Abstract
Doripenem (S-4661), a new parenteral carbapenem, was tested against over 250 clinical isolates, mutants, and transconjugants of Enterobacteriaceae and Acinetobacter spp., selected or derived for their β-lactamase expression characteristics. Imipenem, meropenem, and ertapenem were tested as comparators, along with cephalosporins and piperacillin-tazobactam, by using National Committee for Clinical Laboratory Standards agar dilution methodology. Doripenem MICs were from 0.03 to 0.25 μg/ml for Klebsiella isolates, irrespective of the presence of extended-spectrum β-lactamases (ESBLs) or plasmid-mediated AmpC or hyperproduced K1 β-lactamase. Similarly, MICs of doripenem for both AmpC-inducible and -derepressed Enterobacter isolates were 0.06 to 0.5 μg/ml. ESBL production did not raise the MICs of doripenem for Escherichia coli transconjugants, and studies with known expression mutants confirmed that neither inducible nor depressed AmpC β-lactamase expression was protective in Enterobacter cloacae, Citrobacter freundii, Serratia marcescens, or Morganella morganii. In all of these respects, doripenem resembled meropenem and imipenem, whereas the MICs of ertapenem were raised (but still ≤1 μg/ml) for many ESBL-producing klebsiellas and AmpC-derepressed E. cloacae and C. freundii strains. Resistance to all carbapenems, including doripenem (MICs of mostly 16 to 64 μg/ml, compared with 0.25 to 1 μg/ml for typical strains), was seen in Acinetobacter isolates with metallo-β-lactamases or OXA-carbapenemases. Isolates of Klebsiella and Serratia spp. with IMP, KPC, and SME β-lactamases also were resistant to doripenem (MICs, 8 to >64 μg/ml) and to other carbapenems, although the continued apparent susceptibility (MICs, ≤0.5 μg/ml) of E. coli derivatives with cloned IMP-1 and NMC-A β-lactamases suggested that carbapenem resistance might require other factors besides the enzymes.
Broad-spectrum activity coupled with stability to AmpC and extended-spectrum β-lactamases (ESBLs) makes the carbapenems an extremely attractive class of antibiotics for further development (6). An additional advantage is that carbapenems, unlike extended-spectrum cephalosporins, do not select AmpC-derepressed mutants from inducible populations (3).
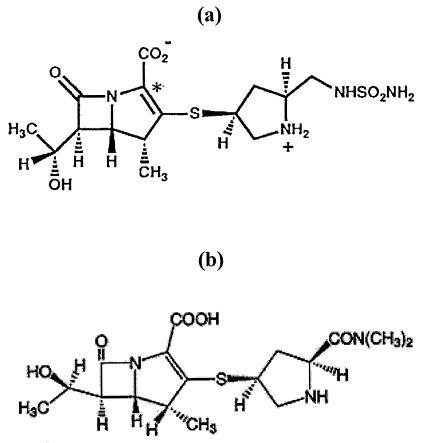
Doripenem (formerly S-4661; Fig. 1) (16, 17, 19) is a novel parenteral carbapenem analogue developed by Shionogi and Co., Ltd. Doripenem displays broad-spectrum activity against gram-positive and gram-negative bacteria both in vitro and in vivo (T. Nishino, M. Otsuki, M. Izawa, Abstr. 36th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-115, 1996; K. Yamaguchi and J. Shimada, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. S-4661, 1997). Like existing carbapenems, it has a trans-configured 6-hydroxyethyl, and like meropenem and ertapenem, but not imipenem, has a 1-β-methyl substituent, which protects against hydrolysis by renal dehydropeptidase I (8). The 2′ substituent is similar to that of meropenem, but is more polar, with a free amino group.
FIG. 1.
Structures of doripenem (a) and meropenem (b).
The present studies assessed the in vitro activity of doripenem against isolates, mutants, and transconjugants of Enterobacteriaceae and Acinetobacter spp. with known β-lactamases and β-lactamase expression, seeking to determine its activity against such strains and to identify whether it shares the excellent β-lactamase stability of existing carbapenems, which were tested in parallel.
MATERIALS AND METHODS
Test strains. (i) Resistant clinical isolates and controls.
The Klebsiella pneumoniae and Klebsiella oxytoca isolates were collected from intensive care unit patients in western and southern Europe during surveys in 1994 (13) and 1997 to 1998 (2). They included 50 isolates inferred to have ESBLs on the basis of ceftazidime/ceftazidime-clavulanate MIC ratios of ≥16, 21 K. oxytoca isolates with hyperproduction of K1 enzyme, and five klebsiellas with plasmid-mediated AmpC enzymes. Ten wild-type Klebsiella isolates, from the same surveys but without these resistance mechanisms, were used as controls: these isolates were susceptible to extended-spectrum cephalosporins at ≤2 μg/ml and gave ceftazidime/ceftazidime-clavulanate MIC ratios of ≤ 4. AmpC-derepressed Enterobacter spp. and cephalosporin-susceptible Enterobacter control strains were obtained from recent British and Irish surveys or were reference submissions. Isolates of carbapenemase-producing Enterobacteriaceae comprised (i) S. marcescens S6 with SME-1 enzyme (24); (ii) recent clinical isolates of K. pneumoniae with KPC-3 enzyme (P. Tierno, Jr., L. Tysall, M.-F. Palepou, K. Young, R. Painter, D. Suber, D. Shungu, L. Silver, K. Inglima, J. Kornblum, N. Woodford, and D. Livermore, Abstr. 43rd Intersci. Conf. Antimicrob. Agents. Chemother., poster C2-50, 2003); and (iii) porin-sufficient and -deficient variants of K. pneumoniae K4181, a clinical isolate from Singapore with the IMP-1 metallo-β-lactamase (T. H. Koh, L. H. Sng, G. S. Babini, N. Woodford, D. M. Livermore, and L. M. Hall, Letter, Antimicrob. Agents Chemother. 45:1939-1940, 2001). The carbapenem-resistant Acinetobacter isolates included those from the United Kingdom and Hong Kong with IMP metallo-β-lactamases (4, 20) and isolates collected worldwide with OXA carbapenemases (1, 5, 14). Carbapenem-susceptible Acinetobacter isolates were controls from a recent United Kingdom survey (7). Escherichia coli ATCC 25922 was a general control.
