SUMMARY
Peripheral tolerance orchestrated by regulatory T cells, dendritic cells (DCs), and mast cells (MCs) has been studied in several models including skin allograft tolerance. We now define a role for MCs in controlling DC behavior (“conditioning”) to facilitate tolerance. Under tolerant conditions, we show that MCs mediated a marked increase in tumor necrosis factor (TNFα)-dependent accumulation of graft-derived DCs in the dLN compared to nontolerant conditions. This increase of DCs in the dLN is due to the local production of granulocyte macrophage colony-stimulating factor (GM-CSF) by MCs that induces a survival advantage of graft-derived DCs. DCs that migrated to the dLN from the tolerant allograft were tolerogenic; i.e., they dominantly suppress T cell responses and control regional immunity. This study underscores the importance of MCs in conditioning DCs to mediate peripheral tolerance and shows a functional impact of peripherally produced TNFα and GM-CSF on the migration and function of tolerogenic DCs.
INTRODUCTION
Mast cells (MCs) populate the mucosa and skin, representing one of the cells earliest to recognize the breach of the host by an invading pathogen. As such, the role of MCs in early innate responses has been well studied (Bischoff and Krämer, 2007; Metz et al., 2008). However, we now know that the early innate response “conditions” the ensuing adaptive response both in a qualitative and quantitative manner to generate appropriate acquired immune responses to control infection. In this context, one of the key immunoregulatory facets of MC function in regulating the development of adaptive immunity is their capacity to regulate the migration of dendritic cells (DCs) from tissue to secondary lymphoid organ. It is well established by the work of Galli and coworkers that tumor necrosis factor α (TNFα) produced by MCs mediates DC migration under inflammatory conditions and in its absence the magnitude of inflammation is ameliorated (Suto et al., 2006). Previously published studies from our lab have shown that MCs are required in a model of skin allograft tolerance mediated by Foxp3+ regulatory T cells (Treg) cells (Lu et al., 2006). It has recently become apparent that, like for inflammation, the trafficking of DCs from tissue to regional secondary lymphoid organs is critical to maintain tolerance as well. It is through the control of DC migration, and the conditioning of tolerogenic DC functions, that MC may exert a profound effect on the maintenance of peripheral tolerance (Dudeck et al., 2011; Ochando et al., 2006).
In this study, we show MCs and MC-derived factors control DC function that is ultimately required for the maintenance of acquired peripheral tolerance. Under inflammatory conditions, MCs are critical for DC migration (Shelburne et al., 2009; Suto et al., 2006). This present study evaluated whether MC also controlled DC migration under tolerogenic conditions and whether this was functionally important in maintaining tolerance. As in inflammatory conditions, we show that DCs from tolerant allografts also migrate in a TNFα-dependent fashion. Upon analysis of the migration of DCs from tolerant tissue, we observed that there was a 3- to 6-fold increased accumulation of DCs in the dLN when compared to the dLN proximal to a syngeneic graft. The migrated DCs were able to dominantly suppress T cell responses even though outnumbered by immune stimulatory DCs in the same dLN. Further studies indicated that the increased accumulation of tolerogenic DCs in the dLN was due to their extended life span. The observation of very high amounts of GM-CSF in allografts of tolerized mice was confirmed to be the mechanism for the enhanced graft survival. Data suggest that MC-derived GM-CSF conditioned the graft-derived tolerogenic DCs by inducing a transcriptional survival program leading to an accumulation in the dLN over time. This finding is shows that MCs play a pivotal role in the conditioning of DCs to mediate tolerance.
RESULTS
DCs Are Critical for the Maintenance of Skin Graft Tolerance
The allograft tolerance induced by donor specific transfusion (DST) and αCD40L is an active process of suppression mediated by the expansion and differentiation of regulatory T (Treg) cells (Quezadaet al., 2003). Sustaining active Treg cell function to maintain the tolerant state may require DCs to trigger antigen-specific Treg cells to mediate suppression. To address the hypothesis as to whether DCs are critical for sustaining allograft tolerance, CD11c-diphtheria toxin receptor mice (Itgax-DTR) were grafted with allogeneic or syngeneic skins. These mice express the DTR under the CD11c promoter and administration of diphtheria toxin (DT) selectively kills cells expressing CD11c (Jung et al., 2002), including DCs. Local treatment of the mice with DT at day 10 post-grafting resulted in rapid rejection of the allograft in pretolerized mice (Figure 1A). Thus, DCs are required for the maintenance of tolerance.
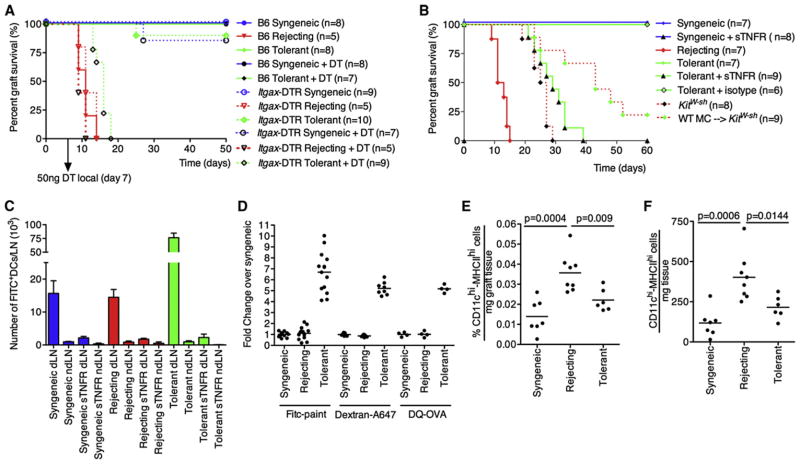
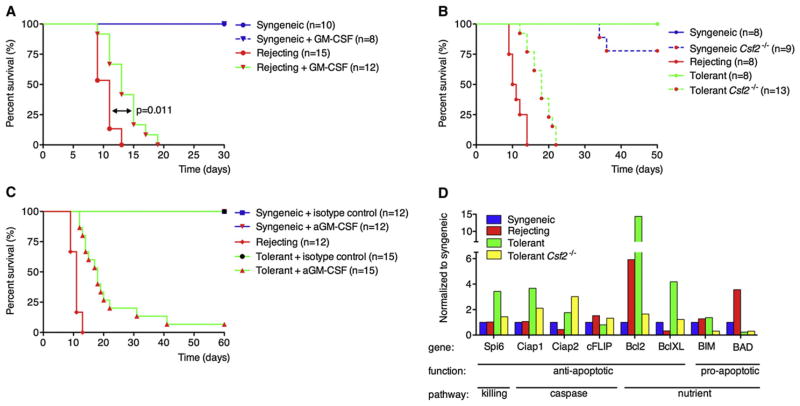
Figure 1. DC Migration Is Required for Establishment of Tolerance.
(A) WT or Itgax-DTR mice were grafted with B6 (syngeneic, syn), F1 skin (rejecting, rej), or F1 skin (tolerant, tol). The tolerant group was pretreated with donor-specific transfusion (DST) and αCD40L. At day 7 postgrafting, DCs were depleted locally by intragraft injection with 50 ng of Diphtheria toxin (DT).
(B) WT mice were grafted as in (A) and DC migration was blocked by intragraft injection of 1 μg of sTNFR-Ig or isotype control at day 1, 3, 5 and 7 post-grafting. Grafts were FITC painted at day 10 post-grafting and 18h later the dLN were analyzed for FITC+ graft-derived DCs.
(C) Mice were grafted as described in (A) and treated with the sTNFR-Ig as in (B). All KitW-sh mice were tolerized with DST and αCD40L. Data are presented as mean ± SEM.
(D) FITC painting (topical application), Dextran-A647 or DQ-OVA (intragraft injections) were compared. The fold change of graft derived DC over control (syngeneic) is depicted.
(E and F) Grafts were collected and weighted before analysis. Relative numbers (E) of total retrieved cells or absolute numbers (F) of DCs per mg graft tissue are shown. Dots in (D)–(F) represent individual mice pooled from at least two independent experiments.
DC Migration and the Role of TNFα in Sustaining Allograft Tolerance
In a vascularized allograft model, it has been shown that the migration of plasmacytoid DCs to the regional LN was essential to sustain peripheral tolerance (Ochando et al., 2006). Figure 1A implicated involvement of DCs in the maintenance of skin allograft tolerance and thus, the importance of DC migration was addressed. To intervene in DC migration, we neutralized TNFα in vivo using soluble TNF receptor-Ig (sTNFR-Ig). Under inflammatory conditions, it has been established that MC-derived TNFα is required for optimal migration of DCs to the dLN and T cell priming (Shelburne et al., 2009; Suto et al., 2006) and we reasoned it would be similarly true under tolerant conditions.
