Abstract
We recently discovered a regulatory mechanism that stimulates production of the multifunctional antimicrobial peptide, cathelicidin antimicrobial peptide (CAMP). In response to subtoxic levels of ER stress, increased sphingosine-1-phosphate (S1P) production activates an NFκB→C/EBPα dependent pathway that enhances CAMP production in cultured human keratinocytes. Since the multifunctional stilbenoid compound, resveratrol (RESV), increases ceramide (Cer) levels, a precursor of S1P, we hypothesized and assessed whether RESV could exploit the same pathway to regulate CAMP production. Accordingly, RESV significantly increased Cer and S1P levels in cultured keratinocytes, paralleled by increased CAMP mRNA/protein expression. Furthermore, topical RESV also increased murine CAMP mRNA/protein expression in mouse skin. Conversely, blockade of Cer→sphingosine→S1P metabolic conversion, with specific inhibitors of ceramidase or sphingosine kinase, attenuated the expected RESV-mediated increase in CAMP expression. The RESV-induced increase in CAMP expression required both NF-κB and C/EBPα transactivation. Moreover, conditioned media from keratinocyte treated with RESV significantly suppressed Staphylococcus aureus growth. Finally, topical RESV, if not coapplied with a specific inhibitor of sphingosine kinase, blocked Staphylococcus aureus invasion into murine skin. These results demonstrate that the dietary stilbenoid, RESV, stimulates S1P signaling of CAMP production through an NF-κB→C/EBPα-dependent mechanism, leading to enhanced antimicrobial defense against exogenous microbial pathogens.
Keywords: antimicrobial peptide, cathelicidin antimicrobial protein, keratinocytes, resveratrol, sphingosine-1-phosphate
INTRODUCTION
Human epidermis is positioned at the interface with the environment, protecting underlying tissues from exogenous microbial pathogens, mechanical damage, and ultraviolet irradiation. These protective mechanisms include the generation of antimicrobial peptides (AMP) that display activity against a broad-spectrum of different pathogens, including Gram-negative and Gram-positive bacteria, fungi, and certain viruses (Dunn et al., 2009; Mendez-Samperio, 2010; Nijnik and Hancock, 2009; Schroder, 2010). In addition to its antimicrobial function, the major AMP, cathelicidin antimicrobial peptide (CAMP), is a multifunctional modulator of cytokine secretion/production, angiogenesis and adaptive immune responses (Lai et al., 2010). Prior studies demonstrated that CAMP expression increases in epithelial tissues, including in epidermal keratinocytes (KC), after external perturbations; e.g., wounding, suberythemagenic UVB irradiation, oxidative stress, and epidermal barrier abrogation (Aberg et al., 2008; Hong et al., 2008; Kim et al., 2009; Mallbris et al., 2010). However, if these external perturbations become excessive, they instead produce cell cycle arrest and apoptosis by increasing endoplasmic reticulum (ER) stress-induced ceramide (Cer) production (Lei et al., 2008). In contrast, subtoxic perturbations produce lower levels of ER stress, which also increases Cer transiently. Some of the increased Cer, generated following subtoxic stress, is metabolized to S1P, which stimulates CAMP production in epithelial tissues, including epidermis, via a (to our knowledge) previously unidentified NF-κB and C/EBPα-mediated pathway (Park et al., 2012). Importantly, this regulatory mechanism operates independently of the well-established vitamin D receptor (VDR)-regulated pathway (Gombart et al., 2005), which instead likely predominates under basal (non-stressed) condition.
Resveratrol (RESV, trans-3, 4, 5-trihydroxystilbene) belongs to a class of phytoalexins, that are synthesized by a restricted number of plants, including berries, peanuts, and red grapes. Notably, the synthesis of RESV in these plants increases in response to external stressors; i.e., infection or UV irradiation (Shakibaei et al., 2009). RESV exerts antioxidant and other anti-inflammatory activities, as well as regulating cellular proliferation, differentiation, Sirt modulation, and mitochondria-initiated apoptosis (Sadruddin and Arora, 2009; Shakibaei et al., 2009). Pertinent to the current studies, RESV also stimulates Cer levels in multiple cell types (Cakir et al., 2011; Dolfini et al., 2007; Signorelli et al., 2009).
We have demonstrated that KC deploy three metabolic mechanisms that protect against Cer-induced apoptosis (Uchida et al., 2010); i.e., Cer-to-glucosylceramide, Cer-to-sphingomyelin (see also (Charruyer et al., 2008)), and ceramidase-mediated hydrolysis of Cer to sphingosine. We showed further that subtoxic external perturbations that induce ER stress and increase cellular Cer production also stimulate metabolic conversion of sphingosine to S1P, leading to enhanced CAMP generation (Park et al., 2012). Here, we show that RESV not only increases Cer production, but also that it initiates downstream conversion of Cer to S1P, leading to stimulation CAMP production in cultured human KC. In addition, we show here that topical RESV stimulates S1P signaling of CAMP production in vivo (murine skin). Finally, we demonstrate that pre-treatment of KC with RESV enhances antimicrobial defense against virulent, exogenous Staphylococcus aureus. Notably RESV itself did not induce ER stress, suggesting that RESV directly stimulates S1P signaling of CAMP expression. These studies illuminate yet-another important, and potentially clinically-beneficial biological activity of RESV; i.e., the ability to enhance epithelial innate immunity through exploitation of an ER stress-initiated pathway.
