Abstract
Recent clinical failures associated with levofloxacin treatment for Streptococcus pneumoniae infections and growing evidence of frequent mutations in the isolate population have led to increased concerns regarding fluoroquinolone resistance. Our objective was to characterize the efficacies of levofloxacin and moxifloxacin against various genotypes of S. pneumoniae after simulated bronchopulmonary exposures. An in vitro model was used to simulate a levofloxacin concentration of 500 mg and a moxifloxacin concentration of 400 mg, which were previously determined to be the concentrations in the epithelial lining fluid of older adults receiving once-daily dosing. The effects of the drugs were tested against six S. pneumoniae containing various mutations. Bacterial density and resistance were quantitatively assessed over 48 h. The S. pneumoniae isolate with no mutation displayed a 4-log reduction in CFU after treatment with both agents and did not develop resistance. Isolates containing the parC or parE mutation or both mutations regrew and developed resistance when they were exposed to levofloxacin, despite an unbound area under the concentration-time curve (AUC):MIC ratio of ∼100. When the isolate containing the parC and gyrA mutations was exposed to levofloxacin, there was a half-log reduction in the number of CFU compared to that for the control, but the isolate subsequently regrew. Likewise, levofloxacin did not kill the isolate containing the parC, gyrA, and parE mutations. Moxifloxacin sustained the killing of all bacterial isolates tested without the development of resistance. Levofloxacin did not sustain bacterial killing and did not prevent the emergence of further resistance in mutants with the parC or parE mutation or both mutations, even though an unbound AUC:MIC ratio for exposure well above the breakpoint of 30 to 40 established in the literature for S. pneumoniae was maintained. Moxifloxacin was effective against all isolates tested, despite the presence of isolates with two- and three-step mutations, for which the MICs were increased.
Streptococcus pneumoniae is the most common organism associated with lower respiratory tract infections and accounts for approximately 50% of all cases of community-acquired pneumonia, 35% of cases of acute sinusitis and acute otitis media, and 20% of chronic bronchitis infections (15, 28). Of growing concern with S. pneumoniae is the increasing levels of resistance to commonly used antimicrobials, namely, the penicillins and other β-lactams, as well as the macrolides. The frequencies of resistance to penicillin and the macrolides among S. pneumoniae strains are estimated to be 40 and 30%, respectively (10). As such, use of one of the antipneumococcal fluoroquinolones (levofloxacin, moxifloxacin, gatifloxacin) as monotherapy for lower respiratory tract infections is fast becoming common practice in both the inpatient and the outpatient settings.
With the increasing use of the antipneumococcal fluoroquinolones, issues surrounding the development of resistance to these agents must be studied. The present rates of fluoroquinolone resistance among S. pneumoniae isolates in the United States and Canada are relatively low. In the United States, the overall rates of fluoroquinolone resistance among 4,650 S. pneumoniae isolates were 1.4% for ciprofloxacin, 0.5% for levofloxacin, 0.3% for gatifloxacin, and 0.3% for moxifloxacin (3). Similar rates of resistance to ciprofloxacin (1.4%), levofloxacin (0.9%), and gatifloxacin or moxifloxacin (<1%) were found in Canada (17). Of perhaps greater concern are the recent clinical failures of levofloxacin treatment of S. pneumoniae infections and growing evidence of frequent mutations in the isolate population. Since 1999, there have been 21 case reports of levofloxacin treatment failures in the United States and Canada (6, 8, 12, 13, 26, 27, 29; N. O. Fishman, B. Suh, L. M. Weigel, B. Lorber, S. Gelone, A. L. Truant, T. D. Gootz, J. D. Christie, and P. H. Edelstein, Abstr. 39th Intersci. Conf. Antimicrob. Agents Chemother., abstr. C1-825, 1999; J. Piper, K. Couch, D. Tuttle, and L. Steele-Moore, Abstr. 41st Intersci. Conf. Antimicrob. Agents Chemother., abstr. L-902, 2001). Furthermore, a recent report (7) of a study with a subset of the levofloxacin-susceptible pneumococcal isolates from the 1999-2000 respiratory season evaluated as part of the TRUST surveillance program suggests that 6.6 and 71% of the S. pneumoniae isolates for which the levofloxacin MICs are 1.0 and 2.0 μg/ml, respectively, contain a first-step parC mutation. Of perhaps more clinical significance was a recent report showing that of 164 unique patient isolates of S. pneumoniae, 29.9% harbored a mutation in either the parC or the gyrA gene, with the majority of isolates (67.3%) having a mutation in the parC locus only. Mutant isolates were found only when the ciprofloxacin MIC was ≥2 μg/ml (3).
