Abstract
WHI-07 [5-bromo-6-methoxy-5,6-dihydro-3′-azidothymidine-5′-(p-bromophenyl)-methoxy alaninyl phosphate] is a novel dual-function aryl phosphate derivative of zidovudine with potent anti-human immunodeficiency virus (HIV) and spermicidal activities. WHI-07 was active against the feline immunodeficiency virus (FIV). This study evaluated whether topical application of WHI-07 as a single agent and in combination with an organometallic vanadium complex, vanadocene dithiocarbamate (VDDTC), via a nontoxic gel microemulsion can block vaginal as well as rectal transmission of feline AIDS (FAIDS) by chronically FIV-infected feline T cells in the natural host model. Genital transmission of FIV was monitored in recipient cats by the appearance of viral antibodies to FIV Gag proteins and by virus isolation of blood leukocytes as measured by FIV reverse transcriptase activity and FIV-specific PCR. Microbicidal activity was considered effective when the treated cats did not show evidence of FIV infection for up to 18 weeks postchallenge. An aggregate analysis of 46 specific-pathogen-free cats revealed that a single dose of the infected cell inoculum efficiently transmitted FIV infection when delivered into the vagina (100%) or rectum (66%). Pretreatment of the vagina or rectum with 2% WHI-07 alone or in combination with 0.25% VDDTC significantly (P = 0.004) protected cats from genital transmission by the highly infectious inoculum (7 million FIVBangston-infected feline T cells). Collectively, using the vaginal and rectal transmucosal model for FAIDS, our studies demonstrated that WHI-07 either alone or in combination with a vanadocene has clinical potential for the development of a dual-function anti-HIV microbicide for sexually active women.
Heterosexual transmission is the main mode of human immunodeficiency virus type 1 (HIV-1) transmission worldwide and accounts for nearly 90% of all HIV infections in women (37, 38, 47, 58). Because most women at risk for HIV infection are of reproductive age, effective use of dual-function microbicide is important to prevent HIV transmission and unintended pregnancies (37, 54, 55). Since semen is an important vehicle for sexual transmission of HIV-1, topical anti-HIV spermicides would ideally provide a female-controlled method of self-protection against HIV as well as prevent pregnancy. However, several anionic polymers and detergent-based dual-function microbicides that are currently undergoing preclinical or clinical development to curb the sexual transmission of HIV exhibit nonspecific antimicrobial as well as spermicidal properties which are an ongoing concern because of their long-term mucosal safety (6). Consequently, new, effective, and mechanism-based microbicides lacking mucosal toxicity are needed.
In a systematic effort to develop a prophylactic contraceptive capable of preventing HIV transmission as well as providing fertility control, our laboratory has previously identified novel aryl phosphate derivatives of the anti-HIV drug 3′-azido-3′-deoxythymidine (zidovudine [ZDV]) with potent anti-HIV and spermicidal activities (7-10). WHI-07 [5-bromo-6-meth-oxy-5,6-dihydro-3′-azidothymidine-5′-(p-bromophenyl)-meth-oxyalaninyl phosphate], an aryl phosphate derivative of 5-bromo-6-methoxy-ZDV with an alanine methyl ester side chain and a bromo substitution in the phenyl ring, was identified as the lead dual-function agent (Fig. 1). Comparative analysis of the anti-HIV and spermicidal activities as a function of WHI-07 concentration revealed sigmoidal dose-response curves, with 50% inhibition of HIV-1 replication at nanomolar concentrations (50% inhibitory concentration [IC50] = 0.005 μM p24 and IC50 = 0.009 μM reverse transcriptase [RT]) and sperm-immobilizing activity at low micromolar concentrations (50% effective concentration = 5 μM) (8). WHI-07 displayed high selective indices against normal human female genital epithelial cells. These dual-function properties of WHI-07 fundamentally differ from those of currently used cytotoxic surfactant microbicides. Furthermore, WHI-07 was rationally designed to undergo intracellular hydrolysis to a monophosphate derivative that is subsequently phosphorylated to give the bioactive triphosphate in a manner that bypasses the enzyme action of thymidine kinase (TK); the latter enzyme activity is compromised in HIV-infected monocytes/macrophages in semen (11). Human leukocytes, female genital tract epithelial cells, and sperm efficiently convert WHI-07 to active ZDV metabolites despite low TK activity (11).
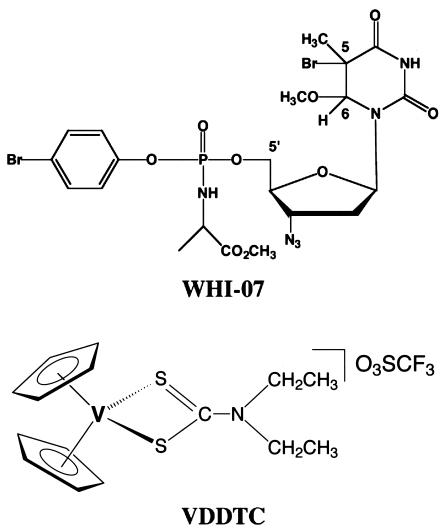
FIG. 1.
Chemical structures of WHI-07 and VDDTC.
WHI-07 was formulated via a nontoxic gel microemulsion for intravaginal use as a potential candidate anti-HIV spermicide. WHI-07-containing gel microemulsion is a potent vaginal contraceptive in the rabbit model (12). In preclinical studies, repetitive intravaginal administration of 2% WHI-07 via a gel microemulsion corresponding to 5.7 × 106 times its in vitro anti-HIV IC50 and 5,700 times its spermicidal 50% effective concentration for up to 13 weeks was not associated with mucosal, systemic, or reproductive toxicity in test animal species (13-16). Repeated intravaginal administration of WHI-07 during the period of major organogenesis at concentrations as high as 2% did not produce teratogenicity or other developmental toxicity in rodent and nonrodent species tested (16). Furthermore, long-term (2-year) intravaginal administration of 2% WHI-07 was not associated with systemic toxicity or increased carcinogenicity in mice (17).
However, it is becoming apparent that no single agent will be able to prevent sexual transmission of HIV long term. Accordingly, we have investigated the in vivo microbicidal efficacy of WHI-07 in combination with vanadocene dithiocarbamate (VDDTC), a bis(cyclopentadienyl) vanadium(IV) complex, as a new class of potent spermicides (20, 21). VDDTC is a disubstituted vanadocene where the two cyclopentadienyl rings are positioned in a tetrahedral symmetry and in a bent conformation with respect to the central vanadium(IV) atom (Fig. 1). The two sulfur atoms of the dithiocarbamate and centroids of the cyclopentadienyl rings in this vanadocene complex with unique contraceptive potential occupy four tetrahedral-like coordination sites about the central vanadium atom (27). In addition, vanadocenes exhibit pleiotropic effects in cells, such as modulation of the cell's redox potential, affect enzymatic phosphorylation, inhibit DNA replication by direct DNA binding and cleavage, and also catalyze the generation of reactive oxygen species (27, 39, 53).
