Abstract
We have demonstrated that 6,7-dihydroxyflavone by itself has only a weak antibacterial effect on methicillin-resistant Staphylococcus aureus (MRSA) but that at concentrations less than MIC it synergistically elevates the susceptibility of clinically isolated MRSA and methicillin-sensitive S. aureus strains to β-lactam antibiotics from 8- to 32,800-fold.
Infections by methicillin-resistant Staphylococcus aureus (MRSA), which occur in both hospitals and the community, present a major therapeutic problem (9). Vancomycin and its derivatives are currently used as antibacterial agents against infectious diseases due to MRSA. However, vancomycin-resistant S. aureus has emerged (2, 7, 18, 19). Thus, the development of a new anti-MRSA agents is urgently needed.
Recently, we found that apigenin, luteolin, and other flavonoids, all having similar chemical structures, are selectively toxic to MRSA and methicillin-sensitive S. aureus (MSSA) (13) and that these flavonoids elevated the susceptibility to β-lactam antibiotics in MRSA (5). We named these compounds intensifiers of β-lactam susceptibility in MRSA. Flavonoids are secondary plant metabolites present in fruit, vegetables, and beverages such as tea and wine, and they possess anti-inflammatory, antiallergic, antiviral, and anticarcinogenic properties (10).
Here we demonstrated that 6,7-dihydroxyflavone dramatically intensifies from 8- to 32,800-fold the susceptibility to β-lactam antibiotics in all clinically isolated strains of MRSA and MSSA examined.
S. aureus strains 1 to 22, 1003, 1010, 1020, and 1032 (clinical isolates) and COL, RN4220, and N315 were used. Some properties of the clinical strains of S. aureus used throughout the present study were determined previously (15).
The MICs of 6,7-dihydroxyflavone (Funakoshi Co. Ltd., Tokyo, Japan) and of antibiotics alone and in combination with 6,7-dihydroxyflavone against the MRSA and MSSA strains were determined as described previously (13). The fractional inhibitory concentration (FIC) was calculated as the MIC of oxacillin and 6,7-dihydroxyflavone in combination divided by the MIC of oxacillin or 6,7-dihydroxyflavone alone (1).
The viable-cell number was determined as follows. MRSA strain 5 (106 CFU/ml) was incubated at 37°C in cation-adjusted Mueller-Hinton (MH) broth containing either 8 μg of methicillin/ml, 25 μg of 6,7-dihydroxyflavone/ml, or both. Viability was confirmed by culturing the cells on MH agar plates for 24 h.
Detection of PBP2a was performed by Western blot analysis. An antibody against PBP2a was prepared with a peptide of PBP2a (CGSKKFEKGMKKLGVGEDIPSDYPFC) (14), which was synthesized by Oriental Yeast Co. (Osaka, Japan) by a solid-phase method. The purity and molecular weight of the peptide were confirmed by reverse-phase high-pressure liquid chromatography and mass analysis (Voyager). An antibody against the peptide conjugated to bovine serum albumin was raised in rabbits by Oriental Yeast Co. MRSA 5 was incubated for 5 h at 37°C in a brain heart infusion broth containing 6,7-dihydroxyflavone (12.5 or 25 μg/ml), and the cell membrane was prepared as described by Oliver et al. (11). After sodium dodecyl sulfate-polyacrylamide gel electrophoresis (8), proteins were transferred electrophoretically to a nitrocellulose membrane (Bio-Rad), essentially as described by Towbin et al. (17). PBP2a was identified by the luminescence method (ECL Plus; Amersham Biosciences) with the antibody against the peptide of PBP2a (6).
We found that 6,7-dihydroxyflavone (Fig. 1) has a weak antibacterial effect on MRSA but that, at a concentration less than its MIC, it elevates the susceptibility to oxacillin (Table 1). The FIC indices of the combination of oxacillin and 6,7-dihydroxyflavone against 20 isolates of MRSA were found to be between 0.251 and 0.504. This result indicates that the effect of the combination of oxacillin and 6,7-dihydroxyflavone is synergistic.
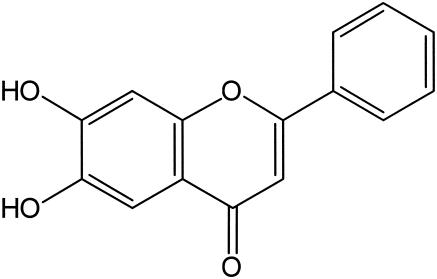
FIG. 1.