(ii) Chromosomal β-lactamase expression mutants.
The isolates of the chromosomal β-lactamase expression mutant series of Enterobacter cloacae, Citrobacter freundii, Serratia marcescens, Morganella morganii, and Proteus vulgaris were described previously (12, 23). Most of these mutant series comprised a β-lactamase-inducible parent isolate together with its AmpC-derepressed and basal mutants, but some lacked an inducible strain, having been derived from isolates that were already derepressed when first cultured from patients. The β-lactamases of these species are AmpC types, except that P. vulgaris has a class A chromosomal enzyme.
(iii) Escherichia coli transconjugants.
Transconjugants of E. coli K-12 J53 and J62, DH5-α, and JM83/109 with various plasmid-mediated β-lactamases were described previously (12) and were prepared by plate or broth mating or, in a few cases, by cloning and transformation.
Susceptibility tests.
MICs were determined by the National Committee for Clinical Laboratory Standards (NCCLS) agar dilution method (18). The drugs tested comprised doripenem (lot no. CF 2066, manufactured by Shionogi and Co., Ltd., Osaka, Japan), ertapenem and imipenem (Merck, Hoddesdon, Hertfordshire, United Kingdom), meropenem (AstraZeneca, Macclesfield, Cheshire, United Kingdom), ceftazidime (GlaxoSmithKline, Stevenage, Hertfordshire, United Kingdom), cefepime (Bristol Myers Squibb, Hounslow, Middlesex, United Kingdom), piperacillin and tazobactam (Wyeth, Taplow, Berkshire, United Kingdom), and ampicillin (Sigma, Poole, Dorset, United Kingdom). Tazobactam was used at a fixed concentration of 4 μg/ml in combination with piperacillin.
RESULTS
Klebsiella spp. with ESBLs, AmpC β-lactamases, or hyperproduced K1 enzyme.
MIC distributions for Klebsiella isolates with ESBLs, plasmid-mediated AmpC enzymes, or hyperproduced K1 β-lactamase are shown in Table 1, together with MICs for control isolates with none of these mechanisms. MICs of doripenem were similarly distributed for all these groups, with modal values of 0.03 to 0.06 μg/ml and with no trend for β-lactamase producers to be less susceptible than the controls. Likewise, the MIC distributions for meropenem and imipenem were independent of ESBLs, AmpC, or hyperproduced K1 enzyme. In contrast, ertapenem MICs for ESBL-positive Klebsiella isolates were scattered from 0.03 to 1 μg/ml, whereas values for ESBL-negative isolates were clustered around 0.03 μg/ml, confirming earlier results that ESBLs are associated with raised (although still low) MICs of this carbapenem (11, 12). ESBL producers were resistant to aztreonam and cephalosporins or had greatly reduced susceptibility, whereas the MIC distribution of piperacillin-tazobactam was bimodal, with some producers susceptible, with MICs of 2 ± 4 to 16 ± 4 μg/ml, while others were highly resistant, with MICs of >64 ± 4 μg/ml. Ampicillin was predictably inactive, because Klebsiella spp. have chromosomal SHV-1 or K1 enzymes that hydrolyze this drug (G. S. Babini and D. M. Livermore, Letter, Antimicrob. Agents Chemother. 44:2230, 2000.).
TABLE 1.
MICs for isolates of Klebsiella and Enterobacter spp. with β-lactamase-mediated resistance
| Isolate (n) and agent | MIC (μg/ml)a
|
||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| ≤0.03 | 0.06 | 0.12 | 0.25 | 0.5 | 1 | 2 | 4 | 8 | 16 | 32 | 64 | >64 | |
| Klebsiella spp. | |||||||||||||
| ESBL producers (50) | |||||||||||||
| Doripenem | 11 | 22 | 12 | 5 | |||||||||
| Meropenem | 22 | 28 | |||||||||||
| Imipenem | 13 | 18 | 12 | 9 | 2 | ||||||||
| Ertapenem | 10 | 31 | 3 | 4 | 1 | 1 | |||||||
| Piperacillin-tazobactam | 4 | 13 | 14 | 2 | 1 | 16 | |||||||
| Aztreonam | 1 | 2 | 4 | 3 | 2 | 38 | |||||||
| Ceftazidime | 1 | 6 | 6 | 37 | |||||||||
| Cefepime | 1 | 2 | 9 | 15 | 12 | 5 | 4 | 0 | 2 | ||||
| Ampicillin | 50 | ||||||||||||
| AmpC producers (5) | |||||||||||||
| Doripenem | 2 | 3 | |||||||||||
| Meropenem | 4 | 1 | |||||||||||
| Imipenem | 1 | 4 | |||||||||||
| Ertapenem | 2 | 3 | |||||||||||
| Piperacillin-tazobactam | 2 | 2 | 1 | ||||||||||
| Aztreonam | 2 | 2 | 1 | ||||||||||
| Ceftazidime | 2 | 3 | |||||||||||
| Cefepime | 1 | 1 | 2 | 1 | |||||||||
