Abstract
This commentary addresses a novel mechanism by which aging-related changes in reproductive hormones could mediate their action in the brain. It presents the evidence that dyotic endocrine signals modulate the expression of tumor necrosis factor (TNF) and related cytokines, and that these cytokines are a functionally important downstream link mediating neurodegeneration and dysfunction. This convergence of dyotic signalling on TNF-mediated degeneration and dysfunction has important implications for understanding the pathophysiology of AD, stroke, and traumatic brain disease, and also for the treatment of these diseases.
Age-related dysregulation of the hypothalamic-pituitary-gonadal (HPG) axis (endocrine dyscrasia) leads to dyotic signalling and the induction of neurodegenerative cascades within the brain (see [1–4] for reviews). Neurodegeneration induced by this aging-related endocrine dyscrasia is mediated via a number of cell cycle-related mechanisms, leading to alterations in the blood-brain barrier [5] and neuronal viability [6–10]. Cell cycle-related biochemical and pathological changes include altered tau phosphorylation and amyloid-b precursor protein (AβPP) metabolism, endoreduplication (polyploidy), mitochondrial biogenesis, upregulation of mitogenic signalling pathways and oxidative stress. All these well-described changes associated with pyramidal neuron autophagy/apoptosis in the Alzheimer’s disease (AD) brain also are observed during the progression of neurons through the cell cycle [2; 11], and are indicative of an aberrant re-entry of post-mitotic neurons into the cell cycle.
Endocrine Dyscrasia and Alzheimer’s Disease
Changes in the brain concentration of HPG axis hormones with age-related endocrine dyscrasia have been demonstrated to induce numerous biochemical, physiological and pathological changes within the brain [2; 11]. In this respect, two groups of hormones have been most closely examined, the gonadotropins and sex steroids. Evidence supporting the elevation in the concentrations of circulating gonadotropins in neurodegeneration is evidenced by 1) total brain concentrations of amyloid-β (Aβ), the major component of amyloid plaques, are increased by luteinizing hormone (LH) and decreased by the anti-gonadotropin leuprolide acetate [12; 13], 2) ovariectomy, which increases circulating gonadotropins, increases brain Aβ load [14], 3). mice over-expressing AβPP but lacking a functional LH receptor (AβPPxLHR−/−) show greatly reduced Aβ deposition in the brain [15], 4) elevated LH/hCG diminishes cognitive function [16; 17], 5) ovariectomy also diminishes cognitive function [14; 18; 19], 6) low LH levels in rodents enhance spatial memory and protect against memory loss [20; 16], 7) leuprolide acetate improves cognition in a murine AβPP transgenic model [13; 19], 8) a phase II clinical trial of leuprolide acetate (http://clinicaltrials.gov/ct/show/nct00076440?orden1/46) demonstrated cognitive stabilization in AD patients over 48 weeks, 9) patients treated with leuprolide acetate for prostate cancer have a 50% reduction in the incidence of AD [21; 22], and 10) these neurodegenerative and cognitive changes are supported by the age-related elevation in gonadotropins and by the increased plasma levels of follicle stimulating hormone (FSH) and LH in men and post-menopausal women with AD above those of age-matched cognitively normal individuals [23; 24].
Evidence supporting the loss of sex steroid signalling in mediating neurodegenerative-like changes is evidenced by findings that, 1) 17β-estradiol (E2) and testosterone have been shown to alter neuronal AβPP processing toward the non-amyloidogenic pathway in both mouse and human cell lines and primary cultures of rat, mouse, and human embryonic cerebrocortical neurons [25–28], 2) ovariectomy, which suppresses circulating levels of estrogens, also has been shown to increase total brain Aβ concentrations in guinea pigs [29] and AβPP transgenic mice (e.g. [30; 26; 31; 32]). Conversely, 17β-estradiol treatment was shown to partially and totally reverse the effects of ovariectomy in guinea pigs [29] and AβPP transgenic mice (e.g. [30]), 3) the negative correlation between serum E2 in women with AD [33]; the negative correlation between serum testosterone in men with AD [23; 34] 4) the improvement in cognition in women with AD treated with E2 in 3 controlled [35–37] and 1 uncontrolled [38] intervention studies; 5) the improvement in cognition in men with AD administered testosterone [39; 40].
Whether these changes induced by 17β-estradiol and testosterone are a direct result of signalling via ER’s or AR’s is unclear. As indicated above, there is mounting evidence to suggest that the effects of 17β-estradiol are actually mediated via gonadotropin signalling [12; 19].
Mechanistically, it has been demonstrated that LH regulates AβPP processing towards the amyloidogenic pathway [12] and that Aβ is in itself a mitogen (see reference [41] and literature cited therein). LH has been shown to mediate mitogenesis since subcutaneous administration of LH induces neurogenesis in the hippocampus of the adult mouse [42], while in sheep there is evidence that GnRH directly, or indirectly via LH, induces neurogenesis in the hippocampus [43]. Elevations in circulating and brain LH (or GnRH) with age-related endocrine dyscrasia could therefore drive the aberrant re-entry of neurons into the cell cycle. Whether this aberrant ‘neurogenesis’ occurs in resident quiescent totipotent stem cells (e.g. in the dentate gyrus), during the process of neurogenesis (migration and differentiation) or in terminally differentiated neurons has yet to be fully resolved.
Changes in perception of the role of inflammation in Alzheimer’s disease
Inflammation as a late step that minimizes disease
Early demonstrations of inflammatory cells in AD brains did not threaten the decades-long primacy of research based on amyloid plaques being the direct primary cause of function loss in AD, since their association with these plaques [44; 45] was interpreted as facilitating plaque removal [46; 47]. This implied that this inflammatory response was to be encouraged, and immunotherapy designed to promote amyloid removal was investigated, albeit with disappointing results [48; 49]. With plaque removal becoming a increasingly questionable goal [50; 51], and a developing awareness that inflammatory mediators induce, and therefore precede, AβPP [52–57] expression and processing towards the amyloidogenic pathways [58], a fresh approach to understanding the sequence and roles of inflammation and amyloid in AD pathogenesis is warranted.
Inflammation as an early step that initiates disease
An alternative approach, that of inflammation preceding and causing amyloid plaque deposition, begun with the work of Griffin and co-workers [59]. In 1989 this group demonstrated, in AD brains, that overexpression of interleukin 1 (IL-1), a cytokine that functionally overlaps with TNF, had a role in amyloid plaque formation. Four years later Tarkowski [60] reported that TNF levels in CSF from 56 individuals who had mild cognitive impairment, when tracked over a period, predicted which were much more likely to develop into frank AD. Newer studies continue to reaffirm this finding, with markers of inflammation showing in serum and CSF before any indications of increased Aβ or tau [61; 62]. Another group took advantage of the increased sensitivity of assaying for soluble TNF receptors rather than TNF itself. They found good evidence for levels of these receptors, which TNF induces, in serum and CSF predicting conversion to clinical AD over a 4–6 year period [63]. A more recent example utilized another acute phase protein, clusterin (apolipoprotein J), and found it to be intimately associated with onset, progression, and severity of this disease [64]. Clusterin, which is induced by TNF [65], was present 10 years earlier than fibrillar Aβ deposition. In addition a new experimental study notably reports that anti-TNF, not TNF, as earlier approaches would have suggested, reduce amyloid plaques in transgenic mice [66]. Taken together, these arguments for inflammation having a key role in the onset, rather than the dissipation, of AD pathology raise the question of what initiates these proinflammatory cytokines increases in the brain in AD.