RESULTS
RESV increases cellular levels of S1P in parallel with enhanced CAMP production
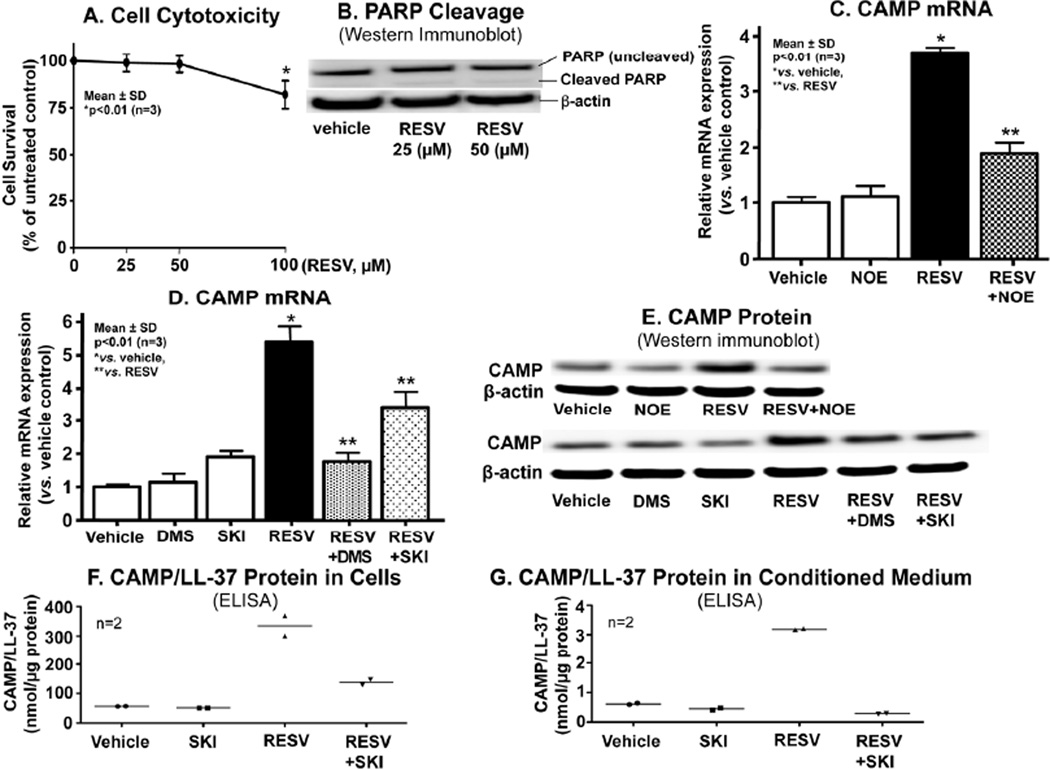
Our prior studies demonstrated that subtoxic levels of ER stress, induced by either external perturbations; e.g., UVB irradiation, or an established pharmacological ER stressor, e.g., thapsigargin, increase not only levels of cellular ceramide (Cer), but also conversion of Cer to its distal metabolite, sphingosine-1-phosphate (S1P), which then stimulated CAMP production (Park et al., 2012). Hence, we first assessed here whether exogenous RESV stimulates production of cellular Cer, as well as its downstream metabolites, without inducing excessive ER stress. Lipid quantification showed a modest, but significant increase in Cer, and large increases in both sphingosine and S1P following treatment of cultured human keratinocytes (KC) with exogenous RESV at concentrations < 50 µM (Table 1). At these RESV concentrations, indicators of apoptosis (i.e., cell viability and PARP cleavage) did not become evident (Figs. 1A and 1B), assuring that these concentrations of RESV are not toxic. Yet, because still-higher concentrations (>100 µM) slightly decreased cell viability (Fig. 1A), we employed RESV at concentrations of < 50 µM in all subsequent studies.
Table 1.
Sphingolipid content in human KC exposed to ER stress
| Lipid Content (pmol/mg protein ± SD) | |||
|---|---|---|---|
| Treatment | Cer | Sphingosine | S1P |
| Vehicle | 736.3 ± 41.2 | 19.3 ± 3.2 | 5.7 ± 0.3 |
| RESV | 818.7 ± 41.9a | 83.4 ± 3.8a | 9.3 ± 0.3a |
Mean + SD.
p<0.01 (n=3) vs. vehicle.
Figure 1.
RESV-mediated increase in S1P is responsible for stimulation of CAMP expression. HaCaT KC pretreated with or without ceramidase (NOE, 25 µM) or SPHK (DMS, 2.5 µM; SKI, 1 µM) inhibitors for 30 mins were incubated exogenous RESV (50 µM or as indicated) for 24 h. Cell viability (A) or PARP cleavage as a measure of apoptosis (B). CAMP mRNA expression assessed by qRT-PCR (C and D). CAMP and LL-37 (an active form of CAMP) protein/peptide levels quantified by Western immunoblot analysis (E) and ELISA, respectively (F and G). Similar results were obtained when the experiment was repeated (triplicate) using different cell preparations.
We next determined whether exogenous RESV stimulates CAMP expression in vitro. Quantitative RT-PCR (qRT-PCR) analysis revealed a significant increase in CAMP mRNA expression in KC after 24 h of exposure to RESV (Figs. 1C and 1D). Consistent with these alterations in CAMP mRNA, Western immunoblot analysis showed that CAMP protein levels also increased following RESV treatment (Fig. 1E). Finally, ELISA analysis of culture supernatant further demonstrated that RESV also enhanced LL-37/CAMP secretion from KC (Figs 1F and 1G). Together, these results indicate that RESV elevates cellular Cer and S1P levels, in parallel with an increase in CAMP/LL-37 production and secretion.
Increased S1P accounts for RESV-mediated enhancement of CAMP production
Our previous studies demonstrated that S1P, but neither Cer nor sphingosine, accounts for the ER-stress-induced increases in CAMP production (Park et al., 2012). Hence, we next investigated whether S1P is the Cer metabolite that accounts for the RESV-mediated upregulation of CAMP. Co-incubation of KC with RESV and N-oleoylethanolamine (NOE), a potent inhibitor of ceramidase, the hydrolytic enzyme that generates sphingosine from Cer, significantly attenuated the expected RESV-induced increase in CAMP mRNA and protein expression (Figs. 1C and 1E). In contrast, addition of NOE alone did not alter CAMP expression. Together, these results suggest that hydrolysis of Cer by ceramidase(s) is required for the RESV-induced stimulation of CAMP expression.
We next determined which distal metabolite of Cer; i.e., sphingosine and/or S1P, is (are) responsible for increased CAMP expression. Inhibition of the conversion of sphingosine to S1P, using dimethylsphingosine (DMS), did not stimulate, but instead significantly attenuated the expected RESV-induced increase in both CAMP mRNA and protein expression (Figs. 1D and 1E), strongly suggesting that sphingosine is not the responsible metabolite.
To elucidate whether S1P is the responsible signal, we next assessed whether RESV stimulates expression of the sphingosine kinase (SPHK1 isoform) that accounts for ER-stressstimulated CAMP expression (Park et al., 2012). Accordingly, qRT-PCR analysis revealed an increase in SPHK1 (1.96 ± 0.09 vs. vehicle control, p<0.01), but not SPHK2 (1.17 ± 0.06 vs. vehicle control), mRNA expression after incubation of KC with RESV, suggesting that RESV stimulates SPHK1 transcription. In contrast, pretreatment of KC with a specific inhibitor of SPHK1, SKI, significantly attenuated the expected RESV-induced stimulation of both CAMP production (Figs. 1D, 1E, and 1F) and secretion (Fig. 1G). Together, these results show that the RESV-induced enhancement of CAMP expression can be attributed to increased S1P generation, resulting from stimulation of SPHK1 expression.
