Abstract
We evaluated B memory responses in healthy adult volunteers who received one oral dose of live-attenuated Shigella flexneri 2a vaccine. LPS-specific BM cells increased from a median of 0 at baseline to 20 spot forming cells (SFC)/106 expanded cells following vaccination (p = 0.008). A strong correlation was found between post-vaccination anti-LPS BM cell counts and peak serum anti-LPS IgG titers (rs = 0.95, p = 0.0003). Increases in BM specific for IpaB approaching significance were also observed. In sum, oral vaccination with live-attenuated S. flexneri 2a elicits BM cells to LPS and IpaB, suggesting that BM responses to Shigella antigens should be further studied as a suitable surrogate of protection in shigellosis.
Keywords: Vaccine, Shigella, B cell memory
1. Introduction
Each year 600,000 children less than 5 years of age living in developing countries are estimated to die from shigellosis [1]. Increased resistance to antimicrobials [2] and potential use of Shigella as a bioterror agent [3] are also of concern. Development of live-attenuated oral Shigella vaccines represents one approach to controlling this disease. Two vaccine candidates, designated CVD 1204 and CVD 1208, were constructed by creating rational deletions in the wild-type Shigella flexneri 2a strain 2457T. CVD 1204 is deleted in guaA (encoding a guanosine monophosphate synthase) and guaB (encoding an inositol monophosphate dehydrogenase), effectively impairing the biosynthesis of guanine nucleotides; CVD 1208 has additional deletions of set and sen genes (that encode Shigella enterotoxins 1 and 2, respectively). Both strains were shown to be safe and immunogenic in Phase 1 trials involving healthy North American adults [4]. Correlates of protection against shigellosis described, heretofore, include serum IgG antibodies [5,6] and peripheral IgA antibody secreting cells (ASC) to serotype specific to lipopolysaccharide (LPS) O-antigen [7,8]. Other antibody responses as well as cell-mediated immunity (CMI) that target conserved antigens such as invasion plasmid antigen (Ipa) B may also contribute to protective immunity [9–12]. A vaccine’s usefulness as a public health tool is strongly influenced by its ability to induce enduring systemic and mucosal antibody responses and to respond anamnestically at later times after antibody titers have fallen. These anamnestic immune responses are generally faster and of higher magnitude than primary responses and depend largely on the presence of B memory (BM) cells [13]. Although relatively long-term humoral and secondary secretory IgA immune responses to LPS in stools have been described in natural Shigella infection as well as in recipients of a live-attenuated Shigella vaccine [14], the presence of BM responses have not been reported. It is important to address this gap in knowledge because of the recently recognized key roles that BM play not only in antibody production to T-cell dependent and independent antigens, but also because of their regulatory and effector functions (e.g., antigen presentation and cytokine production) [15]. In this paper, we evaluate the hypothesis that BM cell responses specific to LPS and IpaB are elicited in volunteers that had seroresponses to CVD 1204 and CVD 1208 live-attenuated Shigella vaccines.
2. Materials and methods
2.1. Specimens
Clinical specimens were obtained from healthy adult volunteers 18–45 years of age from the Baltimore–Washington area that received a single oral dose of S. flexneri 2a ΔguaBA (Center for Vaccine Development [CVD] 1204) or S. flexneri 2a ΔguaBA Δsen Δset (CVD 1208) as previously described [4]. In brief, volunteers received 107, 108, and 109 of each vaccine strain or placebo and had serum obtained on days 0, 7, 14, 28, and 42 as well as peripheral blood mononuclear cells (PBMC) obtained on days 0 and 28 after vaccination. All specimens were cryopreserved as previously described [16] and stored in liquid nitrogen until use. Seroresponse, measured following standard techniques [4], was defined as ≥4-fold rise of antigen-specific antibody pre- to peak post-vaccination. Serum was available to assay nine seroresponders, three non-seroresponders, and two placebo recipients from subjects immunized with 109 cfu of the Shigella strains. For BM assays, 12 LPS seroresponders and 12 non-seroresponders from all three dose cohorts were assayed. All protocols were approved by the Institutional Review Board (IRB) and Good Clinical Practices (GCP) were followed.
2.2. Antigen preparation
LPS and IpaB antigens were prepared from S. flexneri 2a strain 2457T. LPS was purified by the hot aqueous phenol extraction method of Westphal [17]. IpaB was prepared as previously described [18]. Briefly, coding sequences of ipaB were amplified using PCR and inserting into the plasmid vector pACYC-Duet (Novagen, Madison, WI) and co-expressed with its cognant chaperone IpgC on pET15b in Escherichia coli BL21(DE3) as described by Birket et al. [19]. The IpaB was released from IpgC with 1% OPOE.
2.3. PBMC expansion
PBMC expansion was performed as described by Crotty et al. [20]. In brief, PBMC were thawed, washed with complete RPMI 1640 (cRPMI) containing 100 IU/mL penicillin + 100 μg/mL streptomycin (CellGro, Manassas, VA), 2 mM L-glutamine (HyClone, Logan, UT), and 10% heat-inactivated fetal bovine serum (FBS) (BioWhit-taker, Walkersville, MD), and expanded for 5 days in 6-well sterile plates (1 million cells/well) in the presence of 1/100,000 pokeweed mitogen (PWM) (kindly provided by Dr. S. Crotty), 6 μg/mL CpG-2006 (Qiagen/Operon, Huntsville, AL), and 1/10,000 Staphylococcus aureus Cowan (SAC) (Sigma–Aldrich, St. Louis, MO) in cRPMI in a total volume of 2 mL/well. Cells were fed by adding an additional 2 mL of cRPMI after 2–3 days of incubation.