DC migration was measured by painting of skin grafts with FITC at day 10 postgrafting. Graft-derived DCs (CD11c+MHC-II+ FITC+ DCs) in the regional LN of mice bearing a syngeneic graft, a tolerant allograft, or an allograft in the process of rejection were enumerated at 18 hr after application of FITC in combination with sTNFR-Ig. Administration of sTNFR-Ig universally ablated DC migration from syngeneic, allogeneic, and tolerant allografts (Figure 1B). To evaluate whether TNF blockade exerted a functional impact on graft rejection or tolerance, we treated mice with sTNFR-Ig and monitored graft survival. TNF blockade induced acute graft rejection in pre-tolerized mice that normally would have accepted the allograft (Figure 1C). Also, treatment at day 30 postgrafting with sTNFR-Ig led to a break in established tolerance (Figure S1A available online). Taken together, these data are consistent with the hypothesis that TNFα-driven DC migration may be critical to sustain tolerance.
When analyzing the impact of TNFα blockade on DC migration, it was observed that the number of graft-derived DCs from the tolerant allograft was ~6-fold higher than under syngeneic or rejecting conditions (Figure 1D, FITC paint). Trivial explanations for this as FITC drainage to the LN or systemic labeling of DCs was discounted by the fact that TNFα blockade eliminated the appearance of FITC+ DCs in the dLN in all cases (Figure 1B). The possibility of antigen transfer from the migrating DCs to the resident LN DCs was excluded on the basis of the fact that the MFI of the graft-derived DCs was similar to the MFI in the dLN (Figure S1B). Transfer of FITC as a result of representation of migrating DCs is not possible without a reduction in MFI. Finally, the number of DCs in tolerant versus syngeneic in the graft (Figures 1E and 1F) or in the dLN (data not shown) were not grossly different, and actually the rejecting group showed an increase in both relative and absolute numbers of local DCs compared to those in syngeneic and tolerant. Therefore, the number of DCs in tolerant grafts and their migration does not account for the substantial increase of graft derived DCs observed in the regional LN. The increased DC accumulation could be verified by alternative methods of tracking DC migration. Both intragraft injection of Dextran-A647 and DQ-OVA gave a similar result in the fold change over syngeneic controls, although the absolute number of positive DC was reduced (Figure 1D). This also shows that the uptake (dextran-A647) and processing (DQ-OVA) of antigen by graft-derived DCs were not impaired. Additional data to explain the basis for increased accumulation of DCs in tolerant mice will be presented below.
Loss of Graft Tolerance and DC Migration in KitW-sh Mice
The data presented show that DC migration from tolerant allografts is TNFα dependent, and it has been well established that membrane-bound, MC-derived TNFα triggers DC migration during inflammation (Suto et al., 2006). Increased MC numbers have been reported in tolerant allografts (de Vries et al., 2009; Lu et al., 2006), and MC of tolerant, but also syngeneic and rejecting grafts, express membrane-bound TNFα (Figure S1C). Because sTNFR-Ig blocked DC migration (Figure 1B) from tolerant allografts and the MCs expressed membrane-bound TNFα needed for DC migration, we predicted that the absence of MC in pretolerized mice would prohibit the persistence of a tolerant allograft and impair DC migration. As published, pretolerized MC-deficient (KitW-sh) mice begin to lose their grafts at ~20 days (Lu et al., 2006) (Figure 1C). Reconstitution with WT bone marrow-derived MC (BMMCs) can partially restore this defect. For tracking of DC migration, normal and KitW-sh mice grafting was followed by FITC painting at day 10. After 18 hr, the dLNs were analyzed for the presence of graft-derived FITC+ DCs by flow cytometry (Figures 2A and 2B) and histology (Figure 2D). Migration of DCs to the dLN in tolerized KitW-sh mice was profoundly reduced when compared to migration observed in tolerized WT mice (Figure 2B). This reduction could be restored by reconstitution of KitW-sh mice with WT BMMCs to about 70% of what is seen in pre-tolerized C57Bl/6 mice (Figure 2B). The presented data show that there is a MC-dependent, TNFα-dependent increase in graft-derived DCs in the dLNs of tolerant allografts which is critical to maintain allograft survival.
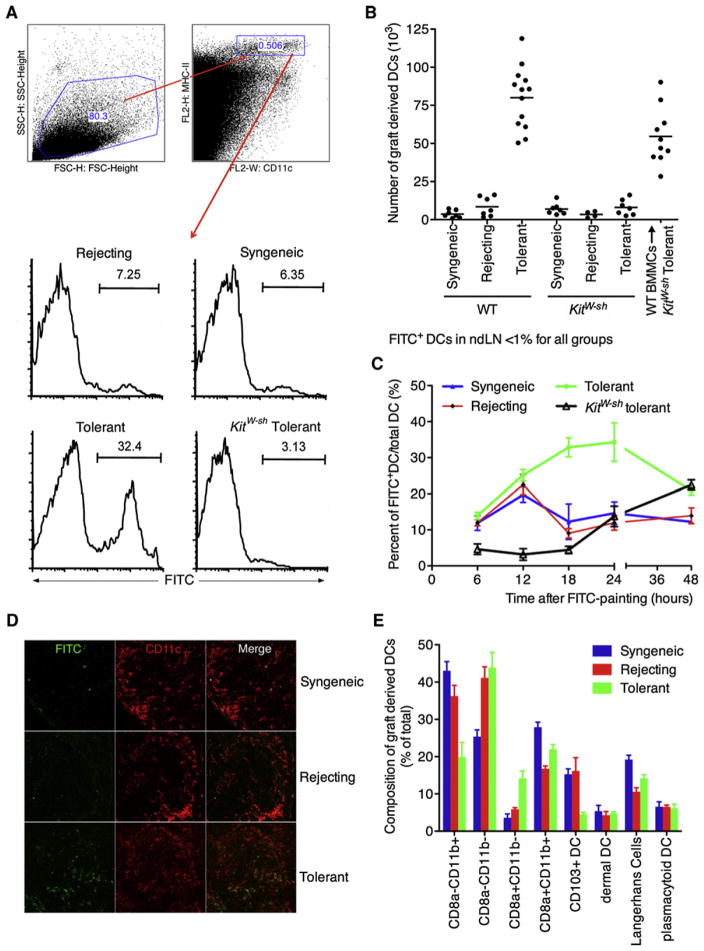
Figure 2. Graft-Derived DCs Accumulate in the Graft dLN under Tolerant Conditions.
(A and B) Grafts of WT or KitW-sh mice were FITC painted at day 10 postgrafting. The graft dLN were collected at 18h later and analyzed for graft derived FITC+ DCs.
(B) Quantification of the total number of FITC+ DCs in the dLN, with every dot representing an individual mouse. Non-dLNs were analyzed and less than 1% of the DCs were FITC positive (data not shown).
(C) Kinetics of graft derived DC accumulation under syngeneic, rejecting and tolerant (WT and KitW-sh) conditions. At day 10 FITC was applied to graft and at different times after FITC painting dLN were analyzed for the presence of FITC+ graft derived DCs.
(D) Histology of the dLN at 18h post FITC painting. Graft derived DCs are FITC+CD11c+.
(E) Composition of the migrating DCs at 12 hr post-FITC painting of day 10 grafts (mean ± SEM).
To generate a more complete profile of DC migration over a longer time period, we monitored graft-derived DC migration to the regional LN over 48 hr (Figure 2C). During the first 12 hr, accumulation of graft-derived DCs was similar between tolerant, syngeneic, and rejecting mice, suggesting that the initial rate of migration is not different between groups. However, after 12 hr, a decline was observed in the number of graft-derived DCs in the syngeneic and rejecting groups whereas the pretolerized mice showed a further increase at 18 hr, which persisted at 24 hr. This confirmed the previous shown data (Figures 1B, 2A, and 2B) that enhanced DC accumulation is observed only under tolerant conditions. Alternatively, KitW-sh mice showed a delayed DC migration, confirming previous studies (Suto et al., 2006). These results show that the initial migratory response of the DCs to the regional LN from all graft types is similar for up to 12 hr. After 12 hr, and up to 48 hr (p48h = 0.0169 compared to syngeneic), the DCs from the tolerant graft appear to accumulate and persist, whereas the DCs from the rejecting and the syngeneic grafts do not. In summary, we showed that there is an increase in both relative and absolute numbers of graft-derived DCs in the dLN under tolerant conditions independent of antigen drainage, antigen transfer, or a higher initial number of DCs in the graft. Further studies addressing the mechanisms of increased DC accumulation in tolerance will be presented below.