Endocrine Dyscrasia Mediates the Expression of Pro-inflammatory Cytokines
Reproductive hormones are well known for their cell growth and differentiation properties. Age-related declines in the production of sex steroids and inhibins by the gonads leads to a decrease in negative feedback inhibition on the hypothalamus and pituitary, resulting in an elevation in the production and circulating concentrations of gonadotropins. Changes in the blood-brain barrier and cell cycle described earlier induced by endocrine dyscrasia may be mediated by alterations in expression of TNF (and Aβ), a molecule with known neurogenic and inflammatory properties.
Gonadotropins
Links between the gonadotropins and TNF in brain function are supported by the findings that TNF, like gonadotropins, is a very pleiotropic cytokine, important in reproductive physiology [67], as well as being a physiological gliotransmitter [68] and central to neurogenesis [69]. Evidence for a role of gonadotropins in regulating TNF expression is demonstrated by the ability of FSH to induce TNF expression in investigations into the illness caused by chronic kidney dialysis [70]. Others, studying the reasons for the exacerbation of rheumatoid arthritis at the onset of menopause, have correlated the high circulating FSH and LH seen at this time with increases in TNF, interleukin-1β (IL-1β) and monocyte chemoattractant protein (MCP)-1 [71]. These data are consistent with the anti-gonadotropic actions of leuprolide rendering it an anti-mitotic and anti-inflammatory agent when it is used to treat endometriosis. In this context leuprolide has been reported to reduce a number of inflammatory cytokines, namely IL-1β [72], IL-6 [73; 74], and MCP-1[75], all of which are induced by TNF [76–78]. Moreover, anti-TNF treatment lowers levels of these cytokines [79; 80; 77].
Sex Steroids
Estradiol has been shown to inhibit the release of TNF from monocytes [81] while both estradiol and progesterone reduce TNF expression in mid-brain astrocytes [82]. In addition, estrogen receptor and estradiol agonists inhibit microglial activation, part of the evidence being reduced production of TNF and similar pro-inflammatory cytokines [83]. The signalling pathways have begun to be studied [84; 85]. In vivo, outcomes are consistent with these steroids inhibiting TNF production through their capacity to negatively feed back on the hypothalamus and lower gonadotropin-releasing hormone, LH and FSH production. Whether sex steroids directly mediate their effects via nuclear steroid receptors, membrane receptors, or via the regulation of gonadotropins or other hormones is unclear. Functionally, estradiol and progesterone can be regarded as anti-TNF agents that act before TNF is generated. These agents have been reported to protect against AD [35–37], stroke [86; 87], and traumatic brain injury [88–92].
Coupling TNF to Endocrine Dyscrasia and the Pathogenesis of Alzheimer’s disease
A considerable basic literature from a number of autonomous groups argues for increased brain TNF [93] having a primary role in the pathogenesis of AD [94–101]. The area has recently been reviewed [102]. It therefore seems plausible from the reasoning in the above paragraphs that these two areas of AD research, hitherto considered unrelated, are in fact the upstream and downstream ends of a single disease mechanism (see Fig. 1). Indeed, both the loss of sex steroids/inhibins and the elevation of GnRH/gonadotropins with age-related endocrine dyscrasia would serve to elevate TNF expression in the brain. This possibility is testable experimentally, and might explain variations in brain TNF levels.
Figure 1.
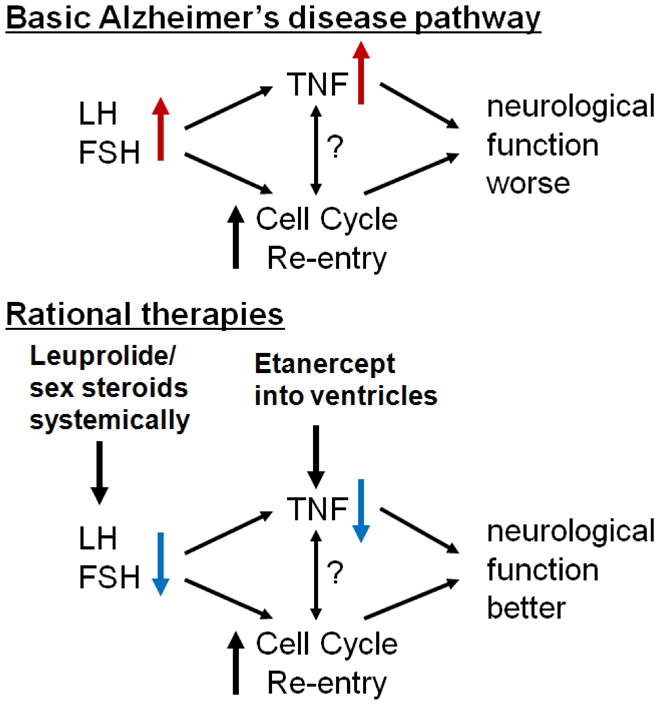
Model of the convergence of dyotic signalling (elevated gonadotropins, suppressed sex steroids) on TNF and cell cycle dynamics as functionally important downstream links mediating neurodegeneration and dysfunction. Based on this model, rational[32] therapies are proposed for the treatment of AD.
Although, as recently reviewed [102], systemic inflammation and trauma may exacerbate pathogenic brain TNF levels in AD, the explanation for the presence of the underlying TNF in AD brains is little discussed, and still relies on arguments concerning viral infections [103], which does not sit well against the apparent non-infectious nature of AD. Induction of TNF by gonadotropins could explain why changes achieved in Aβ deposition and cognition with leuprolide treatment [12; 13], are consistent with those reported for anti-TNF approaches in similar circumstances [97; 104; 101; 66]. At this stage it is not possible to determine whether endocrine dyscrasia is regulating cell cycle signalling independent of TNF. It should be noted, however, that some 15 genes integral to TNF signalling have been found to regulate the G2/M stage of the human cell cycle [105]. Interestingly, a single nucleotide polymorphism in TNF has been associated with the risk of developing AD; that risk was further increased in those individuals positive for APOE E4 [106], a gene involved in cholesterol metabolism and steroidogenesis.
Oxytocin, TNF, anxiety disorder and aggression
Anxiety and aggression are often observed with AD, and after stroke and traumatic brain injury. The literature clearly associates these changes with increased TNF in a range of circumstances. For example, testosterone cells isolated from individuals with a generalized anxiety state generate more TNF on in vitro activation [107], and the ability of endotoxin-triggered human monocytes to generate TNF in vitro associates with aggressiveness [108]. Most strikingly, double TNF receptor knock-out mice essentially lack both anxiety and aggression [109]. These and similar data are consistent with the high anxiolytic capacity of anti-TNF biological agents in patients with rheumatoid arthritis [110] as well as in a mouse model of chronic gastrointestinal inflammation [111]. Inflammatory bowel disease is a well-known cause of anxiety disorder in humans [112]. The well-known anxiolytic actions of progesterone [113] may be mediated via TNF, with the decline in circulating progesterone with endocrine dyscrasia resulting in elevations in TNF. Progesterone has not been tested as a treatment for AD.
In contrast to TNF, oxytocin attenuates experimental anxiety states [114–116], and its levels in human CSF have been inversely associated with life history of aggressive behavior [117]. This is plausibly explained by the ability of oxytocin to reduced production of TNF by monocytes from human volunteers [118]. Evidence for oxytocin being the driving force behind the effects of social interaction (i.c.v. oxytocin receptor inhibitor cancelled the effect), and its insufficiency explaining the effects of social isolation (i.c.v. oxytocin injection cancelled the effect) [119] is compelling. It may also explain, through the capacity of oxytocin to inhibit TNF production [118], why long-term social isolation exacerbates the impairment of spatial working memory in AβPP/PS1 transgenic mice [120]. In addition, social isolation makes experimental stroke worse, decreasing post-stroke survival rate and exacerbating infarct size and edema development [121]. The authors explained their data in terms of IL-6, a cytokine induced by TNF, being higher in the socially isolated animals. So far as we are aware oxytocin has not yet been tested to treat AD, stroke or traumatic brain injury.