The feline immunodeficiency virus (FIV) infection of the domestic cat is an established model for HIV infection in humans (3, 4, 26, 40-42, 44, 46, 59). The purpose of the present study was to examine the ability of WHI-07 either alone or in combination with VDDTC to prevent systemic FIV infection of domestic cats challenged with a vaginal or rectal inoculation of highly infectious FIV-infected feline T cells. Our results provide unprecedented evidence that WHI-07 either alone or in combination with a vanadocene prevents vaginal and/or rectal transmission of FIV in the domestic cat model. WHI-07, because of its potent in vivo contraceptive and microbicidal activities as well as the lack of mucosal, systemic, and reproductive toxicity, shows significant clinical potential as a safe contraceptive anti-HIV microbicide for sexually active women.
MATERIALS AND METHODS
Compounds.
WHI-07 and VDDTC, the structures of which are shown in Fig. 1, were synthesized according to our published procedures (8, 21). WHI-07 differs from ZDV by the presence of 5-bromo and 6-methoxy groups on the thymine ring, a phenyl phosphate group with an alanine side chain, and a bromo substituent on the C-5 position of the phenyl ring. The purity of WHI-07 was >98%, as assessed by the proton (1H), carbon (13C), phosphorous (31P) nuclear magnetic resonance spectra and Fourier-transform infrared spectra. Analytical data for WHI-07 were as follows: calculated C, 36.12; H, 3.90; and N, 12.04; found C, 36.28; H, 4.02; and N, 11.99. The purity of VDDTC [bis(cyclopentadienyl)N,N-diethyl dithiocarbamato triflate salt] as determined by 1H nuclear magnetic resonance, Fourier-transform infrared spectra, UV-visible spectroscopy, and elemental analysis exceeded 99%. Detailed physicochemical analysis confirmed that the dithiocarbamato ligands were attached firmly to the vanadium coordination sphere, and with bis(cyclopentadienyl)vanadium(IV) rings the molecules possess a wedge-like sandwich structure with a tetrahedral coordination about the central vanadium(IV) atom. Nonoxynol-9 (N-9, or IGEPAL CO-630) was kindly supplied by Rhone Poulenc (Cranbury, N.J.).
FIV isolates.
Exogenous infection of T-cell-enriched peripheral blood mononuclear cells (PBMCs) from specific-pathogen-free (SPF) cats as well as feline T-lymphoid cells (FeT-J) inoculated with the Bangston FIV isolate (FIVBangston, subtype B) was as previously described (56). FeT-J is an interleukin-2-independent feline T-cell line highly susceptible to FIV infection (28). In the vaginal and rectal microbicide efficacy study, cats were challenged with an intravaginal or intrarectal inoculum of FeT-J cells infected with the FIVBangston isolate.
In vitro assay of anti-FIV activity of WHI-07.
Phenotypic FIV drug susceptibility studies were performed by measuring the levels of FIV reverse transcriptase (RT) activity in the culture supernatants of T-cell-enriched PBMCs from SPF cats after exogenous infection with FIV, as previously described (50, 60). T-cell-enriched PBMCs were derived from SPF cats by stimulating the Ficoll-Hypaque-separated PBMCs with concanavalin A for 3 days and culturing for an additional 2 weeks in an interleukin-2 (100 U/ml)-supplemented RPMI culture medium in 24-well tissue culture plates, as previously reported (60). T-cell-enriched PBMCs at 106 cells/ml were treated with serial 10-fold dilutions of ZDV (control) or WHI-07 at the same time as FIV inoculation (100 50% tissue culture infective doses). Culture supernatants were harvested at 3- to 4-day intervals for 12 to 18 days, and the cells were recultured in fresh culture medium containing the indicated concentrations of WHI-07 or ZDV. On day 9, cell cultures were tested for FIV replication by measuring the RT activity levels of the culture supernatants (50). Each dilution of the test culture and placebo culture was performed in triplicate, and the experiment was performed three times with PBMCs isolated from different SPF cats. Controls included uninfected PBMCs (negative control), FIV-infected but untreated PBMCs (positive control), and FIV-infected, ZDV-treated PBMCs. The results are presented as the percent control of the mean RT titer using the following formula: percent control replication = 100 × [(mean RT titer of drug-treated culture − mean RT of negative control culture)/(mean RT of untreated positive control culture − mean RT of negative control culture)]. Potential drug toxicity of WHI-07 versus ZDV in these cultures was also monitored by viability and absolute cell count analysis using the trypan blue dye exclusion method (34). A minimum of 500 cells were enumerated to identify the maximum tolerated dose for each drug, which was the maximum dose at which the cultures showed no toxicity.
Intravaginal and intrarectal microbicide efficacy studies. (i) Animals.
Sixty-two SPF adult domestic cats (20 to 26 weeks of age) obtained from Liberty Research, Inc. (Waverley, N.Y.) or Harlan Sprague-Dawley (Indianapolis, Ind.) were used in this study. The animals were housed in accordance with the American Association for Accreditation Laboratory Animal Care standards. All husbandry and experimental contact made with the cats maintained SPF conditions (12-h light-12-h dark photoperiod; 18 to 29°C; 30 to 70% relative humidity). The cats were housed in galvanized gang cages with Plexiglas in a well-ventilated room with no air recirculation. The animal rooms were cleaned daily, and cat litters were changed daily. Cats were provided with dry and wet Purina cat chow and tap water ad libitum. Cats were acclimated to the study room conditions for at least 5 days prior to initiation of the experiment. Animal studies were approved by the Parker Hughes Institute Animal Care and Use Committee, and all animal care procedures conformed to the Guide for the Care and Use of Laboratory Animals (36). Cats were treated at the University of Florida under a contract service agreement between Parker Hughes Institute and the University of Florida. Therefore, cat studies were also approved by the University of Florida Animal Care and Use Committee.
(ii) Vaginal FIV transmission study.