| Ampicillin | 5 | ||||||||||||
| K. oxytoca K1 hyperproducers (21) | |||||||||||||
| Doripenem | 2 | 17 | 1 | 1 | |||||||||
| Meropenem | 5 | 15 | 1 | ||||||||||
| Imipenem | 2 | 11 | 7 | 1 | |||||||||
| Ertapenem | 11 | 8 | 2 | ||||||||||
| Piperacillin-tazobactam | 21 | ||||||||||||
| Aztreonam | 1 | 2 | 1 | 17 | |||||||||
| Ceftazidime | 1 | 7 | 7 | 5 | 1 | ||||||||
| Cefepime | 3 | 1 | 5 | 4 | 6 | 1 | |||||||
| Ampicillin | 21 | ||||||||||||
| Control (10) | |||||||||||||
| Doripenem | 2 | 7 | 1 | ||||||||||
| Meropenem | 10 | ||||||||||||
| Imipenem | 7 | 3 | |||||||||||
| Ertapenem | 10 | ||||||||||||
| Piperacillin-tazobactam | 6 | 2 | 2 | ||||||||||
| Aztreonam | 3 | 5 | 2 | ||||||||||
| Ceftazidime | 5 | 3 | 2 | ||||||||||
| Cefepime | 3 | 5 | 2 | ||||||||||
| Ampicillin | 1 | 9 | |||||||||||
| Enterobacter | |||||||||||||
| AmpC-derepressed (21) | |||||||||||||
| Doripenem | 17 | 2 | 1 | 1 | |||||||||
| Meropenem | 5 | 14 | 2 | ||||||||||
| Imipenem | 7 | 7 | 6 | 1 | |||||||||
| Ertapenem | 1 | 3 | 6 | 7 | 4 | ||||||||
| Piperacillin-tazobactam | 3 | 2 | 6 | 10 | |||||||||
| Aztreonam | 6 | 3 | 4 | 5 | 3 | ||||||||
| Ceftazidime | |||||||||||||
| Cefepime | 2 | 4 | 4 | 1 | 5 | 1 | 1 | 2 | 1 | ||||
| Ampicillin | 21 | ||||||||||||
| AmpC-inducible (10) | |||||||||||||
| Doripenem | 2 | 8 | |||||||||||
| Meropenem | 9 | 1 | |||||||||||
| Imipenem | 1 | 2 | 4 | 2 | 1 | ||||||||
| Ertapenem | 6 | 3 | 1 | ||||||||||
| Piperacillin-tazobactam | 1 | 7 | 2 | ||||||||||
| Aztreonam | 2 | 3 | 2 | 1 | 1 | ||||||||
| Ceftazidime | 1 | 5 | 1 | 2 | 1 | ||||||||
| Cefepime | 2 | 5 | 1 | 1 | |||||||||
| Ampicillin | 2 | 3 | 6 | ||||||||||
Modal MICs are given in boldface.
Enterobacter isolates with derepressed AmpC β-lactamases.
MIC distributions for 21 AmpC-derepressed Enterobacter isolates are shown in Table 1, together with those for 10 control isolates with inducible AmpC. The modal MICs of doripenem (0.06 μg/ml), meropenem (0.03 to 0.06 μg/ml), and imipenem (0.12 to 0.5 μg/ml) were only slightly different between the two groups of organisms, whereas ertapenem MICs for the derepressed organisms were raised four- to eightfold. Predictably, derepression was associated with resistance to ceftazidime, aztreonam, and piperacillin and with reduced susceptibility to cefepime.
Activity against Enterobacteriaceae with carbapenemases.
MICs for isolates with KPC-3, SME-1, and IMP-1 enzymes are shown in Table 2. The four K. pneumoniae isolates with KPC-3 were from separate patients in a single outbreak; the two K. pneumoniae isolates with IMP were variants of a single strain (K4181) and had similar specific activities against imipenem, but differed in expression of a major outer membrane protein, putatively a porin (Koh et al, Letter). All of these isolates were substantially resistant to doripenem (MICs, 8 to >64 μg/ml) and to other carbapenems. MICs of doripenem, like those of other carbapenems, were lower (16 μg/ml compared with >64 μg/ml) for the porin-expressing variant of the IMP-1+ K. pneumoniae strain K4181). Except for S. marcescens S6, with SME-1 enzyme, the carbapenemase-producing strains were broadly resistant to the noncarbapenem agents tested.
TABLE 2.
MICs for wild-type isolates of Enterobacteriaceae with carbapenemases
| Isolate | Species | Phenotype | MIC (μg/ml)a
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DOR | MEM | IMP | ERT | PTZ | AZT | CAZ | CPM | AMP | |||
| CL5761b | Klebsiella spp. | KPC-3 | 16 | 64 | 64 | >64 | >64 | >64 | 64 | 64 | >64 |
| CL5762Ab | Klebsiella spp. | KPC-3 | 8 | 32 | 16 | 16 | >64 | >64 | 64 | 64 | >64 |
| CL5762Bb | Klebsiella spp. | KPC-3 | 32 | 64 | 64 | >64 | >64 | >64 | 64 | 64 | >64 |
| CL5763b | Klebsiella spp. | KPC-3 | 64 | >64 | >64 | >64 | >64 | >64 | >64 | 64 | >64 |
| S6 | S. marcescens | SME-1 | 32 | 32 | >64 | 64 | 8 | 32 | 0.5 | 0.06 | >64 |
| K4181c | K. pneumoniae | IMP-1 porin− | 64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 | >64 |
| K4181c | K. pneumoniae | IMP-1 porin+ | 16 | 16 | 16 | 16 | >64 | >64 | >64 | >64 | >64 |
AMP, ampicillin; AZT, aztreonam; CAZ, ceftazidime; CPM, cefepime; DOR, doripenem; ERT, ertapenem; IMP, imipenem; MEM, meropenem.