Implications for understanding these diseases, and devising therapies
These widespread functional links between the gonadotropins, sex steroids and TNF imply that it is worth investigating all three together when developing an understanding of, and therapy for, neurodegenerative diseases. Anti-TNF agents have been tested in an experimental AD model [66] and open-labeled human trials [122; 123], in an experimental traumatic brain injury model [124], and small, but impressive, open-labeled human stroke trial [125]. In a significant case report of TNF suppression, a patient being treated with the anti-TNF agent etanercept for ankylosing spondylosis prior to T7 complete paraplegia demonstrated remarkable sensory-motor recovery, improving from A to D on the American Spinal Injury Impairment Scale (AIS) within the first year [126].
A point of immediate interest arises from a report that TNF generation in the brain, once initiated, continues there for much longer (months) than it does systemically (hours) [127], exposing a specific target for anti-TNF agents for a much longer period in brain than in the rest of the body. This could rationalize the long reported intervals between stroke onset and apparently successful treatment with an anti-TNF agent [125]. For these reasons synergistic and other therapeutic studies using leuprolide, estrogens, progesterone and oxytocin in conjunction with anti-TNF agents are warranted.
Footnotes
The authors have no conflicts of interest.
References
- 1.Bowen RL, Atwood CS. Living and dying for sex. A theory of aging based on the modulation of cell cycle signaling by reproductive hormones. Gerontology. 2004;50:265–290. doi: 10.1159/000079125. [DOI] [PubMed] [Google Scholar]
- 2.Atwood CS, Meethal SV, Liu T, Wilson AC, Gallego M, Smith MA, Bowen RL. Dysregulation of the hypothalamic-pituitary-gonadal axis with menopause and andropause promotes neurodegenerative senescence. J Neuropathol Exp Neurol. 2005;64:93–103. doi: 10.1093/jnen/64.2.93. [DOI] [PubMed] [Google Scholar]
- 3.Lee HG, Casadesus G, Zhu X, Castellani RJ, McShea A, Perry G, Petersen RB, Bajic V, Smith MA. Cell cycle re-entry mediated neurodegeneration and its treatment role in the pathogenesis of Alzheimer’s disease. Neurochem Int. 2009;54:84–88. doi: 10.1016/j.neuint.2008.10.013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Atwood CS, Bowen RL. The reproductive-cell cycle theory of aging: an update. Exp Gerontol. 2011;46:100–107. doi: 10.1016/j.exger.2010.09.007. [DOI] [PubMed] [Google Scholar]
- 5.Wilson AC, Clemente L, Liu T, Bowen RL, Meethal SV, Atwood CS. Reproductive hormones regulate the selective permeability of the blood-brain barrier. Biochim Biophys Acta. 2008;1782:401–407. doi: 10.1016/j.bbadis.2008.02.011. [DOI] [PubMed] [Google Scholar]
- 6.Arendt T, Holzer M, Gartner U, Bruckner MK. Aberrancies in signal transduction and cell cycle related events in Alzheimer’s disease. J Neural Transm Supp. 1998;54:147–158. doi: 10.1007/978-3-7091-7508-8_14. [DOI] [PubMed] [Google Scholar]
- 7.Raina AK, Monteiro MJ, McShea A, Smith MA. The role of cell cycle-mediated events in Alzheimer’s disease. Int J Exp Pathol. 1999;80:71–76. doi: 10.1046/j.1365-2613.1999.00106.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Neve RL, McPhie DL, Chen Y. Alzheimer’s disease: a dysfunction of the amyloid precursor protein(1) Brain Res. 2000;886:54–66. doi: 10.1016/s0006-8993(00)02869-9. [DOI] [PubMed] [Google Scholar]
- 9.Raina AK, Zhu X, Rottkamp CA, Monteiro M, Takeda A, Smith MA. Cyclin’ toward dementia: cell cycle abnormalities and abortive oncogenesis in Alzheimer disease. J Neurosci Res. 2000;61:128–133. doi: 10.1002/1097-4547(20000715)61:2<128::AID-JNR2>3.0.CO;2-H. [DOI] [PubMed] [Google Scholar]
- 10.Herrup K, Neve R, Ackerman SL, Copani A. Divide and die: cell cycle events as triggers of nerve cell death. J Neurosci. 2004;24:9232–9239. doi: 10.1523/JNEUROSCI.3347-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Meethal SV, Smith MA, Bowen RL, Atwood CS. The gonadotropin connection in Alzheimer’s disease. Endocrine. 2005;26:317–326. doi: 10.1385/ENDO:26:3:317. [DOI] [PubMed] [Google Scholar]
- 12.Bowen RL, Verdile G, Liu T, Parlow AF, Perry G, Smith MA, Martins RN, Atwood CS. Luteinizing hormone, a reproductive regulator that modulates the processing of amyloid-beta precursor protein and amyloid-beta deposition. J Biol Chem. 2004;279:20539–20545. doi: 10.1074/jbc.M311993200. [DOI] [PubMed] [Google Scholar]
- 13.Casadesus G, Webber KM, Atwood CS, Pappolla MA, Perry G, Bowen RL, Smith MA. Luteinizing hormone modulates cognition and amyloid-beta deposition in Alzheimer APP transgenic mice. Biochim Biophys Acta. 2006;1762:447–452. doi: 10.1016/j.bbadis.2006.01.008. [DOI] [PubMed] [Google Scholar]
- 14.Carroll JC, Rosario ER, Chang L, Stanczyk FZ, Oddo S, LaFerla FM, Pike CJ. Progesterone and estrogen regulate Alzheimer-like neuropathology in female 3xTg-AD mice. J Neurosci. 2007;27:13357–13365. doi: 10.1523/JNEUROSCI.2718-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Lin J, Li X, Yuan F, Lin L, Cook CL, Rao Ch V, Lei Z. Genetic ablation of luteinizing hormone receptor improves the amyloid pathology in a mouse model of Alzheimer disease. J Neuropathol Exp Neurol. 2010;69:253–261. doi: 10.1097/NEN.0b013e3181d072cf. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Casadesus G, Milliken EL, Webber KM, Bowen RL, Lei Z, Rao CV, Perry G, Keri RA, Smith MA. Increases in luteinizing hormone are associated with declines in cognitive performance. Mol Cell Endocrinol. 2007;269:107–111. doi: 10.1016/j.mce.2006.06.013. [DOI] [PubMed] [Google Scholar]
- 17.Barron AM, Verdile G, Taddei K, Bates KA, Martins RN. Effect of chronic hCG administration on Alzheimer’s-related cognition and A beta accumulation in PS1KI mice. Endocrinol. 2010;151:5380–5388. doi: 10.1210/en.2009-1168. [DOI] [PubMed] [Google Scholar]
