Abstract
Lesions of the cerebral white matter (WM) result in focal neurobehavioral syndromes, neuropsychiatric phenomena, and dementia. The cerebral WM contains fiber pathways that convey axons linking cerebral cortical areas with each other and with subcortical structures, facilitating the distributed neural circuits that subserve sensorimotor function, intellect, and emotion. Recent neuroanatomical investigations reveal that these neural circuits are topographically linked by five groupings of fiber tracts emanating from every neocortical area: (1) cortico-cortical association fibers; (2) corticostriatal fibers; (3) commissural fibers; and cortico-subcortical pathways to (4) thalamus and (5) pontocerebellar system, brain stem, and/or spinal cord. Lesions of association fibers prevent communication between cortical areas engaged in different domains of behavior. Lesions of subcortical structures or projection/striatal fibers disrupt the contribution of subcortical nodes to behavior. Disconnection syndromes thus result from lesions of the cerebral cortex, subcortical structures, and WM tracts that link the nodes that make up the distributed circuits. The nature and the severity of the clinical manifestations of WM lesions are determined, in large part, by the location of the pathology: discrete neurological and neuropsychiatric symptoms result from focal WM lesions, whereas cognitive impairment across multiple domains—WM dementia—occurs in the setting of diffuse WM disease. We present a detailed review of the conditions affecting WM that produce these neurobehavioral syndromes, and consider the pathophysiology, clinical effects, and broad significance of the effects of aging and vascular compromise on cerebral WM, in an attempt to help further the understanding, diagnosis, and treatment of these disorders.
Keywords: fiber tracts, neuropsychiatry, cognition, demyelination, vascular dementia
The cerebral white matter (WM) was considered in antiquity to be the seat of all sensations, movements, and intellect. It was relegated to relative obscurity as the cerebral cortex ascended to prominence, and cerebral cortical association areas, in particular, came to be regarded as the substrates for cognition.1–3 These notions have required revision. Neurobehavioral disconnection syndromes occur after lesions of selected fiber bundles4,5; dementia can result from lesions confined to the cerebral WM6; and it has become apparent that all neurological function is subserved by distributed neural circuits, in which geographically distant regions in cortical and subcortical nodes are linked together by axonal connections conveyed in the fiber pathways that constitute the cerebral WM.4,5,7–14
Knowledge of the anatomical, functional, and clinical relevance of the WM is thus integral to the understanding of neurological and neuropsychiatric disease. This development is further emphasized by the rapid evolution in magnetic resonance imaging (MRI) techniques that makes it possible to visualize fiber pathways in humans in health and disease.15–19 Here we present an overview of essential anatomy of the cerebral WM; survey several diseases in which the pathology is principally or commonly confined to it; and discuss the clinical manifestations of WM disorders, with an emphasis on neurobehavioral impairments.
Neuroanatomy of WM Pathways
Historical Background
Galen’s (AD 129–130 to 200–201) identification of the corpus callosum20 was perhaps the first recognition of a major fiber bundle, but it was not until the scientific renaissance of the 17th century that it became apparent that the WM was not an amorphous mass but rather consisted of distinct fibers.2,3,21 The gross dissection methodology of investigators in the 19th century led to the identification of distinct fiber fascicles22,23 and the recognition that these bundles could be considered association, projection, or commissural in nature.2,3,24–26 The clinical relevance of association pathways was introduced by Carl Wernicke’s (1848–1900) description of conduction aphasia from what he believed to be the arcuate fasciculus,27 and Joseph Jules Dejerine’s (1849–1917) account of alexia without agraphia from lesions that involved the left occipital pole in addition to the splenium of the corpus callosum.28 Disconnection syndromes were first emphasized in the modern era by Norman Geschwind (1926–1984)4,5 and provided clinical and neuroanatomical impetus to the emergence of behavioral neurology as a discipline. The distributed neural circuitry notion has become fundamental to the understanding of the nervous system in health and disease. It provides a conceptual underpinning to the observation of neurobehavioral deficits that arise not only from cortical lesions but also from lesions of basal ganglia, thalamus, and cerebellum, as well as from the fiber tracts that link cortical areas with each other and with the subcortical nodes.2,29
Organizational Principles
To understand the effects of WM lesions on neurological function, including cognitive and neuropsychiatric impairments, it is essential to know the anatomy of the fiber tracts that it contains. These tracts are aggregations of axons running in close apposition to each other, sharing common cortical and/or subcortical origins and destinations. The great complexity of connections and pathways arising from the cerebral cortex can be reduced to a relatively simple schema (Fig. 1). There is a general principle of brain organization2 that every area of the neocortex is linked with other cortical and subcortical areas by pathways grouped into five fiber bundles, identified as follows.
Figure 1.

Diagram (A) and schema (B) of the principles of organization of white matter fiber pathways emanating from the cerebral cortex. Long association fibers are seen end-on as the stippled area within the white matter of the gyrus. In their course, these fibers either remain confined to the white matter of the gyrus or travel deeper in the white matter of the hemisphere. Short association fibers, or U-fibers, link adjacent gyri. Neighborhood association fibers link nearby regions, usually within the same lobe. Striatal fibers intermingle with the association fibers early in their course, before coursing in the subcallosal fascicle of Muratoff or in the external capsule. Cord fibers segregate into commissural fibers that arise in cortical layers II and III, and the subcortical bundle, which further divides into fibers destined for thalamus arising from cortical layer VI, and those to brain stem and spinal cord in the pontine bundle arising from cortical layer V.2
Association fibers travel to other ipsilateral cortical areas.
Striatal fibers course to the basal ganglia. There is a confluence of fibers (termed the cord) that divides into:
Commissural fibers that pass to the contralateral hemisphere, and another contingent of the cord, the subcortical bundle of projection fibers, that segregates into
Thalamic fibers, and
Pontine fiber fibers that descend to the diencephalon, pons, other brain stem structures, and/or the spinal cord.
We now elaborate on these five classes of fiber tracts and their putative functional properties, because this knowledge is useful when considering the clinical consequence of WM diseases. Many of these tract tracing observations2 are supported by MRI findings in monkey by using diffusion spectrum imaging30 and in human subjects by using diffusion tensor imaging (DTI19,31,32), probabilistic tractrography,33,34 and functional connectivity mapping.35,36 It is likely, therefore, that the observations in monkey will be in general agreement with the anatomical organization of these pathways in humans. See Figure 2.
Figure 2.

Course of the cingulum bundle (CB). (A) Surface views of the ventral (top), medial (middle), and lateral (lower) convexities of the cerebral hemisphere of a rhesus monkey show the trajectory of the CB reflected onto the cortical surface, and the cortical areas that it links, as determined by autoradiographic tract tracing.2 (B) CB fibers in the monkey are shown in this sagittal dimension by using diffusion spectrum magnetic resonance imaging (DSI). CB fibers that intersect a disc (shown by the arrow) course between rostral and caudal cingulate regions and link the cingulate gyrus with the prefrontal and parietal areas. Fibers in the ventral limb of the CB course to the parahippocampal region.30 (C) The course of the CB in human brain is demonstrated using diffusion tensor imaging (DTI), remarkably similar to the findings in monkey.19
Association Fiber Tracts
Association fibers travel to other cortical areas in the same hemisphere. Local association fibers, or U-fibers, travel to adjacent gyri, running immediately beneath the sixth layer. Neighborhood association fibers are directed to nearby regions and are distinguishable from U-fibers by their location. Long association fibers travel in discrete fascicles leading to distant cortical areas in the same hemisphere. These named fiber tracts are the essential anatomic substrates for the interdomain communication between cortical areas that subserve different behaviors, and these deserve particular emphasis (Fig. 3).
Figure 3.

Location of long association fiber pathways in the monkey. The coronal sections in (A) and (B) are taken at the corresponding levels shown on the figure of the lateral hemisphere (top). The fiber bundles are colored for ease of identification. Fiber pathways: AF, arcuate fasciculus; CBd, cingulum bundle dorsal component; CBv, cingulum bundle ventral component; EmC, extreme capsule; FOF, fronto-occipital fascicle; ILF, inferior longitudinal fascicle; MdLF, middle longitudinal fascicle; SLF (I, II, III), superior longitudinal fascicle, subcomponents I, II, and III; UF, uncinate fasciculus. Cerebral sulci: AS, arcuate sulcus; CS, central sulcus; Cing S, cingulate sulcus; IPS, intraparietal sulcus; LF, lateral fissure; PS, principal sulcus; OTS, occipitotemporal sulcus; STS, superior temporal sulcus.2
The superior longitudinal fasciculus (SLF) has three subcomponents.
SLF I lies medially situated in the WM of the superior parietal lobule and the superior frontal gyrus. It links the superior parietal region and adjacent medial parietal cortex in a reciprocal manner with the frontal lobe supplementary and premotor areas. It is thought to play a role in the regulation of higher aspects of motor behavior that require information about body part location, and it may contribute to the initiation of motor activity.
SLF II is more laterally situated and occupies a position in the central core of the hemisphere WM, lateral to the corona radiata and above the Sylvian fissure. It links the caudal inferior parietal lobule (equivalent in human to the angular gyrus) and the parieto-occipital areas, with the posterior part of the dorsolateral and mid-dorsolateral prefrontal cortex. It is thought to serve as the conduit for the neural system subserving visual awareness, the maintenance of attention, and engagement in the environment. It provides a means whereby the prefrontal cortex can regulate the focusing of attention within different parts of space.
SLF III is farther lateral and ventral and is located in the WM of the parietal and frontal operculum. It provides the ventral premotor region and pars opercularis with higher-order somatosensory input, may be crucial for monitoring orofacial and hand actions, and in the human it may be engaged in phonemic and articulatory aspects of language.
The arcuate fasciculus (AF) runs in the WM of the superior temporal gyrus and deep to the upper shoulder of the Sylvian fissure. By linking the caudal temporal lobe with the dorsolateral prefrontal cortex it may be viewed as an auditory spatial bundle, important for the spatial attributes of acoustic stimuli and auditory-related processing. The AF has historically been regarded as linking the posterior (Wernicke) and anterior (Broca) language areas in the human brain and to be involved in conduction aphasia. Our anatomical studies in monkey raise doubts about these anatomical and functional conclusions. This issue is not yet definitively resolved.
The extreme capsule is situated between the claustrum and the insular cortex caudally and between the claustrum and the orbital frontal cortex rostrally. In monkey, the extreme capsule is the principal association pathway linking the middle superior temporal region with the caudal parts of the orbital cortex and the ventral–lateral prefrontal cortex, including area 45. These areas are homologous to the Wernicke and Broca language cortices in human, and thus the extreme capsule (rather than the AF) may have an important role in language.
The middle longitudinal fasciculus (MdLF) is situated within the WM of the caudal inferior parietal lobule and extends into the WM of the superior temporal gyrus. It links several high-level association and paralimbic cortical areas, including the inferior parietal lobule, caudal cingulate gyrus, parahippocampal gyrus, and prefrontal cortex. In the human the MdLF may play a role in language, possibly imbuing linguistic processing with information dealing with spatial organization, memory, and motivational valence.
The uncinate fasciculus occupies the WM of the rostral part of the temporal lobe, the limen insula, and the WM of the orbital and medial frontal cortex. By connecting these temporal and prefrontal areas, the uncinate fasciculus may be a crucial component of the system that regulates emotional responses to auditory stimuli. It may also be involved in attaching emotional valence to visual information, is likely to be an important component of the circuit underlying recognition memory, and is implicated in cognitive tasks that are inextricably linked with emotional associations.37
The inferior longitudinal fasciculus (ILF) is in the WM between the sagittal stratum medially and the parieto-occipital and temporal cortices laterally. It has a vertical limb in the parietal and occipital lobes and a horizontal component contained within the temporal lobe. The ILF is the long association system of the ventral visual pathways in the occipitotemporal cortices. Visual agnosia and prosopagnosia are two clinical situations that may arise from ILF damage.
The fronto-occipital fasciculus (FOF) travels above the body and head of the caudate nucleus and the subcallosal fasciculus of Muratoff (Muratoff bundle [MB]), lateral to the corpus callosum and medial to the corona radiata. It links the parieto-occipital region with dorsal premotor and prefrontal cortices. The FOF is the long association system of the dorsomedial aspects of the dorsal visual stream, and it appears to be an important component of the anatomical substrates involved in peripheral vision and the processing of visual spatial information.
The cingulum bundle (CB) nestles in the WM of the cingulate gyrus. It links the rostral and caudal sectors of the cingulate gyrus with each other, as well as with the dorsolateral, orbital, and medial prefrontal cortices, and the parietal, retrosplenial and ventral temporal cortices (including the parahippocampal gyrus and entorhinal cortex). By virtue of these connections, the CB may facilitate the emotional valence inherent in somatic sensation, nociception, attention, motivation, and memory.2 Cingulectomy, and subsequently bilateral stereotaxic cingulotomy, has achieved the status of established management for certain forms of neuropsychiatric illness, such as obsessive–compulsive disorder, and for intractable pain.38–44
Striatal Fibers
Corticostriatal fibers to the caudate nucleus, putamen, and claustrum are conveyed mainly by the subcallosal fasciculus of Muratoff and the external capsule.
Muratoff Bundle (Subcallosal Fasciculus of Muratoff)
The MB is a semilunar condensed fiber system situated immediately above the head and body of the caudate nucleus. It conveys axons to the striatum principally from association and limbic areas, with some fibers also from the dorsal part of the motor cortex. (There has been confusion about the nature and location of the MB and the FOF. This issue has recently been clarified.2,45)
External Capsule
The external capsule lies between the putamen medially and the claustrum laterally. It conveys fibers from the ventral and medial pre-frontal cortex, ventral premotor cortex, pre-central gyrus, the rostral superior temporal region, and the inferotemporal and preoccipital regions. Projections from primary sensorimotor cortices are directed to the putamen; those from the supplementary motor area and association cortices terminate also in the caudate nucleus.
The MB and external capsule thus convey fibers from sensorimotor, cognitive, and limbic regions of the cerebral cortex to areas within the striatum in a topographically arranged manner. These corticostriatal pathways provide the critical links that enable different regions with the basal ganglia to contribute to motor control, cognition, and emotion.
Cord Fiber System
In addition to association and corticostriatal systems, every cortical region gives rise to a dense aggregation of fibers, termed the cord, which occupies the central core of the WM of the gyrus. The fibers in the cord separate into two distinct segments: a commissural system and projection fibers in the subcortical bundle.
Commissural Fibers
Anterior Commissure
The anterior commissure (AC) traverses the midline in front of the anterior columns of the fornix, above the basal forebrain and beneath the medial and ventral aspect of the anterior limb of the internal capsule. Its fibers link the caudal part of the orbital frontal cortex, the temporal pole, the rostral superior temporal region, the major part of the inferotemporal area, and the parahippocampal gyrus with their counterparts in the opposite hemisphere. In the nonhuman primate the AC is concerned with functional coordination across the hemispheres of highly processed information in the auditory and visual domains, particularly when imbued with mnemonic and limbic valence.
Corpus Callosum
We divide the corpus callosum (CC) into five equal sectors conveying fibers across the hemispheres from the following locations: (1) (rostrum and genu)—fibers from the prefrontal cortex, rostral cingulate region, and supplementary motor area; (2) premotor cortex; (3) ventral premotor region and the motor cortex (face representation most rostral, followed by the hand and the leg), and postcentral gyrus fibers behind the motor fibers; (4) posterior parietal cortex; (5) (splenium)—superior temporal fibers rostrally, inferotemporal and preoccipital fibers caudally. These comments regarding CC topography apply to the midsagittal plane.
Studies of the CC have led to novel understanding of the anatomic underpinnings of perception, attention, memory, language, and reasoning and provided insights into consciousness, self-awareness, and creativity.46–50 Knowledge of CC topography is relevant in the clinical context of callosal section for control of seizures.
Hippocampal Commissures
Three fiber systems link the ventral limbic and paralimbic regions across the hemispheres.
Anterior (uncal and genual) hippocampal fibers are conveyed in the ventral hippocampal commissure—those from the presubiculum, entorhinal cortex, and posterior parahippocampal gyrus in the dorsal hippocampal commissure. The hippocampal decussation conveys fibers from the body of the hippocampal formation to the contralateral septum.51
Projection Fibers
Projection (cortico-subcortical) fibers in the subcortical bundle are conveyed to their destinations via the internal capsule (anterior and posterior limbs) and the sagittal stratum. Each fiber system differentiates further as it progresses in the WM into two principal systems: one destined for thalamus, the other for brain stem and/or spinal cord.
Internal Capsule
The anterior limb of the internal capsule (ICa) conveys fibers from the prefrontal cortex, rostral cingulate region, and supplementary motor area (coursing through the genu of the capsule), principally to the thalamus, hypothalamus, and basis pontis.
The posterior limb of the internal capsule (ICp) conveys descending fibers from the premotor and motor cortices. Face, hand, arm, and leg fibers are arranged in a progressively caudal position. The ICp also conveys descending fibers from the parietal, temporal, and occipital lobes, and the caudal cingulate gyrus. These are topographically arranged within the capsule, in the rostral–caudal and superior–inferior dimensions.
Focal motor and sensory deficits follow infarction of the ICp, and complex behavioral syndromes result from lesions of the genu of the ICa and genu.52–54 Deficits include fluctuating alertness, inattention, memory loss, apathy, abulia, and psychomotor retardation, with neglect of contralateral space and visual–spatial impairment from lesions of the genu in the right hemisphere, and severe verbal memory loss after genu lesions on the left. Deep brain stimulation has been successfully applied to the ICa in some patients with obsessive–compulsive disorder55 and intractable pain.56
Sagittal Stratum
The sagittal stratum (SS) is a major cortico-subcortical WM bundle that conveys fibers from the parietal, occipital, cingulate, and temporal regions to thalamus, basis pontis, and other brain stem structures. It also conveys afferents principally from thalamus to cortex. The SS comprises an internal segment conveying corticofugal fibers efferent from the cortex and an external segment that contains incoming corticopetal fibers. The rostral sector of the SS corresponds to the anteriorly reflected fibers of the Flechsig–Meyer loop, whereas the ventral parts of the midsection of the SS contain the optic radiations and thalamic fibers of the caudal inferior temporal and occipitotemporal areas.
The SS is the equivalent of the internal capsule of the posterior part of the hemispheres. The functional implications are also analogous to those of the ICa and ICp. Whereas damage to the optic radiations in the ventral sector of the SS lead to hemianopsia, damage to the dorsal part of the SS may result in distortion of high-level visual information.
Thalamic Peduncles
Cortico-subcortical fibers enter the thalamus in locations determined by their site of origin. The afferent and efferent fibers between thalamus and cerebral cortex are arrayed around the thalamus and are collectively termed the thalamic peduncles.2
Intrinsic and Extrinsic Cerebellar WM Tracts
There are surprisingly few published details regarding the anatomical organization of cerebellar WM at the systems level, that is, which parts of the cerebellar WM convey afferent and efferent fibers to which specific cerebellar lobules. Further, it has long been suspected that nuclei in the rostral part of the basis pontis project via the middle cerebellar peduncle (MCP) to the posterior lobe of the cerebellum, and those in the caudal basis pontis project to the anterior cerebellum,57 but more precise information concerning MCP organization remains to be elucidated. Similarly, the degree to which there is anatomical and functional differentiation within the superior cerebellar peduncle efferents to thalamus is not presently known. There appears to be topographical organization of function within motor, cognitive, and affective domains in cerebellum,58,59 and therefore defining the WM arrangement of the cerebellar connections with extracerebellar structures is of great interest.
Having completed this overview of cerebral WM anatomy, we now proceed to a consideration of diseases that afflict the cerebral WM either in isolation or as the principal site of pathology.
Diseases of the Cerebral WM
Disorders of cerebral WM are common at any age and in many clinical settings. The history in a particular patient, the results of the clinical examination, and specifically targeted laboratory investigations will often lead to the correct diagnosis. MRI has proven invaluable in the study of these disorders because it discloses structural aspects of WM systems in vivo with great clarity. The most useful means of classifying WM disorders is by careful analysis of the specific neuropathology, which reveals an impressive range of diseases, injuries, and intoxications to which the WM is vulnerable (Table 1). Few disorders damage only the WM, and there is usually some combination of gray matter (GM) and WM neuropathology. However, all the entities we discuss here feature prominent or exclusive WM involvement, and we highlight the contribution of these changes while not dismissing the importance of GM pathology. Neuropathology is central for understanding etiology and improving treatment, but the location of the WM damage is more directly pertinent than its etiology for studying brain–behavior relationships. The categories of WM disorder and selected examples of each are discussed, along with an account of their salient neurobehavioral manifestations, followed by consideration of the effects of aging and vascular disease on cerebral WM.
TABLE 1.
Cerebral White Matter Disorders
| Genetic | Leukodystrophies (e.g., adrenoleukodystrophy, metachromatic leukodystrophy, globoid cell leukodystrophy) |
| Vanishing white matter disease | |
| Alexander’s disease | |
| Adult-onset leukodystrophy with neuroaxonal spheroids | |
| Mitochondrial encephalopathy with lactic acid and stroke (MELAS) | |
| Fragile X tremor-ataxia syndrome | |
| Aminoacidurias (e.g., phenylketonuria) | |
| Phakomatoses (e.g., neurofibromatosis) | |
| Mucopolysaccharidoses | |
| Myotonic dystrophy | |
| Callosal agenesis | |
| Demyelinative | Multiple sclerosis |
| Acute disseminated encephalomyelitis | |
| Acute hemorrhagic encephalomyelitis | |
| Schilder’s disease | |
| Marburg’s disease | |
| Balo’s concentric sclerosis | |
| Infectious | HIV and AIDS dementia complex |
| Progressive multifocal leukoencephalopathy | |
| Subacute sclerosing panencephalitis | |
| Progressive rubella panencephalitis | |
| Varicella zoster encephalitis | |
| Cytomegalovirus encephalitis | |
| Lyme encephalopathy | |
| Inflammatory | Systemic lupus erythematosus |
| Behcet’s disease | |
| Sjögren’s syndrome | |
| Wegener’s granulomatosis | |
| Temporal arteritis | |
| Polyarteritis nodosa | |
| Scleroderma | |
| Isolated angiitis of the central nervous system | |
| Sarcoidosis | |
| Toxic | Cranial irradiation |
| Therapeutic drugs (e.g., methotrexate, BCNU, cyclophosphamide) | |
| Drugs of abuse (e.g., toluene, heroin) | |
| Alcohol (Marchiafava–Bignami disease) | |
| Environmental toxins (e.g., carbon monoxide) | |
| Metabolic | Cobalamin deficiency |
| Folate deficiency | |
| Central pontine myelinolysis | |
| Hypoxic–ischemic injury | |
| Posterior reversible encephalopathy syndrome | |
| Hypertensive encephalopathy/eclampsia | |
| High-altitude cerebral edema | |
| Vascular | Binswanger’s disease |
| CADASIL | |
| Leukoaraiosis | |
| Cerebral amyloid angiopathy | |
| Intravascular lymphoma | |
| White matter disease of prematurity | |
| Migraine | |
| Traumatic | Traumatic brain injury (diffuse axonal injury) |
| Shaken baby syndrome | |
| Corpus callosotomy | |
| Focal lesions of WM tracts (e.g., fornix transection, splenium of CC tumor) | |
| Neoplastic | Gliomatosis cerebri |
| Diffusely infiltrative gliomas | |
| Lymphomatosis cerebri | |
| Langerhans cell histiocytosis | |
| Focal white matter tumors | |
| Hydrocephalic | Early hydrocephalus |
| Normal pressure hydrocephalus | |
| Degenerative | White matter changes in Alzheimer disease |
| Effects of aging on myelin |
Genetic Diseases
The leukodystrophies are a heterogeneous group of genetic diseases involving dysmyelination as a result of substrate accumulation due to enzymatic defects. This group includes adrenoleukodystrophy, inherited in an X-linked recessive manner, and metachromatic leukodystrophy, globoid cell leukodystrophy, and vanishing WM disease, which are autosomal recessive (Table 2). These disorders are more common than previously recognized, in large part because of the improved detection with advances in MRI techniques and appropriate genetic analyses. Collectively, their incidence rivals that of multiple sclerosis. The prevalence of adrenoleukodystrophy alone is 1 in 17,000, about 20,000 patients in the United States.60 Other inherited disorders we consider here are adult-onset leukodystrophy with neuroaxonal spheroids, mitochondrial encephalopathy with lactic acidosis and stroke-like episodes, and fragile X–associated tremor ataxia syndrome.
TABLE 2.
Biochemistry and Genetics of X-linked Adrenoleukodystrophy (X-ALD), Metachromatic Leukodystrophy (MLD), Globoid Cell Leukodystrophy (GLD), and Vanishing White Matter Disease (VWMD)
| Variable | X-ALD | MLD | GLD | VWMD |
|---|---|---|---|---|
| Affected gene | ABCD1 | ASA gene | GALC gene | Any of 1–5 subunits of eIF2B |
| Gene locus | Xq28 | 22q13 | 14q31 | Several |
| Enzyme/protein | ALDP | Aryl-sulfatase A* | GALC | eIF2B |
| Substrate | VLCFA | Sulfatide | Galactosylceramide | Heat shock and other proteins |
Rare cases due to saposin B deficiency.
X-linked adrenoleukodystrophy (X-ALD) is characterized by impaired ability to degrade very long-chain fatty acids (VLCFAs) that causes malfunction of the adrenal cortex and nervous system myelin.61 It presents in childhood in approximately 35% of patients. Affected boys develop normally until 4–8 years of age and then suffer dementia and progressive neurologic decline that leads to a vegetative state and death. More than 90% have adrenal in-sufficiency. The disorder presents as adreno-myeloneuropathy in young adulthood in 35%–40% of patients, characterized by progressive paraparesis and sphincter disturbances due to involvement of the long tracts in the spinal cord. Rapidly progressive inflammatory demyelination develops in 20% of these patients, leading to death in 1–2 years,62,63 a pattern that is similar to that encountered in the childhood form of cerebral X-ALD. This presentation of cerebral X-ALD in adulthood may manifest with impaired psychomotor speed, spatial cognition, memory, and executive functions, whereas those with MRI evidence of severe cerebral disease have global and language impairment as well.64 These deficits are highly correlated with degree of brain MRI involvement. We have seen this disease (Schmahmann, Eichler unpublished) produce a relentlessly progressive dementia in a man in his sixth decade, with inattention, amnesia, impaired cognitive flexibility and problem-solving skills, and visual spatial disorganization, progressing to stereotyped nonmeaningful but complex behaviors, relentless wandering, perseveration, apraxia and posterior aphasia with fluent jargon, impaired comprehension, and poor repetition. In this case there was relative sparing of elementary motor features, normal reflexes, and plantar responses, but striking release phenomena (palmar grasp, snout, root, suck) were present.
Presymptomatic cerebral involvement in X-ALD can be detected on neuroimaging.65 Eighty percent of patients show symmetric, posterior parietal, and occipital periventricular WM lesions,66 with a characteristic garland of gadolinium contrast enhancement67 (Fig. 4A), and increased choline (Ch) and decreased N -acetyl aspartate (NAA) on MRI spectroscopy (MRS) in WM that appears normal on conventional MRI or DTI.68,69 Inflammatory demyelination of the brain is prominent, commencing in the center of the CC where the fiber bundles are most tightly packed, and spreading into the periventricular WM70 in a parieto-occipital (about 80%) or frontoparietal (20%) distribution. The inflammation lies behind the leading edge of demyelination and therefore is probably a response to the primary dysmyelinative process. Recent evidence suggests that microglial apoptosis may precede the demyelination.71
Figure 4.

