Abstract
Pain from cancer can be severe, difficult to treat, and greatly diminishes patients’ quality of life. It is therefore important to gain new information on the mechanisms of cancer pain and develop new treatment strategies. We have used a murine model of bone cancer pain to investigate underlying peripheral neural mechanisms and novel treatment approaches. In this model, implantation of fibrosarcoma cells into and around the calcaneous bone produces mechanical and thermal hyperalgesia in mice. C-fiber nociceptors in tumor-bearing mice develop spontaneous ongoing activity and sensitization to thermal stimuli. However, it is unclear whether sensitization of nociceptors to mechanical stimuli underlies the mechanical hyperalgesia seen in tumor-bearing mice. We therefore examined responses of C-fiber nociceptors to suprathreshold mechanical stimuli in tumor-bearing mice and found they did not differ from those of C-nociceptors in control mice. Thus, sensitization of C-fiber nociceptors to mechanical stimulation does not appear to underlie tumor-evoked mechanical hyperalgesia in this murine model of bone cancer pain.
We also examined the effect of the non-selective cannabinoid receptor agonist, WIN 55, 212-2, on spontaneous activity and responses evoked by mechanical stimuli of C-fiber nociceptors innervating the tumor-bearing paw. Selective CB1 and CB2 antagonists were administered to determine the contribution of each receptor subtype to the effects of WIN 55,212-2. Intraplantar administration of WIN 55,212-2 attenuated spontaneous discharge and responses evoked by mechanical stimulation of C-fiber nociceptors. These effects were inhibited by prior intraplantar administration of selective CB1 (AM281) or CB2 (AM630) receptor antagonists but not by vehicle. These results indicate that activation of either CB1 or CB2 receptors reduced the spontaneous activity of C-fiber nociceptors associated with tumor growth as well as their evoked responses. Our results provide further evidence that activation of peripheral cannabinoid receptors may be a useful target for the treatment of cancer pain.
Keywords: Peripheral nerve, tibial nerve, mouse, electrophysiology, AM281, AM630
1. Introduction
Of the estimated 1.6 million new cancer patients diagnosed each year, between 30 and 60% will develop chronic pain related to tumor growth and/or treatment (van den Beuken-van Everdingen et al., 2007; Howlader et al., 2011). More than a third of these individuals will report pain of moderate or severe intensity, which can significantly impact quality of life (van den Beuken-van Everdingen et al., 2007). Cancer pain, particularly pain associated with bone metastases, may not respond well to standard therapies and can be debilitating with many patients experiencing breakthrough pain (Zeppetella et al., 2000; Mercadante & Portenoy, 2001; Meuser et al., 2001; Mercadante et al., 2010). In patients diagnosed with primary or metastatic bone cancer, more than 60% with chronic pain classified it as severe (Rustøen et al., 2005). Evaluating and managing cancer pain is a major clinical challenge. Progress towards understanding the pathophysiological changes associated with bone cancer pain is essential for the development of effective novel therapies.
We have used a reliable model of bone cancer pain in the mouse (Cain et al., 2001; Wacnik et al., 2001) to investigate neural mechanisms that contribute to cancer pain. Implantation of osteolytic fibrosarcoma cells into and around the calcaneous bone produced mechanical, heat, and cold hyperalgesia as the cells proliferated and the tumor enlarged. In mice with visible tumor growth and hyperalgesia, we found that C-fiber, but not A-fiber, nociceptors innervating the tumor-bearing paw developed spontaneous activity and enhanced responses to heat and cold stimuli. It is not known, however, whether nociceptors become sensitized to mechanical stimuli during tumor growth. In our earlier studies, it was found that mechanical response thresholds of C-fiber nociceptors were unchanged during tumor development, but responses evoked by suprathreshold mechanical stimuli were not evaluated (Cain et al., 2001).
Cannabinoids may be a useful treatment or adjuvant for cancer pain. Cannabinoids produce antinociception through activation of cannabinoid receptors, referred to as CB1 and CB2 (Griffin et al., 2000; Fride and Mechoulam, 1993). CB1 receptors are associated with the peripheral and central nervous systems whereas CB2 receptors are primarily located on immune cells (Facci et al., 1995; Galiegue et al., 1995; Munro et al., 1993). Cannabinoid receptor agonists have been shown to reduce hyperalgesia in animal models of inflammatory (Calignano et al., 1998; Moss and Johnson, 1980; Elmes et al., 2005; Clayton et al., 2002; Richardson et al., 1998b; Martin et al., 1999), neuropathic (Ibrahim et al., 2003; Bridges et al., 2001; Fox et al., 2001; Herzberg et al., 1997), capsaicin (Johanek et al., 2001; Rukwied et al., 2003; Ko & Woods, 1999; Li et al., 1999; Richardson et al., 1998a, b) and cancer pain (Kehl et al., 2003; Hamamoto et al., 2007; Potenzieri et al., 2008a; Hald et al., 2008; Khasabova et al., 2011). Importantly, administration of cannabinoids into the tumor-bearing paw decreased mechanical hyperalgesia through activation of cannabinoid receptors located in the periphery (Khasabova et al., 2011; Potenzieri et al., 2008b), suggesting that cannabinoids decreased mechanically-evoked responses of nociceptors. However, it is unknown whether cannabinoids decreased mechanically-evoked responses that were increased due to sensitization. We therefore examined the effects of cannabinoids on responses of C-fiber nociceptors. The goals of the present study were to determine: 1) whether C-fiber nociceptors develop enhanced responses to suprathreshold mechanical stimuli during tumor growth, and 2) whether spontaneous activity and mechanically-evoked responses of C-fiber nociceptors are decreased by local administration of cannabinoids. We utilized the non-selective cannabinoid receptor agonist, WIN 55, 212-2, at a dose previously shown to be effective in reducing tumor-evoked mechanical hyperalgesia (Potenzieri et al., 2008b). In addition, specific CB1 and CB2 antagonists were administered to determine the contribution of each receptor subtype to the overall impact of WIN 55,212-2.
2. Experimental Procedures
2.1 Subjects
Eighty-two adult (8–10 weeks old) male C3H/He mice (National Institutes of Health), housed in cages of 3–4 on a 12 h light/dark cycle, were used. Sixty-two mice were implanted with fibrosarcoma cells and 20 naïve mice served as normal controls (in which C-fiber nociceptors were studied but did not receive any drug injection). All subjects had free access to food and water. Behavioral testing occurred between 8:00 am and 12:00 pm. All protocols and procedures were approved by the University of Minnesota Institutional Animal Care and Use Committee and were conducted according to the guidelines established by the International Association for the Study of Pain (Zimmerman, 1983).
2.2 Implantation of cancer cells
NCTC clone 2472 fibrosarcoma cells, obtained from the American Type Culture Collection (Manassas, VA), were maintained in 75 cm2 flasks in NCTC medium at pH 7.35 with 10% horse serum and grown to confluence. They were fed and passed one time weekly by a 1:6–9 split ratio. When prepared for implantation, the medium was poured off and rinsed with PBS. Trypsin was added for 5–10 min to detach cells from the flask. Enzymatic action was stopped with a sufficient volume of medium. Cells were then counted via hemocytometer pelletted, re-suspended and rinsed in PBS, pelletted a second time, then re-suspended in PBS for implantation at a concentration of 2 × 105 2472 cells/10 μl.
Mice were briefly anesthetized using isoflurane (2–3%) and cancer cells were injected unilaterally into and around the calcaneous bone of the left hind paw ing a 29 g needle. Subjects were monitored for motor impairment following recovery from anesthesia and returned to their home cage at the completion of injections. None of the mice in the current study demonstrated motor impairment following implantation.
2.3 Assessment of mechanical hyperalgesia
Mice were habituated to the testing room for at least one week prior to obtaining behavioral measures. Mice were placed into individual glass containers (11 cm L × 6.5 cm W × 5.5 cm H) on a raised wire mesh surface and allowed to habituate for 30–40 min. Mechanical hyperalgesia was evaluated using a calibrated von Frey monofilament (0.4 g, 3.9 mN) applied to the plantar surface of the hind paw through the mesh screen. Pressure was applied until the filament bent slightly, and it was held against the skin for 1–2 s. A withdrawal response was indicated by rapid removal of the hind paw from the monofilament, which was occasionally followed by rapid flinching and/or licking of the plantar surface. The monofilament was applied to each hind paw ten times at intervals of at least 5 s, and the total number of withdrawal responses was recorded for each hind paw.
Baseline measurements were taken over a three day period prior to tumor cell implantation. Tumor cells were implanted approximately 24 h after the last testing session. Testing did not occur for the first three days after injection to allow mice to recover from the procedure. Testing resumed on post-implantation day (PID) 4 followed by PID 6 and PID 8 during which time mechanical hyperalgesia typically developed. Starting on PID 11, daily testing occurred just prior to electrophysiological experiments. Mice were used for electrophysiological studies when the frequency of withdrawal responses for the tumor-bearing paw reached a minimum of 70%, typically between PID 11 and 16. Mice that did not exhibit withdrawal response frequencies of at least 70% following tumor implantation were not used (less than 10% of mice).
