178:2589–2598. doi: 10.4049/jimmunol.178.4.2589. [DOI] [PubMed] [Google Scholar]
- Wahl SM. Transforming growth factor-beta: innately bipolar. Curr Opin Immunol. 2007;19:55–62. doi: 10.1016/j.coi.2006.11.008. [DOI] [PubMed] [Google Scholar]
- Weber MS, Prod’homme T, Youssef S, Dunn SE, Rundle CD, Lee L, Patarroyo JC, Stuve O, Sobel RA, Steinman L, Zamvil SS. Type II monocytes modulate T cell-mediated central nervous system autoimmune disease. Nat Med. 2007;13:935–943. doi: 10.1038/nm1620. [DOI] [PubMed] [Google Scholar]
- Wiesemann E, Deb M, Trebst C, Hemmer B, Stangel M, Windhagen A. Effects of interferon-beta on co-signaling molecules: upregulation of CD40, CD86 and PD-L2 on monocytes in relation to clinical response to interferon-beta treatment in patients with multiple sclerosis. Mult Scler. 2008;14:166–176. doi: 10.1177/1352458507081342. [DOI] [PubMed] [Google Scholar]
- Wolf SD, Dittel BN, Hardardottir F, Janeway CA., Jr Experimental autoimmune encephalomyelitis induction in genetically B cell-deficient mice. J Exp Med. 1996;184:2271–2278. doi: 10.1084/jem.184.6.2271. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wurster AL, Tanaka T, Grusby MJ. The biology of Stat4 and Stat6. Oncogene. 2000;19:2577–2584. doi: 10.1038/sj.onc.1203485. [DOI] [PubMed] [Google Scholar]
- Xiu Y, Wong CP, Bouaziz JD, Hamaguchi Y, Wang Y, Pop SM, Tisch RM, Tedder TF. B lymphocyte depletion by CD20 monoclonal antibody prevents diabetes in nonobese diabetic mice despite isotype-specific differences in Fc gamma R effector functions. J Immunol. 2008;180:2863–2875. doi: 10.4049/jimmunol.180.5.2863. [DOI] [PubMed] [Google Scholar]
- Yanaba K, Bouaziz JD, Haas KM, Poe JC, Fujimoto M, Tedder TF. A regulatory B cell subset with a unique CD1dhiCD5+ phenotype controls T cell-dependent inflammatory responses. Immunity. 2008;28:639–650. doi: 10.1016/j.immuni.2008.03.017. [DOI] [PubMed] [Google Scholar]
- Young DA, Lowe LD, Booth SS, Whitters MJ, Nicholson L, Kuchroo VK, Collins M. IL-4, IL-10, IL-13, and TGF-beta from an altered peptide ligand-specific Th2 cell clone down-regulate adoptive transfer of experimental autoimmune encephalomyelitis. J Immunol. 2000;164:3563–3572. doi: 10.4049/jimmunol.164.7.3563. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.