Chemical structure of 6,7-dihydroxyflavone.
TABLE 1.
MICs and FIC indices of oxacillin in the presence of 6,7-dihydroxyflavone against MRSA and MSSA
| Strain no. | MIC (μg/ml)
|
FIC indexa | |||
|---|---|---|---|---|---|
| 6,7-Dihydroxy- flavone alone | Oxacillin
|
||||
| Alone | With 6,7-dihydroxy- flavone at:
|
||||
| 12.5 μg/ml | 25 μg/ml | ||||
| MRSA | |||||
| 1 | 50 | 512 | 4 | 2 | 0.258 |
| 2 | 25 | 512 | 0.25 | NDb | 0.500 |
| 3 | 50 | 256 | 0.5 | 0.25 | 0.252 |
| 4 | 25 | 512 | 2 | 0.13 | 0.504 |
| 5 | 50 | 512 | 0.5 | <0.06 | 0.251 |
| 6 | 50 | 128 | 0.5 | 0.25 | 0.254 |
| 7 | 25 | 512 | 0.25 | ND | 0.500 |
| 8 | 50 | 512 | 1 | 0.25 | 0.252 |
| 9 | 25 | 512 | 0.25 | ND | 0.500 |
| 10 | 50 | 128 | 0.25 | <0.06 | 0.252 |
| 12 | 50 | 512 | 1 | 1 | 0.252 |
| 13 | 50 | 512 | 2 | 1 | 0.254 |
| 16 | 25 | 512 | 2 | ND | 0.504 |
| 17 | 25 | 128 | 0.5 | ND | 0.504 |
| 18 | 25 | 512 | 1 | ND | 0.502 |
| 19 | 25 | 512 | 1 | ND | 0.502 |
| 20 | 50 | 64 | 0.13 | <0.06 | 0.252 |
| 21 | 50 | 128 | 0.13 | 0.13 | 0.251 |
| 22 | 50 | 128 | 0.25 | 0.13 | 0.252 |
| COL | 50 | 512 | 1 | 0.25 | 0.252 |
| N315 | 50 | 64 | <0.06 | <0.06 | 0.251 |
| MSSA | |||||
| 1003 | 50 | 0.5 | 0.13 | <0.06 | 0.510 |
| 1010 | 50 | 0.5 | 0.13 | 0.13 | 0.510 |
| 1020 | 50 | 0.25 | <0.06 | <0.06 | 0.490 |
| 1032 | 50 | 0.25 | <0.06 | <0.06 | 0.490 |
| ATCC 6538 | 50 | <0.06 | <0.06 | <0.06 | 1.250 |
| RN4220 | 25 | 0.13 | <0.06 | ND | 0.962 |
FIC index ≤ 0.5, synergy effect; 0.5 < FIC index ≤ 1, additive effect.
ND, not determined.
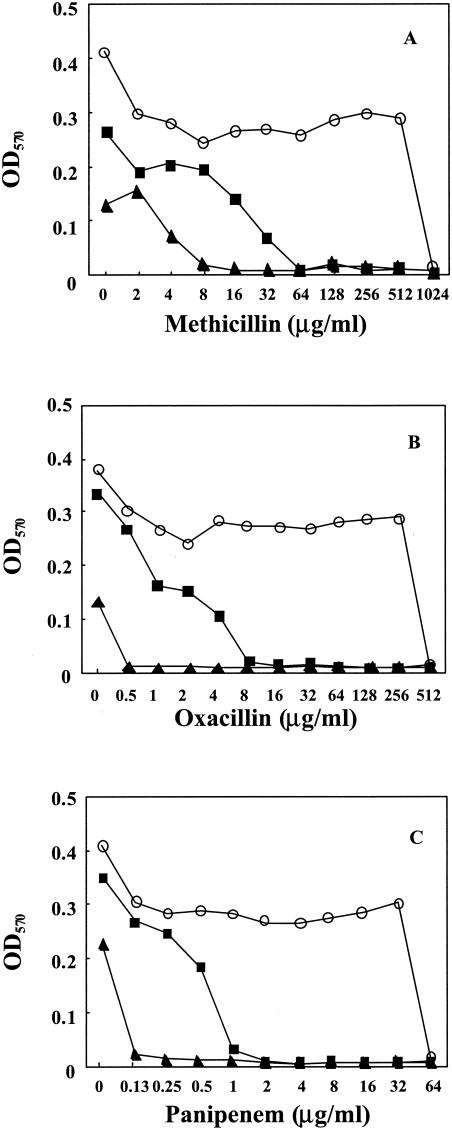
We also found that 6,7-dihydroxyflavone dramatically intensifies from 8- to 32,800-fold the susceptibility of MRSA strains to β-lactams such as methicillin, cephapirin, panipenem, and cefotaxime in all strains of MRSA and MSSA examined (Table 2). However, 6,7-dihydroxyflavone had no effect on the susceptibility to the other antibiotics indicated in Table 2 except that it also increased the susceptibility to streptomycin, but only weakly. Figure 2 shows the synergistic effects of β-lactams and 6,7-dihydroxyflavone in the growth of MRSA 5. In addition, 6,7-dihydroxyflavone dose dependently inhibited the growth of MRSA in the absence of β-lactams (Fig. 2).