2.4 Surgical procedures and electrophysiological recording
Mice were anesthetized using acepromazine maleate (20 mg/kg, i.p.) and sodium pentobarbital (48 mg/kg, i.p.). The level of anesthesia was evaluated by applying pressure to the right hind paw or tail and/or testing for corneal reflexes. Supplemental doses of sodium pentobarbital were administered as needed. At the completion of the experiment, mice were euthanized by an overdose of sodium pentobarbital.
Once adequately anesthetized, the hair around the left hind leg was removed and an incision was made in the skin over the gastrocnemius muscle, which was dissected and removed to access the tibial nerve. The skin was then sutured to a stainless steel ring (1.3 cm inner diameter) to form a pool that was filled with mineral oil. Dental impression material (COE-FLEX, GC America) was applied to the skin and around the ring to prevent oil from leaking out of the pool during the experiment and to stabilize the hind paw.
After the impression material had cured (~10 min), the nerve was gently dissected from surrounding tissue and placed on a small mirror platform in order to perform fine dissection of nerve fibers. The epineurium was removed allowing small bundles of fibers to be cut proximally, teased into fine filaments using fine forceps, and placed on a silver wire recording electrode. Action potentials from individual fibers were amplified, audiomonitored, and visualized on an oscilloscope and PC using Spike 2.0 software (CED, Cambridge, UK). Nociceptors were initially identified by mild pinching and/or applying pressure to the glabrous skin of the hind paw. Von Frey monofilaments were used to identify the precise location of the receptive field, which was marked on the skin with a felt-tip pen. C-fiber nociceptors with ongoing spontaneous discharge were preferentially studied in tumor-bearing mice.
Conduction velocity (CV) was determined for each fiber. The fiber was electrically stimulated by inserting two fine pin electrodes under the skin outside the receptive field. Beginning with a voltage below threshold, electrical pulses (200 μs) were delivered every 2 s until the response threshold was reached, and the conduction velocity was calculated using a stimulus of 1.5 times the threshold value. The CV for each fiber was determined by dividing the conduction distance (distance from RF to recording electrode in mm) by the latency to the action potential. Fibers with CV of 1.3 m/s or less were classified as C-fibers.
2.5 Electrophysiological responses of nociceptors
Once a C-fiber nociceptor was isolated, the rate of SPONTANEOUS ACTIVITY was determined for a period of two minutes before any testing and just prior to drug (or vehicle) administration. Mechanical response thresholds were obtained using a set of calibrated von Frey monofilaments. The receptive field was stimulated multiple times with a single filament, and if no response was elicited, the next higher force (or lower force if there was a response) was applied. Response threshold was defined as the lowest force that elicited a response on 50% or more of the trials.
Responses evoked by suprathreshold mechanical stimuli were determined using a single suprathreshold von Frey monofilament that delivered a force of 147 mN. This monofilament was applied three times, each for a duration of 5 s with an inter-stimulus interval of 60 sec. The response to the von Frey stimulus was defined as the mean number of evoked action potentials from the three trials.
A Peltier device (contact area 1 cm2) was used to deliver heat stimuli to the skin. Beginning at a base temperature of 32°C, stimuli of 34°C to 50°C were delivered in ascending order of 2°C. Each stimulu’s was applied for 5 s, and a 60 s interstimulus interaval was utilized. The temperature at which the fiber first responded was considered the heat threshold. Nociceptors that did not respond to any heat stimuli were classified as C-mechanonociceptors (CM) while those that responded to heat were classified as C-mechanoheat nociceptors (CMH). We were unable to apply heat to some fibers due to the location of the receptive field.
2.6 Drug preparation and administration
The non-selective cannabinoid receptor agonist, (R)-(+)-[2,3-Dihydro-5-methyl-3-(4-morpholinylmethyl)pyrrolo[1,2,3-de]-1,4-benzoxazin-6-yl]-1-naphthalenylmeth anone mesylate (WIN 55,212-2), the CB1 receptor antagonist 1-(2,4-Dichlorophenyl)-5-(4-iodophenyl)-4-methyl-N-4-morpholinyl-1H-pyrazole-3-carboxamide (AM281), and the CB2 receptor antagonist 6-Iodo-2-methyl-1-[2-(4-morpholinyl)ethyl]-1H-indol-3-yl](4-methoxyphenyl)methanone (AM 630) were purchased from Tocris Bioscience (Ellisville, MO). A 100 mM stock solution of WIN 55,212-2 was prepared in a vehicle of 90% saline, 5% DMSO, and 5% Tween 80, and 25 mM stock solutions of AM281 and AM630 were prepared using the same vehicle solution. Drugs were diluted to the appropriate concentration in sterile physiological saline prior to injection. WIN 55,212-2 was administered at a dose of 10 μg (in 10 μl) and the antagonists were administered at a dose of 1 μg in the same volume. All injections were made using a 0.3 ml syringe with a 28 g needle. Care was taken to insert the needle outside the receptive field to avoid damaging the receptive field.
2.7 Experimental design
Following baseline measures of spontaneous activity, mechanical response threshold, and responses to the suprathreshold von Frey monofilament, WIN 55,212-2 or vehicle alone was injected into the receptive field. To determine the contributions of CB1 and CB2 receptors to the effects of WIN 55,212-2, the CB1 and CB2 receptor antagonists, AM281 and AM630, respectively, were administered 5 min prior to injection of WIN 55,212-2. In addition, some nociceptors (n=6) were given an injection of vehicle alone 5 min prior to the injection of WIN 55,212-2 to ensure that the initial injection did not alter the effect of the agonist. Spontaneous activity, mechanical response threshold, and responses to suprathreshold mechanical stimulation were determined before and at 15, 30, 45 and 60 min after injection of WIN 55,212-2 or vehicle.
2.8 Data analyses
In behavioral studies, the frequency of withdrawal evoked by the 3.9 mN von Frey monofilament was expressed as the percentage of positive responses out of 10 trials for both the left (tumor-bearing) and right (normal) hind paws. A two-way analysis of variance (ANOVA) with repeated measures was used to compare the difference between hind paws before and after fibrosarcoma cell implantation. Fisher’s LSD post-hoc tests were used to determine differences between the groups at specific time points.
Several characteristics of C-fiber nociceptors isolated from both tumor-bearing and naïve control mice were compared. The proportion of different fiber types (i.e., CM vs. CMH) was assessed using a Chi-square analysis. The Mann-Whitney U test was used to assess the difference in the median levels of spontaneous discharge of nociceptors between groups due to the variable rate of spontaneous activity among C-fiber nociceptors from tumor-bearing mice and the lack of spontaneous activity in most C-fiber nociceptors from control mice. Response thresholds (mN) of C-fibers were compared between tumor-bearing and control mice using Chi-square analysis of the frequency distribution of forces, since the forces produced by the von Frey monofilaments are not linear increments in force and there was much greater variability among response thresholds for tumor-bearing mice. Comparisons in the mean number of impulses evoked by the suprathreshold von Frey monofilament between tumor-bearing and control mice were made using an independent t-test. Evoked responses of each fiber were determined by subtracting the number of spontaneous impulses during the 5 sec immediately preceding each stimulus from the number of impulses evoked during the stimulus.
In order to confirm that the use of multiple injections did not alter the effect of the agonist, spontaneous activity of C-fiber nociceptors and responses evoked by mechanical stimuli were compared following injection with WIN 55,212-2 alone or at 5 min following a vehicle injection. Two-way ANOVAs with repeated measures were used to evaluate the rate of ongoing spontaneous activity and responses evoked by the suprathrehold von Frey monofilament. The Kruskall-Wallis one-way ANOVA for ranks was used to determine differences between groups in mechanical response threshold. For C-fiber nociceptors that received two injections, the mean rate of spontaneous activity immediately before the vehicle injection was compared to the mean rate of spontaneous activity immediately prior to the injection of WIN 55,212-2 using a dependent t-test.
To determine the effects of vehicle, WIN 55,212-2, or WIN 55,212-2 in combination with AM281 or AM630 on spontaneous activity of C-fiber nociceptors and the number of impulses evoked by the suprathreshold von Frey monofilament in tumor-bearing mice, two-way ANOVA with repeated measures were used. Where indicated, post-hoc evaluations (Fisher’s LSD) were performed to determine differences between the groups at specific time points. The Kruskall-Wallis one-way ANOVA for ranked data was used to determine differences in mechanical response thresholds of C-fiber nociceptors among the groups at each time point.
All data are expressed as mean ± SEM or median ± interquartile range (IQR).
3. Results
3.1 Development of mechanical hyperalgesia in tumor-bearing mice
A total of 57 mice developed mechanical hyperalgesia. There was a significant difference in withdrawal response frequency between tumor-bearing and control hind paws (F1,56 = 1317.30, p<.001) and over time (F5,280 = 127.83, p<.001) and a significant interaction between paw and time (F5,280 = 134.80, p<.001). While there were no differences between paws in the frequency of withdrawal prior to cancer cell implantation (baseline), response frequencies for the tumor-bearing paw became greater starting on PID 4, continued to increase through PID 11, and remained elevated prior to the electrophysiology experiment. Withdrawal response frequencies for the tumor-bearing paw approached 70% by PID 8 and typically exceeded 80% at later times (see Figure 1).
