Abstract
BACKGROUND
The authors have recently shown that a majority of patients with myelodysplastic syndrome (MDS) classified by the International Prognostic Scoring System as lower risk die without transformation to acute myelogenous leukemia (AML). The cause of death (COD) of these patients is not well understood. Identifying the COD could help to guide early therapy decisions.
METHODS
The authors retrospectively analyzed the COD in a cohort of 273 deceased patients with lower-risk MDS according to the International Prognostic Scoring System at presentation to The University of Texas M. D. Anderson Cancer Center from 1980 to 2004. MDS-related death was defined as infection, bleeding, transformation to AML, or disease progression. Remaining CODs were classified as non–MDS-related.
RESULTS
Median age at presentation was 66 years (range, 19-88 years). Overall median survival was 59 weeks (range, 1-831 weeks). All French-American-British leukemia classification subgroups were represented. The percentage of International Prognostic Scoring System low and intermediate-1 groups were 21% and 79%, respectively. The most common cytogenetic abnormality (9%) was del(5q). Patients received supportive care only. The COD was identified as MDS-related in 230 of 273 (84%) patients. The most common disease-related CODs were infection (38%), transformation to AML (15%), and hemorrhage (13%). The most frequent non–disease-related COD was cardiovascular events (19 of 43 patients).
CONCLUSIONS
The majority of patients with low- or intermediate-1 risk MDS will die because of causes related to their underlying disease. Although these results need to be validated in different populations, early therapeutic intervention could be considered in the management of these patients to improve survival.
Keywords: myelodysplastic syndrome, mortality, International Prognostic Scoring System, cause of death
The International Prognostic Scoring System is commonly used to predict survival rates and progression to acute myelogenous leukemia (AML) in patients with myelodysplastic syndrome (MDS).1 The International Prognostic Scoring System categorizes patients into lower (low/intermediate-1) versus higher (intermediate-2/high) risk groups. Approximately ⅔ of patients with MDS have lower-risk disease at the time of initial diagnosis.2,3
We studied the outcomes of patients with lower-risk MDS who presented to a cancer center. On the basis of that analysis, we developed a prognostic scoring system for lower-risk MDS3 that stratifies patient prognosis based on 7 adverse prognostic factors. Depending on the number of those factors, 4-year survival ranged from 78% to <10%. Importantly, further analysis showed that only 10% of patients had transformed to AML, but 50% had died. The cause of death (COD) among this subgroup of lower-risk patients with poor outcome is not well understood. The understanding of the natural history of disease in patients with lower-risk MDS may have significant implications for their clinical management.
The median age at diagnosis of patients with MDS is approximately 75 years.4 Currently, a large fraction of MDS patients receives supportive care (such as transfusions and growth factors) only, rather than disease-specific therapy. This is likely because, until recently, no effective therapy was available for MDS. In light of the recent development of effective drugs against MDS,5-7 it is important to determine whether the COD in this elderly population is because of age-related comorbidities or directly associated with MDS. This information could be used to guide intervention in patients with lower-risk MDS who are identified as having a poor outcome and might benefit from early therapeutic intervention.
The aim of the present study was to establish the COD in a cohort of MDS patients with low- or intermediate-1 risk disease at presentation and to examine whether the CODs have changed over time in this patient group.
MATERIALS AND METHODS
Patient Selection
The institutional review board at The University of Texas M. D. Anderson Cancer Center (MDACC) approved this retrospective analysis. All adults with a diagnosis of MDS referred from 1980 through 2004 to MDACC (N = 1279) were reviewed. A total of n = 903 patients had died during this time period. Only deceased patients with low/intermediate-1 risk features as defined by the International Prognostic Scoring System1 at initial presentation at MDACC who had a known COD (as defined below) were included in this study. A total of n = 273 patients met the inclusion criteria. Clinical, pathological, and laboratory information were retrieved from a database maintained by the Department of Leukemia at MDACC. Morphology was based on the French-American-British (FAB) system.8 Transformation to AML was defined as presence of ≥30% myeloblasts in the bone marrow.