- 18.Frye CA, Duffy CK, Walf AA. Estrogens and progestins enhance spatial learning of intact and ovariectomized rats in the object placement task. Neurobiol Learn Mem. 2007;88:208–216. doi: 10.1016/j.nlm.2007.04.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Bryan KJ, Mudd JC, Richardson SL, Chang J, Lee HG, Zhu X, Smith MA, Casadesus G. Down-regulation of serum gonadotropins is as effective as estrogen replacement at improving menopause-associated cognitive deficits. J Neurochem. 2010;112:870–881. doi: 10.1111/j.1471-4159.2009.06502.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Berry A, Tomidokoro Y, Ghiso J, Thornton J. Human chorionic gonadotropin (a luteinizing hormone homologue) decreases spatial memory and increases brain amyloid-beta levels in female rats. Horm Behav. 2008;54:143–152. doi: 10.1016/j.yhbeh.2008.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Bowen RL, Beaird H, Atwood CS, Smith MA, Rimm AA. Men treated for prostate cancer have a decresed incidence of dementia. 9th International Congress on Alzheimer’s Disease 2004 [Google Scholar]
- 22.D’Amico AV, Braccioforte MH, Moran BJ, Chen MH. Luteinizing-hormone releasing hormone therapy and the risk of death from Alzheimer disease. Alzheimer’s Dis Assoc Disord. 2010;24:85–89. doi: 10.1097/wad.0b013e31819cb8f4. [DOI] [PubMed] [Google Scholar]
- 23.Bowen RL, Isley JP, Atkinson RL. An association of elevated serum gonadotropin concentrations and Alzheimer disease? J Neuroendocrinol. 2000;12:351–354. doi: 10.1046/j.1365-2826.2000.00461.x. [DOI] [PubMed] [Google Scholar]
- 24.Short RA, Bowen RL, O’Brien PC, Graff Radford NR. Elevated gonadotropin levels in patients with Alzheimer disease. Mayo Clin Proc. 2001;76:906–909. doi: 10.4065/76.9.906. [DOI] [PubMed] [Google Scholar]
- 25.Jaffe K, Blanco ME. Involvement of amino acids, opioids, nitric oxide, and NMDA receptors in learning and memory consolidation in crickets. Pharmacol Biochem Behav. 1994;47:493–496. doi: 10.1016/0091-3057(94)90149-x. [DOI] [PubMed] [Google Scholar]
- 26.Xu H, Gouras GK, Greenfield JP, Vincent B, Naslund J, Mazzarelli L, Fried G, Jovanovic JN, Seeger M, Relkin NR, Liao F, Checler F, Buxbaum JD, Chait BT, Thinakaran G, Sisodia SS, Wang R, Greengard P, Gandy S. Estrogen reduces neuronal generation of Alzheimer beta-amyloid peptides. Nat Med. 1998;4:447–451. doi: 10.1038/nm0498-447. [DOI] [PubMed] [Google Scholar]
- 27.Chang D, Kwan J, Timiras PS. Estrogens influence growth, maturation, and amyloid beta-peptide production in neuroblastoma cells and in a beta-APP transfected kidney 293 cell line. Adv Exp Med Biol. 1997;429:261–271. doi: 10.1007/978-1-4757-9551-6_19. [DOI] [PubMed] [Google Scholar]
- 28.Manthey D, Heck S, Engert S, Behl C. Estrogen induces a rapid secretion of amyloid beta precursor protein via the mitogen-activated protein kinase pathway. Eur J Biochem. 2001;268:4285–4291. doi: 10.1046/j.1432-1327.2001.02346.x. [DOI] [PubMed] [Google Scholar]
- 29.Petanceska SS, Nagy V, Frail D, Gandy S. Ovariectomy and 17beta-estradiol modulate the levels of Alzheimer’s amyloid beta peptides in brain. Exp Gerontol. 2000;35:1317–1325. doi: 10.1016/s0531-5565(00)00157-1. [DOI] [PubMed] [Google Scholar]
- 30.Zheng H, Xu H, Uljon SN, Gross R, Hardy K, Gaynor J, Lafrancois J, Simpkins J, Refolo LM, Petanceska S, Wang R, Duff K. Modulation of A(beta) peptides by estrogen in mouse models. J Neurochem. 2002;80:191–196. doi: 10.1046/j.0022-3042.2001.00690.x. [DOI] [PubMed] [Google Scholar]
- 31.Gouras GK, Xu H, Gross RS, Greenfield JP, Hai B, Wang R, Greengard P. Testosterone reduces neuronal secretion of Alzheimer’s beta-amyloid peptides. Proc Natl Acad Sci U S A. 2000;97:1202–1205. doi: 10.1073/pnas.97.3.1202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Pike CJ, Carroll JC, Rosario ER, Barron AM. Protective actions of sex steroid hormones in Alzheimer’s disease. Front Neuroendocrinol. 2009;30:239–258. doi: 10.1016/j.yfrne.2009.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Manly JJ, Merchant CA, Jacobs DM, Small SA, Bell K, Ferin M, Mayeux R. Endogenous estrogen levels and Alzheimer’s disease among postmenopausal women. Neurology. 2000;54:833–837. doi: 10.1212/wnl.54.4.833. [DOI] [PubMed] [Google Scholar]
- 34.Hogervorst E, Bandelow S, Combrinck M, Smith AD. Low free testosterone is an independent risk factor for Alzheimer’s disease. Exp Gerontol. 2004;39:1633–1639. doi: 10.1016/j.exger.2004.06.019. [DOI] [PubMed] [Google Scholar]
- 35.Honjo H, Ogino Y, Naitoh K, Urabe M, Kitawaki J, Yasuda J, Yamamoto T, Ishihara S, Okada H, Yonezawa T, et al. In vivo effects by estrone sulfate on the central nervous system-senile dementia (Alzheimer’s type) J Steroid Biochem. 1989;34:521–525. doi: 10.1016/0022-4731(89)90137-4. [DOI] [PubMed] [Google Scholar]
- 36.Asthana S, Craft S, Baker LD, Raskind MA, Birnbaum RS, Lofgreen CP, Veith RC, Plymate SR. Cognitive and neuroendocrine response to transdermal estrogen in postmenopausal women with Alzheimer’s disease: results of a placebo-controlled, double-blind, pilot study. Psychoneuroendocrinology. 1999;24:657–677. doi: 10.1016/s0306-4530(99)00020-7. [DOI] [PubMed] [Google Scholar]
- 37.Asthana S, Baker LD, Craft S, Stanczyk FZ, Veith RC, Raskind MA, Plymate SR. High-dose estradiol improves cognition for women with AD: results of a randomized study. Neurology. 2001;57:605–612. doi: 10.1212/wnl.57.4.605. [DOI] [PubMed] [Google Scholar]
- 38.Fillit H, Weinreb H, Cholst I, Luine V, McEwen B, Amador R, Zabriskie J. Observations in a preliminary open trial of estradiol therapy for senile dementia-Alzheimer’s type. Psychoneuroendocrinology. 1986;11:337–345. doi: 10.1016/0306-4530(86)90019-3. [DOI] [PubMed] [Google Scholar]
- 39.Tan RS, Pu SJ. A pilot study on the effects of testosterone in hypogonadal aging male patients with Alzheimer’s disease. Aging Male. 2003;6:13–17. [PubMed] [Google Scholar]
- 40.Cherrier MM, Matsumoto AM, Amory JK, Asthana S, Bremner W, Peskind ER, Raskind MA, Craft S. Testosterone improves spatial memory in men with Alzheimer disease and mild cognitive impairment. Neurology. 2005;64:2063–2068. doi: 10.1212/01.WNL.0000165995.98986.F1. [DOI] [PubMed] [Google Scholar]