Figure 1.
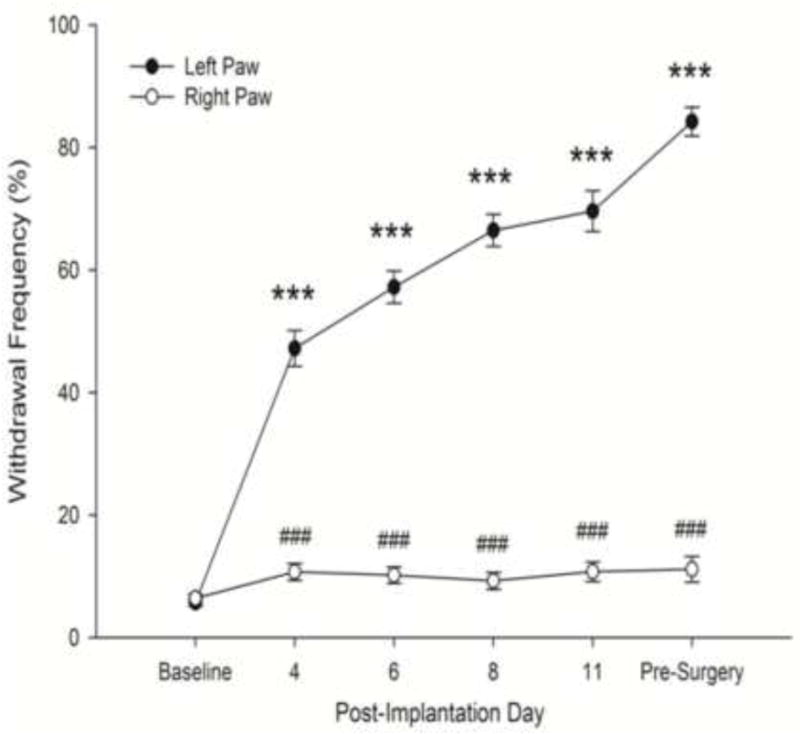
Changes in the frequency of paw withdrawal to mechanical stimulation following implantation of cancer cells into the left hind paw. Tumor development was accompanied by an increase in the frequency of paw withdrawals to application of the von Frey monofilament (3.9 mN bending force) to the plantar surface of the hind paw, while no changes occurred in response rates of the normal, right hind paw. The pre-surgery time point refers to the time just prior to the electrophysiology experiment. Data are expressed as mean ± SEM. *** p<.001 vs. Baseline, ###p<.001 vs. left paw
3.2 General characteristics of C-fiber nociceptors in control and tumor-bearing mice
A total of 34 C-fiber nociceptors were studied in 20 control mice and 70 C-fiber nociceptors were studied in 57 tumor-bearing mice. Of all nociceptors studied, 61 (58.7%) were classified CM, 16 (15.4%) as CMH, and 27 (26.0%) were unable to be tested for heat sensitivity. There was no difference in the proportion of CM and CMH fibers present between the two groups (X² = 0.51, n.s.). General characteristics of C-fibers for both tumor-bearing and control mice, including conduction velocity, rates of spontaneous activity, and responses to mechanical stimuli are presented in Table 1. Conduction velocities of C-fiber nociceptors in tumor-bearing mice did not differ from those in control mice. There were clear differences in spontaneous activity between tumor-bearing and control mice. Nearly all (97.1%) C-fiber nociceptors studied in tumor-bearing mice exhibited ongoing, spontaneous activity whereas only 8.8% of C-fiber nociceptors in control mice exhibited spontaneous activity. In addition, the median rate of spontaneous activity in C-fiber nociceptors isolated from tumor-bearing mice was higher than that of control mice (Mann-Whitney U = 110.50, n1 = 34, n2 = 70, p<.001). The median mechanical response threshold of C-fiber nociceptors in tumor-bearing mice tended to be higher than the median response threshold in control mice; however, there were no differences in the distribution of threshold values between the two groups (X² = 0.66, n.s.). There was greater variability in response thresholds among C-fiber nociceptors isolated in tumor-bearing mice, and this may have contributed to the difference in median values. There was not a difference between the two groups in responses evoked by suprathreshold mechanical stimuli (t88 = 1.27, n.s.).
Table 1.
Characteristics of C-fiber nociceptors isolated in tumor-bearing vs. control mice.
| Tumor | Control | |
|---|---|---|
| CV (m/s) | ||
| Mean ± SEM | 0.36 ± 0.04 | 0.34 ± .03 |
| Min | 0.12 | 0.13 |
| Max | 1.30 | 1.00 |
| SA1 (Hz) | ||
| Fibers with SA | 68 (97.1%) | 3 (8.8%) |
| Median rate ±IQR | 0.28 ± 0.34 | 0.00 ± .00 |
| Min | 0.00 | 0.00 |
| Max | 1.62 | 0.24 |
| Threshold (mN) | ||
| Median ±IQR | 39.2 ± 25.5 | 13.7 ± 9.8 |
| Suprathreshold2 | ||
| Mean ± SEM | 29.4 ± 2.7 | 36.0 ± 3.1 |
SA: Spontaneous Activity
Number of impulses/stimulus
A total of 16 C-fiber nociceptors (12 from tumor-bearing mice and 4 from control mice) were found to be sensitive to heat stimuli. The mean heat thresholds for tumor-bearing (40.8 ± 0.8°C) and control mice (43.0 ± 1.0°C) were comparable to those found in our earlier study (Cain et al., 2001), with heat thresholds from C-fiber nociceptors in tumor-bearing mice tending to be lower than those in control mice.
Spontaneous activity of C-fiber nociceptors and responses evoked by mechanical stimuli were compared following injection with WIN 55,212-2 alone (n = 12) or 5 min following a vehicle injection (n = 6) to confirm that the use of multiple injections did not alter the effect of the agonist. A prior injection of vehicle did not change the effects of a subsequent injection of WIN 55,212-2. There were no differences between the groups in the effect of WIN 55,212-2 on spontaneous activity (F1,16 = .63, n.s.), mechanical response thresholds (all Kruskall-Wallis[3[ <10, n.s.), or the number of impulses evoked by the suprathreshold von Frey monofilament (F1,23 = .50, n.s). In addition, there were no differences in the mean rate of spontaneous activity prior to the injection of vehicle (.43 ± .09 Hz) and just prior to the subsequent injection of WIN 55,212-2 (.39 ± .12 Hz; t4 = 1.03, n.s.). Therefore, data for these groups were combined for the remaining analyses.
3.3 WIN 55,212-2 reduced spontaneous discharge in C-fiber nociceptors
To assess the effect of vehicle or WIN 55,212-2 on spontaneous activity, we used only C-fiber nociceptors that had an ongoing discharge rate of at least 0.05 Hz. Forty-three fibers met this criterion, and the mean rate of spontaneous activity for these fibers before any injection was 0.38 ±.05 Hz.
The rate of spontaneous activity of C-fiber nociceptors differed among the four drug treatment groups (F3,39 = 4.88, p<.01), but the rate of spontaneous activity did not change over time (F4,156 = 1.51, n.s.). Injection of WIN 55, 212-2 (n = 18) reduced the rate of spontaneous activity (Figure 2). The rate of spontaneous activity in C-fiber nociceptors injected with WIN 55,212-2 differed from the vehicle-treated group at all time points after injection. Treatment with vehicle (n = 10) did not alter the rate of spontaneous activity at any time point. In addition, treatment with either AM281 (n = 6) or AM630 (n = 9) blocked the inhibitory effect of WIN 55,212-2 on spontaneous activity, and the mean rates of spontaneous activity of C-fiber nociceptors for these groups were higher than those treated with WIN 55,212-2 alone at 45 and/or 60 min after injection. Both antagonists had a similar effect in blocking the inhibitory effect of WIN 55,212-2 on the rate of spontaneous activity.
Figure 2.