Determination of COD
The following sources were used to determine the COD: final progress note (28%), autopsy report (4%), death certificate (4%), information from the MDACC leukemia database (54%), and correspondence (such as a physician letter or physician telephone note) (12%). The US Social Security Death Index was used for death verification. Causes of death coded as related to MDS included infections, hemorrhage, transformation to AML, and progression of disease (defined as worsening cytopenia without transformation to AML). All other CODs were classified as non–MDS-related (eg, other malignancies). Validation of the COD was performed in a random sample of all patients by 2 independent physicians.
Statistical Analysis
Overall median survival was measured as the time from presentation to MDACC to the time of death. Chi-square test was used to describe trends in differences of COD over time. Overall survival by decade was calculated by Kaplan-Meier and log-rank test.9
RESULTS
Patient Characteristics
Patient characteristics are shown in Table 1. Median age at presentation was 66 years (range, 19-88 years). Overall median survival was 59 weeks (range, 1-831 weeks). By FAB classification, all subgroups were identified (refractory anemia = 41%, refractory anemia with ringed sidero-blasts = 6%, refractory anemia with excess blasts = 29%, refractory anemia with excess blasts in transformation = 12%, chronic myelomonocytic leukemia = 12%). By International Prognostic Scoring System score, most patients were intermediate-1 (intermediate-1 = 79% and low = 21%). Most patients had diploid cytogenetics (58%), and the most common cytogenetic abnormality was deletion of chromosome 5q (9%). Progression to AML before death occurred in 15% of the patients.
Table 1.
Patient Characteristics
| Characteristic | Median (Range) |
|---|---|
| Age, y | 66 (19-88) |
| Hemoglobin, g/dL | 9.6 (3.2-15.4) |
| Platelet count, × 103/L | 61 (2-692) |
| WBC, × 103/L | 3.7 (0.6-99) |
| ANC, × 103/μL | 1.8 (0.04-46) |
| Serum creatinine, mg/dL | 1 (0.4-2.8) |
| Bilirubin, mg/dL | 0.7 (0.1-6.5) |
| Albumin, g/dL | 3.9 (1.7-5.2) |
| Bone marrow blasts, % | 3 (0-10) |
| No. (%) | |
| Men | 205 (75) |
| Cytogenetics | |
| Diploid | 161 (59) |
| –Y | 8 (3) |
| –5q | 25 (9) |
| –7 | 7 (3) |
| –5/–7 | 2 (1) |
| Trisomy 8 | 17 (6) |
| –20q | 11 (4) |
| Complex | 23 (8) |
| Miscellaneous | 22 (8) |
| FAB classification | |
| RA | 114 (42) |
| RARS | 16 (6) |
| RAEB | 79 (29) |
| RAEBT | 34 (12) |
| CMML | 33 (12) |
| IPSS | |
| Low | 57 (21) |
| Intermediate-1 | 209 (77) |
| NS | 10 (4) |
WBC indicates white blood cell count; ANC, absolute neutrophil count; FAB, French-American-British; RA, refractory anemia; RARS, refractory anemia with ringed sideroblasts; RAEB, refractory anemia with excess blasts; RAEBT, refractory anemia with excess blasts in transformation; CMML, chronic myelomonocytic leukemia; IPSS, International Prognostic Scoring System; NS, not specified.
Causes of Death
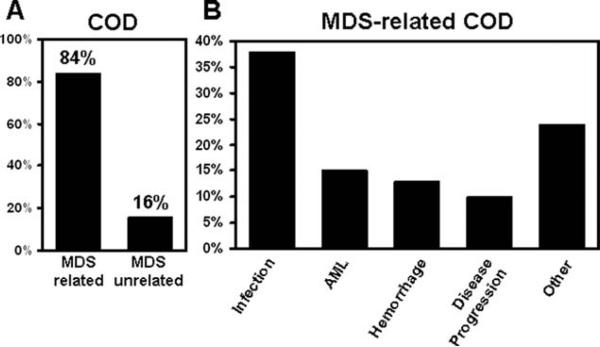
MDS-related COD was defined as infection, hemorrhage, transformation to AML, and progression of disease. On the basis of this definition, COD was identified as MDS-related in 230 of 273 (84%) patients (Fig. 1A).
Figure 1.
(A) Of 273 patients with a known cause of death (COD), 230 (84%) died of myelodysplastic syndrome (MDS)-related causes versus 43 (16%) patients who died of unrelated causes. (B) Among patients with a known COD related to MDS (n = 230), the majority (n = 104) died of infections, followed by transformation to acute myelogenous leukemia (AML)(n = 40) and hemorrhage (n = 35).