- 41.Porayette P, Gallego MJ, Kaltcheva MM, Bowen RL, Vadakkadath Meethal S, Atwood CS. Differential processing of amyloid-beta precursor protein directs human embryonic stem cell proliferation and differentiation into neuronal precursor cells. J Biol Chem. 2009;284:23806–23817. doi: 10.1074/jbc.M109.026328. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Mak GK, Enwere EK, Gregg C, Pakarainen T, Poutanen M, Huhtaniemi I, Weiss S. Male pheromone-stimulated neurogenesis in the adult female brain: possible role in mating behavior. Nat Neurosci. 2007;10:1003–1011. doi: 10.1038/nn1928. [DOI] [PubMed] [Google Scholar]
- 43.Hawken PA, Jorre TJ, Rodger J, Esmaili T, Blache D, Martin GB. Rapid induction of cell proliferation in the adult female ungulate brain (Ovis aries) associated with activation of the reproductive axis by exposure to unfamiliar males. Biol Reprod. 2009;80:1146–1151. doi: 10.1095/biolreprod.108.075341. [DOI] [PubMed] [Google Scholar]
- 44.Haga S, Akai K, Ishii T. Demonstration of microglial cells in and around senile (neuritic) plaques in the Alzheimer brain. An immunohistochemical study using a novel monoclonal antibody. Acta Neuropathol. 1989;77:569–575. doi: 10.1007/BF00687883. [DOI] [PubMed] [Google Scholar]
- 45.Itagaki S, McGeer PL, Akiyama H, Zhu S, Selkoe D. Relationship of microglia and astrocytes to amyloid deposits of Alzheimer disease. J Neuroimmunol. 1989;24:173–182. doi: 10.1016/0165-5728(89)90115-x. [DOI] [PubMed] [Google Scholar]
- 46.Akiyama H, Kondo H, Mori H, Kametani F, Nishimura T, Ikeda K, Kato M, McGeer PL. The amino-terminally truncated forms of amyloid beta-protein in brain macrophages in the ischemic lesions of Alzheimer’s disease patients. Neurosci Lett. 1996;219:115–118. doi: 10.1016/s0304-3940(96)13197-9. [DOI] [PubMed] [Google Scholar]
- 47.Schenk D, Barbour R, Dunn W, Gordon G, Grajeda H, Guido T, Hu K, Huang J, Johnson Wood K, Khan K, Kholodenko D, Lee M, Liao Z, Lieberburg I, Motter R, Mutter L, Soriano F, Shopp G, Vasquez N, Vandevert C, Walker S, Wogulis M, Yednock T, Games D, Seubert P. Immunization with amyloid-beta attenuates Alzheimer-disease-like pathology in the PDAPP mouse. Nature. 1999;400:173–177. doi: 10.1038/22124. [DOI] [PubMed] [Google Scholar]
- 48.Bishop GM, Robinson SR, Smith MA, Perry G, Atwood CS. Call for Elan to publish Alzheimer’s trial details. Nature. 2002;416:677. doi: 10.1038/416677d. [DOI] [PubMed] [Google Scholar]
- 49.Smith MA, Atwood CS, Joseph JA, Perry G. Predicting the failure of amyloid-beta vaccine. Lancet. 2002;359:1864–1865. doi: 10.1016/S0140-6736(02)08695-6. [DOI] [PubMed] [Google Scholar]
- 50.Holmes C, Boche D, Wilkinson D, Yadegarfar G, Hopkins V, Bayer A, Jones RW, Bullock R, Love S, Neal JW, Zotova E, Nicoll JA. Long-term effects of Abeta42 immunisation in Alzheimer’s disease: follow-up of a randomised, placebo-controlled phase I trial. Lancet. 2008;372:216–223. doi: 10.1016/S0140-6736(08)61075-2. [DOI] [PubMed] [Google Scholar]
- 51.Atwood CS. Amyloid-beta aggregation as a protective acute-phase response to injury/neurodegeneration: A barrier function for amyloid-beta deposits. In: Bucciantini SRaM., editor. Functional Amyloid Aggregation. Kerala, India: Research Signpost; 2010. pp. 115–134. [Google Scholar]
- 52.Brugg B, Dubreuil YL, Huber G, Wollman EE, Delhaye Bouchaud N, Mariani J. Inflammatory processes induce beta-amyloid precursor protein changes in mouse brain. Proc Natl Acad Sci U S A. 1995;92:3032–3035. doi: 10.1073/pnas.92.7.3032. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 53.Buxbaum JD, Liu KN, Luo Y, Slack JL, Stocking KL, Peschon JJ, Johnson RS, Castner BJ, Cerretti DP, Black RA. Evidence that tumor necrosis factor alpha converting enzyme is involved in regulated alpha-secretase cleavage of the Alzheimer amyloid protein precursor. J Biol Chem. 1998;273:27765–27767. doi: 10.1074/jbc.273.43.27765. [DOI] [PubMed] [Google Scholar]
- 54.Goldgaber D, Harris HW, Hla T, Maciag T, Donnelly RJ, Jacobsen JS, Vitek MP, Gajdusek DC. Interleukin 1 regulates synthesis of amyloid beta-protein precursor mRNA in human endothelial cells. Proc Natl Acad Sci U S A. 1989;86:7606–76010. doi: 10.1073/pnas.86.19.7606. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Ge YW, Lahiri DK. Regulation of promoter activity of the APP gene by cytokines and growth factors: implications in Alzheimer’s disease. Ann N Y Acad Sci. 2002;973:463–467. doi: 10.1111/j.1749-6632.2002.tb04684.x. [DOI] [PubMed] [Google Scholar]
- 56.Schmidt J, Barthel K, Wrede A, Salajegheh M, Bahr M, Dalakas MC. Interrelation of inflammation and APP in sIBM: IL-1 beta induces accumulation of beta-amyloid in skeletal muscle. Brain. 2008;131:1228–1240. doi: 10.1093/brain/awn053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Sommer G, Kralisch S, Lipfert J, Weise S, Krause K, Jessnitzer B, Lossner U, Bluher M, Stumvoll M, Fasshauer M. Amyloid precursor protein expression is induced by tumor necrosis factor alpha in 3T3-L1 adipocytes. J Cell Biochem. 2009;108:1418–1422. doi: 10.1002/jcb.22382. [DOI] [PubMed] [Google Scholar]
- 58.Liao YF, Wang BJ, Cheng HT, Kuo LH, Wolfe MS. Tumor necrosis factor-alpha, interleukin-1beta, and interferon-gamma stimulate gamma-secretase-mediated cleavage of amyloid precursor protein through a JNK-dependent MAPK pathway. J Biol Chem. 2004;279:49523–49532. doi: 10.1074/jbc.M402034200. [DOI] [PubMed] [Google Scholar]
- 59.Griffin WS, Stanley LC, Ling C, White L, MacLeod V, Perrot LJ, White CL, 3rd, Araoz C. Brain interleukin 1 and S-100 immunoreactivity are elevated in Down syndrome and Alzheimer disease. Proc Natl Acad Sci U S A. 1989;86:7611–7615. doi: 10.1073/pnas.86.19.7611. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Tarkowski E, Andreasen N, Tarkowski A, Blennow K. Intrathecal inflammation precedes development of Alzheimer’s disease. J Neurol Neurosurg Psych. 2003;74:1200–1205. doi: 10.1136/jnnp.74.9.1200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 61.Laurin D, David Curb J, Masaki KH, White LR, Launer LJ. Midlife C-reactive protein and risk of cognitive decline: a 31-year follow-up. Neurobiol Aging. 2009;30:1724–1727. doi: 10.1016/j.neurobiolaging.2008.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 62.Schuitemaker A, Dik MG, Veerhuis R, Scheltens P, Schoonenboom NS, Hack CE, Blankenstein MA, Jonker C. Inflammatory markers in AD and MCI patients with different biomarker profiles. Neurobiol Aging. 2009;30:1885–1889. doi: 10.1016/j.neurobiolaging.2008.01.014. [DOI] [PubMed] [Google Scholar]