Differences in Phenotypes of Migrating Graft-Derived DC Subsets from Tolerant versus Rejecting or Syngeneic Grafts
We performed studies to evaluate the origins and phenotypes of the graft-derived DCs. To determine if the migrating DCs were of donor or host origin, we grafted Ly5.2 mice with Ly5.1 grafts. After FITC painting, we analyzed the dLN for the presence of either Ly5.1 or Ly5.2 DCs. At 18 hr after FITC painting, virtually no Ly5.1 DCs were observed, suggesting that the accumulating DCs are host derived (Figure S2). Additionally, no differences in the surface marker expression of MHC-II, CD80, CD86, and CD40 were observed (data not shown).
The six major populations of migratory DCs present in the skin dLN were quantified by phenotypic analysis of surface markers. The broad differentiation was based on expression of CD8α, in which CD8α+ are from lymphoid origin and the CD8α− are myeloid. Differentiation of Langerhans cells (LHCs) was determined on the basis of the expression of CD326 and CD103 in which the CD103+ DCs are “professional” CD8 T cell presenters (del Rio et al., 2007). Analysis of DC subsets in the dLN of tolerant mice showed that the CD103+ DCs were greatly diminished under tolerizing conditions (Figure 2E). Interestingly, the lymphoid DC subset (CD8α+CD11b−), known to be able to cross-present antigen, was increased. In contrast, the myeloid sub-population (CD8α−CD11b+) showed a reduction (Figure 2E). No Gr1+CD11b+ “myeloid suppressor” DCs (<0.5%) were found within the migrating population of DCs (data not shown). Furthermore, no substantive differences were found in the dermal DC, LHC, or plasmacytoid DC at this time point. Altogether, this results shows that under tolerant conditions, there is a skewing of the early migrating DCs toward the lymphoid subsets at the expense of both myeloid and CD103+ DCs.
Graft-Derived DCs but Not Other DCs in the Graft dLN of Tolerized Mice Are Dominantly Suppressive
Central to the ability of the DCs to maintain tolerance is their capacity to functionally silence alloreactivity. First, the ability of the graft-derived DCs to act as proficient APCs to elicit T cell responses was evaluated. Graft-derived Ly5.1+DCs (by sorting MHCIIhighCD11c+FITC+ cells) were pulsed with ISQ (OVA peptide recognized by OT-II transgenic T cells) and cocultured with CFSE-labeled OVA-specific Ly5.2+ OT-II transgenic T cells (OTII T cells). As expected, OTII T cell proliferation was observed when syngeneic and rejecting graft-derived DCs were used (Figure 3A). However, when graft-derived DCs from tolerized mice (Tolerant (graft)) were used greatly reduced T cell responses were observed. Thus, DCs from a tolerant graft are ineffective APCs.
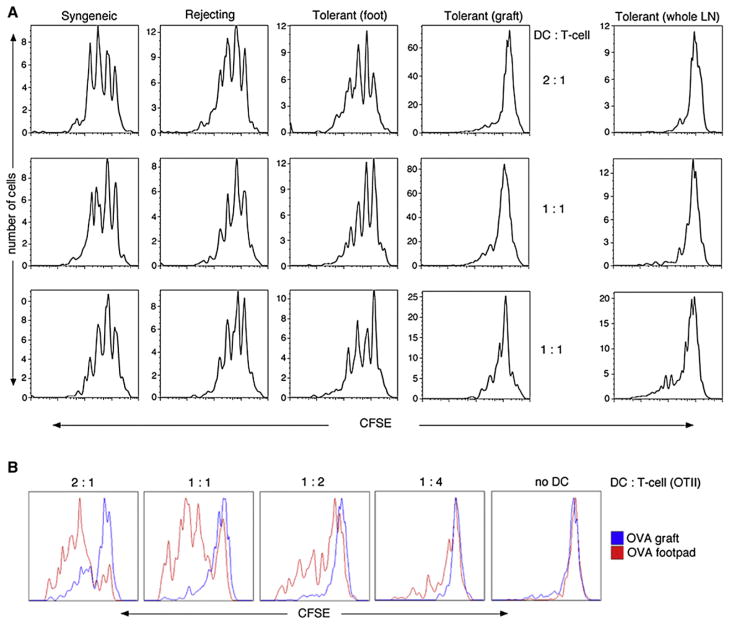
Figure 3. Graft-Derived DCs Are Dominantly Suppressive, whereas Non-Graft-Derived DCs within the Same dLN Still Can Activate T Cells.
(A) Graft-derived Ly5.1+FITC+ DCs (“Syngeneic,” “Rejecting,” and “Tolerant (graft)”) were sorted and ex vivo pulsed with OVA-peptide (ISQ). Alternatively, DCs within the same dLN that were derived from FITC painting the foot were isolated (“Tolerant (foot)”). The non-graft-derived DCs were labeled by FITC painting of the front footpad of the allografted and tolerized mice. Subsequently, the ISQ-pulsed DCs were cocultured for 4 days at different ratios with CFSE labeled OVA-specific TCR transgenic OTII Ly5.2+ T cells. After 4 days, CFSE dye dilution was analyzed within the CD4+Ly5.2+ population. In order to address whether the graft-derived DCs were dominantly suppressive, all dLN DCs without FITC painting (“Tolerant (whole LN)”) were pulsed with ISQ.
(B) Mice were tolerized and at day 10 post grafting and locally treated by either intragraft or front footpad injection of OVA-Alum. This treatment would ensure OVA being processed and presented by either the graft-derived DCs or non-graft-derived DCs. Like in (A), for both treatments axillary and inguinal were used as dLNs. Whole dLNs were collected and the purified DCs were cultured as in (A). Shown are representative histograms of four independent experiments with 3 or 4 mice per group.
Second, the ability of DCs derived from a tolerant allograft to suppress the proliferation of T cells when in the presence of other (non-FITC+) APCs was assessed. As such, in lieu of sorting graft-derived DCs, whole dLN DCs (containing both graft-derived DCs and LN-resident DCs) from tolerized mice without FITC painting were used as a source of APCs. Suppression was observed in the LN draining the tolerant allograft (Figure 3A, tolerant [whole LN]). As expected, DCs in the dLN from mice with syngeneic grafts or rejecting allografts showed robust proliferation (Figure S3). Therefore, in a pool of both immune stimulatoryand tolerogenic DC, suppression is dominant in this model.
Third, we determined whether the coexistence within the dLN of tolerogenic DCs and other DCs permanently ablated APC function of all DCs within that LN. The axillary and inguinal LNs not only drain the graft but also other parts of the skin, including the forelegs. One group of pretolerized mice received FITC painting on the front footpads, which allowed the isolation of a population of FITC+ DCs from a LN that also contained populations of tolerogenic DCs from the nearby tolerant allograft. FITC+ DCs (derived from the front footpads) were purified by cell sorting, pulsed with ISQ, and cocultured with OT-II T cells. Interestingly, the FITC+ DCs derived from outside the graft, but within the same dLN, showed robust proliferation of OT-II T cells (Figure 3A; tolerant [foot]). This demonstrates that within the same dLN, the presence of tolerogenic DCs does not permanently incapacitate the APC activities of other DCs present in the LN.
A final verification that DCs are imprinted differentially in a tolerant site versus a nontolerant site was derived from the following studies as presented in Figure 3B: OVA was injected in either the footpad (nontolerant site; “OVA-Footpad”) or in a tolerant allograft (tolerant site; OVA-Graft) and whole dLN DCs were purified and cocultured with CFSE-labeled OT-II T cells. When OVA was processed by DCs in vivo at a location outside the transplant, robust proliferation was observed (red histograms). However, when OVA was processed by DCs in the tolerant, allogeneic skin graft, T cell proliferation was suppressed (blue histograms). Thus, the functional status of tissue-derived DCs within the regional LN is imprinted by the site of tissue origin.
