Abstract
Persistent growth forms of Chlamydia pneumoniae have been associated with chronic infections in vivo. We investigated the effects of first-line therapeutics on the induction of persistence by monitoring recoverable organisms, gene expression of membrane proteins, and morphology. We found that all of the antibiotics tested have distinct and subinhibitory concentrations at which they induce persistence.
The chlamydial biphasic developmental cycle can be altered toward persistence by addition of gamma interferon or penicillin G or deprivation of essential nutrients (1, 2). Persistent chlamydiae fail to complete development from reticulate bodies (RBs) into infectious elementary bodies (EBs) but retain metabolic activity. Persistent Chlamydia pneumoniae RBs are enlarged and morphologically aberrant and form small inclusions. Persistent chlamydiae exhibit characteristic gene and protein expression profiles showing reduced levels of outer membrane proteins like the major outer membrane protein Omp1 or OmcB (2). Chlamydiae may be reactivated from persistence by removal of the inducing stimulus. Several studies performed with cell cultures have indicated that persistent C. pneumoniae may be refractory to killing by antibiotics (4, 6). Although persistence as defined in in vitro models has not been demonstrated unequivocally in vivo, it is known that C. pneumoniae can cause long-lasting and chronic pulmonary infections that do not respond to treatment with otherwise chlamydicidal drugs (4, 5). These infections are inferred to be caused by persistent growth forms. To determine if clinically relevant antichlamydial drugs might induce a persistent growth state, we investigated whether first-line therapeutics, including macrolides, tetracyclines, rifampin, and quinolones, induce persistence.
HeLa cells were infected with C. pneumoniae AR-39 (3), and various concentrations of ciprofloxacin, doxycycline, erythromycin, or rifampin were added. Chlamydiae of a first set of two identically treated cultures were harvested after 72 h and passed on to fresh HeLa cells for enumeration of viable inclusion-forming units (IFU) by immunofluorescence assay. The second set of infected cells was cultured for an additional 48 h in antibiotic-free medium and subsequently passed on to fresh HeLa monolayers. Expression of 16S rRNA, pmpB, and omcB mRNAs was analyzed at 72 h under normal growth conditions, at 72 h in the presence of 0.38 μg of ciprofloxacin per ml or 0.05 μg of doxycycline per ml, and at 48 h after recovery from antimicrobial treatment by a quantitative reverse transcription-PCR assay with TaqMan primer-probe sets. C. pneumoniae grown for 72 h in presence of 0.05 μg of doxycycline per ml or 0.38 μg of ciprofloxacin per ml or in the absence of antibiotics was stained with a fluorescein isothiocyanate-labeled, genus-specific anti-lipopolysaccharide antibody (Imagen; Dako) for laser scanning microscopy or processed for electron microscopy in accordance with standard procedures.
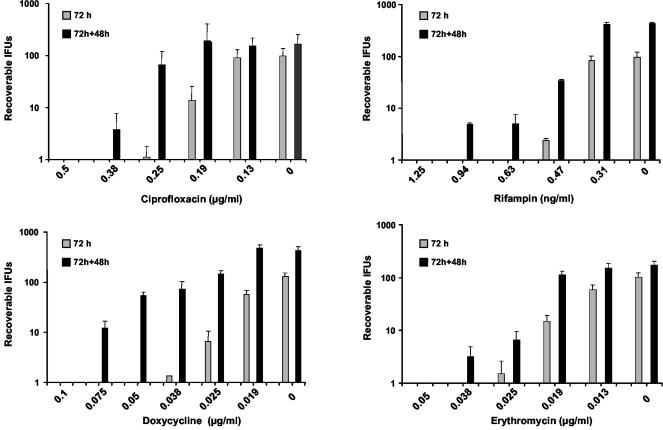
Lack of infectious progeny and reversibility are two major characteristics of persistence. We compared the numbers of IFU recovered 72 h after initial treatment with ciprofloxacin, doxycycline, rifampin, or erythromycin (treated) to the numbers recovered from parallel cultures following an additional 48 h of incubation in antibiotic-free medium (reactivation) (Fig. 1). The antibiotic concentrations used in these experiments were chosen to include a range of concentrations that yielded total clearance of the bacteria in some cases to small doses that had no effect on the organisms. No viable EBs were produced 72 h after infection in the presence of 0.5 μg of ciprofloxacin per ml, nor were any cultivable organisms present following 48 h of reactivation in antibiotic-free medium. When treated with 0.38 μg of ciprofloxacin per ml, a few organisms were recovered from reactivated samples, but none were recovered from treated samples. At 0.25 and 0.19 μg/ml, increasing numbers of inclusions were counted in both the sample that was antibiotic treated for 72 h and the reactivated sample, with a significantly higher number in the