MRI appearance of (A) X-linked adrenoleukodystrophy (X-ALD), T1-weighted image post-gadolinium; (B) metachromatic leukodystrophy (MLD), FLAIR image; (C) globoid cell leukodystrophy (GLD), T2-weighted image; and (D) vanishing white matter disease (VWMD), T1-weighted image.
Metachromatic leukodystrophy (MLD) is a lysosomal storage disorder resulting from a deficiency of aryl sulfatase A leading to a defect in the desulfation of 3-0-sulfogalactosyl lipids and intracellular accumulation of sulfatides.72 It occurs in about one per 40,000 live births.73 Late infantile MLD is most common, usually appearing between 18 and 24 months.74 The juvenile form emerges between 4 and 16 years.75 The adult form begins after 16 years of age.76 Symptoms vary by age of onset (Mahmood and Eichler, unpublished). Children usually present with gait disturbance and develop ataxia, spastic quadriplegia, and optic atrophy as they progress to a decerebrate state. Progression in adults is slower, and psychosis, behavioral disturbances, and dementia are the major presenting features.77,78 MRI reveals involvement of the periventricular WM, centrum semiovale, genu and splenium of the CC, ICp, descending pyramidal tracts, claustrum, and occasionally cerebellar WM (Fig. 4B). Subcortical U-fibers are usually spared.79 Active lesions do not enhance, although areas that have previously undergone massive dysmyelination can show punctuate striated (tigroid) enhancement,79 corresponding to patchy areas of preserved myelin.80
Globoid cell leukodystrophy (GLD), also known as Krabbe’s Disease, is caused by deficiency of the enzyme galactosyl ceramidase (GALC) that is responsible for converting galactosylceramide into galactose and ceramide. The absence of GALC leads to the accumulation of galactosylceramide as well as psychosine, a cytotoxic byproduct of galactosylceramide. Galactosylceramide accumulation prompts a macrophagocytic response.81 Psychosine accumulation is thought to poison cells and lead to oligodendrocyte cell death.82 Incidence is estimated at one per 100,000 births.83 Infantile GLD presents in the first 6 months of life with hyperirritability, increased muscular tone, fever, and developmental arrest, leading to further cognitive decline, myoclonus, opisthotonus, nystagmus, and optic atrophy. Patients rarely survive beyond 2 years. In an estimated 10% of cases83 symptoms begin after the patient has begun to walk; these are considered late onset.84,85 Reports of adult-onset cases have increased in recent years, presenting with slowly evolving hemiparesis, intellectual impairment, cerebellar ataxia, and visual failure, and, in a few instances, with spastic paraplegia and increased T2 MRI signal along the corticospinal tracts.86 Early imaging reveals symmetrical involvement of the basal ganglia, thalami, and posterior aspect of the centrum semiovale87 (Fig. 4C). The later stages of the disease are characterized by dramatic cerebral and cerebellar atrophy. In the cerebellum, the dentate nuclei and WM are usually involved. Contrast enhancement has been reported in the lumbosacral nerve roots, but not elsewhere, setting this entity apart from X-ALD.88
In the neuropathology of both MLD and GLD, central WM is reduced to the point of cavitation, replaced by marked gliosis.75,89–91 Both disorders show dysmyelination of peripheral nervous system with histiocytic infiltration. In MLD the cerebellar WM is also affected, together with loss of granule and Purkinje cells.91 MLD acquires its name from the abundant sulfatide granules in macrophages that take on their characteristic metachromatic hue after treatment with acidified cresyl violet. In GLD, the pathognomonic multinucleated globoid cells are actually dysmorphic macrophages, engorged with undigested galactosylceramide.
Vanishing white matter disease (VWMD) can be caused by a defect in any one of the five subunits of eukaryotic initiation factor 2B (eIF2B),92,93 a highly conserved, ubiquitously expressed protein that plays an essential role in the initiation of protein synthesis. Clinical symptoms begin in the first few years, after normal or mildly delayed early development. Symptoms include ataxia and seizures, often occurring after fever or minor head trauma. The course is chronic and progressive, with episodic declines after stressors such as fever, head trauma, or periods of fright. Patients usually survive only a few years past the clinical onset, although survival into adulthood has been described.94,95 MRI shows vanishing of WM over time, best recognized on proton density and fluid-attenuated inversion recovery (FLAIR) images (Fig. 4D). Contrast enhancement has not been reported. The cerebellar WM and brain stem show varying degrees of involvement. Imaging abnormalities are found even in presymptomatic individuals.96 Autopsy confirms WM rarefaction and cystic degeneration. The cerebral WM is diffusely affected with a consistency that ranges from gelatinous to cavitary.97 The frontoparietal regions are most severely affected, with myelin pallor, thinning, and cystic changes. Axonal loss varies with the degree of cavitation. GM is largely unaffected. An inflammatory response is notably absent—a failure of astrogliosis may be responsible for the cavitated appearance.
Adult-onset leukodystrophy with neuroaxonal spheroids (AOLNS) is a familial or sporadic disorder characterized radiographically by symmetric, bilateral, T2-hyperintense, and T1-hypointense MRI signal involving frontal lobe WM (Fig. 5A). Neuropathologic examination demonstrates a severe leukodystrophy with myelin and axonal loss, gliosis, macrophages, and axonal spheroids, with early and severe frontal WM involvement, and complete sparing of cerebral cortical neurons98,99 (Fig. 5B–D). The etiology is unknown, although in our series we detected abnormalities in some mitochondrial enzymes, and in one patient, electron transport chain analysis revealed equivocal complex 1 deficiency, suggesting mitochondrial dysfunction.
Figure 5.

Imaging and pathology in a patient with adult-onset leukodystrophy with neuroaxonal spheroids. (A) FLAIR MRI in the axial plane showing confluent high signal in the periventricular, deep, and subcortical white matter of the frontal and parietal lobes extending through the splenium of the corpus callosum. (B) Gross pathology of a coronal section of the cerebral hemisphere, showing gliosis in the centrum semiovale (arrow) and internal capsule (arrowhead). (C) Several neuroaxonal spheroids on microscopic analysis of frontal white matter (original magnification, ×20; Luxol fast blue hematoxylin and eosin stain). (D) Neurofilament immunostain of white matter reveals mild loss of axons and an axonal spheroid (original magnification, ×20).99
The disorder usually presents with executive system dysfunction and other neurobehavioral deficits, progressing to dementia. The extent and degree of change outside the frontal lobe correlates with disease duration. The WM containing long association tracts interconnecting parietal, temporal, and occipital lobes with the frontal lobe are affected early and most severely. In contrast, projection pathways are spared until late in the illness, as exemplified in a patient whose cortical blindness corresponded to the late pathological changes in the SS that contains the optic radiations.99 This dichotomy of early dementia, but late failure of gait, strength, dexterity and sensation, provides an interesting glimpse into the clinicopathological distinction between association and projection fiber tract involvement in AOLNS, and the functional contributions of these different WM tracts.
Mitochondrial encephalopathy with lactic acidosis and strokelike episodes (MELAS) was initially described in patients with normal early development and short stature, who developed seizures, hemiparesis, and hemianopia or cortical blindness, and in whom ragged red fibers were evident on muscle biopsy.100 Criteria for diagnosis101 are strokelike episodes before age 40 (not confined to vascular territories); encephalopathy characterized by seizures, dementia, or both; with lactic acidosis and/or ragged-red fibers. Recurrent headache or vomiting may be present. The disease is most commonly maternally inherited through the mitochondrial DNA, and in 70%–80% of MELAS patients the enzymatic defect is a complex I deficiency and, to a lesser degree, a complex IV deficiency, associated with a point mutation at 3243 in the tRNA Leu (UUR) region. Periventricular and diffuse WM hyperintensities, as well as areas of cortical infarction and cerebral edema, are seen on MRI102 (Fig. 6), consistent with the pathology showing diffuse gliosis of cerebral and cerebellar WM, and diffuse atrophy of the cerebral and cerebellar cortices.103 Dementia and psychosis may be the initial clinical manifestation of MELAS. In one published case104 a young woman presented with headaches, confusion, aphasia, and apraxia, followed some years later by temper tantrums, aggressive and paranoid behavior, disinhibition, and ideas of reference. In our patient,105 a man in his 40s presented with memory loss, social withdrawal, hallucinations, paranoia, impaired planning and strategy formation, and a right homonymous hemianopsia. Over the ensuing decade, his frontal lobe syndrome remained problematic and the dementia progressed, but only mild motor slowing appeared. MRI currently shows volume loss with multiple scattered WM T2 and FLAIR hyperintensities.
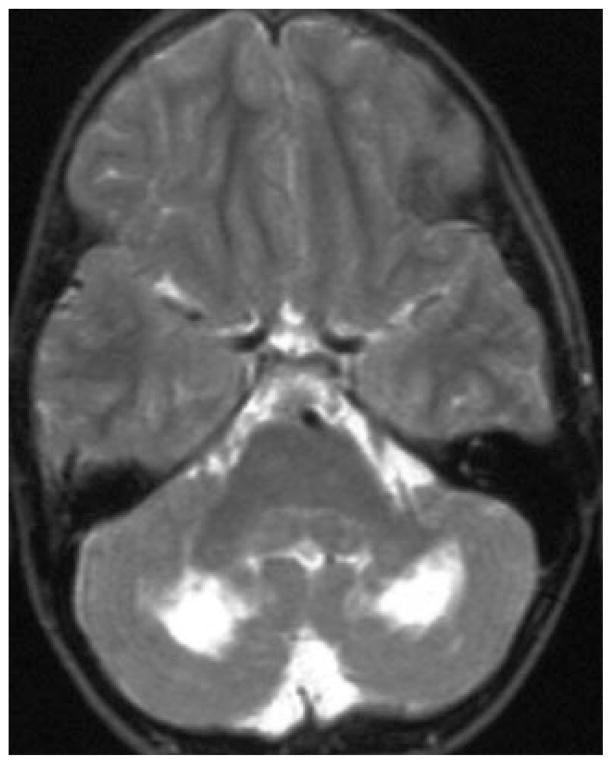
Figure 6.

FLAIR MRI in a patient with mitochondrial encephalopathy with lactic acidosis and stroke-like episodes (MELAS).2
Fragile X–associated tremor ataxia syndrome (FX-TAS) is an adult-onset neurodegenerative disorder that affects carriers, principally males, of premutation alleles (55–200 CGG repeats) of the fragile X mental retardation 1 (FMR1) gene, with a powerful predictive relationship between the length of the CGG repeat and the neurological and neuropathological involvement.106–108 Patients present in older adulthood primarily with gait ataxia and intention tremor. Progressive cognitive decline is characterized by impaired executive function, working memory, intelligence, declarative learning and memory, information processing speed, temporal sequencing, and visuospatial functioning, but language is spared.109 The MRI pattern of WM pathology in FXTAS is distinctive (Fig. 7): increased T2 signal in the MCP is typical, and cerebellar and cerebral WM changes are also consistently observed.107 Neuropathology reveals marked abnormalities in cerebral and cerebellar WM, dramatically enlarged inclusion-bearing astrocytes in cerebral WM, and widespread intranuclear and astroglial inclusions in brain, cranial nerve nuclei, and autonomic neurons of the spinal cord. Spongiosis is present in the MCPs. Cerebral WM can be severely affected both grossly and microscopically, with parenchymal pallor and spongiosis. Periventricular WM is generally spared.108 Greco et al.108 postulate that in the setting of normal cortical thickness and neuronal counts, neuronal and/or glial dysfunction causes or contributes to the clinical presentation. Involvement of the MCP is interesting in light of the fact that the MCP conveys essentially all cerebral cortical input (including associative and paralimbic) to the cerebellum,110 and the cerebellum contributes not only to motor control but also to the modulation of cognition and emotion.58,59 When the deafferentation of cerebellum by the MCP lesions is added to the massive disruption of cerebral long association fiber tracts evident in pathological studies of FXTAS, the cognitive decline becomes readily understandable.
Figure 7.

MRI features of fragile X–associated tremor ataxia syndrome (FXTAS). White matter pallor is seen in the cerebellar parenchyma (A), as well as in the middle cerebellar peduncles (B).
Demyelinative Diseases
Multiple sclerosis (MS) is an inflammatory disease of myelin, but it may also damage axons, conferring a worse prognosis.111 In terms of higher function, MS has recently been better appreciated as a source of cognitive and emotional impairment, recalling the initial insights of Jean-Martin Charcot (1825–1893).112 As recently as 1970, cognitive impairment of any degree in MS was thought to occur in about 5% of patients,113 but community-based neuropsychological studies place this figure in the 40%–50% range.114 Dementia may occur, with an estimated prevalence as high as 23%.115 Cognitive impairments in MS also include a wide range of focal neurobehavioral syndromes and neuropsychiatric disturbances.116 The source of cognitive impairment appears to be related primarily to WM involvement (Fig. 8), because many studies find at least modest correlations between extent of MRI WM damage and the degree of cognitive loss.116 Subtle WM pathology may not be detected by conventional MRI, but more sophisticated MRI techniques (diffusion-weighted imaging [DWI], FLAIR sequences, ultrahigh field strength, magnetization transfer, and magnetic resonance spectroscopy [MRS]117,118) have documented abnormalities in the normal-appearing WM. Cerebral cortical demyelination is also present in MS,119 raising the possibility that cognitive impairment may result from this aspect of the disease. Whereas a contribution of cortical demyelination is plausible, this remains uncertain because the cortical lesion load in MS may be limited and therefore have minimal effect on cognition.120 Given that demyelination in large fiber tracts probably exerts a far greater effect on the distributed neural networks subserving higher functions,121 the main determinant of cognitive dysfunction in MS appears to be WM demyelination.
Figure 8.