Graft-Derived DCs of Tolerized Mice Show Increased Survival
The enhanced survival of tolerogenic DCs in the dLN was considered a potential mechanism for the increased numbers of DCs in the dLN of tolerant allografts. In order to address this, we performed an in vivo pulse chase experiment by first FITC painting the graft and after 3 hr blocking migration by an intragraft injection of sTNFR-Ig. We have shown that this blocks all DC migration (Figure 1B). Therefore, only DCs that migrated during the first 3 hr after topical application of FITC would be reaching the graft dLN. Accumulation of FITC+ DCs was still observed with a peak at 12 hr under tolerant conditions whereas both syngeneic and rejecting showed the highest number of graft-derived DCs in the dLN at 9 hr after FITC painting (Figure 4A). However, a steep decline was observed in all groups, most likely because TNFα is also a survival factor for LHCs and DCs (Koch et al., 1990; Ludewig et al., 1995). Nonetheless, the number of graft-derived DCs under tolerant conditions in the dLN was still increased. In conclusion, this suggests that graft-derived, tolerogenic DCs accumulate in the dLN as a result of enhanced persistence.
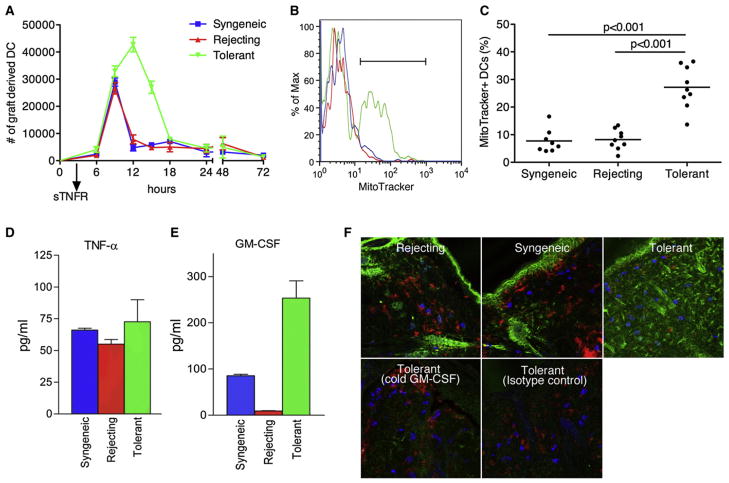
Figure 4. Graft-Derived Tolerogenic DCs Show Enhanced Survival.
(A) At day 10 after receipt of the skin graft, local application of FITC (pulse) was followed by intragraft injection of sTNFR-Ig 3h later to block DC migration. At different time points after FITC painting and sTNFR-Ig blockade, dLNs were collected. The DCs that migrated during the initial 3 hr were quantified (chase). Pooled data from three independent experiments are shown.
(B and C) Because at 12 hr post-FITC painting a split was observed in graft-derived DC accumulation (Figure 2B), DCs were collected at this time point and analyzed for survival by means of MitoTracker (B) and quantified on the basis of the percentage of MitoTracker+ cells (C). Pooled data from thtrr independent experiments with every dot represents a single mouse.
(D and E) Grafts were collected, weighted, and incubated in plain HBSS. Supernatant was collected and analyzed for the presence of TNFα (D) and GM-CSF (E) by ELISA. Pooled data from three independent experiments presented as mean ± SD.
(F) Skin grafts were stained with GM-CSF antibody (green) and costained with CD11c (red) for DCs and CD117 (blue) for MCs. To confirm specificity of the antibody, we either incubated slides with an isotype control or preincubated them with unlabeled GM-CSF antibody of the same clone (“cold GM-CSF”).
Studies were performed to address whether this persistence of DCs in the dLN of tolerant allografts was due to enhanced survival. Graft-derived DCs were analyzed at 12 hr after FITC painting, given that at this point a strong decline in graft-derived DCs was observed in rejecting and syngeneic conditions compared to pretolerized mice (Figure 2C). The graft-derived DCs from tolerized mice showed a higher percentage of viable cells as shown by the use of MitoTracker, a dye that stains active mitochondria (Figure 4B). Within the live gate, ~10% of the graft-derived DCs in the syngeneic showed to be MitoTracker positive, meaning that 90% of the DCs were in the process of undergoing apoptosis. In contrast, under tolerant conditions this was ~25% (Figure 4C). This result was confirmed by AnnexinV staining. The relative change of AnnexinV versus MitoTracker compared to syngeneic was 0.97 versus 1.07 for rejecting and 2.48 versus 3.31 for tolerant. Both MitoTracker and AnnexinV staining suggest that there is a survival advantage of DCs migrating from the immunosuppressive environment of the tolerant graft, leading to a regional accumulation of DCs in the dLN of the accepted allograft over time.
GM-CSF, a DC Survival Factor, Is Abundantly Present within Tolerant Allogeneic Skin Grafts
The prior findings suggested that the cytokine environment of the tolerant allograft might condition the DCs with enhanced longevity. As such, differences in the cytokine milieu among syngeneic, rejecting, and tolerant allografts were investigated with regard to known survival factors for DCs. TNFα clearly showed an impact on the survival of DCs in general (Figure 4A); however, the local concentration was similar between the three different groups (Figure 4D). Another survival factor for DCs is GM-CSF. Luminex as well as ELISA analysis of GM-CSF in the grafts showed markedly increased concentration in tolerant allografts (Figure 4E). Staining of day 10 grafts showed a distinct pattern of GM-CSF only in the tolerant graft (Figure 4F). This could be blocked by preincubation of unlabeled GM-CSF antibody (cold GM-CSF). The observed widespread and not clearly cell-associated staining pattern is most likely caused by the binding of GM-CSF to extracellular heparin containing proteoglycans, which is required for optimal function of this cytokine (Alvarez-Silva and Borojevic, 1996; Modrowski et al., 1998). Considering that we observed changes in the subsets of the migrating DCs (Figure 2E), a possible explanation could be that the subsets had a different sensitivity to GM-CSF, giving survival advantage to one but not the other subsets. We therefore tested purified splenic DCs for potential subset-specific survival in response to GM-CSF. Interestingly, we found that CD11b−CD8α+ DCs accumulated over time at the expense of both CD11b+CD8α− and CD103+ DCs. However, this was independent of GM-CSF because a similar pattern was found in the absence of GM-CSF (Figure S4). The results further strengthened the hypothesis that the accumulation of tolerogenic DCs in the dLN was due to a survival advantage when compared to syngeneic or rejecting conditions and that GM-CSF potentially could provide this survival signal.
GM-CSF Is Necessary for Graft Tolerance
GM-CSF has been repeatedly shown to be involved in the differentiation and survival of DCs in vitro and in vivo (Sallusto and Lanzavecchia, 1994). High amounts of GM-CSF were observed only under tolerant conditions (Figures 4E and 4F), yet the necessity for graft tolerance was not yet clear. In the first experiment, we therefore supplemented GM-CSF by four intragraft injections during the first week after grafting in mice with either syngeneic or allogeneic (rejecting) grafts. Supplementing GM-CSF even for this brief period led to a significant shift (p = 0.011) in graft survival without any treatment prior to grafting (Figure 5A). Conversely, blocking GM-CSF locally with the GM-CSF antibody in either syngeneic or allogeneic (tolerant) grafts during the first week after grafting induced rapid rejection of the tolerant graft in 80% of the mice before day 25 (Figure 5B). The introduction of GM-CSF as well as antibody treatment had no impact on graft survival in syngeneic controls.
Figure 5. GM-CSF Is Required for Tolerance by Inducing Prosurvival Genes in Graft Derived DCs.
(A) Recombinant GM-CSF (50 μl; 800 ng/ml) was injected locally in the graft during the first week after grafting (days 1, 3, 5, and 7) to mice that received either B6 (syngeneic) or F1 (rejecting) skin graft.
(B) GM-CSF (50 μl anti-GMSCF; 10 μg/ml) was blocked locally in syngeneic and tolerized mice at days 1, 3, 5, and 7 and graft survival monitored.
(C) WT or Csf2−/− mice were grafted with syngeneic (B6) or allogeneic (F1) skins and/or tolerized with αCD40L plus DST. Graft rejection was followed for 60 days after grafting.
(D) Graft-derived DCs were purified by sorting and analyzed for genes involved in apoptosis and survival by RT-PCR. For clarity labeling shows which major pathway of apoptosis is affected, and whether the gene is pro- or antiapoptotic. Shown is one of two experiments with similar results.
In addition, we found that the majority of pretolerized GM-CSF-deficient (Csf2−/−) mice acutely rejected the allograft before day 20 postgrafting (Figure 5C), whereas tolerized WT mice normally accept the allograft for over 80 days. To exclude a possible impact of the absence of GM-CSF on the number of MCs, we tested several locations known to harbor MCs. This revealed that Csf2−/− mice have normal numbers of MC in all tested tissues (Figure S5). Thus, the observed rejection in these mice is not due to a reduction in MCs. In summary, we showed that GM-CSF is indispensible for graft tolerance.