The first issue surrounding the prevention of the development of resistance in S. pneumoniae involves maintenance of adequate levels of exposure to the fluoroquinolone. For the fluoroquinolones, the pharmacodynamic parameter that best predicts exposure and that best correlates with the outcome is the area under the concentration-time curve (AUC):MIC ratio. In vitro, in vivo, and clinical trials have determined that maintenance of an unbound AUC:MIC ratio of 30 to 40 maximizes the efficacies of fluoroquinolones against S. pneumoniae (1, 14, 16, 21). In addition to exposure of the organism to the antimicrobial agent, the target binding site and the genotypic profile of the organism are becoming increasingly important. S. pneumoniae isolates with chromosomal mutations in either the parC or the parE region of topoisomerase IV or the gyrA or gyrB region of DNA gyrase display low-level resistance, whereas isolates with second-step mutations involving alterations in both the parC (parE) and gyrA (gyrB) regions have high-level fluoroquinolone resistance. These mechanisms of resistance are more likely to affect the older antipneumococcal fluoroquinolone levofloxacin, since it binds primarily to DNA gyrase or DNA topoisomerase IV, while the new fluoroquinolones, moxifloxacin and gatifloxacin, bind strongly to both DNA gyrase and topoisomerase IV (18, 24, 25). This could perhaps begin to explain the clinical failures associated with levofloxacin.
To study this relationship, we used an in vitro model which simulated the steady-state bronchopulmonary pharmacokinetic profiles of levofloxacin and moxifloxacin in patients receiving conventional once-daily dosing regimens to examine the rate of killing and the development of resistance among S. pneumoniae isolates with various genotypic mutations.
MATERIALS AND METHODS
Bacterial strains and susceptibility testing.
Six S. pneumoniae isolates with various genotypic profiles (kindly provided by G. V. Doern, University of Iowa, Iowa City) were selected for inclusion (Table 1). Genotypic confirmation was performed by the DNA amplification and sequencing procedures previously described by Doern and colleagues (3). Isolates containing parC and parE mutations were considered first-step mutants, as the parE mutation is considered silent when the mutation involves an isoleucine-to-valine substitution at position 460. MICs were determined by use of a microdilution technique by the method of the National Committee for Clinical Laboratory Standards (22).
TABLE 1.
Preexperimental phenotypic and genotypic profiles of the S. pneumoniae isolates
| S. pneumoniae isolate | Mutation locus or loci for levofloxacin | Levofloxacin MIC (μg/ml) | Mutation locus or loci for moxifloxacin | Moxifloxacin MIC (μg/ml) |
|---|---|---|---|---|
| 53 | None | 1.0 | None | 0.125 |
| 1911 | parC + gyrA | 16 | parC + gyrA | 2 |
| 93 | parC + gyrA + parE | 128 | parC + gyrA + parE | 8 |
| 1312 | parC + parE | 2 | parC + parE | 0.25 |
| 1386 | parC + parE | 2 (32)a | NAb | (4) |
| 1610 | parC | 2 (32) | NA | (4) |
The values in parentheses are the postexperimental MICs (see Table 4).
NA, not applicable, as in vitro experimentation was not performed with these isolates and moxifloxacin.
Antibiotics.
The following antibiotics were used: levofloxacin for intravenous injection (25 mg/ml; lot E1213; expiration date, December 2004; Ortho-McNeil) and moxifloxacin standard powder (BAY 12-8039; potency, 87.8%; lot 661093E; expiration date, September 2004; Bayer Corporation). All drugs were used before the labeled expiration date.
Bacterial growth medium.
Cation-adjusted Mueller-Hinton broth (CAMHB; Becton Dickinson, Sparks, Md.) supplemented with 2.5 to 5% lysed horse blood (LHB; Remel, Lenexa, Kans.) was used as the bacterial growth medium in all in vitro model experiments. The volumes of growth medium used in each of the models with levofloxacin and moxifloxacin were 1,000 and 300 ml, respectively, on the basis of half-life and flow-rate calculations. Trypticase soy agar plates (diameter, 100 mm) with 5% sheep blood were used for quantitative determinations. Mueller-Hinton agar plates (diameter, 100 mm) with 2.5 to 5% LHB containing levofloxacin and moxifloxacin at concentrations of two, four, or eight times the MIC or no drug were used for quantitative determinations of resistance.
In vitro model.
The in vitro model used in this study has been described previously (9). By using a central compartment model, bacteria were exposed to changing concentrations of antibiotics to simulate the pharmacokinetic parameters of the drugs in the human bronchopulmonary region previously determined with patients receiving conventional once-daily dosing regimens of levofloxacin and moxifloxacin (4). Each experiment consisted of four independent models (three models of antibiotic treatment and one growth control model), which were run simultaneously for all organisms and treatment regimens. The models were placed in a 37°C temperature-controlled circulating water bath for optimal temperature control, and magnetic stirring bars were used in each model to ensure adequate mixing of all contents. Fresh CAMHB supplemented with LHB was continuously pumped into each of the models with a peristaltic pump at rates which simulated the elimination half-lives of the test antibiotics obtained in the study of pharmacokinetics of the drugs in the human bronchopulmonary region mentioned above (4). The levofloxacin half-lives from 0 to 12 and 12 to 24 h were 6 and 10 h, respectively. The moxifloxacin half-life was 20 h.
Studies were conducted over 48 h with levofloxacin and all S. pneumoniae isolates and with moxifloxacin and S. pneumoniae isolates 53, 1911, 93, and 1312. A starting inoculum, prepared as four independent starting inocula, of 106 CFU/ml was prepared from an overnight culture of the test isolate for all model experiments. To ensure that the bacteria were in logarithmic growth phase prior to antimicrobial exposure, experiments were started 0.5 h after inoculation of the bacteria into the models.