Sixteen SPF cats were used for intravaginal dosing of FIV-infected FeT-J cells. These 16 cats in subgroups of four were given intravaginal inoculations of increasing doses of FIVBangston-infected FeT-J cells (5 × 103 to 5 × 106 cells/0.4 ml). Blood was obtained from these cats at 1, 2, 3, 5, 7, and 10 weeks after exposure to the virus. FIV infection in PBMCs was documented by virus isolation coupled with RT assays (VI-RT) and FIV-specific PCR analysis, as previously described (1, 25). Serum (1:25) was analyzed for antibody response to major FIV Gag proteins p26 and p15 by FIV immunoblotting (43, 51).
Cats were considered positive for FIV if one of the following criteria was met: (i) sera from two different bleeding dates were positive by Western blotting (WB); (ii) a single WB result and a single VI-RT result were positive with or without a PCR positive result (on different bleeding dates); (iii) mononuclear cells from two different bleeding dates were positive by VI-RT; and (iv) mononuclear cells from two different bleeding dates were positive by VI-PCR with the same tissue source. The WB result was considered positive if the p26 (major core) band was stronger than both the preserum band and the negative control for the specific blot. Although PCR of culture-amplified cells can sometimes detect FIV infection earlier than WB and VI-RT, PCR has a greater chance of being false positive or false negative. VI-RT was considered positive for the particular bleeding date if positive RT values were obtained on at least two culture harvest days. The RT values were considered positive if the value was ≥10,000 cpm/ml.
(iii) Gel microemulsion formulation.
Due to the lipophilic nature of WHI-07, we developed a submicron (30 to 80 nm) particle size microemulsion-based formulation to achieve as much as 2% WHI-07 for intravaginal or intrarectal use. Microemulsions appear to have the ability to deliver larger amounts of topically applied agents into the mucosa than traditional vehicles because they provide a better reservoir for a poorly soluble drug through their capacity for enhanced solubilization (18). A microemulsion-based system with high solubilizing capacity for WHI-07 was identified through systematic mapping of ternary-phase diagrams and drug solubilization studies. Based on these studies, an effective drug solubilization method for vaginal bioavailability in a clinically applicable gel was composed of Phospholipon 90G and Captex 300 as the oil phase with Pluronic F68 and Cremophor EL as surfactants, propylene glycol and polyethylene glycol 200 as cosurfactants, and water as a carrier. Polymer suspensions of SeaSpen PF and Viscarin GP-209 carrageenans were selected as additives to the microemulsion to obtain a gel with desirable viscosity containing up to 2% WHI-07 with high thickening capability and compatibility with microemulsions. WHI-07 was stable in the gel microemulsion formulation. A corresponding control gel microemulsion using the ingredients described for the lead formulation without WHI-07 was used as the placebo formulation.
In addition to WHI-07, we evaluated the potential microbicidal activity of VDDTC, which was selected from 45 organovanadium(IV) complexes that were synthesized and tested for spermicidal and cytotoxic activities (19-21). VDDTC was tested either alone or in combination with WHI-07 via gel microemulsion.
(iv) Intravaginal and intrarectal gel application and virus inoculation.
For the vaginal microbicide efficacy study, 10 cats received 0.4 ml of 5% N-9 gel (35), 5 cats received 0.4 ml of 2% WHI-07 gel microemulsion, 10 cats received 0.4 ml of 0.25% VDDTC in gel microemulsion, 5 cats received 0.4 ml of gel microemulsion containing 2% WHI-07 plus 0.25% VDDTC, and 10 cats treated with vehicle alone served as positive control.
For the rectal microbicide efficacy study, three cats received 0.3 ml of 2% WHI-07 gel microemulsion and three cats received an equal volume of vehicle. The formulations were administered intravaginally or intrarectally using a blunt-tipped pipette in cats sedated with a combination of ketamine, atropine, and acepromazine. All cats that received a vaginal or rectal treatment were followed 1 to 2 min later by vaginal or rectal inoculation of FIVBangston-infected feline lymphoid (FeT-J) cells (7 × 106 cells in 0.2 ml of cell culture supernatant) using a 20-gauge feeding needle.
Blood samples were collected before and after intravaginal or intrarectal inoculation at 3, 6, 9, 12, 15, and 18 weeks for complete blood cell counts, serum antiviral antibody assays, and collection of PBMCs for virus isolation and FIV-specific PCR from all treated cats. Anti-FIV antibodies were determined by WB analysis (43, 51). The WB result was considered positive if the p26 (major core) band was stronger than both the preserum band and negative control for the specific blot. FIV infection was documented by testing the cells for proviral DNA by FIV gag-specific and env-specific PCR at the termination of the cocultures (1, 25). The RT values were considered positive if the value was ≥10,000 cpm/ml. Animals were electively sacrificed 18 weeks after vaginal or rectal inoculation with FIV-infected cells.
(v) Statistical analysis.
Statistical significance was determined with the use of Fisher's exact test to compare the number of animals infected between test and control groups during the same experiment. P values of less than 0.05 were regarded as significant.
RESULTS
In vivo cat model for the transmucosal transmission of FIV.
We first established a cat model for the transmucosal transmission of AIDS for evaluation of the preventive antiretroviral properties of WHI-07. Domestic cats can be infected with FIV via vaginal and rectal routes using cell-associated or cell-free inocula (4, 5, 29, 31, 41, 42, 44, 45). Since the efficiency of transmucosal transmission is dependent on cell tropism of the virus, we employed the dual-tropic strain FIVBangston (subtype B), which exhibits considerable macrophage tropism (43). To this end, 16 SPF cats in subgroups of 4 were challenged intravaginally with various cell doses of the feline T-cell line FeT-J chronically infected with FIVBangston. FIV infection was monitored at 1, 2, 3, 5, 7, and 10 weeks after exposure to the infectious cell inoculum. Intravaginal inoculation with 5 × 106 FIVBangston-carrying FeT-J cells resulted in FIV infection in four of four cats. These cats became consistently seropositive with antibodies against the major FIV Gag proteins p26 and p15 and expressed detectable levels of FIV gag provirus DNA in their PBMCs, and FIV could be isolated from their PBMCs (Table 1). Thus, domestic cats acquire FIV infection after intravaginal inoculation of FIVBangston-infected FeT-J cells.
TABLE 1.
Virologic and serological status of cats given vaginal inoculation of FIV-infected T cells
| Intravaginal inoculum (cells) | No. positive/no. of animals tested
|
||
|---|---|---|---|
| FIV antibodiesa | FIV isolationb | Proviral DNAc | |
| 5 × 106d | 4/4 | 4/4 | 4/4 |
| 5 × 104 | 1/4 | 1/4 | 1/4 |
| 5 × 103 | 0/4 | 0/4 | 0/4 |
FIV-specific immunoglobulin G antibodies in plasma (1:25) were detected by WB analysis.