RESV increases mCAMP expression in murine epidermis
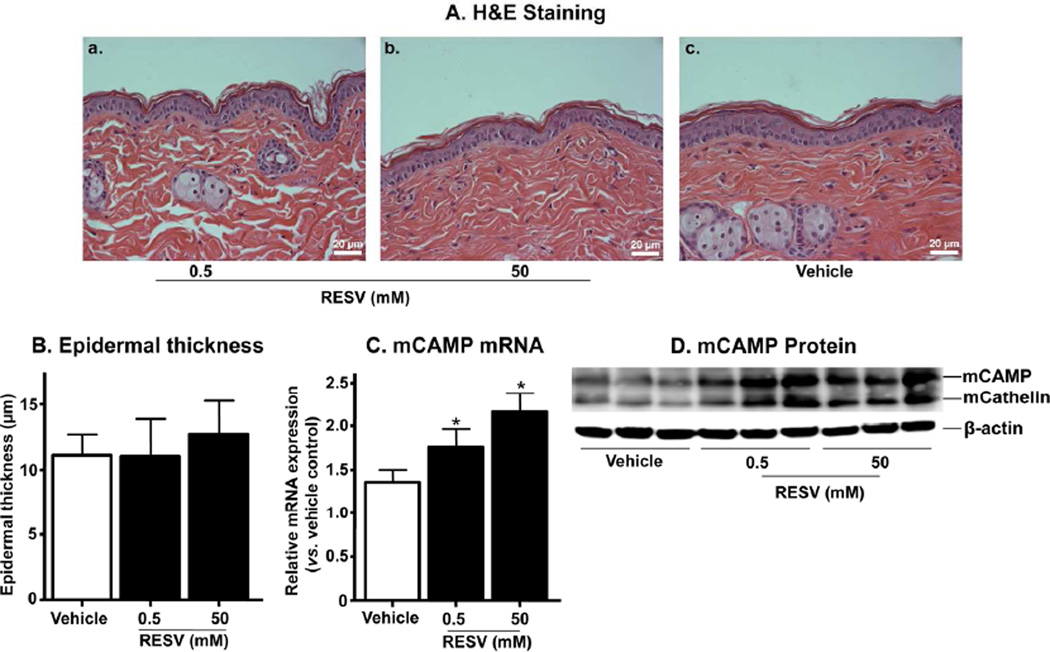
Prior studies showed that murine CAMP (mCAMP), the murine homologue of CAMP, increases in normal mouse skin following exposure to subtoxic levels of ER stress (Park et al., 2011), also via S1P-stimulated signaling (Park et al., 2012). Hence, we next investigated whether mCAMP expression increases in murine epidermis after topical RESV treatment. Morphological evidence of toxicity, such as changes in epidermal thickness or inflammation did not become evident in murine skin after treatment with topical RESV (0.5–50 mM [2.5–250 nmole/cm2]) (Figs. 2A and 2B). Yet at these doses, topical RESV increased both mCAMP mRNA and protein expression in murine epidermis (Figs. 2C and 2D). Together, these results validate our in vitro studies by showing that topical RESV also stimulates mCAMP production in epidermis.
Figure 2.
Topical RESV increases mCAMP expression in normal murine skin. The flanks of mice were treated with topical applications of RESV or vehicle (ethanol) alone. H&E staining (A). Epidermal thickness (B). mCAMP mRNA (C) and protein expression (D), respectively. Similar results were obtained when the experiment was repeated (in duplicate) using different skin preparations. Scare bar = 20 µm.
RESV-induced stimulation of CAMP expression requires NF-κB and C/EBPα activation
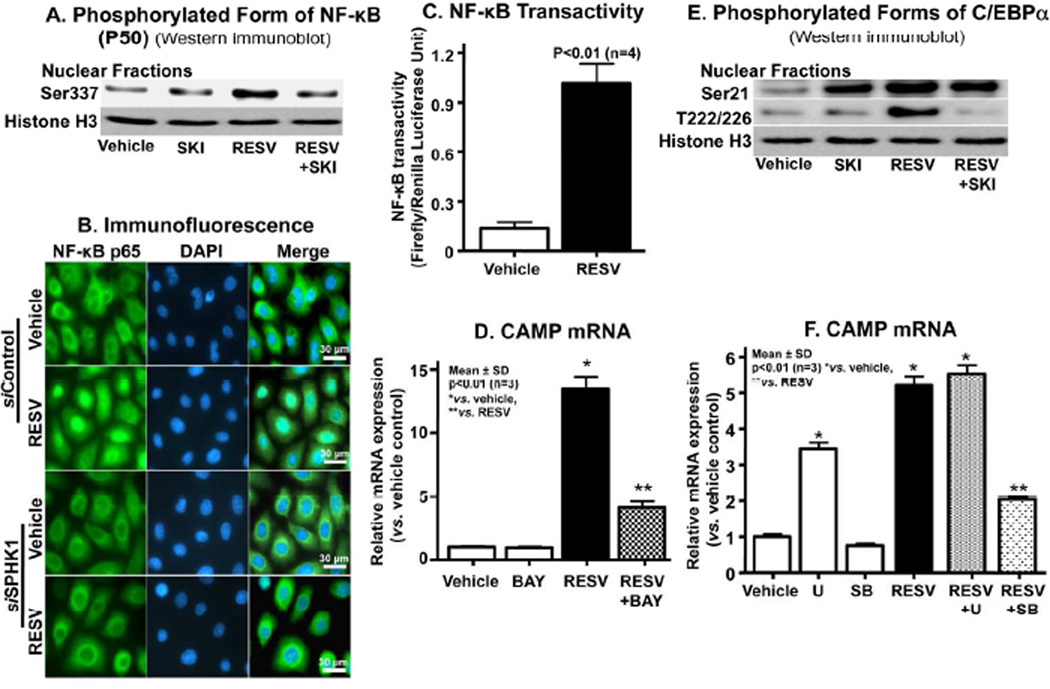
Our recent studies indicated that NF-κB-mediated C/EBPα activation is a downstream signal of increased S1P-dependent CAMP expression (Park et al., 2012). Therefore, we next assessed changes in NF-κB phosphorylation after RESV exposure. After incubations with RESV, but not vehicle alone, additional phosphorylated NF-κB1 (p50) levels increased, while conversely, blockade of the conversion of sphingosine to S1P by SKI attenuated the expected increase in phospho-NF-κB generation (Fig. 3A). In addition, the silencing of SPHK1 using siRNA significantly attenuated RESV-induced nuclear translocation of NF-κB (Fig. 3B). Moreover, an NF-κB reporter assay revealed a significant increase in NF-κB transactivity after treatment of KC with RESV (Fig. 3C), while conversely, inhibition of NF-κB activation, using a specific inhibitor of NF-κB, BAY11-7082, attenuated the RESV-induced upregulation of CAMP expression (Fig. 3D). Together, these results indicate that the RESV-induced increase in S1P activates NF-κB, leading to increased CAMP expression.