Multiple isolates from a single outbreak.
Porin-expressing and deficient variants of the same strain (10).
Activity versus chromosomal β-lactamase-inducibility mutants of Enterobacteriaceae.
Derepression of AmpC in laboratory mutants was not associated with any general increase in the MICs of doripenem, imipenem, and meropenem (Table 3). Minor exceptions were derepression-associated rises in the doripenem MIC for the C. freundii C12 and M. morganii M6 series, although not in any other C. freundii or M. morganii series. MICs of doripenem, imipenem, and meropenem for the AmpC-deficient mutants were equal to or slightly below those for the inducible and derepressed variants, further confirming that AmpC enzymes did not protect significantly, irrespective of their mode of expression. MICs of ertapenem were mostly raised for the derepressed variants in the C. freundii and E. cloacae series, often by a factor of four- to eightfold (Table 3). Derepression of AmpC in E. cloacae and C. freundii also was associated with sharply raised MICs of aztreonam and ceftazidime and with smaller rises in the MICs of cefepime, whereas the behavior of piperacillin-tazobactam varied with the strain. Derepression in S. marcescens was associated with raised MICs of aztreonam, ceftazidime, and (although less so) cefepime; derepression in M. morganii was associated with resistance to ceftazidime but not aztreonam, cefepime, or piperacillin-tazobactam. Derepression in of P. vulgaris conferred only small rises in the MICs of the compounds tested here, but it has more effect on cefotaxime and ceftriaxone (22).
TABLE 3.
MICs for AmpC inducibility mutant series of Enterobacteriaceae
| Straina | MIC (μg/ml)b
|
||||||||
|---|---|---|---|---|---|---|---|---|---|
| DOR | MEM | IMP | ERT | PTZ | AZT | CAZ | CPM | AMP | |
| C. freundii | |||||||||
| C2 | 0.06 | 0.03 | 0.5 | 0.015 | 2 | 0.25 | 0.5 | 0.06 | >64 |
| C2-CON | 0.06 | 0.03 | 0.25 | 0.125 | 32 | 16 | 32 | 1 | >64 |
| C2-DEF | 0.06 | 0.015 | 0.25 | <0.008 | 2 | 0.125 | 0.5 | 0.06 | 16 |
| C4 | 0.125 | 0.03 | 1 | 0.015 | 16 | 1 | 2 | 0.06 | >64 |
| C4-CON | 0.03 | 0.03 | 0.5 | 0.03 | 4 | 4 | 32 | 0.125 | >64 |
| C4-DEF | 0.06 | 0.03 | 0.5 | <0.008 | 1 | 0.125 | 1 | <0.03 | 32 |
| C10 | 0.03 | 0.015 | 0.5 | <0.008 | 4 | 0.06 | 0.25 | <0.03 | 32 |
| C10-CON | 0.03 | 0.03 | 0.5 | 0.03 | 8 | 4 | 32 | 0.06 | >64 |
| C10-DEF | 0.03 | 0.015 | 0.06 | <0.008 | 1 | 0.06 | 0.125 | <0.03 | 4 |
| C12 | 0.03 | 0.015 | 0.25 | <0.008 | 4 | 0.125 | 0.5 | <0.03 | 16 |
| C12-CON | 0.125 | 0.06 | 1 | 0.06 | 8 | 4 | 32 | 0.06 | >64 |
| C12-DEF | <0.008 | <0.008 | 0.06 | <0.008 | 1 | 0.06 | 0.25 | <0.03 | 4 |
| E. cloacae | |||||||||
| 84-CON | 0.06 | 0.06 | 0.25 | 0.5 | 32 | 64 | >64 | 2 | >64 |
| 84-DEF | 0.03 | 0.015 | 0.25 | <0.008 | 4 | 0.5 | 1 | 0.06 | 64 |
| 100-CON | 0.06 | 0.03 | 0.25 | 0.015 | 2 | 2 | 2 | 0.06 | >64 |
| 100-DEF | <0.008 | <0.008 | <0.03 | <0.008 | <0.125 | <0.03 | <0.03 | <0.03 | 0.5 |
| 684 | 0.06 | 0.03 | 0.5 | 0.03 | 4 | 0.125 | 0.5 | 0.125 | >64 |
| 684-CON | 0.06 | 0.06 | 0.25 | 0.25 | 32 | 32 | 64 | 1 | >64 |
| 684-DEF | 0.06 | 0.015 | 0.25 | <0.008 | 2 | 0.06 | 0.125 | <0.03 | 4 |
| S. marcescens | |||||||||
| S2 | 0.06 | 0.03 | 2 | 0.03 | 16 | 0.125 | 0.125 | 0.06 | >64 |
| S2-CON | 0.06 | 0.03 | 1 | 0.03 | >64 | 4 | 2 | 1 | >64 |
| S2-DEF | 0.06 | 0.03 | 2 | 0.015 | 16 | 0.125 | 0.25 | 0.06 | >64 |
| S7 | 0.06 | 0.03 | 0.25 | 0.03 | 16 | 0.125 | 0.125 | 0.125 | >64 |
| S7-CON | 0.06 | 0.03 | 1 | 0.03 | 32 | 8 | 2 | 1 | >64 |
| S7-DEF | 0.125 | 0.06 | 2 | 0.03 | 16 | 0.125 | 0.25 | 0.125 | >64 |
| M. morganii | |||||||||
| M1 | 0.5 | 0.125 | 4 | 0.03 | 0.25 | <0.03 | 0.06 | <0.03 | >64 |
| M1-CON | 0.5 | 0.125 | 2 | 0.03 | <0.125 | 0.5 | 4 | <0.03 | >64 |
| M1-DEF | 0.25 | 0.06 | 1 | <0.008 | 0.25 | <0.03 | 0.06 | <0.03 | 4 |
| M3-CON | 0.5 | 0.125 | 2 | 0.03 | 0.5 | 0.5 | 16 | <0.03 | >64 |
| M3-DEF | 0.25 | 0.06 | 2 | 0.015 | 0.25 | <0.03 | 0.06 | <0.03 | 2 |
| M6 | 0.06 | 0.03 | 1 | 0.015 | 0.25 | <0.03 | 0.06 | <0.03 | 64 |
| M6-CON | 0.5 | 0.125 | 2 | 0.03 | 0.25 | 0.05 | 8 | <0.03 | >64 |
| M6-DEF | 0.25 | 0.06 | 1 | 0.015 | 0.25 | <0.03 | 0.06 | <0.03 | 4 |