Levofloxacin and moxifloxacin were added to the models at concentrations that simulated the concentrations found in human epithelial lining fluid (4). The simulated peak and trough levofloxacin concentrations were 15.23 ± 4.53 and 2.94 ± 1.74 μg/ml, respectively. The simulated peak and trough moxifloxacin concentrations were 11.66 ± 11.90 and 5.71 ± 6.3 μg/ml, respectively. To confirm the simulation of the pharmacokinetic parameters in the human bronchopulmonary region, samples were taken throughout the duration of the model experiment, and samples were stored at −80°C until they were assayed for their drug concentrations.
To assess bacterial density over time, samples were obtained from each model and serially diluted in CAMHB. Aliquots of each diluted sample were plated in duplicate for quantitative culture. The volume of the aliquots used for determination of bacterial counts was either 10 or 100 μl, depending on the dilution used. After 24 h of incubation at 37°C, the change in the log10 number of CFU per milliliter over the 48-h interval was calculated, and time-kill curves were constructed by plotting the log10 number of CFU per milliliter against time. The limit of quantification was 101 CFU/ml.
To assess the development of resistance over time for each of the test organisms, samples were obtained from each model at 0, 8, 24, and 48 h and plated in duplicate for quantitative culture on Mueller-Hinton agar with 2.5 to 5% LHB containing levofloxacin or moxifloxacin at concentrations of two, four, and eight times the MIC or no drug. The development of resistance was further examined by postexperimental MIC and genotypic profile determinations for S. pneumoniae isolates 1386 and 1610.
Antibiotic concentration determinations.
Samples of CAMHB supplemented with LHB taken from each of the treatment models were assayed for levofloxacin and moxifloxacin concentrations. Samples were analyzed by a validated ion-paired high-performance liquid chromatography (HPLC) method as described previously (20), with modifications. CAMHB with LHB was used to prepare standards, check samples, and dilute samples as required. For the moxifloxacin assay, a Waters Associates (Milford, Mass.) 515 pump was equipped with a Nucleosil 100 C18 column (10 μm; 4.6 by 250 mm; Alletech Associates, Deerfield, Ill.) and a μBondapak C18 Guard-pak precolumn (Waters Associates). A programmable fluorescence detector (emission, 418 nm; excitation, 295 nm; range, 0.1 absorbance units, full scale; model 980; Applied Biosystems, Foster City, Calif.) was used to detect the analytes. The mobile phase consisted of a mixture of 0.01 M sodium phosphate buffer with 0.01 M tetrabutylammonium hydrogen sulfate and acetonitrile (80:20) filtered through a 0.22-mm-pore-size filter. The flow rate was 1.3 ml/min. A 150-μl aliquot of a standard, quality control, or unknown sample and a 50-μl aliquot of an internal standard (gatifloxacin at 2.0 μg/ml) were placed in a labeled tube. A 600-μl volume of acetonitrile was added to each tube, and the tubes were vortexed for 30 s. The supernatants were transferred to a clean tube and dried under a stream of nitrogen at 40°C. The residual material was reconstituted in a 200 μl of 0.01 N HCl. The solution was vortexed and transferred to WISP vials for injection into a WISP 717 Plus autosampler (Waters Associates). A chromatography data system (EZChrome Elite; Scientific Software, San Ramon, Calif.) was used for data acquisition. The moxifloxacin HPLC assay was linear (r = 0.9995 to 1.0000) over a concentration range of 1.0 to 50.0 μg/ml. The intraday quality control samples (n = 10) with concentrations of 2.0 and 40.0 μg/ml had coefficients of variation (CVs) of 0.6694 and 0.8059%, respectively. The interday quality control samples (n = 7) with concentrations of 2.0 and 40.0 μg/ml had CVs of 0.47 and 0.89%, respectively.
Levofloxacin standards were prepared in a manner similar to that described above for moxifloxacin, and the concentrations were assayed by a validated HPLC procedure (23). The assay was linear (r = 0.9995 to 1.0000) over a concentration range of 1.0 to 20.0 μg/ml. The intraday quality control samples (n = 11) with concentrations of 2.0 and 18.0 μg/ml had CVs of 1.93 and 2.38%, respectively. The interday quality control samples (n = 7) with concentrations of 2.0 and 18.0 μg/ml had CVs of 1.46 and 0.84%, respectively.
Pharmacokinetic and pharmacodynamic analyses.
The target values of the pharmacokinetic parameters for the human bronchopulmonary region were selected prior to initiation of the study. By using actual drug concentration data from each set of experiments, the maximum (peak) and minimum (trough) concentrations, as well as the AUCs, were determined for each antibiotic by noncompartmental methods. The AUC values were calculated by the trapezoidal method. The AUC from 0 to 24 h (AUC0-24):MIC ratio was determined by using experimental pharmacokinetic and screening MIC data.
RESULTS
Susceptibility testing.
Table 1 shows the genotypic profiles as well as the preexperimental MICs of levofloxacin and moxifloxacin for the six S. pneumoniae isolates used in this study.
Pharmacokinetic analysis.