- 63.Buchhave P, Zetterberg H, Blennow K, Minthon L, Janciauskiene S, Hansson O. Soluble TNF receptors are associated with Abeta metabolism and conversion to dementia in subjects with mild cognitive impairment. Neurobiol Aging. 2009;450:56–59. doi: 10.1016/j.neurobiolaging.2008.10.012. [DOI] [PubMed] [Google Scholar]
- 64.Thambisetty M, Simmons A, Velayudhan L, Hye A, Campbell J, Zhang Y, Wahlund LO, Westman E, Kinsey A, Guntert A, Proitsi P, Powell J, Causevic M, Killick R, Lunnon K, Lynham S, Broadstock M, Choudhry F, Howlett DR, Williams RJ, Sharp SI, Mitchelmore C, Tunnard C, Leung R, Foy C, O’Brien D, Breen G, Furney SJ, Ward M, Kloszewska I, Mecocci P, Soininen H, Tsolaki M, Vellas B, Hodges A, Murphy DG, Parkins S, Richardson JC, Resnick SM, Ferrucci L, Wong DF, Zhou Y, Muehlboeck S, Evans A, Francis PT, Spenger C, Lovestone S. Association of plasma clusterin concentration with severity, pathology, and progression in Alzheimer’s disease. Arch Gen Psychiatry. 2010;67:739–748. doi: 10.1001/archgenpsychiatry.2010.78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 65.Hardardottir I, Kunitake ST, Moser AH, Doerrler WT, Rapp JH, Grunfeld C, Feingold KR. Endotoxin and cytokines increase hepatic messenger RNA levels and serum concentrations of apolipoprotein J (clusterin) in Syrian hamsters. J Clin Invest. 1994;94:1304–1309. doi: 10.1172/JCI117449. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Shi JQ, Shen W, Chen J, Wang BR, Zhong LL, Zhu YW, Zhu HQ, Zhang QQ, Zhang YD, Xu J. Anti-TNF-alpha reduces amyloid plaques and tau phosphorylation and induces CD11c-positive dendritic-like cell in the APP/PS1 transgenic mouse brains. Brain Res. 2011;1368:239–247. doi: 10.1016/j.brainres.2010.10.053. [DOI] [PubMed] [Google Scholar]
- 67.Haider S, Knofler M. Human tumour necrosis factor: physiological and pathological roles in placenta and endometrium. Placenta. 2009;30:111–123. doi: 10.1016/j.placenta.2008.10.012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 68.Beattie EC, Stellwagen D, Morishita W, Bresnahan JC, Ha BK, Von Zastrow M, Beattie MS, Malenka RC. Control of synaptic strength by glial TNFalpha. Science. 2002;295:2282–2285. doi: 10.1126/science.1067859. [DOI] [PubMed] [Google Scholar]
- 69.Bernardino L, Agasse F, Silva B, Ferreira R, Grade S, Malva JO. Tumor necrosis factor-alpha modulates survival, proliferation, and neuronal differentiation in neonatal subventricular zone cell cultures. Stem Cells. 2008;26:2361–2371. doi: 10.1634/stemcells.2007-0914. [DOI] [PubMed] [Google Scholar]
- 70.Iqbal J, Sun L, Kumar TR, Blair HC, Zaidi M. Follicle-stimulating hormone stimulates TNF production from immune cells to enhance osteoblast and osteoclast formation. Proc Natl Acad Sci U S A. 2006;103:14925–14930. doi: 10.1073/pnas.0606805103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 71.Kass AS, Lea TE, Torjesen PA, Gulseth HC, Forre OT. The association of luteinizing hormone and follicle-stimulating hormone with cytokines and markers of disease activity in rheumatoid arthritis: a case-control study. Scand J Rheumatol. 2010;39:109–117. doi: 10.3109/03009740903270607. [DOI] [PubMed] [Google Scholar]
- 72.Meresman GF, Bilotas MA, Lombardi E, Tesone M, Sueldo C, Baranao RI. Effect of GnRH analogues on apoptosis and release of interleukin-1beta and vascular endothelial growth factor in endometrial cell cultures from patients with endometriosis. Hum Reprod. 2003;18:1767–1771. doi: 10.1093/humrep/deg356. [DOI] [PubMed] [Google Scholar]
- 73.Ferreira RA, Vieira CS, Rosa ESJC, Rosa e Silva AC, Nogueira AA, Ferriani RA. Effects of the levonorgestrel-releasing intrauterine system on cardiovascular risk markers in patients with endometriosis: a comparative study with the GnRH analogue. Contraception. 2010;81:117–122. doi: 10.1016/j.contraception.2009.08.003. [DOI] [PubMed] [Google Scholar]
- 74.Ficicioglu C, Kumbak B, Akcin O, Attar R, Yildirim G, Yesildaglar N. Comparison of follicular fluid and serum cytokine concentrations in women undergoing assisted reproductive treatment with GnRH agonist long and antagonist protocols. Gynecol Endocrinol. 2010;26:181–186. doi: 10.1080/09513590903215557. [DOI] [PubMed] [Google Scholar]
- 75.Khan KN, Kitajima M, Hiraki K, Fujishita A, Sekine I, Ishimaru T, Masuzaki H. Changes in tissue inflammation, angiogenesis and apoptosis in endometriosis, adenomyosis and uterine myoma after GnRH agonist therapy. Hum Reprod. 2010;25:642–653. doi: 10.1093/humrep/dep437. [DOI] [PubMed] [Google Scholar]
- 76.Shalaby MR, Waage A, Aarden L, Espevik T. Endotoxin, tumor necrosis factor-α and interleukin-1 induce interleukin-6 production in vivo. Clin Immunol Immunopathol. 1989;53:488–498. doi: 10.1016/0090-1229(89)90010-x. [DOI] [PubMed] [Google Scholar]
- 77.Charles P, Elliott MJ, Davis D, Potter A, Kalden JR, Antoni C, Breedveld FC, Smolen JS, Eberl G, deWoody K, Feldmann M, Maini RN. Regulation of cytokines, cytokine inhibitors, and acute-phase proteins following anti-TNF-alpha therapy in rheumatoid arthritis. J Immunol. 1999;163:1521–1528. [PubMed] [Google Scholar]
- 78.Mueller L, von Seggern L, Schumacher J, Goumas F, Wilms C, Braun F, Broering DC. TNF-alpha similarly induces IL-6 and MCP-1 in fibroblasts from colorectal liver metastases and normal liver fibroblasts. Biochem Biophys Res Commun. 2010;397:586–591. doi: 10.1016/j.bbrc.2010.05.163. [DOI] [PubMed] [Google Scholar]
- 79.Redl H, Schlag G, Paul E, Bahrami S, Buurman WA, Strieter RM, Kunkel SL, Davies J, Foulkes R. Endogenous modulators of TNF and IL-1 response are under partial control of TNF in baboon bacteremia. Am J Physiol. 1996;40:R1193–R1198. doi: 10.1152/ajpregu.1996.271.5.R1193. [DOI] [PubMed] [Google Scholar]
- 80.Brennan FM, Chantry D, Jackson A, Maini R, Feldmann M. Inhibitory effect of TNF alpha antibodies on synovial cell interleukin-1 production in rheumatoid arthritis. Lancet. 1989;2:244–247. doi: 10.1016/s0140-6736(89)90430-3. [DOI] [PubMed] [Google Scholar]