Graft-Derived Tolerogenic DCs Show a GM-CSF-Dependent Upregulation of Antiapoptotic Molecules
It is known that the Csf2−/− mice have ~20% reduction in the overall number of DCs (Kingston et al., 2009; Ni and O’Neill, 2001) and that GM-CSF induces a survival program in DCs protecting them from apoptosis (Chen et al., 2007). To address whether GM-CSF impacted survival of DCs in vivo, we sorted graft-derived DCs from the dLN. Genes known to be involved in survival were analyzed by RT-PCR. Downregulation of the proapoptotic BAD and upregulation of the antiapoptotic genes Bcl2 and BclXL were observed in graft-derived DCs of tolerized mice compared to DCs derived from rejecting allogeneic or syngeneic grafts (Figure 5D). Interestingly, Spi-6, known to protect against Gzb-mediated killing, was also upregulated albeit modestly. The caspase pathway does not seem to be affected. Altogether, it seems that GM-CSF renders DCs less sensitive to apoptosis by nutrient and growth factor deprivation and provides some protection against GzB-mediated killing.
MCs Can Produce Large Amounts of GM-CSF and Sustain DC Survival In Vitro
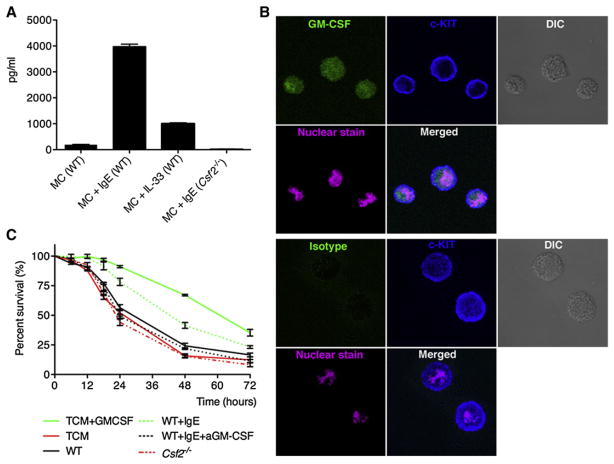
MCs can produce GM-CSF in response to activation by various mechanisms, among which is IgE opsonization. This leads to maturation and activation without degranulation as long as the IgE-specific antigen is not present. However, the pool of IgE in both mouse and man is heterogeneous and each clone can induce a different response when binding to the FcεRI on MCs (Kitaura et al., 2003). Additionally, IL-33 has been shown to mature human MCs without degranulation (Allakhverdi et al., 2007), inducing the production of a variety of immunological mediators including GM-CSF. We therefore first wanted to determine whether BMMCs could produce GM-CSF in response to DNP-specific IgE (clone A3B1) and IL-33 as a positive control. Normal “resting” BMMCs produced very small amounts of GM-CSF, which increased 8-fold by the addition of IL33. However, activation with IgE induced an almost 20-fold increase compared to WT controls (Figure 6A). As expected, IgE activated BMMCs from the Csf2−/− mouse showed no production of GM-CSF. Staining of the IgE-activated and subsequently monensin-treated BMMCs showed a distinct intracellular staining of GM-CSF (Figure 6B). This shows that MCs when activated can produce copious amounts of GM-CSF in vitro.
Figure 6. In Vitro-Cultured MCs Can Produce Large Amounts of Functional GM-CSF when Activated.
(A) GM-CSF production by BMMCs from either WT or Csf2−/− mice after stimulation with nothing, 1 μg/ml of DNP-IgE, or 10 ng/ml recombinant IL-33 for 24 hr.
(B) Confocal images of DNP-IgE stimulated MCs. MCs were treated for 12 hr with DNP-IgE and subsequently for 2 hr in the presence of monensin for intracellular accumulation of GM-CSF (green). Surface staining was done with cKit (blue) and nuclear staining with Hoechst (pink). Pictures were recorded at 63× magnification.
(C) Supernatant of (A) was used for maintaining bead-purified splenic DCs. For 72 hr, DCs were monitored for survival by flow cytometry (MitoTracker+). As a positive control, complete RPMI was supplemented with 20 ng/ml GM-CSF (TCM+GMCSF). For negative controls, complete RPMI or conditioned media from IgE stimulated Csf2−/− MCs was used. Data presented as mean ± SEM are pooled from four independent experiments and had duplicates within each experiment.
To directly assess whether MC can promote DC survival, we created cocultures of BMMCs and splenic DCs; however, the coculture led to the release of the granular content of the MCs and negatively impacted the viability of the DC. Therefore, DC culture media was conditioned by growing BMMCs for 24 hr in the absence or presence of IgE for activation. Normal DC culture conditions included the addition of GM-CSF (positive control) and showed a gradual decline of viability with ~30% survival at 72 hr from MitoTracker staining. In the absence of GM-CSF this was ~10% (Figure 6C). BMMCs without stimulation showed a similar trend as the negative control as did IgE activated BMMCs from the Csf2−/− mice. However, when supernatants of WT BMMCs activated with IgE were used, an enhanced survival of the DCs was observed compared to no GM-CSF (Figure 6C). Similar survival data was obtained by microscopic analysis of the DCs based on trypan blue exclusion (Figure S6). This shows that MCs are indeed capable of producing a sufficient amount of biologically active GM-CSF to prolong DC survival, at least in vitro.
MC-Derived GM-CSF Is Needed for Graft Tolerance
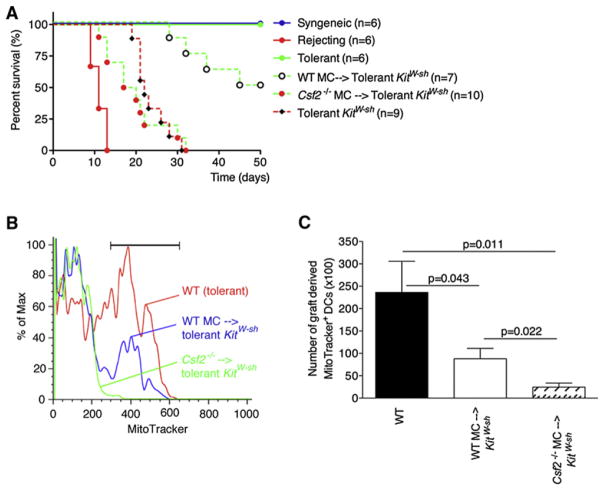
The next question we addressed is whether MC derived GM-CSF is important for the establishment of graft tolerance by increasing the resistance to apoptosis of graft-derived DCs in vivo. To implicate MC derived GM-CSF, we locally reconstituted KitW-sh mice with either WT or Csf2−/− BMMCs. After 8–10 weeks the mice were tolerized and grafted with allogeneic skin. Control B6 mice were age matched and showed a normal pattern of rejection (allogeneic) or acceptance (syngeneic, tolerant allogeneic). KitW-sh mice reconstituted with WT BMMCs showed a gradual loss of tolerance (Figure 7A). However, 8 out of 10 KitW-sh mice receiving the Csf2−/− MC rejected the grafts before day 30. Surprisingly, Csf2−/− MC reconstituted mice rejected their grafts faster than non-reconstituted KitW-sh, suggesting that these MC produce other mediators besides GM-CSF that may be detrimental to graft survival in the absence of GM-CSF.
Figure 7. MC-Derived GM-CSF Is Required for Graft Tolerance by Increasing Longevity of Tolerogenic Graft-Derived DCs.
(A) KitW-sh mice were locally reconstituted with either WT or Csf2−/− BMMCs. Mice were tolerized and grafted 8–10 weeks later and monitored for graft rejection.
(B and C) KitW-sh mice were reconstituted and grafted as in (A). At 10 days postgrafting, FITC was applied to the graft to label the graft-derived DCs. After 12 hr, dLNs were collected and stained with MitoTracker so that the viability of the graft-derived DC could be evaluated. Shown are a representative histogram (B) and accompanying quantification (C). Data were pooled from two independent experiments (n = 10/group, mean ± SEM).