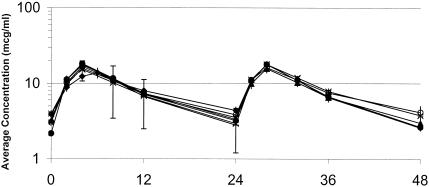
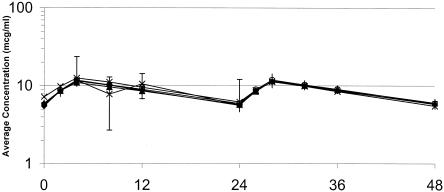
The target values of the pharmacokinetic parameters and the mean values of the experimental pharmacokinetic data are summarized in Table 2. The values of the pharmacokinetic parameters for levofloxacin and moxifloxacin observed in the model were similar to the target values. The actual concentrations of levofloxacin obtained in the model were, on average, 14% higher than the target concentrations. Graphical depictions of the target and actual concentrations of levofloxacin and moxifloxacin are presented in Fig. 1 and 2, respectively.
TABLE 2.
Simulated dosing regimens, target human epithelial lining fluid drug concentrations, and corresponding pharmacokinetic profiles observed in in vitro simulations
| Antibiotic (regimen), parameter, and isolate | Peak concn (μg/ml)a | Trough concn (μg/ml)b | AUC0-24 (μg · h/ml)c |
|---|---|---|---|
| Levofloxacin (500 mg every 24 h) | |||
| Target valuec (mean [±SD]) | 15.23 (4.53) | 2.94 (1.74) | 180 |
| Actual value (mean [±SD])d | |||
| 53 | 13.79 (2.11) | 4.38 (0.50) | 268 (34) |
| 1911 | 17.52 (1.58) | 3.33 (0.50) | 203 (22) |
| 93 | 15.82 (0.20) | 3.52 (0.20) | 193 (4) |
| 1312 | 16.75 (1.52) | 3.83 (0.15) | 200 (15) |
| 1386 | 18.19 (0.21) | 3.20 (0.58) | 202 (18) |
| 1610 | 17.88 (0.66) | 2.80 (0.39) | 194 (5) |
| Moxifloxacin (400 mg every 24 h) | |||
| Target valuec (mean [±SD]) | 11.66 (11.9) | 5.71 (6.3) | 208 |
| Actual value (mean [±SD])d | |||
| 53 | 11.47 (0.17) | 5.62 (0.23) | 201 (1) |
| 1911 | 10.97 (0.29) | 5.76 (0.55) | 198 (9) |
| 93 | 12.51 (0.16) | 6.21 (0.46) | 222 (4) |
| 1312 | 11.73 (0.52) | 5.92 (0.35) | 199 (17) |
Values were obtained at 4 h after dose administration.
Values were obtained at 24 h after dose administration.
Adapted from reference 4.
Values are presented as the means for the three treatment models.
FIG. 1.
Human epithelial lining fluid (ELF) levofloxacin concentrations and the corresponding pharmacokinetic profiles observed for the isolates in the in vitro simulations. Error bars represent the standard deviations for the patient epithelial lining fluid levofloxacin concentrations. The values on the x axis are times (in hours). ⧫, isolate 53; ×, isolate 93; +, isolate 1610; ○, isolate 1312; *, concentration in patient epithelial lining fluid; ▴, isolate 1911; •, isolate 1386.
FIG. 2.
Human epithelial lining fluid moxifloxacin concentrations and the corresponding pharmacokinetic profile observed for the isolates in the in vitro simulations. Error bars represent the standard deviation for the patient epithelial lining fluid moxifloxacin concentrations. The values on the x axis are times (in hours). ⧫, isolate 53; ○, isolate 1312; ▴, isolate 1911; ×, isolate 93; *, concentration in patient epithelial lining fluid.
Bactericidal activity.
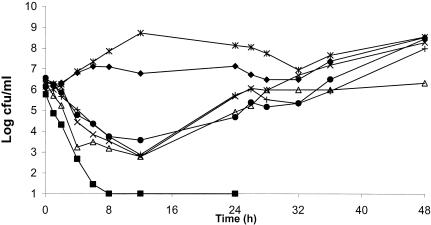
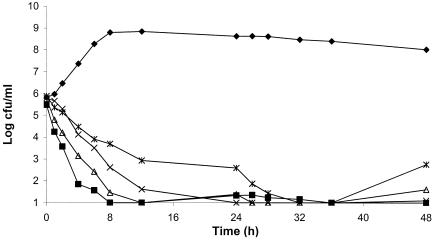
The average bacterial density of the starting inoculum was 1.7 × 106 ± 1.9× 106 CFU/ml. Figures 3 and 4 summarize the resultant killing curves for levofloxacin and moxifloxacin, respectively. Data are plotted as the means for levofloxacin and moxifloxacin in the three treatment models as well as the mean for the growth control for all isolates tested.
FIG. 3.
Antimicrobial efficacies of levofloxacin against S. pneumoniae isolates after exposure to simulated concentrations in human epithelial fluid. ⧫, control (n = 6); ▪, isolate 53; ▵, isolate 1312; ×, isolate 1911; *, isolate 93; +, isolate 1610; •, isolate 1386.
FIG. 4.
Antimicrobial efficacy of moxifloxacin against S. pneumoniae after exposure to simulated concentrations in human epithelial fluid. ⧫, control (n = 4); ▪, isolate 53; ▵, isolate 1312; ×, isolate 1911; *, isolate 93.