FLAIR MRI in multiple sclerosis. (A) White matter hyperintensity perpendicular to the lateral ventricle (Dawson’s finger), shown by the arrow. (B) In a second case, the focal area of hyperintensity (arrow) corresponded to the initial clinical presentation.2
Acute disseminated encephalomyelitis (ADEM) is another inflammatory demyelinative disease, probably postinfectious and autoimmune in origin. It is generally monophasic, but repeated episodes have been described. Diagnostic criteria do not reliably distinguish ADEM from first presentations of relapsing diseases such as MS and neuromyelitis optica,122,123 but ADEM can be aggressive, massively disseminated, and life threatening.124 Four patterns of cerebral involvement in ADEM have been described based on MRI findings: (1) lesions of less than 5 mm; (2) large, confluent, or tumefactive lesions, with perilesional edema and mass effect; (3) additional bithalamic involvement; and (4) acute hemorrhagic encephalomyelitis (AHEM) with hemorrhage identified in the large demyelinative lesion.125 ADEM can be treated, sometimes with dramatic success, using immune-modulating agents such as intravenous immunoglobulin (IVIG). The presentation depends on the location of the pathology. One of our recent patients presented with inability to find her shoe with the left foot, the beginning of a hemineglect syndrome from a right parieto-occipital WM lesion that heralded disseminated, asymmetric, bihemispheric demyelination; her deficits responded immediately to IVIG (Fig. 9). A second young woman with AHEM presented with hemianopsia related to the posterior location of the initial pathology. She evolved to hemispheric edema requiring craniotomy for herniation before she came to our attention and recovered with IVIG treatment.
Figure 9.

MRI features of acute disseminated encephalomyelitis (ADEM). (A) Coronal T1-weighted postgadolinium image showing enhancing lesions in the right more than left hemispheres. (B) Axial zero-B MRI demonstration of the multiple lesions. (C) FLAIR MRI 6 months after marked clinical recovery shows much improved areas of hyperintensity.
Infectious Diseases
Some nervous system infections have a predilection for the cerebral WM and include prominent neurobehavioral sequelae.
AIDS dementia complex (ADC) commonly has WM abnormalities on MRI, and WM pallor is an early neuropathological finding.126 Rarely, fatal and fulminant leukoencephalopathy can be seen as the only manifestation of human immunodeficiency virus (HIV) infection.127 Involvement of the basal ganglia is also evident in ADC, and the initial reports of dementia in AIDS stressed the subcortical profile of the dementia syndrome,128 analogous to that in patients with subcortical dementias such as Huntington’s and Parkinson’s diseases. The role of WM dysfunction is not easily dismissed, however, in light of evidence that MRI WM changes (Fig. 10) improve in parallel with cognitive decline in patients with successful treatment of dementia.129–131 Advanced neuroimaging illuminates this issue. Tensor-based morphometry in HIV/AIDS patients showed that, whereas atrophy was widespread in the brain, only WM tissue loss correlated with cognitive impairment.132 The neuropathology of ADC is still being elucidated, but evidence supports the role of WM dysfunction in neurobehavioral dysfunction. This issue highlights a more general need for studies that delineate the relative contributions of subcortical GM and WM dysfunction to the pathogenesis of dementia.
Figure 10.

FLAIR MRI showing hyperintensities in prefrontal white matter in a patient with HIV and cognitive impairment.2
Progressive multifocal leukoencephalopathy (PML) is an opportunistic demyelinative infection of immunocompromised patients, caused by a human polyomavirus, JC virus, that attacks the myelin-producing oligodendrocyte.133,134 PML was previously recognized in the setting of immune compromise after organ transplantation, bone marrow–derived tumors, and chemotherapy, until the worldwide HIV/AIDS pandemic produced an explosion of cases. Interest in the relevance of this disorder for a new patient demographic has emerged with the report that PML occurred in some MS patients treated with the adhesion-molecule α-integrin inhibitor natalizumab.135 The clinical manifestations vary greatly, depending on the location of the demyelination. Focal elementary findings include hemianopsia, cortical blindness, hemiparesis, and cerebellar motor symptoms of ataxia and dysarthria. Cognitive presentations include frontal lobe syndromes and aphasia, progressing to quadriparesis, mutism, and unresponsiveness. The virus has a predilection for subcortical U-fibers, but cortical demyelination appears to be integral to the process, together with macrophage and microglial activation.136 MRI findings of widespread, asymmetric, nonenhancing infiltrative lesions without mass effect137 (Fig. 11) may also be located in subcortical gray nuclei, because the axons conveyed in WM tracts course to, and terminate in, these GM destinations, and because there is probably intrinsic pathology of axons and neurites in the GM. Cerebellar WM may be involved early,138 and brain stem disease is also described.139 Multiple locations of abnormal findings on MRI and pathological observation are expected, and the disease has a relentlessly progressive course, although limited advances have been made in AIDS patients by using highly active antiretroviral therapeutic regimens.140
Figure 11.
MRI features of progressive multifocal leukoencephalopathy (PML). (A) T2-weighted image shows involvement of white matter of the right occipital region (arrow), accounting for the hemianopsia in this HIV-positive patient. (B) FLAIR MRI in a patient with systemic lymphoma and PML, demonstrating confluent prefrontal white matter lesion spreading across the genu of the corpus callosum (arrow), and additional lesions affecting local association fibers of the right prefrontal and parieto-occipital cortices (arrowheads). (C, D) Axial FLAIR images in an HIV-positive patient showing confluent subcortical and deep white matter involvement by PML. (Panels A and B are from reference 2.)
Autoimmune Inflammatory Diseases
These central nervous system diseases are similar to infectious diseases in that their pathology cannot be assigned only to the cerebral WM. Nevertheless, growing evidence implicates a role for WM involvement in neurobehavioral dysfunction.
Systemic lupus erythematosus (SLE) is the best-studied example and proves illustrative. Neuropsychiatric lupus refers to a diverse group of syndromes in SLE patients that includes cognitive dysfunction,141 and milder cognitive impairment can be noted even in SLE patients without overt neurologic disease.142 MRI WM hyperintensities are common, related to vasculopathy and presumably autoimmune factors, and a relationship between dementia and leukoencephalopathy in SLE has been suggested.143 Data from studies with MRS have shown that, even in SLE patients with normal WM on conventional MRI and no neuropsychiatric features, subtle cognitive impairment correlates with increased WM Ch, but not with the neuronal marker NAA or hippocampal atrophy.144 Support is thus accumulating for a contribution of cerebral myelin damage to cognitive impairment in SLE. Other proposed pathogenic factors in SLE, such as autoimmune mediators including antiphospholipid antibodies, proinflammatory cytokines, and anti–N -methyl-D-aspartate receptor antibodies, also merit study.
Toxic Leukoencephalopathy
Many toxic brain disorders preferentially affect the cerebral WM.145 A spectrum of severity has been described, ranging from mild, reversible confusion, to coma and death, with concomitant MRI and neuropathological WM changes.145 Cranial irradiation and cancer chemotherapeutic drugs, most notably methotrexate146,147 (Fig. 12), are leukotoxic, an effect that complicates the treatment of many malignancies.
Figure 12.

FLAIR MRI in the axial plane of a patient with cognitive decline after receiving methotrexate.2
Toluene leukoencephalopathy (TL) is an intriguing disorder that convincingly illustrates the ability of pure WM damage to produce dementia.148–152 Toluene (methylbenzene) is a common household and industrial solvent and is the major solvent in spray paint. It is abused by millions of people worldwide for its euphorigenic effect, an abuse that has a lifetime prevalence in the United States estimated at 18%.152 The intentional inhalation of toluene, often for years without respite, results in a dramatic syndrome of dementia, ataxia, and other neurologic signs.149,150 The effects are readily detectable on MRI and include diffuse cerebral and cerebellar WM hyperintensity (Fig. 13). The degree of cerebral involvement strongly correlates with the severity of dementia, which is the most prominent manifestation of the syndrome.148,150 Autopsy studies of TL reveal selective myelin loss that spares the cerebral cortex, neuronal cell bodies, and even axons in all but the most severe cases.151,152 TL thus ex-emplifies the toxic WM disorders and stands out as a convincing example of WM dementia (WMD).6,116,153
Figure 13.
T2-weighted MRI appearance in the axial plane of toluene encephalopathy in two patients (A, B).
Inhalation of heated heroin vapor (colloquially termed “chasing the dragon”) produces a devastating, progressive spongiform leukoencephalopathy. The MRI appearance154–156 is highly suggestive, if not pathognomonic (Fig. 14). Cocaine use may produce similar findings, including symmetric and widespread involvement of the posterior cerebral hemispheric WM, cerebellar WM, splenium of the CC, and brain stem (medial lemniscus and lateral brain stem), with sparing of the deep cerebellar nuclei. MRS in areas of parenchymal damage demonstrates elevated lactate and myoinositol, reduced NAA and creatine, normal to slightly decreased Ch, and normal lipid peak. Neuropathologically this is WM spongiform degeneration with relative sparing of U-fibers, whereas electron microscopy reveals intramyelinic vacuolation with splitting of intraperiod lines. Preservation of axons with no evidence of Wallerian degeneration, inflammatory cellular reaction, or demyelination is taken to indicate that axons may be relatively spared, consistent with the degree of recovery in some cases.154 Clinical manifestations include cerebellar motor findings of ataxia, dysmetria and dysarthria, bradykinesia, rigidity, and hypophonia, and the syndrome may progress over weeks to pseudobulbar palsy, akinetic mutism, decorticate posturing, and spastic quadriparesis. Death occurs in approximately 20% of cases. Clinical and MRI findings can progress after cessation of drug use, indicating that the toxic exposure precipitates an evolving injury. The lack of concordance between MRI perfusion and spectroscopy may reflect impaired energy metabolism at the cellular level. The lactate peak on MRS; mitochondrial swelling and distended endoplasmic reticulum in oligodendrocytes on autopsy; and apparent response to antioxidants and mitochondrial cofactors such as vitamin E, vitamin C, and coenzyme Q suggest mitochondrial dysfunction as a basis for this entity.154,155,157
Figure 14.

MRI scans after heroin inhalation, known colloquially as “chasing the dragon.” FLAIR images in the axial plane (A–D). Corresponding 1H MRS imaging spectra in two of the images show characteristic lactate peak and decreased NAA.154
Other toxin-induced spongiform leukoencephalopathies with fluid accumulation restricted to myelin sheaths include those precipitated by cuprizone, ethidium bromide, actinomycin D, triethyl tin, hexachlorophene, isonicotinic acid, hydrazine, and cycloleucine.154,158
Metabolic Disorders
A diverse group of metabolic disturbances features WM neuropathology and a variety of neurobehavioral syndromes. In some patients, metabolic disturbances coexist with toxic disorders (including methotrexate) to produce a clinical and MRI picture known as posterior reversible leukoencephalopathy syndrome159 that also occurs in patients with hypertension, including those with preeclampsia.
Deficiency of cobalamin (vitamin B12) can lead to dementia with a prominent WM component radiologically and pathologically. Cobalamin is important in the maintenance of normal myelin, and its deficiency results in subacute combined degeneration (SCD) of the spinal cord. Cobalamin deficiency may also cause perivascular degeneration of myelinated fibers in the cerebrum that is identical to the WM pathology in SCD, and these brain lesions probably account for dementia.160 Because cobalamin deficiency may produce dementia that is easily correctable with treatment, vitamin B12 screening is routine in the evaluation of dementia. Well-documented cases of WM lesions and dementia have improved after parenteral treatment with vitamin B12.161,162
Hypoxic ischemic encephalopathy itself may produce a delayed, diffuse leukoencephalopathy.163,164 We described a woman who suffered presumed cardiac arrest, was reportedly comatose for 2 days, and then recovered well, only to develop confusion, gait difficulty, and incontinence over the ensuing 2 weeks. She was mute and unable to follow commands and had right hemianopsia, arms held in a flexion, although she could move her legs, with spasticity and hyperreflexia in all extremities. MRI showed extensive, symmetric WM T2 and FLAIR hyperintensities, and DWI and apparent diffusion coefficient mapping revealed restricted diffusion of the WM165 (Fig. 15). Demyelination has been proposed as a pathophysiological mechanism in these cases, accounting for both latency to onset and variable prognosis. A proposed mechanism is that the demyelination might be triggered by selective vulnerability of the WM to hypoxic injury, resulting from its widely spaced arterioles and lack of anastamoses.165 Delayed leukoencephalopathy in the setting of hypoxic encephalopathy has also been associated with carbon monoxide poisoning,166,167 but exposure to the toxin is not a prerequisite.
Figure 15.

Axial MRI in delayed leukoencephalopathy after hypoxic–ischemic insult. (A) FLAIR image shows extensive, symmetric white matter hyperintensities with relative sparing of subcortical white matter. (B) Diffusion-weighted imaging shows restricted diffusion of the white matter abnormalities, confirmed on (C), apparent diffusion coefficient mapping.165
Trauma
Traumatic brain injury (TBI) is a major source of neurobehavioral disability estimated to affect 1.4 million Americans per year.168 Of all the major neuropathological complications of TBI (cortical contusion, intracerebral hemorrhage, subdural hematoma, epidural hematoma, penetrating injury, hypoxic–ischemic damage), arguably the most important is the WM lesion known as diffuse axonal injury (DAI),169,170 or WM shearing injury. DAI involves primarily the brain stem, cerebral hemispheres, and CC and is likely to be ubiquitous in TBI.116,171 Both myelin and axons are highly vulnerable to DAI, and this injury disrupts distributed neural networks by disconnecting widespread cortical and subcortical regions. DAI has been linked with acute effects such as loss of consciousness, as well as chronic sequelae including persistent attentional, executive, comportment, and memory disturbances. These deficits may occur with DAI in all degrees of TBI severity, from concussion to the vegetative state.172,173 Damage to the frontal lobe WM appears to be particularly detrimental to long-term outcome, interfering with comportment, occupational function, and community reintegration.116
Neoplasms
Central nervous system tumors have been considered problematic for investigating brain–behavior relationships because their often wide extent, mass effect, and associated edema can complicate precise localization of the neuropathology. However, with improved neuroimaging, neurobehavioral effects of many cerebral tumors can be studied using detailed clinical–neuropathological correlations.174
Gliomas can be particularly illustrative in terms of their effects on WM tracts because they originate primarily in WM and spread via WM tracts to other regions.175,176 Gliomatosis cerebri (GC), a diffusely infiltrative astrocytic malignancy with a clear predilection for cerebral WM, can be seen by MRI to spread via inter- and interhemispheric WM pathways177 (Fig. 16). Neurobehavioral features leading to progressive dementia are the most common presenting, and persistent, clinical manifestations of this tumor. This scenario underscores the conclusion that selective WM dysfunction is sufficient to produce clinically significant cognitive and emotional disturbances.
Figure 16.
Coronal T1-weighted image in a patient with gliomatosis cerebri. Note the spread of tumor along white matter planes.
Cerebral lymphoma may demonstrate a clinical propensity similar to GC when it takes the diffusely infiltrative form of lymphomatosis cerebri.178 Study of brain tumors producing neurobehavioral changes related to WM dysfunction deserves more attention, particularly as more powerful neuroimaging modalities make it possible to identify the location and spread of these tumors throughout their course.
Langerhans’ cell histiocytosis (LCH) is a disorder of unknown cause characterized by proliferation of the Langerhans’ cell—a bone marrow–derived, antigen-presenting dendritic cell. It may affect the nervous system, notably the hypothalamic–pituitary region, leading to diabetes insipidus and other endocrinopathies. It may also be located in the pons, cerebellum, basal ganglia, and cerebral WM179,180 (Fig. 17). The cerebellar lesions are characterized as neurodegenerative and exhibit a profound inflammatory process dominated by CD8-reactive lymphocytes, associated with tissue degeneration, microglial activation, and gliosis.181 We have seen two patients with LCH (unpublished; and case 8b in reference 182), in whom, on MRI, the disorder appears isolated within cerebellar WM. The cerebellar motor syndrome is troublesome but is overshadowed by cognitive and neuropsychiatric dysfunction. In one patient, high T2 signal on MRI was isolated to the cerebellar WM during childhood; images during the teenage years demonstrated pancerebellar atrophy and attenuated cerebellar WM. The patient had been placed in special-education classes because of cognitive impairment, and his behaviors were perseverative, impulsive, self-absorbed, immature, and unreliable. He demonstrated poor judgment, took unnecessary risks, engaged in inappropriate interactions, and was “his own worst enemy.” He was alternately agitated, tearful, and sarcastic, and he had a cerebellar motor syndrome of moderate severity. An earlier report of a patient with LCH involving cerebellar WM also reported significant deficits in global cognitive scores, memory, attention and concentration, and perceptual–organizational capabilities, along with substantial emotional and behavioral problems.183 These behaviors fall within the domain of the cerebellar cognitive affective syndrome and its neuropsychiatric manifestations,182,184,185 and they probably reflect involvement of the nonmotor region of cerebellum in the posterior lobe.
Figure 17.
T2-weighted axial MRI in a patient with Langerhans cell histiocytosis, showing hyperintense signal abnormality in the white matter of the cerebellum.182
Hydrocephalus
Whether originating early or late in life, hydrocephalus exerts its most prominent neuropathological effects on cerebral WM.186,187 Cortical damage is uncommon, occurring only late in the course. Injury to the deep GM is also less prominent than WM injury, indicating that the cognitive effects of hydrocephalus are related primarily to tract damage, at least at the time when diagnostic and treatment issues are most crucial. Periventricular WM is compromised by the excess volume of ventricular cerebrospinal fluid. In patients with normal pressure hydrocephalus (NPH)188 characterized by the clinical triad of dementia, urinary incontinence, and gait impairment, treatment with ventriculoperitoneal shunt can be most effective.189,190 Improvement is not universal, particularly in older patients with coexistent ischemic damage in the WM191 or concomitant Alzheimer’s disease (AD).192 The reversibility of NPH, at least early in the course before widespread GM damage has occurred, likely results from the significant ability of compromised WM to recover.
Aging, Vascular Disease, and WM Lesions
Aged monkeys lose WM within the cerebral cortex and subcortical regions193,194 and display memory impairment on tasks of spatial and visual recognition that correlates with the extent of degeneration of myelinated fibers in cortex and WM.195 There is now a vigorous field of investigation into the WM changes that characterize the aging process, as well as the relevance of these findings for speed of information processing, cognition, and dementia in humans.
WM Hyperintensities in the Elderly: MRI Observations
Computed tomography (CT) and MRI have led to an increased recognition of the prevalence of WM lesions in the elderly. Termed leukoaraiosis (WM rarefaction) by Hachinski et al.196,197 (Fig. 18), these findings were initially thought to be a radiographic manifestation of Binswanger’s disease (Fig. 19). It is now appreciated that these lesions are extremely prevalent both in successful aging and in aging associated with cognitive decline.
Figure 18.

Leukoaraiosis is visible as (A) white matter hypodensity on CT and (B) white matter hyperintensity on FLAIR MRI in the same patient.
Figure 19.