Infectious FIV was isolated from PBMCs.
FIV proviral DNA was detected by nested PCR using FIV gag-specific primers.
FeT-JBangston-infected feline cells.
WHI-07 prevents transmucosal transmission of FIV in the cat model.
The in vitro anti-FIV activity of WHI-07 in comparison to that of ZDV was determined by measuring FIV RT activity in culture supernatants. WHI-07 inhibited the in vitro replication of FIVBangston in feline PBMCs from SPF cats in a concentration-dependent fashion, with an IC50[RT] of 0.12 μM (Table 2). At 10 μM, >90% inhibition of FIV replication was achieved with both WHI-07 and ZDV without apparent cytotoxicity.
TABLE 2.
In vitro anti-FIV activity of WHI-07a
| Treatment | FIV-RT activity
|
% Cell viabilityb | |
|---|---|---|---|
| Mean cpm ± SD | % Inhibition | ||
| None (positive control) | 186,016 ± 12,908c | NAd | 57 ± 4 |
| None (negative control) | 2,009 ± 216 | NA | 67 ± 2 |
| WHI-07, 0.01 μM | 162,496 ± 11,426 | 12.8 | ND |
| WHI-07, 0.1 μM | 103,702 ± 2,481 | 44.7 | ND |
| WHI-07, 1.0 μM | 48,435 ± 3,458 | 73.6 | ND |
| WHI-07, 10.0 μM | 12,388 ± 5194 | 94.3 | 65 ± 2 |
| ZDV, 10.0 μM | 2,459 ± 439 | 99.7 | 61 ± 3 |
T-cell-enriched PBMCs from SPF cats were exogenously infected with FIV and then cultured for 9 days at 37°C in the presence or absence of WHI-07. On day 9, FIV replication was monitored by measurement of RT activity in the culture supernatants. Controls included PBMCs (negative control), FIV-infected but untreated PBMCs (positive control), and FIV-infected and ZDV-treated PBMCs.
Cell viability was determined by trypan blue dye exclusion assay by counting a minimum of 500 cells. ND, not done.
Mean ± standard deviation (n = 3).
NA, not applicable.
We next examined the ability of 2% WHI-07 either alone or in combination with 0.25% VDDTC via a nontoxic gel microemulsion to prevent the transmucosal transmission of FIVBangston in domestic cats challenged with an intravaginal inoculum of 7 × 106 FIVBangston-infected FeT-J cells (Table 3). Systemic FIV infection was monitored at 3, 6, 9, 12, 15, and 18 weeks after intravaginal exposure to chronically FIV-infected feline T cells by the appearance of viral antibodies to FIV Gag proteins by WB, VI-RT, and FIV-specific PCR. FIV infection was detected by VI-RT and PCR as early as 3 weeks after intravaginal inoculation of 7 × 106 cells. By 6 weeks postchallenge, systemic FIV infection was determined by VI-RT and PCR as well as by WB. All cats that became positive by WB analysis were persistently infected with FIV throughout the duration (18 weeks) of the study.
TABLE 3.
Effectiveness of WHI-07 gel microemulsion in protecting cats from vaginal FIV transmissiona
| Intravaginal pretreatment | Inoculum (cells) | No. infected/total no. challenged (% of total)
|
P valuee | ||
|---|---|---|---|---|---|
| WBb | VI-RTc | VI-PCRd | |||
| Vehicle | 7 × 106 | 10/10 (100)d | 10/10 (100) | 10/10 (100) | |
| 5% N-9 | 7 × 106 | 6/10 (60) | 6/10 (60) | 6/10 (60) | 0.09 |
| 2% WHI-07 | 7 × 106 | 2/5 (40) | 2/5 (40) | 2/5 (40) | 0.02 |
| 0.25% VDDTC | 7 × 106 | 8/10 (80) | 8/10 (80) | 8/10 (80) | 0.47 |
| 2% WHI-07 + 0.25% VDDTC | 7 × 106 | 1/5 (20) | 1/5 (20) | 1/5 (20) | 0.004 |
SPF cats received a vaginal gel (0.4 ml) treatment followed 1 to 2 min later by an intravaginal inoculation of FIVBangston-infected feline lymphoid (FeT-J) cells (7 × 106 cells in 0.2 ml). Systemic FIV infection was monitored at 3, 6, 9, 12, 15, and 18 weeks after intravaginal exposure to chronically FIV-infected feline T cells by the appearance of viral antibodies to FIV Gag proteins by WB and by VI-RT and FIV-specific PCR.
WB analysis of FIV Gag proteins p26 and p15 in blood plasma.
VI-RT, virus isolation of PBMCs measured by FIV RT activity.
VI-PCR, PCR analysis of FIV infection.
Vehicle control versus test (Fisher's exact test).
Whereas 10 of 10 control cats treated with the vehicle became FIV infected within 3 to 6 weeks after inoculation, only 2 of 5 cats receiving vaginal instillation of a 2% WHI-07-containing gel microemulsion showed evidence of FIV infection by 18 weeks after inoculation (P = 0.02; Fisher's exact test). By comparison, 6 of 10 cats treated with a 5% N-9 gel acquired FIV infection after the same intravaginal FIV challenge (P = 0.09). Similarly, 8 of 10 cats treated with a 0.25% VDDTC-containing gel microemulsion became FIV infected. Cats treated with VDDTC alone did not differ from controls (P = 0.47). Notably, only one of five cats treated with a gel microemulsion containing 2% WHI-07 plus 0.25% VDDTC became FIV infected, albeit with a decreased FIV titer. Thus, the use of a gel microemulsion containing WHI-07 either alone or in combination with VDDTC was significantly (P = 0.004) more effective in preventing the transvaginal transmission of FIV in domestic cats. The treated cats remained consistently negative for systemic FIV infection during the 18-week monitoring period.
We also examined the ability of WHI-07 to prevent the transrectal transmission of FIV. Under similar conditions, two of three cats challenged with an intrarectal inoculum of 7 × 106 FeT-J cells infected with FIVBangston became FIV positive, whereas none of the three 2% WHI-07-treated cats became positive for FIV throughout the 18-week duration of the study (Table 4).
TABLE 4.