Moxifloxacin was observed to have a rapid bactericidal effect (3-log reduction) against all isolates tested. The effect of moxifloxacin against isolates 1386 and 1610, which contained both the parC and parE mutations and the parC mutation, respectively, was not tested, as moxifloxacin produced complete eradication of isolate 1312 containing the parC and parE mutations and so testing of these isolates was not considered necessary. The densities of isolates 53 and 1312 declined to the limit of detection (101 CFU/ml) during the initial 8 to 12 h, with no regrowth or development of resistance observed over the remaining 48 h. For the isolate containing a two-step mutation (isolate 1911), the density declined to the limit of detection by 24 h, with no subsequent regrowth or development of resistance. The density of the isolate containing a three-step mutation (isolate 93) declined to the limit of detection by 32 h, with a regrowth of 3 logs by 48 h but no development of resistance.
Levofloxacin produced complete killing of isolate 53, which had no genotypic mutations, by 8 h, with no regrowth or the development of resistance. For those isolates containing the parC or parE mutation or both mutations (isolates 1312, 1386, and 1610), there was an approximately 2.5-log reduction by 12 h, with regrowth starting at 12 h and the development of resistance to concentrations of eight times the MIC by 48 h. For the isolate containing the parC and gyrA mutations, levofloxacin produced a 3-log reduction during the first 12 h, with subsequent regrowth to the level in the control model. Levofloxacin did not produce any bacterial killing of the isolate containing a three-step mutation (parC, gyrA, parE), as the growth of the isolate throughout the dosing regimen was similar to that of the isolate in the control model.
Detection of resistance.
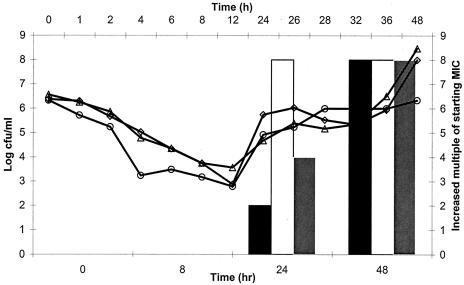
None of the S. pneumoniae isolates tested developed resistance to moxifloxacin (Table 3). Isolates containing the parC or parE mutation or both mutations (isolates 1312, 1386, and 1610) developed resistance to levofloxacin at 24 and 48 h (Fig. 5). All three isolates grew on agar containing levofloxacin at concentrations up to eight times the MIC at 48 h. Resistant mutants of each of the three isolates emerged in all three levofloxacin treatment models during each independent run. The levofloxacin killing curves for these three isolates clearly reflected the observed changes in the MICs, since the regrowth of each isolate was noted. The postexperimental MICs and genotypic determinations for isolates 1386 and 1610 are presented in Table 4. For both isolates the ciprofloxacin, levofloxacin, moxifloxacin, and gatifloxacin MICs increased 8, 16, 16, and 32 times, respectively, in the postexposure determinations. Likewise, the genotypic profile of isolate 1386 changed from parC parE at 0 h to parC parE gyrA at 48 h. For isolate 1610, the genotypic profile changed from parC at 0 h to parC parE gyrA at 48 h. The postexposure MICs for the isolates originally containing two- and three-step mutations (isolates 1911 and 93) did not increase.
TABLE 3.
Simulated pharmacodynamics of moxifloxacin and levofloxacin in epithelial lining fluid against S. pneumoniae isolates with various genotypic profiles
| Antibiotic and isolate | AUC:MIC ratio | Avg (range) change in log10 CFU at:
|
Change in multiple of the MIC at:
|
||
|---|---|---|---|---|---|
| 24 h | 48 h | 24 h | 48 h | ||
| Levofloxacin | |||||
| 53 | 268 | −4.72 (none) | −4.72 (none) | NCa | NC |
| 1911 | 13 | −0.63 (−1.94 to 0.53) | +1.97 (1.78 to 2.23) | NC | NC |
| 93 | 1.5 | +2.19 (2.03 to 2.25) | +2.61 (2.52 to 2.65) | NC | NC |
| 1312 | 100 | −1.41 (−1.47 to −1.33) | 0 (−0.33 to 0.67) | 2 | 8 |
| 1386 | 101 | −1.88 (−2.56 to −1.0) | +1.91 (1.89 to 1.95) | 8 | 8 |
| 1610 | 97 | −0.62 (−0.85 to −0.37) | +1.63 (none) | 4 | 8 |
| Moxifloxacin | |||||
| 53 | 804 | −4.21 (−4.54 to −3.54) | −4.54 (none) | NC | NC |
| 1911 | 99 | −4.81 (none) | −4.71 (−4.87 to −4.57) | NC | NC |
| 93 | 28 | −3.31 (−3.83 to −2.92) | −3.14 (−4.83 to −2.20) | NC | NC |
| 1312 | 797 | −4.38 (−4.76 to −3.76) | −4.15 (−4.76 to −3.76) | NC | NC |
NC, no change.
FIG. 5.
Antibacterial efficacies and resistance profiles for S. pneumoniae containing the parC or parE mutation or both mutations after exposure to simulated human epithelial lining fluid levofloxacin concentrations. ▪, MIC for isolate 1312; ○, isolate 1312; □, MIC for isolate 1386; ▵, isolate 1386; shaded square, MIC for isolate 1610; ⋄, isolate 1610.