TABLE 2.
MICs of various antibacterial agents for MRSA and MSSA in the absence or presence of 6,7-dihydroxyflavone
| Strain no. | MIC (μg/ml) ofa:
|
|||||||||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MET
|
HAP
|
PAPM
|
CTX
|
VAN
|
STR
|
OFX
|
TET
|
ERY
|
KAN
|
|||||||||||
| − | + | − | + | − | + | − | + | − | + | − | + | − | + | − | + | − | + | − | + | |
| MRSA | ||||||||||||||||||||
| 1 | >1,024 | 4 | 128 | 0.063 | 128 | 0.031 | 1,024 | 64 | 1 | 1 | 32 | 8 | 16 | 16 | 0.5 | 0.5 | >1,024 | >1,024 | >1,024 | >1,024 |
| 2 | >1,024 | 0.25 | 128 | 0.0078 | 128 | 0.0078 | 512 | 0.25 | 1 | 0.5 | 64 | 16 | 128 | 128 | 0.5 | 0.5 | >1,024 | >1,024 | >1,024 | >1,024 |
| 3 | 1,024 | 0.25 | 64 | 0.5 | 64 | 0.063 | 1,024 | 0.25 | 2 | 1 | 64 | 16 | 32 | 64 | 0.5 | 0.5 | >1,024 | >1,024 | >1,024 | >1,024 |
| 4 | >1,024 | 4 | 64 | 4 | 64 | 0.13 | 1,024 | 16 | 2 | 2 | 32 | 8 | 16 | 16 | 128 | 128 | >1,024 | >1,024 | >1,024 | >1,024 |
| 5 | >1,024 | 0.25 | 128 | 0.0039 | 64 | 0.0039 | 1,024 | 8 | 1 | 1 | 32 | 16 | 32 | 32 | 0.5 | 0.5 | >1,024 | >1,024 | >1,024 | >1,024 |
| 6 | 1,024 | 8 | 64 | 0.25 | 64 | 0.031 | 1,024 | 0.25 | 2 | 0.5 | 64 | 16 | 64 | 32 | 0.5 | 0.5 | >1,024 | >1,024 | >1,024 | >1,024 |
| 7 | >1,024 | 4 | 64 | 8 | 128 | 0.063 | 1,024 | 8 | 2 | 1 | 64 | 16 | 32 | 32 | 0.5 | 0.5 | >1,024 | >1,024 | >1,024 | >1,024 |
| 8 | >1,024 | 4 | 64 | 8 | 64 | 0.13 | 1,024 | 16 | 2 | 1 | 64 | 16 | 32 | 32 | 0.5 | 0.5 | 0.25 | 0.25 | >1,024 | >1,024 |
| 9 | >1,024 | 2 | 64 | 0.002 | 64 | 0.002 | 512 | 0.25 | 2 | 0.5 | 32 | 8 | 1 | 1 | 128 | 128 | >1,024 | >1,024 | >1,024 | >1,024 |
| 10 | 512 | 2 | 64 | 0.016 | 8 | 0.0078 | 512 | 0.25 | 1 | 1 | 32 | 16 | 512 | 256 | 0.5 | 0.5 | >1,024 | >1,024 | >1,024 | >1,024 |
| 12 | >1,024 | 4 | 128 | 0.25 | 64 | 0.13 | 1,024 | 32 | 2 | 1 | 64 | 8 | 1 | 1 | 0.5 | 0.5 | >1,024 | >1,024 | >1,024 | >1,024 |
| 13 | >1,024 | 4 | 128 | 2 | 128 | 0.13 | 1,024 | 32 | 2 | 1 | 64 | 16 | 1 | 1 | 0.5 | 0.5 | >1,024 | >1,024 | >1,024 | >1,024 |
| 16 | >1,024 | 8 | 64 | 8 | 64 | 0.13 | 512 | 32 | 1 | 1 | 64 | 16 | 32 | 32 | 0.5 | 0.5 | >1,024 | >1,024 | >1,024 | >1,024 |