Figure 3.
NF-κB-C/EBPα activation is required for RESV-induced upregulation of CAMP expression. HaCaT KC were pretreated or transfected with/without a specific inhibitor of SPHK1, SKI (1 µM), NF-κB inhibitor (BAY11-7082, 2 µM), MAP kinase inhibitors ([U0126, ERK inhibitor], SB [SB201290, p38MAP kinase inhibitor]) or SPHK1-siRNA followed incubating with RESV (50 µM). Phosphorylated forms of NF-κB or C/EBPα (either Ser-21 or Thr-222/226) in nuclear fractions were assessed by Western immunoblot analysis (A and E). RESV-induced nuclear translocation of NF-κB was determined by immunohistochemistry (B). NF-κB transactivation was assessed using a luciferase reporter assay (C). CAMP mRNA (D and F). Similar results were obtained when the experiment was repeated (duplicate) using different cell preparations. Scare bar = 30 µm.
To further delineate the mechanism by which RESV increases CAMP production, we next assessed C/EBPα activation, a distal step in S1P-induced CAMP upregulation (Park et al., 2012), assessed by C/EBPα phosphorylation (at both Ser-21 and Thr-222/226). After incubation with RESV, Western immunoblot analysis of C/EBPα demonstrated increased phosphorylation of both the Ser-21 and Thr-222/226 sites in a KC nuclear fraction (Fig. 3E). In contrast, the addition of SKI selectively decreased the phosphorylation of Thr-222/226 sites, while the Ser-21 phosphorylation site remained unaffected (Fig. 3E). These results suggest that C/EBPα phosphorylation serves as a key downstream transcriptional signal for the RESV-stimulated CAMP production (Fig. 5).
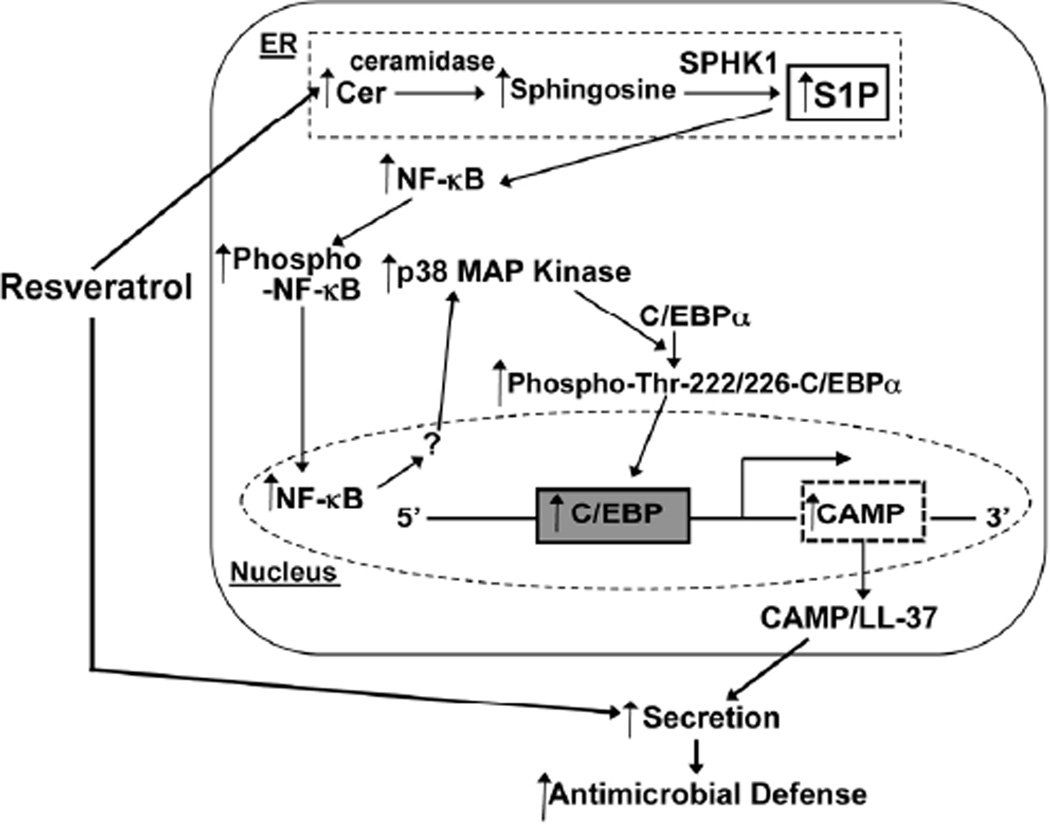
Figure 5.
Proposed mechanism of RESV-mediated stimulation of CAMP production in KC. RESV stimulates cellular Cer production and also increases Cer metabolic conversion to sphigosine and then SIP by SPHKI (but not SPHK2). SIP activates NF-κB-phosphorylation, leading to translocation of phospho-NF-κB to nucleus. NF-κB increases p38MAP kinase activation that stimulates C/EBPα by its phosphorylation of Thr 222/226, rather than Ser-21 sites. C/EBPα binds to the 5'-upstream promoter region of CAMP to upregulate CAMP expression. RESV also stimulates CAMP/LL-37 secretion from cells, resulting in enhanced antimicrobial defense in epidermis.
Our prior studies showed that p38 mitogen-activated protein (MAP) kinases, which phosphorylates C/EBPα, also is required for the activation of C/EBPα in response to ER stress (Park et al., 2011). Accordingly, a p38MAP kinase-specific inhibitor, SB201290, significantly diminished the expected RESV-induced increase in CAMP mRNA expression (Fig. 3F). In contrast, an inhibitor of ERK (extracellular-signal regulated kinase) (U0126) did not attenuate the RESV-induced increase in CAMP expression. Together, these results show that not only C/EBPα phosphorylation, but also MAP kinase are required for RESV-stimulated increase in CAMP production.