2.4. BM assays
96-Well ELISPOT MAHA (Millipore, Billerica, MA) plates were coated in triplicate with LPS 5 μg/mL, IpaB 0.5 μg/mL, and total goat anti-human IgG 5 μg/mL in phosphate buffered saline (PBS) overnight at 4 °C, blocked with 1% bovine serum albumin (BSA) (Sigma) in RPMI for 2 h at 37 °C, washed with PBS, and incubated with 105 expanded PBMC per well for wells coated with LPS and IpaB. Dilutions for total IgG wells were performed starting with 25,000 cells/well and continuing with serial twofold dilutions to 391 cells. Cells were incubated for 6 h at 37 °C and 5% CO2, washed with PBS + 0.05% Tween 20 (PBST) followed by PBS and incubated with mouse anti-human PAN IgG biotin conjugated antibody (Hybridoma Reagent Laboratory, Baltimore, MD) overnight at 4 °C, washed with PBST and PBS, and labeled with horseradish peroxidase (HRP) conjugated Avidin D (Vector Laboratories, Burlingame, CA) for 1 h at room temperature (RT). 50 μl true blue peroxidase substrate (KPL, Gaithersburg, MD) was added per well for 8 min at RT before the reaction was stopped with ddH2O. ELISPOTs were read using an automated ELISPOT reader (BioReader 3000, Immunobiosys, The Colony, TX) by a blinded investigator. The final results are presented as numbers of spot forming cells (SFC)/106 expanded cells, as well as % SFC/total IgG SFC.
2.5. Flow cytometry
Expanded and unexpanded PBMC were washed with 1% FBS in PBS and labeled with fluorochrome-labeled monoclonal antibodies as follows: IgG-FITC (clone G18-145, BD Pharmingen, Franklin Lakes, NJ), CD19-PE-Cy5 (clone J4.119, Beckman Coulter, Fullerton, CA), CD27-PE (clone M-T271, BD Pharmingen), and CD3-PE-Cy7 (clone UCHT1, Beckman Coulter) in volumes of 50 μl for 20–30 min at 4 °C, washed with 1% FBS in PBS, and fixed in 300 μl of 1% formaldehyde. Events were acquired on a MoFlow flow cytometer/cell sorter (Beckman Coulter) and analyzed using WinList 5.0 (Verity Software House, Topsham, ME) software.
2.6. Avidity assays
Two 96-well U-bottom ELISA plates (Thermo Labsystems, Franklin, MA) were coated in triplicate in carbonate coating buffer, pH 9.6 with 5 μg/mL LPS and 0.5 μg/mL IpaB at 37 °C for 3 h, washed with PBST, blocked with 10% Carnation instant non-fat dry milk overnight at 4 °C, washed with PBST, incubated with serially diluted serum specimens for 1 h at 37 °C, and washed with PBST. One plate was treated with PBST and one with 6 M urea in PBST for 1 h. Both plates were subsequently washed with PBST, incubated with goat anti-human HRP-labeled antibody (MP Biomedicals, Solon, OH) 1:5000 for 1 h at 37 °C, washed with PBST, and developed with TMB microwell peroxidase substrate system (KPL) for 15 min at RT. Development was stopped by the addition of 1 M phosphoric acid. Plates were read using the Multiskan Ascent Microplate Reader (Thermo Labsystems) at 450 nm. Linear regression curves were calculated for each serum sample and titers were determined as the reciprocal of the serum dilution that produces an optical density (OD) of 0.2 above the blank, and reported as ELISA units (EU)/mL. Results are expressed as avidity index (AI), which was calculated by dividing the EU/mL of specimens incubated with urea divided by the EU/mL of specimens in the absence of urea [21].
2.7. Statistical analysis
To ensure that the expansion of PBMC was adequate to allow for the detection of low numbers of antigen-specific BM cells, we performed preparatory optimization experiments and determined that it was important to include as an exclusion criterion the presence of ≤20,000 total IgG SFC/106 expanded cells detected by ELISPOT after incubation with SAC, PWM, and CpG-2006. Thus, expanded PBMC preparations that did not fulfill this criterion were excluded from analysis. The median number of SFC from triplicate runs was used in the statistical analyses; SFC over negative control wells were included in the analysis. In our assays the limit of detection of antigen-specific/total expanded cells was 1 in 100,000 (0.001%), whereas the limit of detection of antigen-specific/total IgG secreting cells was determined by the maximum number of total IgG SFC for each individual volunteer in anti-IgG coated ELISPOT plates. The latter ranged from an acceptable minimum of 0.005% (i.e., 1 specific SFC in the 20,000 total IgG SFC/106 expanded cell cutoff) to 0.0005% in a volunteer that had 214,186 total IgG SFC/106 expanded cells.
Microsoft® Office Excel 2003, Sigmastat Version 9.0, and STATA Version 9.0 were used for statistical analysis. All hypotheses were evaluated using non-parametric two-sided tests. Pre- and post-vaccination results were paired. Antigen-specific SFC/106 expanded cells were divided by total IgG SFC as well as adjusted for defined cell populations including CD19+ CD3− [total B] and CD19+ CD27+ CD3− [BM] by multiplying the SFC/total IgG by the percent CD19+ CD3− cells or CD19+ CD27+ CD3−cells. The Wilcoxon signed rank test was used to assess continuous pre- to post-vaccination antigen-specific BM responses among all cell populations. Receiver operator curve (ROC) analysis was used to optimally dichotomize BM responses. Correlations between seroresponse and BM response were performed using Spearman ρ for continuous variables and Fisher’s exact test for dichotomous variables.