MC-Derived GM-CSF Increased Longevity of Graft-Derived DCs
Finally, we wanted to address whether DC survival was altered when GM-CSF was lacking in the MC compartment only. KitW-sh mice were reconstituted with either WT or Csf2−/− BMMCs, tolerized, and grafted. At day 10 postgrafting, FITC painting was applied and 12 hr later the graft dLNs were collected and analyzed for the number of graft-derived FITC+ DCs and their viability by means of flow cytometry. Reconstitution with WT MCs did not restore the survival of DCs to the extent of WT mice, mean of 5.8% ± 1.7% versus 14.9% ± 4.1%, respectively, probably because of a lower density of MCs, defined as CD45+cKit+FcεRI+ (Figure S7A), in the skin of locally reconstituted mice compared to WT mice. However, the Csf2−/− MC reconstituted KitW-sh mice showed a significant decrease of DC survival compared to WT MC reconstituted KitW-sh mice (mean of 5.8% ± 1.7% versus 2.0% ± 0.7% respectively; p = 0.022) even though the reconstitution efficiency was the same between these two groups (Figure S7B). It should be noted that the total number of cells recovered in the Csf2−/− MC reconstituted mice was lower and thus the total number of recovered FITC+ DCs was also reduced in these mice, this was reflected in the absolute number of MitoTracker+ DC (Figure 7C). Together, these results show that MC-derived GM-CSF has an important role in the survival of graft-derived DCs and that the increased resistance to apoptosis of graft-derived DCs in tolerized mice is required for the establishment of graft tolerance.
DISCUSSION
The findings presented in this study show that MC are indispensable in conditioning DCs to mediate tolerance. First, we show that the MC-dependent, TNFα-dependent migration of DCs from the tolerant allograft to the dLN is required for the maintenance of tolerance. We have not documented that MC-derived TNFα is critical in our studies, but prior studies have clearly demonstrated MC-derived TNFα controls DC migration (Shelburne et al., 2009; Suto et al., 2006). Second, DCs in the dLN of a tolerant allograft accumulate in a MC-dependent process. Third, the DCs from tolerant allografts, but not DCs from a syngeneic graft, are tolerogenic, i.e., incapable of inducing T cell proliferation. Furthermore, the graft-derived DCs are dominantly suppressive even though DCs that present in the same dLN are still able to induce T cell proliferation when tested independently. Fourth, production of GM-CSF from MCs is critical for graft tolerance and conditions the enhanced survival of DCs in tolerant allografts, which accounts for their increased accumulation in the dLN.
Numerous lines of evidence presented suggest that DC migration is critical for maintenance of graft tolerance. DT-mediated depletion of DCs in our model elicited a breakdown in graft tolerance, establishing the involvement of DCs after the initial induction of tolerance with DST and αCD154. This suggests that the “dominant tolerance,” known to be mediated by Foxp3+ Treg cells in this system (Quezada et al., 2005), is in part mediated through the persistent effects of DCs. Furthermore, the breakdown in tolerance with the depletion of DCs is consistent with the recently recognized function of DCs in maintaining peripheral self tolerance (Lutz and Kurts, 2009). From previous studies, it was shown that under inflammatory conditions, MC-derived TNFα was required for optimal DC migration (Shelburne et al., 2009; Suto et al., 2006), and therefore, we considered the role of TNFα in MC-induced DC migration in tolerance. By FITC painting, the data presented clearly show that the regional blockade of TNFα prohibited DC migration and facilitated a breakdown in graft tolerance. Although we did not specifically establish that TNFα production from MCs was involved in graft survival or in controlling DC migration, in the absence of MC, graft tolerance could not be established and DC migration was ablated. Therefore, we presume that MC-derived TNFα is responsible for DC migration both in tolerance and as previously established in inflammation (Shelburne et al., 2009; Suto et al., 2006).
In addition to the need for DC migration, graft-derived DCs from tolerant allografts accumulated in higher numbers in the regional LN when compared to those derived from syngeneic or rejecting allografts. After excluding many possible reasons for the observed increase, we concluded that the most likely explanation for this phenomenon was increased DC survival. Recently, it was shown that an increase of GM-CSF in the uterine endometrium at coitus is required for suppression of T cell responses needed to protect the fetus from being rejected by the mother (Moldenhauer et al., 2010). Maternal tolerance is similar to the acquired tolerance to an allograft. This comparison was further strengthened by our finding that the concentration of GM-CSF in grafts of pretolerized mice was markedly increased as measured by Luminex and ELISA. Therefore, GM-CSF was a potential target important for the induction and maintenance of acquired immune tolerance.
Production of GM-CSF by MC in tolerant allografts appears to play a pivotal role in sustaining graft survival. Besides ELISA, histological analysis also indicated heightened levels of GM-CSF in tolerant allografts, but failed to show cell-specific association. This might be due to the nature of GM-CSF that belongs to the group of heparin-binding growth factors. Besides directly binding of GM-CSF to the GM-CSF receptor, it can also be presented by binding to heparan-containing proteoglycans present on other cells, such as skin fibroblasts and stromal cells. This modulates the biological activity of GM-CSF (Alvarez-Silva and Borojevic, 1996; Modrowski et al., 1998) and explains the observed staining pattern of GM-CSF in our histological studies. Presentation of high levels of GM-CSF by skin fibroblast has shown to inhibit activation and proliferation of bone marrow-derived progenitor cells, whereas low levels of presentation induced activation and proliferation (Carvalho et al., 2000). Thus, the observed low levels of GM-CSF in rejecting allografts could potentially activate DCs but will have minimal influence on their survival. In contrast, the high levels of GM-CSF in tolerized mice could potentially suppress maturation of the graft-derived DCs, rendering them more tolerogenic and additionally increasing their longevity. Because the histology proved inconclusive with regard to MCs being a possible source of GM-CSF, we resorted to analysis of in vitro-cultured BMMCs. After activation of these MCs, we detected intracellular GM-CSF accompanied by copious amounts of GM-CSF in the culture supernatant. Furthermore, enhanced survival was observed when freshly isolated splenic DCs were cultured in this supernatant.
Studies were undertaken to evaluate the molecular basis for the enhanced survival of DCs from tolerant allografts. These studies looked at the expression of molecules involved in apoptosis. There are three main pathways that induce apoptosis, which are intertwined (Chen et al., 2007; Faderl et al., 2003). First, granzyme B (Gzb)-mediated killing by Treg and CD8 Teff cells has been shown to reduce the number of DCs in the tumor dLN and suppress immune responses (Cao et al., 2007). Gzb-mediated killing can be evaded by upregulation of the serpin protease inhibitor Spi6 (Medema et al., 2001). Given that we had previously shown that skin graft tolerance is maintained by Gzb+ Treg cells (Gondek et al., 2008), it was not surprising to see an upregulation of Spi6 in the graft-derived DC. Second, activation of the caspase pathway was not affected in our model, even though in vitro studies showed upregulation of the suppressors Ciap1 and C-FLIP due to the presence of GM-CSF (Faderl et al., 2003; Leverkus et al., 2000). Third, the nutrient deprivation pathway was the most strikingly affected. In concordance with in vitro-cultured DCs, GM-CSF induced the expression of both the antiapoptotic molecules Bcl2 and BclXL, in conjunction with the downregulation of the proapoptotic molecule BAD. This would suggest that in the transplant there might a local depletion of one or more of the essential amino acids, which has been shown to be an important mechanism to induce and maintain tolerance (Cobbold et al., 2009).
To further address the importance of GM-CSF in allograft tolerance, we performed a series of experiments manipulating GM-CSF in vivo. Removing GM-CSF by either blocking antibodies or by using Csf2−/− mice in pretolerized mice induced rejection whereas introducing GM-CSF to untreated mice with an allograft could delay rejection. This confirms that GM-CSF is required for the maintenance of tolerance. Additionally, GM-CSF has a profound impact on one of the major producers of GM-CSF outside the bone marrow, the MC. The presence of GM-CSF has shown to downregulate cKit and FcεRI and levels of tryptase and histamine in in vitro-cultured MCs (Welker et al., 2001). This would suggest that the MCs in the graft are less sensitive to degranulation due to the high concentration of local GM-CSF. This further contributes to maintenance of tolerance because previously we showed that degranulation is detrimental for graft survival (de Vries et al., 2009).
In 2006, we showed that MCs are required for the induction and maintenance of graft tolerance (Lu et al., 2006). This study provides a mechanistic and molecular explanation for this requirement by functionally linking MCs to DCs and showing that MCs are pivotal cells in conditioning DCs to mediate tolerance.