RESV treatment increases epithelial defense against S. aureus
Finally, we assessed the functional relevance of these mechanistic studies by asking whether RESV-induced stimulation of CAMP production in vitro enhances antimicrobial defense against a virulent microbial pathogen, i.e., S. aureus (ΔmprF strain). Conditioned media, collected from KC previously treated with RESV, but neither media alone, nor media treated with vehicle, suppressed S. aureus growth (Fig. 4A). Pertinently, the potency of RESV was comparable to the levels of growth inhibition achieved with exogenous synthetic LL-37 (0.05 mM [250 pmole/cm2]), which served as a positive control in this experiments (Fig. 4C).
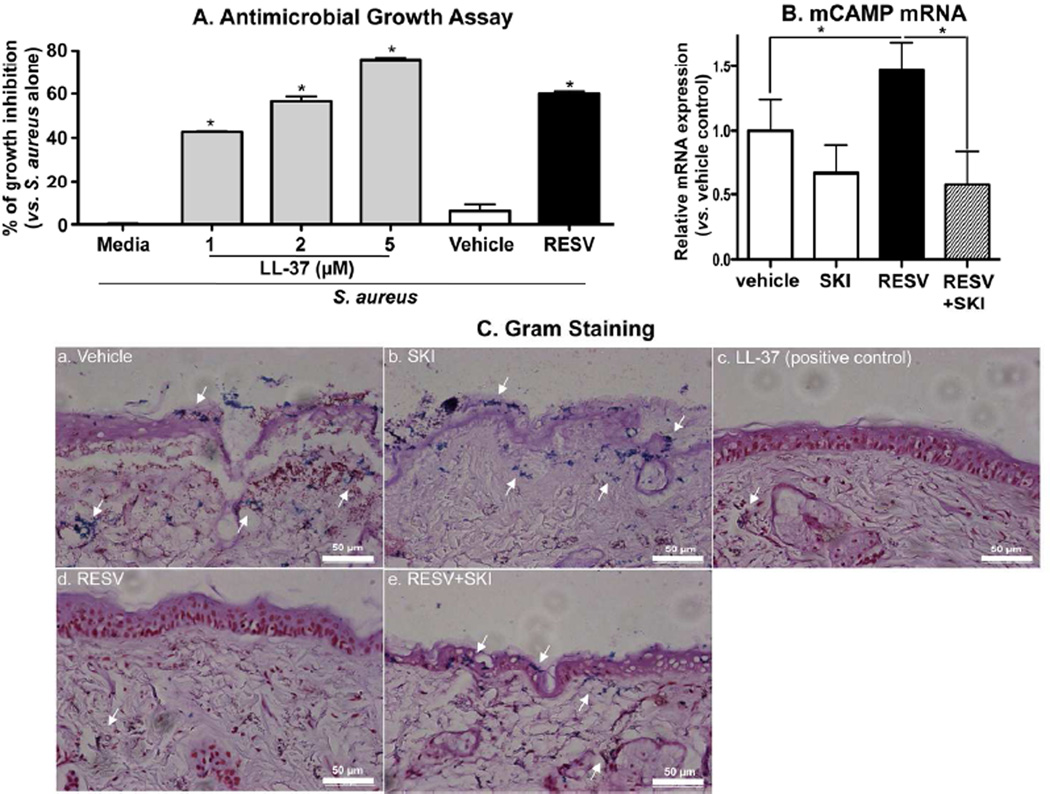
Figure 4.
RESV enhanced antimicrobial defense through increased CAMP production: Growth inhibition of S. aureus. S. aureus were incubated for the indicated times with LL-37 or conditioned medium of HaCaT KC treated with RESV (50 µM) (A). Bacterial invasion studies (ex vivo) (B and C): S. aureus were epicutaneously applied to a full-thickness pieces of murine skin (n=2) treated with RESV (50 mM [250 nmole/cm2]), SKI (1 µM), LL-37 RESV (50 µM [250 pmole/cm2]) and/or vehicle twice daily for 3 days, followed by incubation for 24 h at 37°C. CAMP mRNA expression in skin was assessed by qRT-PCR (B). Bacterial invasion/growth into murine skin was assessed by gram staining (counter staining with H&E) (C). Scare bar = 50 µm.
Finally, we investigated whether murine skin, pretreated with topical RESV, blocks the invasion of virulent S. aureus. Since normal epidermis, with its competent permeability and antimicrobial barrier, prevents S. aureus invasion, we also assessed invasions into skin where barrier had been compromised by topical oxazolone. Again topical RESV significantly increased mCAMP mRNA expression, and this induction was blocked by coapplications of a specific sphingosine kinase inhibitor, SKI (Fig. 4B). Gram staining showed that S. aureus invaded deeply into vehicle and SKI-treated skin (indicated by arrows in Fig. 4C). In contrast, as in our prior that showed increased epidermal S1P production by topical C2Cer (Park et al., 2012), murine skin that had been treated with topical RESV resisted S. aureus invasion, while conversely, S. aureus invasion become evident in skin cotreated with RESV plus SKI (Fig. 4C). Together, these results indicate that the RESV-stimulated increase in CAMP production and secretion generates sufficient mCAMP to interdict highly-virulent bacterial pathogen.
DISCUSSION
The plant stilbenoid, RESV, has multiple, potentially-beneficial biological activities, not only as an antioxidant due to its stilbene structure (Sadruddin and Arora, 2009; Sundaresan et al., 2011), but also as an; i) activator of SIRT 1 coding of the NAD-dependent deacetylase, sirtuin-1; ii) inhibitor of proinflammatory cell adhesion molecule expression; iii) stimulator of endolethelial nitric oxide synthase (eNOS) activity; and iv) inhibitor of platelet aggregation. Because RESV can modulate multiple cellular functions, its activity is being explored in multiple clinical settings, including as an anti-inflammatory agent, immune modulator, for treatment of metabolic syndrome, and to prevent cellular senescence (Baile et al., 2011; Mercken et al., 2012; Szkudelski and Szkudelska, 2011). Our studies demonstrate an additional potential clinical niche for RESV; i.e., as a potent modulator of innate immunity through stimulation of CAMP production. While high levels of ER stress stimulates induced apoptosis, at lower, subtoxic levels, Cer is effectively metabolized to S1P, a mechanism that rescues KC from cell death (Uchida et al., 2010), while simultaneously stimulating CAMP production (Park et al., 2012). Since apoptosis was not evident in our present studies, a similar S1P-mediated rescue mechanism likely accounts for the stimulation of CAMP production by RESV.