3. Results
To determine if BM cells are present in seroresponders and correlate with the magnitude of the seroresponse we determined the presence or absence of BM cells among volunteers that received oral live Shigella vaccines CVD 1204 and CVD 1208 and either did (seroresponders) or did not (non-seroresponders) mount fourfold or higher increases in serum IgG antibody recognizing LPS or IpaB-post-vaccination, when compared to baseline. Because IgG+ BM cells represent only ~1% of all freshly collected PBMC and antigen-specific IgG+ BM cells comprise only ~1% of all IgG+ BM cells, expansion of PBMC with mitogens including (PWM and SAC) and CpG is necessary to induce antibody secretion and to detect at least 10 SFC when 100,000 PBMC are plated [20]. As described in Section 2, to ensure the integrity of data and to optimize the sensitivity and reproducibility of the assay to identify antigen-specific BM cells, specimens that did not show adequate numbers (at least 20,000 total IgG SFC/106 expanded cells) were eliminated from analyses.
3.1. Flow cytometric analysis of expanded cells
We collected flow cytometry data in 22 of the volunteers. The proportions of CD19+ CD3− [total B] cells, CD19+ CD27+ CD3− [BM] cells, and CD19+ CD27+ IgG+ CD3− [IgG+ BM] cells pre- and post-vaccination were similar (~11%, ~3%, and ~0.7%, respectively). In vitro expansion resulted in ~3-fold increase in total B cells, ~5-fold increase in BM and ~10-fold increase in IgG+ BM cells (Table 1 and Fig. 1). As can be seen in Fig. 1, expansion resulted in a marked increase in the proportions of BM (i.e., CD19+ CD27+ CD3− cells) and in those BM expressing IgG. Of the subjects studied, nine LPS seroresponders and eight non-seroresponders had evidence of adequate expansion and were analyzed for the presence of specific BM cells; of the nine LPS seroresponders, seven were also IpaB seroresponders. Specimens that were excluded due to inadequate numbers of expanded cells (<20,000 total IgG SFC/106) by the ELISPOT had similar proportions of total B, BM and IgG+ BM cells before expansion as volunteers who were included in the analysis. However, they had considerably fewer PBMC numbers before and after expansion, as well as a suboptimal expansion (~2-fold increase in total B, ~3-fold increase in BM, and ~5-fold increase in IgG+ BM cells; data not shown), indicating that these cells were not functionally optimal.
Table 1. Cell populations before and after expansion and vaccinationa.
| Expansion (n = 22) | Vaccination (n = 22) | # PBMC (×106)b | % CD19+ | % CD19+ CD27+ | % CD19+ CD27+ IgG+ |
|---|---|---|---|---|---|
| Pre | Pre | 4.9 (3.8–6.1) | 11.4 (9–13.7) | 2.8 (2.0–3.5) | 0.7 (0.4–1.0) |
| Pre | Post | 4.6 (3.6–5.7) | 11.4 (8.8–13.9) | 2.9 (2.1–3.8) | 0.8 (0.4–1.2) |
| Post | Pre | 5.1 (3.6–6.7) | 32.6 (27.9–37.4) | 15.3 (12.8–17.9) | 7.2 (5.4–9.0) |
| Post | Post | 4.1 (2.6–5.3) | 29.5 (23.4–35.6) | 15.2 (11.8–18.6) | 7.1 (5.2–9.1) |
PBMC from recipients of CVD 1204, CVD 1208 or placebo were counted and analyzed by flow cytometry before and after expansion and vaccination; all cells were gated on CD3−. Results are expressed as mean (95% C.I.).
Number of PBMC seeded for expansion or recovered after expansion.
Fig. 1.
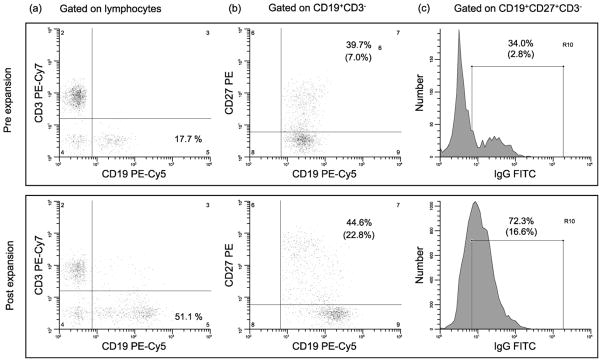
Representative flow cytometric analysis pre- and post-expansion. Staining of PBMC performed before and after expansion in a representative volunteer; gated on the lymphocyte region as defined by forward versus side light scatter (a); gated on CD19+ CD3− (b); gated on CD19+ CD27+ CD3− (c); in parentheses under the percentages of cells in selected regions are the % of positive cells in the corresponding region as related to the number of cells in the lymphocyte region.
3.2. Evaluation of total and specific ASC after expansion of BM cells
BM cells increased from a median of 0 SFC/106 expanded cells pre-vaccination to a median of 20 SFC/106 expanded cells post-vaccination for LPS seroresponders (p = 0.008 by Wilcoxon signed rank) and a median of 0 SFC/106 expanded cells pre-vaccination to a median of 23 SFC/106 expanded cells post-vaccination for IpaB seroresponders (p = 0.062 by Wilcoxon signed rank). The median percentages of antigen-specific SFC as a proportion of median total IgG SFC showed increases from 0% pre-vaccination to 0.02% post-vaccination for LPS and 0% pre-vaccination to 0.03% post-vaccination for IpaB (Fig. 2). The observed p-values were similar to those recorded when analyzing the numbers of SFC/106 expanded cells (data not shown). A representative example of ELISPOT data is shown in Fig. 3. Individuals who were not seroresponders did not exhibit a statistically significant increase in antigen-specific BM responses pre- to post-vaccination (data not shown). BM responses were seen exclusively among seroresponders irrespective of whether they received CVD 1204 or CVD 1208 (data not shown).