The most common disease-related COD was infection (38% of all deaths), followed by transformation to AML (15%) and hemorrhage (13%) (Fig. 1B). The single most common infectious COD was pneumonia (13%). Pneumonia and sepsis accounted for 40% and 38%, respectively, of all infections. In 23% of the cases, the site of infection was not further specified. A microorganism could be identified in 30% of the patients with pneumonia. Among those, the proportion of fungal, bacterial, and viral pneumonia was 58%, 25%, and 17%, respectively. Fatal hemorrhage occurred in the central nervous system in 26%, and gastrointestinal bleeding and pulmonary hemorrhage each were responsible for 24% of bleeding-related deaths. Infection, fatal hemorrhage, and transformation to AML constituted 96% of all known causes of MDS-related deaths; the remainder were classified as disease progression, which includes progressive bone marrow failure without transformation to AML. In some cases, the COD in the chart was documented as either MDS or progressive disease without further specification.
The most frequent non–disease-related COD was cardiovascular events (19 of 273 patients, 7%). Of note, 8 of 273 (3%) patients died of a secondary neoplasm. Table 2 shows CODs classified by organ system.
Table 2.
Causes of Death
| Cause of Death | No. | % |
|---|---|---|
| Cardiovascular | ||
| Cardiac (NOS) | 1 | 0.4 |
| CHF | 9 | 3.3 |
| MI | 9 | 3.3 |
| Total | 19 | 7 |
| Gastrointestinal | ||
| GI hemorrhage | 8 | 2.9 |
| Hepatic failure | 4 | 1.5 |
| Mesenteric ischemia | 1 | 0.4 |
| Necrotic bowel | 1 | 0.4 |
| Total | 14 | 5.1 |
| Hematologic | ||
| Bone marrow failure | 1 | 0.4 |
| GVHD | 2 | 0.7 |
| Hemorrhage | 9 | 3.3 |
| Leukemia/AML | 40 | 14.7 |
| Lymphoma | 2 | 0.7 |
| Mastocytosis | 1 | 0.4 |
| MDS | 18 | 6.6 |
| Myeloproliferative disease | 3 | 1.1 |
| Pancytopenia | 2 | 0.7 |
| Progressive disease | 10 | 3.7 |
| Total | 88 | 32.2 |
| Infectious | ||
| Brain infection | 2 | 0.7 |
| Heart infection | 1 | 0.4 |
| Infection (NOS) | 23 | 8.4 |
| Pneumonia | 40 | 14.7 |
| Sepsis | 38 | 13.9 |
| Total | 104 | 38.1 |
| Neurologic | ||
| CNS hemorrhage | 9 | 3.3 |
| CVA | 5 | 1.8 |
| Total | 14 | 5.1 |
| Oncologic | ||
| Cancer (NOS) | 2 | 0.7 |
| Melanoma | 1 | 0.4 |
| Liver cancer | 1 | 0.4 |
| Lung cancer | 2 | 0.7 |
| Renal cell cancer | 1 | 0.4 |
| Sarcoma | 1 | 0.4 |
| Total | 8 | 2.9 |
| Other causes | ||
| Aspiration from oral hemorrhage | 1 | 0.4 |
| Gunshot wound | 1 | 0.4 |
| Iron intoxication | 1 | 0.4 |
| Suicide | 1 | 0.4 |
| Total | 4 | 1.5 |
| Pulmonary | ||
| MOF | 3 | 1.1 |
| Pulmonary hemorrhage | 8 | 2.9 |
| Respiratory arrest | 9 | 3.3 |
| Total | 20 | 7.3 |
| Renal | ||
| Renal failure | 2 | 0.7 |
| Total | 2 | 0.7 |
NOS indicates not otherwise specified; CHF, congestive heart failure; MI, myocardial infarction; GI, gastrointestinal; GVHD, graft versus host disease; AML acute myelogenous leukemia; MDS, myelodysplastic syndrome; CNS, central nervous system; CVA, cerebrovascular accident; MOF, multiorgan failure.