EXPERIMENTAL PROCEDURES
Mice
C57Bl/6 (Ly5.1+ or Ly5.2+), CB6F1, C57Bl/6-TCRb5.2, C57Bl/6J.KitW-sh (Jackson Laboratory, Bar Harbor, Maine, USA) mice were used. The Ly5.2+ mice were bred onto OTII CD4-Tg mice. B6.Itgax-DTR mice were a kind gift of J.R. Conejo-Garcia (Dartmouth Medical School, Lebanon, NH, USA). B6.Csf2−/− mice were provided by J.A. Whitsett (University of Cincinnati College of Medicine, Cincinnati, Ohio, USA). All animal protocols were approved by the Institutional Animal Care and Use Committee of Dartmouth College.
Skin Graft Model
Skin grafting was performed in accordance with the procedure described previously (Quezada et al., 2005).
In Vivo Tracking of DC Migration
For migration studies, 8 μl of 5 mg/ml FITC-paint was applied directly onto the preshaved graft, whereas Dextran-A647 and DQ-OVA were injected subcutaneously (s.c.) in the graft. DC migration from the graft was blocked by local injection of 50 μl of sTNFR-Ig (20 μg/ml). For DC depletion, Itgax-DTR mice were locally treated with 10 ng of DT.
Cytokine Analysis
Day 10 grafts were incubated in HBSS (500 mg graft tissue/ml) for 1 hr at 37°C. Cytokines in the supernatant were analyzed by multiplex analysis and reconfirmed by ELISA. GM-CSF production by MCs in vitro was measured by ELISA.
Immunohistochemistry
dLN were collected at 18 hr after FITC painting of day 10 grafts and compared to graft dLN of day 11 grafts without FITC painting. Slides were stained with CD11c and Hoechst. Skin sections were stained with cKit, CD11c, and GM-CSF. For cytospins, week 6 BMMCs were activated with 1 μg/ml of DNP-IgE for 12 hr followed by 2 hr in the presence of monensin. Cells were stained with cKit, FcεRI, GM-CSF, and Hoechst as nuclear stain. GM-CSF staining controls included isotype (Rat IgG2b-FITC) and preincubation with unlabelled GM-CSF. All slides were recorded by confocal microscopy (LSM 510Meta, Zeiss).
Dendritic Cell Phenotyping and Functionality
Day 10 graft derived FITC+ DCs 12h post-FITC painting were analyzed for six major subsets known to be present in skin dLNs. Survival potential of DCs subsets in response to GM-CSF was assessed in vitro over time. For functionality studies, dLN were collected after FITC painting and Ly5.1+CD11c+ MHCIIhighFITC+ DCs were sorted (FACSAria) yielding purities of over 98%. DCs were pulsed with ISQ (1 hr, 37°C), washed, and plated at different ratios with 40.103 naive CFSE-labeled Ly5.2+ OT-II T cells and analyzed for CFSE dye dilution on day 4. For both dominant suppression and antigen-specific suppression, DCs from whole LN were sorted. For antigen-specific suppression in vivo, mice were injected with 50 μl OVA-Alum (1 mg/ml) either in the graft or the front footpad prior to DC sorting.
In Vitro Analysis of MC-Induced DC Survival
WT and Csf2−/− BMMCs were cultured for 3 weeks with 20 ng/ml recombinant IL-3 and subsequently for 3–5 weeks with rIL-3 and 50 ng/ml rSCF. MCs were plated at a concentration of 1.106/ml in the presence or absence of 1 μg/ml DNP-IgE or 10 ng/ml rIL-33 for activation. Purified splenic DCs were plated in the different MC-conditioned media at 40.103/well. As positive control, DCs were cultured with 20 ng/ml rGM-CSF whereas for negative control, this was omitted. DCs were analyzed for cell death by MitoTracker deep red and trypan blue.
In Vivo Analysis of DC Survival
Grafts of syngeneic, rejecting, and tolerized mice were FITC painted and dLNs were analyzed at 3, 6, 9, 12, 15, 24, 48, and 72 hr after FITC painting for the presence of graft-derived DC by flow cytometry either in the presence or absence of sTNFR-Ig. Additionally FITC+ DCs were analyzed at 12 hr post-FITC painting for viability by MitoTracker staining.
RT-PCR on Graft-Derived DCs
FITC+ graft-derived DCs were purified at day 10 postgrafting and 12 hr after FITC painting. DCs were sorted directly into lysis buffer (Ambion). RNA was purified and amplified before analysis by RT-PCR (MessageAmp II aRNA Amplification kit, Applied Biosciences). Purity was evaluated after each step.
In Vivo Studies on the Role of MC-Derived GMCSF in DC Survival
Mice were grafted with either syngeneic or allogeneic grafts without prior treatment. At day 1, 3, 5, and 7 mice received a local intragraft injection of 50 μl recombinant GM-CSF (800 ng/ml). Graft rejection was followed for 30 days after grafting. Conversely, tolerized animals and syngeneic controls received 50 μl αGMSCF (10 μg/ml) at the same days so that GM-CSF was blocked locally. Additionally, Csf2−/− mice were grafted with syngeneic or allogeneic skins (rejecting and tolerant). To specifically address the role of MCs, we reconstituted KitW-sh mice locally with 6–8 weeks of cultured WT or Csf2−/− BMMCs (Lu, et al., 2006). After 8 weeks, mice were tolerized and grafted. After each experiment, mice were analyzed for the presence of cKit+FcεRIhigh cells so that MC reconstitution could be confirmed. Mice that failed to reconstitute were excluded from the studies.
Statistics
All statistics have been calculated with the Prism 4.03 software package (GraphPad Software). Luminex data are shown as mean ± SD, whereas other data are expressed as mean ± SEM. Statistical significance was calculated for a 95% confidence interval (p < 0.05). Statistical methods are further specified in the Supplemental Experimental Procedures.
Supplementary Material
Acknowledgments
The authors acknowledge support of National Institutes of Health grant (R01AT005382, AI048667, United States), “The Wellcome Trust,” MRC Centre for Transplantation (United Kingdom), National Institute for Health Research (NIHR) Biomedical Research Centre (BRC) at Guy’s and St Thomas’ NHS Foundation Trust and King’s College London and the Agency of Science, Technology and Research (A*STAR, Singapore). E.C.N. is supported by a postdoctoral fellowship from National Multiple Sclerosis Society. C.O. is recipient of a postdoctoral fellowship from BECAS Chile.
Footnotes
Supplemental Information includes seven figures and Supplemental Experimental Procedures and can be found with this article online at doi:10.1016/ j.immuni.2011.09.012.