Effectiveness of WHI-07 gel microemulsion in protecting cats from rectal FIV transmissiona
| Intrarectal pretreatment | Inoculum (cells) | No. infected/total no. challenged (% of total)
|
P valuee | ||
|---|---|---|---|---|---|
| WBb | VI-RTc | VI-PCRd | |||
| Vehicle | 7 × 106 | 2/3 (66) | 2/3 (66) | 2/3 (66) | |
| 2% WHI-07 | 7 × 106 | 0/3 (0) | 0/3 (0) | 0/3 (0) | 0.40 |
SPF cats received a rectal gel (0.3 ml) treatment followed 1 to 2 min later by an intrarectal inoculation of FIVBangston-infected feline lymphoid (FeT-J) cells (7 × 106 cells in 0.2 ml). Systemic FIV infection was monitored at 3, 6, 9, 12, 15, and 18 weeks after intrarectal exposure to chronically FIV-infected feline T cells by the appearance of viral antibodies to FIV Gag proteins by WB and by VI-RT and FIV-specific PCR.
WB analysis of FIV Gag proteins p26 and p15 in blood plasma.
VI-RT, virus isolation of PBMCs measured by FIV RT activity.
VI-PCR, PCR analysis of FIV infection.
Vehicle control versus test (Fisher's exact test).
DISCUSSION
In earlier studies using rabbits, the gel microemulsion of the dual-function antiretroviral spermicide WHI-07 was found to exhibit significant contraceptive activity which was superior to the potency of the commercial formulation of the detergent-type spermicide N-9 (12). Our present study extends our previous preclinical work on WHI-07 and provides unprecedented experimental evidence that WHI-07 either alone or in combination with a vanadocene is capable of preventing the transvaginal as well as transrectal transmission of FIV in domestic cats.
WHI-07, a cell-permeable aryl phosphate derivative of bromo-methoxy-ZDV, was designed to bypass the rate-limiting TK dependency of ZDV activation (11). WHI-07 exhibits enhanced lipophilicity and superior pharmacokinetics and retains full activity in TK-deficient cells when compared with ZDV (9, 22). In addition to being a potent retroviral RT inhibitor, WHI-07 induces a rapid and dramatic depletion of all cellular nucleoside diphosphate and triphosphate pools, which would contribute to the overall reduction of nucleic acid synthesis, viral replication, and cell death (57). The chelated vanadocene complex VDDTC is an effective spermicide (21, 27). Both WHI-07 and VDDTC exhibit potent spermicidal activities at low micromolar concentrations, with high selective indices against human female genital tract epithelial cells, and lack mucosal toxicity following repeated intravaginal administration (9, 22-24). VDDTC has a unique configurational preference which alters the membrane by intercalation and induces Fe2+-initiated lipid peroxidation (32, 33). The striking antiretroviral efficacy of the combination of WHI-07 and VDDTC is most likely due to the potent antiviral activity of WHI-07 as well as the combined effects of WHI-07 and the organovanadium complex on key cellular pathways essential for nucleic acid synthesis and viral replication.
Experimental transmucosal infection in cats with FIV is a valuable model for testing the in vivo efficacy of potential anti-HIV microbicides. Although FIV can be transmitted experimentally in both a cell-associated and cell-free manner through the vaginal or rectal mucosa (4, 5, 29, 31, 41, 42, 44, 45), the efficiency of FIV infection via the vaginal or rectal mucosal route is dependent upon the expression of specific chemokine receptor molecules (5, 26, 42). Our comparative studies using two dual-tropic strains with considerable macrophage tropism (FIVUK8 and FIVBangston) showed marked differences with respect to the ability to induce systemic FIV infection via the vaginal route (data not shown). In our study, FIVBangston infection could be established transmucosally with relative high efficiency via the vaginal route using a high dose of infected FeT-J cells. Control FIV-positive cats inoculated via the vaginal or rectal route with a high dose of FIVBangston-infected FeT-J cells expressed detectable levels of FIV gag provirus DNA in PBMCs. By WB analysis, plasma from these cats demonstrated a strong anti-FIV antibody response against major FIV gag proteins p26 and p15 detectable at 2 to 3 weeks postchallenge and remained consistently positive for antibodies throughout the 18-week monitoring period. Since FIV rapidly crosses mucous membranes following mucosal exposure and is rapidly transported via dendritic and T cells to systemic lymphoid tissues (42), in these preliminary efficacy studies the time period between gel treatment and cell inoculation was minimized to maximize in vivo drug availability. Microbicidal activity was considered effective when mononuclear cells of the treated cats were negative for FIV, and these cats did not display FIV-associated hematologic abnormalities for up to 18 weeks postchallenge.
The dose of WHI-07 and/or VDDTC used for the vaginal microbicide efficacy studies was based on the lack of local, systemic, and reproductive toxicity observed in two 13-week subchronic studies and a 2-year carcinogenicity study of the gel microemulsion with and without 2% WHI-07 which were performed in mice (13-17, 23, 24). Since no adverse effects were observed with the highest concentration of WHI-07 or VDDTC tested in mice and rabbits, we selected the 2% and 0.25% doses, respectively, for the intravaginal microbicide efficacy study. Despite the well-known bioavailability of the drugs in gel microemulsion, failure to achieve 100% inhibition of vaginal transmission even with the optimum dose of WHI-07 or VDDTC used could in part be due to incomplete mixing of cell inoculum with the test gel or inadequate distribution of the test gel in the vagina. Although WHI-07 completely prevented the rectal transmission of FIV, the number of animals in each group was too small for statistical significance. The use of carrageenan as an excipient in the gel microemulsion did not exhibit in vivo anti-FIV activity.
It has been found that currently used vaginal delivery systems (gels, foams, creams, suppositories, and tablets) break down almost immediately following insertion into the vaginal cavity and have minimal bioadherence to the vaginal walls (18). This is believed to be due to their water miscibility and/or their lack of physical stability at body temperature. Thus, they exhibit limited effectiveness. Poor bioavailability may lead to ineffective therapy, the need for higher dosing, and/or undesirable side effects. Microemulsions appear to have the ability to deliver larger amounts of topically applied WHI-07 and VDDTC into the genital mucosa than traditional vehicles because of their capacity for enhanced solubilization (52). The drug dissolved rather than suspended in the vehicle is in a form for immediate absorption and is generally more rapidly and more effectively absorbed. The microemulsion-based lipophilic and vaginal spermicides WHI-07 and VDDTC appear to offer several benefits for vaginal delivery, including increased absorption, potent contraceptive activity, and decreased toxicity.
