Abstract
OBJECTIVE
To determine whether sleep-disordered breathing (SDB) is more prevalent among women with preeclampsia than among normotensive controls
STUDY DESIGN
Preeclamptic patients admitted to the hospital for observation and normotensive, gestational age matched controls hospitalized for obstetrical indications other than preeclampsia were recruited for an overnight sleep evaluation. Watch-PAT100, a validated wrist-mounted, ambulatory device designed to diagnose SDB, was used to complete all sleep studies.
RESULTS
Twenty preeclamptic patients and 20 controls were recruited. Preeclamptic subjects had a higher mean BMI (32.6± 9.5 vs. 24.5 ± 3.5, P=0.001). Preeclamptic subjects had higher mean respiratory disturbance (RDI, mean difference 4.9 events/hour of sleep), apnea hypopnea (AHI, mean difference 5.7 events/hour of sleep) and oxygen desaturation (ODI, mean difference 4.5 events/hour of sleep) indices, however these differences did not reach statistical significance. Preeclamptic subjects were more likely to have more severe forms of SDB compared to controls (ODI ≥ 5, 20% vs. 0%, p=.047).
CONCLUSION
Compared to normotensive controls, preeclamptic subjects experience more SDB events and a greater degree of nocturnal hypoxemia. Further research is needed to determine if SBD, independent of BMI, is a significant contributing factor to the risk of developing preeclampsia
Introduction
Sleep disordered breathing (SDB) refers to a group of disorders characterized by abnormal respiratory patterns (e.g., apneas, hypopneas) or abnormal gas exchange (e.g., hypoxia) during sleep.1–2 Obstructive sleep apnea (OSA), the most common type of SDB, is characterized by airway narrowing during sleep that leads to respiratory disruption, hypoxia, and nocturnal arousals. In the non-pregnant population SDB has been associated with an increased risk of developing hypertension and coronary heart disease.3–5 Self-reported snoring and daytime sleepiness are two symptoms of SDB, and while studies have shown that these sleep related complaints are common in pregnancy,6–9 there are very few studies that have adequately evaluated the relationship of SDB in pregnancy to preeclampsia.
While the underlying etiology of preeclampsia remains unclear, placental hypoperfusion and the subsequent generation of reactive oxygen species and inflammatory mediators has been implicated in its pathogenesis.10–11 SDB, which is characterized by episodes of intermittent hypoxia and sympathetic stimulation, consequently may result in abnormal placental perfusion and the subsequent production of circulating factors that alter endothelial cell function. By these mechanisms, there is biologic plausibility for SDB to be a risk factor for the development of preeclampsia.
The objective of this study was to objectively evaluate SDB in a group of preeclamptic women compared to normotensive controls.
Methods
This was a single center, prospective case-control study at an urban, tertiary-care maternity hospital. We recruited preeclamptic patients admitted to the hospital for observation and normotensive, gestational age matched controls hospitalized for obstetrical indications other than preeclampsia. Women were excluded if their primary care provider deemed them too unstable to be expectantly managed on the antepartum floor. The study was approved by the institutional review board and informed consent was obtained.
Preeclampsia was defined and classified by the criteria established by the National High Blood Pressure Education Program Working Group.12 Criteria for diagnosis of preeclampsia were blood pressure of ≥ 140mmHg systolic or ≥ 90mmHg diastolic that occurs after 20 weeks gestation in a woman with previously normal blood pressure and proteinuria of 0.3 grams or higher in a 24-hour urine specimen. Preeclampsia was considered severe if one or more of the following was present: blood pressure of ≥ 160mmHg systolic or ≥ 110mmHg diastolic on two occasions at least 6 hours apart, proteinuria of 5g or higher in a 24-hour collection, urine output of less than 500cc over 24 hours, elevated liver enzymes, thrombocytopenia, fetal growth restriction, or maternal symptoms (e.g. severe headache, visual changes, right upper-quadrant pain). Superimposed preeclampsia was defined as new-onset proteinuria or a sudden increase in baseline proteinuria in woman with hypertension before 20 weeks gestation.