| 17 | >1,024 | 16 | 64 | 1 | 32 | 0.031 | 512 | 4 | 2 | 2 | 32 | 16 | 1 | 1 | 128 | 128 | >1,024 | >1,024 | >1,024 | >1,024 |
| 18 | >1,024 | 8 | 32 | 0.5 | 32 | 0.031 | 512 | 4 | 2 | 2 | 32 | 16 | 1 | 1 | 128 | 64 | >1,024 | >1,024 | >1,024 | >1,024 |
| 19 | >1,024 | 32 | 64 | 0.25 | 32 | 0.13 | 1,024 | 8 | 2 | 1 | 64 | 8 | 1 | 1 | 128 | 128 | >1,024 | >1,024 | >1,024 | >1,024 |
| 20 | 64 | 2 | 16 | 0.031 | 2 | 0.0039 | 32 | 0.25 | 1 | 1 | 64 | 16 | 32 | 32 | 256 | 256 | 0.25 | 0.25 | >1,024 | >1,024 |
| 21 | 128 | 0.25 | 32 | 0.25 | 4 | 0.016 | 256 | 0.25 | 1 | 1 | 32 | 8 | 1 | 1 | 0.5 | 0.5 | 0.25 | 0.25 | 16 | 16 |
| 22 | 128 | 2 | 16 | 0.25 | 4 | 0.016 | 128 | 0.25 | 1 | 1 | 32 | 8 | 1 | 1 | 0.5 | 0.5 | 0.25 | 0.25 | 16 | 16 |
| COL | >1,024 | 4 | 64 | 0.5 | 64 | 0.031 | 1,024 | 0.25 | 2 | 1 | >1,024 | >1,024 | 2 | 2 | 128 | 128 | 0.13 | 0.13 | 16 | 16 |
| N315 | 8 | 2 | 32 | 0.002 | 8 | 0.002 | 256 | 0.25 | 0.5 | 0.25 | 32 | 4 | 1 | 1 | 0.25 | 0.25 | >1,024 | >1,024 | 512 | 512 |
| MSSA | ||||||||||||||||||||
| 1003 | 2 | 0.25 | 0.25 | 0.063 | 0.063 | 0.016 | 2 | 0.25 | 1 | 0.5 | 32 | 8 | 1 | 1 | 0.25 | 0.25 | 0.13 | 0.13 | 16 | 8 |
| 1010 | 1 | 0.25 | 0.5 | 0.13 | 0.063 | 0.0078 | 2 | 0.25 | 1 | 1 | 32 | 16 | 1 | 2 | 0.5 | 0.5 | 0.25 | 0.25 | >1,024 | 1,024 |
| 1020 | 1 | 0.25 | 0.13 | 0.002 | 0.031 | 0.0039 | 0.25 | 0.25 | 1 | 0.5 | 32 | 16 | 1 | 1 | 0.5 | 0.5 | 0.13 | 0.13 | 16 | 8 |
| 1032 | 1 | 0.25 | 0.25 | 0.0039 | 0.063 | 0.0039 | 2 | 0.25 | 1 | 1 | 32 | 32 | 1 | 1 | 0.5 | 0.5 | 0.25 | 0.25 | 16 | 16 |
| ATCC 6538 | 1 | 0.25 | 0.13 | 0.0039 | 0.031 | 0.002 | 0.25 | 0.25 | 1 | 1 | 32 | 16 | 1 | 1 | 0.5 | 0.5 | 0.25 | 0.13 | 16 | 16 |
| RN4220 | 2 | 0.25 | 0.13 | 0.002 | 0.031 | 0.002 | 0.25 | 0.0078 | 1 | 0.25 | 32 | 4 | 1 | 1 | 0.5 | 0.5 | 0.25 | 0.25 | 16 | 8 |
MET, methicillin; HAP, cephapirin; PAPM, panipenem; CTX, cefotaxime; VAN, vancomycin; STR, streptomycin; OFX, ofloxacin; TET, tetracycline; ERY, erythromycin; KAN, kanamycin. −, alone; +, with 12.5 μg of 6,7-dihydroxyflavone/ml.