Our prior studies demonstrated that non-toxic levels of external perturbation; i.e, subtoxic UVB irradiation, as well as ER stress itself increase Cer production, followed by the metabolic conversion of Cer to S1P leading to stimulation of CAMP production (Park et al., 2012; Uchida et al., 2010). In these studies, we show that RESV exploits this pathway by directly increasing cellular Cer levels. Pertinently, RESV increases catalytic activity, but not mRNA expression of serine palmitoyl transferase (SPT) (Scarlatti et al., 2003), the enzyme that catalyzes the initial step in de novo Cer synthesis. Since in the present study we also found that RESV did not stimulate mRNA levels for either SPT1 or SPT2 (not shown), RESV likely stimulates catalytic activity of SPT. Increased Cer then is followed by accelerated production of S1P with SPHK1, but not SPHK2, accounting for the enhanced metabolic conversion of sphingosine to S1P production by RESV. RESV like ER stressors appears to increase S1P production by stimulation of SPHK1 mRNA expression (Park et al., 2012). Finally, we demonstrated further that RESV upregulates CAMP production through the same S1P→NF-κB→C/EBPα mechanism that we recently characterized in epithelial cells, including both human and murine KC (Park et al., 2012) (Fig. 5).
To assess the potential relevance of these findings, we then showed that the RESV-induced increase CAMP production enhances antimicrobial defense both (in cultured KC) and murine skin exposed to exogenous S. aureus. Yet, stilbene derivatives, including RESV, display direct antimicrobial activity against pathogens, such as S. aureus, Enterococcus faecalis, and Psedomonas aeruginosa (Chan, 2002). Moreover, the sphingoid base itself is a potent antimicrobial cationic lipid (Bibel et al., 1992). However, we showed that inhibition of S1P production attenuates antimicrobial activity against S. aureus (Fig. 4). Hence, increased CAMP by RESV stimulation of S1P production likely accounts for enhanced antimicrobial defense in KC.
As noted above, RESV is a potent activator of sirtuin (Sirt) 1, a nicotinamide adenine dinucleotide (NAD+)-dependent deacetylase (a class III histone deacetylase [HDAC]). Although histone 4 acetylation is required for induction of CAMP expression by the VDR-dependent mechanism (Schauber et al., 2008), Sirt1 does not preferentially catalyze histone 4, suggesting that RESV unlikely attenuates the VDR pathway. Moreover, Sirt1 deacetylase transcription factors, including forkhead box O1 (FoxO1), stimulated by RESV leads to an increase in transcription of target gene(s) by recruitment and formation of a complex with C/EBPα (Qiao and Shao, 2006). Hence, while C/EBPα is responsible for downstream stimulation of S1Pmediated CAMP production, RESV-induced Sirt activation could additively stimulate CAMP expression.
In summary, we demonstrate that previously unidentified important biological role of RESV, i.e., RESV enhances antimicrobial defense through an innate immune element, CAMP via stimulation of S1P-mediated NF-κB→C/EBPα mechanism, in epidermis.
MATERIALS AND METHODS
Cell culture
Immortalized, nontransformed (HaCaT) KC, derived from human epidermis (a gift from Dr. N. Fusenig [Heidelberg, Germany]) were grown as described previously (Uchida et al., 2002). Culture medium was switched to serum-free KC growth medium containing 0.07 mM calcium chloride and growth supplements (Invitrogen, Carlsbad, CA) one day prior to resveratrol (RESV) treatment. Cell toxicities, including apoptosis were assessed by poly(ADP-ribose) polymerase (PARP) cleavage as well as a viability assay kit (CCK-8) (Dojindo, Rockville, MD) based on WST-8 formazan dye (Uchida et al., 2010, Fuda, 2007).
Animal experiments
Female hairless (hr/hr) mice, aged 6–8 weeks old, were purchased from Charles River laboratories (Wilmington, MA, USA) and maintained in a temperature- and humidity-controlled room, and given standard laboratory food and tab water ad libitum (under an Institutional Animal Care and Use Committee [San Francisco Veterans Administration Medical Center]-approved protocol). Mice were treated topically on the flanks with RESV (0.5–50 mM [2.5–250 nmole /cm2]) or vehicle (ethanol) alone twice dailies for 3 days. The Change in the overall morphology was assessed by Hematoxylin & Eosin (H&E) staining, as reported (Park et al., 2011).
Measurement of intercellular levels of sphingolipids
The levels of cellular Cer, sphingosine, and S1P were quantitated using an HPLC system equipped with a fluorometrical detector system (JASCO, Tokyo, Japan), as described previously (Park et al., 2011). Sphingolipids are expressed as pmol per mg protein.
Quantitative real-time polymerase chain reaction analysis
Quantitative real-time polymerase chain reaction (qRT-PCR) was performed using cDNA prepared from mRNA fractions of cell lysates, as described previously (Park et al., 2011; Uchida et al., 2010) mRNA expression was normalized to levels of GAPDH.
Western immunoblot analysis
Western immunoblot analysis was performed as described previously (Park et al., 2011). The following antibodies were used: Anti-human or -mouse β-actin (Abcam, Cambrige, MA) antibody, anti-Cathelicidin (CAMP) (LifeSpan Biosciences, Seattle, WA), anti-phospho NF-κB1 (p50) or anti-NF-κB1 p65 (Santa Cruz Biotech, Santa Cruz, CA), anti-Histone H3, antiphosphoSer-21 or -phosphoThr-222/226 C/EBPα (Cell Signaling, Boston, MA), or anti-PARP (Sciences, Franklin Lakes, NJ).
ELISA for LL-37 quantification
LL-37 content of cell lysates and conditioned medium of KC incubated RESV was determined by ELISA kit (Hycult Biotech Inc., Plymouth Meeting, PA) in accordance with the manufacture’s instructions.
siRNA transfections and immunohistochemistry
HaCaT KC were transfected with 20 nM siRNA for SPHK1 (Invitrogen), as previously (Park et al., 2011). Cells were treated with RESV or vehicle for 60 min. NF-κB is distributed using anti-NF-κB p65 (Santa Cruz Biotech.) and anti-rabbit IgG conjugated with fluorescein isothiocyanate (Invitrogen). Cells were counterstained with the nuclear marker 4′,6-diaminido-2-phenylindole (DAPI) (Vector Laboratories, Burlingame, CA).