Case and control subjects were asked to complete an overnight sleep evaluation with the Watch-PAT100 (Itamar medical Ltd., Israel, Figure 1) during one night of their hospital stay. The patients were instructed on how to set up the wrist worn device and how to initiate the study when they were ready to go to sleep. Using a peripheral arterial tonometry (PAT) finger plethysmograph and a standard SpO2 probe, the Watch-Pat 100 records the PAT signal, heart rate, oxyhemoglobin saturation, as well as actigraphy from the inbuilt actigraph. Sleep time is estimated using an inbuilt actigraph.13 Analysis of these signals allows for the determination of a respiratory disturbance index (RDI), an apnea hypopnea index (AHI) and an oxygen desaturation index (ODI), all of which are measures of SDB. Respiratory events are identified by digital vasoconstriction, which is mediated by α-adrenergic receptors that are exquisitely sensitive to surges in sympathetic activity that accompany SDB respiratory events (apneas and hypopneas). Vasoconstriction results in an attenuated PAT signal. The Watch-PAT proprietary software algorithm was used to analyze the PAT signal amplitude along with the heart rate and SpO2 to estimate the RDI, AHI and ODI. Specifically, an RDI event is scored if one of the following 3 criteria is met: (1) PAT amplitude reduction occurred with acceleration in the pulse rate or increase in wrist activity; (2) PAT amplitude reduction occurred with ≥ 3% oxyhemoglobin desaturation; or (3) ≥ 4% oxyhemoglobin desaturation occurred.14 The AHI includes only the last two of these events while the ODI incorporates only the third. Studies in non-pregnant populations have shown that the respiratory indices derived from the Watch-PAT are strongly correlated with those obtained from standard polysomnography (PSG), and have demonstrated that the Watch-PAT is an accurate and reliable ambulatory method for the detection of SDB.14–17 Moreover, O’Brien et al recently published data comparing Watch-PAT to full PSG in pregnant subjects. Their results indicate that among pregnant women, Watch-PAT AHI correlated very well with PSG AHI( r=0.76, p<.0001) and that the Watch-PAT has excellent sensitivity (88%) and specificity (86%) for identification of SDB (AHI≥5).18
Figure 1.
Watch-PAT100
We compared Watch-PAT derived mean RDI, AHI and ODI values between cases and controls We also grouped RDI, AHI and ODI into severity categories (0–4.9, 5–9.9, 10–14.9 and ≥15) and examined the distribution between cases and controls. At an alpha of 0.05 with a power of 80%, a total of 40 patients (20 cases and 20 controls) were required to demonstrate a 5 point mean difference (with a standard deviation of 5.5) in any SDB index. Analyses were performed using a two-tailed independent Student t test, χ2, Fisher exact test, and the χ2 test for trend, as appropriate. Statistical analyses were performed using SPSS 14.0 statistical software (SPSS Inc., Chicago, IL).
Results
A total of 49 subjects were consented to participate in the sleep study. Three preeclamptic and one control subject were excluded from analysis because they had invalid sleep studies (i.e., PAT or SpO2 probe did not record). One preeclamptic and one control were excluded because they removed the device soon after initiating the study because of difficulty falling asleep with the device, and thus no results were available. One preeclamptic subject had to remove the device soon after initiating the study because a change in her clinical condition mandated expeditious delivery. Two preeclamptic subjects were ultimately ruled out for preeclampsia and diagnosed with other conditions (chronic hypertension without superimposed preeclampsia, pheochromocytoma) after the sleep study took place. The remaining twenty preeclamptic patients and 20 controls had valid sleep studies. Seven (35%) of the preeclamptic patients had mild disease, 9 (45%) met severe criteria and 4 (20%) had superimposed preeclampsia. Of the 20 control patients, 7 (35%) were hospitalized for threatened preterm labor, 6 (30%) for placenta previa or suspected abruption, and 7 (35%) for preterm premature rupture of membranes. There were no significant differences between cases and controls with respect to maternal age, race, and gestational age at the time of the sleep study (Table 1). Body mass index (BMI), both pre-pregnancy and the time of the sleep study, was higher in preeclamptic subjects compared to controls (Table 1).
Table 1.