FIG. 2.
Synergistic inhibition of growth by 6,7-dihydroxyflavone and methicillin (A), oxacillin (B), or panipenem (C) in MRSA 5. Bacteria were cultured at 37°C in cation-adjusted MH broth, to which was added increasing amounts of 6,7-dihydroxyflavone and the indicated concentrations of methicillin, oxacillin, and panipenem. The bacterial growth was detected as the change in optical density at 570 nm (OD570) at 24 h. Levels of 6,7-dihydroxyflavone: 0 (control; ○), 12.5 (▪), and 25 μg/ml (▴).
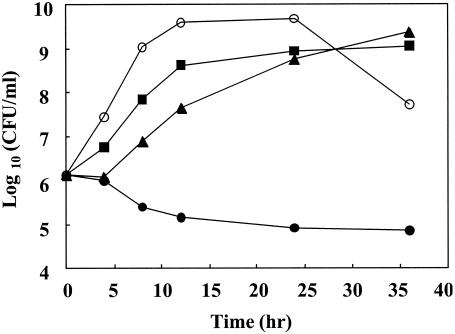
We also examined the effects of 6,7-dihydroxyflavone and methicillin on MRSA viability. The growth rate of MRSA was slightly inhibited in the presence of 25 μg of 6,7-dihydroxyflavone/ml. Methicillin alone (8 μg/ml) had little effect on growth. In contrast, a combination of 25 μg of 6,7-dihydroxyflavone/ml and 8 μg of methicillin/ml, the concentration of which was much lower than the MIC (1,024 μg/ml), greatly decreased the viable-cell number (Fig. 3). Therefore, these data clearly demonstrate that the combination of methicillin and 6,7-dihydroxyflavone results in synergistic bactericidal activity against MRSA.
FIG. 3.
Time-kill curves for 6,7-dihydroxyflavone and methicillin used against MRSA 5. Overnight cultures were diluted to approximately 106 CFU/ml with cation-adjusted MH broth containing either 8 μg of methicillin (▪)/ml, 25 μg of 6,7-dihydroxyflavone (▴)/ml, or both (•). The control experiment (○) was performed by using culture medium without methicillin and 6,7-dihydroxyflavone. Bacteria were incubated at 37°C for 0, 4, 8, 12, 24, and 36 h, and viability was then confirmed by culturing the cells on MH agar plates for 24 h.
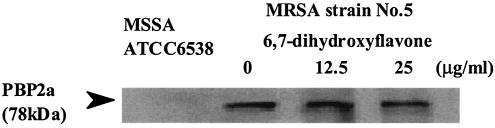
MRSA is resistant to all β-lactams because of the acquisition of a mecA gene cassette, which produces an additional transpeptidase called PBP2a. We therefore examined the effects of 6,7-dihydroxyflavone on the amount of PBP2a by using the antibody against the peptide of PBP2a. Figure 4 clearly demonstrates that 6,7-dihydroxyflavone had no effect on the amount of PBP2a in strain 5.
FIG. 4.
Effects of 6,7-dihydroxyflavone on the amount of PBP2a in the membrane fraction of MRSA 5. MRSA 5 cells were collected at 5 h of incubation after adding a final concentration of 0, 12.5, or 25 μg/ml of 6,7-dihydroxyflavone, and the membrane fraction (10 μg of protein) was then loaded by sodium dodecyl sulfate-polyacrylamide gel electrophoresis and subjected to Western blotting analysis. PBP2′ was detected by use of ECL Plus.