Fig. 2.
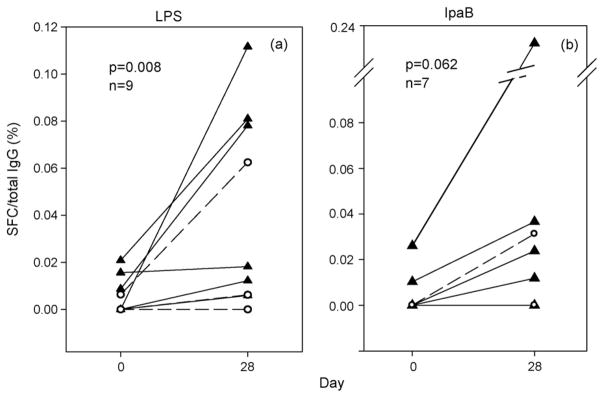
Antigen-specific IgG BM cell responses. Shown are recipients of CVD 1204 (triangles) and CVD 1208 (open circles) who mounted a ≥4-fold rise of anti-LPS IgG pre- to post-vaccination and had evidence of appropriate BM expansion in vitro; LPS (a) and IpaB (b) ELISPOT performed on days 0 and 28; comparisons made by Wilcoxon signed rank test for 1204 and 1208 combined. Results are expressed as the % of specific SFC per total IgG+ expanded cell populations.
Fig. 3.
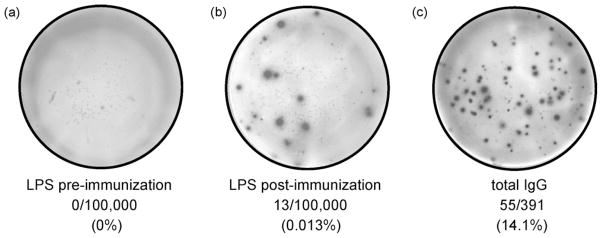
Representative ELISPOT analysis pre- and post-expansion. 100,000 expanded PBMC of a representative volunteer were plated in ELISPOT wells coated with LPS pre-vaccination (a) and post-vaccination (b). (c) Total IgG spots in a representative well of ELISPOT plates coated with anti-IgG and seeded with 391 expanded PBMC from a post-vaccination specimen.
3.3. Correlation of BM responses with seroresponses
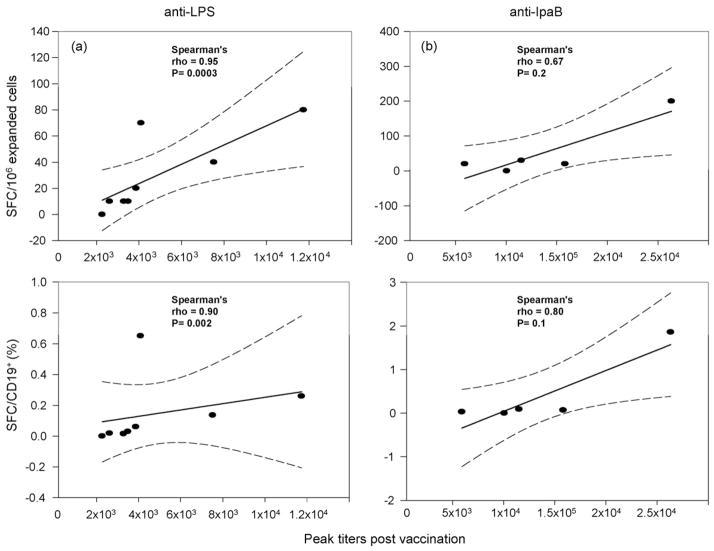
The correlation coefficient comparing antigen-specific SFC/106 expanded cells on day 28 to peak seroresponse post-vaccination was 0.95 (p = 0.0003 by Spearman ρ) for LPS and 0.67 (p = 0.2 by Spearman ρ) for IpaB (Table 2). Correlations were not significant on day 0. Analyzing the data using the numbers of LPS-specific BM cells among total IgG+ BM cells or adjusting for specific cell populations, including CD19+, CD19+ CD27+, and CD19+ CD27+ IgG+ BM cells did not significantly alter the correlations (Table 2). Interestingly, adjusting for CD19+ cells slightly reduced the correlation for LPS and improved the correlation for IpaB (Fig. 4). The likely explanation for this effect is that one outlier for LPS (very strong responder) exhibited fewer CD19+ B cells resulting in a higher percentage of specific SFC/CD19+ cells, thereby decreasing the correlation. Excluding this volunteer (who was an LPS seroresponder, but not an IpaB responder) from the analysis and adjusting for subpopulations had a small positive effect on the observed IpaB correlations. ROC analysis utilizing maximal correct classification to compare BM response to ≥4-fold seroresponse revealed optimal cutoffs of ≥10 SFC/106 expanded cells and ≥0.006% antigen-specific SFC/total SFC for LPS as well as ≥20 SFC/106 expanded cells and ≥0.01% antigen-specific SFC/total SFC for IpaB.