Demographics of participants
| Cases (N = 20) | Controls (N = 20) | P | |
|---|---|---|---|
| Age | 26.7± 7.2 | 28.7 ± 6.3 | 0.4 |
| Gestational age | 31.4 ±3.8 | 31.3 ± 2.4 | 0.4 |
| Pre-pregnancy BMI | 32.6± 9.5 | 24.5 ± 3.5 | 0.001 |
| Current BMI | 36.4±9.7 | 28.7±3.9 | 0.002 |
| Race/ethnicity | |||
| Caucasian | 6 (30%) | 6 (30%) | 0.3 |
| Black | 13 (65%) | 10 (50%) | |
| Hispanic | 0(0%) | 3 (15%) | |
| Other | 1 (5%) | 1(5%) |
All data are presented as mean ± standard deviation or N (%)
Preeclamptic subjects had higher mean RDI (mean difference 4.9), AHI (mean difference 5.7) and ODI (mean difference 4.5) values compared to controls. However, the differences did not reach statistical significance (Table 2). As predicted in our a priori power calculations, the mean difference in AHI was > 5; however, given the greater than anticipated standard deviation value for the cases, our p value was > 0.05.
Table 2.
Mean RDI, AHI and ODI results
| Cases (N = 20) | Controls (N = 20) | P | |
|---|---|---|---|
| RDI | 10.3±13.8 | 5.4 ±3.1 | 0.1 |
| AHI | 7.8±14.2 | 2.1 ±2.7 | 0.1 |
| ODI | 4.9 ± 10.7 | 0.3 ± 0.5 | 0.07 |
All data are presented as mean ± standard deviation
Figures 2–4 depict the distribution of RDI, AHI and ODI among cases and controls. Preeclamptic subjects were more likely to have values indicative of moderate to severe disease. The most striking finding was in regard to analysis of the ODI. Twenty percent of women with preeclampsia experienced greater than or equal to five desaturation (≥4%) events per hour of sleep (ODI ≥ 5) while none of the normotensive controls demonstrated this degree of nocturnal hypoxemia (p=0.047).
Figure 2.
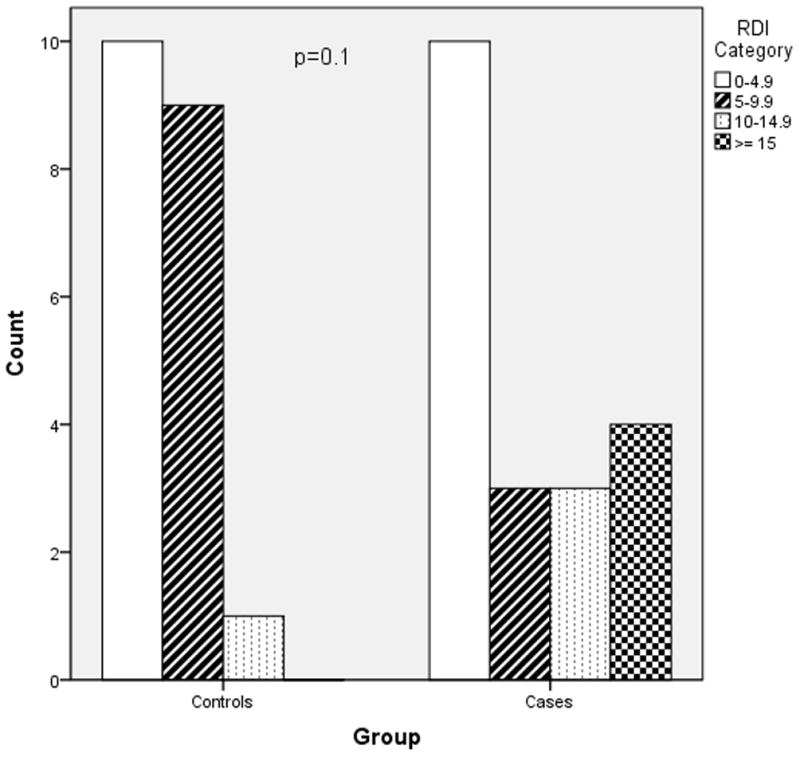
RDI severity in cases versus controls
Figure 4.