Guz et al. (4) have demonstrated that flavonolignan and flavone derivatives are potent inhibitors of the NorA multidrug resistance pump (12) in S. aureus. However, Table 2 clearly demonstrates that 6,7-dihydroxyflavone had no effect on the susceptibility to ofloxacin of MRSA strains. Furthermore, we have confirmed that 6,7-dihydroxyflavone has no effect on the uptake of oxacillin in MRSA 5 (Y. Miwa, H. Shibata, and T. Higuti, unpublished work).
Alternatively, we propose a model for the mechanism of high resistance of MRSA to β-lactams and the massive reduction in the β-lactam MICs caused by transposons (16) or flavone and its derivatives. In this model, (i) PBP2a has a low affinity not only for the β-lactam ring of β-lactams but also for d-Ala-d-Ala in N-acetylmuramyl-pentapeptide because the β-lactam ring mimics the d-Ala-d-Ala moiety of the normal substrate. Thus (ii) PBP2a can cross-link between N-acetylmuramyl-pentapeptide and pentaglycine only when MRSA has been mutated such that there is an elevated concentration of N-acetylmuramyl-pentapeptide on the nascent cross wall (septum) as the main center of linear wall growth of the staphylococcal cell (3). Furthermore, (iii) the inhibition of peptidoglycan biosynthesis could decrease the concentration of N-acetylmuramyl-pentapeptide and/or pentaglycine on the nascent cross wall, such that PBP2a with low affinity for the d-Ala-d-Ala would not be able to participate in the cross-linking of peptidoglycans and such that the other four penicillin binding proteins with high affinity for the substrates would take part in the cross-linking. This could be a reason why flavone and its derivatives caused a massive reduction in the methicillin MIC even though they retained an intact PBP2a.
However, further in vitro and in vivo studies are required to confirm the model and also the relevancy of the combination therapy with 6,7-dihydroxyflavone and β-lactam antibiotics to multidrug-resistant MRSA infection.
Acknowledgments
This work was supported in part by grants-in-aid for scientific research (no. 11558085 and 14390038) to T.H. from the Ministry of Education, Science, and Culture of Japan; by a fund for the Originative Study Result Fostering Project (no. 0552) from the Japan Science and Technology Corporation; and by a grant from Alps Pharmaceutical Industries Co., Ltd.
We thank the late Toru Usui, Kyoto Microbiological Institute, Kyoto, Japan, John J. Iandolo, University of Oklahoma Health Sciences Center, Hitoshi Komatsuzawa, Hiroshima University Graduate School of Biomedical Sciences, Hiroshima, Japan, and Keiichi Hiramatsu, Juntendo University, Tokyo, Japan, for providing us with S. aureus clinical strains MRSA 1 to 22, COL, RN4220, and N315. We also thank Sankyo Co., Ltd., Tokyo, for providing panipenem.
REFERENCES
- 1.Bajaksouzian, S., M. A. Visalli, M. R. Jacobs, and P. C. Appelbaum. 1997. Activities of levofloxacin, ofloxacin, and ciprofloxacin alone and in combination with amikacin against acinetobacters, as determined by checkerboard and time-kill studies. Antimicrob. Agents Chemother. 41:1073-1076. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Cui, L., X. Ma, K. Sato, K. Okuma, F. C. Tenover, E. M. Mamizuka, C. G. Gemmell, M. N. Kim, M. C. Ploy, N. E. Solh, V. Ferraz, and K. Hiramatsu. 2003. Cell wall thickening is a common feature of vancomycin resistance in Staphylococcus aureus. J. Clin. Microbiol. 41:5-14. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Giesbrecht, P., T. Kersten, H. Maidhof, and J. Wecke. 1998. Staphylococcal cell wall: morphogenesis and fatal variations in the presence of penicillin. Microbiol. Mol. Biol. Rev. 62:1371-1414. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Guz, N. R., F. R. Stermitz, J. B. Johnson, T. D. Beeson, S. Willen, J. F. Hsiang, and K. Lewis. 2001. Flavonolignan and flavone inhibitors of a Staphylococcus aureus multidrug resistance pump: structure-activity relationships. J. Med. Chem. 44:261-268. [DOI] [PubMed] [Google Scholar]
- 5.Higuchi, T., Y. Sato, and S. Murasugi. September 2001. Use of flavone derivatives for induction of β-lactam-sensitivity of MRSA. U.S. patent 6,294,526 B1.