| P. vulgaris | |||||||||
| VA1 | 0.5 | 0.125 | 4 | 0.03 | 1 | 0.06 | 0.125 | 0.125 | >64 |
| VA1-CON | 0.25 | 0.06 | 2 | 0.015 | 0.5 | 0.06 | 0.125 | 0.125 | >64 |
| VA1-DEF | 0.125 | 0.03 | 0.5 | <0.008 | 0.5 | <0.03 | <0.03 | <0.03 | 32 |
| V2 | 0.25 | 0.06 | 2 | 0.015 | 1 | <0.03 | 0.06 | 0.125 | >64 |
| V2-CON | 0.125 | 0.03 | 1 | 0.015 | 1 | 0.06 | 0.125 | 0.25 | >64 |
| V2-DEF | 0.125 | 0.03 | 1 | 0.015 | 0.5 | <0.03 | <0.03 | 0.06 | 32 |
| V3 | 0.25 | 0.06 | 2 | 0.015 | 0.5 | <0.03 | <0.03 | 0.06 | >64 |
| V3-CON | 0.25 | 0.06 | 2 | 0.015 | 0.5 | 0.125 | 0.125 | 0.25 | >64 |
| V3-DEF | 0.25 | 0.06 | 1 | 0.015 | 0.5 | 0.06 | 0.06 | 0.06 | 32 |
CON, constitutively hyperproducing AmpC enzyme irrespective of induction; DEF, deficient: making only a trace amount of AmpC enzyme irrespective of induction.
Abbreviations for antibiotics are as defined in Table 2.
E. coli transconjugants.
MICs for E. coli transconjugants and their host strains are shown in Table 4. Transconjugants prepared by plate and broth mating were in E. coli K-12 J53 or J62 hosts: those with the IMP-1 and NMC-A carbapenemases, which required cloning, were prepared in DH5-α and JM109, respectively. None of the TEM, SHV, or OXA enzymes affected activity of the carbapenems, and MICs of these agents were as frequently slightly below as they were slightly above those for the plasmid-free hosts. In contrast, the MICs of ampicillin were raised for all the transconjugants, and those of the cephalosporins were raised for transconjugants with TEM- and SHV-type ESBLs. The TEM-1 producer was surprisingly resistant to piperacillin-tazobactam, perhaps reflecting its high level of enzyme synthesis. E. coli derivatives with the cloned IMP and NMC carbapenemases showed small but perceptible increases in MICs of all of the carbapenems, supporting the view that these enzymes offer only limited protection in fully-permeable organisms such as these hosts. IMP-1 was also associated with resistance to ceftazidime and slightly raised MICs of cefepime and piperacillin-tazobactam. NMC-A, in contrast, was associated with small rises in the MICs of aztreonam and piperacillin-tazobactam, but not those of ceftazidime and cefepime.
TABLE 4.
MICs for E. coli strains with plasmid-mediated and cloned β-lactamases
| Host | β-Lactamase | MIC (μg/ml)a
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| DOR | MEM | IMP | ERT | PTZ | AZT | CAZ | CPM | AMP | ||
| Plasmid carrying | ||||||||||
| J53 | OXA-1 | 0.015 | 0.015 | 0.125 | <0.008 | 2 | 0.06 | 0.125 | 0.25 | >64 |
| J53 | OXA-2 | 0.015 | 0.015 | 0.125 | <0.008 | 4 | 0.06 | 0.25 | 0.06 | >64 |
| J53 | OXA-3 | 0.03 | 0.03 | 0.25 | 0.015 | 4 | 0.125 | 0.5 | <0.03 | >64 |
| J62 | OXA-10 | 0.03 | 0.015 | 0.125 | 0.015 | 2 | 0.125 | 0.125 | 0.03 | >64 |
| J53 | SHV-1 | 0.06 | 0.015 | 0.25 | <0.008 | 32 | 0.125 | 0.5 | 0.125 | >64 |
| J53 | SHV-2 | 0.03 | 0.03 | 0.125 | 0.25 | >64 | 16 | 32 | 32 | >64 |
| J53 | SHV-3 | <0.008 | <0.008 | <0.03 | <0.008 | 0.5 | <0.03 | 0.06 | <0.03 | 64 |
| J53 | SHV-4 | 0.125 | 0.03 | 1 | 0.015 | 8 | >64 | >64 | 8 | >64 |
| J53 | SHV-5 | 0.06 | 0.03 | 0.5 | 0.015 | 4 | >64 | >64 | 2 | >64 |
| J62 | TEM-1 | 0.06 | 0.03 | 0.5 | 0.015 | 64 | 0.125 | 0.25 | 0.125 | >64 |
| J53 | TEM-2 | 0.015 | <0.008 | <0.03 | <0.008 | 1 | 0.06 | 0.06 | 0.06 | >64 |
| J62 | TEM-3 | 0.03 | 0.015 | 0.125 | 0.015 | 2 | 4 | 16 | 0.5 | >64 |
| J53 | TEM-6 | 0.03 | 0.015 | 0.125 | 0.015 | 4 | 16 | >64 | 1 | >64 |
| J53 | TEM-9 | 0.06 | 0.03 | 0.25 | 0.03 | 4 | >64 | >64 | 16 | >64 |
| J53 | TEM-10 | 0.06 | 0.03 | 0.125 | 0.015 | 4 | 64 | >64 | 2 | >64 |
| JM109 | NMC-A | 0.5 | 1 | 2 | 2 | 16 | 1 | 0.125 | <0.03 | 64 |
| DH5-α | IMP-1 | 0.125 | 0.125 | 0.5 | 0.125 | 8 | <0.03 | 16 | 0.25 | 32 |
| Plasmid free | ||||||||||
| DH5-α | R- | <0.004 | 0.03 | <0.015 | 0.008 | 2 | <0.03 | 0.06 | <0.03 | 2 |
| J53-1 | R- | 0.03 | 0.03 | 1 | 0.008 | 4 | 0.125 | 0.125 | 0.06 | 2 |
| J62-1 | R- | 0.06 | 0.03 | 0.25 | 0.008 | 4 | <0.03 | 0.125 | <0.03 | 2 |
| JM83b | R- | 0.03 | 0.03 | 0.25 | 0.008 | 4 | <0.03 | 0.125 | <0.03 | 2 |
Abbreviations are as defined in Table 2.