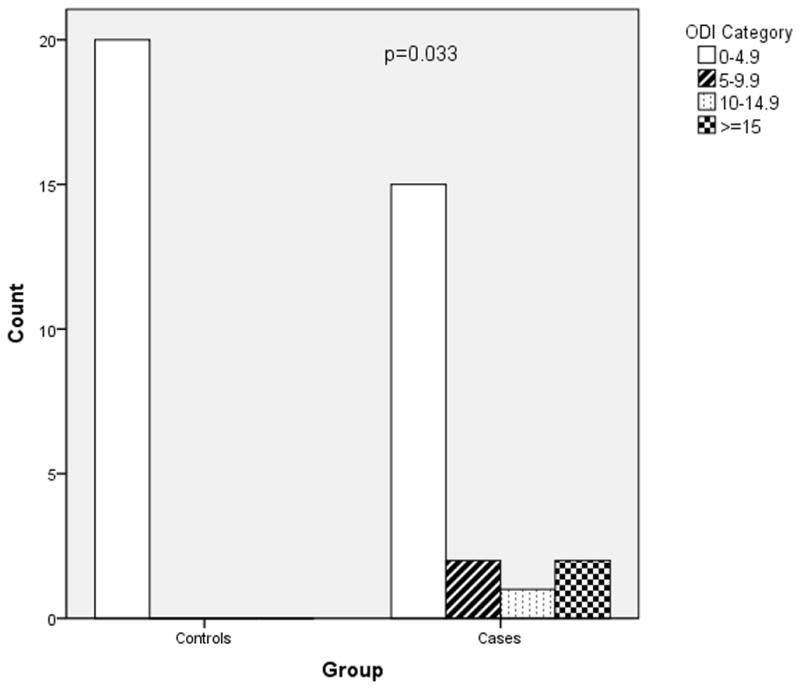
ODI severity in cases versus controls
Discussion
The results of this study indicate that compared to normotensive controls, preeclamptic subjects experience more SDB events and a greater degree of nocturnal hypoxemia. Our study is unique in that our preeclamptic subjects were all preterm, with a gestational-age range of 25–36 weeks and a mean gestational age of 31.4 ± 2 weeks. This is an especially important population to study because of the high maternal and neonatal morbidity associated with preterm preeclampsia.19 Despite decades of research, as of yet there exists no screening tests to accurately predict preeclampsia nor treatment strategies to effectively prevent the disease. If further observational studies confirm our findings, the rationale would exist for performing clinical studies to evaluate, for women with SDB, interventions for the prevention and or treatment of preeclampsia.
Preeclampsia complicates 2–8% of pregnancies and is associated with an increased risk of intrauterine growth restriction, placental abruption, preterm birth and stillbirth.20 Maternal complications of preeclampsia include seizures, stroke, renal failure, liver dysfunction, cardiac dysfunction and pulmonary edema. Studies also suggest that women who develop preeclampsia are at increased risk of cardiovascular sequelae (e.g., coronary artery disease, stroke) later in life.21–22 The possibility that SDB is associated with pregnancy complications, and in particular preeclampsia, is biologically plausible. In non-pregnant populations SDB has been associated with incident hypertension.5 Moreover, SDB has been linked to enhanced inflammatory and oxidative stress responses, endothelial damage and metabolic derangements. 23–24 These same biological pathways have been associated with adverse pregnancy outcomes. Several studies have shown that markers of inflammation, oxidative stress, and endothelial dysfunction are increased in women who develop preeclampsia.25–27 Recent data also suggest that insulin resistance and dysregulation of adipose secreted hormones (e.g., leptin), physiologic changes linked to SDB, also may contribute to the pathophysiology of preeclampsia.28–29
Furthermore, data are now emerging suggesting a relationship between SDB during pregnancy and preeclampsia. In a large cross sectional study, Bourjeily et al found that snoring, the most common SDB symptom, was associated with gestational hypertension/preeclampsia even after adjusting for multiple factors including BMI at delivery (aOR 2.3, 95% CI 1.4–4).30 While objective assessments of SDB’s effect on preeclampsia risk are limited, most confirm our findings. Three case control studies have reported higher rates of objectively-assessed SDB among women with preeclampsia compared to normotensive controls, and two of these studies reported a positive relationship independent of BMI.31–33 In a retrospective cohort study Louis et al reported that preeclampsia rates were highest among women with PSG-diagnosed SDB compared to obese controls without SDB (19% vs. 10%) but this difference was not statistically significant.34 In the largest retrospective cohort of women with PSG-confirmed SDB (n=791), Chen at al reported that SDB was associated with an increased risk of preeclampsia (aOR 1.6, 95% CI 2.16, 11.26). However, this Taiwanese database study lacked BMI data, and the reported obesity rate was only 1.6%.35 Louis et al recently published data from an observational study (N=175) demonstrating a more than two-fold increase in the risk of preeclampsia among obese women with sleep study confirmed SDB, compared to obese women without SDB.36 This study had no gestational age criteria for SDB assessment, with some assessments occurring in late pregnancy (mean gestational age at sleep study 21±8 weeks).