FLAIR MRI of a patient with Binswanger’s encephalopathy. Hyperintense signal abnormality is seen at periventricular zones, white matter immediately beneath cortex, splenium of the CC, and internal and external/extreme capsule regions. Multiple hypodensities consistent with lacunar infarcts are also seen in the basal ganglia and thalamus.2
Definition of WM Hyperintensities
WM lesions can be visualized on CT as areas of hypoattenuation (Fig. 18). MRI has greater sensitivity and reveals WM lesions that may not be identified on CT and appear as hyper-intensity (WMH) on T2-weighted and FLAIR images. These WMHs are distinguished from infarction by the absence of well-defined hypointensity on T1-weighted images. Periventricular regions are most commonly affected, particularly around the frontal and occipital horns. In severe cases there is a halo of WMH surrounding the lateral ventricles198 (Fig. 19), and a variable extent of discrete ovoid subcortical WMH. MRI measurements of water proton diffusion taken using apparent diffusion coefficient mapping show increased diffusivity within the lesions.199 These features are not disease specific, however, because they reflect an increased concentration of water within the affected tissue. The most common cerebral small-vessel pathologies associated with WMH are related to hypertension, diabetes, atherosclerosis, and cerebral amyloid angiopathy. Rare vascular diseases associated with WMH include Fabry’s disease and hereditary mutations of the COL4A1 gene.
WMHs in the Elderly: Pathophysiology and Clinical Features
Multiple lines of evidence suggest that vascular pathology is the main cause of most of the age-related WMHs, once other neurological diseases are excluded. Histopathology shows demyelination with various degrees of axonal loss and gliosis, consistent with injury to the myelin or oligodendrocyte, but this has not helped determine the underlying causes.200 CT and MRI findings are visually more dramatic than gross or routine microscopic pathology, but there is good correlation between imaging and pathology when using myelin stains that reveal relative myelin loss.200 Arteriosclerosis or microinfarction may be present, but careful studies of the vascular system with serial sections are rarely performed.
Epidemiology of WMH
Prospective, population-based cohort studies (Framingham Study,201 Rotterdam Study,202,203 Cardiovascular Health Study204) have elucidated the epidemiology of WMH. One study using a sensitive ordinal scale for grading WMH severity205 found that more than 95% of persons older than 70 years have detectable WMH on MRI. Consequently, studies of WMH in older persons are focused on determining variability in extent of WM lesions rather than their presence or absence. The strongest risk factors for greater extent of WMH are age, hypertension, diabetes, and smoking,203,204 whereas systemic measures of atherosclerosis, such as internal carotid artery plaques, are weakly associated. Retinal vascular changes206 and indices of renal function207 are closely associated with WMH, possibly reflecting the presence of shared risk factors for small vessel disease. Serum studies show associations between WMH, or their progression, and markers of endothelial dysfunction (serum homocysteine and intercellular adhesion molecule 1),208 thrombogenesis (thrombomodulin and fibrinogen),209,210 inflammation (C-reactive protein),208 and antioxidant levels.210 A link with β-amyloid metabolism is shown by associations with either increased serum A-β211,212 or decreased cerebrospinal fluid A-β.213 The basis for these findings is unknown but might be related to the presence of cerebral amyloid angiopathy (CAA).211 Despite these known risk factors, much of the variance in age-related WMH remains unexplained and may be accounted for by genetic factors.214
Pathophysiology of WMH: Small-vessel Disease
A strong relationship with cerebrovascular disease is shown by robust associations between WMH burden and history of ischemic215 or hemorrhagic stroke,216 ischemic stroke evolution,217 incidence of new ischemic215,218 or hemorrhagic stroke,219–221 and presence and incidence of silent brain infarcts.222 These relationships with stroke and infarction are not accounted for by shared vascular risk factors. Treatment of hypertension, the strongest modifiable risk factor for cerebrovascular disease, with an angiotensin-converting enzyme inhibitor and thiazide diuretic, was associated with reduced WMH progression,223 whereas treatment with a 3-hydroxy-3-methyl-glutaryl (HMG) CoA reductase inhibitor (statin) had no effect.224
Cerebral small-vessel disease is thought to cause ischemia through vascular stenosis, occlusion, or impaired reactivity producing the WM changes. Tissue pathology consists only of nonspecific injury without evidence of frank infarction, although lesions show immunoreactivity for hypoxia-inducible factor 1, which is expressed in the presence of ischemia.225 Hemispheric WM blood supply is derived predominantly from penetrating branches of the middle cerebral artery stem or from penetrating branches of circumferential arteries coursing over the hemispheric surface.226 The few millimeters of WM adjacent to the wall of the lateral ventricle represent a distal endzone territory of blood supply from the choroidal arteries. Blood flow studies show this to be a low-perfusion region, and the fact that it is the most frequent site of WMH involvement possibly reflects a vulnerability to blood flow reduction.227 Brain regions with higher burden of WMH in demented subjects show decreased blood flow and metabolism, as well as increased oxygen extraction indicative of hypoperfusion.228–232 Blood flow disturbances are less severe in the nondemented.233
Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy (CADASIL) is caused by mutations in the notch 3 gene234 that lead to hyalinization and thickening of the arterial media of small blood vessels in the brain. Other organs are not affected, although asymptomatic vascular changes can be detected on skin biopsy.235 Studies in CADASIL patients provide strong evidence that cerebral small-vessel disease can cause WMH. MRI reveals lacunar infarcts with extensive WMH burden (Fig. 20). The anterior temporal WM and external capsule are frequently involved—sites uncommonly affected by sporadic age-related WMH236—making CADASIL unusual in that it has a relatively specific spatial distribution of lesions. CADASIL causes impaired cognition and progressive dementia.237,238 Affected individuals present in their 30s and 40s with migraines, memory loss, psychiatric symptoms, or stroke.239 Notably, however, studies of radiographic correlates of cognition in CADASIL show that WMH alone is not associated with global cognitive function after controlling for volume of lacunar infarcts,238 indicating that tissue infarction may be required to produce more severe forms of cognitive impairment. This is exemplified by a 41-year-old patient (Schmahmann, unpublished) with notch 3–confirmed presymptomatic CADASIL, whose cognition is presently entirely preserved in the setting of diffuse and prominent WMH.
Figure 20.

MRI appearance of white matter changes in axial sections of patients with CADASIL. (A, B) FLAIR MRI in an asymptomatic 39-year-old, notch 3 gene positive with family history of early stroke, whose imaging findings were incidentally noted. Characteristic temporal lobe white matter involvement is highlighted (arrows). (C) FLAIR MRI in a patient with clinically established CADASIL. (D) T2-weighted MRI in a patient with notch 3 gene and pathologically proven disease.
Cerebral amyloid angiopathy (CAA) is characterized by amyloid deposition in the media and adventitia of small arteries of the cerebral cortex and meninges. Rare hereditary cases may be caused by mutations in the amyloid precursor protein, resulting in deposition of β-amyloid, or by mutations in other genes including cystatin C, transthyretin, and gelsolin.240 Affected individuals present in their 30s and 40s with cognitive impairment or intracerebral hemorrhage. Extensive WMH are typically present. Unlike CADASIL, CAA also exists as a sporadic disease. In contrast to hereditary CAA, sporadic CAA appears to be exclusively a disease of β-amyloid. It is a major cause of intracerebral hemorrhage in the elderly241 (Fig. 21). Because the cerebral vascular pathology is almost exclusively limited to cerebral cortex, CAA-related hemorrhages occur in lobar brain regions (i.e., within the cortex or at the cortico–subcortical junction) but not in deep hemispheric brain regions such as the putamen or thalamus.242 The presence of multiple or recurrent lobar brain hemorrhages, in the absence of coagulopathy or other secondary causes such as vascular malformations, is highly specific for the presence of CAA pathology.241 MRI with gradient echo sequence is sensitive to the presence of small hemosiderin deposits from previous hemorrhages, also called microbleeds, and can suggest the diagnosis of CAA.241
Figure 21.

MRI in the axial plane in cerebral amyloid angiopathy. (A) Gradient echo MRI demonstrating multiple punctuate areas of hemorrhage (microbleeds, arrow) at the cortico–subcortical junctions. (B) MRI FLAIR sequence in a patient with lobar intraparenchymal hemorrhage in the left occipital lobe (double arrows), as well as periventricular WMH (single arrow) and subcortical WMH (arrowheads).221
There is increasing recognition that sporadic CAA is associated with cognitive dysfunction, even though many patients with CAA-related intracerebral hemorrhage do not have severe cognitive impairment or dementia.243 A population-based autopsy study showed that CAA pathology was associated with ante-mortem cognitive performance, controlling for the extent of AD pathology.244 These subjects did not have symptomatic stroke. The same study showed that the prevalence of CAA in those older than 80 years is more than 10%,244 which is much greater than the population prevalence of symptomatic brain hemorrhage but may be similar to the population prevalence of asymptomatic lobar microbleeds.245 These data suggest that CAA contributes to cognitive decline in the elderly and that the clinical effect of CAA is not limited to those with stroke. WMH burden is high in CAA and is associated with cognitive impairment independent of stroke.221
In contrast to CADASIL, there is no typical distribution of WMH suggestive of CAA.227 WMHs appear to be a marker of CAA disease burden and progression, because they are associated with the number of lobar microbleeds221 and predict new symptomatic intra-cerebral hemorrhages221 and asymptomatic lobar microbleeds.246 An interesting feature of CAA-related WMH is that the site of tissue pathology in the WM is remote from the site of vessel pathology in the cortex, potentially suggesting a flow-related mechanism of injury. DWI shows abnormalities in water diffusivity in brain regions not typically involved by WMH, suggesting that tissue microstructural changes may be more widespread than the changes in T2 hyperintensity.247,248 The recent advent of molecular imaging of β-amyloid, using Pittsburgh compound B and other ligands,249 offers the opportunity to address the relationship between extent and location of WMH, and extent and location of β-amyloid deposition.
Age-related WM Lesions, Cognition, and Behavior
An association between WMH and cognitive dysfunction has long been recognized.196 Research studies and clinical practice show, however, that only a modest amount of variance in cognition performance is explained by WMH. This conclusion is not surprising, perhaps, given the large amount of cognitive performance that remains unexplained by currently recognized brain pathologies, including AD.250 Practicing clinicians are familiar with the situation where a patient displays considerable incidentally discovered MRI WMH despite apparently normal cognition. Although within-individual decline from previous performance levels may be underappreciated, it appears that some individuals can compensate for high WMH burden through unknown mechanisms.
Population-based studies of aging report a relationship between WMH volume on MRI, determined by ordinal scales or by volumetric analysis, and cognitive performance, determined by psychological testing.201,204,251,252 These populations were free of dementia and stroke at study onset. Longitudinal follow-up shows that those with higher baseline lesion burden have greater subsequent decline in test performance.253,254 Further, those with higher WMH progression on follow-up MRI have greater decline in test performance than those with less lesion progression.253–255 WM lesions are associated with subjective impression of cognitive performance, even in those with psychological test performance in the reference range, supporting their relevance to clinical practice.256 There are few data to show whether a critical threshold of lesion severity exists, below which WMH can be considered insignificant and above which they should be considered clinically relevant to cognitive performance.
WMH and Risk of Cognitive Change
Higher WMH burden is associated with the transition from normal cognition to mild cognitive impairment (MCI),257,258 but not from MCI to dementia.258,259 Whereas the severity of periventricular WMH predicts future dementia, predominantly AD, this relationship is reduced to a trend when also controlling for other MRI measures, such as brain atrophy.260 Thus, MRI-identified WMH lesions appear to be sufficient to cause mild forms of cognitive dysfunction but rarely cause dementia in the absence of other brain pathologies. Nonetheless, these lesions have public health relevance because they are ubiquitous with aging, and cumulative disability across the aging population may be large. By causing mild cognitive dysfunction, WM lesions may decrease cognitive reserve and predispose to dementia in the presence of additional brain pathologies. Autopsy-based studies of dementia emphasize the coexistence of vascular and AD pathology,261 and these WMH lesions and other vascular pathologies may account for some of the otherwise unexplained variation between cognitive performance and burden of AD pathology.
WM Changes in AD
Cerebral atrophy and loss of WM are marked in the later stages of AD,262,263 and cerebral WM lesions in AD have the appearance of incomplete infarction.264,265 Three mechanisms have been proposed for the WM findings in AD.
First, CAA occurs in up to 98% of AD cases and causes microvascular alterations, including WM ischemia and lacunar infarction.266 Deposition of congophilic β-amyloid in cerebral arteries and arterioles predisposes to lobar hemorrhage and ischemic WM lesions via occlusive vascular disease,266 and AD patients may have microbleeds on MRI, but they are not at increased risk of lobar hemorrhage.267 The clinical effect of CAA-related WM lesions in AD is an area of active investigation because early evidence indicates that the amount of CAA pathology correlates with cognitive performance.268
Next is the controversial area of mixed AD and vascular dementia (VaD). Leukoaraiosis is probably of ischemic origin226 and probably lies on a continuum with Binswanger’s disease,269,270 but its frequent presence in AD brains raises the question of a vascular contribution to AD. AD and VaD have been regarded as distinguishable clinically and neuropathologically, but substantial overlap of AD and VaD is now acknowledged.271 Cerebrovascular risk factors have recently been suggested to contribute etiologically to AD,271 but these factors may simply reflect the co-occurrence of common age-related conditions rather than causal relationships.271 Although much overlap exists, AD and VaD can be differentiated clinically when present in pure form.
A third explanation for WM changes in AD is Wallerian degeneration from loss of neocortical pyramidal neurons in affected cortical areas. More severe LA seen in AD patients has been suggested to result from Wallerian degeneration,272 and DTI studies of AD have found microstructural WM damage in the CC and temporal, frontal, and parietal lobe WM consistent with Wallerian degeneration due to neocortical neuronal loss.273 Disconnection of cerebral association areas from related cortical and subcortical regions by these WM changes may thus be an additional burden in AD and mixed dementia.
Nature of Cognitive Impairment Associated with WMHs
WMHs most severely affect information processing speed and executive function.201,251,254,274–280 Memory is affected to a lesser degree,255,276,281 and therefore diagnostic instruments that are heavily weighted toward memory, such as the Mini-Mental State Exam, may underestimate the degree of dysfunction. However, the type of memory impairment may be critical because evidence suggests that WM disorders tend to display impaired memory retrieval rather than encoding, and WMH may be more usefully studied with measures that differentiate these memory components.116 Impaired connectivity is presumed to be the mechanism by which WMHs cause cognitive dysfunction, although direct evidence to support this hypothesis is relatively scarce.281 Some studies suggest that periventricular WMHs are of greater significance than subcortical lesions, perhaps reflecting the importance of long association fibers in brain networks subserving cognition, as discussed earlier.251
A substantial literature links WMH with depressive symptoms and major depressive disorder in the elderly.282,283 Cognitive impairments associated with WMH, including impaired processing speed, are also described in depression. The association between WMH and depression does not seem to be restricted to those with mild cognitive impairment, dementia, or pseudodementia. This finding has given rise to a vascular depression hypothesis of late-onset depression,284 supported by the observation that response to antidepressant treatment may be worse in those with higher burden of WMH.285,286 This clinical scenario also raises the issue of pseudodepression, that is, apathy and abulia from WM disease masquerading as a primary affective disorder.
Studies examining the relationship between cognition and WMH have been almost entirely limited to global measures of WMH volume or global WMH severity. However, if WMHs do cause WM dysfunction by disrupting specific WM tracts, then these lesions in specific anatomic locations, rather than global extent, should more closely be linked with neuropsychological test performance. One study correlated frontal and temporal WMHs with executive function and memory, respectively,274 but the use of large regions of interest encompassing the entire lobar WM did not allow for more precise localization of the WM tracts potentially involved. Another approach was to grade abnormalities in a specific WM pathway related to memory.287 Some investigators have used anatomically coregistered images to produce statistical parametric maps of WM regions where WMH frequency correlates with depressive symptoms.288,289 WM lesions in the prefrontal cortex have been associated with impaired functional MRI activation of dorsal prefrontal cortex.281 Also, several studies have attempted to link regional WMH, grouped by lobe, with regional cortical metabolism or perfusion.277,290 In general, there has been agreement that frontal lobe hypometabolism is a feature of subcortical small-vessel disease including WMH,277,290–293 and a link with frontal WMH has sometimes been found292 but not uniformly established.290
Synopsis of Neurobehavioral Syndromes of Cerebral WM
The disorders that lead to alterations in cerebral WM are remarkably heterogeneous, but they may reasonably be considered as a group with respect to their neurobehavioral manifestations. The available literature indicates that these disorders are associated with focal neurobehavioral syndromes, neuropsychiatric conditions, and cognitive dysfunction or dementia.
Focal Neurobehavioral Syndromes
The neurobehavioral syndromes related to focal WM lesions are familiar to neurologists from the classic literature describing aphasia, apraxia, agnosia, callosal disconnection, and related syndromes.4,5,116 Most result from stroke, although occasionally focal tumors and demyelinative plaques are responsible. These cases are comparatively rare; well-defined, isolated focal WM lesions that correlate convincingly with a given neurobehavioral deficit are unusual. These are exemplified by neglect syndromes from lesions in the anterior limb and genu of the IC2,52,53 (Fig. 22A), pseudothalamic pain from lesions of the parietal WM deep to SII294 (Fig. 22B), frontal behavioral disturbances in Marchiafava–Bignami disease of the CC,295 fornix lesions that impair memory,296,297 alexia without agraphia from the classic dual lesion (splenium and left occipital pole WM28) as well as from a single subcortical lesion undercutting Wernicke’s area2 (Fig. 22D and E), volitional facial paresis from premotor subcortical lesions,2 visual loss from the WM lesions of posterior reversible encephalopathy syndrome (Fig. 22C), as well as the elementary deficits of visual loss from lesions of the geniculocalcarine pathway,298 and sensory loss299 and weakness300 from lesions of the posterior limb of the IC. Behavioral neurology is founded on observations of this kind, which remain paradigmatic of the lesion method as applied to WM as well as GM regions.301
Figure 22.
Focal WM lesions with neurobehavioral manifestations. (A) Lacune in the genu of the right internal capsule (arrow) on CT presenting with hemineglect.2,52 (B) Diagram of the WM lesion responsible for parietal pseudothalamic pain syndrome, thought to disrupt the second somatosensory cortex from thalamus.297 (C) FLAIR MRI of posterior reversible encephalopathy syndrome producing visual loss.2 (D, E) Focal WM lesion consisting of metastatic melanoma with surrounding edema, producing alexia without agraphia.
The location of the WM lesion affects the degree of recovery from deficit. Patients with aphasia recover more slowly when the lesion involves the area between the CC medially, the corona radiata laterally, and the caudate nucleus ventrally.302 This is the territory of the (1) Muratoff Bundle immediately above the head and body of the caudate nucleus, that transmits fibers from dorsal cortical areas to the caudate nucleus, and (2) of the fronto-occipital fasciculus that links the dorsal and medial prestriate and posterior parietal cortices with the dorsolateral prefrontal cortex. This finding provides support for the notion that location of lesion (i.e., which specific WM tracts are damaged) is crucial in recovery from aphasia. It also emphasizes the importance of intact communication between the cortical and subcortical nodes in the distributed neural circuits that support language processing.
Neuropsychiatric Syndromes
Several neuropsychiatric symptoms have been described in association with cerebral WM pathology. Although these presentations are diverse and etiologically puzzling, a postulated link between WM abnormalities and psychiatric dysfunction is common in the literature.116 These associations have been pursued in two complementary ways. First, this idea has been pursued by exploring psychiatric phenomena in WM disorders, as in the case of MS, in which depression, mania, psychosis, and euphoria have all been examined.116 Second, interest has developed in the potential relevance of WM dysfunction in psychiatric diseases, as exemplified by the study of myelin dysfunction in schizophrenia303 and by a study showing abnormalities in the uncinate fasciculus in patients with schizophrenia and schizotypal personality disorder.304
In addition to acquired and genetically determined diseases, some neuropsychiatric disorders have been postulated to result from disruption of the natural phenomenon of pruning of excess axons in the developing brain.305,306 This association has been observed in boys with early infantile autism in whom the volume of cerebral WM is greater than in age-matched control subjects.307 Pruning of axonal connections during development appears necessary for optimal sculpting of neural circuits. Persistence into adulthood of excess and chaotically organized fiber systems may be as detrimental to healthy cognition as the loss of axonal connections is in the mature brain.
WM Dementia
The most important neurobehavioral syndrome related to cerebral WM damage is WM dementia (WMD). This category was formally introduced in 1988 in an effort to define the dementia syndrome that occurs in patients with widespread cerebral WM involvement.6 All the WM disorders can produce WMD (Fig. 23), representing an important source of disability, although milder cognitive dysfunction may be the presenting feature. WMD can be difficult to detect because early neurobehavioral features are often subtle, elemental neurologic manifestations are variably present, and establishing the diagnosis of the primary WM disorder can be challenging,116 but attention to this syndrome and its earliest appearance are key clinical goals. A profile of neurobehavioral features typical of WMD has been postulated to include executive dysfunction, memory retrieval deficit, visuospatial impairment, and psychiatric disorder, with relatively preserved language, normal extrapyramidal function, and normal procedural memory. WMD is thus distinct both from cortical dementia, in which memory encoding and language are usually impaired,308 and from subcortical GM dementia, in which extrapyramidal function and procedural memory are typically affected.309 Given the impressive number of WM disorders at all ages that produce WMD and other neurobehavioral syndromes, an awareness of the role of WM in cognition and behavior will enhance understanding of brain–behavior relationships and improve patient care.
Figure 23.