Related to JM109, used as a host for NMC-A β-lactamase.
Acinetobacter spp. with carbapenemases.
MICs for Acinetobacter isolates are summarized in Table 5. All of the carbapenemase producers were resistant to doripenem as well as to other carbapenems (MICs, 8 to >64 μg/ml). Most were resistant to all of the comparator β-lactams too, although two (A1411 with IMP-1 and 74510 with IMP-4) were susceptible to piperacillin-tazobactam, perhaps due to inherent susceptibility to tazobactam, and several had marginal susceptibility (MICs of 8 μg/ml) to aztreonam and cefepime. The control Acinetobacter strains, which lacked carbapenemases, were susceptible to doripenem as well as imipenem and meropenem at 0.12 to 2 μg/ml, were less susceptible to ertapenem (MIC, 1 to 16 μg/ml), and showed wide MIC ranges for the other comparators, with frank resistance being frequent.
TABLE 5.
MICs for Acinetobacter isolates with carbapenemases
| Isolate no. | Carba- penemase | Source (reference) | MIC (μg/ml)a
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| DOR | MEM | IMP | ERT | PTZ | AZT | CAZ | CPM | AMP | |||
| A1411 | IMP-1 | United Kingdom (20) | 16 | 16 | 8 | 32 | <0.125 | 8 | 64 | 16 | 16 |
| 74510 | IMP-4 | Hong Kong (4) | 32 | 32 | 32 | >64 | 2 | 16 | >64 | 64 | >64 |
| 116 | OXA27 | Singapore (1) | 32 | 32 | 64 | >64 | >64 | 32 | 64 | 16 | >64 |
| 327009 | OXA25 | Spain (1) | >64 | >64 | >64 | >64 | >64 | 64 | >64 | 64 | >64 |
| 4737 | OXA26 | Belgium (1) | >64 | >64 | >64 | >64 | >64 | 32 | >64 | 16 | >64 |
| SM28 | OXA40 | Spain (14) | >64 | >64 | >64 | >64 | >64 | 64 | >64 | 64 | >64 |
| 14BR | OXA23 | Brazil (5) | 32 | 32 | >64 | >64 | >64 | 64 | >64 | 64 | >64 |
| 2002BR | OXA23 | Brazil (5) | 32 | 32 | >64 | >64 | >64 | 64 | >64 | >64 | >64 |
| 450BR | OXA23 | Brazil (5) | 32 | 32 | >64 | >64 | >64 | 32 | >64 | >64 | >64 |
| 564BR | OXA23 | Brazil (5) | 32 | 32 | >64 | >64 | >64 | 32 | >64 | >64 | >64 |
| 936BR | OXA23 | Brazil (5) | 64 | 64 | >64 | >64 | >64 | 32 | >64 | >64 | >64 |
| 801BR | OXA23 | Brazil (5) | 32 | 32 | 64 | >64 | >64 | 32 | >64 | 64 | >64 |
| 869BR | OXA23 | Brazil (5) | 32 | 32 | >64 | >64 | >64 | 64 | >64 | 64 | >64 |
| 4131BR | OXA23 | Brazil (5) | 64 | 64 | >64 | >64 | >64 | 32 | >64 | 64 | >64 |
| Carbapenemase-negative controls (n = 10) | |||||||||||
| Range | United Kingdom (7) | 0.25-2 | 0.25-2 | 0.12-2 | 1-16 | 0.12->64 | 1->64 | 1->64 | 2-64 | 2->64 | |
| Mode(s) | United Kingdom (7) | 0.5 | 1 | 0.5 | 4 | 64 | 16 | 4-8 | 4-8 | >64 | |
Abbreviations are as defined in Table 2.