Because semen is an important vehicle for sexual transmission of HIV-1 (30, 48, 61), WHI-07 either alone or in combination with a vanadocene could provide protection by inactivating HIV-1 or preventing HIV-1 from replicating either in semen or the infected host cells that line the vaginal wall. Although antiretroviral drugs can greatly reduce the HIV viral load in semen, they are not foolproof in preventing HIV infection via sexual contact (2, 49). A substantial percentage of HIV-positive men have active, potentially infectious viruses in their semen, even after 6 months of therapy. Hence, significant proportions of men who undergo therapy and subsequently feel well remain potentially infectious and therefore continue to pose a public health risk unless they monitor their sexual behavior carefully. The results of our present study point to the potential utility of a WHI-07 gel microemulsion as a prophylactic antiviral agent.
In conclusion, WHI-07 either alone or in combination with a vanadocene via a gel microemulsion shows potential as a vaginal and rectal microbicide for the prevention of the sexual transmission of HIV, which is thought to be transmitted primarily by infected cells present in semen. The rapid intracellular delivery of bioactive nucleotides by WHI-07 despite low or absent TK activity has clinical advantage for curbing the sexual transmission of HIV by leukocytes and germ cells in semen. The demonstrated lack of mucosal, systemic, reproductive, or developmental toxicity in test animal species as well as the lack of tumorigenic potential of WHI-07 in long-term carcinogenicity studies indicate that WHI-07 displays the very properties required of an ideal spermicidal microbicide and warrant further preclinical research and development.
Acknowledgments
We thank R. Pu and J. K. Yamamoto (University of Florida) for their services under the FIV contract research agreement between the Parker Hughes Institute and the University of Florida.
This work was supported in part by National Institutes of Health grants HD 37357, HD 42884, HD 42889, and AI 54352 and American Foundation for AIDS Research grant 02667.
REFERENCES
- 1.Arai, M., D. D. Earl, and J. K. Yamamoto. 2002. Is AZT/3TC therapy effective against FIV infection or immunopathogenesis? Vet. Immunol. Immunopathol. 85:189-204. [DOI] [PubMed] [Google Scholar]
- 2.Barroso, P. F., M. Schechter, P. Gupta, C. Bressan, A. Bomfim, and L. H. Harrison. 2003. Adherence to antiretroviral therapy and persistence of HIV RNA in semen. J. Acquir. Immune Defic. Syndr. 32:435-440. [DOI] [PubMed] [Google Scholar]
- 3.Bendinelli, M., M. Pistello, D. Matteucci, S. Lombardi, F. Baldinotti, P. Bandecchi, R. Ghilarducci, L. Ceccherini-Nelli, C. Garzelli, and A. Poli. 1993. Small animal model of AIDS and the feline immunodeficiency virus. Adv. Exp. Med. Biol. 335:189-202. [DOI] [PubMed] [Google Scholar]
- 4.Bishop, S. A., C. R. Stokes, T. J. Gruffydd-Jones, C. V. Whiting, and D. A. Harbour. 1996. Vaginal and rectal infection of cats with feline immunodeficiency virus. Vet. Microbiol. 51:217-227. [DOI] [PubMed] [Google Scholar]
- 5.Burkhard, M. J., L. A. Obert, L. L. O'Neil, L. J. Diehl, and E. A. Hoover. 1997. Mucosal transmission of cell-associated and cell-free feline immunodeficiency virus. AIDS Res. Hum. Retrovir. 13:347-355. [DOI] [PubMed] [Google Scholar]
- 6.D'Cruz, O. J., and F. M. Uckun. 2004. Clinical development of microbicides for the prevention of HIV infection. Curr. Pharm. Des. 10:315-336. [DOI] [PubMed]
- 7.D'Cruz, O. J., T. K. Venkatachalam, Z. Zhu, M.-L. Shih, and F. M. Uckun. 1998. Bromo-methoxy and aryl phosphate derivatives of azidothymidine are dual function spermicides with potent anti-HIV activity. Biol. Reprod. 59:503-515. [DOI] [PubMed] [Google Scholar]
- 8.D'Cruz, O. J., M.-J. Shih, S. H. Yiv, C.-L. Chen, and F. M. Uckun. 1999. Synthesis, characterization and preclinical formulation of a novel phenyl phosphate derivative of bromo-methoxy zidovudine (compound WHI-07) with potent anti-HIV and spermicidal activities. Mol. Hum. Reprod. 5:421-432. [DOI] [PubMed] [Google Scholar]
- 9.D'Cruz, O. J., and F. M. Uckun. 2002. Pre-clinical safety evaluation of novel nucleoside analogue-based dual-function microbicides (WHI-05 and WHI-07). J. Antimicrob. Chemother. 50:793-803. [DOI] [PubMed] [Google Scholar]
- 10.D'Cruz, O. J., T. K. Venkatachalam, and F. M. Uckun. 2000. Structural requirements for potent human spermicidal activity of dual-function aryl phosphate derivative of bromo-methoxy zidovudine (compound WHI-07). Biol. Reprod. 62:37-44. [DOI] [PubMed] [Google Scholar]
- 11.D'Cruz, O. J., T. K. Venkatachalam, and F. M. Uckun. 2001. Thymidine kinase-independent intracellular delivery of bioactive nucleotides by aryl phosphate derivatives of bromo-methoxy zidovudine (compounds WHI-05 and WHI-07) in normal human genital tract epithelial cells and sperm. Biol. Reprod. 64:51-59. [DOI] [PubMed] [Google Scholar]
- 12.D'Cruz, O. J., and F. M. Uckun. 2003. Contraceptive activity of a spermicidal aryl phosphate derivative of bromo-methoxy zidovudine (compound WHI-07) in rabbits. Fertil. Steril. 79:864-872. [DOI] [PubMed] [Google Scholar]
- 13.D'Cruz, O. J., B. Waurzyniak, S. H. Yiv, and F. M. Uckun. 2000. Evaluation of subchronic (13-week) and reproductive toxicity potential of intravaginal gel-microemulsion formulation of a dual-function phenyl phosphate derivative of bromo-methoxy zidovudine (compound WHI-07) in B6C3F1 mice. J. Appl. Toxicol. 20:319-325. [DOI] [PubMed] [Google Scholar]
- 14.D'Cruz, O. J., and F. M. Uckun. 2001. Short-term (13-week) toxicity study of 5-bromo-6-methoxy-5,6-dihydro-3′-azidothymidine-5′-(p-bromophenyl) methoxyalaninyl phosphate (WHI-07), a novel anti-HIV and contraceptive agent, in B6C3F1 mice. Toxicol. Sci. 60:373-378. [DOI] [PubMed] [Google Scholar]