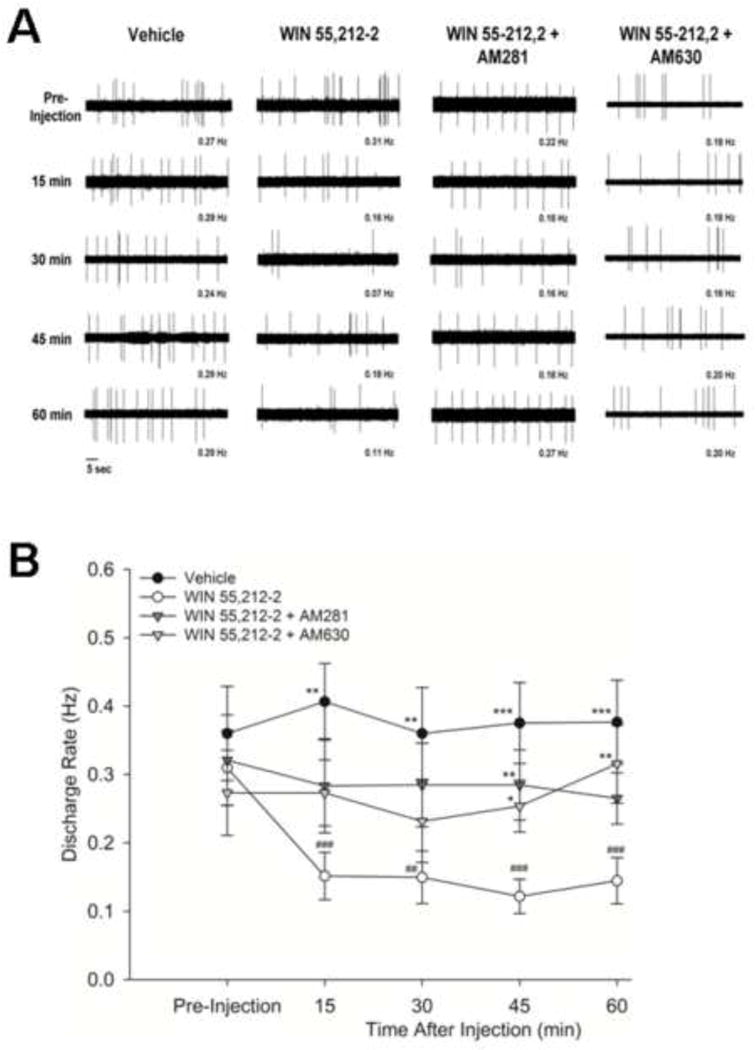
Effect of WIN 55,212-2 on the rate of spontaneous discharge of C-fiber nociceptors. (A) Representative examples of spontaneous discharge from separate nociceptors in tumor-bearing mice before (pre-injection) and at 15, 30, 45 and 60 min after intraplantar administration of vehicle, WIN 55,212-2, WIN 55,212-2 + AM281, and WIN 55,212-2 + AM630.(B) Mean (±SEM) rates of spontaneous activity in tumor-bearing mice before and at 15, 30, 45 and 60 min after intraplantar administration of vehicle, WIN 55,212-2, WIN 55,212-2 + AM281, and WIN 55,212-2 + AM630. Vehicle treatment did not alter the rate of spontaneous activity whereas treatment with WIN 55,212-2 produced a significant decrease in spontaneous activity throughout the post-injection testing period. Administration of AM281 or AM630 prevented the decrease in spontaneous activity produced by WIN 55,212-2. * p<.05, ** p≤.01, *** p≤.001 vs. WIN 55,212-2; ## p<.01, ### p≤.001 vs. pre-injection value.
3.4 Effects of WIN 55,212-2 on mechanical sensitivity of C-fiber nociceptors
The effect of WIN 55,212-2 on mechanical sensitivity was studied in 56 C-fiber nociceptors. No differences in response thresholds were found among the groups before injection or at any time after injection (data not shown). The median threshold values for each group before injection were 19.6 ± 29.4 mN (vehicle; n = 15), 39.6 ± 29.4 mN (WIN 55,2122; n = 24), 19.6 ± 25.5 mN (WIN 55,212 + AM281; n = 7) and 39.2 ± 60.3 mN (WIN 55,212-2 + AM630; n = 10).
As shown in Figure 3, administration of WIN 55,212-2 decreased the number of impulses evoked by the suprathreshold von Frey monofilament (F1,52 = 3.19, p<.05). There were significant differences in the number of impulses evoked over time (F4,208 = 2.56, p<.05), as well as a significant drug by time interaction (F4,208 = 4.78, p<.001). Treatment with WIN 55,212-2 (n = 25) reduced the number of impulses evoked by the von Frey monofilament at all time points after injection as compared to baseline (pre-injection) values. Treatment with vehicle (n = 14) or WIN 55,212-2 in combination with AM281 (n = 7) or AM630 (n = 10) did not result in any changes in the evoked number of impulses at any time. The number of impulses evoked by the suprathreshold von Frey monofilament following administration of vehicle alone or the antagonists combined with WIN 55,212-2 was higher than those evoked following WIN 55,212-2 alone for up to 60 min after injection. Thus, AM 281 and AM630 blocked the decrease in the evoked response to suprathreshold mechanical stimuli produced by the administration of WIN 55,212-2, indicating that the effect of WIN 55,212-2 occurred through both CB1 and CB2 receptors.
Figure 3.
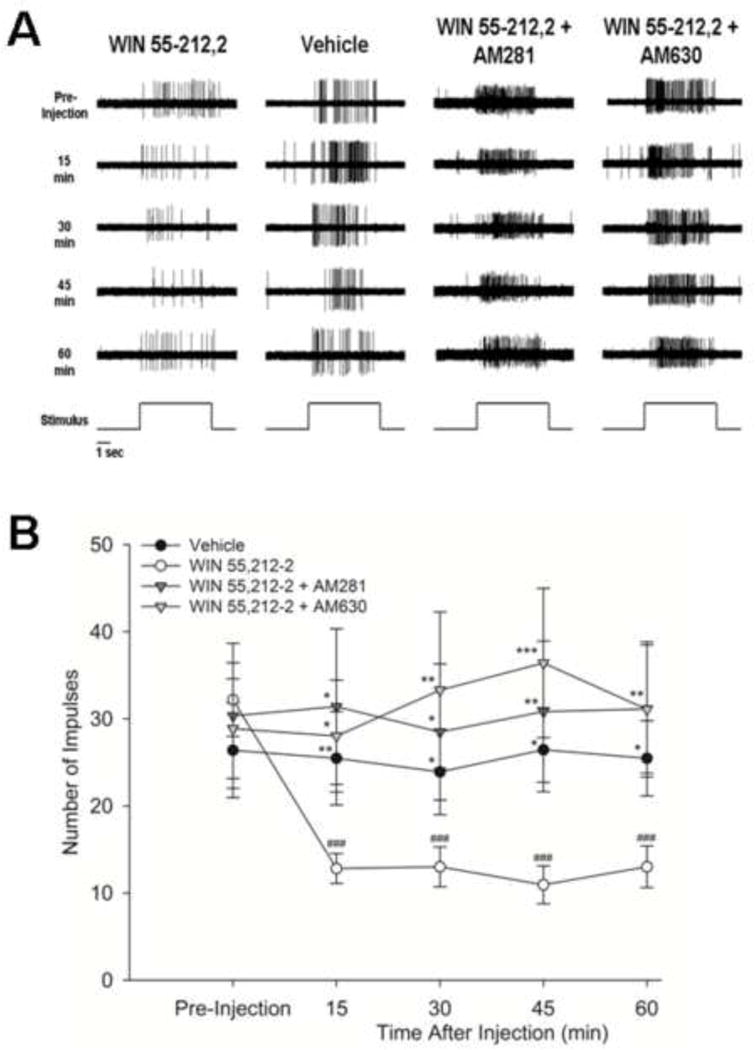
Effect of WIN 55,212-2 on responses of C-fiber nociceptors evoked by suprathreshold mechanical stimulation. (A) Representative examples of nociceptor responses evoked by 147 mN before (pre-injection) and at 15, 30, 45 and 60 min after intraplantar administration of vehicle, WIN 55,212-2, WIN 55,212-2 + AM281, and WIN 55,212-2 + AM630. The time of application of the stimulus is shown at the bottom of each column. (B) Mean (±SEM) number of evoked impulses before and at 15, 30, 45 and 60 min after intraplantar administration of vehicle, WIN 55,212-2, WIN 55,212-2 + AM281, and WIN 55,212-2 + AM630. Evoked responses were not changed following injection of vehicle but decreased following WIN 55,212-2. This was blocked by pretreatment with AM281 or AM630. * p<.05, ** p≤.01, *** p<.001 vs. WIN 55,212-2; ### p<.001 vs. pre-injection value.
4. Discussion
Activation of cannabinoid receptors in the periphery has been shown to attenuate hyperalgesia associated with bone cancer pain and other chronic pain conditions (Hald et al., 2008; Saghafi et al., 2011; Walker & Huang, 2002; Rice et al., 2002). Using a murine model of cancer pain in which fibrosarcoma cells are implanted into and around the calcaneous bone of one hind paw, we have shown that local administration of synthetic or endogenous cannabinoids or increasing the availability of endogenous cannabinoids by inhibiting their hydrolysis attenuated tumor-evoked mechanical hyperalgesia (Khasabova et al., 2008, 2011; Potenzieri et al., 2008). In order to evaluate the underlying mechanisms associated with the antihyperalgesic properties of cannabinoids in this model, we examined the effects of the synthetic cannabinoid receptor agonist WIN 55,2122 on response properties of C-fiber nociceptors. In earlier studies we demonstrated that C-, but not Aδ-nociceptors, located in skin overlying tumor growth developed spontaneous activity and increased responses to heat and cold stimuli (Cain et al., 2001). Here we show that WIN 55,212-2 decreased spontaneous activity and mechanicallyievoked responses of C-fiber nociceptors, and this occurred through both CB1 and CB2 receptors. Modulation of C-fiber activity by CB1 and CB2 receptors is consistent with our earlier behavioral studies following peripheral administration of synthetic and endogenous cannabinoids (Khasabova et al., 2008, 2011; Potenzieri et al., 2008).