reactivated sample (P < 0.05). The largest difference in IFU counts between treated and reactivated cultures occurred when 0.25 μg of ciprofloxacin per ml was used, with approximately 60-fold more organisms detected in the reactivated sample. Cultures treated with 0.13 μg of ciprofloxacin per ml and reactivated were not different from the nontreated control (0 μg/ml) at 72 h or at 72 h plus 48 h. In summary, three concentrations of ciprofloxacin yielded differences between treated and reactivated cultures greater than in the antibiotic-free control (0.38, 0.25, and 0.19 μg/ml), with one concentration (0.38 μg/ml) showing no recoverable organisms after 72 h. Similar results were obtained with doxycycline, rifampin, and erythromycin. The persistence-inducing effect was greatest for doxycycline with five concentrations (0.075 to 0.019 μg/ml) showing differences between 72 h and the reactivated samples larger than in the untreated control cultures, including two concentrations (0.075 and 0.05 μg/ml) that exhibited no recoverable organisms at 72 h. We further studied the expression of the cysteine-rich 60-kDa outer membrane protein gene (omcB, Cpn 0557) and the outer membrane protein G gene (pmpG, Cpn 0444) under the influence of subinhibitory concentrations of ciprofloxacin (0.38 μg/ml) and doxycycline (0.05 μg/ml). In the presence of ciprofloxacin, omcB and pmpG were downregulated 5.9- and 4.1-fold, respectively, compared to untreated samples at the 72-h postinfection time point. Similarly, doxycycline-treated samples expressed 4.9- and 5.2-fold fewer omcB and pmpG transcripts, respectively, than did control cultures at the same time point. After recovery for 48 h in antibiotic-free medium, omcB and pmpG expression returned to levels similar to those seen in control untreated cultures at the 72-h time point (data not shown). We then studied the effect of subinhibitory concentrations of ciprofloxacin (0.38 μg/ml) and doxycycline (0.05 μg/ml) on chlamydial morphology (Fig. 2). Anti-lipopolysaccharide staining showed large, typically shaped inclusions after 72 h under normal growth conditions. Cultures treated with antibiotics contained significantly smaller inclusions. Electron microscopy of untreated cells revealed large numbers of EBs in the 72-h postinfection inclusions. In contrast, inclusions in antibiotic-treated cultures were significantly smaller and contained no EBs and only few RBs. RBs were atypical in morphology and roughly three to five times the size of the RBs found under normal culture conditions.
FIG. 1.
Numbers of IFU recoverable from parallel cultures after 72 h in the presence of antibiotics and after an additional 48 h of reactivation in antibiotic-free medium. Total numbers of recoverable IFU are indicated on the y axis. Concentrations of antibiotics used are on the x axis. The antibiotic used in each experiment is indicated below the x axis. Organisms not cultivable in the presence of the antibiotic (72 h) but recoverable after replacement of the medium (72 h + 48 h) were deemed to be persistent.
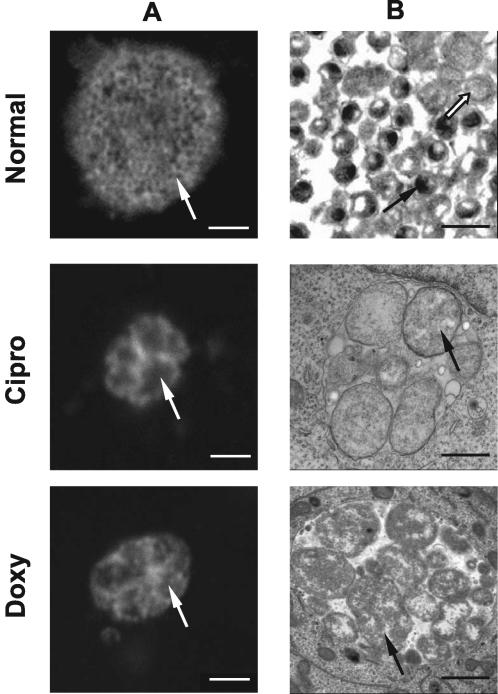
FIG. 2.
Immunofluorescence and electron microscopy of inclusion morphology in the presence and absence of persistence-inducing antibiotics. C. pneumoniae inclusions grown in the presence or absence of antibiotics for 72 h postinfection were visualized by confocal immunofluorescence (column A) or electron (column B) microscopy. Confocal microscopy of normally grown C. pneumoniae revealed large inclusions with granular staining structures (white arrow) that corresponded to EBs (black arrow) visible in a similar sample visualized by electron microscopy. Only few regular-size RBs are visible (white arrow). In contrast, doxycycline (Doxy)- and ciprofloxacin (Cipro)-treated cultures exhibited altered morphology at 72 h postinfection. Inclusions were smaller. Enlarged RB structures were evident in both confocal (white arrows) and electron (black arrows) microscopy micrographs of these samples. EBs were lacking in the antibiotic-treated samples, and the visible RBs were roughly three to five times the size of regular RBs under normal growth conditions. Scale bars: A, 2 μm; B, 1 μm.