Table 2. Correlations of BM assay results with peak serologic responsesa.
| Day 0
|
Day 28
|
|||
|---|---|---|---|---|
| ρc | pc | ρc | pc | |
| LPS (n = 8)b | ||||
| SFC/106 expanded cells | 0.51 | 0.2 | 0.95 | 0.0003 |
| SFC/total IgG+ | 0.55 | 0.2 | 0.95 | 0.0003 |
| SFC/CD19+ | 0.52 | 0.2 | 0.90 | 0.002 |
| SFC/CD19+ CD27+ | 0.55 | 0.2 | 0.90 | 0.002 |
| SFC/CD19+ CD27+ IgG+ | 0.55 | 0.2 | 0.93 | 0.001 |
| IpaB (n = 5)b | ||||
| SFC/106 expanded cells | 0.67 | 0.2 | 0.67 | 0.2 |
| SFC/total IgG+ | 0.67 | 0.2 | 0.8 | 0.1 |
| SFC/CD19+ | 0.67 | 0.2 | 0.8 | 0.1 |
| SFC/CD19+ CD27+ | 0.67 | 0.2 | 0.8 | 0.1 |
| SFC/CD19+ CD27+ IgG+ | 0.67 | 0.2 | 0.8 | 0.1 |
Statistically significant differences (p < 0.05) are bolded.
Only recipients of CVD 1204 and CVD 1208 who mounted a fourfold or higher rise of anti-LPS IgG pre- to post-vaccination and had evidence of appropriate BM expansion in vitro were included in this analysis. In these volunteers anti-LPS and anti-IpaB SFC were adjusted for defined B subpopulations and correlated with the corresponding peak antigen-specific serological response before and after immunization.
Flow cytometry data available for 8/9 LPS seroresponders and 5/7 IpaB seroresponders.
Determined by Spearman ρ comparing BM response to peak serum titer.
Fig. 4.
Correlation of anti-LPS SFC versus peak anti-LPS titer. Recipients of CVD 1204 and CVD 1208 who mounted a ≥4-fold rise of anti-LPS IgG pre- to post-vaccination and had evidence of appropriate BM expansion in vitro were included in the analysis. Peak serum titers to LPS (a) and IpaB (b) were plotted against the number of specific SFC/106 expanded cells (upper panels) and the % of specific SFC in CD19+ expanded cell populations (lower panels) from PBMC obtained 28 days after immunization; dashed lines represent the 95% confidence interval.
3.4. Avidity maturation
An important hypothesis to evaluate is whether the volunteers who seroresponded to LPS or IpaB produced antibody that possessed increased avidity over time and to determine whether increases in avidity correlate with the presence of BM cells. To this end, we measured avidity of the anti-LPS and anti-IpaB antibody responses by measuring the ability of urea, a chaotropic agent, to dissociate antibody binding [21]. Preliminary optimization experiments indicated that treatment with 6 M urea for 1 h resulted in the dissociation of 30–70% of bound antibody, suggesting that this assay was appropriate to detect changes in avidity over time (data not shown). However, antibody avidity remained unchanged from day 0 (pre-vaccination) through days 7, 14, 21, 28 and 42 post-vaccination (Fig. 5).
Fig. 5.
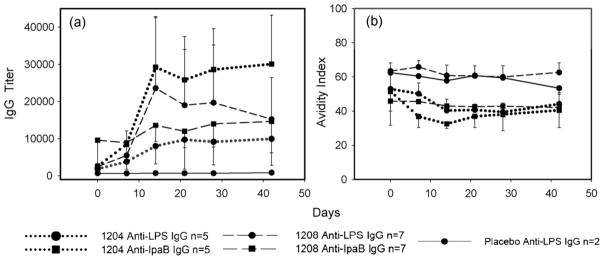
Avidity maturation of IgG anti-LPS and IpaB seroresponses elicited by immunization with CVD 1204 and CVD 1208 strains. Shown are recipients of CVD 1204 (dotted), CVD 1208 (dashed) and placebo (solid). Sera were drawn before (day 0) and at 7, 14, 21, 28 and 42 days after immunization. IgG titers to LPS (circles) and IpaB (squares) were determined by ELISA (a). 6 M urea for 1 h was used as a chaotrope agent to measure avidity indices (b); error bars depict standard errors.
4. Discussion
Vaccination with one dose of an oral live-attenuated Shigella vaccine results in antigen-specific BM responses among subjects who mount fourfold or higher specific antibody responses, with a high degree of correlation between specific BM cells and serum antibody titers. While the median BM response to LPS 28 days post-vaccination is significantly increased over baseline, the response to IpaB only exhibited a trend approaching significance. The small number of IpaB seroresponders likely contributed to a diminished ability to demonstrate statistical significance.