- 15.D'Cruz, O. J., and F. M. Uckun. 2001. Lack of adverse effects on fertility of female CD-1 mice exposed to repetitive intravaginal gel-microemulsion formulation of a dual-function anti-HIV agent: aryl phosphate derivative of bromo-methoxy-zidovudine (compound WHI-07). J. Appl. Toxicol. 21:317-322. [DOI] [PubMed] [Google Scholar]
- 16.D'Cruz, O. J., D. Erbeck, and F. M. Uckun. 2003. Developmental toxicology studies of WHI-07, a novel nucleoside analogue-based dual-function microbicide, administered intravaginally to rabbits. Toxicol. Pathol. 31:698-708. [DOI] [PubMed] [Google Scholar]
- 17.D'Cruz, O. J., D. Erbeck, B. Waurzyniak, and F. M. Uckun. 2002. Two-year toxicity and carcinogenicity studies of B6C3F1 mice with 5-bromo-6-methoxy-5,6-dihydro-3′-azidothymidine-5′-(p-bromophenyl) methoxyalaninyl phosphate (WHI-07), a novel anti-HIV and contraceptive agent. Toxicology 179:61-77. [DOI] [PubMed] [Google Scholar]
- 18.D'Cruz, O. J., and F. M. Uckun. 2001. Gel-microemulsions as vaginal spermicides and intravaginal drug delivery vehicles. Contraception 64:113-123. [DOI] [PubMed] [Google Scholar]
- 19.D'Cruz, O. J., Y. Dong, and F. M. Uckun. 1999. Spermicidal activity of oxovanadium(IV) complexes of 1,10-phenanthroline, 2,2′-bipyridyl, 5′-bromo-2′-hydroxyacetophenone and derivatives in humans. Biol. Reprod. 60:435-444. [DOI] [PubMed] [Google Scholar]
- 20.D'Cruz, O. J., P. Ghosh, and F. M. Uckun. 1998. Spermicidal activity of metallocene complexes containing vanadium(IV) in humans. Biol. Reprod. 58:1515-1526. [DOI] [PubMed] [Google Scholar]
- 21.D'Cruz, O. J., P. Ghosh, and F. M. Uckun. 1998. Spermicidal activity of chelated complexes of bis(cyclopentadienyl)vanadium(IV). Mol. Hum. Reprod. 4:683-693. [DOI] [PubMed] [Google Scholar]
- 22.D'Cruz, O. J., and F. M. Uckun. Preclinical overview of WHI-07, a novel nucleoside analog-based dual-function microbicide. Curr. Med. Chem., in press.
- 23.D'Cruz, O. J., and F. M. Uckun. 2001. Intravaginal toxicity studies of a gel-microemulsion formulation of spermicidal vanadocenes in rabbits. Toxicol. Appl. Pharmacol. 170:104-112. [DOI] [PubMed] [Google Scholar]
- 24.D'Cruz, O. J., B. Waurzyniak, and F. M. Uckun. 2001. Subchronic (13-week) toxicity studies of intravaginal administration of spermicidal vanadocene dithiocarbamate in mice. Contraception 64:177-185. [DOI] [PubMed] [Google Scholar]
- 25.Diehl, L. J., C. K. Mathiason-Dubard, L. L. O'Neill, and E. A. Hoover. 1995. Longitudinal assessment of feline immunodeficiency virus kinetics in plasma by use of a quantitative competitive reverse transcriptase PCR. J. Virol. 69:2328-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.English, R. V., P. Nelson, C. M. Johnson, M. Nasisse, W. A. Tompkins, and M. B. Tompkins. 1994. Development of clinical disease in cats experimentally infected with feline immunodeficiency virus. J. Infect. Dis. 170:543-552. [DOI] [PubMed] [Google Scholar]
- 27.Ghosh, P., S. Ghosh, O. J. D'Cruz, and F. M. Uckun. 1998. Structural and biological characterization of a novel spermicidal vanadium(IV) complex: bis(π-cyclopentadienyl)-N,N-diethyl dithiocarbamato vanadium(IV) tetrafluoro borate, [VCp2(DeDtc)](BF4). J. Inorg. Biochem. 72:89-98. [DOI] [PubMed] [Google Scholar]
- 28.Hohdatsu, T., H. Hirabayashi, K. Motokawa, and H. Koyama. 1996. Comparative study of the cell tropism of feline immunodeficiency virus isolates of subtypes A, B and D classified on the basis of the env gene V3-V5 sequence. J. Gen. Virol. 77:93-100. [DOI] [PubMed] [Google Scholar]
- 29.Jordan, H. L., J. Howard, W. A. Tompkins, and S. Kennedy-Stoskopf. 1995. Detection of feline immunodeficiency virus in semen from seropositive domestic cats (Felis catus). J. Virol. 69:7328-7333. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Kalichman, S. C., M. Cage, T. Barnett, P. Tharnish, D. Rompa, J. Austin, W. Luke, J. O'Mowrey, and R. F. Schinazi. 2001. Human immunodeficiency virus in semen and plasma: investigation of sexual transmission risk behavioral correlates. AIDS Res. Hum. Retrovir. 17:1695-1703. [DOI] [PubMed] [Google Scholar]
- 31.Kohmoto, M., Y. Ikeda, E. Sato, Y. Nishimura, Y. Inoshima, M. Shimojima, Y. Tohya, T. Mikami, and T. Miyazawa. 2003. Experimental mucosal infection with molecularly cloned feline immunodeficiency viruses. Clin. Diagn. Lab. Immunol. 10:185-188. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32.Kotchevar, A. T., P. Ghosh, and F. M. Uckun. 1998. Interactions of vanadocene(IV)-chelated complexes with artificial membranes. J. Phys. Chem. B 102:10925-10930. [Google Scholar]
- 33.Kotchevar, A. T., P. Ghosh, D. DuMez, and F. M. Uckun. 2000. Induction of aerobic peroxidation of liposomal membranes by bis(cyclopentadienyl)-vanadium(IV) (acetylacetonate) complexes. J. Inorg. Biochem. 83:151-160. [DOI] [PubMed] [Google Scholar]
- 34.Mishell, B. B., S. M. Shiigi, C. Henry, E. L. Chan, J. North, R. Gallily, M. Slomich, K. Miller, J. Marbrook, D. Parks, and A. H. Good. 1980. Preparation of mouse cell suspensions, p. 3-27. In B. B. Mishell and S. M. Shiigi (ed.), Selected methods in cellular immunology. W. H. Freeman and Co., San Francisco, Calif.
- 35.Moench, T. R., K. J. Whaley, T. D. Mandrell, B. D. Bishop, C. J. Witt, and R. A. Cone. 1993. The cat/feline immunodeficiency virus model for transmucosal transmission of AIDS: nonoxynol-9 contraceptive jelly blocks transmission by an infected cell inoculum. AIDS 7:797-802. [DOI] [PubMed] [Google Scholar]
- 36.National Research Council. 1996. Guide for the care and use of laboratory animals. National Academy Press, Washington, D.C.