TABLE 4.
Postexperimental MICs and genotypic profiles for the S. pneumoniae isolates after exposure to levofloxacin at concentrations found in epithelial lining fluid
| Isolate and time studied (genotypic profile) | MIC (μg/ml)
|
|||
|---|---|---|---|---|
| Levofloxacin | Ciprofloxacin | Moxifloxacin | Gatifloxacin | |
| 1386 | ||||
| Preexperimental (parC + parE) | 2 | 8 | 0.25 | 0.5 |
| Postexperimental (parC + parE + gyrA) | 32 | >64 | 4 | 16 |
| 1610 | ||||
| Preexperimental (parC) | 2 | 8 | 0.25 | 0.5 |
| Postexperimental (parC + parE + gyrA) | 32 | >64 | 4 | 16 |
Pharmacodynamic analysis.
The results of the pharmacodynamic analysis are summarized in Table 3. Moxifloxacin produced complete bactericidal activity (3-log reduction) against all isolates tested, despite the presence of two- and three-step mutations with the accompanying increased MICs and unbound AUC:MIC ratios that decreased to approximately 30. It was noted, however, that both the rate and the extent of killing increased with higher AUC:MIC ratios.
Regrowth and resistance to levofloxacin occurred with isolates with the parC or parE mutation or both mutations, even though the unbound AUC:MIC ratio was 100, a level of exposure that is well above the breakpoint of 30 to 40 established in the literature for the treatment of S. pneumoniae infections. Exposures for the isolates containing two- and three-step mutations (isolates 1911 and 93, respectively) were well below the previously established breakpoints, and as such, bacterial killing was not observed.
DISCUSSION
The in vitro study described here was performed with the aim of determining the relationship between simulated concentrations of levofloxacin and moxifloxacin in the human bronchopulmonary region and activities against S. pneumoniae isolates with various genotypic profiles. We found that levofloxacin did not sustain bacterial killing and did not prevent the emergence of further resistance in mutants with a parC or parE mutation or both mutations, even though the unbound AUC:MIC ratio for exposure was maintained well above the breakpoint of 30 to 40 established in the literature (1, 14, 16, 21). Conversely, moxifloxacin was effective against all isolates tested, despite the presence of two- and three-step mutations with the accompanying increased MICs.
Our results highlight some important points regarding differences in the potencies of fluoroquinolones in terms of maximal effectiveness and the prevention of resistance. These findings seem to match those of Blondeau and colleagues (2), who found that moxifloxacin, but not levofloxacin, was effective at mutant prevention concentrations that were below the drug concentrations achievable in serum. Furthermore, it is becoming evident that not all fluoroquinolones are equal and, as such, should not have the same pharmacodynamic breakpoints.
Previous in vitro modeling studies have determined that maintenance of an unbound AUC:MIC ratio for exposure of 30 to 40 correlates with high rates of bacterial killing (14, 16). Those studies, however, were performed with S. pneumoniae isolates that were susceptible to the fluoroquinolones and that did not have identifiable underlying genetic mutations. Furthermore, the simulated fluoroquinolone concentrations were those achieved in serum and not the concentrations at the site of infection, the bronchopulmonary region. To our knowledge, this is the first study to have used an in vitro model to simulate the concentrations of levofloxacin and moxifloxacin in the human bronchopulmonary region and to test their activities against S. pneumoniae isolates with various genetic mutations.
An in vitro study conducted by Ibrahim and colleagues (11) examined the efficacy of levofloxacin against an S. pneumoniae isolate containing a gyrA mutation and two parE mutations. The investigators found that levofloxacin, dosed to achieve an AUC:MIC ratio of ≥35, was able to eradicate the resistant isolate. These findings may be limited in that the investigators used Todd-Hewitt broth supplemented with 0.5% yeast extract, which is not the optimal growth medium for S. pneumoniae and, as such, could have affected the outcome. Furthermore, the resistant isolate studied may not have been optimal for determination of the potency of levofloxacin against mutant isolates since levofloxacin is not affected as much by mutations in the gyrA locus as it is by mutations in the parC locus.
The results found in our study seem to correlate with those of an in vivo study conducted by Croisier and colleagues (5), in which levofloxacin was ineffective when mutant S. pneumoniae isolates with a parC mutation and parC and gyrA mutations and for which the MIC was ≥2.0 μg/ml were used. That study also found that resistant mutants of these isolates appeared when they had a preexisting parC mutation. Of further interest is an in vitro study conducted by Madaras-Kelly and colleagues (19), in which levofloxacin retained no antimicrobial effect against a parC mutant with phenotypic expression of fluoroquinolone efflux. Conversely, moxifloxacin exhibited greater antimicrobial effects against all mutant isolates tested in this study.
The major limitation of our study is that we did not test levofloxacin-resistant isolates with eightfold lower doses of moxifloxacin, which would have yielded the same AUC:MIC ratio produced by levofloxacin, in order to see if moxifloxacin treatment would also result in the development of resistance in these isolates. Likewise, we did not expose isolate 93 to a 20-fold higher dose of levofloxacin to see if levofloxacin would be effective at higher levels of exposure, as was the case for moxifloxacin. These studies are pending and will be useful in further elucidating the AUC:MIC ratio necessary for the fluoroquinolones.