In the present study, we characterized C. pneumoniae persistence by four main criteria as previously defined in studies of the similar phenomenon in C. trachomatis (1): inability to produce infectious EBs, altered gene expression, aberrant morphology, and reversibility of persistence upon removal of the persistence-inducing stimulus. Lack of infectious progeny at 72 h, a time point when the normal developmental cycle is completed, is clearly indicative of persistence. Reactivation from persistence was analyzed by allowing C. pneumoniae 48 h to recover in antibiotic-free medium following the initial 72-h period of infection in the presence of antibiotics. All of the antibiotics tested exhibited at least one concentration at which all of the viable chlamydiae existed in a persistent state. Nevertheless, recovery from persistence at this concentration was not complete. Antibiotic treatment reduced the infectious progeny by about 95%. At slightly lower concentrations, recovery increased, but not all of the organisms were in a persistent state. We further characterized persistence by analyzing gene expression and morphology under the influence of ciprofloxacin and doxycycline. Expression of pmpG and omcB is amplified during the recondensation of RBs to EBs at the end of the normal chlamydial growth cycle. Thus, expression of these genes was monitored to evaluate persistence because persistent organisms are blocked in the final RB-to-EB transition. Both genes were downregulated in the presence of antibiotics, an observation that is consistent with the apparent absence of EBs, as determined by microscopy, in the treated cultures. After recovery in antibiotic-free medium, expression of these late cycle genes increased to levels seen during the end of a normal developmental cycle. This is indicative of the transformation of aberrant RBs into infectious EBs and correlates with the increase in recoverable organisms in cell culture. The morphology of RBs in the noncultivable state described in this report, observed by microscopy, further supports our interpretation that the chlamydiae were truly persistent. Inclusions formed by persistent chlamydiae have previously been reported to be of decreased size and to contain only enlarged, atypical RBs (1).
The effects of antibiotics vary with concentration from killing to noneffectiveness. Induction of persistence is only one outcome in the middle of this spectrum. In this respect, persistence resembles bacteriostasis. Several antibiotics, such as sulfonamides and tetracyclines, inhibit the growth of, but do not kill, most bacteria. Although the underlying mechanisms inducing bacteriostasis and persistence may differ, the two may have similar consequences in vivo. Without additional support from the host immune system, both persistent and static bacteria remain viable and may resume normal growth in changed conditions. Unlike most bacteria, the intracellular niche of persistent chlamydiae partially protects them from the immune system and thus can lead to complete failure of therapy. We have shown in this in vitro study that subinhibitory concentrations of antibiotics can induce persistence. We speculate that during initiation of therapy, owing to reduced tissue penetration or noncompliance with dosing regimens, these subinhibitory concentrations may well be achieved in vivo. Insufficient therapy at the beginning of the infection may aggravate the course and result in refractoriness to further antimicrobial treatment.
Acknowledgments
J.G. was supported by a grant from the Deutsche Forschungsgemeinschaft (GI 344/2-1).
We thank D. Nelson for critical reading of the manuscript.
REFERENCES
- 1.Beatty, W. L., R. P. Morrison, and G. I. Byrne. 1994. Persistent chlamydiae: from cell culture to a paradigm for chlamydial pathogenesis. Microbiol. Rev. 58:686-699. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Beatty, W. L., G. I. Byrne, and R. P. Morrison. 1993. Morphologic and antigenic characterization of interferon gamma-mediated persistent Chlamydia trachomatis infection in vitro. Proc. Natl. Acad. Sci. USA 90:3998-4002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Gieffers, J., R. J. Belland, W. Whitmire, S. Ouellette, D. Crane, M. Maass, G. I. Byrne, and H. D. Caldwell. 2002. Isolation of Chlamydia pneumoniae clonal variants by a focus-forming assay. Infect. Immun. 70:5827-5834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gieffers, J., H. Fullgraf, J. Jahn, M. Klinger, K. Dalhoff, H. A. Katus, W. Solbach, and M. Maass. 2001. Chlamydia pneumoniae infection in circulating human monocytes is refractory to antibiotic treatment. Circulation 103:351-356. [DOI] [PubMed] [Google Scholar]
- 5.Hammerschlag, M. R., K. Chirgwin, and P. M. Roblin. 1992. Persistent infection with Chlamydia pneumoniae following acute respiratory illness. Clin. Infect. Dis. 14:178-182. [DOI] [PubMed] [Google Scholar]
- 6.Kutlin, A., P. M. Roblin, and M. R. Hammerschlag. 1999. In vitro activities of azithromycin and ofloxacin against Chlamydia pneumoniae in a continuous-infection model. Antimicrob. Agents Chemother. 43:2268-2272. [DOI] [PMC free article] [PubMed] [Google Scholar]