FLAIR MRI in the axial plane of an 80-year-old man with slowly evolving WM dementia. No single cause has been identified for the cognitive decline or WM hyperintensities.2
Summary and Conclusion
The distributed neural circuits that subserve behavior are topographically linked in a highly precise manner by five major groupings of fiber tracts: cortico–cortical association fibers; corticostriatal fibers; commissural fibers across the hemispheres; and cortico–subcortical pathways linking cerebral cortex to thalamus, the pontocerebellar system, and the brain stem and spinal cord. Lesions of association fibers prevent communication between cerebral cortical areas engaged in different domains of behavior. Lesions of subcortical structures, or the projection/striatal fibers that link them with the cerebral cortex, disrupt the contribution of subcortical nodes to the ultimate behavior. Disconnection syndromes may thus be regarded as resulting not only from lesions of the cerebral cortex but also from lesions of subcortical structures themselves, and of the WM tracts that link the nodes that make up the distributed circuits. The nature and the severity of the clinical manifestations of subcortical and WM lesions are determined, in large part, by the location, extent, and timing of onset of the underlying pathology. Discrete neurological and neuropsychiatric symptoms result from focal WM lesions. Cognitive impairment across multiple domains—WMD—is now recognized in the setting of diffuse WM disease. Unresolved issues relating to the significance and prevention of WMH in the elderly require further study. We hope that this synthetic review of WM diseases and their neurobehavioral manifestations may further the understanding, diagnosis, and treatment of these disorders.
Acknowledgments
Supported in part by National Institute of Mental Health grant 1R01MH067980 and the Birmingham Foundation. The assistance of Jason MacMore, BA, is gratefully acknowledged.
Footnotes
Conflicts of Interest
The authors declare no conflicts of interest.
References
- 1.Neuburger M. Die historische Entwicklung der experimentellen Gehirn- und Rückenmarksphysiologie vor Flourens. Ferdinand Enke Verlag, Stuttgart. Translated and edited, with additional material, by Edwin Clarke (1981) The Historical Development of Experimental Brain and Spinal Cord Physiology before Flourens. Johns Hopkins University Press; Baltimore and London: 1897. [Google Scholar]
- 2.Schmahmann JD, Pandya DN. Fiber Pathways of the Brain. Oxford University Press; New York: 2006. [Google Scholar]
- 3.Schmahmann JD, Pandya DN. Cerebral white matter–historical evolution of facts and notions concerning the organization of the fiber pathways of the brain. J Hist Neurosci. 2007;16:237–267. doi: 10.1080/09647040500495896. [DOI] [PubMed] [Google Scholar]
- 4.Geschwind N. Disconnexion syndromes in animals and man. I. Brain. 1965;88:237–294. doi: 10.1093/brain/88.2.237. [DOI] [PubMed] [Google Scholar]
- 5.Geschwind N. Disconnexion syndromes in animals and man. II. Brain. 1965;88:585–644. doi: 10.1093/brain/88.3.585. [DOI] [PubMed] [Google Scholar]
- 6.Filley CM, Franklin GM, Heaton RK, Rosenberg NL. White matter dementia. Clinical disorders and implications. Neuropsychiatry Neuropsychol Behav Neurol. 1988;1:239–254. [Google Scholar]
- 7.Nauta WJH. Some efferent connections of the prefrontal cortex in the monkey. In: Waren JM, Akert K, editors. The Frontal Granular Cortex and Behavior. McGraw-Hill; New York: 1964. pp. 397–409. [Google Scholar]
- 8.Luria AR. Higher cortical functions in man. Prefaces to the English. In: Teuber H-L, Pribram KH, editors. Authorized Translation from the Russian, by Basil Haigh. Basic Books; New York: 1966. [Google Scholar]
- 9.Pandya DN, Kuypers HG. Cortico-cortical connections in the rhesus monkey. Brain Res. 1969;13:13–36. doi: 10.1016/0006-8993(69)90141-3. [DOI] [PubMed] [Google Scholar]
- 10.Jones EG, Powell TP. An anatomical study of converging sensory pathways within the cerebral cortex of the monkey. Brain. 1970;93:793–820. doi: 10.1093/brain/93.4.793. [DOI] [PubMed] [Google Scholar]
- 11.Mesulam MM. A cortical network for directed attention and unilateral neglect. Ann Neurol. 1981;10:309–325. doi: 10.1002/ana.410100402. [DOI] [PubMed] [Google Scholar]
- 12.Mesulam M-M. Principles of Behavioral and Cognitive Neurology. 2. Oxford University Press; New York: 2000. [Google Scholar]
- 13.Ungerleider LG, Mishkin M. Two cortical visual systems. In: Ingle DJ, Goodale MA, Mansfield RJW, editors. Analysis of Visual Behavior. MIT Press; Cambridge, MA: 1982. pp. 549–586. [Google Scholar]
- 14.Goldman-Rakic PS. Topography of cognition: parallel distributed networks in primate association cortex. Annu Rev Neurosci. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- 15.Basser PJ, Mattiello J, LeBihan D. MR diffusion tensor spectroscopy and imaging. Biophys J. 1994;66:259–267. doi: 10.1016/S0006-3495(94)80775-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Wedeen VJ, David TL, Weiskoff RM, et al. White matter connectivity explored by MRI. Hum Brain Mapp. 1995;(Suppl 1):36. [Google Scholar]
- 17.Wedeen VJ, Wang RP, Schmahmann JD, et al. Diffusion spectrum magnetic resonance imaging (DSI) tractography of fiber crossings. NeuroImage. 2008;41:1267–1277. doi: 10.1016/j.neuroimage.2008.03.036. [DOI] [PubMed] [Google Scholar]
- 18.Conturo TE, Lori NF, Cull TS, et al. Tracking neuronal fiber pathways in the living human brain. Proc Natl Acad Sci USA. 1999;96:10422–10427. doi: 10.1073/pnas.96.18.10422. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Catani M, Howard RJ, Pajevic S, Jones DK. Virtual in vivo interactive dissection of white matter fasciculi in the human brain. NeuroImage. 2002;17:77–94. doi: 10.1006/nimg.2002.1136. [DOI] [PubMed] [Google Scholar]
- 20.Garrison FH. In: History of neurology. Revised and enlarged with a bibliography of classical, original, and standard works in neurology. McHenry Lawrence C., Jr, editor. Thomas; Springfield, IL: 1969. foreword by Derek E. Denny-Brown. [Google Scholar]
- 21.Steno (Stensen) N. Parisiis edito 1669. Latinitate donata, opera & studio Guidonis Fanosii; 1671. Dissertatio de cerebri anatome, spectatissimis viris dd Societatis apud dominum Thevenot collectae, dictata, atque è gallico exemplari. [Google Scholar]
- 22.Reil JC. Archiv für die Physiologie. Halle, Curtschen Buchhandlung. 1809;9:136–208. [Google Scholar]
- 23.Burdach KF. Zweyter Band, 1822. Leipzig: 1819–1826. Vom Baue und Leben des Gehirns. (CF) [Google Scholar]; Dritter band. Leipzig: 1826. In der Dyk’schen Buch-handlung. [Google Scholar]
- 24.Gall FJ, Spurzheim G. Anatomie et Physiologie du Systeme Nerveux en general, et du Cerveau en particulier. Atlas. Paris: Chez F. Schoell; 1810. [Google Scholar]
- 25.Meynert T. Vom Gerhirne der Säugethiere. In: Stricker S, editor. Handbuch der Lehre von den Geweben des Menschen und der Thiere. Vol. 2. Leipzig: Englemann; 1871–1872. pp. 694–808. Translated by Henry Power in three volumes (1870–1873) [Google Scholar]; Meynert T. The brain of mammals. In: Stricker S, editor. Manual of human and comparative histology. Vol. 2. New Sydenham Society; London: 1872. pp. 367–537. [Google Scholar]
- 26.Dejerine JJ. Anatomie des Centres Nerveux. Rueff et Cie; Paris: 1895. [Google Scholar]
- 27.Wernicke C. Der aphasische Symptomencomplex. Eine psychologische Studie auf anatomischer Basis. Breslau; Cohn: 1874. [Google Scholar]
- 28.Dejerine JJ. Contribution à l’étude anatomopathologique et clinique des différentes variétés de cécité verbale. Mém Soc Biol. 1892;4:61–90. [Google Scholar]
- 29.Schmahmann JD, Pandya DN. Disconnection syndromes of basal ganglia, thalamus and cerebrocerebellar systems. Cortex. 2008;44:1037–1066. doi: 10.1016/j.cortex.2008.04.004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Schmahmann JD, Pandya DN, Wang R, et al. Association fiber pathways of the brain: pParallel observations from diffusion spectrum imaging and autoradiography. Brain. 2007;130:630–653. doi: 10.1093/brain/awl359. [DOI] [PubMed] [Google Scholar]
- 31.Makris N, Worth AJ, Sorensen AG, et al. Morphometry of in vivo human white matter association pathways with diffusion-weighted magnetic resonance imaging. Ann Neurol. 1997;42:951–962. doi: 10.1002/ana.410420617. [DOI] [PubMed] [Google Scholar]
- 32.Makris N, Kennedy DN, McInerney S, et al. Segmentation of subcomponents within the superior longitudinal fascicle in humans: a quantitative, in vivo, DT-MRI study. Cereb Cortex. 2005;15:854–869. doi: 10.1093/cercor/bhh186. [DOI] [PubMed] [Google Scholar]
- 33.Léhericy S, Ducros M, Krainik A, et al. 3-D diffusion tensor axonal tracking shows distinct SMA and pre-SMA projections to the human striatum. Cereb Cortex. 2004;14:1302–1309. doi: 10.1093/cercor/bhh091. [DOI] [PubMed] [Google Scholar]
- 34.Johansen-Berg H, Behrens TE, Sillery E, et al. Functional-anatomical validation and individual variation of diffusion tractography-based segmentation of the human thalamus. Cereb Cortex. 2005;15:31–39. doi: 10.1093/cercor/bhh105. [DOI] [PubMed] [Google Scholar]
- 35.Raichle ME, MacLeod AM, Snyder AZ, et al. A default mode of brain function. Proc Natl Acad Sci USA. 2001;98:676–682. doi: 10.1073/pnas.98.2.676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Greicius MD, Supekar K, Menon V, Dougherty RF. Resting-State Functional Connectivity Reflects Structural Connectivity in the Default Mode Network. Cereb Cortex. 2008 Apr 9; doi: 10.1093/cercor/bhn059. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Ghashghaei HT, Barbas H. Pathways for emotion: interactions of prefrontal and anterior temporal pathways in the amygdala of the rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- 38.Foltz EL, White LE. Pain “relief” by frontal cingulumotomy. J Neurosurg. 1962;19:89–100. doi: 10.3171/jns.1962.19.2.0089. [DOI] [PubMed] [Google Scholar]
- 39.Ballantine HT, Cassidy WL, Flanagan NB, Marino R., Jr Stereotaxic anterior cingulotomy for neuropsychiatric illness and intractable pain. J Neurosurg. 1967;26:488–495. doi: 10.3171/jns.1967.26.5.0488. [DOI] [PubMed] [Google Scholar]
- 40.Ballantine HT, Bouckoms AJ, Thomas EK, Gitiunas IE. Treatment of psychiatric illness by stereotactic cingulotomy. Biol Psychiatry. 1987;22L:807–819. doi: 10.1016/0006-3223(87)90080-1. [DOI] [PubMed] [Google Scholar]
- 41.Jenike MA, Baer L, Ballantine HT, et al. Cingulotomy for refractory obsessive-compulsive disorder. Arch Gen Psychiatry. 1991;48:548–555. doi: 10.1001/archpsyc.1991.01810300060009. [DOI] [PubMed] [Google Scholar]
- 42.Spangler WJ, Cosgrove GR, Ballantine HT, et al. Magnetic resonance image-guided stereotactic cingulotomy for intractable psychiatric disease. Neurosurgery. 1996;38:1071–1078. [PubMed] [Google Scholar]
- 43.Price BH, Baral I, Cosgrove GR, et al. Improvement in severe self-mutilation following limbic leucotomy: A series of 5 consecutive cases. J Clin Psychiatry. 2001;62:925–932. doi: 10.4088/jcp.v62n1202. [DOI] [PubMed] [Google Scholar]
- 44.Cosgrove GR, Rauch SL. Stereotactic cingulotomy. Neurosurg Clin N Am. 2003;14:225–235. doi: 10.1016/s1042-3680(02)00115-8. [DOI] [PubMed] [Google Scholar]
- 45.Schmahmann JD, Pandya DN. The complex history of the fronto-occipital fasciculus. J Hist Neurosci. 2007;16:362–377. doi: 10.1080/09647040600620468. [DOI] [PubMed] [Google Scholar]
- 46.Gazzaniga MS. The human brain is actually two brains, each capable of advanced mental functions. When the cerebrum is divided surgically, it is as if the cranium contained two separate spheres of consciousness. Sci Am. 1967;217:24–29. [PubMed] [Google Scholar]
- 47.Gazzaniga MS. Cerebral specialization and interhemispheric communication: does the corpus callosum enable the human condition? Brain. 2000;123:1293–1326. doi: 10.1093/brain/123.7.1293. [DOI] [PubMed] [Google Scholar]
- 48.Sperry RW. The great cerebral commissure. Sci Am. 1964;210:42–52. doi: 10.1038/scientificamerican0164-42. [DOI] [PubMed] [Google Scholar]
- 49.Sperry R. Consciousness, personal identity and the divided brain. Neuropsychologia. 1984;22:661–673. doi: 10.1016/0028-3932(84)90093-9. [DOI] [PubMed] [Google Scholar]
- 50.Bogen JE, Bogen GM. Creativity and the corpus callosum. Psychiatr Clin North Am. 1988;11:293–301. [PubMed] [Google Scholar]
- 51.Demeter S, Rosene DL, Van Hoesen GW. Interhemispheric pathways of the hippocampal formation, presubiculum, and entorhinal and posterior parahippocampal cortices in the rhesus monkey: the structure and organization of the hippocampal commissures. J Comp Neurol. 1985;233:30–47. doi: 10.1002/cne.902330104. [DOI] [PubMed] [Google Scholar]
- 52.Schmahmann JD. Boston Society of Neurology and Psychiatry. 1984. Hemi-inattention from right hemisphere subcortical infarction. [Google Scholar]
- 53.Tatemichi TK, Desmond DW, Prohovnik I, et al. Confusion and memory loss from capsular genu infarction: a thalamocortical disconnection syndrome? Neurology. 1992;42:196–1979. doi: 10.1212/wnl.42.10.1966. [DOI] [PubMed] [Google Scholar]
- 54.Chukwudelunzu FE, Meschia JF, Graff-Radford NR, Lucas JA. Extensive metabolic and neuropsychological abnormalities associated with discrete infarction of the genu of the internal capsule. J Neurol Neurosurg Psychiatry. 2001;71:658–662. doi: 10.1136/jnnp.71.5.658. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 55.Anderson D, Ahmed A. Treatment of patients with intractable obsessive-compulsive disorder with anterior capsular stimulation. Case report. J Neurosurg. 2003;98:1104–1108. doi: 10.3171/jns.2003.98.5.1104. [DOI] [PubMed] [Google Scholar]
- 56.Kumar K, Toth C, Nath RK. Deep brain stimulation for intractable pain: a 15-year experience. Neurosurgery. 1997;40:736–746. doi: 10.1097/00006123-199704000-00015. [DOI] [PubMed] [Google Scholar]
- 57.von Bechterew W. Zur Anatomie der Schenkel des Kleinhirns, insbesondere der Brückenarme. Neurologisches Centralblatt. 1885;4:121–125. [Google Scholar]
- 58.Schmahmann JD. An emerging concept: The cerebellar contribution to higher function. Arch Neurol. 1991;48:1178–1187. doi: 10.1001/archneur.1991.00530230086029. [DOI] [PubMed] [Google Scholar]
- 59.Schmahmann JD. Disorders of the cerebellum. Ataxia, dysmetria of thought, and the cerebellar cognitive affective syndrome. J Neuropsychiatry Clin Neurosci. 2004;16:367–378. doi: 10.1176/jnp.16.3.367. [DOI] [PubMed] [Google Scholar]
- 60.Bezman L, Moser AB, Raymond GV, et al. Adrenoleukodystrophy: incidence, new mutation rate, and results of extended family screening. Ann Neurol. 2001;49:512–517. [PubMed] [Google Scholar]
- 61.Moser H, Smith K, Watkins P, et al. X-linked adrenoleukodystrophy. In: Scriver C, et al., editors. The Metabolic and Molecular Bases of Inherited Disease. McGraw-Hill; New York: 2000. pp. 3257–3301. [Google Scholar]
- 62.van Geel BM, Bezman L, Loes DJ, et al. Evolution of phenotypes in adult male patients with X-linked adrenoleukodystrophy. Ann Neurol. 2001;49:186–194. doi: 10.1002/1531-8249(20010201)49:2<186::aid-ana38>3.0.co;2-r. [DOI] [PubMed] [Google Scholar]
- 63.Eichler FS, Mahmood A, Loes D, et al. Magnetic resonance imaging detection of lesion progression in adult patients with X-linked adrenoleukodystrophy. Arch Neurol. 2007:659–664. doi: 10.1001/archneur.64.5.659. [DOI] [PubMed] [Google Scholar]
- 64.Edwin D, Speedie LJ, Kohler W, et al. Cognitive and brain magnetic resonance imaging findings in adrenomyeloneuropathy. Ann Neurol. 1996;40:675–678. doi: 10.1002/ana.410400419. [DOI] [PubMed] [Google Scholar]
- 65.Aubourg P, Sellier N, Chaussain JL, Kalifa G. MRI detects cerebral involvement in neurologically asymptomatic patients with adrenoleukodystrophy. Neurology. 1989;39:1619–1621. doi: 10.1212/wnl.39.12.1619. [DOI] [PubMed] [Google Scholar]
- 66.Melhem ER, Barker PB, Raymond GV, Moser HW. X-linked adrenoleukodystrophy in children: review of genetic, clinical, and MR imaging characteristics. AJR Am J Roentgenol. 1999;173:1575–1581. doi: 10.2214/ajr.173.6.10584804. [DOI] [PubMed] [Google Scholar]
- 67.Melhem ER, Loes DJ, Georgiades CS, et al. X-linked adrenoleukodystrophy: the role of contrast-enhanced MR imaging in predicting disease progression. AJNR Am J Neuroradiol. 2000;21:839–844. [PMC free article] [PubMed] [Google Scholar]
- 68.Eichler FS, Barker PB, Cox C, et al. Proton MR spectroscopic imaging predicts lesion progression on MRI in X-linked adrenoleukodystrophy. Neurology. 2002;58:901–907. doi: 10.1212/wnl.58.6.901. [DOI] [PubMed] [Google Scholar]
- 69.Eichler FS, Itoh R, Barker PB, et al. Proton MR spectroscopic and diffusion tensor brain MR imaging in X-linked adrenoleukodystrophy: initial experience. Radiology. 2002;225:245–252. doi: 10.1148/radiol.2251011040. [DOI] [PubMed] [Google Scholar]
- 70.Schaumburg HH, Powers JM, Raine CS, et al. Adrenoleukodystrophy. A clinical and pathological study of 17 cases. Arch Neurol. 1975;32:577–591. doi: 10.1001/archneur.1975.00490510033001. [DOI] [PubMed] [Google Scholar]
- 71.Eichler FS, Ren JQ, Cossoy M, et al. Is microglial apoptosis an early pathogenic change in cerebral X-ALD? Ann Neurol. 2008;63:729–742. doi: 10.1002/ana.21391. [DOI] [PubMed] [Google Scholar]
- 72.Austin JH. Metachromatic sulfatides in cerebral white matter and kidney. Proc Soc Exp Biol Med. 1959;100:361–364. doi: 10.3181/00379727-100-24627. [DOI] [PubMed] [Google Scholar]
- 73.Heinisch U, Zlotogora J, Kafert S, Gieselmann V. Multiple mutations are responsible for the high frequency of metachromatic leukodystrophy in a small geographic area. Am J Hum Genet. 1995;56:51–57. [PMC free article] [PubMed] [Google Scholar]
- 74.Hagberg B. Clinical aspects of globoid cell and metachromatic leukodystrophies. Birth Defects Orig Artic Ser. 1971;7:103–112. [PubMed] [Google Scholar]
- 75.Von Hirsch T, Peiffer J. Histological methods in differential diagnosis of leukodystrophy from lipoidosis. Arch Psychiatr Nervenkr Z Gesamte Neurol Psychiatr. 1955;194:88–104. doi: 10.1007/BF00392351. [DOI] [PubMed] [Google Scholar]