Dual-luciferase reporter assay for NF-κB transcriptional activity
Transcriptional activities of NF-κB was assessed using a Reporter Kits (SABiosciences, Frederick, MD), as described previously (Park et al., 2011).
Antimicrobial assay
Antimicrobial activity of conditioned medium collected from KC against Staphylococcus (S.) aureus (ΔmprF strain) was assessed as described previously (Bernard and Gallo, 2010).
S. aureus invasion assay
S. aureus invasion assay was performed, as we reported previously (Park et al., 2011). Briefly, full-thickness pieces of murine skin treated with RESV, SKI, and/or vehicle (propylene glycol: ethanol, 7:3) were harvested from hairless mice (24 week-old, female, hr/hr, n=5) under an Institutional Animal Care and Use Committee (San Francisco Veterans Affairs Medical Center)-approved protocol. Epidermal permeability barrier was attenuated by topical application of topical oxazolone (1%) once every other day for five times. Mice were treated with resveratrol twice dailies for the last three days, during the last three oxazolone treatments, prior to harvesting skin. Skin was placed on filter paper dermis side down, and maintained at the airmedium interface in KC growth medium (as above). S. aureus (ISP479C) (Siboo et al., 2001) in PBS or PBS was epicutaneously applied (20 µl/cm2), followed by incubation for 24 h at 37°C in 5% CO2 in air. Gram staining was performed to assess S. aureus invasion (Park et al., 2011).
Statistical Analyses
Statistical comparisons were performed using an unpaired Student t Test.
Acknowledgments
The authors thank Drs. Paul M. Sullam and Ho Seong Seo (Northern California Institute For Research and Education and Veterans Affairs Medical Center, San Francisco, California) for technical support and advice in bacterial invasion studies. The authors thank to Ms. Sally Pennypacker for technical support in cell culture. We thank Ms. Joan Wakefield superb editorial assistance. This study was supported by National Rosacea Society and National Institutes of Health Grant AR051077 and AR062025 (the National Institute of Arthritis and Musculoskeletal and Skin Diseases) (to YU). The content is solely the responsibility of the authors and does not necessarily represent the official views of the National Institutes of Health.
Abbreviations
- AMP
antimicrobial peptide
- CAMP
human cathelicidin antimicrobial protein
- CRAMP
cathelin-related antimicrobial peptide
- Cer
ceramide
- ER
endoplasmic reticulum
- KC
keratinocytes
- RESV
resveratrol
- qRT-PCR
quantitative real-time polymerase chain reaction
- S1P
sphingosine-1-phosphate
- VDR
vitamin D receptor
Footnotes
Conflict of Interest
The authors state no conflict of interest.
REFERENCES
- Aberg KM, Man MQ, Gallo RL, Ganz T, Crumrine D, Brown BE, et al. Co-regulation and interdependence of the mammalian epidermal permeability and antimicrobial barriers. J Invest Dermatol. 2008;128:917–925. doi: 10.1038/sj.jid.5701099. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baile CA, Yang JY, Rayalam S, Hartzell DL, Lai CY, Andersen C, et al. Effect of resveratrol on fat mobilization. Annals of the New York Academy of Sciences. 2011;1215:40–47. doi: 10.1111/j.1749-6632.2010.05845.x. [DOI] [PubMed] [Google Scholar]
- Bernard JJ, Gallo RL. Cyclooxygenase-2 enhances antimicrobial peptide expression and killing of Staphylococcus aureus. J Immunol. 2010;185:6535–6544. doi: 10.4049/jimmunol.1002009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bibel DJ, Aly R, Shinefield HR. Antimicrobial activity of sphingosines. J Invest Dermatol. 1992;98:269–273. doi: 10.1111/1523-1747.ep12497842. [DOI] [PubMed] [Google Scholar]
- Cakir Z, Saydam G, Sahin F, Baran Y. The roles of bioactive sphingolipids in resveratrol-induced apoptosis in HL60: acute myeloid leukemia cells. J Cancer Res Clin Oncol. 2011;137:279–286. doi: 10.1007/s00432-010-0884-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chan MM. Antimicrobial effect of resveratrol on dermatophytes and bacterial pathogens of the skin. Biochem Pharmacol. 2002;63:99–104. doi: 10.1016/s0006-2952(01)00886-3. [DOI] [PubMed] [Google Scholar]
- Charruyer A, Bell SM, Kawano M, Douangpanya S, Yen TY, Macher BA, et al. Decreased ceramide transport protein, cert, function alters sphingomyelin production following UVB irradiation. J Biol Chem. 2008;283:16682–16692. doi: 10.1074/jbc.M800799200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dolfini E, Roncoroni L, Dogliotti E, Sala G, Erba E, Sacchi N, et al. Resveratrol impairs the formation of MDA-MB-231 multicellular tumor spheroids concomitant with ceramide accumulation. Cancer Lett. 2007;249:143–147. doi: 10.1016/j.canlet.2006.08.013. [DOI] [PubMed] [Google Scholar]
- Dunn C, Peppa D, Khanna P, Nebbia G, Jones M, Brendish N, et al. Temporal analysis of early immune responses in patients with acute hepatitis B virus infection. Gastroenterol. 2009;137:1289–1300. doi: 10.1053/j.gastro.2009.06.054. [DOI] [PubMed] [Google Scholar]
- Garg SK, Volpe E, Palmieri G, Mattei M, Galati D, Martino A, et al. Sphingosine 1-phosphate induces antimicrobial activity both in vitro and in vivo. J Infect Dis. 2004;189:2129–2138. doi: 10.1086/386286. [DOI] [PubMed] [Google Scholar]
- Gombart AF, Borregaard N, Koeffler HP. Human cathelicidin antimicrobial peptide (CAMP) gene is a direct target of the vitamin D receptor and is strongly up-regulated in myeloid cells by 1,25-dihydroxyvitamin D3. FASEB J. 2005;19:1067–1077. doi: 10.1096/fj.04-3284com. [DOI] [PubMed] [Google Scholar]