Trends in Causes of Death Over Time
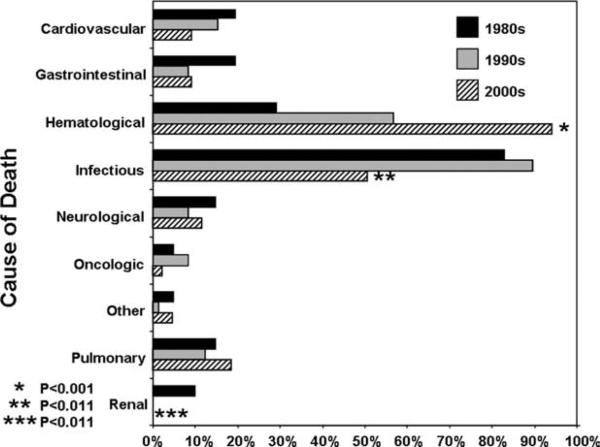
As shown above, infections accounted for the majority of the MDS-related deaths in our patient cohort. Given the introduction of better antibiotic prophylaxis, growth factors, and new therapeutic drugs for MDS, we hypothesized that over time a downward shift would be expected with regard to infections as the major COD in MDS patients. As shown in Figure 2, when comparing the trend over 3 decades, there was a statistically significant decrease in the proportion of infections as COD (P < .011) and renal disease as COD (P < .003). Conversely, hematological COD (bone marrow failure, pancytopenia, and progressive disease) significantly increased (P < .001). Hematological COD increased from 6 cases reported during the 1980s to 41 cases noted in the 1990s and 41 cases from 2000 to 2006 (P < .001). Parallel to this trend, transformation to AML as a COD also increased over time (P = .017). The remaining causes of death were stable in each decade (Fig. 2).
Figure 2.
Distribution of cause of death by decade is shown. P values shown are for the decade of the 2000s compared with previous decades.
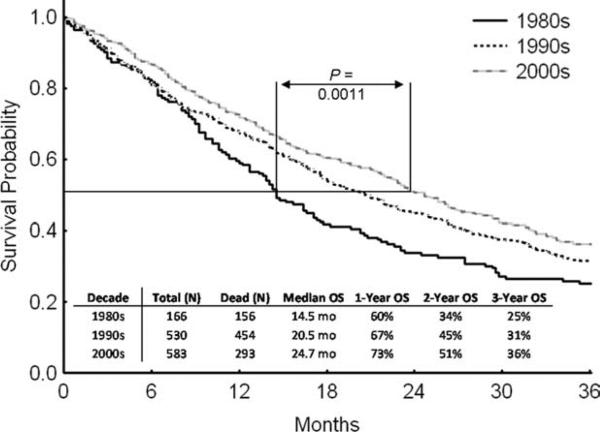
Overall, an increase in median survival (P < .004) was noted from 1980 to 2006 (Fig. 3). This reduction in mortality occurred possibly as a result of a combination of several factors, as no single factor has been conclusively shown to affect overall survival in this population, including the introduction of growth factors for cytopenias (erythropoietin-stimulating agents and granulocyte-stimulating factors) and better antimicrobial agents (both used as prophylaxis and during acute infection).
Figure 3.
Survival is shown by decade. OS indicates overall survival.
DISCUSSION
We have recently demonstrated, based on a patient cohort from MDACC, that patients with International Prognostic Scoring System low- and intermediate-1 risk MDS can be divided in 3 categories with significant differences in median survival. Whereas patients in risk category 1 had a median survival of >80 months, median survival sharply declined to 26.6 and 14.2 months for categories 2 and 3, respectively. We also showed that only 20% of the cohort fell into the favorable category 1, meaning that on average 8 of 10 patients with lower-risk MDS will have a significantly shortened life expectancy.
Historically, most clinicians have provided supportive care only to International Prognostic Scoring System lower-risk patients. Treatment usually is not initiated until there is evidence of disease progression (increased percentage of marrow blasts or progressive pancytopenia, transfusion needs). This approach is guided by data indicating that International Prognostic Scoring System lower-risk patients, depending on their age, will have an estimated survival of 2.4 to 11.8 years.1 Our previous study provided evidence that patients classified by the International Prognostic Scoring System as lower risk represent a heterogeneous group regarding outcome. Until recently, this knowledge would not have led to any changes in clinical practice, because no effective MDS-specific therapy was available. Since 2002, this might have changed with the emergence of 3 approved drugs to treat MDS. 5-Azacitidine,7 5-aza-2′-deoxycitidine,5 and lenalidomide have activity in MDS. But only the latter is clearly approved for lower-risk MDS and in patients with transfusion-dependent anemia and chromo-some 5q abnormalities.