In our earlier electrophysiological studies, mechanical response thresholds of C-fiber nociceptors in tumor-bearing mice were not lower than those of C-fiber nociceptors in control mice (Cain et al., 2001). However, it could be that responses to suprathreshold mechanical stimuli of spontaneously active C-fiber nociceptors were elevated and thereby contributed to mechanical hyperalgesia. In the present study we examined evoked responses of C-fiber nociceptors in mice with tumor-evoked mechanical hyperalgesia and found that C-fiber nociceptors were not sensitized to mechanical stimuli. Although we cannot rule out the possibility that mechanically-insensitive nociceptors developed mechanical sensitivity as a result of tumor growth, our data suggest that mechanical hyperalgesia in this model of cancer pain may result from central sensitization. Indeed, we have shown that wide dynamic range neurons in the dorsal horn of the spinal cord exhibited enhanced responses to mechanical stimuli (Khasabov et al., 2007). Attenuation of tumor-evoked mechanical hyperalgesia by peripheral administration of WIN 55,212-2 appears to be mediated by reduced spontaneous activity in C-fiber nociceptors which may decrease the C-fiber drive to maintain the sensitization of nociceptive dorsal horn neurons, and by a decrease in responses of C-fibers evoked by mechanical stimuli. Endogenous cannabinoids activating CB1 receptors on nociceptors may modulate the responsiveness of these nociceptors to mechanical and thermal stimuli under normal conditions. Genetic deletion of CB1 receptors on nociceptors lowered response thresholds of nociceptors and increased inflammatory pain (Agarwal et al., 2007), and intraplantar administration of a CB1 receptor antagonist in naïve mice produced mechanical hyperalgesia (Khasabova et al., 2008). Changes in expression or sensitivity of CB1 or CB2 receptors associated with tumor growth (see below) may have prevented the sensitization of nociceptors to mechanical stimuli in tumor-bearing mice.
There are several potential mechanisms by which activation of CB1 and CB2 receptors following WIN 55,212-2 reduced responses of C-fiber nociceptors. One possibility is that increased expression and function of cannabinoid receptors on C-fiber nociceptors and dorsal root ganglion (DRG) neurons makes these nociceptors more responsive to peripheral administration of WIN 55,212-2. CB1 receptors are located on primary afferent neurons (Hohmann and Herkenham, 1999; Ahluwalia et al., 2000, 2002; Salio et al., 2002; Bridges et al., 2003; Price et al., 2003; Agarwal et al., 2007) and CB1 protein expression was increased in DRG ipsilateral to the tumor-bearing paw and in DRG neurons that were co-cultured with the fibrosarcoma cells (Khasabova et al., 2008). Increased expression of CB1 receptor protein in DRG was also found in a mouse model of skin cancer (Guerrero et al., 2008). Activation of CB1 receptors on DRG neurons inhibited Na+ and Ca2+ channels (Khasabova et al., 2002, 2004; Kim et al., 2005; Vasquez and Lewis, 1999) and this may have contributed the decrease in spontaneous activity and responses to mechanical stimuli in C-fiber nociceptors observed in the present study.
We found that CB2 receptors also contributed to the inhibitory effects of WIN 55,212-2 on nociceptor activity, which is consistent with our behavioral studies (Potenzieri et al., 2008). Like CB1 receptors, peripheral CB2 receptors have been shown to play a role in cannabinoid-evoked antihyperalgesia in models of inflammatory (Guindon et al., 2007) and neuropathic pain (Desroches et al., 2008), and activation of CB2 receptors during inflammation reduced C-fiber evoked activity of nociceptive neurons in the spinal cord (Nackley et al., 2004). In the skin, CB2 receptors are associated with keratinocytes (Casanova et al., 2003; Ibrahim et al., 2005) and immune cells (Munro et al., 1993; Klein et al., 2003). In the model of cancer pain used in the present studies, the expression of CB2 receptors and the endogenous cannabinoid 2-arachidonoyl glycerol was increased in the skin of the tumor-bearing hind paw (Khasabova et al., 2011). It is therefore likely that CB2 receptor activation attenuated evoked responses of C-fiber nociceptors and reduced tumor-evoked hyperalgesia by inhibiting the release of substances from keratinocytes and immune cells that excite and sensitize nociceptors. For example, endothelin-1 is released from keratinocytes (Yohn et al., 1993) and we showed in our earlier studies that this peptide contributed to spontaneous activity and sensitization of C-fiber nociceptors to heat following tumor growth via ETA receptors (Hamamoto et al., 2008).
There are additional mechanisms by which WIN 55, 212-2 decreased nociceptor activity in the present study. One possibility, for example, is through modulation of purinergic signaling which has been implicated in contributing to cancer pain (see review, Falk et al., 2012). Adenosine triphosphate (ATP) has been found in high concentrations in tumor cells (Maehara et al., 1987), and its release following cell damage may excite nociceptors (Burnstock, 1996). Cannabinoids were shown to decrease ATP-evoked currents in nodose, dorsal root and trigeminal ganglion neurons via CB1 receptors (Krishtal et al., 2006; Shen et al., 2007). Importantly, tumor growth increased the expression of P2X3 receptors on epidermal nerves in plantar skin overlying the tumor (Gilchrist et al., 2005) and in DRG (Wu et al., 2012), and blockade of P2X3 receptors reduced hyperalgesia in a model of bone cancer pain (Kaan et al., 2010). Consistent with results of the present study and with the notion that WIN 55, 2112-2 may have attenuated responses of C-fiber nociceptors by interfering with purinergic signaling, cannabinoids were shown to decrease mechanically-evoked activity of urinary bladder afferent fibers and it was suggested that this occurred through an interaction of CB1 and P2X3 receptors (Walczak et al., 2009).
Cannabinoids have also been shown to modulate transient receptor potential (TRP) channels, including TRP ankyrin 1 (TRPA1) and TRP vanilloid 1 (TRPV1) (De Petrocellis et al., 2011), which are important transducers of noxious stimuli in nociceptors (see reviews, Basbaum et al., 2009; Stucky et al., 2009). For example, cannabinoids inhibited TRPV1-mediated currents in sensory neurons (Akopian et al., 2008; Sántha et al., 2010; Patwardhan et al., 2006). Importantly, mRNA for the TRPV1 channel was increased in the DRG in this (Khasabova et al., 2007) and other models (Miiyama et al., 2007) of cancer pain, and hyperalgesia associated with tumor growth was decreased by TRPV1 antagonists (Ghilardi et al., 2995; Menendez et al., 2006; Shinoda et al., 2008). Collectively, these studies highlight the importance of these channels in cancer-evoked hyperalgesia and suggest that desensitization of TRP channels by cannabinoids represents a downstream pathway by which cannabinoids can decrease responses of nociceptors to reduce cancer pain.
Based on the antihyperalgisic properties of cannabinoid agonists and compounds that enhance endogenous cannabinoid activity in animal models of persistent pain, there is increasing interest in exploiting the cannabinoid system for pain management. Clinical studies have provided some support for the use of cannabinoids for analgesia in humans. Cannabinoid agonists attenuated post-operative (Raft et al., 1977) and neuropathic pain (Nurmikko et al., 2007) and enhanced the analgesic efficacy of opioids (Yesilyurt et al., 2003). Two small clinical studies of the efficacy of delta-9-tetrohydrocannabinol for the treatment of advanced cancer pain found that patients reported pain relief comparable to codeine (Noyes et al., 1975a, b; 1976). Unfortunately, higher doses produced sedation and other side effects such as dizziness, drowsiness, blurred vision and impaired cognitive functioning that would limit the usefulness of systemic cannabinoids for long term therapy. Restricting cannabinoids to the periphery would minimize unwanted side effects, and the contribution of peripheral CB1 and CB2 receptors to antihyperalgesia may offer unique opportunities for managing pain that is difficult to treat, such as cancer pain. We have shown, for example, that simultaneous activation of peripheral CB1 and CB2 receptors had a synergistic effect in reducing tumor-evoked hyperalgesia (Khasabova et al., 2011). These results provide additional rationale for targeting both CB1 and CB2 receptors in the periphery for the management of cancer pain and perhaps for other persistent pain states as well.
Tumor growth in mouse hind paw produced ongoing activity in C-fiber nociceptors.
The cannabinoid receptor agonist, WIN 55,212-2, reduced C-fiber activity.
Effects of WIN 55,212-2 were reduced by CB1 or CB2 receptor antagonists.
Further evidence that peripheral CB receptors may be targets to treat cancer pain.