The presence of a B memory response can be assessed by documenting: (1) an anamnestic secondary immune response which occurs more quickly and reaches a higher titer than the primary response, (2) avidity maturation, and (3) the presence of BM cells [22]. BM cells have been described in humans who have received vaccines known to induce a T-cell dependent response such as viral vaccines including smallpox [23] and rotavirus [24] as well as vaccines that have had a bacterial polysaccharide conjugated to a protein, such as pneumococcal [25] and meningococcal [26] conjugate vaccines. The techniques utilized to study BM cells include ELISPOT, where PBMC are expanded ex vivo to increase the number of BM cells and to induce antibody secretion [20], as well as flow cytometry by which fluorochrome-labeled antigen as well as cell surface markers allow the identification of antigen-specific BM cells [24]. In the present studies we used flow cytometry to determine the proportions of phenotypically defined total B, BM and IgG+ BM cell populations before and after expansion. This information helped to validate the BM cell ELISPOT assays and provided the rationale for limiting the analyses to specimens that showed evidence of appropriate expansion. By documenting a 10-fold increase in expanded CD19+ CD27+ IgG+ BM cell populations and eliminating specimens that exhibited suboptimal expansion of functional cells (defined as ≤20,000 total IgG SFC/106 expanded cells), we were able to report BM responses as low as 0.02% antigen-specific SFC/total IgG SFC post-vaccination for the LPS polysaccharide antigen (i.e., 1:5000) and 0.03% for the protein antigen IpaB (i.e., 1:3333). These proportions of antigen-specific BM cells are similar to those previously reported in vaccines who received parenteral viral vaccines such as the highly immunogenic and efficacious recombinant hepatitis B vaccine, which elicited a median of 0.07% hepatitis B surface antigen-specific IgG secreting cells over the total IgG secreting cells [27] as well as the diphtheria and tetanus toxoids, which elicited specific B cells in the range of 0.01–1% [28]. To our knowledge, this is the first report of a BM cell response to LPS in recipients of an oral live-attenuated bacterial vaccine.
Evidence of BM cells specifically recognizing LPS in this setting suggests that the immune system processes LPS, a type 1 T-cell independent antigen, differently when presented in the context of a live-attenuated bacterial vaccine than when presented in the context of a parenteral polysaccharide vaccine not conventionally thought to induce BM. Another explanation for the presence of BM cells specific to LPS is that they are indeed T-independent. Despite the long held belief that B cell memory does not occur without T-cell help, recent evidence suggests that phenotypically distinct subsets of BM cells are generated in response to carbohydrates in a T-cell independent manner [22,29]. Interestingly, the BM cells generated independently of T-cells do not undergo high-level somatic hypermutation and avidity maturation [30], which may help explain the lack of avidity maturation seen in our study. The possibility that vaccination with a live-attenuated Shigella vaccine resulted in a polyclonal stimulation of BM cells [31] is less likely, as the total IgG SFC would be expected to increase with polyclonal stimulation.
Reports of correlations between antigen-specific serum titers and antigen-specific BM cells have not been consistent. Leyendeckers and colleagues did not find a correlation between IgG specific to tetanus toxin and CD19+ IgG+ cells in humans at various time points after vaccination [32] whereas Crotty and Tuaillon reported a moderate correlation between antigen-specific serum IgG and antigen-specific BM cells in humans at various time points after receiving smallpox or hepatitis B vaccines [23,27]. We believe that the reason for a very high correlation between the peak antigen-specific serum IgG and antigen-specific BM cells is the timing at which the BM cells were collected and the exclusion of specimens that did not reveal evidence of optimal expansion. The kinetics of antigen-specific BM cells suggest that their presence in the peripheral circulation is higher at 1 month than at 7 days or 1 year [26]. Thus, attempts to correlate antigen-specific serum anti-body responses with antigen-specific BM cells at time points other than day 28 are likely to reveal lower correlations. Additionally, including specimens that show evidence of inadequate expansion would decrease the sensitivity of identifying antigen-specific BM cells, also decreasing the correlation with antigen-specific antibody responses. Of note, in spite of the strong correlations observed between antibody levels and BM responses, not every seropositive subject exhibited a specific BM response. This is likely the result of methodological shortcomings, notably the inability to plate more than 100,000 expanded cells/well. Future studies in which sufficient cells are available to Plate 200,000 or higher cell numbers/well, as well as the development of better methods of expanding BM cells and their measurement at several time points will allow us to establish the validity of this hypothesis. Interestingly, adjusting for phenotype of the antigen-specific SFC detected by ELISPOT by multiplying the 100,000 expanded cells by the percent CD19+ cells, CD19+ CD27+ cells, or CD19+ CD27+, IgG+ cells did not have a major effect on the correlations as all (CD19+, CD19+ CD27+, and CD19+ CD27+ IgG+) subsets of antigen-specific SFC were increased 28 days post-vaccination and correlated with seroresponses.
A weakness of these studies is the small number of volunteers available in this convenience sample. Although this did not preclude us from observing strong, statistically significant, specific anti-LPS BM responses and associations with anti-LPS antibody levels, it is likely that the small sample size provided insufficient power to adequately estimate the presence and association of anti-IpaB BM cells and seroresponses. This limitation is a function of utilizing leftover specimens from old clinical trials to test new hypotheses and poses a problem mainly if the null hypothesis is not rejected. Future studies will address this issue by evaluating larger numbers of vaccines. In upcoming studies we plan to evaluate the presence of BM over several months to investigate the longevity of these responses. We will also undertake more detailed phenotypic flow cytometric analyses involving the use of monoclonal antibodies to molecules present in the surface and/or intracellularly in BM cells to further define the effector BM subsets (e.g., IgD, IgA, IgM, activation markers such as CD38, and gut homing markers such as integrin α4/β7 to start exploring the homing potential of BM elicited by immunization with attenuated strains of Shigella).
In sum, we observed that oral vaccination with live-attenuated S. flexneri 2a elicits detectable BM cells to LPS and IpaB 28 days after antigen exposure. A strong correlation between anti-LPS BM cells and peak anti-LPS antibody responses advances the possibility that BM cells may be an important indicator for long-term humoral immunity and a candidate surrogate of protection in shigellosis that merits further study.
Acknowledgments
We thank the volunteers for participating in the clinical trial, the clinical and regulatory staff at CVD, Mardi Reymann for providing technical advice and Drs. Marcela Pasetti and William Blackwelder for helpful discussions. Support for this research was provided by NIH R01-AI057927 (to M.B.S.), K23-AI065759 (to J.S.) and N01-AI25461 (VTEU, to M.M.L.).