- 6.Higuti, T., T. Negama, M. Takigawa, J. Uchida, T. Yamane, T. Asai, I. Tani, K. Oeda, M. Shimizu, K. Nakamura, and H. Ohkawa. 1988. A hydrophobic protein, chargerin II, purified from rat liver mitochondria is encoded in the unidentified reading frame A6L of mitochondrial DNA. J. Biol. Chem. 263:6772-6776. [PubMed] [Google Scholar]
- 7.Hiramatsu, K., N. Aritaka, H. Hanaki, S. Kawasaki, Y. Hosoda, S. Hori, Y. Fukuchi, and I. Kobayashi. 1997. Dissemination in Japanese hospitals of strains of Staphylococcus aureus heterogeneously resistant to vancomycin. Lancet 350:1670-1673. [DOI] [PubMed] [Google Scholar]
- 8.Laemmli, U. K. 1970. Cleavage of structural proteins during the assembly of the head of bacteriophage T4. Nature 227:680-685. [DOI] [PubMed] [Google Scholar]
- 9.Levy, S. B. 1998. The challenge of antibiotic resistance. Sci. Am. 278:46-53. [DOI] [PubMed] [Google Scholar]
- 10.Nijveldt, R. J., E. Nood, D. E. Hoorn, P. G. Boelens, K. Norren, and P. A. Leeuwen. 2001. Flavonoids: a review of probable mechanisms of action and potential applications. Am. J. Clin. Nutr. 74:418-425. [DOI] [PubMed] [Google Scholar]
- 11.Oliver, B., B. Wolfgang, and P. Gerhard. 1997. Regulation of β-lactamase synthesis as a novel site of action for suppression of methicillin resistance in Staphylococcus aureus. Zentbl. Bakteriol. 285:413-430. [DOI] [PubMed] [Google Scholar]
- 12.Putman, M., H. W. van Veen, and W. N. Konings. 2000. Molecular properties of bacterial multidrug transporters. Microbiol. Mol. Biol. Rev. 64:672-693. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Sato, Y., S. Suzaki, T. Nishikawa, M. Kihara, H. Shibata, and T. Higuti. 2000. Phytochemical flavones isolated from Scutellaria barbata and antibacterial activity against methicillin-resistant Staphylococcus aureus. J. Ethnopharmacol. 72:483-488. [DOI] [PubMed] [Google Scholar]
- 14.Sekiguchi, K., M. Saito, and R. Yajima. 1995. Detection of methicillin-resistant Staphylococcus aureus (MRSA) with antibodies against synthetic peptides derived from penicillin-binding protein 2′. Microbiol. Immunol. 39:545-550. [DOI] [PubMed] [Google Scholar]
- 15.Shibata, H., C. Shirakata, H. Kawasaki, Y. Sato, T. Kuwahara, Y. Ohnishi, N. Arakaki, and T. Higuti. 2003. Flavone markedly affects phenotypic expression of β-lactam resistance in methicillin-resistant Staphylococcus aureus strains isolated clinically. Biol. Pharm. Bull. 26:1478-1483. [DOI] [PubMed] [Google Scholar]
- 16.Tomasz, A. 2000. The staphylococcal cell wall, p. 351-360. In V. A. Fischetti, R. P. Novick, J. J. Ferretti, D. A. Portnoy, and J. I. Rood (ed.), Gram-positive pathogens. American Society for Microbiology, Washington, D.C.
- 17.Towbin, H., T. Staehelin, and J. Gordon. 1979. Electrophoretic transfer of proteins from polyacrylamide gels to nitrocellulose sheets: procedure and some applications. Proc. Natl. Acad. Sci. USA 76:4350-4354. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Weigel, L. M. 2002. High-level vancomycin resistance in a clinical isolate of Staphylococcus aureus, p. 16. In Proceedings of the 10th International Symposium on Staphylococci and Staphylococcal Infections.
- 19.Wootton, M., R. A. Howe, T. R. Walsh, P. M. Bennett, and A. P. MacGowan. 2002. In vitro activity of 21 antimicrobials against vancomycin-resistant Staphylococcus aureus (VRSA) and hetero VRSA (hVRSA). J. Antimicrob. Chemother. 50:755-766. [DOI] [PubMed] [Google Scholar]