Our study has several limitations. First, while we were able to match our cases and controls for maternal age and gestational age, our preeclamptic patients had a significantly higher BMI both pre-pregnancy (self-reported) and measured at the time of the sleep study. Obesity increases the risk of developing SDB as well as the risk of developing preeclampsia.37–39 Therefore, BMI is an important confounder we were not able to control for given our relatively small sample size. Larger prospective studies are needed to assess whether SDB, independent of BMI, increases the risk of preeclampsia, or if there is a significant interaction between SDB and obesity that modulates an individual’s risk of preeclampsia. Secondly, we were only able to evaluate preeclamptic patients who were admitted to the hospital and deemed stable for inpatient observation. Term patients with mild or severe disease were usually delivered immediately upon presentation and therefore we were not able to recruit them for an overnight sleep study. Similarly, preterm patients presenting with severe disease were often not eligible because they were deemed too sick or unstable to be expectantly managed. Consequently, our preeclamptic population consisted of preterm patients with mild disease, or severe/superimposed disease that was considered stable enough to manage expectantly as an inpatient. Therefore, our study results cannot be generalized to all preeclamptic patients, and perhaps only certain subgroups of preeclampsia are associated with SDB. Finally, this was a case-control study, and while an association between SDB and preeclampsia was demonstrated, we cannot infer causality. In fact, the physiologic changes of preeclampsia, particularly the increased interstitial fluid, can result in airway edema and increase the likelihood of nocturnal airway obstruction and SDB.40–41 In reality, the relationship between SDB and preeclampsia is likely bi-directional and only large prospective studies with serial, objective assessments of SDB starting in early pregnancy will allow for a clear understanding of this complex relationship.
In summary, data are now emerging suggesting a positive relationship between SDB and preeclampsia; however, the current evidence is limited. Many studies, including ours, were not able to control for obesity, or clearly define a temporal relationship between SDB and the subsequent development of preeclampsia. There are no large studies that have examined SDB in pregnancy using prospective, serial objective measures. Further research is needed to determine if SBD, independent of BMI, is a significant contributing factor to preeclampsia, and whether higher levels of nocturnal desaturation in preeclamptic women may increase the risk of adverse maternal and neonatal outcomes.
Figure 3.
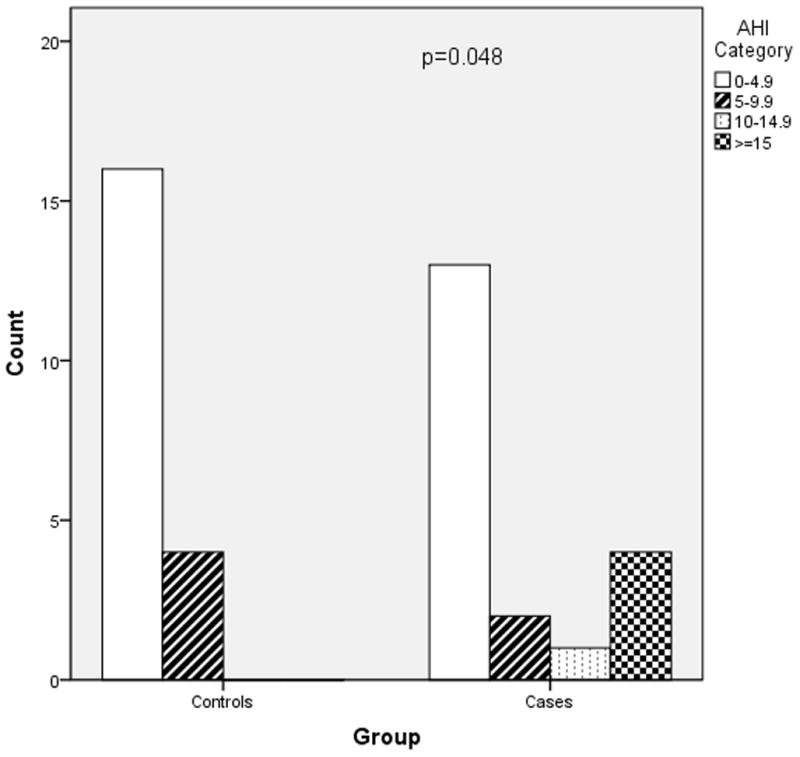
AHI severity in cases versus controls
Acknowledgments
Financial Support: NIH/NICHD 1K12HD050121, Preeclampsia Foundation Vision Grant, Northwestern Memorial Foundation Dixon Translational Research Initiative
Footnotes
DISCLOSURE: The authors report no conflict of interest