Contributor Information
J.K. Simon, Email: jsimon@medicine.umaryland.edu.
M.B. Sztein, Email: msztein@medicine.umaryland.edu.
References
- 1.Kotloff KL, Winickoff JP, Ivanoff B, Clemens JD, Swerdlow DL, Sansonetti PJ, et al. Global burden of Shigella infections: implications for vaccine development and implementation of control strategies. Bull World Health Organ. 1999;77(8):651–66. [PMC free article] [PubMed] [Google Scholar]
- 2.Pickering LK. Antimicrobial resistance among enteric pathogens. Semin Pediatr Infect Dis. 2004;15(April 2):71–7. doi: 10.1053/j.spid.2004.01.009. [DOI] [PubMed] [Google Scholar]
- 3.Bioterrorism Agents/Diseases. Center for Disease Control and Prevention Website. 2004 Nov 19; http://www.bt.cdc.gov/agent/agentlist.asp.
- 4.Kotloff KL, Pasetti MF, Barry EM, Nataro JP, Wasserman SS, Sztein MB, et al. Deletion in the Shigella enterotoxin genes further attenuates Shigella flexneri 2a bearing guanine auxotrophy in a phase 1 trial of CVD 1204 and CVD 1208. J Infect Dis. 2004;190(November 10):1745–54. doi: 10.1086/424680. [DOI] [PubMed] [Google Scholar]
- 5.Black RE, Levine MM, Clements ML, Losonsky G, Herrington D, Berman S, et al. Prevention of shigellosis by a Salmonella typhi–Shigella sonnei bivalent vaccine. J Infect Dis. 1987;155(June 6):1260–5. doi: 10.1093/infdis/155.6.1260. [DOI] [PubMed] [Google Scholar]
- 6.Cohen D, Block C, Green MS, Lowell G, Ofek I. Immunoglobulin M, A, and G antibody response to lipopolysaccharide O antigen in symptomatic and asymptomatic Shigella infections. J Clin Microbiol. 1989;27(January 1):162–7. doi: 10.1128/jcm.27.1.162-167.1989. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Kotloff KL, Losonsky GA, Nataro JP, Wasserman SS, Hale TL, Taylor DN, et al. Evaluation of the safety, immunogenicity, and efficacy in healthy adults of four doses of live oral hybrid Escherichia coli–Shigella flexneri 2a vaccine strain EcSf2a-2. Vaccine. 1995;13(April 5):495–502. doi: 10.1016/0264-410x(94)00011-b. [DOI] [PubMed] [Google Scholar]
- 8.Levine MM, Kotloff KL, Barry EM, Pasetti MF, Sztein MB. Clinical trials of Shigella vaccines: two steps forward and one step back on a long, hard road. Nat Rev Microbiol. 2007;5(July 7):540–53. doi: 10.1038/nrmicro1662. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Oaks EV, Hale TL, Formal SB. Serum immune response to Shigella protein antigens in rhesus monkeys and humans infected with Shigella spp. Infect Immun. 1986;53(July 1):57–63. doi: 10.1128/iai.53.1.57-63.1986. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Oberhelman RA, Kopecko DJ, Salazar-Lindo E, Gotuzzo E, Buysse JM, Venkatesan MM, et al. Prospective study of systemic and mucosal immune responses in dysenteric patients to specific Shigella invasion plasmid antigens and lipopolysaccharides. Infect Immun. 1991;59(July 7):2341–50. doi: 10.1128/iai.59.7.2341-2350.1991. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Barzu S, Nato F, Rouyre S, Mazie JC, Sansonetti P, Phalipon A. Characterization of B-cell epitopes on IpaB, an invasion-associated antigen of Shigella flexneri: identification of an immunodominant domain recognized during natural infection. Infect Immun. 1993;61(September 9):3825–31. doi: 10.1128/iai.61.9.3825-3831.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Samandari T, Kotloff KL, Losonsky GA, Picking GA, Sansonetti WD, Levine PJ, et al. Production of IFN-gamma and IL-10 to Shigella invasins by mononuclear cells from volunteers orally inoculated with a Shiga toxin-deleted Shigella dysenteriae type 1 strain. J Immunol. 2000;164(January 4):2221–32. doi: 10.4049/jimmunol.164.4.2221. [DOI] [PubMed] [Google Scholar]
- 13.Traggiai E, Puzone R, Lanzavecchia A. Antigen dependent and independent mechanisms that sustain serum antibody levels. Vaccine. 2003;21(June Suppl 2):S35–7. doi: 10.1016/s0264-410x(03)00198-1. [DOI] [PubMed] [Google Scholar]
- 14.Li A, Karnell A, Huan PT, Cam PD, Minh NB, Tram LN, et al. Safety and immunogenicity of the live oral auxotrophic Shigella flexneri SFL124 in adult Vietnamese volunteers. Vaccine. 1993;11(2):180–9. doi: 10.1016/0264-410x(93)90015-p. [DOI] [PubMed] [Google Scholar]
- 15.Sanz I, Wei C, Lee FE, Anolik J. Phenotypic and functional heterogeneity of human memory B cells. Semin Immunol. 2008;20(February 1):67–82. doi: 10.1016/j.smim.2007.12.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Sztein MB, Wasserman SS, Tacket CO, Edelman R, Hone D, Lindberg AA, et al. Cytokine production patterns and lymphoproliferative responses in volunteers orally immunized with attenuated vaccine strains of Salmonella typhi. J Infect Dis. 1994;170(December 6):1508–17. doi: 10.1093/infdis/170.6.1508. [DOI] [PubMed] [Google Scholar]