In conclusion, our findings suggest that not all fluoroquinolones have the same AUC:MIC ratio needed to maximize antimicrobial killing and prevent the emergence of resistance. The concentrations of levofloxacin found in the bronchopulmonary region of humans did not produce bacterial eradication in the presence of first-step mutants of S. pneumoniae, despite exposure to high concentrations. Moreover, for these isolates inadequate levofloxacin exposures resulted in the emergence of high-level phenotypic and genotypic resistance profiles. Although an in vitro model offers the worst-case scenario for a given antimicrobial, since it does not have the benefit of an intact immune system that can aid with eradication of the infection, this technique has been widely used for pharmacodynamic profiling of the fluoroquinolones and has previously been shown to be predictive of treatment outcomes in humans. Further study is necessary to determine the magnitude of the AUC:MIC ratio for levofloxacin that is required to ensure sufficient antimicrobial effects against S. pneumoniae isolates containing parC mutations. While the conventional breakpoints of 30 to 40 for the unbound AUC:MIC ratio appear to be in question for levofloxacin, this level of exposure was found to be appropriate for moxifloxacin, as the compound displayed potent antimicrobial effects against all isolates.
Acknowledgments
We thank Gary Doern for assistance with pre- and postexperimental genotypic profile determinations.
This work was supported by an unrestricted educational grant from Bayer Pharmaceuticals.
REFERENCES
- 1.Ambrose, P. G., D. M. Grasela, T. H. Grasela, J. Passarell, H. B. Mayer, and P. F. Pierce. 2001. Pharmacodynamic assessment of fluoroquinolones against Streptococcus pneumoniae in patients with community-acquired respiratory tract infections. Antimicrob. Agents Chemother. 45:2793-2797. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Blondeau, J. M., X. Zhao, G. Hansen, and K. Drlica. 2001. Mutant prevention concentrations of fluoroquinolones for clinical isolates of Streptococcus pneumoniae. Antimicrob. Agents Chemother. 45:433-438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Brueggemann, A. B., S. L. Coffman, P. Rhomberg, H. Huynh, L. Almer, A. Nilius, R. Flamm, and G. V. Doern. 2002. Fluoroquinolone resistance in Streptococcus pneumoniae in United States since 1994-1995. Antimicrob. Agents Chemother. 46:680-688. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Capitano, B., H. M. Mattoes, E. Shore, A. O'Brien, S. Braman, C. Sutherland, and D. P. Nicolau. Steady state intrapulmonary concentrations of moxifloxacin, levofloxacin, and azithromycin in older adults. Chest, in press. [DOI] [PubMed]
- 5.Croisier, D., P. Chavanet, C. Lequeu, A. Ahanou, A. Nierlich, C. Neuwirth, L. Piroth, M. Duong, M. Buisson, and H. Portier. 2002. Efficacy and pharmacodynamics of simulated human-like treatment with levofloxacin on experimental pneumonia induced with penicillin-resistant pneumococci with various susceptibilities to fluoroquinolones. J. Antimicrob. Chemother. 50:349-360. [DOI] [PubMed] [Google Scholar]
- 6.Davidson, R., R. Cavalcanti, J. L. Brunton, D. J. Blast, J. C. de Azavedo, P. Kibsey, C. Fleming, and D. W. Low. 2002. Resistance to levofloxacin and failure of treatment of pneumococcal pneumonia. N. Engl. J. Med. 346:747-750. [DOI] [PubMed] [Google Scholar]
- 7.Davies, T. A., A. Evangelista, S. Pfleger, K. Bush, D. F. Sahm, and R. Goldschmidt. 2002. Prevalence of single mutations in topoisomerase type II genes among levofloxacin-susceptible clinical isolates of Streptococcus pneumoniae isolated in the United States in 1992-1996 and 1999-2000. Antimicrob. Agents Chemother. 46:119-124. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Empey, P. E., H. R. Jennings, A. C. Thornton, R. P. Rapp, and M. E. Evans. 2001. Levofloxacin failure in a patient with pneumococcal pneumonia. Ann. Pharmacother. 35:687-690. [DOI] [PubMed] [Google Scholar]
- 9.Garrison, M. W., K. Vance-Bryan, T. A. Larson, J. P. Toscano, and J. C. Rotschafer. 1990. Assessment of effects of protein binding on daptomycin and vancomycin killing of Staphylococcus aureus by using an in vitro pharmacodynamic model. Antimicrob. Agents Chemother. 34:1925-1931. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Hoban, D., K. Waites, and D. Felmingham. 2003. Antimicrobial susceptibility of community-acquired respiratory tract pathogens in North America in 1999-2000: findings of the PROTEKT surveillance study. Diagn. Microbiol. Infect. Dis. 45:251-259. [DOI] [PubMed] [Google Scholar]