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hauri P, editor. The International Classification of Sleep Disorders, Diagnostic and Coding Manual. Westchester, IL: American Academy of Sleep Medicine; 2005. [Google Scholar]
- 2.Iber C, Ancoli-Israel S, Chesson A, Quan S. The AASM manual for the scoring of sleep and associated events: rules, terminology and technical specifications. Westchester, IL: American Academy of Sleep Medicine; 2007. [Google Scholar]
- 3.Hung J, Whitford EG, Parsons RW, Hillman DR. Association of sleep apnoea with myocardial infarction in men. Lancet. 1990 Aug 4;336(8710):261–264. doi: 10.1016/0140-6736(90)91799-g. [DOI] [PubMed] [Google Scholar]
- 4.Mooe T, Rabben T, Wiklund U, Franklin KA, Eriksson P. Sleep-disordered breathing in women: occurrence and association with coronary artery disease. Am J Med. 1996 Sep;101(3):251–256. doi: 10.1016/S0002-9343(96)00122-2. [DOI] [PubMed] [Google Scholar]
- 5.Peppard PE, Young T, Palta M, Skatrud J. Prospective study of the association between sleep-disordered breathing and hypertension. N Engl J Med. 2000 May 11;342(19):1378–1384. doi: 10.1056/NEJM200005113421901. [DOI] [PubMed] [Google Scholar]
- 6.Hedman C, Pohjasvaara T, Tolonen U, Suhonen-Malm AS, Myllyla VV. Effects of pregnancy on mothers’ sleep. Sleep medicine. 2002 Jan;3(1):37–42. doi: 10.1016/s1389-9457(01)00130-7. [DOI] [PubMed] [Google Scholar]
- 7.Leung PL, Hui DS, Leung TN, Yuen PM, Lau TK. Sleep disturbances in Chinese pregnant women. Bjog. 2005 Nov;112(11):1568–1571. doi: 10.1111/j.1471-0528.2005.00737.x. [DOI] [PubMed] [Google Scholar]
- 8.Loube DI, Poceta JS, Morales MC, Peacock MD, Mitler MM. Self-reported snoring in pregnancy. Association with fetal outcome. Chest. 1996 Apr;109(4):885–889. doi: 10.1378/chest.109.4.885. [DOI] [PubMed] [Google Scholar]
- 9.Pien GW, Fife D, Pack AI, Nkwuo JE, Schwab RJ. Changes in symptoms of sleep-disordered breathing during pregnancy. Sleep. 2005 Oct 1;28(10):1299–1305. doi: 10.1093/sleep/28.10.1299. [DOI] [PubMed] [Google Scholar]
- 10.Ness RB, Roberts JM. Heterogeneous causes constituting the single syndrome of preeclampsia: a hypothesis and its implications. Am J Obstet Gynecol. 1996 Nov;175(5):1365–1370. doi: 10.1016/s0002-9378(96)70056-x. [DOI] [PubMed] [Google Scholar]
- 11.Redmond C, Sargent I. Latest Advances in Understanding Preeclampsia. Science. 2005;208:1582–1594. doi: 10.1126/science.1111726. [DOI] [PubMed] [Google Scholar]
- 12.Report of the National High Blood Pressure Education Program Working Group on High Blood Pressure in Pregnancy. Am J Obstet Gynecol. 2000 Jul;183(1):S1–S22. [PubMed] [Google Scholar]
- 13.Hedner J, Pillar G, Pittman SD, Zou D, Grote L, White DP. A novel adaptive wrist actigraphy algorithm for sleep-wake assessment in sleep apnea patients. Sleep. 2004 Dec 15;27(8):1560–1566. doi: 10.1093/sleep/27.8.1560. [DOI] [PubMed] [Google Scholar]