- 76.Austin J, Armstrong D, Fouch S, et al. Metachromatic leukodystrophy (MLD). VIII. MLD in adults: diagnosis and pathogenesis. Arch Neurol. 1968;18:225–240. doi: 10.1001/archneur.1968.00470330015001. [DOI] [PubMed] [Google Scholar]
- 77.Filley CM, Gross KF. Psychosis with cerebral white matter disease. Neuropsychiatry Neuropsychol Behav Neurol. 1992;5:119–125. [Google Scholar]
- 78.Hyde TM, Ziegler JC, Weinberger DR. Psychiatric disturbances in metachromatic leukodystrophy. Insights into the neurobiology of psychosis. Arch Neurol. 1992;49:401–406. doi: 10.1001/archneur.1992.00530280095028. [DOI] [PubMed] [Google Scholar]
- 79.Faerber EN, Melvin J, Smergel EM. MRI appearances of metachromatic leukodystrophy. Pediatr Radiol. 1999;29:669–672. doi: 10.1007/s002470050672. [DOI] [PubMed] [Google Scholar]
- 80.Van Der Voorn JP, Kamphorst W, van der Knaap MS, Powers JM. The leukoencephalopathy of infantile GM1gangliosidosis: oligo-dendrocytic loss and axonal dysfunction. Acta Neuropathol (Berl) 2004;107:539–545. doi: 10.1007/s00401-004-0848-9. [DOI] [PubMed] [Google Scholar]
- 81.Kobayashi T, Shinnoh N, Goto I, et al. Galactosylceramide- and lactosylceramide-loading studies in cultured fibroblasts from normal individuals and patients with globoid cell leukodystrophy (Krabbe’s disease) and GM1 gangliosidosis. Biochim Biophys Acta. 1985;835:456–464. [PubMed] [Google Scholar]
- 82.Miyatake T, Suzuki K. Globoid cell leukodystrophy: additional deficiency of psychosine galactosidase. Biochem Biophys Res Commun. 1972;48:539–543. doi: 10.1016/0006-291x(72)90381-6. [DOI] [PubMed] [Google Scholar]
- 83.Wenger D, Suzuki K, Suzuki YS. Galactosylceramide lipidosis: Globoid cell leukodystrophy (Krabbe Disease) In: Scriver C, et al., editors. The Metabolic and Molecular Basis of Inherited Disease. McGraw-Hill; New York: 2000. pp. 3669–3694. [Google Scholar]
- 84.Lyon G, Hagberg B, Evrard P, et al. Symptomatology of late onset Krabbe’s leukodystrophy: the European experience. Dev Neurosci. 1991;13:240–244. doi: 10.1159/000112167. [DOI] [PubMed] [Google Scholar]
- 85.Barone R, Bruhl K, Stoeter P, et al. Clinical and neuroradiological findings in classic infantile and late-onset globoid-cell leukodystrophy (Krabbe disease) Am J Med Genet. 1996;63:209–217. doi: 10.1002/(SICI)1096-8628(19960503)63:1<209::AID-AJMG37>3.0.CO;2-Q. [DOI] [PubMed] [Google Scholar]
- 86.Farina L, Bizzi A, Finocchiaro G, et al. MR imaging and proton MR spectroscopy in adult Krabbe disease. AJNR Am J Neuroradiol. 2000;21:1478–1482. [PMC free article] [PubMed] [Google Scholar]
- 87.Baram TZ, Goldman AM, Percy AK. Krabbe disease: specific MRI and CT findings. Neurology. 1986;36:111–115. doi: 10.1212/wnl.36.1.111. [DOI] [PubMed] [Google Scholar]
- 88.Nagar VA, Ursekar MA, Krishnan P, Jankharia BG. Krabbe disease: unusual MRI findings. Pediatr Radiol. 2006;36:61–64. doi: 10.1007/s00247-005-0008-y. [DOI] [PubMed] [Google Scholar]
- 89.Powers JM. A neuropathologic overview of the neurodystrophies and neurolipidoses. In: Moser HW, editor. Neurodystrophies and Neurolipidosis. Vol. 22. Elsevier Science B.V; 1996. [Google Scholar]
- 90.Sourander P, Hansson HA, Olsson Y, Svennerholm L. Experimental studies on the pathogenesis of leucodystrophies. II. The effect of sphingolipids on various cell types in cultures from the nervous system. Acta Neuropathol (Berl) 1966;6:231–242. doi: 10.1007/BF00687853. [DOI] [PubMed] [Google Scholar]
- 91.Takashima S, Matsui A, Fujii Y, Nakamura H. Clinicopathological differences between juvenile and late infantile metachromatic leukodystrophy. Brain Dev. 1981;3:365–374. doi: 10.1016/s0387-7604(81)80065-4. [DOI] [PubMed] [Google Scholar]
- 92.Van Der Knaap MS, Leegwater PA, Konst AA, et al. Mutations in each of the five subunits of translation initiation factor eIF2B can cause leukoencephalopathy with vanishing white matter. Ann Neurol. 2002;51:264–270. doi: 10.1002/ana.10112. [DOI] [PubMed] [Google Scholar]
- 93.Van Der Knaap MS, Pronk JC, Scheper GC. Vanishing white matter disease. Lancet Neurol. 2006;5:413–423. doi: 10.1016/S1474-4422(06)70440-9. [DOI] [PubMed] [Google Scholar]
- 94.Hanefeld F, Holzbach U, Kruse B, et al. Diffuse white matter disease in three children: an encephalopathy with unique features on magnetic resonance imaging and proton magnetic resonance spectroscopy. Neuropediatrics. 1993;24:244–248. doi: 10.1055/s-2008-1071551. [DOI] [PubMed] [Google Scholar]
- 95.Schiffmann R, Moller JR, Trapp BD, et al. Childhood ataxia with diffuse central nervous system hypomyelination. Ann Neurol. 1994;35:331–340. doi: 10.1002/ana.410350314. [DOI] [PubMed] [Google Scholar]
- 96.Scheper GC, Mulder J, Kleijn M, et al. Inactivation of eIF2B and phosphorylation of PHAS-I in heat-shocked rat hepatoma cells. J Biol Chem. 1997;272:26850–26856. doi: 10.1074/jbc.272.43.26850. [DOI] [PubMed] [Google Scholar]
- 97.Van Haren K, van der Voorn JP, Peterson DR, et al. The life and death of oligodendrocytes in vanishing white matter disease. J Neuropathol Exp Neurol. 2004;63:618–630. doi: 10.1093/jnen/63.6.618. [DOI] [PubMed] [Google Scholar]
- 98.Marotti JD, Tobias S, Fratkin JD. Adult onset leukodystrophy with neuroaxonal spheroids and pigmented glia: Report of a family, historical perspective, and review of the literature. Acta Neuropathol (Berl) 2004;107:481–488. doi: 10.1007/s00401-004-0847-x. [DOI] [PubMed] [Google Scholar]
- 99.Freeman SH, Hyman BT, Sims KB, et al. Adult onset leukodystrophy with neuroaxonal spheroids: clinical, neuroimaging and neuropathologic observations. Brain Pathol. 2008 Apr 15; doi: 10.1111/j.1750-3639.2008.00163.x. [Epub ahead of print] [DOI] [PMC free article] [PubMed] [Google Scholar]
- 100.Pavlakis SG, Phillips PC, DiMauro S, et al. Mitochondrial myopathy, encephalopathy, lactic acidosis, and strokelike episodes: a distinctive clinical syndrome. Ann Neurol. 1984;16:481–488. doi: 10.1002/ana.410160409. [DOI] [PubMed] [Google Scholar]
- 101.Hirano M, Ricci E, Koenigsberger MR, et al. Melas: an original case and clinical criteria for diagnosis. Neuromuscul Disord. 1992;2:125–135. doi: 10.1016/0960-8966(92)90045-8. [DOI] [PubMed] [Google Scholar]
- 102.Castillo M, Kwock L, Green C. MELAS syndrome: imaging and proton MR spectroscopic findings. AJNR Am J Neuroradiol. 1995;16:233–239. [PMC free article] [PubMed] [Google Scholar]
- 103.Tanahashi C, Nakayama A, Yoshida M, et al. MELAS with the mitochondrial DNA 3243 point mutation: a neuropathological study. Acta Neuropathol. 2000;99:31–38. doi: 10.1007/pl00007403. [DOI] [PubMed] [Google Scholar]
- 104.Thomeer EC, Verhoeven WM, van de Vlasakker CJ, Klompenhouwer JL. Psychiatric symptoms in MELAS; a case report. J Neurol Neurosurg Psychiatry. 1998;64:692–693. doi: 10.1136/jnnp.64.5.692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 105.Kim HG, Schmahmann JD, Sims K, et al. A neuropsychiatric presentation of mitochondrial myopathy, encephalopathy, lactic acidosis and stroke-like episodes. Med Psychiatry. 1999;2:3–9. [Google Scholar]
- 106.Leehey MA, Munhoz RP, Lang AE, et al. The fragile X premutation presenting as essential tremor. Arch Neurol. 2003;60:117–121. doi: 10.1001/archneur.60.1.117. [DOI] [PubMed] [Google Scholar]
- 107.Jacquemont S, Hagerman RJ, Leehey M, et al. Fragile X premutation tremor/ataxia syndrome: molecular, clinical, and neuroimaging correlates. Am J Hum Genet. 2003;72:869–878. doi: 10.1086/374321. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 108.Greco CM, Berman RF, Martin RM, et al. Neuropathology of fragile X-associated tremor/ataxia syndrome (FXTAS) Brain. 2006;129:243–255. doi: 10.1093/brain/awh683. [DOI] [PubMed] [Google Scholar]
- 109.Grigsby J, Brega AG, Engle K, et al. Cognitive profile of fragile X premutation carriers with and without fragile X-associated tremor/ataxia syndrome. Neuropsychology. 2008;22:48–60. doi: 10.1037/0894-4105.22.1.48. [DOI] [PubMed] [Google Scholar]
- 110.Schmahmann JD, Pandya DN. The cerebrocerebellar system. In: Schmahmann JD, editor. The Cerebellum and Cognition. Academic Press; San Diego: 1997. [Google Scholar]; Int Rev Neurobiol. 41:31–60. doi: 10.1016/s0074-7742(08)60346-3. [DOI] [PubMed] [Google Scholar]
- 111.Medana IM, Esiri MM. Axonal damage: a key predictor of outcome in human CNS diseases. Brain. 2003;126:515–530. doi: 10.1093/brain/awg061. [DOI] [PubMed] [Google Scholar]
- 112.Charcot JM. Lectures on the diseases of the nervous system delivered at La Salpêtrière. New Sydenham Society; London: 1877. [Google Scholar]
- 113.Kurtzke JF. Neurologic impairment in multiple sclerosis and the Disability Status Scale. Acta Neurol Scand. 1970;46:493–512. doi: 10.1111/j.1600-0404.1970.tb05808.x. [DOI] [PubMed] [Google Scholar]
- 114.Rao SM, Leo GJ, Bernardin L, Unverzagt F. Cognitive dysfunction in multiple sclerosis. I. Frequency, patterns, and prediction. Neurology. 1991;41:685–691. doi: 10.1212/wnl.41.5.685. [DOI] [PubMed] [Google Scholar]
- 115.Boerner RJ, Kapfhammer HP. Psychopathological changes and cognitive impairment in encephalomyelitis disseminata. Eur Arch Clin Neurosci. 1999;249:96–102. doi: 10.1007/s004060050072. [DOI] [PubMed] [Google Scholar]
- 116.Filley CM. The Behavioral Neurology of White Matter. Oxford University Press; New York: 2001a. [Google Scholar]
- 117.Wattjes MP, Harzheim M, Lutterbey GG, et al. Prognostic value of high-field proton magnetic resonance spectroscopy in patients presenting with clinically isolated syndromes suggestive of multiple sclerosis. Neuroradiology. 2008;50:123–129. doi: 10.1007/s00234-007-0325-y. [DOI] [PubMed] [Google Scholar]
- 118.Zivadinov R, Stosic M, Cox JL, et al. The place of conventional MRI and newly emerging MRI techniques in monitoring different aspects of treatment outcome. J Neurol. 2008;255(Suppl 1):61–74. doi: 10.1007/s00415-008-1009-1. [DOI] [PubMed] [Google Scholar]
- 119.Stadelmann C, Albert M, Wegner C, Brück W. Cortical pathology in multiple sclerosis. Curr Opin Neurol. 2008;1:229–234. doi: 10.1097/01.wco.0000318863.65635.9a. [DOI] [PubMed] [Google Scholar]
- 120.Catalaa I, Fulton JC, Zhang X, et al. MR imaging quantitation of gray matter involvement in multiple sclerosis and its correlation with disability measures and neurocognitive testing. AJNR Am J Neuroradiol. 1999;20:1613–1618. [PMC free article] [PubMed] [Google Scholar]
- 121.Filley CM. Neurobehavioral Anatomy. 2. University Press of Colorado; Boulder, CO: 2001. [Google Scholar]
- 122.de Seze J, Debouverie M, Zephir H, et al. Acute fulminant demyelinating disease: a descriptive study of 60 patients. Arch Neurol. 2007;64:1426–1432. doi: 10.1001/archneur.64.10.1426. [DOI] [PubMed] [Google Scholar]
- 123.Young NP, Weinshenker BG, Lucchinetti CF. Acute disseminated encephalomyelitis: current understanding and controversies. Semin Neurol. 2008;28:84–94. doi: 10.1055/s-2007-1019130. [DOI] [PubMed] [Google Scholar]
- 124.Sonneville R, Demeret S, Klein I, et al. Acute disseminated encephalomyelitis in the intensive care unit: clinical features and outcome of 20 adults. Intensive Care Med. 2008;34:528–532. doi: 10.1007/s00134-007-0926-2. [DOI] [PubMed] [Google Scholar]
- 125.Tenembaum S, Chitnis T, Ness J, Hahn JS International Pediatric MS Study Group . Acute disseminated encephalomyelitis. Neurology. 2007;68(16 Suppl 2):S23–S36. doi: 10.1212/01.wnl.0000259404.51352.7f. [DOI] [PubMed] [Google Scholar]
- 126.Navia BA, Cho ES, Petito CK, Price RW. The AIDS dementia complex: II. Neuropathology. Ann Neurol. 1986;19:525–535. doi: 10.1002/ana.410190603. [DOI] [PubMed] [Google Scholar]
- 127.Jones HR, Ho DD, Forgacs P, et al. Acute fulminating fatal leukoencephalopathy as the only mainfestation of human immunodeficiency virus infection. Ann Neurol. 1988;23:519–522. doi: 10.1002/ana.410230515. [DOI] [PubMed] [Google Scholar]
- 128.Navia BA, Jordan BD, Price RW. The AIDS dementia complex: I. Clinical features. Ann Neurol. 1986;19:517–524. doi: 10.1002/ana.410190602. [DOI] [PubMed] [Google Scholar]
- 129.Tozzi V, Narciso P, Galgani S, et al. Effects of zidovudine in 30 patients with mild to end-stage AIDS dementia complex. AIDS. 1993;7:683–692. doi: 10.1097/00002030-199305000-00012. [DOI] [PubMed] [Google Scholar]
- 130.Fillippi CG, Sze G, Farber SJ, et al. Regression of HIV encephalopathy and basal ganglia signal intensity abnormality at MR imaging in patient with AIDS after the initiation of protease inhibitor therapy. Radiology. 1998;206:491–498. doi: 10.1148/radiology.206.2.9457204. [DOI] [PubMed] [Google Scholar]
- 131.Thurnher MM, Schindler EG, Thurnher SA, et al. Highly active antiretroviral therapy for patients with AIDS dementia complex: effect on MR imaging findings and clinical course. AJNR Am J Neuroradiol. 2000;21:670–678. [PMC free article] [PubMed] [Google Scholar]
- 132.Chiang MC, Dutton RA, Hayashi KM, et al. 3D patterns of brain atrophy in HIV/AIDS visualized using tensor-based morphometry. NeuroImage. 2007;34:44–60. doi: 10.1016/j.neuroimage.2006.08.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 133.Greenlee JE. Progressive multifocal leucoencephalopathy in the era of natalizumab: a review and discussion of the implications. Int MS J. 2006 Nov;13:100–107. [PubMed] [Google Scholar]
- 134.von Einsiedel RW, Fife TD, Aksamit AJ, et al. Progressive multifocal leukoencephalopathy in AIDS: a clinicopathologic study and review of the literature. J Neurol. 1993;240:391–406. doi: 10.1007/BF00867351. [DOI] [PubMed] [Google Scholar]
- 135.Langer-Gould A, Atlas SW, Green AJ, et al. Progressive multifocal leukoencephalopathy in a patient treated with natalizumab. N Engl J Med. 2005;353:375–381. doi: 10.1056/NEJMoa051847. [DOI] [PubMed] [Google Scholar]
- 136.Moll NM, Rietsch AM, Ransohoff AJ, et al. Cortical demyelination in PML and MS: Similarities and differences. Neurology. 2008;70:336–343. doi: 10.1212/01.WNL.0000284601.54436.e4. [DOI] [PubMed] [Google Scholar]
- 137.Garrels K, Kucharczyk W, Wortzman G, Shandling M. Progressive multifocal leukoencephalopathy: clinical and MR response to treatment. AJNR Am J Neuroradiol. 1996;17:597–600. [PMC free article] [PubMed] [Google Scholar]
- 138.Arai Y, Tsutsui Y, Nagashima K, et al. Autopsy case of the cerebellar form of progressive multifocal leukoencephalopathy without immunodeficiency. Neuropathology. 2002;22:48–56. doi: 10.1046/j.0919-6544.2001.00424.x. [DOI] [PubMed] [Google Scholar]
- 139.Kastrup O, Maschke M, Diener HC, Wanke I. Progressive multifocal leukoencephalopathy limited to the brain stem. Neuroradiology. 2002;44:227–229. doi: 10.1007/s00234-001-0714-6. [DOI] [PubMed] [Google Scholar]
- 140.Giancola ML, Rizzi EB, Lorenzini P, et al. Progressive multifocal leukoencephalopathy in HIV-infected patients in the era of HAART: radiological features at diagnosis and follow-up and correlation with clinical variables. AIDS Res Hum Retroviruses. 2008;24:155–162. doi: 10.1089/aid.2006.0252. [DOI] [PubMed] [Google Scholar]
- 141.West SG. Neuropsychiatric lupus. Rheum Dis Clin North Am. 1994;20:129–158. [PubMed] [Google Scholar]
- 142.Kozora E, Thompson LL, West SG, Kotzin BL. Analysis of cognitive and psychological deficits in systemic lupus erythematosus patients without overt central nervous system disease. Arthritis Rheum. 1996;39:2035–2045. doi: 10.1002/art.1780391213. [DOI] [PubMed] [Google Scholar]
- 143.Kirk A, Kertesz A, Polk MJ. Dementia with leukoencephalopathy in systemic lupus erythematosus. Can J Neurol Sci. 1991;18:344–348. doi: 10.1017/s0317167100031929. [DOI] [PubMed] [Google Scholar]
- 144.Kozora E, Arciniegas DB, Filley CM, et al. Cognition, MRS neurometabolites, and MRI volumetrics in non-neuropsychiatric systemic lupus erythematosus. Cogn Behav Neurol. 2005;8:159–162. doi: 10.1097/01.wnn.0000181543.05064.4b. [DOI] [PubMed] [Google Scholar]
- 145.Filley CM, Kleinschmidt-DeMasters BK. Toxic leukoencephalopathy. N Engl J Med. 2001;345:425–432. doi: 10.1056/NEJM200108093450606. [DOI] [PubMed] [Google Scholar]
- 146.Reddick WE, Glass JO, Helton KJ, et al. Prevalence of leukoencephalopathy in children treated for acute lymphoblastic leukemia with high-dose methotrexate. AJNR Am J Neuroradiol. 2005;26:1263–1269. [PMC free article] [PubMed] [Google Scholar]
- 147.Haykin ME, Gorman M, van Hoff J, et al. Diffusion-weighted MRI correlates of subacute methotrexate-related neurotoxicity. J Neurooncol. 2006;76:153–157. doi: 10.1007/s11060-005-4569-2. [DOI] [PubMed] [Google Scholar]
- 148.Hormes JT, Filley CM, Rosenberg NL. Neurologic sequelae of chronic solvent vapor abuse. Neurology. 1986;36:698–702. doi: 10.1212/wnl.36.5.698. [DOI] [PubMed] [Google Scholar]
- 149.Rosenberg NL, Spitz MC, Filley CM, et al. Central nervous system effects of chronic toluene abuse—clinical, brainstem evoked response and magnetic resonance imaging studies. Neurotoxicol Teratol. 1988;10:489–495. doi: 10.1016/0892-0362(88)90014-1. [DOI] [PubMed] [Google Scholar]
- 150.Filley CM, Heaton RK, Rosenberg NL. White matter dementia in chronic toluene abuse. Neurology. 1990;40:532–534. doi: 10.1212/wnl.40.3_part_1.532. [DOI] [PubMed] [Google Scholar]
- 151.Rosenberg NL, Kleinschmidt-DeMasters BK, Davis KA, et al. Toluene abuse causes diffuse central nervous system white matter changes. Ann Neurol. 1988;23:611–614. doi: 10.1002/ana.410230614. [DOI] [PubMed] [Google Scholar]
- 152.Filley CM, Halliday W, Kleinschmidt-DeMasters BK. The effects of toluene on the central nervous system. J Neuropathol Exp Neurol. 2004;63:1–12. doi: 10.1093/jnen/63.1.1. [DOI] [PubMed] [Google Scholar]
- 153.Filley CM. The behavioral neurology of cerebral white matter. Neurology. 1998;50:1535–1540. doi: 10.1212/wnl.50.6.1535. [DOI] [PubMed] [Google Scholar]
- 154.Kriegstein AR, Shungu DC, Millar WS, et al. Leukoencephalopathy and raised brain lactate from heroin vapor inhalation (“chasing the dragon”) Neurology. 1999;10:1765–1773. doi: 10.1212/wnl.53.8.1765. [DOI] [PubMed] [Google Scholar]
- 155.Bartlett E, Mikulis DJ. Chasing “chasing the dragon” with MRI: leukoencephalopathy in drug abuse. Br J Radiol. 2005;78:997–1004. doi: 10.1259/bjr/61535842. [DOI] [PubMed] [Google Scholar]
