Abstract
Observational data suggest that lower folate status is associated with an increased risk of colorectal neoplasia, implying that folate may be useful as a chemopreventive agent. We conducted a combined analysis of three large randomized trials of folic acid supplementation for the prevention of metachronous adenomas in patients with an adenoma history. Participants included 2,632 men and women with a history of adenomas randomized to either 0.5 or 1.0 mg/day of folic acid or placebo, and who had a follow-up endoscopy 6 to 42 months after randomization (mean=30.6 (standard deviation=8.1) months). We used random-effects meta-analysis to estimate risk ratios (RR’s) and 95% confidence intervals (CI). The RR comparing folic acid vs. placebo was 0.98 (95% CI=0.82–1.17) for all adenomas and 1.06 (95% CI=0.81–1.39) for advanced lesions. Folic acid was associated with a non-significant decreased risk of any adenoma among subjects in the lowest quartile of baseline plasma folate (≤11 nmol/L) and no effect among individuals in the highest quartile (>29 nmol/L, p for trend = 0.17). There was a non-significant trend of decreasing risk of any adenoma associated with folic acid supplements with increasing alcohol intake. During the early follow-up reported here, more deaths occurred in the placebo group than in the folic acid group (1.7% vs. 0.5%, p=.002). In conclusion, after up to 3.5 years of folic acid use, there is no clear decrease or increase in the occurrence of new adenomas in patients with a history of adenoma.
Keywords: Folic acid, supplementation, colorectal adenomas, clinical trial
Introduction
Observational studies have suggested that lower folate status is associated with a higher risk of colorectal adenomas and cancer.1–3 However, some epidemiological4–6 and animal studies7–10 have provided evidence suggesting that folic acid supplementation and high blood levels of folate may be associated with an increased risk of colorectal cancer. Clinical trials of supplementation are the best design to clarify the actual effects of folate on risk of colorectal neoplasia.
Data from five randomized clinical trials of folic acid supplementation for the prevention of colorectal adenomas among high-risk individuals (with an adenoma history) have been reported. Two small trials reported at least suggestions of benefit,11, 12 but this was not observed in two much larger trials, the Aspirin/Folate Polyp Prevention Study (AFPPS) and the United Kingdom Colorectal Adenoma Prevention (ukCAP) trial13, 14. A third large trial, the Nurses’ Health Study/Health Professionals Follow-up Study (NHS/HPFS) folic acid polyp prevention trial, showed no overall risk reduction, but suggestions of decreased risks among those with higher alcohol intake and lower folate levels at baseline15. Interestingly, the AFPPS trial found indications of an increased risk for advanced lesions and multiple adenomas with prolonged treatment and follow-up13.
To obtain more precise clinical evidence on the effects of folic acid supplementation on the occurrence of new adenomas in patients with a history of adenomas, we conducted a pooled analysis of the three large adenoma trials, focusing on 3.5 years of treatment, which encompassed virtually all of the treatment and follow-up across trials. To address the possibility that there may be subgroups of individuals who may be more susceptible to the effects of folic acid in the large bowel, we also conducted a stratified analysis by selected patient characteristics and dietary exposures.
Methods
Study Design
From literature searches in PubMed using keywords: “folic acid”, “trial” and “colorectal adenoma”, and by contacting colleagues, we identified three placebo-controlled randomized trials with more than 500 randomized subjects that investigated folic acid in any dose as a chemopreventive agent for large-bowel adenomas (Table 1).
Table 1.
