Abstract
A double-site enzyme-linked lactate dehydrogenase enzyme immunodetection assay was tested against field isolates of Plasmodium falciparum for assessing in vitro drug susceptibilities to a wide range of antimalarial drugs. Its sensitivity allowed the use of parasite densities as low as 200 parasites/μl of blood. Being a nonisotopic, colorimetric assay, it lies within the capabilities of a modest laboratory at the district level.
Multidrug resistance in Plasmodium falciparum remains one of the leading problems facing effective malaria control in tropical countries, particularly in Southeast Asia (1, 3, 5, 6). Until the time when molecular markers can reliably be used to determine levels of drug resistance in an area, heavy reliance will remain on in vivo drug efficacy studies and in vitro assays to determine drug susceptibilities in clinical isolates.
A double-site enzyme-linked lactate dehydrogenase enzyme immunodetection (DELI) assay has recently been developed (2) for assessing in vitro drug susceptibility that relies on the detection of P. falciparum-specific lactate dehydrogenase. The DELI method offers some unique advantages over the present standard isotopic assay. Its sensitivity allows the measurement of drug response with very low parasite densities (as low as 0.005% in one study [4]). In addition, since it is a nonisotopic, enzyme-linked immunosorbent assay (ELISA), equipment is simplified to the needs of running a straightforward ELISA.
To further evaluate the potential of the DELI microtest for the monitoring of in vitro drug susceptibilities of field isolates, we compared the DELI assay to the present standard isotopic microtest for the susceptibilities first of the K1 laboratory strain and then of 86 fresh clinical isolates to eight antimalarial drugs (chloroquine, quinine, mefloquine, lumefantrine, artesunate, dihydroartemisinin, atovaquone, and doxycycline).
Eighty-six fresh isolates for the assay comparison were obtained between March 2001 and June 2002 from patients with acute falciparum malaria attending the clinics of the Shoklo Malaria Research Unit.
For the isotopic microtest (7), the fresh isolates were adjusted to an optimum density of 0.5 to 1.0% parasitized erythrocytes and a hematocrit of 1.5% with freshly washed group O erythrocytes and complete RPMI medium with 10% heat-inactivated heterologous sera or commercial AB sera.
For the DELI microtest (2), the infected red blood cells were diluted in culture medium at the same hematocrit (1.5%) as that used for the isotopic microtest but at a parasitemia level of 0.2% and were distributed in the same manner in the appropriate antimalarial predosed plates. The plates were incubated at 37°C in the presence of 5% CO2, 5% O2, and 90% N2 and were frozen after 42 h.
Concentration-response data were analyzed by a nonlinear regression function to determine the 50% effective concentration (EC50), defined as the concentration of the drug which inhibited 50% of (i) the uptake of [3H]hypoxanthine into the nucleoprotein of the parasites (for the isotopic assay) or (ii) the production of lactate dehydrogenase as determined by optical density values from test wells compared to those obtained from drug-free control wells (for the DELI method).
The effect of parasite density on EC50s was assessed in three field isolates for chloroquine, quinine, mefloquine, and artesunate. There was a nonsignificant trend for lower EC50s with decreasing parasitemias (0.5 to 0.005% infected red blood cells) for each drug and which proved almost significant for chloroquine (r = 0.55, P = 0.053) and artesunate (r = 0.53, P = 0.064). Where the parasite density was known and high enough to necessitate dilution, optimal responses were consistently obtained when the parasitemia level was adjusted to 0.1 to 0.2% (approximately 4,000 to 8,000 parasites/μl of blood).
Table 1 shows a comparison of the EC50s for the K1 parasite clone determined by the DELI and isotopic assays. The DELI assay showed a greater variability than did the isotopic assay and a nonsignificant trend to overestimate in vitro responses to all drugs.
TABLE 1.