References
- Allakhverdi Z, Smith DE, Comeau MR, Delespesse G. Cutting edge: The ST2 ligand IL-33 potently activates and drives maturation of human mast cells. J Immunol. 2007;179:2051–2054. doi: 10.4049/jimmunol.179.4.2051. [DOI] [PubMed] [Google Scholar]
- Alvarez-Silva M, Borojevic R. GM-CSF and IL-3 activities in schistosomal liver granulomas are controlled by stroma-associated heparan sulfate proteoglycans. J Leukoc Biol. 1996;59:435–441. doi: 10.1002/jlb.59.3.435. [DOI] [PubMed] [Google Scholar]
- Bischoff SC, Krämer S. Human mast cells, bacteria, and intestinal immunity. Immunol Rev. 2007;217:329–337. doi: 10.1111/j.1600-065X.2007.00523.x. [DOI] [PubMed] [Google Scholar]
- Cao X, Cai SF, Fehniger TA, Song J, Collins LI, Piwnica-Worms DR, Ley TJ. Granzyme B and perforin are important for regulatory T cell-mediated suppression of tumor clearance. Immunity. 2007;27:635–646. doi: 10.1016/j.immuni.2007.08.014. [DOI] [PubMed] [Google Scholar]
- Carvalho MA, Arcanjo K, Silva LC, Borojevic R. The capacity of connective tissue stromas to sustain myelopoiesis depends both upon the growth factors and the local intercellular environment. Biol Cell. 2000;92:605–614. doi: 10.1016/s0248-4900(01)01113-3. [DOI] [PubMed] [Google Scholar]
- Chen M, Huang L, Shabier Z, Wang J. Regulation of the lifespan in dendritic cell subsets. Mol Immunol. 2007;44:2558–2565. doi: 10.1016/j.molimm.2006.12.020. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cobbold SP, Adams E, Farquhar CA, Nolan KF, Howie D, Lui KO, Fairchild PJ, Mellor AL, Ron D, Waldmann H. Infectious tolerance via the consumption of essential amino acids and mTOR signaling. Proc Natl Acad Sci USA. 2009;106:12055–12060. doi: 10.1073/pnas.0903919106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- del Rio ML, Rodriguez-Barbosa JI, Kremmer E, Forster R. CD103- and CD103+ bronchial lymph node dendritic cells are specialized in presenting and cross-presenting innocuous antigen to CD4+ and CD8+ T cells. J Immunol. 2007;178:6861–6866. doi: 10.4049/jimmunol.178.11.6861. [DOI] [PubMed] [Google Scholar]
- de Vries VC, Wasiuk A, Bennett KA, Benson MJ, Elgueta R, Waldschmidt TJ, Noelle RJ. Mast cell degranulation breaks peripheral tolerance. Am J Transplant. 2009;9:2270–2280. doi: 10.1111/j.1600-6143.2009.02755.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dudeck A, Suender CA, Kostka SL, von Stebut E, Maurer M. Mast cells promote Th1 and Th17 responses by modulating dendritic cell maturation and function. Eur J Immunol. 2011;41:1883–1893. doi: 10.1002/eji.201040994. [DOI] [PubMed] [Google Scholar]
- Faderl S, Harris D, Van Q, Kantarjian HM, Talpaz M, Estrov Z. Granulocyte-macrophage colony-stimulating factor (GM-CSF) induces antiapoptotic and proapoptotic signals in acute myeloid leukemia. Blood. 2003;102:630–637. doi: 10.1182/blood-2002-06-1890. [DOI] [PubMed] [Google Scholar]
- Gondek DC, Devries V, Nowak EC, Lu LF, Bennett KA, Scott ZA, Noelle RJ. Transplantation survival is maintained by granzyme B+ regulatory cells and adaptive regulatory T cells. J Immunol. 2008;181:4752–4760. doi: 10.4049/jimmunol.181.7.4752. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jung S, Unutmaz D, Wong P, Sano G, De los Santos K, Sparwasser T, Wu S, Vuthoori S, Ko K, Zavala F, et al. In vivo depletion of CD11c+ dendritic cells abrogates priming of CD8+ T cells by exogenous cell-associated antigens. Immunity. 2002;17:211–220. doi: 10.1016/s1074-7613(02)00365-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kingston D, Schmid MA, Onai N, Obata-Onai A, Baumjohann D, Manz MG. The concerted action of GM-CSF and Flt3-ligand on in vivo dendritic cell homeostasis. Blood. 2009;114:835–843. doi: 10.1182/blood-2009-02-206318. [DOI] [PubMed] [Google Scholar]
- Kitaura J, Song J, Tsai M, Asai K, Maeda-Yamamoto M, Mocsai A, Kawakami Y, Liu FT, Lowell CA, Barisas BG, et al. Evidence that IgE molecules mediate a spectrum of effects on mast cell survival and activation via aggregation of the FcepsilonRI. Proc Natl Acad Sci USA. 2003;100:12911–12916. doi: 10.1073/pnas.1735525100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koch F, Heufler C, Kämpgen E, Schneeweiss D, Böck G, Schuler G. Tumor necrosis factor alpha maintains the viability of murine epidermal Langerhans cells in culture, but in contrast to granulocyte/macrophage colony-stimulating factor, without inducing their functional maturation. J Exp Med. 1990;171:159–171. doi: 10.1084/jem.171.1.159. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leverkus M, Walczak H, McLellan A, Fries HW, Terbeck G, Bröcker EB, Kämpgen E. Maturation of dendritic cells leads to up-regulation of cellular FLICE-inhibitory protein and concomitant down-regulation of death ligand-mediated apoptosis. Blood. 2000;96:2628–2631. [PubMed] [Google Scholar]
- Lu LF, Lind EF, Gondek DC, Bennett KA, Gleeson MW, Pino-Lagos K, Scott ZA, Coyle AJ, Reed JL, Van Snick J, et al. Mast cells are essential intermediaries in regulatory T-cell tolerance. Nature. 2006;442:997–1002. doi: 10.1038/nature05010. [DOI] [PubMed] [Google Scholar]
- Ludewig B, Graf D, Gelderblom HR, Becker Y, Kroczek RA, Pauli G. Spontaneous apoptosis of dendritic cells is efficiently inhibited by TRAP (CD40-ligand) and TNF-alpha, but strongly enhanced by interleukin-10. Eur J Immunol. 1995;25:1943–1950. doi: 10.1002/eji.1830250722. [DOI] [PubMed] [Google Scholar]
- Lutz MB, Kurts C. Induction of peripheral CD4+ T-cell tolerance and CD8+ T-cell cross-tolerance by dendritic cells. Eur J Immunol. 2009;39:2325–2330. doi: 10.1002/eji.200939548. [DOI] [PubMed] [Google Scholar]
- Medema JP, Schuurhuis DH, Rea D, van Tongeren J, de Jong J, Bres SA, Laban S, Toes RE, Toebes M, Schumacher TN, et al. Expression of the serpin serine protease inhibitor 6 protects dendritic cells from cytotoxic T lymphocyte-induced apoptosis: Differential modulation by T helper type 1 and type 2 cells. J Exp Med. 2001;194:657–667. doi: 10.1084/jem.194.5.657. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Metz M, Siebenhaar F, Maurer M. Mast cell functions in the innate skin immune system. Immunobiology. 2008;213:251–260. doi: 10.1016/j.imbio.2007.10.017. [DOI] [PubMed] [Google Scholar]
- Modrowski D, Lomri A, Marie PJ. Glycosaminoglycans bind granulocyte-macrophage colony-stimulating factor and modulate its mitogenic activity and signaling in human osteoblastic cells. J Cell Physiol. 1998;177:187–195. doi: 10.1002/(SICI)1097-4652(199810)177:1<187::AID-JCP19>3.0.CO;2-A. [DOI] [PubMed] [Google Scholar]
- Moldenhauer LM, Keenihan SN, Hayball JD, Robertson SA. GM-CSF is an essential regulator of T cell activation competence in uterine dendritic cells during early pregnancy in mice. J Immunol. 2010;185:7085–7096. doi: 10.4049/jimmunol.1001374. [DOI] [PubMed] [Google Scholar]
- Ni K, O’Neill HC. Development of dendritic cells from GM-CSF−/− mice in vitro : GM-CSF enhances production and survival of cells. Dev Immunol. 2001;8:133–146. doi: 10.1155/2001/68024. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochando JC, Homma C, Yang Y, Hidalgo A, Garin A, Tacke F, Angeli V, Li Y, Boros P, Ding Y, et al. Alloantigen-presenting plasmacytoid dendritic cells mediate tolerance to vascularized grafts. Nat Immunol. 2006;7:652–662. doi: 10.1038/ni1333. [DOI] [PubMed] [Google Scholar]
- Quezada SA, Fuller B, Jarvinen LZ, Gonzalez M, Blazar BR, Rudensky AY, Strom TB, Noelle RJ. Mechanisms of donor-specific transfusion tolerance: Preemptive induction of clonal T-cell exhaustion via indirect presentation. Blood. 2003;102:1920–1926. doi: 10.1182/blood-2003-02-0586. [DOI] [PubMed] [Google Scholar]
- Quezada SA, Bennett K, Blazar BR, Rudensky AY, Sakaguchi S, Noelle RJ. Analysis of the underlying cellular mechanisms of anti-CD154-induced graft tolerance: The interplay of clonal anergy and immune regulation. J Immunol. 2005;175:771–779. doi: 10.4049/jimmunol.175.2.771. [DOI] [PubMed] [Google Scholar]
- Sallusto F, Lanzavecchia A. Efficient presentation of soluble antigen by cultured human dendritic cells is maintained by granulocyte/macrophage colony-stimulating factor plus interleukin 4 and downregulated by tumor necrosis factor alpha. J Exp Med. 1994;179:1109–1118. doi: 10.1084/jem.179.4.1109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shelburne CP, Nakano H, St John AL, Chan C, McLachlan JB, Gunn MD, Staats HF, Abraham SN. Mast cells augment adaptive immunity by orchestrating dendritic cell trafficking through infected tissues. Cell Host Microbe. 2009;6:331–342. doi: 10.1016/j.chom.2009.09.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suto H, Nakae S, Kakurai M, Sedgwick JD, Tsai M, Galli SJ. Mast cell-associated TNF promotes dendritic cell migration. J Immunol. 2006;176:4102–4112. doi: 10.4049/jimmunol.176.7.4102. [DOI] [PubMed] [Google Scholar]
- Welker P, Grabbe J, Zuberbier T, Grützkau A, Henz BM. GM-CSF downmodulates c-kit, Fc(epsilon)RI(alpha) and GM-CSF receptor expression as well as histamine and tryptase levels in cultured human mast cells. Arch Dermatol Res. 2001;293:249–258. doi: 10.1007/s004030100225. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.