Acknowledgments
The authors want to thank Drs. Darryl Hamamoto, Iryna Khasabova and Glenn Giesler for critically reading an earlier version of the manuscript. This work was supported by NIH grants DA011471 and CA091007. Megan Uhelski was supported by NIDA training grant T32 DA07234.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Agarwal N, Pacher P, Tegeder I, Amaya F, Constantin CE, Brenner GJ, Rubino T, Michalski CW, Marsicano G, Monory K, Mackie K, Marian C, Batkai S, Parolaro D, Fischer MJ, Reeh P, Kunos G, Kress M, Lutz B, Woolf CJ, Kuner R. Cannabinoids mediate analgesia largely via peripheral type 1 cannabinoid receptors in nociceptors. Nature Neurosci. 2007;10:870–879. doi: 10.1038/nn1916. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ahluwalia J, Urban L, Bevan S, Capogna M, Nagy I. Cannabinoid 1 receptors are expressed by nerve growth factor- and glial cell-derived neurotrophic factor-responsive primary sensory neurons. Neurosci. 2002;110:747–753. doi: 10.1016/s0306-4522(01)00601-7. [DOI] [PubMed] [Google Scholar]
- Ahluwalia J, Urban L, Capogna M, Bevan S, Nagy I. Cannabinoid 1 receptors in nociceptive primary sensory neurons. Neurosci. 2000;100:685–688. doi: 10.1016/s0306-4522(00)00389-4. [DOI] [PubMed] [Google Scholar]
- Akopian AN, Ruparel NB, Patwardhan A, Hargreaves KM. Cannabinoids desensitize capsaicin and mustard oil responses in sensory neurons via TRPA1 activation. J Neurosci. 2008;28(5):1064–1075. doi: 10.1523/JNEUROSCI.1565-06.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basbaum AI, Bautista DM, Scherrer G, Julius D. Cellular and molecular mechanisms of pain. Cell. 2009;139(2):267–284. doi: 10.1016/j.cell.2009.09.028. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bridges D, Ahmad K, Rice ASC. The synthetic cannabinoid WIN55,212-2 attenuates hyperalgesia and allodynia in a rat model of neuropathic pain. Brit J Pharmacol. 2001;133:586–594. doi: 10.1038/sj.bjp.0704110. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Burnstock G. A unifying purinergic hypothesis for the initiation of pain. Lancet. 1996;347:1604–1605. doi: 10.1016/s0140-6736(96)91082-x. [DOI] [PubMed] [Google Scholar]
- Bridges D, Rice AS, Egertova M, Elphick MR, Winter J, Michael GJ. Localization of cannabinoid receptor 1 in rat dorsal root ganglion using in situ hybridization and immunohistochemistry. Neurosci. 2003;119(3):803–812. doi: 10.1016/s0306-4522(03)00200-8. [DOI] [PubMed] [Google Scholar]
- Cain DM, Wacnik PW, Eikmeier L, Beitz A, Wilcox GL, Simone DA. Functional interactions between tumor and peripheral nerve in a model of cancer pain in the mouse. Pain Med. 2001;2(1):15–23. doi: 10.1046/j.1526-4637.2001.002001015.x. [DOI] [PubMed] [Google Scholar]
- Calignano A, La Rana G, Giuffida A, Piomelli D. Control of pain initiation by endogenous cannabinoids. Nature. 1998;394:277–281. doi: 10.1038/28393. [DOI] [PubMed] [Google Scholar]
- Casanova ML, Blazquez C, MartinezPalacio J, Villanueva C, Fernandez-Acenero MJ, Huffman JW, Jorcano JL, Guzman M. Inhibition of skin tumor growth and angiogenesis in vivo by activation of cannabinoid receptors. J Clin Invest. 2003;111:43–50. doi: 10.1172/JCI16116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clayton N, Marshall FH, Bountra C, O’Shaughnessy CT. CB1 and CB2 cannabinoid receptors are implicated in inflammatory pain. Pain. 2002;96:253–260. doi: 10.1016/S0304-3959(01)00454-7. [DOI] [PubMed] [Google Scholar]
- De Petrocellis L, Ligresti A, Moriello AS, Allarà M, Bisogno T, Petrosino S, Stott CG, Di Marzo V. Effects of cannabinoids and cannabinoid-enriched Cannabis extracts on TRP channels and endocannabinoid metabolic enzymes. Br J Pharmacol. 2011;163(7):1479–1494. doi: 10.1111/j.1476-5381.2010.01166.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Desroches J, Guindon J, Lambert C, Beaulieu P. Modulation of the anti-nociceptive effects of 2-arachidononyl glycerol by peripherally administered FAAH and MGL inhibitors in a neuropathic pain model. Brit J Pharmacol. 2008;155:913–924. doi: 10.1038/bjp.2008.322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmes SJR, Winyard LA, Medhurst SJ, Clayon NM, Wilson AW, Kendall DA, Chapman V. Activation of CB1 and CB2 receptors attenuates the induction and maintenance of inflammatory pain in the rat. Pain. 2005;118:327–335. doi: 10.1016/j.pain.2005.09.005. [DOI] [PubMed] [Google Scholar]
- Falk S, Uldall M, Heegaard AM. The role of purinergic receptors in cancer-induced bone pain. J Osteoporos. 2012 doi: 10.1155/2012/758181. published online October 3, 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Facci L, Dal Toso R, Romanello S, Buriani A, Skaper SD, Leon A. Mast cells express a peripheral cannabinoid receptor with differential sensitivity to anandamide and palmitoylethanolamide. P Natl Acad Sci USA. 1995;92(8):3376–3380. doi: 10.1073/pnas.92.8.3376. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fox A, Kesingland A, Gentry C, McNair K, Patel S, Urban L, James I. The role of central and peripheral cannabinoid receptors in the antihyperalgesic activity of cannabinoids in a model of neuropathic pain. Pain. 2001;92:91–100. doi: 10.1016/s0304-3959(00)00474-7. [DOI] [PubMed] [Google Scholar]
- Fride R, Mechoulam R. Pharmacological activity of the cannabinoid receptor agonist, anandamide, a brain constituent. Eur J Pharmacol. 1993;231(2):313–314. doi: 10.1016/0014-2999(93)90468-w. [DOI] [PubMed] [Google Scholar]
- Galiegue S, Mary S, Marchand J, Dussossoy D, Carriere D, Carayon P, Bouaboula M, Shire D, Fur GLE, Casellas P. Expression of central and peripheral cannabinoid receptors in human immune tissues and leukocyte populations. Eur J Biochem. 1995;232(1):54–61. doi: 10.1111/j.1432-1033.1995.tb20780.x. [DOI] [PubMed] [Google Scholar]
- Gilchrist LS, Cain DM, Harding-Rose C, Kov AN, Wendelschafer-Crabb G, Kennedy WR, Simone DA. Re-organization of P2X3 receptor localization on epidermal nerve fibers in a murine model of cancer pain. Brain Res. 2005;1044:197–205. doi: 10.1016/j.brainres.2005.02.081. [DOI] [PubMed] [Google Scholar]
- Griffin G, Tao Q, Abood ME. Predictors of Quality of Life in Oncology Outpatients with Pain from Bone Metastasis. J Pharmacol Exp Ther. 2000;292(3):886–894. [PubMed] [Google Scholar]
- Guerrero AV, Quang P, Dekker N, Jordan RCK, Schmidt BL. Peripheral cannabinoids attenuate carcinoma-induced nociception in mice. Neurosci Lett. 2008;433:77–81. doi: 10.1016/j.neulet.2007.12.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guindon J, Desroches J, Beaulieu P. The antinociceptive effects of intraplantar injections of 2-arachidononyl glycerol are mediated by cannabinoid CB2 receptors. Brit J Pharmacol. 2007;150:693–701. doi: 10.1038/sj.bjp.0706990. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hald A, Ding M, Egerod C, Hansen RR, Konradsen D, Jorgensen SG, Atalay B, Nasser A, Bjerrum OJ, Heegaard A-M. Differential effects of repeated low dose treatment with the cannabinoid agonist WIN 55,212-2 in experimental models of bone cancer pain and neuropathic pain. Pharmacol Biochem Beh. 2008;91:38–46. doi: 10.1016/j.pbb.2008.04.021. [DOI] [PubMed] [Google Scholar]