- 37.National Institute of Allergy and Infectious Diseases.2002. Fact sheet: HIV infection in women. [Online.] http://www.niaid.nih.gov/factsheets/womenhiv.htm.
- 38.National Institute of Allergy and Infectious Diseases.2003. Fact sheet: HIV/AIDS statistics. [Online.] http://biodefense.niaid.nih.gov/factsheets/aidsstat.htm.
- 39.Nechay, B. R. 1982. Mechanisms of action of vanadium. Annu. Rev. Pharmacol. Toxicol. 24:501-524. [DOI] [PubMed] [Google Scholar]
- 40.North, T. W., G. L. North, and N. C. Pedersen. 1989. Feline immunodeficiency virus, a model for reverse transcriptase-targeted chemotherapy for acquired immunodeficiency syndrome. Antimicrob. Agents Chemother. 34:1505-1507. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.Obert, L. A., and E. A. Hoover. 2000. Feline immunodeficiency virus clade C mucosal transmission and disease courses. AIDS Res. Hum. Retrovir. 16:677-688. [DOI] [PubMed] [Google Scholar]
- 42.Obert, L. A., and E. A. Hoover. 2002. Early pathogenesis of transmucosal feline immunodeficiency virus infection. J. Virol. 76:6311-6322. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Okada, S., R. Pu, E. Young, W. V. Stoffs, and J. K. Yamamoto. 1994. Superinfection of cats with feline immunodeficiency virus subtypes A and B. AIDS Res. Hum. Retrovir. 10:1739-1746. [DOI] [PubMed] [Google Scholar]
- 44.O'Neil, L. L., M. J. Burkhard, and E. A. Hoover. 1996. Frequent perinatal transmission of feline immunodeficiency virus by chronically infected cats. J. Virol. 70:2894-2901. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.O'Neil, L. L., M. J. Burkhard, L. J. Diehl, and E. A. Hoover. 1995. Vertical transmission of feline immunodeficiency virus. AIDS Res. Hum. Retrovir. 1:171-182. [DOI] [PubMed] [Google Scholar]
- 46.Pedersen, N. C., J. K. Yamamoto, T. Ishida, and H. Hansen. 1989. Feline immunodeficiency virus infection. Vet. Immunol. Immunopathol. 21:111-129. [DOI] [PubMed] [Google Scholar]
- 47.Piot, P., M. Bartos, P. D. Ghys, N. Walker, and B. Schwartlander. 2001. The global impact of HIV/AIDS. Nature 410:968-973. [DOI] [PubMed] [Google Scholar]
- 48.Quayle, A. J., C. Xu, K. H. Mayer, and D. J. Anderson. 1997. T lymphocytes and macrophages, but not motile spermatozoa, are a significant source of human immunodeficiency virus in semen. J. Infect. Dis. 176:960-968. [DOI] [PubMed] [Google Scholar]
- 49.Quinn, T. C., M. J. Wawer, N. Sewankambo, D. Serwadda, C. Li, F. Wabwire-Mangen, M. O. Meehan, T. Lutalo, R. H. Gray, et al. 2000. Viral load and heterosexual transmission of human immunodeficiency virus type 1. N. Engl. J. Med. 342:921-929. [DOI] [PubMed] [Google Scholar]
- 50.Rey, M. A., B. Spire, D. Dormont, F. Barre-Sinoussi, L. Montagnier, and J. C. Chermann. 1984. Characterization of the RNA dependent DNA polymerase of a new human T-lymphotropic retrovirus. Biochem. Biophys. Res. Commun. 121:126-133. [DOI] [PubMed] [Google Scholar]
- 51.Tanabe, T., and J. K. Yamamoto. 2001. Phenotypic and functional characteristics of FIV infection in the bone marrow stroma. Virology 282:113-122. [DOI] [PubMed] [Google Scholar]
- 52.Tenjaria, S. 1999. Microemulsions: an overview and pharmaceutical applications. Crit. Rev. Ther. Drug Carrier Syst. 16:461-521. [PubMed] [Google Scholar]
- 53.Tsiani, E., and I. G. Fantus. 1997. Vanadium compounds: biological actions and potential as pharmacological agents. Trends Endocrinol. Metab. 8:51-58. [DOI] [PubMed] [Google Scholar]
- 54.Turpin, J. A. 2002. Considerations and development of topical microbicides to inhibit the sexual transmission of HIV. Expert Opin. Investig. Drugs 11:1077-1097. [DOI] [PubMed] [Google Scholar]
- 55.Uckun, F. M., and O. J. D'Cruz. 1999. Prophylactic contraceptives for HIV/AIDS. Hum. Reprod. Update 5:506-514. [DOI] [PubMed] [Google Scholar]
- 56.Uckun, F. M., C. L. Chen, P. Samuel, S. Pendergrass, T. K. Venkatachalam, B. Waurzyniak, and S. Qazi. 2003. In vivo antiretroviral activity of stampidine in chronically feline immunodeficiency virus-infected cats. Antimicrob. Agents Chemother. 47:1233-1240. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 57.Uckun, F. M., H.-L. Tai, and O. J. D'Cruz. Antileukemic activity and cellular metabolism of the aryl phosphate derivative of bromo-methoxy zidovudine (compound WHI-07). Arzneimittelforschung, in press. [DOI] [PubMed]
- 58.United Nations Program on HIV/AIDS. 2001. Report on global HIV/AIDS epidemic. [Online.] http://www.unaids.org.
- 59.Willett, B. J., J. N. Flynn, and M. J. Hosie. 1997. FIV infection of the domestic cat: an animal model for AIDS. Immunol. Today 18:182-189. [DOI] [PubMed] [Google Scholar]
- 60.Yamamoto, J. K., N. C. Pederson, E. W. Ho, T. Okuda, and G. H. Theilen. 1988. Feline immunodeficiency syndrome—a comparison between feline T-lymphotropic lentivirus and feline leukemia virus. Leukemia 2:204-215. [PubMed] [Google Scholar]
- 61.Zhang, H., G. Dornadula, M. Beumont, L. Livornese, Jr., B. Van Uitert, K. Henning, and R. J. Pomerantz. 1988. Human immunodeficiency virus type 1 in the semen of men receiving highly active antiretroviral therapy. N. Engl. J. Med. 339:1803-1809. [DOI] [PubMed] [Google Scholar]