Little is known about the specific cause of death in lower International Prognostic Scoring System score patients. Given the advanced age at presentation, MDS-specific treatment would likely be of little or no benefit if most deaths were because of age-related comorbidities. In the present study, we observed that in fact 85% of all deaths within our patient population with International Prognostic Scoring System low or intermediate-1 scores occurred as a result of the underlying disease rather than other unrelated causes. It should be noted that this group of patients did not received any specific form of therapy for their MDS, and therefore the data presented here reflect the natural course of patients with lower-risk disease without specific intervention. In line with previous reports,10 the majority of MDS-related mortality was caused by infections. Our study also provides evidence for changing trends in COD, with the proportion of infections declining over the past 3 decades, whereas hemato-logical causes have been steadily rising. Of importance, we also detected an improvement in survival with time. Although we cannot account for any specific intervention that resulted in this phenomenon, this might be explained by improved supportive care, including more aggressive transfusion approaches, use of growth factors for cytopenic patients more recently, as well as more effective antimicrobial agents used both in prophylaxis and during active infection.
We also demonstrated that cardiac events were the main cause of death in patients who presumably died of non–MDS-related causes. Transfusional hemosiderosis, a well-described poor prognostic marker in MDS, may contribute to cardiomyopathy.11 Because the determination of the underlying cause of cardiomyopathy is difficult without biopsy, it is possible that in a significant number of patients who died from cardiac causes the death was a result of their MDS. Therefore, the actual MDS-related COD in our patient cohort may be in excess of 85%.
In light of the data presented here, consideration should be given to early therapeutic intervention for lower-risk patients who may have poor prognostic features, with the goal of improving survival and quality of life. This could include the use of lower dose/schedules of hypomethylating agents, combinations of lenalidomide with growth factors, or newer investigational approaches for this group of patients.
Another important issue is to determine whether the non-MDS CODs were similar to the most frequent CODs in non-MDS populations. According to the Centers for Disease Control and Prevention, the top 3 CODs in the United States (2006) for the age group of 65 years and older are, in descending order: cardiovascular disease (29%), malignancies (22%), and cerebrovascular insults (6.7%).12 In that sense, non–MDS-related CODs in our series match those of the general population. This analysis is complicated by the finding that intracranial hemorrhage and cardiomyopathy could also be MDS-related.
There are some limitations to the current study. The most important related to the cohort of patients studied here. To perform the detailed retrospective analysis performed here, we analyzed a group of patients evaluated at 1 major referral center. It is possible that the characteristics of these patients were worse than other patients treated at other centers, and that cause of death could be different in patients with lower-risk disease earlier in the course of their disease or not referred to a tertiary care center. For instance, the median survival of the patients included in our study was somewhat shorter than previously reported for lower-risk patients. This could be explained by the finding that survival measured in our study reflects survival from time of referral to our center rather than from time of MDS diagnosis. This is a problem inherent to the nature of referrals to a large cancer center and cannot be controlled in most circumstances, and it is probably also difficult to control time of initial identification of cytopenia to referral to a hematologist. But the definitions in survival times should not affect the actual cause of death, and therefore not affect the conclusions drawn from our results. Another limitation might arise from the finding that patients referred to our institution will likely have more complicated clinical features and might not be representative of low-risk patients in general. Because all patients included in this study were either International Prognostic Scoring System low or intermediate-1, the conclusion can still be drawn that there are patients with poor outcome within the low-risk cohort, and most of these will die of MDS-related causes. It should also be noted that although we observed a trend toward fewer kidney-related deaths, the number of patients is too small to draw any conclusions in this regard. Another limitation relates to our not having data regarding platelet count and alloimmunization status of patients who died from bleeding complications. These data would be of interest to review our current transfusion strategies in patients with MDS at risk for bleeding. We also realize that for a fraction of our patients, the documenting physician summarized the COD as, for example, “bone marrow failure,” “MDS,” or “disease progression,” all which might include any of the MDS-related sequelae such as infection or hemorrhage. This is a clear difficulty of this type of retrospective chart-review that cannot be overcome.