- Hong SP, Kim MJ, Jung MY, Jeon H, Goo J, Ahn SK, et al. Biopositive effects of lowdose UVB on epidermis: coordinate upregulation of antimicrobial peptides and permeability barrier reinforcement. J Invest Dermatol. 2008;128:2880–2887. doi: 10.1038/jid.2008.169. [DOI] [PubMed] [Google Scholar]
- Kim BJ, Rho YK, Lee HI, Jeong MS, Li K, Seo SJ, et al. The effect of calcipotriol on the expression of human beta defensin-2 and LL-37 in cultured human keratinocytes. Clin & Develop Immunol. 2009;2009:645898. doi: 10.1155/2009/645898. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lai Y, Cogen AL, Radek KA, Park HJ, Macleod DT, Leichtle A, et al. Activation of TLR2 by a small molecule produced by Staphylococcus epidermidis increases antimicrobial defense against bacterial skin infections. J Invest Dermatol. 2010;130:2211–2221. doi: 10.1038/jid.2010.123. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lei X, Zhang S, Bohrer A, Ramanadham S. Calcium-independent phospholipase A2 (iPLA2 beta)-mediated ceramide generation plays a key role in the cross-talk between the endoplasmic reticulum (ER) and mitochondria during ER stress-induced insulin-secreting cell apoptosis. J Biol Chem. 2008;283:34819–34832. doi: 10.1074/jbc.M807409200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallbris L, Carlen L, Wei T, Heilborn J, Nilsson MF, Granath F, et al. Injury downregulates the expression of the human cathelicidin protein hCAP18/LL-37 in atopic dermatitis. Exp Dermatol. 2010;19:442–449. doi: 10.1111/j.1600-0625.2009.00918.x. [DOI] [PubMed] [Google Scholar]
- Mendez-Samperio P. The human cathelicidin hCAP18/LL-37: a multifunctional peptide involved in mycobacterial infections. Peptides. 2010;31:1791–1798. doi: 10.1016/j.peptides.2010.06.016. [DOI] [PubMed] [Google Scholar]
- Mercken EM, Carboneau BA, Krzysik-Walker SM, de Cabo R. Of mice and men: the benefits of caloric restriction, exercise, and mimetics. Ageing research reviews. 2012;11:390–398. doi: 10.1016/j.arr.2011.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Nijnik A, Hancock RE. The roles of cathelicidin LL-37 in immune defences and novel clinical applications. Curr Opin Hematol. 2009;16:41–47. doi: 10.1097/moh.0b013e32831ac517. [DOI] [PubMed] [Google Scholar]
- Park K, Elias PM, Oda Y, Mackenzie D, Mauro T, Holleran WM, et al. Regulation of Cathelicidin Antimicrobial Peptide Expression by an Endoplasmic Reticulum (ER) Stress Signaling, Vitamin D Receptor-independent Pathway. J Biol Chem. 2011;286:34121–34130. doi: 10.1074/jbc.M111.250431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park K, Elias PM, Shin KO, Lee YM, Hupe M, Borkowski AW, et al. A Novel Role of Lipid Species, Sphingosine-1-Phosphate, in Epithelial Innate Immunity. Molecular and cellular biology. 2012 doi: 10.1128/MCB.01103-12. http://www.ncbi.nlm.nih.gov/pubmed/23230267. [DOI] [PMC free article] [PubMed]
- Qiao L, Shao J. SIRT1 regulates adiponectin gene expression through Foxo1-C/enhancer-binding protein alpha transcriptional complex. J Biol Chem. 2006;281:39915–39924. doi: 10.1074/jbc.M607215200. [DOI] [PubMed] [Google Scholar]
- Sadruddin S, Arora R. Resveratrol: biologic and therapeutic implications. J Cardiometab Syndr. 2009;4:102–106. doi: 10.1111/j.1559-4572.2008.00039.x. [DOI] [PubMed] [Google Scholar]
- Scarlatti F, Sala G, Somenzi G, Signorelli P, Sacchi N, Ghidoni R. Resveratrol induces growth inhibition and apoptosis in metastatic breast cancer cells via de novo ceramide signaling. Faseb J. 2003;17:2339–2341. doi: 10.1096/fj.03-0292fje. [DOI] [PubMed] [Google Scholar]
- Schauber J, Oda Y, Buchau AS, Yun QC, Steinmeyer A, Zugel U, et al. Histone acetylation in keratinocytes enables control of the expression of cathelicidin and CD14 by 1,25-dihydroxyvitamin D3. J Invest Dermatol. 2008;128:816–824. doi: 10.1038/sj.jid.5701102. [DOI] [PubMed] [Google Scholar]
- Schroder JM. The role of keratinocytes in defense against infection. Curr Opin Infect Dis. 2010;23:106–110. doi: 10.1097/QCO.0b013e328335b004. [DOI] [PubMed] [Google Scholar]
- Shakibaei M, Harikumar KB, Aggarwal BB. Resveratrol addiction: to die or not to die. Mol Nutr Food Res. 2009;53:115–128. doi: 10.1002/mnfr.200800148. [DOI] [PubMed] [Google Scholar]
- Siboo IR, Cheung AL, Bayer AS, Sullam PM. Clumping factor A mediates binding of Staphylococcus aureus to human platelets. Infect Immun. 2001;69:3120–3127. doi: 10.1128/IAI.69.5.3120-3127.2001. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Signorelli P, Munoz-Olaya JM, Gagliostro V, Casas J, Ghidoni R, Fabrias G. Dihydroceramide intracellular increase in response to resveratrol treatment mediates autophagy in gastric cancer cells. Cancer Lett. 2009;282:238–243. doi: 10.1016/j.canlet.2009.03.020. [DOI] [PubMed] [Google Scholar]
- Sundaresan NR, Pillai VB, Gupta MP. Emerging roles of SIRT1 deacetylase in regulating cardiomyocyte survival and hypertrophy. J Mol Cell Cardiol. 2011;51:614–618. doi: 10.1016/j.yjmcc.2011.01.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szkudelski T, Szkudelska K. Anti-diabetic effects of resveratrol. Annals of the New York Academy of Sciences. 2011;1215:34–39. doi: 10.1111/j.1749-6632.2010.05844.x. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Houben E, Park K, Douangpanya S, Lee YM, Wu BX, et al. Hydrolytic pathway protects against ceramide-induced apoptosis in keratinocytes exposed to UVB. J Invest Dermatol. 2010;130:2472–2480. doi: 10.1038/jid.2010.153. [DOI] [PubMed] [Google Scholar]
- Uchida Y, Murata S, Schmuth M, Behne MJ, Lee JD, Ichikawa S, et al. Glucosylceramide synthesis and synthase expression protect against ceramide-induced stress. J Lipid Res. 2002;43:1293–1302. [PubMed] [Google Scholar]