Comparative EC50s of eight drugs for the K1 parasite clone
| Druga | DELI assay
|
Isotopic assay
|
||||||||
|---|---|---|---|---|---|---|---|---|---|---|
| No. of isolates | EC50 (ng/ml)
|
% CVc | No. of isolates | EC50 (ng/ml)
|
% CVc | |||||
| Meanb | 95% CId | Range | Meanb | 95% CId | Range | |||||
| Chloroquine | 6 | 218.5 | 170.4-280.2 | 173.1-340.3 | 26.8 | 6 | 188.1 | 180.2-196.4 | 175.0-192.3 | 4.4 |
| Quinine | 6 | 275.4 | 213.3-356.6 | 183.9-393.5 | 23.8 | 6 | 261.16 | 241.9-282.0 | 238.3-286.2 | 7.7 |
| Mefloquine | 6 | 13.7 | 8.7-21.5 | 7.6-19.7 | 36.5 | 6 | 14.79 | 12.5-17.4 | 11.7-17.8 | 13.8 |
| Lumefantrine | 6 | 5.3 | 4.0-7.0 | 3.3-6.8 | 23.1 | 6 | 5.15 | 4.6-5.8 | 4.3-5.8 | 10.1 |
| Artesunate | 6 | 1.02 | 0.95-1.44 | 0.95-1.65 | 21.5 | 6 | 0.982 | 0.79-1.22 | 0.74-1.23 | 19.6 |
| Dihydroartemisinin | 6 | 1.23 | 0.98-1.56 | 0.91-1.72 | 22.5 | 6 | 1.058 | 0.91-1.24 | 0.97-1.42 | 17.6 |
| Atovaquone | 4 | 3.22 | 0.98-10.10 | 3.2-5.6 | 53.5 | 6 | 3.82 | 3.13-4.67 | 2.93-4.88 | 17.5 |
| Doxycycline | 6 | 10,266 | 8,362-12,604 | 7,177-11,530 | 17.8 | 6 | 8,925 | 7,942-10,030 | 7,737-10,590 | 12.3 |
For all drugs, there were no significant differences between the two methods (P > 0.1, paired t test).
Geometric mean 50% inhibitory concentration in vitro.
CV, coefficient of variation.
CI, confidence interval.
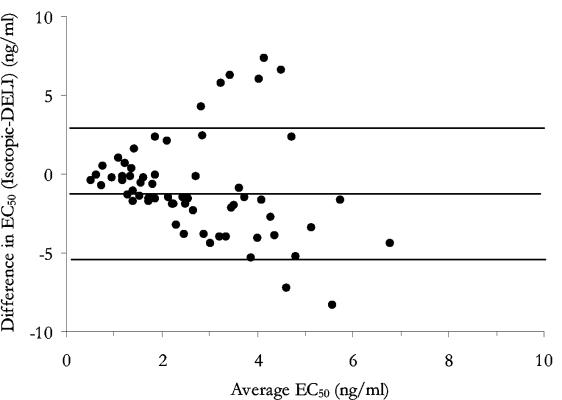
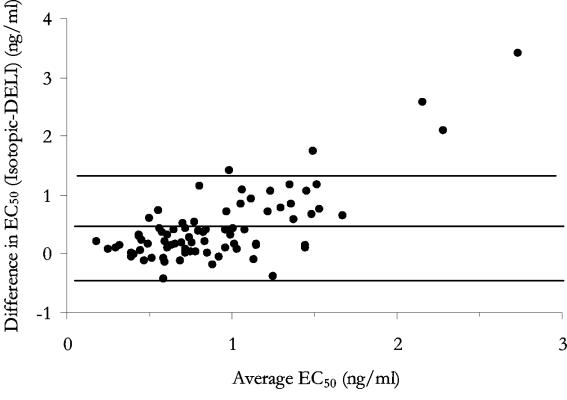
Eighty-six field isolates were assessed by both assay methods for susceptibilities to eight antimalarial drugs (Table 2). Atovaquone and doxycycline provided the least reliable estimations of EC50s. For atovaquone, 14 of 86 isolates (16%) produced a poor dose response with the DELI assay compared with 9 of 86 (11%) for the isotopic assay (χ20.05, 2 = 15.1, P < 0.001). For doxycycline, only 2 of 86 isolates (2%) were unable to be assessed by the DELI assay, compared to 29 of 86 isolates (25%) by the isotopic assay (χ20.05, 2 = 20.5, P < 0.001). These differences suggest that the successful interpretation of dose response with these two drugs is influenced by the assay used, with the DELI assay proving the more reliable in the case of doxycycline. Overall, geometric mean EC50s were significantly higher by the DELI assay for chloroquine, lumefantrine, and atovaquone but lower for artesunate and dihydroartemisinin. No differences in mean EC50s were seen between the two assays for quinine, mefloquine, and doxycycline. Differences between the two methods became more apparent at higher EC50s, particularly for artesunate, where levels were underestimated by the DELI assay (Fig. 1) (n = 79, rs = −0.732, P < 0.001) and atovaquone, showing a tendency to overestimate responses (Fig. 2) (n = 64, rs = 0.793, P < 0.001).
TABLE 2.