Summary of Clinical Trial Designs
| AFPPS (N = 1021) | NHS/HPFS (N = 672) | ukCAP (N = 939) | |
|---|---|---|---|
| Years of recruitment | 1994–1998 | 1996–1999 | 1997–2001 |
|
| |||
| Population | Recent history of colorectal adenomas (within last 16 months); no remaining lesions in large bowel within 6 months | History of colorectal adenomas | Recent history of colorectal adenomas (within 6 months); no remaining lesions in large bowel within 6 months |
|
| |||
| Recruitment sites | United States and Canada | United States | United Kingdom and Denmark |
|
| |||
| Treatment | Placebo vs. 1 mg folic acid | Placebo vs. 1 mg folic acid | Placebo vs. 0.5 mg folic acid |
|
| |||
| Compliance (% pills taken throughout the duration of the trial) | (n=983) Placebo: 90.3% Folic acid: 92.1% |
NA | (n=838) Placebo: 77.5% Folic Acid: 80.4% |
|
| |||
| Compliance (Change in folate levels)* | (n=845) Placebo: 6.2 ± 18.8 Folic Acid: 50.4 ± 38.1 |
(n=480) Placebo: 18.7 ± 19.5 Folic Acid: 68.1 ± 57.2 |
NA |
| Central Pathology Review | Yes | No | No |
| Proportion of subjects with follow-up colonoscopy within 42 months | 92% | 60% | 65% |
AFPPS, Aspirin/Folate Polyp Prevention Study; NHS/HPFS, Nurses’ Health Study/Health Professional Follow-up Study; ukCAP, United Kingdom Colorectal Adenoma Prevention
For NHS, compliance is measured approximately mid-point during the trial; for AFPPS, compliance is measured at year 3
Aspirin/Folate Polyp Prevention Study (AFPPS)
The design and findings from the AFPPS13, 16 have been previously reported. In brief, potential participants were recruited between July 1994 and March 1998 at nine clinical centers in the U.S. and Canada. Eligible individuals aged 21–80 years had at least one of the following: one or more histologically confirmed adenomas removed within 3 months before recruitment, one or more histologically confirmed adenomas removed within 16 months before recruitment and a lifetime history of two or more confirmed adenomas, or a histologically confirmed adenoma at least 1.0 cm in diameter removed within 16 months before recruitment. Subjects were required to have had a complete colonoscopy documenting no remaining polyps in the large bowel within 6 months of study entry. The trial had a two-by-three factorial design, studying 1 mg of folic acid or placebo and aspirin 81 or 325 mg/day or placebo. Originally, the trial was designed to investigate only aspirin, but shortly after enrollment began, it was expanded to examine folic acid; 100 individuals were randomized before the folic acid component was initiated. The period of treatment and follow-up was originally planned to be 3 years; however, because of concern that the beneficial effects of folic acid supplementation might take longer to emerge,17 participants were asked to continue randomized study treatment for a second colonoscopic surveillance cycle (usually 3 or 5 years) after the initially planned 3 year interval. All participants agreed not to take folic acid supplements (including multivitamins) during the treatment periods of the trial. The study provided multivitamins without folic acid to those participants who wanted to continue multivitamin use. Among those individuals who completed follow-up questionnaires during the first 3 years, folate use was reported by 13.5% of patients overall (12.6% in placebo, 14.4% in folic acid groups).
In total, 1,021 individuals were randomized to folic acid or placebo; 940 participants (92.1%) underwent one or more follow-up endoscopies at least 6 months following randomization up to 42 months later.