- 156.Offiah C, Hall E. Heroin-induced leukoencephalopathy: characterization using MRI, diffusion-weighted imaging, and MR spectroscopy. Clin Radiol. 2008;63:146–152. doi: 10.1016/j.crad.2007.07.021. [DOI] [PubMed] [Google Scholar]
- 157.Wolters EC, van Wijngaarden GK, Stam FC, et al. Leucoencephalopathy after inhaling “heroin” pyrolysate. Lancet. 1982;2:1233–1237. doi: 10.1016/s0140-6736(82)90101-5. [DOI] [PubMed] [Google Scholar]
- 158.Powell HC, Meyers RR, Lampert PW. Edema in Neurotoxic Injury. Williams & Wilkins; Baltimore: 1980. [Google Scholar]
- 159.Hinchey J, Chaves C, Appignani B, et al. A reversible posterior leukoencephalopathy syndrome. N Engl J Med. 1996;334:494–500. doi: 10.1056/NEJM199602223340803. [DOI] [PubMed] [Google Scholar]
- 160.Adams RD, Kubik CS. Subacute degeneration of the brain in pernicious anemia. N Engl J Med. 1944;231:1–9. [Google Scholar]
- 161.Chatterjee A, Yapundich R, Palmer CA, et al. Leukoencephalopathy associated with cobalamin deficiency. Neurology. 1996;46:832–834. doi: 10.1212/wnl.46.3.832. [DOI] [PubMed] [Google Scholar]
- 162.Stojsavljević N, Lević Z, Drulović J, Dragutinović G. A 44-month clinical-brain MRI follow-up in a patient with B12 deficiency. Neurology. 1997;49:878–881. doi: 10.1212/wnl.49.3.878. [DOI] [PubMed] [Google Scholar]
- 163.Lee HB, Lyketsos CG. Delayed post-hypoxic leukoencephalopathy. Psychosomatics. 2001;42:530–533. doi: 10.1176/appi.psy.42.6.530. [DOI] [PubMed] [Google Scholar]
- 164.Molloy S, Soh C, Williams TL. Reversible delayed posthypoxic leukoencephalopathy. AJNR Am J Neuroradiol. 2006;27:1763–1765. [PMC free article] [PubMed] [Google Scholar]
- 165.Chen-Plotkin AS, Pau KT, Schmahmann JD. Delayed leukoencephalopathy after hypoxic-ischemic injury. Arch Neurol. 2008;65:144–145. doi: 10.1001/archneurol.2007.7. [DOI] [PubMed] [Google Scholar]
- 166.Choi IS. Delayed neurologic sequelae in carbon monoxide intoxication. Arch Neurol. 1983;40:433–435. doi: 10.1001/archneur.1983.04050070063016. [DOI] [PubMed] [Google Scholar]
- 167.Sandson TA, Lilly RB, Sodkol M. Kluver-Bucy syndrome associated with delayed post-anoxic leukoencephalopathy following carbon monoxide poisoning. J Neurol Neurosurg Psychiatry. 1988;51:156–157. doi: 10.1136/jnnp.51.1.156. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 168.Langlois J, Rutland-Brown W, Thomas K. Traumatic Brain Injury in the United States: Emergency Department Visits, Hospitalizations and Deaths. Centers for Disease Control and Prevention, National Center for Injury Prevention; Atlanta, GA: 2004. [Google Scholar]
- 169.Adams JH, Graham DI, Murray LS, Scott G. Diffuse axonal injury due to nonmissile head injury: an analysis of 45 cases. Ann Neurol. 1982;12:557–563. doi: 10.1002/ana.410120610. [DOI] [PubMed] [Google Scholar]
- 170.Smith DH, Meaney DF, Shull WH. Diffuse axonal injury in head trauma. J Head Trauma Rehabil. 2003;18:307–316. doi: 10.1097/00001199-200307000-00003. [DOI] [PubMed] [Google Scholar]
- 171.Kraus MF, Susmaras T, Caughlin BP, et al. White matter injury and cognition in chronic traumatic brain injury: a diffusion tensor imaging study. Brain. 2007;130:2508–2519. doi: 10.1093/brain/awm216. [DOI] [PubMed] [Google Scholar]
- 172.Alexander MP. Mild traumatic brain injury: pathophysiology, natural history, and clinical management. Neurology. 1995;45:1252–1260. doi: 10.1212/wnl.45.7.1253. [DOI] [PubMed] [Google Scholar]
- 173.Adams JH, Graham DI, Jennett B. The neuropathology of the vegetative state after an acute brain insult. Brain. 2000;123:1327–1338. doi: 10.1093/brain/123.7.1327. [DOI] [PubMed] [Google Scholar]
- 174.Filley CM, Kleinschmidt-DeMasters BK. Neurobehavioral presentations of brain neoplasms. West J Med. 1995;163:19–25. [PMC free article] [PubMed] [Google Scholar]
- 175.Giese A, Westphal M. Glioma invasion in the central nervous system. Neurosurgery. 1996;39:235–250. doi: 10.1097/00006123-199608000-00001. [DOI] [PubMed] [Google Scholar]
- 176.Geer CP, Grossman SA. Interstitial flow along white matter tracts: a potentially important mechanism for the dissemination of primary brain tumors. J Neurooncol. 1997;32:193–201. doi: 10.1023/a:1005761031077. [DOI] [PubMed] [Google Scholar]
- 177.Filley CM, Kleinschmidt-DeMasters BK, Lillehei KO, et al. Gliomatosis cerebri: neurobe-havioral and neuropathological observations. Cogn Behav Neurol. 2003;16:149–159. doi: 10.1097/00146965-200309000-00002. [DOI] [PubMed] [Google Scholar]
- 178.Rollins KE, Kleinschmidt-DeMasters BK, Corboy JR, et al. Lymphomatosis cerebri as a cause of white matter dementia. Hum Pathol. 2005;36:282–290. doi: 10.1016/j.humpath.2005.01.014. [DOI] [PubMed] [Google Scholar]
- 179.Grois N, Barkovich AJ, Rosenau W, Ablin AR. Central nervous system disease associated with Langerhans’ cell histiocytosis. Am J Pediatr Hematol Oncol. 1993;15:245–254. doi: 10.1097/00043426-199305000-00014. [DOI] [PubMed] [Google Scholar]
- 180.Grois NG, Favara BE, Mostbeck GH, Prayer D. Central nervous system disease in Langerhans cell histiocytosis. Hematol Oncol Clin North Am. 1998;12:287–305. doi: 10.1016/s0889-8588(05)70511-6. [DOI] [PubMed] [Google Scholar]
- 181.Grois N, Prayer D, Prosch H, Lassmann H CNS LCH Co-operative Group. Neuropathology of CNS disease in Langerhans cell histiocytosis. Brain. 2005;128:829–838. doi: 10.1093/brain/awh403. [DOI] [PubMed] [Google Scholar]
- 182.Schmahmann JD, Weilburg JB, Sherman JC. The neuropsychiatry of the cerebellum – insights from the clinic. Cerebellum. 2007;6:254–267. doi: 10.1080/14734220701490995. [DOI] [PubMed] [Google Scholar]
- 183.Whitsett SF, Kneppers K, Coppes MJ, Egeler RM. Neuropsychologic deficits in children with Langerhans cell histiocytosis. Med Pediatr Oncol. 1999;33:486–492. doi: 10.1002/(sici)1096-911x(199911)33:5<486::aid-mpo9>3.0.co;2-j. [DOI] [PubMed] [Google Scholar]
- 184.Schmahmann JD, Sherman JC. The cerebellar cognitive affective syndrome. Brain. 1998;121:561–579. doi: 10.1093/brain/121.4.561. [DOI] [PubMed] [Google Scholar]
- 185.Levisohn L, Cronin-Golomb A, Schmahmann JD. Neuropsychological consequences of cerebellar tumour resection in children: cerebellar cognitive affective syndrome in a paediatric population. Brain. 2000;123:1041–1050. doi: 10.1093/brain/123.5.1041. [DOI] [PubMed] [Google Scholar]
- 186.Del Bigio MR. Neuropathological changes caused by hydrocephalus. Acta Neuropathol. 1993;85:573–585. doi: 10.1007/BF00334666. [DOI] [PubMed] [Google Scholar]
- 187.Del Bigio MR, da Silva MC, Drake JM, Tuor UI. Acute and chronic cerebral white matter damage in neonatal hydrocephalus. Can J Neurol Sci. 1994;21:299–305. doi: 10.1017/s0317167100040865. [DOI] [PubMed] [Google Scholar]
- 188.Adams RD, Fisher CM, Hakim S, et al. Symptomatic occult hydrocephalus with “normal” cerebrospinal-fluid pressure. N Engl J Med. 1965;273:117–126. doi: 10.1056/NEJM196507152730301. [DOI] [PubMed] [Google Scholar]
- 189.Schwarzschild M, Rordorf G, Bekken K, et al. Normal-pressure hydrocephalus with misleading features of irreversible dementias: a case report. J Geriatr Psychiatry Neurol. 1997;10:51–54. doi: 10.1177/089198879701000202. [DOI] [PubMed] [Google Scholar]
- 190.Marmarou A, Young HF, Aygok GA, et al. Diagnosis and management of idiopathic normal-pressure hydrocephalus: a prospective study in 151 patients. J Neurosurg. 2005;102:987–997. doi: 10.3171/jns.2005.102.6.0987. [DOI] [PubMed] [Google Scholar]
- 191.Earnest MP, Fahn S, Karp JH, Rowland LP. Normal pressure hydrocephalus and hypertensive cerebrovascular disease. Arch Neurol. 1974;31:262–266. doi: 10.1001/archneur.1974.00490400076009. [DOI] [PubMed] [Google Scholar]
- 192.Bech RA, Juhler M, Waldemar G, et al. Frontal brain and leptomeningeal biopsy specimens correlated with cerebrospinal fluid outflow resistance and B-wave activity in patients suspected of normal-pressure hydrocephalus. Neurosurgery. 1997;40:497–502. doi: 10.1097/00006123-199703000-00013. [DOI] [PubMed] [Google Scholar]
- 193.Peters A, Leahu D, Moss MB, McNally KJ. The effects of aging on area 46 of the frontal cortex of the rhesus monkey. Cereb Cortex. 1994;4:621–635. doi: 10.1093/cercor/4.6.621. [DOI] [PubMed] [Google Scholar]
- 194.Peters A. The effects of normal aging on myelin and nerve fibers: a review. J Neurocytol. 2002;31:581–593. doi: 10.1023/a:1025731309829. [DOI] [PubMed] [Google Scholar]
- 195.Makris N, Papadimitriou GM, Van Der Kouwe A, et al. Frontal connections and cognitive changes in normal aging rhesus monkeys: a DTI study. Neurobiol Aging. 2007;28:1556–1567. doi: 10.1016/j.neurobiolaging.2006.07.005. [DOI] [PubMed] [Google Scholar]
- 196.Hachinski VC, Potter P, Merskey H. Leuko-araiosis: an ancient term for a new problem. Can J Neurol Sci. 1986;13(4 Suppl):533–534. doi: 10.1017/s0317167100037264. [DOI] [PubMed] [Google Scholar]
- 197.Hachinski VC, Potter P, Merskey H. Leuko-araiosis. Arch Neurol. 1987;44:21–23. doi: 10.1001/archneur.1987.00520130013009. [DOI] [PubMed] [Google Scholar]
- 198.DeCarli C, Fletcher E, Ramey V, et al. Anatomical mapping of white matter hyperintensities (WMH): exploring the relationships between periventricular WMH, deep WMH, and total WMH burden. Stroke. 2005;36:50–55. doi: 10.1161/01.STR.0000150668.58689.f2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 199.O’Sullivan M, Summers PE, Jones DK, et al. Normal-appearing white matter in ischemic leukoaraiosis: a diffusion tensor MRI study. Neurology. 2001;57:2307–2310. doi: 10.1212/wnl.57.12.2307. [DOI] [PubMed] [Google Scholar]
- 200.Fernando MS, O’Brien JT, Perry RH, et al. Comparison of the pathology of cerebral white matter with post-mortem magnetic resonance imaging (MRI) in the elderly brain. Neuropathol Appl Neurobiol. 2004;30:385–395. doi: 10.1111/j.1365-2990.2004.00550.x. [DOI] [PubMed] [Google Scholar]
- 201.Au R, Massaro JM, Wolf PA, et al. Association of white matter hyperintensity volume with decreased cognitive functioning: the Framingham Heart Study. Arch Neurol. 2006;63:246–250. doi: 10.1001/archneur.63.2.246. [DOI] [PubMed] [Google Scholar]
- 202.de Leeuw FE, de Groot JC, Bots ML, et al. Carotid atherosclerosis and cerebral white matter lesions in a population based magnetic resonance imaging study. J Neurol. 2000;247:291–296. doi: 10.1007/s004150050586. [DOI] [PubMed] [Google Scholar]
- 203.de Leeuw FE, de Groot JC, Oudkerk M, et al. Hypertension and cerebral white matter lesions in a prospective cohort study. Brain. 2002;125(Pt 4):765–772. doi: 10.1093/brain/awf077. [DOI] [PubMed] [Google Scholar]
- 204.Longstreth WT, Jr, Manolio TA, Arnold A, et al. Clinical correlates of white matter findings on cranial magnetic resonance imaging of 3301 elderly people. The Cardiovascular Health Study. Stroke. 1996;27:1274–1282. doi: 10.1161/01.str.27.8.1274. [DOI] [PubMed] [Google Scholar]
- 205.de Leeuw FE, de Groot JC, Achten E, et al. Prevalence of cerebral white matter lesions in elderly people: a population based magnetic resonance imaging study. The Rotterdam Scan Study. J Neurol Neurosurg Psychiatry. 2001;70:9–14. doi: 10.1136/jnnp.70.1.9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 206.Longstreth W, Jr, Larsen EK, Klein R, et al. Associations between findings on cranial magnetic resonance imaging and retinal photography in the elderly: the Cardiovascular Health Study. Am J Epidemiol. 2007;165:78–84. doi: 10.1093/aje/kwj350. [DOI] [PubMed] [Google Scholar]
- 207.Khatri M, Wright CB, Nickolas TL, et al. Chronic kidney disease is associated with white matter hyperintensity volume: the Northern Manhattan Study (NOMAS) Stroke. 2007;38:3121–3126. doi: 10.1161/STROKEAHA.107.493593. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 208.Hassan A, Hunt BJ, O’Sullivan M, et al. Homocysteine is a risk factor for cerebral small vessel disease, acting via endothelial dysfunction. Brain. 2004;127(Pt 1):212–219. doi: 10.1093/brain/awh023. [DOI] [PubMed] [Google Scholar]
- 209.Breteler MM, van Swieten JC, Bots ML, et al. Cerebral white matter lesions, vascular risk factors, and cognitive function in a population-based study: the Rotterdam Study. Neurology. 1994;44:1246–1252. doi: 10.1212/wnl.44.7.1246. [DOI] [PubMed] [Google Scholar]
- 210.Schmidt R, Fazekas F, Hayn M, et al. Risk factors for microangiopathy-related cerebral damage in the Austrian stroke prevention study. J Neurol Sci. 1997;152:15–21. doi: 10.1016/s0022-510x(97)00137-8. [DOI] [PubMed] [Google Scholar]
- 211.Gurol ME, Irizarry MC, Smith EE, et al. Plasma beta-amyloid and white matter lesions in AD, MCI, and cerebral amyloid angiopathy. Neurology. 2006;66:23–29. doi: 10.1212/01.wnl.0000191403.95453.6a. [DOI] [PubMed] [Google Scholar]
- 212.van Dijk EJ, Prins ND, Vermeer SE, et al. Plasma amyloid beta, apolipoprotein E, lacunar infarcts, and white matter lesions. Ann Neurol. 2004;55:570–575. doi: 10.1002/ana.20050. [DOI] [PubMed] [Google Scholar]
- 213.Stenset V, Johnsen L, Kocot D, et al. Associations between white matter lesions, cerebrovascular risk factors, and low CSF Abeta42. Neurology. 2006;67:830–833. doi: 10.1212/01.wnl.0000234030.77831.5a. [DOI] [PubMed] [Google Scholar]
- 214.Carmelli D, DeCarli C, Swan GE, et al. Evidence for genetic variance in white matter hyperintensity volume in normal elderly male twins. Stroke. 1998;29:1177–1181. doi: 10.1161/01.str.29.6.1177. [DOI] [PubMed] [Google Scholar]
- 215.Vermeer SE, Hollander M, van Dijk EJ, et al. Silent brain infarcts and white matter lesions increase stroke risk in the general population: the Rotterdam Scan Study. Stroke. 2003;34:1126–1129. doi: 10.1161/01.STR.0000068408.82115.D2. [DOI] [PubMed] [Google Scholar]
- 216.Smith EE, Rosand J, Knudsen KA, et al. Leukoaraiosis is associated with warfarin-related hemorrhage following ischemic stroke. Neurology. 2002;59:193–197. doi: 10.1212/wnl.59.2.193. [DOI] [PubMed] [Google Scholar]
- 217.Ay H, Arsava EM, Rosand J, et al. Severity of leukoaraiosis and susceptibility to infarct growth in acute stroke. Stroke. 2008;39:1409–1413. doi: 10.1161/STROKEAHA.107.501932. [DOI] [PubMed] [Google Scholar]
- 218.Kuller LH, Longstreth WT, Jr, Arnold AM, et al. White matter hyperintensity on cranial magnetic resonance imaging: a predictor of stroke. Stroke. 2004;35:1821–1825. doi: 10.1161/01.STR.0000132193.35955.69. [DOI] [PubMed] [Google Scholar]
- 219.Gorter JW. Major bleeding during anticoagulation after cerebral ischemia: patterns and risk factors. Stroke Prevention In Reversible Ischemia Trial (SPIRIT). European Atrial Fibrillation Trial (EAFT) study groups. Neurology. 1999;53:1319–1327. doi: 10.1212/wnl.53.6.1319. [DOI] [PubMed] [Google Scholar]
- 220.Neumann-Haefelin T, Hoelig S, Berkefeld J, et al. Leukoaraiosis is a risk factor for symptomatic intracerebral hemorrhage after thrombolysis for acute stroke. Stroke. 2006;37:2463–2466. doi: 10.1161/01.STR.0000239321.53203.ea. [DOI] [PubMed] [Google Scholar]
- 221.Smith EE, Gurol ME, Eng JA, et al. White matter lesions, cognition, and recurrent hemorrhage in lobar intracerebral hemorrhage. Neurology. 2004;63:1606–1612. doi: 10.1212/01.wnl.0000142966.22886.20. [DOI] [PubMed] [Google Scholar]
- 222.Longstreth WT, Jr, Dulberg C, Manolio TA, et al. Incidence, manifestations, and predictors of brain infarcts defined by serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2002;33:2376–2382. doi: 10.1161/01.str.0000032241.58727.49. [DOI] [PubMed] [Google Scholar]
- 223.Dufouil C, Chalmers J, Coskun O, et al. Effects of blood pressure lowering on cerebral white matter hyperintensities in patients with stroke: the PROGRESS (Perindopril Protection Against Recurrent Stroke Study) Magnetic Resonance Imaging Substudy. Circulation. 2005;112:1644–1650. doi: 10.1161/CIRCULATIONAHA.104.501163. [DOI] [PubMed] [Google Scholar]
- 224.ten Dam VH, van den Heuvel DM, van Buchem MA, et al. Effect of pravastatin on cerebral infarcts and white matter lesions. Neurology. 2005;64:1807–1809. doi: 10.1212/01.WNL.0000161844.00797.73. [DOI] [PubMed] [Google Scholar]
- 225.Fernando MS, Simpson JE, Matthews F, et al. White matter lesions in an unselected cohort of the elderly: molecular pathology suggests origin from chronic hypoperfusion injury. Stroke. 2006;37:1391–1398. doi: 10.1161/01.STR.0000221308.94473.14. [DOI] [PubMed] [Google Scholar]
- 226.Pantoni L, Garcia JH. Pathogenesis of leukoaraiosis: a review. Stroke. 1997;28:652–659. doi: 10.1161/01.str.28.3.652. [DOI] [PubMed] [Google Scholar]
- 227.Holland CM, Smith EE, Csapo I, et al. Spatial distribution of white-matter hyperintensities in Alzheimer disease, cerebral amyloid angiopathy, and healthy aging. Stroke. 2008;39:1127–1133. doi: 10.1161/STROKEAHA.107.497438. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 228.De Reuck J, Decoo D, Marchau M, et al. Positron emission tomography in vascular dementia. J Neurol Sci. 1998;154:55–61. doi: 10.1016/s0022-510x(97)00213-x. [DOI] [PubMed] [Google Scholar]
- 229.De Reuck J, Decoo D, Strijckmans K, Lemahieu I. Does the severity of leukoaraiosis contribute to senile dementia? A comparative computerized and positron emission tomographic study. Eur Neurol. 1992;32:199–205. doi: 10.1159/000116822. [DOI] [PubMed] [Google Scholar]
- 230.Hatazawa J, Shimosegawa E, Satoh T, et al. Subcortical hypoperfusion associated with asymptomatic white matter lesions on magnetic resonance imaging. Stroke. 1997;28:1944–1947. doi: 10.1161/01.str.28.10.1944. [DOI] [PubMed] [Google Scholar]
- 231.Tohgi H, Yonezawa H, Takahashi S, et al. Cerebral blood flow and oxygen metabolism in senile dementia of Alzheimer’s type and vascular dementia with deep white matter changes. Neuroradiology. 1998;40:131–137. doi: 10.1007/s002340050553. [DOI] [PubMed] [Google Scholar]
- 232.Yao H, Sadoshima S, Ibayashi S, et al. Leukoaraiosis and dementia in hypertensive patients. Stroke. 1992;23:1673–1677. doi: 10.1161/01.str.23.11.1673. [DOI] [PubMed] [Google Scholar]
- 233.ten Dam VH, van den Heuvel DM, de Craen AJ, et al. Decline in total cerebral blood flow is linked with increase in periventricular but not deep white matter hyperintensities. Radiology. 2007;243:198–203. doi: 10.1148/radiol.2431052111. [DOI] [PubMed] [Google Scholar]