- 11.Ibrahim, K. H., L. B. Hovde, G. Ross, B. Gunderson, D. H. Wright, and J. C. Rotschafer. 2002. Microbiologic effectiveness of time- or concentration-based dosing strategies in Streptococcus pneumoniae. Diagn. Microbiol. Infect. Dis. 44:265-271. [DOI] [PubMed] [Google Scholar]
- 12.Kays, M. B., D. W. Smith, M. F. Wack, and G. A. Denys. 2002. Levofloxacin treatment failure in a patient with fluoroquinolone-resistant Streptococcus pneumoniae pneumonia. Pharmacotherapy 22:395-399. [DOI] [PubMed] [Google Scholar]
- 13.Kuehnert, M. J., F. S. Nolte, and C. A. Perlino. 1999. Fluoroquinolone resistance in Streptococcus pneumoniae. Ann. Intern. Med. 131:312-313. [DOI] [PubMed] [Google Scholar]
- 14.Lacey, M. K., W. Lu, A. Xu, P. R. Tessier, D. P. Nicolau, R. Quintiliani, and C. H. Nightingale. 1999. Pharmacodynamic comparisons of levofloxacin, ciprofloxacin, and ampicillin against Streptococcus pneumoniae in an in vitro model of infection. Antimicrob. Agents Chemother. 43:672-677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.LaForce, D. M. 1992. Antibacterial therapy for lower respiratory tract infections in adults: a review. Clin. Infect. Dis. 14(Suppl. 2):S233-S237. [DOI] [PubMed] [Google Scholar]
- 16.Lister, P. D., and C. C. Sanders. 1999. Pharmacodynamics of trovafloxacin, ofloxacin, and ciprofloxacin against Streptococcus pneumoniae in an in vitro pharmacokinetic model. J. Antimicrob. Chemother. 43:79-86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Low, D. E., J. Azavedo, K. Weiss, T. Mazzulli, M. Kuhn, D. Church, K. Forward, G. Zhanel, A. Simor, and A. McGeer. 2002. Antimicrobial resistance among clinical isolates of Streptococcus pneumoniae in Canada during 2000. Antimicrob. Agents Chemother. 46:1295-1301. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lu, T., X. Zhao, and K. Drlica. 1999. Gatifloxacin activity against quinolone-resistant gyrase: allele-specific enhancement of bacteriostatic activities by the C-8 methoxy group. Antimicrob. Agents Chemother. 43:2969-2974. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Madaras-Kelly, K. J., C. Daniels, M. Hegbloom, and M. Thompson. 2002. Pharmacodynamic characterization of efflux and topoisomerase IV-mediated fluoroquinolone resistance in Streptococcus pneumoniae. J. Antimicrob. Chemother. 50:211-218. [DOI] [PubMed] [Google Scholar]
- 20.Marangos, M. N., A. T. Skoutelis, C. H. Nightingale, Z. Zhu, A. G. Psyrogiannis, D. P. Nicolau, H. P. Bassaris, and R. Quintiliani. 1995. Absorption of ciprofloxacin in patients with diabetic gastroparesis. Antimicrob. Agents Chemother. 39:2161-2163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Mattoes, H. M., M. Banevicius, D. Li, C. Turley, D. Xuan, C. H. Nightingale, and D. P. Nicolau. 2001. Pharmacodynamic assessment of gatifloxacin against Streptococcus pneumoniae. Antimicrob. Agents Chemother. 45:2092-2097. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.National Committee for Clinical Laboratory Standards. 1997. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 4th ed. Approved standard. NCCLS document M7-A4, vol. 17, no. 2, p. 10-13. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 23.Onyeji, C. O., K. Q. Bui, R. C. Owens, Jr., D. P. Nicolau, R. Quintilian, and C. H. Nightingale. 1999. Comparative efficacies of levofloxacin and ciprofloxacin against Streptococcus pneumoniae in a mouse model of experimental septicemia. Int. J. Antimicrob. Agents 12:107-114. [DOI] [PubMed] [Google Scholar]
- 24.Pesotva, E., J. J. Millichap, G. A. Noskin, and L. R. Peterson. 2000. Intracellular targets of moxifloxacin: a comparison with other fluoroquinolones. J. Antimicrob. Chemother. 45:583-590. [DOI] [PubMed] [Google Scholar]
- 25.Peterson, L. R. 2001. Quinolone molecular structure-activity relationships: what have we learned about improving antibacterial activity. Clin. Infect. Dis. 33(Suppl. 3):S180-S186. [DOI] [PubMed] [Google Scholar]
- 26.Ross, J. J., M. G. Worthington, and S. L. Gorbach. 2002. Resistance to levofloxacin and failure of treatment of pneumoccal pneumonia. N. Engl. J. Med. 347:65-66. [DOI] [PubMed] [Google Scholar]
- 27.Urban, C., N. Rahman, X. Zhao, N. Mariano, S. Segal-Maurer, K. Drlica, and J. J. Rahal. 2001. Fluoroquinolone-resistant Streptococcus pneumoniae associated with levofloxacin therapy. J. Infect. Dis. 184:794-798. [DOI] [PubMed] [Google Scholar]
- 28.Willett, L. R., J. L. Carson, and J. W. Williams, Jr. 1994. Current diagnosis and management of sinusitis. J. Gen. Intern. Med. 9:38-45. [DOI] [PubMed] [Google Scholar]
- 29.Wortmann, G. W., and S. P. Bennett. 1999. Fatal meningitis due to levofloxacin-resistant Streptococcus pneumoniae. Clin. Infect. Dis. 29:1599-1600. [DOI] [PubMed] [Google Scholar]