- 14.Pittman SD, Ayas NT, MacDonald MM, Malhotra A, Fogel RB, White DP. Using a wrist-worn device based on peripheral arterial tonometry to diagnose obstructive sleep apnea: in-laboratory and ambulatory validation. Sleep. 2004 Aug 1;27(5):923–933. doi: 10.1093/sleep/27.5.923. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Zou D, Grote L, Peker Y, Lindblad U, Hedner J. Validation a portable monitoring device for sleep apnea diagnosis in a population based cohort using synchronized home polysomnography. Sleep. 2006 Mar 1;29(3):367–374. doi: 10.1093/sleep/29.3.367. [DOI] [PubMed] [Google Scholar]
- 16.Bar A, Pillar G, Dvir I, Sheffy J, Schnall RP, Lavie P. Evaluation of a portable device based on peripheral arterial tone for unattended home sleep studies. Chest. 2003 Mar;123(3):695–703. doi: 10.1378/chest.123.3.695. [DOI] [PubMed] [Google Scholar]
- 17.Ayas NT, Pittman S, MacDonald M, White DP. Assessment of a wrist-worn device in the detection of obstructive sleep apnea. Sleep Med. 2003 Sep;4(5):435–442. doi: 10.1016/s1389-9457(03)00111-4. [DOI] [PubMed] [Google Scholar]
- 18.O’Brien LM, Bullough AS, Shelgikar AV, Chames MC, Armitage R, Chervin RD. Validation of Watch-PAT-200 against polysomnography during pregnancy. J Clin Sleep Med. 2012 Jun 15;8(3):287–294. doi: 10.5664/jcsm.1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Norwitz ER, Funai EF. Expectant management of severe preeclampsia remote from term: hope for the best, but expect the worst. Am J Obstet Gynecol. 2008 Sep;199(3):209–212. doi: 10.1016/j.ajog.2008.06.084. [DOI] [PubMed] [Google Scholar]
- 20.Geographic variation in the incidence of hypertension in pregnancy. World Health Organization International Collaborative Study of Hypertensive Disorders of Pregnancy. Am J Obstet Gynecol. 1988 Jan;158(1):80–83. [PubMed] [Google Scholar]
- 21.McDonald SD, Malinowski A, Zhou Q, Yusuf S, Devereaux PJ. Cardiovascular sequelae of preeclampsia/eclampsia: a systematic review and meta-analyses. Am Heart J. 2008 Nov;156(5):918–930. doi: 10.1016/j.ahj.2008.06.042. [DOI] [PubMed] [Google Scholar]
- 22.Kajantie E, Eriksson JG, Osmond C, Thornburg K, Barker DJ. Pre-eclampsia is associated with increased risk of stroke in the adult offspring: the Helsinki birth cohort study. Stroke. 2009 Apr;40(4):1176–1180. doi: 10.1161/STROKEAHA.108.538025. [DOI] [PubMed] [Google Scholar]
- 23.Jelic S, Le Jemtel TH. Inflammation, oxidative stress, and the vascular endothelium in obstructive sleep apnea. Trends Cardiovasc Med. 2008 Oct;18(7):253–260. doi: 10.1016/j.tcm.2008.11.008. [DOI] [PubMed] [Google Scholar]
- 24.Dempsey JA, Veasey SC, Morgan BJ, O’Donnell CP. Pathophysiology of sleep apnea. Physiol Rev. 2010 Jan;90(1):47–112. doi: 10.1152/physrev.00043.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Bernardi F, Guolo F, Bortolin T, Petronilho F, Dal-Pizzol F. Oxidative stress and inflammatory markers in normal pregnancy and preeclampsia. J Obstet Gynaecol Res. 2008 Dec;34(6):948–951. doi: 10.1111/j.1447-0756.2008.00803.x. [DOI] [PubMed] [Google Scholar]
- 26.Hubel CA. Oxidative stress in the pathogenesis of preeclampsia. Proc Soc Exp Biol Med. 1999 Dec;222(3):222–235. doi: 10.1177/153537029922200305. [DOI] [PubMed] [Google Scholar]
- 27.Challis JR, Lockwood CJ, Myatt L, Norman JE, Strauss JF, 3rd, Petraglia F. Inflammation and pregnancy. Reprod Sci. 2009 Feb;16(2):206–215. doi: 10.1177/1933719108329095. [DOI] [PubMed] [Google Scholar]