Finally, identifying the cause of death as MDS-associated does not necessarily allow the conclusion that early intervention will reduce mortality. Because potential disease-altering therapy was only recently approved for MDS, the vast majority of the patients in our cohort did not receive such therapy, and therefore the current study is not able to draw conclusions regarding the benefit of early intervention. Prospective randomized trials will be needed to establish the clinical benefit of early therapy in low-risk MDS.
In conclusion, we have shown herein that the majority of patients with low-risk MDS will die of causes related to their underlying malignancy. Identification of patients at risk for poor outcome and subsequent early intervention could potentially lead to improved survival in the future.
CONFLICT OF INTEREST DISCLOSURES
Supported by National Cancer
Institute Grant 5PO11CA108631 and the Leukemia and Lymphoma Society.
REFERENCES
- 1.Greenberg P, Cox C, LeBeau MM, Fenaux P, et al. International scoring system for evaluating prognosis in myelodysplastic syndromes. Blood. 1997;89:2079–2088. [PubMed] [Google Scholar]
- 2.Rollison DE, Howlader N, Smith MT, et al. Epidemiology of myelodysplastic syndromes and chronic myeloproliferative disorders in the United States, 2001-2004, using data from the NAACCR and SEER programs. Blood. 2008;112:45–52. doi: 10.1182/blood-2008-01-134858. [DOI] [PubMed] [Google Scholar]
- 3.Garcia-Manero G, Shan J, Faderl S, et al. A prognostic score for patients with lower risk myelodysplastic syndrome. Leukemia. 2008;22:538–543. doi: 10.1038/sj.leu.2405070. [DOI] [PubMed] [Google Scholar]
- 4.Ma X, Does M, Raza A, Mayne ST. Myelodysplastic syndromes: incidence and survival in the United States. Cancer. 2007;109:1536–1542. doi: 10.1002/cncr.22570. [DOI] [PubMed] [Google Scholar]
- 5.Kantarjian H, Issa JP, Rosenfeld CS, et al. Decitabine improves patient outcomes in myelodysplastic syndromes: results of a phase III randomized study. Cancer. 2006;106:1794–1803. doi: 10.1002/cncr.21792. [DOI] [PubMed] [Google Scholar]
- 6.List A, Dewald G, Bennett J, et al. Lenalidomide in the myelodysplastic syndrome with chromosome 5q deletion. N Engl J Med. 2006;355:1456–1465. doi: 10.1056/NEJMoa061292. [DOI] [PubMed] [Google Scholar]
- 7.Silverman LR, Demakos EP, Peterson BL, et al. Randomized controlled trial of azacitidine in patients with the myelodysplastic syndrome: a study of the cancer and leukemia group B. J Clin Oncol. 2002;20:2429–2440. doi: 10.1200/JCO.2002.04.117. [DOI] [PubMed] [Google Scholar]
- 8.Bennett JM, Catovsky D, Daniel MT, et al. Proposals for the classification of the myelodysplastic syndromes. Br J Haematol. 1982;51:189–199. [PubMed] [Google Scholar]
- 9.Kaplan E, Meier P. Nonparametric estimation from incomplete observations. J Am Stat Assoc. 1958;53:457–481. [Google Scholar]
- 10.Cunningham I, MacCallum SJ, Nicholls MD, et al. The myelodysplastic syndromes: an analysis of prognostic factors in 226 cases from a single institution. Br J Haematol. 1995;90:602–606. doi: 10.1111/j.1365-2141.1995.tb05590.x. [DOI] [PubMed] [Google Scholar]
- 11.Jabbour E, Kantarjian HM, Koller C, Taher A. Red blood cell transfusions and iron overload in the treatment of patients with myelodysplastic syndromes. Cancer. 2008;112:1089–1095. doi: 10.1002/cncr.23280. [DOI] [PubMed] [Google Scholar]
- 12.Centers for Disease Control Ten Leading Causes of Death, United States 2006, All Races, Both Sexes. [May 1, 2009]. Available at: http://webapp.cdc.gov/cgi-bin/broker.exe.