- Hamamoto DT, Giridharagopalan S, Simone DA. Acute and chronic administration of the cannabinoid receptor agonist CP 55,940 attenuates tumor-evoked hyperalgesia. Eur J Pharmacol. 2007;558:73–87. doi: 10.1016/j.ejphar.2006.11.062. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamamoto DT, Khasabov SG, Cain DM, Simone DA. Tumor-evoked sensitization of nociceptors: A role for endothelin. J Neurophysiol. 2008;100:2300–2311. doi: 10.1152/jn.01337.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Herzberg U, Eliav E, Bennett GJ, Kopin IJ. The analgesic effects of R(+)-WIN 55,212-2 mesylate, a high affinity cannabinoid agonist, in a rat model of neuropathic pain. Neurosci Lett. 1997;221:157–160. doi: 10.1016/s0304-3940(96)13308-5. [DOI] [PubMed] [Google Scholar]
- Hohmann AG, Herkenham M. Localization of central cannabinoid CB1 receptor messenger RNA in neuronal subpopulations of rat dorsal root ganglia: a double-label in situ hybridization study. Neurosci. 1999;90:923–931. doi: 10.1016/s0306-4522(98)00524-7. [DOI] [PubMed] [Google Scholar]
- Howlader N, Noone AM, Krapcho M, Neyman N, Aminou R, Waldron W, Altekruse SF, Kosary CL, Ruhl J, Tatalovich Z, Cho H, Mariotto A, Eisner MP, Lewis DR, Chen HS, Feuer EJ, Cronin KA, Edwards BK, editors. SEER Cancer Statistics Review, 1975–2008. National Cancer Institute; Bethesda, MD: 2011. http://seer.cancer.gov/csr/1975_2008/, based on November 2010 SEER data submission, posted to the SEER web site. [Google Scholar]
- Ibrahim MM, Deng H, Zvonok A, Cockayne DA, Kwan J, Mata HP, Vanderah TW, Lai J, Porreca F, Makriyannis A, Malan TP., Jr Activation of CB2 cannabinoid receptors by AM1241 inhibits experimental neuropathic pain: Pain inhibition by receptors not present in the CNS. Proc Natl Acad Sci USA. 2003;100:10529–10533. doi: 10.1073/pnas.1834309100. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim MM, Porreca F, Lai J, Albrecht PJ, Rice FL, Khodorova A, Davar G, Makriyannis A, Vanderah TW, Mata HP, Malan TP., Jr CB2 cannabinoid receptor activation produces antinociception by stimulating peripheral release of endogenous opioids. Brit J Pharmacol. 2005;102:3093–3098. doi: 10.1073/pnas.0409888102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Johanek LM, Heitmiller DR, Turner M, Nader N, Hodges J, Simone DA. Cannabinoids attenuate capsaicin-evoked hyperalgesia through spinal and peripheral mechanisms. Pain. 2001;93:303–315. doi: 10.1016/S0304-3959(01)00336-0. [DOI] [PubMed] [Google Scholar]
- Kaan TK, Yip PK, Patel S, Davies M, Marchand F, Cockayne DA, Nunn PA, Dickenson AH, Ford AP, Zhong Y, Malcangio M, McMahon SB. Systemic blockade of P2X3 and P2X2/3 receptors attenuates bone cancer pain behaviour in rats. Brain. 2010;133:2549–2564. doi: 10.1093/brain/awq194. [DOI] [PubMed] [Google Scholar]
- Kehl LJ, Hamamoto DT, Wacnik PW, Croft DL, Norsted BD, Wilcox GL, Simone DA. A cannabinoid agonist differentially attenuates deep tissue hyperalgesia in animal models of cancer and inflammatory muscle pain. Pain. 2003;103:175–186. doi: 10.1016/s0304-3959(02)00450-5. [DOI] [PubMed] [Google Scholar]
- Khasabova IA, Chandiramani A, Harding-Rose C, Simone DA, Seybold VS. Increasing 2-arachidonoyl glycerol signaling in the periphery attenuates mechanical hyperalgesia in a model of bone cancer pain. Pharmacol Res. 2011;64:60–67. doi: 10.1016/j.phrs.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasabova IA, Chandiramani A, Harding-Rose C, Simone DA, Seybold VS. Increasing 2-arachidonoyl glycerol signaling in the periphery attenuates mechanical hyperalgesia in a model of bone cancer pain. Pharmacol Res. 2008;64:60–67. doi: 10.1016/j.phrs.2011.03.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasabova IA, Harding-Rose C, Simone DA, Seybold VS. Differential effects of CB1 and opioid agonists on two populations of adult rat dorsal root ganglion neurons. J Neurosci. 2004;18:1744–1753. doi: 10.1523/JNEUROSCI.4298-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasabova IA, Khasabov SG, Harding-Rose C, Coicou LG, Seybold BA, Lindberg AE, Steevens CD, Simone DA, Seybold VS. A decrease in anandamide signaling contributes to the maintenance of cutaneous mechanical hyperalgesia in a model of bone cancer pain. J Neurosci. 2008;29:11141–11152. doi: 10.1523/JNEUROSCI.2847-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khasabova IA, Simone DA, Seybold VS. Cannabinoids attenuate depolarization-dependent Ca2+ influx in intermediate-size primary afferent neurons of adult rats. Neurosci. 2002;115:613–625. doi: 10.1016/s0306-4522(02)00449-9. [DOI] [PubMed] [Google Scholar]
- Khasabova IA, Stucky CL, Harding-Rose C, Eikmeier L, Beitz AJ, Coicou LG, Hanson AE, Simone DA, Seybold VS. Chemical interactions between fibrosarcoma cancer cells and sensory neurons contribute to cancer pain. J Neurosci. 2007;19:10289–10298. doi: 10.1523/JNEUROSCI.2851-07.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim HI, Kim TH, Shin YK, Lee CS, Park M, Song J-H. Anandamide suppression of Na+ currents in rat dorsal root ganglion neurons. Brain Res. 2005;1062:39–47. doi: 10.1016/j.brainres.2005.09.004. [DOI] [PubMed] [Google Scholar]
- Klein TW, Newton C, Larsen K, Lu L, Perkins I, Nong L, Friedman H. The cannabinoid system and immune modulation. J Leukocyte Biol. 2003;74:486–496. doi: 10.1189/jlb.0303101. [DOI] [PubMed] [Google Scholar]
- Ko M, Woods JH. Local administration of Δ9-tetrahydrocannabinol attenuates capsaicin-induced thermal nociception in rhesus monkeys: a peripheral cannabinoid action. Psychopharmacol. 1999;143:322–326. doi: 10.1007/s002130050955. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishtal O, Lozovaya N, Fedorenko A, Savelyev I, Chizhmakov I. The agonists for nociceptors are ubiquitous, but the modulators are specific: P2X receptors in the sensory neurons are modulated by cannabinoids. Pflugers Arch. 2006;453(3):353–360. doi: 10.1007/s00424-006-0094-1. [DOI] [PubMed] [Google Scholar]
- Li J, Daughters RS, Bullis C, Bengiamin R, Stucky MW, Brennan J, Simone DA. The cannabinoid receptor agonist WIN 55,212-2 mesylate blocks the development of hyperalgesia produced by capsaicin in rats. Pain. 1999;81:25–33. doi: 10.1016/s0304-3959(98)00263-2. [DOI] [PubMed] [Google Scholar]
- Maehara Y, Kusumoto H, Anai H, Kusumoto T, Sugimachi K. Human tumor tissues have higher ATP contents than normal tissues. Clin Chim Acta. 1987;169:341–343. doi: 10.1016/0009-8981(87)90337-8. [DOI] [PubMed] [Google Scholar]
- Martin WJ, Loo CM, Basbaum AI. Spinal cannabinoids are anti-allodynic in rats with persistent inflammation. Pain. 1999;82:199–205. doi: 10.1016/S0304-3959(99)00045-7. [DOI] [PubMed] [Google Scholar]
- Mercadante S, Portenoy RK. Opioid poorly-responsive cancer pain. Part 1: Clinical considerations. J Pain Symptom Manage. 2001;21(2):144–150. doi: 10.1016/s0885-3924(00)00228-1. [DOI] [PubMed] [Google Scholar]
- Mercadante S, Zagonel V, Breda E, Arcara C, Gebbia V, Porzio G, Aielli F, David F, Gammucci T, Narducci F, Lanzetta G, Restuccia R, Lembo A, Passeri V, Virzi V, Casuccio A. Breakthrough pain in oncology: A longitudinal study. J Pain Symptom Manage. 2010;40(2):183–190. doi: 10.1016/j.jpainsymman.2010.01.010. [DOI] [PubMed] [Google Scholar]
- Meuser T, Pietruck C, Radbruch L, Stute P, Lehmann KA, Grond S. Symptoms during cancer pain treatment following WHO-guidelines: A longitudinal follow-up study of symptom prevalence, severity and etiology. Pain. 2001;93:247–257. doi: 10.1016/S0304-3959(01)00324-4. [DOI] [PubMed] [Google Scholar]
- Moss DE, Johnson RL. Tonic analgesic effects of Δ9-tetrahydrocannabinol as measured with the formalin test. Eur J Pharmacol. 1980;61:313–315. doi: 10.1016/0014-2999(80)90134-x. [DOI] [PubMed] [Google Scholar]
- Munro S, Thomas KL, Abu-Shaar M. Molecular characterization of a peripheral receptor for cannabinoids. Nature. 1993;365:61–65. doi: 10.1038/365061a0. [DOI] [PubMed] [Google Scholar]