DISCUSSION
Doripenem behaved very similarly to meropenem, which it resembles in structure (19). Its MICs for control wild-type Enterobacteriaceae were identical (±1 dilution) to those of meropenem, were slightly lower than those of ertapenem, and were four- to eightfold below those of imipenem. MICs for isolates, transconjugants, and mutants with (i) classical TEM, SHV, and OXA enzymes; (ii) TEM and SHV ESBLs; (iii) plasmid-mediated or stably derepressed AmpC enzymes; and (iv) hyperproduced K1 enzyme were no higher than those for control strains lacking these characteristics. In all of these aspects, doripenem behaved similarly to meropenem and imipenem, whereas MICs of ertapenem for many ESBL producers and AmpC-derepressed Enterobacter and C. freundii isolates and mutants were raised. This difference between ertapenem and other carbapenems is in keeping with previous results (9, 11, 12). Unlike with cephalosporins, however, there is no substantial inoculum effect for ESBL producers with ertapenem (12), and there is no trend toward reduced susceptibility among laboratory transconjugants with ESBLs (Table 4). Production of ESBLs and hyperproduction of K1 and AmpC enzymes was associated with the expected ranges of resistance to cephalosporins, aztreonam, and piperacillin-tazobactam; minor differences between the MICs published here and those found previously (for example, see reference 23) probably reflect use of NCCLS methodology here versus local or British Society for Antimicrobial Chemotherapy methodology previously.
Isolates of Enterobacteriaceae with IMP, KPC, and SME carbapenemases were resistant to doripenem, imipenem, ertapenem, and meropenem and—in most cases—also to other β-lactams. It is therefore reasonable to assume that doripenem, like other carbapenems, is a substrate for these enzymes. In contrast to the carbapenem resistance of these carbapenemase-producing isolates, E. coli derivatives with cloned IMP-1 and NMC-A showed only small reductions in susceptibility to carbapenems. This difference presumably reflects the greater permeability of the E. coli host strains; the DH5-α recipient used for IMP-1, in particular, was exquisitely susceptible to β-lactams, implying considerable permeability. More generally, it has been noted that many blaIMP and blaVIM-positive Enterobacteriaceae (and some nonfermenters) remain susceptible to established carbapenems at 1 to 2 μg/ml, implying that substantive resistance requires additional factors (21), Notable too, in context, was the fact that the porin-deficient variant of strain K. pneumoniae K4181 (IMP-1+) was at least eightfold more resistant to doripenem and other carbapenems than its porin-expressing variant (Table 2). Carbapenemases have been very slow to emerge in Enterobacteriaceae, perhaps because they fail to give resistance except in such impermeable organisms, meaning that bacteria must sequentially acquire multiple mechanisms and go through a series of selection cycles to achieve significant resistance. Thus, only 59 out of 1.42 million Enterobacteriaceae reported from >250 U.S. hospitals between 1996 to 2002 to the The Surveillance Network surveillance were indicated to be imipenem resistant (10), and carbapenems remain remarkably effective drugs for the treatment of the serious infections caused by Enterobacteriaceae, even 18 years after the launch of imipenem.
In Acinetobacter spp., in contrast, there is a growing problem with carbapenem resistance: some of it associated with metallo-β-lactamases, some with OXA-carbapenemases, and some with carbapenemase-independent mechanisms (10), perhaps entailing target change, impermeability, or efflux. Doripenem, like meropenem and imipenem, was consistently active against carbapenemase-negative Acinetobacter isolates, but lacked activity against those with metallo- and OXA carbapenemases.
The present data support and extend the findings of Tsuji et al. (19), who reported that doripenem (then S-4661) had very similar in vitro activity to meropenem against a range of bacterial species, but who did not examine isolates with known modes of antibiotic resistance. Compared with imipenem, both doripenem and meropenem have greater anti-Pseudomonas and anti-Proteus activities, although with the disadvantage of being recognized by pseudomonal efflux systems (15). Ertapenem is fundamentally different in being relatively less active against nonfermenters and slightly more affected by AmpC and ESBLs (11). It is likely that doripenem will provide an alternative to imipenem and meropenem as a parenteral agent for the treatment of severe infections, including those caused by multiresistant pathogens. It is also being developed in a nebulized formulation for treatment of infections in the cystic fibrosis lung.
REFERENCES
- 1.Afzal-Shah, M., N. Woodford, and D. M. Livermore. 2001. Characterization of OXA-25, OXA-26 and OXA-27, molecular class D β-lactamases associated with carbapenem resistance in clinical isolates of Acinetobacter baumannii. Antimicrob. Agents Chemother. 45:583-588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Babini, G. S., and D. M. Livermore. 2000. Antimicrobial resistance amongst Klebsiella spp. collected from intensive care units in Southern and Western Europe in 1997-1998. J. Antimicrob. Chemother. 45:183-189. [DOI] [PubMed] [Google Scholar]
- 3.Chow, J. W., M. J. Fine, D. M. Shlaes, J. P. Quinn, D. C. Hooper, M. P. Johnson, R. Ramphal, M. M. Wagener, D. K. Miyashiro, and V. L. Yu. 1991. Enterobacter bacteremia: clinical features and emergence of antibiotic resistance during therapy. Ann. Intern. Med. 115:585-590. [DOI] [PubMed] [Google Scholar]