- 81.Ralston SH, Russell RG, Gowen M. Estrogen inhibits release of tumor necrosis factor from peripheral blood mononuclear cells in postmenopausal women. J Bone Miner Res. 1990;5:983–988. doi: 10.1002/jbmr.5650050912. [DOI] [PubMed] [Google Scholar]
- 82.Kipp M, Karakaya S, Johann S, Kampmann E, Mey J, Beyer C. Oestrogen and progesterone reduce lipopolysaccharide-induced expression of tumour necrosis factor-alpha and interleukin-18 in midbrain astrocytes. J Neuroendocrinol. 2007;19:819–822. doi: 10.1111/j.1365-2826.2007.01588.x. [DOI] [PubMed] [Google Scholar]
- 83.Smith JA, Das A, Butler JT, Ray SK, Banik NL. Estrogen or estrogen receptor agonist inhibits lipopolysaccharide-induced microglial activation and death. Neurochem Res. 2010 doi: 10.1007/s11064-010-0336-7. in press. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Srivastava S, Weitzmann MN, Cenci S, Ross FP, Adler S, Pacifici R. Estrogen decreases TNF gene expression by blocking JNK activity and the resulting production of c-Jun and JunD. J Clin Invest. 1999;104:503–513. doi: 10.1172/JCI7094. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 85.Hsu SM, Chen YC, Jiang MC. 17 beta-estradiol inhibits tumor necrosis factor-alpha-induced nuclear factor-kappa B activation by increasing nuclear factor-kappa B p105 level in MCF-7 breast cancer cells. Biochem Biophys Res Commun. 2000;279:47–52. doi: 10.1006/bbrc.2000.3891. [DOI] [PubMed] [Google Scholar]
- 86.Jiang N, Chopp M, Stein D, Feit H. Progesterone is neuroprotective after transient middle cerebral artery occlusion in male rats. Brain Res. 1996;735:101–107. doi: 10.1016/0006-8993(96)00605-1. [DOI] [PubMed] [Google Scholar]
- 87.Yang SH, Liu R, Wu SS, Simpkins JW. The use of estrogens and related compounds in the treatment of damage from cerebral ischemia. Ann N Y Acad Sci. 2003;1007:101–107. doi: 10.1196/annals.1286.010. [DOI] [PubMed] [Google Scholar]
- 88.Stein DG. Brain damage, sex hormones and recovery: a new role for progesterone and estrogen? Trends Neurosci. 2001;24:386–391. doi: 10.1016/s0166-2236(00)01821-x. [DOI] [PubMed] [Google Scholar]
- 89.Stein DG, Hoffman SW. Estrogen and progesterone as neuroprotective agents in the treatment of acute brain injuries. Pediatr Rehabil. 2003;6:13–22. doi: 10.1080/1363849031000095279. [DOI] [PubMed] [Google Scholar]
- 90.Pan DS, Liu WG, Yang XF, Cao F. Inhibitory effect of progesterone on inflammatory factors after experimental traumatic brain injury. Biomed Environ Sci. 2007;20:432–438. [PubMed] [Google Scholar]
- 91.Stein DG, Wright DW. Progesterone in the clinical treatment of acute traumatic brain injury. Expert Opin Investig Drugs. 2010;19:847–857. doi: 10.1517/13543784.2010.489549. [DOI] [PubMed] [Google Scholar]
- 92.Yi KD, Perez E, Yang S, Liu R, Covey DF, Simpkins JW. The assessment of non-feminizing estrogens for use in neuroprotection. Brain Res. 2010;1379:61–70. doi: 10.1016/j.brainres.2010.11.058. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 93.Tarkowski E, Liljeroth AM, Minthon L, Tarkowski A, Wallin A, Blennow K. Cerebral pattern of pro- and anti-inflammatory cytokines in dementias. Brain Res Bull. 2003;61:255–260. doi: 10.1016/s0361-9230(03)00088-1. [DOI] [PubMed] [Google Scholar]
- 94.McNaull BB, Todd S, McGuinness B, Passmore AP. Inflammation and anti-Inflammatory strategies for Alzheimer’s disease - a mini-review. Gerontology. 2010;56:3–14. doi: 10.1159/000237873. [DOI] [PubMed] [Google Scholar]
- 95.Gorlovoy P, Larionov S, Pham TT, Neumann H. Accumulation of tau induced in neurites by microglial proinflammatory mediators. FASEB J. 2009;23:2502–2513. doi: 10.1096/fj.08-123877. [DOI] [PubMed] [Google Scholar]
- 96.Janelsins MC, Mastrangelo MA, Park KM, Sudol KL, Narrow WC, Oddo S, Laferla FM, Callahan LM, Federoff HJ, Bowers WJ. Chronic neuron-specific tumor necrosis factor expression enhances the local inflammatory environment ultimately leading to neuronal death in 3xTg-AD mice. Am J Pathol. 2008;173:1768–1782. doi: 10.2353/ajpath.2008.080528. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 97.McAlpine FE, Lee JK, Harms AS, Ruhn KA, Blurton Jones M, Hong J, Das P, Golde TE, LaFerla FM, Oddo S, Blesch A, Tansey MG. Inhibition of soluble TNF signaling in a mouse model of Alzheimer’s disease prevents pre-plaque amyloid-associated neuropathology. Neurobiol Dis. 2009;34:163–177. doi: 10.1016/j.nbd.2009.01.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.McCoy MK, Tansey MG. TNF signaling inhibition in the CNS: implications for normal brain function and neurodegenerative disease. J Neuroinflammation. 2008;5:45. doi: 10.1186/1742-2094-5-45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 99.Tweedie D, Sambamurti K, Greig NH. TNF-alpha inhibition as a treatment strategy for neurodegenerative disorders: new drug candidates and targets. Curr Alzheimer Res. 2007;4:378–385. doi: 10.2174/156720507781788873. [DOI] [PubMed] [Google Scholar]
- 100.van Gool WA, van de Beek D, Eikelenboom P. Systemic infection and delirium: when cytokines and acetylcholine collide. Lancet. 2010;375:773–775. doi: 10.1016/S0140-6736(09)61158-2. [DOI] [PubMed] [Google Scholar]
- 101.Medeiros R, Figueiredo CP, Pandolfo P, Duarte FS, Prediger RD, Passos GF, Calixto JB. The role of TNF-alpha signaling pathway on COX-2 upregulation and cognitive decline induced by beta-amyloid peptide. Behav Brain Res. 2010;209:165–173. doi: 10.1016/j.bbr.2010.01.040. [DOI] [PubMed] [Google Scholar]
- 102.Clark IA, Alleva LM, Vissel B. The roles of TNF in brain dysfunction and disease. Pharmacol Ther. 2010;128:519–548. doi: 10.1016/j.pharmthera.2010.08.007. [DOI] [PubMed] [Google Scholar]
- 103.Carter CJ. Interactions between the products of the Herpes simplex genome and Alzheimer’s disease susceptibility genes: relevance to pathological-signalling cascades. Neurochem Int. 2008;52:920–934. doi: 10.1016/j.neuint.2007.11.003. [DOI] [PubMed] [Google Scholar]