Comparative EC50s of eight drugs for field isolates
| Drug | No. of isolates | EC50 (ng/ml)
|
P | |||||
|---|---|---|---|---|---|---|---|---|
| DELI assay
|
Isotopic assay
|
|||||||
| Meana | 95% CIb | Range | Meana | 95% CIb | Range | |||
| Chloroquine | 82 | 86.8 | 80.2-93.9 | 29.4-202.7 | 78.0 | 70.8-85.8 | 29.0-264.7 | 0.017 |
| Quinine | 81 | 229.8 | 202.1-261.3 | 42.7-731.8 | 208.7 | 181.1-240.7 | 31.0-1,336.7 | NSd |
| Mefloquine | 82 | 22.7 | 19.1-27.1 | 1.98-104.4 | 24.7 | 20.4-29.8 | 2.70-160.0 | NS |
| Lumefantrine | 75 | 26.0 | 22.4-30.2 | 3.64-126.8 | 21.6 | 18.4-25.4 | 4.20-106.6 | 0.008 |
| Artesunate | 79 | 0.61 | 0.54-0.69 | 0.08-1.44 | 0.96 | 0.84-0.92 | 0.28-4.43 | 0.000 |
| DHAc | 79 | 0.69 | 0.58-0.82 | 0.10-7.96 | 1.13 | 0.97-1.31 | 0.19-6.92 | 0.000 |
| Atovaquone | 64 | 2.39 | 1.9-3.0 | 0.31-27.7 | 1.75 | 1.4-2.1 | 0.34-11.5 | 0.047 |
| Doxycycline | 59 | 5,887 | 4,617-7,504 | 555-18,530 | 5,383 | 4,460-6,498 | 433-34,996 | NS |
Geometric mean 50% inhibitory concentration in vitro.
CI, confidence interval.
DHA, dihydroartemisinin.
NS, not significant.
FIG. 1.
Differences between responses to artesunate in vitro plotted against their means with 95% limits of agreement.
FIG. 2.
Differences between responses to atovaquone in vitro plotted against their means with 95% limits of agreement.
This study was prompted by a desire to have an assay that could provide a relatively fast and accurate assessment of in vitro drug susceptibility patterns of parasite populations and that could be conducted in a modestly equipped laboratory at the district level. This implied an assay that was sufficiently sensitive to allow the use of virtually any fresh isolate without regard to parasite density. We believe that the DELI assay adequately fits these criteria and will prove a useful tool for monitoring drug susceptibility patterns in areas where malaria is endemic once the coated antibody plate and reagent kit (ELISA-malaria antigen test; Diamed AG, 1785 Cressier s/Morat, Switzerland) become commercially available.
REFERENCES
- 1.Brockman, A., R. N. Price, M. van Vugt, D. G. Heppner, D. Walsh, P. Sookto, T. Wimonwattrawatee, S. Looareesuwan, N. J. White, and F. Nosten. 2000. Plasmodium falciparum antimalarial drug susceptibility on the north-western border of Thailand during five years of extensive use of artesunate-mefloquine. Trans. R. Soc. Trop. Med. Hyg. 94:537-544. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Druilhe, P., A. Moreno, C. Blanc, P. H. Brasseur, and P. Jacquier. 2001. A colorimetric in vitro sensitivity assay for Plasmodium falciparum based on a highly sensitive double-site LDH antigen capture ELISA. Am. J. Trop. Med. Hyg. 64:233-241. [DOI] [PubMed] [Google Scholar]
- 3.McGready, R., and F. Nosten. 1999. The Thai-Burmese border: drug studies of Plasmodium falciparum in pregnancy. Ann. Trop. Med. Parasitol. 93(Suppl. I):S19-S23. [DOI] [PubMed] [Google Scholar]
- 4.Moreno, A., P. Brasseur, N. Cuzin-Ouattara, C. Blanc, and P. Druilhe. 2001. Evaluation under field conditions of the colourimetric DELI-microtest for the assessment of Plasmodium falciparum drug resistance. Trans. R. Soc. Trop. Med. Hyg. 95:100-103. [DOI] [PubMed] [Google Scholar]
- 5.Nosten, F., F. O. ter Kuile, T. Chongsuphajaisiddhi, C. Luxemburger, H. K. Webster, M. Edstein, L. Phaipun, K. L. Thew, and N. J. White. 1991. Mefloquine-resistant falciparum malaria on the Thai-Burmese border. Lancet 337:1140-1143. [DOI] [PubMed] [Google Scholar]
- 6.Price, R. N., F. Nosten, C. Luxemburger, M. van Vugt, L. Phaipun, T. Chongsuphajaisiddhi, and N. J. White. 1997. Artesunate/mefloquine treatment of multi-drug resistant falciparum malaria. Trans. R. Soc. Trop. Med. Hyg. 91:574-577. [DOI] [PubMed] [Google Scholar]
- 7.Webster, H. K., E. F. Boudreau, K. Pavanand, K. Yongvanitchit, and L. W. Pang. 1985. Antimalarial drug susceptibility testing of Plasmodium falciparum in Thailand using a microdilution radioisotope method. Am. J. Trop. Med. Hyg. 34:228-235. [DOI] [PubMed] [Google Scholar]