- 28.Miehle K, Stepan H, Fasshauer M. Leptin, adiponectin and other adipokines in gestational diabetes mellitus and pre-eclampsia. Clin Endocrinol (Oxf) 2012 Jan;76(1):2–11. doi: 10.1111/j.1365-2265.2011.04234.x. [DOI] [PubMed] [Google Scholar]
- 29.Landon MB, Spong CY, Thom E, et al. A multicenter, randomized trial of treatment for mild gestational diabetes. N Engl J Med. 2009 Oct 1;361(14):1339–1348. doi: 10.1056/NEJMoa0902430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Bourjeily G, Raker CA, Chalhoub M, Miller MA. Pregnancy and fetal outcomes of symptoms of sleep-disordered breathing. Eur Respir J. Oct;36(4):849–855. doi: 10.1183/09031936.00021810. [DOI] [PubMed] [Google Scholar]
- 31.Champagne K, Schwartzman K, Opatrny L, et al. Obstructive sleep apnoea and its association with gestational hypertension. Eur Respir J. 2009 Mar;33(3):559–565. doi: 10.1183/09031936.00122607. [DOI] [PubMed] [Google Scholar]
- 32.Reid J, Skomro R, Cotton D, et al. Pregnant women with gestational hypertension may have a high frequency of sleep disordered breathing. Sleep. 2011 Aug;34(8):1033–1038. doi: 10.5665/SLEEP.1156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Yinon D, Lowenstein L, Suraya S, et al. Pre-eclampsia is associated with sleep-disordered breathing and endothelial dysfunction. Eur Respir J. 2006 Feb;27(2):328–333. doi: 10.1183/09031936.06.00010905. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Louis JM, Auckley D, Sokol RJ, Mercer BM. Maternal and neonatal morbidities associated with obstructive sleep apnea complicating pregnancy. Am J Obstet Gynecol. 2010 Mar;202(3):261, e261–265. doi: 10.1016/j.ajog.2009.10.867. [DOI] [PubMed] [Google Scholar]
- 35.Chen YH, Kang JH, Lin CC, Wang IT, Keller JJ, Lin HC. Obstructive sleep apnea and the risk of adverse pregnancy outcomes. Am J Obstet Gynecol. 2011 Sep 16; doi: 10.1016/j.ajog.2011.09.006. [DOI] [PubMed] [Google Scholar]
- 36.Louis J, Auckley D, Miladinovic B, et al. Perinatal Outcomes Associated With Obstructive Sleep Apnea in Obese Pregnant Women. Obstet Gynecol. 2012 Nov;120(5):1085–1092. doi: 10.1097/AOG.0b013e31826eb9d8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Peppard PE, Young T, Palta M, Dempsey J, Skatrud J. Longitudinal study of moderate weight change and sleep-disordered breathing. JAMA. 2000 Dec 20;284(23):3015–3021. doi: 10.1001/jama.284.23.3015. [DOI] [PubMed] [Google Scholar]
- 38.Bodnar LM, Kaufman JS. Body mass index and preeclampsia. Epidemiology. 2004 Mar;15(2):252–253. doi: 10.1097/01.ede.0000112145.70380.a2. [DOI] [PubMed] [Google Scholar]
- 39.ACOG Committee Opinion number 315, September 2005. Obesity in pregnancy. Obstet Gynecol. 2005 Sep;106(3):671–675. doi: 10.1097/00006250-200509000-00054. [DOI] [PubMed] [Google Scholar]
- 40.Santiago JR, Nolledo MS, Kinzler W, Santiago TV. Sleep and sleep disorders in pregnancy. Ann Intern Med. 2001 Mar 6;134(5):396–408. doi: 10.7326/0003-4819-134-5-200103060-00012. [DOI] [PubMed] [Google Scholar]
- 41.Sahota PK, Jain SS, Dhand R. Sleep disorders in pregnancy. Curr Opin Pulm Med. 2003 Nov;9(6):477–483. doi: 10.1097/00063198-200311000-00005. [DOI] [PubMed] [Google Scholar]