- 234.Joutel A, Corpechot C, Ducros A, et al. Notch3 mutations in CADASIL, a hereditary adult-onset condition causing stroke and dementia. Nature. 1996;383:707–710. doi: 10.1038/383707a0. [DOI] [PubMed] [Google Scholar]
- 235.Mayer M, Straube A, Bruening R, et al. Muscle and skin biopsies are a sensitive diagnostic tool in the diagnosis of CADASIL. J Neurol. 1999;246:526–532. doi: 10.1007/s004150050398. [DOI] [PubMed] [Google Scholar]
- 236.Singhal S, Rich P, Markus HS. The spatial distribution of MR imaging abnormalities in cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy and their relationship to age and clinical features. AJNR Am J Neuroradiol. 2005;26:2481–2487. [PMC free article] [PubMed] [Google Scholar]
- 237.Harris JG, Filley CM. CADASIL: Neuropsychological findings in three generations of an affected family. J Int Neuropsychol Soc. 2001;7:768–774. doi: 10.1017/s135561770176612x. [DOI] [PubMed] [Google Scholar]
- 238.Viswanathan A, Gschwendtner A, Guichard JP, et al. Lacunar lesions are independently associated with disability and cognitive impairment in CADASIL. Neurology. 2007;69:172–179. doi: 10.1212/01.wnl.0000265221.05610.70. [DOI] [PubMed] [Google Scholar]
- 239.Chabriat H, Vahedi K, Iba-Zizen MT, et al. Clinical spectrum of CADASIL: a study of 7 families. Cerebral autosomal dominant arteriopathy with subcortical infarcts and leukoencephalopathy. Lancet. 1995;346:934–939. doi: 10.1016/s0140-6736(95)91557-5. [DOI] [PubMed] [Google Scholar]
- 240.Zhang-Nunes SX, Maat-Schieman ML, van Duinen SG, et al. The cerebral beta-amyloid angiopathies: hereditary and sporadic. Brain Pathol. 2006;16:30–39. doi: 10.1111/j.1750-3639.2006.tb00559.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 241.Knudsen KA, Rosand J, Karluk D, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Neurology. 2001;56:537–539. doi: 10.1212/wnl.56.4.537. [DOI] [PubMed] [Google Scholar]
- 242.Smith EE, Greenberg SM. Clinical diagnosis of cerebral amyloid angiopathy: validation of the Boston criteria. Curr Atheroscler Rep. 2003;5:260–266. doi: 10.1007/s11883-003-0048-4. [DOI] [PubMed] [Google Scholar]
- 243.Greenberg SM, Gurol ME, Rosand J, Smith EE. Amyloid angiopathy-related vascular cognitive impairment. Stroke. 2004;35(11 Suppl 1):2616–2619. doi: 10.1161/01.STR.0000143224.36527.44. [DOI] [PubMed] [Google Scholar]
- 244.Neuropathology Group of the Medical Research Council Cognitive Function Ageing Study (MRC CFAS) Pathological correlates of late-onset dementia in a multicentre, community-based population in England and Wales. Lancet. 2001;357:169–175. doi: 10.1016/s0140-6736(00)03589-3. [DOI] [PubMed] [Google Scholar]
- 245.Vernooij MW, Van Der Lugt A, Ikram MA, et al. Prevalence and risk factors of cerebral microbleeds: the Rotterdam Scan Study. Neurology. 2008;70:1208–1214. doi: 10.1212/01.wnl.0000307750.41970.d9. [DOI] [PubMed] [Google Scholar]
- 246.Chen YW, Gurol ME, Rosand J, et al. Progression of white matter lesions and hemorrhages in cerebral amyloid angiopathy. Neurology. 2006;67:83–87. doi: 10.1212/01.wnl.0000223613.57229.24. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 247.Salat DH, Smith EE, Tuch DS, et al. White matter alterations in cerebral amyloid angiopathy measured by diffusion tensor imaging. Stroke. 2006;37:1759–1764. doi: 10.1161/01.STR.0000227328.86353.a7. [DOI] [PubMed] [Google Scholar]
- 248.Viswanathan A, Patel P, Rahman R, et al. Tissue microstructural changes are independently associated with pre-index cognitive impairment in survivors of lobar intracerebral hemorrhage. Stroke. 2008;39:1988–1992. doi: 10.1161/STROKEAHA.107.509091. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 249.Johnson KA, Gregas M, Becker JA, et al. Imaging of amyloid burden and distribution in cerebral amyloid angiopathy. Ann Neurol. 2007;62:229–234. doi: 10.1002/ana.21164. [DOI] [PubMed] [Google Scholar]
- 250.Arriagada PV, Growdon JH, Hedley-Whyte ET, Hyman BT. Neurofibrillary tangles but not senile plaques parallel duration and severity of Alzheimer’s disease. Neurology. 1992;42(3 Pt 1):631–639. doi: 10.1212/wnl.42.3.631. [DOI] [PubMed] [Google Scholar]
- 251.de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and cognitive function: the Rotterdam Scan Study. Ann Neurol. 2000;47:145–151. doi: 10.1002/1531-8249(200002)47:2<145::aid-ana3>3.3.co;2-g. [DOI] [PubMed] [Google Scholar]
- 252.Mosley TH, Jr, Knopman DS, Catellier DJ, et al. Cerebral MRI findings and cognitive functioning: the Atherosclerosis Risk in Communities study. Neurology. 2005;64:2056–2062. doi: 10.1212/01.WNL.0000165985.97397.88. [DOI] [PubMed] [Google Scholar]
- 253.Longstreth WT, Jr, Arnold AM, Beauchamp NJ, Jr, et al. Incidence, manifestations, and predictors of worsening white matter on serial cranial magnetic resonance imaging in the elderly: the Cardiovascular Health Study. Stroke. 2005;36:56–61. doi: 10.1161/01.STR.0000149625.99732.69. [DOI] [PubMed] [Google Scholar]
- 254.Prins ND, van Dijk EJ, den Heijer T, et al. Cerebral small-vessel disease and decline in information processing speed, executive function and memory. Brain. 2005;128(Pt 9):2034–2041. doi: 10.1093/brain/awh553. [DOI] [PubMed] [Google Scholar]
- 255.Schmidt R, Ropele S, Enzinger C, et al. White matter lesion progression, brain atrophy, and cognitive decline: the Austrian Stroke Prevention study. Ann Neurol. 2005;58:610–616. doi: 10.1002/ana.20630. [DOI] [PubMed] [Google Scholar]
- 256.de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and subjective cognitive dysfunction: the Rotterdam Scan Study. Neurology. 2001;56:1539–1545. doi: 10.1212/wnl.56.11.1539. [DOI] [PubMed] [Google Scholar]
- 257.Lopez OL, Jagust WJ, Dulberg C, et al. Risk factors for mild cognitive impairment in the Cardiovascular Health Study Cognition Study: part 2. Arch Neurol. 2003;60:1394–1399. doi: 10.1001/archneur.60.10.1394. [DOI] [PubMed] [Google Scholar]
- 258.Smith EE, Egorova S, Blacker D, et al. Magnetic resonance imaging white matter hyperintensities and brain volume in the prediction of mild cognitive impairment and dementia. Arch Neurol. 2008;65:94–100. doi: 10.1001/archneurol.2007.23. [DOI] [PubMed] [Google Scholar]
- 259.DeCarli C, Mungas D, Harvey D, et al. Memory impairment, but not cerebrovascular disease, predicts progression of MCI to dementia. Neurology. 2004;63:220–227. doi: 10.1212/01.wnl.0000130531.90205.ef. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 260.Vermeer SE, Prins ND, den Heijer T, et al. Silent brain infarcts and the risk of dementia and cognitive decline. N Engl J Med. 2003;348:1215–1222. doi: 10.1056/NEJMoa022066. [DOI] [PubMed] [Google Scholar]
- 261.Schneider JA, Arvanitakis Z, Bang W, Bennett DA. Mixed brain pathologies account for most dementia cases in community-dwelling older persons. Neurology. 2007;69:2197–2204. doi: 10.1212/01.wnl.0000271090.28148.24. [DOI] [PubMed] [Google Scholar]
- 262.Hubbard BM, Anderson JM. A quantitative study of cerebral atrophy in old age and senile dementia. J Neurol Sci. 1981;50:135–145. doi: 10.1016/0022-510x(81)90048-4. [DOI] [PubMed] [Google Scholar]
- 263.Brinkman SD, Sarwar M, Levin HS, Morris HH., 3rd Quantitative indexes of computed tomography in dementia and normal aging. Radiology. 1981;138:89–92. doi: 10.1148/radiology.138.1.7455102. [DOI] [PubMed] [Google Scholar]
- 264.Brun A, Englund E. A white matter disorder in dementia of the Alzheimer type: a pathoanatomical study. Ann Neurol. 1986;19:253–262. doi: 10.1002/ana.410190306. [DOI] [PubMed] [Google Scholar]
- 265.Englund E, Brun A. White matter changes in dementia of Alzheimer’s type: the difference in vulnerability between cell compartments. Histopathology. 1990;16:433–439. doi: 10.1111/j.1365-2559.1990.tb01542.x. [DOI] [PubMed] [Google Scholar]
- 266.Jellinger KA. Alzheimer’s disease and cerebrovascular pathology: an update. J Neural Transm. 2002;109:813–836. doi: 10.1007/s007020200068. [DOI] [PubMed] [Google Scholar]
- 267.Atri A, Locascio JJ, Lin JM, et al. Prevalence and effects of lobar microhemorrhages in early-stage dementia. Neurodegener Dis. 2005;2:305–312. doi: 10.1159/000092317. [DOI] [PubMed] [Google Scholar]
- 268.Pfeifer LA, White LR, Ross GW, et al. Cerebral amyloid angiopathy and cognitive function: the HAAS autopsy study. Neurology. 2002;58:1629–1634. doi: 10.1212/wnl.58.11.1629. [DOI] [PubMed] [Google Scholar]
- 269.Román GC. Binswanger disease: the history of a silent epidemic. Ann N Y Acad Sci. 2000;903:19–23. doi: 10.1111/j.1749-6632.2000.tb06345.x. [DOI] [PubMed] [Google Scholar]
- 270.Román GC. From UBOs to Binswanger’s disease. Impact of magnetic resonance imaging on vascular dementia research. Stroke. 1996;27:1269–1273. doi: 10.1161/01.str.27.8.1269. [DOI] [PubMed] [Google Scholar]
- 271.Selnes OA, Vinters HV. Vascular cognitive impairment. Nat Clin Pract Neurol. 2006;2:538–547. doi: 10.1038/ncpneuro0294. [DOI] [PubMed] [Google Scholar]
- 272.Leys D, Pruvo JP, Parent M, et al. Could Wallerian degeneration contribute to “leukoaraiosis” in subjects free of any vascular disorder? J Neurol Neurosurg Psychiatry. 1991;54:46–50. doi: 10.1136/jnnp.54.1.46. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 273.Bozzali M, Falini A, Francheschi M, et al. White matter damage in Alzheimer’s disease assessed in vivo using diffusion tensor magnetic resonance imaging. J Neurol Neurosurg Psychiatry. 2002;72:742–746. doi: 10.1136/jnnp.72.6.742. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 274.Burton EJ, Kenny RA, O’Brien J, et al. White matter hyperintensities are associated with impairment of memory, attention, and global cognitive performance in older stroke patients. Stroke. 2004;35:1270–1275. doi: 10.1161/01.STR.0000126041.99024.86. [DOI] [PubMed] [Google Scholar]
- 275.Marshall GA, Hendrickson R, Kaufer DI, et al. Cognitive correlates of brain MRI subcortical signal hyperintensities in non-demented elderly. Int J Geriatr Psychiatry. 2006;21:32–35. doi: 10.1002/gps.1419. [DOI] [PubMed] [Google Scholar]
- 276.Nordahl CW, Ranganath C, Yonelinas AP, et al. Different mechanisms of episodic memory failure in mild cognitive impairment. Neuropsychologia. 2005;43:1688–1697. doi: 10.1016/j.neuropsychologia.2005.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 277.Reed BR, Eberling JL, Mungas D, et al. Effects of white matter lesions and lacunes on cortical function. Arch Neurol. 2004;61:1545–1550. doi: 10.1001/archneur.61.10.1545. [DOI] [PubMed] [Google Scholar]
- 278.Soderlund H, Nilsson LG, Berger K, et al. Cerebral changes on MRI and cognitive function: the CASCADE study. Neurobiol Aging. 2006;27:16–23. doi: 10.1016/j.neurobiolaging.2004.12.008. [DOI] [PubMed] [Google Scholar]
- 279.Van Den Heuvel DM, ten Dam VH, de Craen AJ, et al. Increase in periventricular white matter hyperintensities parallels decline in mental processing speed in a non-demented elderly population. J Neurol Neurosurg Psychiatry. 2006;77:149–153. doi: 10.1136/jnnp.2005.070193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 280.Ylikoski R, Ylikoski A, Erkinjuntti T, et al. White matter changes in healthy elderly persons correlate with attention and speed of mental processing. Arch Neurol. 1993;50:818–824. doi: 10.1001/archneur.1993.00540080029009. [DOI] [PubMed] [Google Scholar]
- 281.Nordahl CW, Ranganath C, Yonelinas AP, et al. White matter changes compromise prefrontal cortex function in healthy elderly individuals. J Cogn Neurosci. 2006;18:418–429. doi: 10.1162/089892906775990552. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 282.de Groot JC, de Leeuw FE, Oudkerk M, et al. Cerebral white matter lesions and depressive symptoms in elderly adults. Arch Gen Psychiatry. 2000;57:1071–1076. doi: 10.1001/archpsyc.57.11.1071. [DOI] [PubMed] [Google Scholar]
- 283.Firbank MJ, O’Brien JT, Pakrasi S, et al. White matter hyperintensities and depression–preliminary results from the LADIS study. Int J Geriatr Psychiatry. 2005;20:674–679. doi: 10.1002/gps.1342. [DOI] [PubMed] [Google Scholar]
- 284.Thomas AJ, O’Brien JT, Davis S, et al. Ischemic basis for deep white matter hyperintensities in major depression: a neuropathological study. Arch Gen Psychiatry. 2002;59:785–792. doi: 10.1001/archpsyc.59.9.785. [DOI] [PubMed] [Google Scholar]
- 285.Iosifescu DV, Renshaw PF, Lyoo IK, et al. Brain white-matter hyperintensities and treatment outcome in major depressive disorder. Br J Psychiatry. 2006;188:180–185. doi: 10.1192/bjp.188.2.180. [DOI] [PubMed] [Google Scholar]
- 286.Taylor WD, Steffens DC, MacFall JR, et al. White matter hyperintensity progression and late-life depression outcomes. Arch Gen Psychiatry. 2003;60:1090–1096. doi: 10.1001/archpsyc.60.11.1090. [DOI] [PubMed] [Google Scholar]
- 287.Bocti C, Swartz RH, Gao FQ, et al. A new visual rating scale to assess strategic white matter hyperintensities within cholinergic pathways in dementia. Stroke. 2005;36:2126–2131. doi: 10.1161/01.STR.0000183615.07936.b6. [DOI] [PubMed] [Google Scholar]
- 288.Sheline YI, Price JL, Vaishnavi SN, et al. Regional white matter hyperintensity burden in automated segmentation distinguishes late-life depressed subjects from comparison subjects matched for vascular risk factors. Am J Psychiatry. 2008;165:524–532. doi: 10.1176/appi.ajp.2007.07010175. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 289.Taylor WD, MacFall JR, Steffens DC, et al. Localization of age-associated white matter hyperintensities in late-life depression. Prog Neuropsychopharmacol Biol Psychiatry. 2003;27:539–544. doi: 10.1016/S0278-5846(02)00358-5. [DOI] [PubMed] [Google Scholar]
- 290.Tullberg M, Fletcher E, DeCarli C, et al. White matter lesions impair frontal lobe function regardless of their location. Neurology. 2004;63:246–253. doi: 10.1212/01.wnl.0000130530.55104.b5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 291.Starkstein SE, Sabe L, Vazquez S, et al. Neuropsychological, psychiatric, and cerebral perfusion correlates of leukoaraiosis in Alzheimer’s disease. J Neurol Neurosurg Psychiatry. 1997;63:66–73. doi: 10.1136/jnnp.63.1.66. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 292.Sultzer DL, Mahler ME, Cummings JL, et al. Cortical abnormalities associated with subcortical lesions in vascular dementia. Clinical and position emission tomographic findings. Arch Neurol. 1995;52:773–780. doi: 10.1001/archneur.1995.00540320049012. [DOI] [PubMed] [Google Scholar]
- 293.Yao H, Sadoshima S, Kuwabara Y, et al. Cerebral blood flow and oxygen metabolism in patients with vascular dementia of the Binswanger type. Stroke. 1990;21:1694–1699. doi: 10.1161/01.str.21.12.1694. [DOI] [PubMed] [Google Scholar]
- 294.Schmahmann JD, Leifer D. Parietal pseudothalamic pain syndrome. Clinical features and anatomical correlates. Arch Neurol. 1992;49:1032–1037. doi: 10.1001/archneur.1992.00530340048017. [DOI] [PubMed] [Google Scholar]
- 295.Leventhal CM, Baringer JR, Arnason BG, Fisher CM. A case of Marchiafava-Bignami disease with clinical recovery. Trans Am Neurol Assoc. 1965;90:87–91. [PubMed] [Google Scholar]
- 296.Heilman KM, Sypert GW. Korsakoff ’s syndrome resulting from bilateral fornix lesions. Neurology. 1977;27:490–493. doi: 10.1212/wnl.27.5.490. [DOI] [PubMed] [Google Scholar]
- 297.D’Esposito M, Verfaellie M, Alexander MP, Katz DI. Amnesia following traumatic bilateral fornix transection. Neurology. 1995;45:1546–1550. doi: 10.1212/wnl.45.8.1546. [DOI] [PubMed] [Google Scholar]
- 298.Polyak S. In: The Vertebrate Visual System. Klüver H, editor. University of Chicago Press; Chicago, IL: 1957. [Google Scholar]
- 299.Groothuis DR, Duncan GW, Fisher CM. The human thalamocortical sensory path in the internal capsule: evidence from a small capsular hemorrhage causing a pure sensory stroke. Ann Neurol. 1977;2:328–331. doi: 10.1002/ana.410020412. [DOI] [PubMed] [Google Scholar]
- 300.Fisher CM. Capsular infarcts: the underlying vascular lesions. Arch Neurol. 1979;36:65–73. doi: 10.1001/archneur.1979.00500380035003. [DOI] [PubMed] [Google Scholar]
- 301.Aralasmak A, Ulmer JL, Kocak M, et al. Association, commissural, and projection pathways and their functional deficit reported in literature. J Comput Assist Tomogr. 2006;30:695–715. doi: 10.1097/01.rct.0000226397.43235.8b. [DOI] [PubMed] [Google Scholar]
- 302.Naeser MA, Palumbo CL, Helm-Estabrooks N, et al. Severe nonfluency in aphasia. Role of the medial subcallosal fasciculus and other white matter pathways in recovery of spontaneous speech. Brain. 1989;112:1–38. doi: 10.1093/brain/112.1.1. [DOI] [PubMed] [Google Scholar]
- 303.Davis KL, Stewart DG, Friedman JI, et al. White matter changes in schizophrenia: evidence for myelin related dysfunction. Arch Gen Psychiatry. 2003;60:443–456. doi: 10.1001/archpsyc.60.5.443. [DOI] [PubMed] [Google Scholar]
- 304.Nakamura M, McCarley RW, Kubicki M, et al. Fronto-temporal disconnectivity in schizotypal personality disorder: a diffusion tensor imaging study. Biol Psychiatry. 2005;58:468–478. doi: 10.1016/j.biopsych.2005.04.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 305.Lamantia AS, Rakic P. Cytological and quantitative characteristics of four cerebral commissures in the rhesus monkey. J Comp Neurol. 1990;291:520–537. doi: 10.1002/cne.902910404. [DOI] [PubMed] [Google Scholar]
- 306.Innocenti GM. Exuberant development of connections, and its possible permissive role in cortical evolution. Trends Neurosci. 1995;18:397–402. doi: 10.1016/0166-2236(95)93936-r. [DOI] [PubMed] [Google Scholar]
- 307.Herbert MR, Ziegler DA, Deutsch CK, et al. Dissociations of cerebral cortex, subcortical and cerebral white matter volumes in autistic boys. Brain. 2003;126:1182–1192. doi: 10.1093/brain/awg110. [DOI] [PubMed] [Google Scholar]
- 308.Filley CM, Heaton RK, Nelson LM, et al. A comparison of dementia in Alzheimer’s disease and multiple sclerosis. Arch Neurol. 1989;46:157–161. doi: 10.1001/archneur.1989.00520380061013. [DOI] [PubMed] [Google Scholar]
- 309.Lafosse JM, Corboy JR, Leehey MA, et al. MS vs. HD: Can white matter and subcortical gray matter pathology be distinguished neuropsychologically? J Clin Exp Neuropsychol. 2007;29:142–154. doi: 10.1080/13803390600582438. [DOI] [PubMed] [Google Scholar]