- 104.Tobinick EL, Chen K, Chen X. Rapid intracerebroventricular delivery of Cu-DOTA-etanercept after peripheral administration demonstrated by PET imaging. BMC Res Notes. 2009;2:28. doi: 10.1186/1756-0500-2-28. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Mukherji M, Bell R, Supekova L, Wang Y, Orth AP, Batalov S, Miraglia L, Huesken D, Lange J, Martin C, Sahasrabudhe S, Reinhardt M, Natt F, Hall J, Mickanin C, Labow M, Chanda SK, Cho CY, Schultz PG. Genome-wide functional analysis of human cell-cycle regulators. Proc Natl Acad Sci U S A. 2006;103:14819–14824. doi: 10.1073/pnas.0604320103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 106.Di Bona D, Candore G, Franceschi C, Licastro F, Colonna Romano G, Camma C, Lio D, Caruso C. Systematic review by meta-analyses on the possible role of TNF-alpha polymorphisms in association with Alzheimer’s disease. Brain Res Rev. 2009;61:60–68. doi: 10.1016/j.brainresrev.2009.05.001. [DOI] [PubMed] [Google Scholar]
- 107.Vieira MM, Ferreira TB, Pacheco PA, Barros PO, Almeida CR, Araujo Lima CF, Silva Filho RG, Hygino J, Andrade RM, Linhares UC, Andrade AF, Bento CA. Enhanced Th17 phenotype in individuals with generalized anxiety disorder. J Neuroimmunol. 2010;229:212–218. doi: 10.1016/j.jneuroim.2010.07.018. [DOI] [PubMed] [Google Scholar]
- 108.Suarez EC, Lewis JG, Kuhn C. The relation of aggression, hostility, and anger to lipopolysaccharide-stimulated tumor necrosis factor (TNF)-alpha by blood monocytes from normal men. Brain Behav Immun. 2002;16:675–684. doi: 10.1016/s0889-1591(02)00019-3. [DOI] [PubMed] [Google Scholar]
- 109.Patel A, Siegel A, Zalcman SS. Lack of aggression and anxiolytic-like behavior in TNF receptor (TNF-R1 and TNF-R2) deficient mice. Brain Behav Immun. 2010 doi: 10.1016/j.bbi.2010.1005.1005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Uguz F, Akman C, Kucuksarac S, Tufekci O. Anti-tumor necrosis factor-alpha therapy is associated with less frequent mood and anxiety disorders in patients with rheumatoid arthritis. Psychiatry Clin Neurosci. 2009;63:50–55. doi: 10.1111/j.1440-1819.2008.01905.x. [DOI] [PubMed] [Google Scholar]
- 111.Bercik P, Verdu EF, Foster JA, Macri J, Potter M, Huang X, Malinowski P, Jackson W, Blennerhassett P, Neufeld KA, Lu J, Khan W, Corthesy Theulaz I, Cherbut C, Bergonzelli GE, Collins SM. Chronic gastrointestinal inflammation induces anxiety-like behavior and alters central nervous system biochemistry in mice. Gastroenterology. 2010;139:2102–2112. doi: 10.1053/j.gastro.2010.06.063. [DOI] [PubMed] [Google Scholar]
- 112.Andrews H, Barczak P, Allan RN. Psychiatric illness in patients with inflammatory bowel disease. Gut. 1987;28:1600–1604. doi: 10.1136/gut.28.12.1600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 113.Rodriguez-Sierra JF, Hagley MT, Hendricks SE. Anxiolytic effects of progesterone are sexually dimorphic. Life Sci. 1986;38:1841–1845. doi: 10.1016/0024-3205(86)90139-6. [DOI] [PubMed] [Google Scholar]
- 114.Blume A, Bosch OJ, Miklos S, Torner L, Wales L, Waldherr M, Neumann ID. Oxytocin reduces anxiety via ERK1/2 activation: local effect within the rat hypothalamic paraventricular nucleus. Eur J Neurosci. 2008;27:1947–1956. doi: 10.1111/j.1460-9568.2008.06184.x. [DOI] [PubMed] [Google Scholar]
- 115.Missig G, Ayers LW, Schulkin J, Rosen JB. Oxytocin reduces background anxiety in a fear-potentiated startle paradigm. Neuropsychopharmacology. 2010;35:2607–2616. doi: 10.1038/npp.2010.155. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 116.Slattery DA, Neumann ID. Chronic icv oxytocin attenuates the pathological high anxiety state of selectively bred Wistar rats. Neuropharmacol. 2010;58:56–61. doi: 10.1016/j.neuropharm.2009.06.038. [DOI] [PubMed] [Google Scholar]
- 117.Lee R, Ferris C, Van de Kar LD, Coccaro EF. Cerebrospinal fluid oxytocin, life history of aggression, and personality disorder. Psychoneuroendocrinology. 2009;34:1567–1573. doi: 10.1016/j.psyneuen.2009.06.002. [DOI] [PubMed] [Google Scholar]
- 118.Clodi M, Vila G, Geyeregger R, Riedl M, Stulnig TM, Struck J, Luger TA, Luger A. Oxytocin alleviates the neuroendocrine and cytokine response to bacterial endotoxin in healthy men. Am J Physiol. 2008;295:E686–E691. doi: 10.1152/ajpendo.90263.2008. [DOI] [PubMed] [Google Scholar]
- 119.Norman GJ, Karelina K, Morris JS, Zhang N, Cochran M, Courtney DeVries A. Social interaction prevents the development of depressive-like behavior post nerve injury in mice: a potential role for oxytocin. Psychosom Med. 2010;72:519–526. doi: 10.1097/PSY.0b013e3181de8678. [DOI] [PubMed] [Google Scholar]
- 120.Huang HJ, Liang KC, Ke HC, Chang YY, Hsieh Li HM. Long-term social isolation exacerbates the impairment of spatial working memory in APP/PS1 transgenic mice. Brain Res. 2011;2011:150–160. doi: 10.1016/j.brainres.2010.11.043. [DOI] [PubMed] [Google Scholar]
- 121.Karelina K, Norman GJ, Zhang N, Morris JS, Peng H, DeVries AC. Social isolation alters neuroinflammatory response to stroke. Proc Natl Acad Sci U S A. 2009;106:5895–5900. doi: 10.1073/pnas.0810737106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 122.Tobinick EL, Gross H, Weinberger A, Cohen H. TNF-alpha modulation for treatment of Alzheimer’s disease: A 6- month pilot study. MedGenMed Neurol Neurosurg. 2006;8:25. [PMC free article] [PubMed] [Google Scholar]
- 123.Tobinick EL, Gross H. Rapid cognitive improvement in Alzheimer’s disease following perispinal etanercept administration. J Neuroinflamm. 2008;5:2. doi: 10.1186/1742-2094-5-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 124.Chio CC, Lin JW, Chang MW, Wang CC, Yang CZ, Chang CP. Therapeutic evaluation of etanercept in a model of traumatic brain injury. J Neurochem. 2010;115:921–929. doi: 10.1111/j.1471-4159.2010.06969.x. [DOI] [PubMed] [Google Scholar]
- 125.Tobinick E. Rapid improvement of chronic stroke deficits after perispinal etanercept: three consecutive cases. CNS Drugs. 2011;25:145–155. doi: 10.2165/11588400-000000000-00000. [DOI] [PubMed] [Google Scholar]
- 126.Dinomais M, Stana L, Egon G, Richard I, Menei P. Significant recovery of motor function in a patient with complete T7 paraplegia receiving etanercept. J Rehabil Med. 2009;41:286–288. doi: 10.2340/16501977-0329. [DOI] [PubMed] [Google Scholar]
- 127.Qin LY, Wu XF, Block ML, Liu YX, Bresse GR, Hong JS, Knapp DJ, Crews FT. Systemic LPS causes chronic neuroinflammation and progressive neurodegeneration. Glia. 2007;55:453–462. doi: 10.1002/glia.20467. [DOI] [PMC free article] [PubMed] [Google Scholar]