- Nackley AG, Zvonok AM, Makriyannis A, Hohmann AG. Activation of cannabinoid CB2 receptors suppresses C-fiber responses and windup in spinal wide dynamic range neurons in the absence and presence of inflammation. J Neurophysiol. 2004;92:3562–3574. doi: 10.1152/jn.00886.2003. [DOI] [PubMed] [Google Scholar]
- Noyes R, Jr, Brunk SF, Avery DH, Canter A. Psychologic effects of oral delta-9-tetrahydrocannabinol in advanced cancer patients. Compr Psychiatry. 1976;17(5):641–646. doi: 10.1016/s0010-440x(76)80008-9. [DOI] [PubMed] [Google Scholar]
- Noyes, Brunk SF, Avery JA, Canter AC. The analgesic properties of delta-9-tetrahydrocannabinol. Clin Pharmacol Ther. 1975b;18:84–89. doi: 10.1002/cpt197518184. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Noyes R, Jr, Brunk SF, Baram DA, Canter A. Analgesic effect of delta-9-tetrahydrocannabinol. J Clin Pharmacol. 1975;15:139–143. doi: 10.1002/j.1552-4604.1975.tb02348.x. [DOI] [PubMed] [Google Scholar]
- Nurmikko TJ, Serpell MG, Hoggart B, Toomey PJ, Morlion BJ, Haines D. Sativex successfully treats neuropathic pain characterised by allodynia: A randomised, double-blind, placebo-controlled clinical trial. Pain. 2007;133:210–220. doi: 10.1016/j.pain.2007.08.028. [DOI] [PubMed] [Google Scholar]
- Patwardhan AM, Jeske NA, Price TJ, Gamper N, Akopian AN, Hargreaves KM. The cannabinoid WIN 55,212-2 inhibits transient receptor potential vanilloid 1 (TRPV1) and evokes peripheral antihyperalgesia via calcineurin. Proc Natl Acad Sci. 2006;103(30):11393–11398. doi: 10.1073/pnas.0603861103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenzieri C, Brink TS, Pacharinsak C, Simone DA. Cannabinoid modulation of cutaneous Aδ nociceptors during inflammation. J Neurophysiol. 2008a;100:2794–2806. doi: 10.1152/jn.90809.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Potenzieri C, Harding-Rose C, Simone DA. The cannabinoid receptor agonist, WIN 55,212-2, attenuates tumor-evoked hyperalgesia through peripheral mechanisms. Brain Res. 2008b;1215:69–75. doi: 10.1016/j.brainres.2008.03.063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Price TJ, Helesic G, Parghi D, Hargreaves KM, Flores CM. The neuronal distribution of cannabinoid receptor type 1 in the trigeminal ganglion of the rat. Neurosci. 2003;120:155–162. doi: 10.1016/S0306-4522(03)00333-6. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raft D, Gregg J, Ghia J, Harris L. Effects of intravenous tetrahydrocannabinol on experimental and surgical pain. Psychological correlates of the analgesic response. Clin Pharmacol Ther. 1977;21:26–33. doi: 10.1002/cpt197721126. [DOI] [PubMed] [Google Scholar]
- Rice ASC, Farquhar-Smith WP, Nagy I. Endocannabinoids and pain: spinal and peripheral analgesia in inflammation and neuropathy. Prostag Leukotr Ess. 2002;66:243–256. doi: 10.1054/plef.2001.0362. [DOI] [PubMed] [Google Scholar]
- Richardson JD, Aanonsen L, Hargreaves KM. Antihyperalgesic effects of spinal cannabinoids. Eur J Pharmacol. 1998a;345:145–153. doi: 10.1016/s0014-2999(97)01621-x. [DOI] [PubMed] [Google Scholar]
- Richardson JD, Kilo S, Hargreaves KM. Cannabinoids reduce hyperalgesia and inflammation via interaction with peripheral CB1 receptors. Pain. 1998b;75:111–119. doi: 10.1016/S0304-3959(97)00213-3. [DOI] [PubMed] [Google Scholar]
- Rukwied R, Watkinson A, McGlone F, Dvorak M. Cannabinoid agonists attenuate capsaicin-induced responses in human skin. Pain. 2003;102:283–288. doi: 10.1016/S0304-3959(02)00401-3. [DOI] [PubMed] [Google Scholar]
- Rustøen T, Moum T, Padilla G, Paul S, Miaskowski C. Predictors of quality of life in oncology outpatients with pain from bone metastasis. J Pain Symptom Manag. 2005;30(3):234–242. doi: 10.1016/j.jpainsymman.2005.04.002. [DOI] [PubMed] [Google Scholar]
- Saghafi N, Lam DK, Schmidt BL. Cannabinoids attenuate cancer pain and proliferation in a mouse model. Neurosci Lett. 2011;488:247–251. doi: 10.1016/j.neulet.2010.11.039. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Salio C, Cottone E, Conrath M, Franzoni MF. CB1 cannabinoid receptors in amphibian spinal cord: relationships with some nociception markers. J Chem Neuroanat. 2002;24:153–162. doi: 10.1016/s0891-0618(02)00040-6. [DOI] [PubMed] [Google Scholar]
- Sántha P, Jenes A, Somogyi C, Nagy I. The endogenous cannabinoid anandamide inhibits transient receptor potential vanilloid type 1 receptor-mediated currents in rat cultured primary sensory neurons. Acta Physiol Hung. 2010;97(2):149–158. doi: 10.1556/APhysiol.97.2010.2.1. [DOI] [PubMed] [Google Scholar]
- Shen JJ, Liu CJ, Li A, Hu XW, Lu YL, Chen L, Zhou Y, Liu LJ. Cannabinoids inhibit ATP-activated currents in rat trigeminal ganglionic neurons. Sheng Li Xue Bao. 2007;59(6):745–752. [PubMed] [Google Scholar]
- Stucky CL, Dubin AE, Jeske NA, Malin SA, McKemy DD, Story GM. Roles of transient receptor potential channels in pain. Brain Res Rev. 2009;60(1):2–23. doi: 10.1016/j.brainresrev.2008.12.018. [DOI] [PMC free article] [PubMed] [Google Scholar]
- van den Beuken-van Everdingen MHJ, de Rijke JM, Kessels AG, Schouten HC, van Kleef M, Patijn J. Prevalence of pain in patients with cancer: a systematic review of the past 40 years. Ann Oncol. 2007;18(9):1437–1449. doi: 10.1093/annonc/mdm056. [DOI] [PubMed] [Google Scholar]
- Vasquez C, Lewis DL. The CB1 cannabinoid receptor can sequester G-proteins, making them unavailable to couple to other receptors. J Neurosci. 1999;1:9271–9280. doi: 10.1523/JNEUROSCI.19-21-09271.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wacnik PW, Eikmeier LJ, Ruggles TR, Ramnaraine ML, Walcheck BK, Beitz AJ, Wilcox GL. Functional interactions between tumor and peripheral nerve: Morphology, a gogen identification, and behavioral characterization of a new murine model of cancer pain. J Neurosci. 2001;21(23):9355–9366. doi: 10.1523/JNEUROSCI.21-23-09355.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Walczak JS, Price TJ, Cervero F. Cannabinoid CB1 receptors are expressed in the mouse urinary bladder and their activation modulates afferent bladder activity. Neurosci. 2009;159(3):1154–1163. doi: 10.1016/j.neuroscience.2009.01.050. [DOI] [PubMed] [Google Scholar]
- Walker JM, Huang SM. Cannabinoid analgesia. Pharmacol & Ther. 2002;95:127–135. doi: 10.1016/s0163-7258(02)00252-8. [DOI] [PubMed] [Google Scholar]
- Wu JX, Xu MY, Miao XR, Lu ZJ, Yuan XM, Li XQ, Yu WF. Functional up-regulation of P2X3 receptors in dorsal root ganglion in a rat model of bone cancer pain. Eur J Pain. 2012 doi: 10.1002/j.1532-2149.2012.00149.x. [DOI] [PubMed] [Google Scholar]
- Yesilyurt O, Dogrul A, Gul H, Seyrek M, Kusmez O, Ozkan Y, Yildiz O. Topical cannabinoid enhances topical morphine antinociception. Pain. 2003;105:303–308. doi: 10.1016/s0304-3959(03)00245-8. [DOI] [PubMed] [Google Scholar]
- Yohn JJ, Morelli JG, Walchak SJ, Rundell KB, Norris DA, Zamora MR. Cultured human keratinocytes synthesize and secrete endothelin-1. J Investigat Dermatol. 1993;100:23–26. doi: 10.1111/1523-1747.ep12349932. [DOI] [PubMed] [Google Scholar]
- Zeppetella G, O’Doherty CA, Collins S. Prevalence and characteristics of breakthrough pain in cancer patients admitted to a hospice. J Pain Symptom Manag. 2000;20(2):87–92. doi: 10.1016/s0885-3924(00)00161-5. [DOI] [PubMed] [Google Scholar]
- Zimmerman M. Ethical guidelines for investigation of experimental pain in conscious animals. Pain. 1983;16:109–110. doi: 10.1016/0304-3959(83)90201-4. [DOI] [PubMed] [Google Scholar]