- 17.Westphal O, Jann K, Himmelspach K. Chemistry and immunochemistry of bacterial lipopolysaccharides as cell wall antigens and endotoxins. Prog Allergy. 1983;33:9–39. [PubMed] [Google Scholar]
- 18.Picking WL, Mertz JA, Marquart ME, Picking WD. Cloning, expression, and affinity purification of recombinant Shigella flexneri invasion plasmid antigens IpaB and IpaC. Protein Expr Purif. 1996;8(December 4):401–8. doi: 10.1006/prep.1996.0117. [DOI] [PubMed] [Google Scholar]
- 19.Birket SE, Harrington AT, Espina M, Smith ND, Terry CM, Darboe N, et al. Preparation and characterization of translocator/chaperone complexes and their component proteins from Shigella flexneri. Biochemistry. 2007;46(July 27):8128–37. doi: 10.1021/bi700099c. [DOI] [PubMed] [Google Scholar]
- 20.Crotty S, Aubert RD, Glidewell J, Ahmed R. Tracking human antigen-specific memory B cells: a sensitive and generalized ELISPOT system. J Immunol Methods. 2004;286(March 1–2):111–22. doi: 10.1016/j.jim.2003.12.015. [DOI] [PubMed] [Google Scholar]
- 21.Polanec J, Seppala I, Rousseau S, Hedman K. Evaluation of protein-denaturing immunoassays for avidity of immunoglobulin G to rubella virus. J Clin Lab Anal. 1994;8(1):16–21. doi: 10.1002/jcla.1860080105. [DOI] [PubMed] [Google Scholar]
- 22.Tarlinton D. B-cell memory: are subsets necessary? Nat Rev Immunol. 2006;6(October 10):785–90. doi: 10.1038/nri1938. [DOI] [PubMed] [Google Scholar]
- 23.Crotty S, Felgner P, Davies H, Glidewell J, Villarreal L, Ahmed R. Cutting edge: long-term B cell memory in humans after smallpox vaccination. J Immunol. 2003;171(November 10):4969–73. doi: 10.4049/jimmunol.171.10.4969. [DOI] [PubMed] [Google Scholar]
- 24.Rojas OL, Caicedo L, Guzman C, Rodriguez LS, Castaneda J, Uribe L, et al. Evaluation of circulating intestinally committed memory B cells in children vaccinated with attenuated human rotavirus vaccine. Viral Immunol. 2007;20(2):300–11. doi: 10.1089/vim.2006.0105. [DOI] [PubMed] [Google Scholar]
- 25.Clutterbuck EA, Salt P, Oh S, Marchant A, Beverley P, Pollard AJ. The kinetics and phenotype of the human B-cell response following immunization with a heptavalent pneumococcal-CRM conjugate vaccine. Immunology. 2006;119(November 3):328–37. doi: 10.1111/j.1365-2567.2006.02436.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Kelly DF, Snape MD, Clutterbuck EA, Green S, Snowden C, Diggle L, et al. CRM197-conjugated serogroup C meningococcal capsular polysaccharide, but not the native polysaccharide, induces persistent antigen-specific memory B cells. Blood. 2006;108(October 8):2642–7. doi: 10.1182/blood-2006-01-009282. [DOI] [PubMed] [Google Scholar]
- 27.Tuaillon E, Tabaa YA, Petitjean G, Huguet MF, Pajeaux G, Fondere JM, et al. Detection of memory B lymphocytes specific to hepatitis B virus (HBV) surface antigen (HBsAg) from HBsAg-vaccinated or HBV-immunized subjects by ELISPOT assay. J Immunol Methods. 2006;315(August 1–2):144–52. doi: 10.1016/j.jim.2006.07.016. [DOI] [PubMed] [Google Scholar]
- 28.Nanan R, Heinrich D, Frosch M, Kreth HW. Acute and long-term effects of booster immunisation on frequencies of antigen-specific memory B-lymphocytes. Vaccine. 2001;20(November 3–4):498–504. doi: 10.1016/s0264-410x(01)00328-0. [DOI] [PubMed] [Google Scholar]
- 29.Obukhanych TV, Nussenzweig MC. T-independent type II immune responses generate memory B cells. J Exp Med. 2006;203(February 2):305–10. doi: 10.1084/jem.20052036. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Toellner KM, Jenkinson WE, Taylor DR, Khan M, Sze DM, Sansom DM, et al. Low-level hypermutation in T cell-independent germinal centers compared with high mutation rates associated with T cell-dependent germinal centers. J Exp Med. 2002;195(February 3):383–9. doi: 10.1084/jem.20011112. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Lanzavecchia A, Bernasconi N, Traggiai E, Ruprecht CR, Corti D, Sallusto F. Understanding and making use of human memory B cells. Immunol Rev. 2006;211(June):303–9. doi: 10.1111/j.0105-2896.2006.00403.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Leyendeckers H, Odendahl M, Lohndorf A, Irsch J, Spangfort M, Miltenyi S, et al. Correlation analysis between frequencies of circulating antigen-specific IgG-bearing memory B cells and serum titers of antigen-specific IgG. Eur J Immunol. 1999;29(April 4):1406–17. doi: 10.1002/(SICI)1521-4141(199904)29:04<1406::AID-IMMU1406>3.0.CO;2-P. [DOI] [PubMed] [Google Scholar]