- 4.Chu, Y.-W., M. Afzal-Shah, E. T. S. Houang, M.-F. I. Palepou, D. J. Lyon, N. Woodford, and D. M. Livermore. 2001. IMP-4, a novel metallo-β-lactamase from nosocomial Acinetobacter spp. collected in Hong Kong between 1994 and 1998. Antimicrob. Agents Chemother. 45:710-714. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Dalla Costa, L. M., J. M. Coelho, H. A. Souza, M. E. S. Castro, C. J. N. Stier, K. L. Bragagnolo, A. Rea-Neto, S. R. Penteado-Filho, D. M. Livermore, and N. Woodford. 2003. Outbreak of carbapenem-resistant Acinetobacter baumannii producing OXA-23 enzyme in Curitiba, Brazil. J. Clin. Microbiol. 41:3403-3406. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Edwards, J. R., and M. J. Betts. 2000. Carbapenems: the pinnacle of the β-lactam antibiotics or room for improvement? J. Antimicrob. Chemother. 45:1-4. [DOI] [PubMed] [Google Scholar]
- 7.Henwood, C. J., T. Gatward, M. Warner, D. James, M. W. Stockdale, R. Spence, K. J. Towner, D. M. Livermore, and N. Woodford. 2002. Antibiotic resistance among clinical isolates of Acinetobacter in the United Kingdom and in-vitro evaluation of tigecycline (GAR-936). J. Antimicrob. Chemother. 49:479-487. [DOI] [PubMed] [Google Scholar]
- 8.Iso, Y., T. Irie, Y. Nishino, K. Motokawa, and Y. Nishitani. 1996. A novel 1 β-methylcarbapenem antibiotic, S-4661. Synthesis and structure-activity relationships of 2-(5-substituted pyrrolidin-3-ylthio)-1 β-methylcarbapenems. J. Antibiot. (Tokyo) 49:199-209. [DOI] [PubMed] [Google Scholar]
- 9.Kohler, J., K. L. Dorso, K. Young, G. G. Hammond, H. Rosen, H. Kropp, and L. L. Silver. 1999. In vitro activities of the potent, broad-spectrum carbapenem MK-0826 (L-749,345) against broad-spectrum β-lactamase- and extended-spectrum β-lactamase-producing Klebsiella pneumoniae and Escherichia coli clinical isolates. Antimicrob. Agents Chemother. 43:1170-1176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Livermore, D. M. 2003. Threat from the pink corner. Ann. Med. 35:226-234. [DOI] [PubMed] [Google Scholar]
- 11.Livermore, D. M., A. M. Sefton, and G. M. Scott. 2003. Properties and potential of ertapenem. J. Antimicrob Chemother. 52:331-344. [DOI] [PubMed]
- 12.Livermore, D. M., K. J. Oakton, M. W. Carter, and M. Warner. 2001. Activity of ertapenem (MK-0826) versus Enterobacteriaceae with potent β-lactamases. Antimicrob. Agents Chemother. 45:2831-2837. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Livermore, D. M., and M. Yuan. 1996. Antibiotic resistance and production of extended-spectrum β-lactamases amongst Klebsiella spp. from intensive care units in Europe. J. Antimicrob. Chemother. 38:409-424. [DOI] [PubMed] [Google Scholar]
- 14.Lopez-Otsoa, F., L. Gallego, K. J. Towner, L. Tysall, N. Woodford, and D. M. Livermore. 2002. Endemic carbapenem resistance associated with OXA-40 carbapenemase among Acinetobacter baumannii isolates from a hospital in northern Spain. J. Clin. Microbiol. 40:4741-4743. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Masuda, N., E. Sakagawa, S. Ohya, N. Gotoh, H. Tsujimoto, and T. Nishino. 2000. Substrate specificities of MexAB-OprM, MexCD-OprJ, and MexXY-OprM efflux pumps in Pseudomonas aeruginosa. Antimicrob. Agents Chemother. 44:3322-3327. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Mikamo, H., K. Izumi, Y. X. Hua, Y. Hayasaki, Y. Sato, and T. Tamaya. 2000. In vitro and in vivo antibacterial activities of a new injectable carbapenem, S-4661, against gynaecological pathogens. J. Antimicrob. Chemother. 46:471-474. [DOI] [PubMed] [Google Scholar]
- 17.Mori, M., M. Hikida, T. Nishihara, T. Nasu, and S. Mitsuhashi. 1996. Comparative stability of carbapenem and penem antibiotics to human recombinant dehydropeptidase-I. J. Antimicrob. Chemother. 37:1034-1036. [DOI] [PubMed] [Google Scholar]
- 18.National Committee for Clinical Laboratory Standards. 2003. Methods of dilution antimicrobial susceptibility testing of bacteria that grow aerobically. M07-A6. Approved standard, 6th ed. National Committee for Clinical Laboratory Standards, Villanova, Pa.
- 19.Tsuji, M., Y. Ishii, A. Ohno, S. Miyazaki, and K. Yamaguchi. 1998. In vitro and in vivo antibacterial activities of S-4661, a new carbapenem. Antimicrob. Agents Chemother. 42:94-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Tysall, L., M. W. Stockdale, P. R. Chadwick, M. F. Palepou, K. J. Towner, D. M. Livermore, and N. Woodford. 2002. IMP-1 carbapenemase detected in an Acinetobacter clinical isolate from the United Kingdom. J. Antimicrob. Chemother. 49:217-218. [DOI] [PubMed] [Google Scholar]
- 21.Yan, J. J., W. C. Ko, C. L. Chuang, and J. J. Wu. 2002. Metallo-β-lactamase-producing Enterobacteriaceae isolates in a university hospital in Taiwan: prevalence of IMP-8 in Enterobacter cloacae and first identification of VIM-2 in Citrobacter freundii. J. Antimicrob. Chemother. 50:503-511. [DOI] [PubMed] [Google Scholar]
- 22.Yang, Y., and D. M. Livermore. 1988. Chromosomal β-lactamase expression and resistance to β-lactam antibiotics in Proteus vulgaris and Morganella morganii. Antimicrob. Agents Chemother. 32:1385-1391. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Yang, Y. J., and D. M. Livermore. 1989. Interactions of meropenem with class I chromosomal β-lactamases. J. Antimicrob. Chemother. 24(Suppl. A):207-217. [DOI] [PubMed] [Google Scholar]
- 24.Yang, Y. J., P. Wu, and D. M. Livermore. 1990. Biochemical characterization of a β-lactamase that hydrolyzes penems and carbapenems from two Serratia marcescens isolates. Antimicrob. Agents Chemother. 34:755-758. [DOI] [PMC free article] [PubMed] [Google Scholar]