Nurses’ Health Study/Health Professionals Follow-Up Study (NHS/HPFS)
Details of the NHS/HPFS Polyp Prevention Study are outlined elsewhere18. In brief, subjects participating in two large observational U.S. cohort studies (the Nurses’ Health Study (NHS)19 or the Health Professionals Follow-Up Study (HPFS)20) who were aged 50–78 years and who had a lifetime history of at least one histologically-confirmed adenomatous polyp were eligible to participate. Contrary to the AFPPS and ukCAP trials, the NHS/HPFS folic acid prevention trial was conducted entirely though the postal system. Subjects were recruited from 1996 to 1999 and randomized to receive either a folic acid tablet (1 mg) or a placebo tablet to be taken once daily for approximately 3 years. The length of each subject’s colonoscopic surveillance interval was determined by the endoscopist recommendation. After 3 years on study, subjects were given the opportunity to extend participation for 2 to 3 ½ additional years; 49% of all randomized participants consented to do so. All participants agreed not to take folic acid supplements (including multivitamins) during the treatment period of the trial. The study did not quantify folic acid use among participants during the treatment period. The participants were asked to provide informed consent to review medical records for all endoscopic procedures during the study period and for all histopathologic reports regarding any polyp removed. The first participants were randomized in May 1996; 672 subjects were ultimately randomized. A total of 402 (59.8%) had one or more follow-up examinations and pathological confirmations between 6 and 42 months following start of pill-taking. Due to the implementation of folic acid fortification of certain foods during the course of the trial, over time folate levels (baseline vs. mid-trial) increased significantly in all participants with available measurements on folate (non-fasting) at baseline and mid-trial. However, the overall increase in folate levels was more pronounced in the folic acid than in the placebo group (mean (SD) ng/mL: baseline vs. mid-trial: placebo: 9.3 (6.2) vs. 17.0 (7.9), p<0.05, FA: 9.7 (6.8) vs. 39.2 (24.8), p<0.05)18 suggesting reasonable pill compliance among participants who had donated two blood samples.
United Kingdom Colorectal Adenoma Prevention (ukCAP) Trial
In the ukCAP trial,14 eligible patients were individuals aged less than 75 years old who had an adenoma (at least 0.5 cm) diagnosed within 6 months of study entry (or previously if one or more adenomas of any size were removed within 6 months of randomization) and documentation of no remaining polyps in the bowel. Participants were recruited from 10 clinical centers in the United Kingdom and Denmark. Randomization was to aspirin (300 mg or placebo), and to folic acid (0.5 mg or placebo) using a 2 × 2 factorial design. Recruitment for this trial occurred between December 1997 and November 2001. In total there were 47 patients reporting folic acid use at baseline of which 41 took these in the form of multivitamins (4.7% in the folic acid group and 5.3% in the placebo group). All participants were discouraged from taking multivitamins during the trial. (Only 47/939 participants were taking multivitamins at study entry.) Follow-up endoscopy was scheduled as per clinical recommendation, typically three years after the qualifying examination. The ukCAP entered 939 patients; 615 (65.5%) had a surveillance endoscopy at least 6 months after randomization up to 42 months later.
Data Collection
Virtually all patients in the AFPPS completed a questionnaire at baseline regarding personal characteristics, medical history and lifestyle habits, and many patients in the ukCAP study provided similar information. In the NHS/HPFS, these data were obtained from the parent observational studies21, 22. Dietary intake of folate was collected using food frequency questionnaires in the AFPPS and ukCAP at baseline, and from a food frequency questionnaire closest in time to randomization in the NHS/HPFS. Food frequency questionnaires used in the AFPPS and NHS/HPFS accounted for changes in folate composition (in mg) due to folic acid fortification in the United States. In the ukCAP, there was no adjustment in folate composition because their food supply is not fortified with folic acid.
We obtained participant-level data from each trial, including randomized folic acid or placebo treatment group; age at study entry (in years at last birthday); sex; race; baseline body mass index (BMI); number of baseline adenomas before randomization; family history of colorectal cancer; baseline smoking status; occurrence and date of death during the treatment period; occurrence and date of diagnosis of myocardial infarctions, strokes, or new cancer diagnoses during the treatment period; and timing and outcome of each large bowel endoscopy follow-up examination during the treatment period, including the type, size, and location of each adenoma found.
Blood samples were obtained from subjects at baseline from non-fasting participants except in the ukCAP. Plasma levels of folate were determined by standard methods in the AFPPS23 and NHS/HPFS24. Subject-reported pill-taking compliance was collected in the AFPPS and ukCAP trials. The NHS/HPFS collected folate levels at midtrial as well as data on self reported dropouts18.
Available data were sent in a de-identified data file to Dartmouth Medical School for analysis as a combined dataset. This study was determined to be exempt from IRB review by the Dartmouth College Human Subjects Review Committee.
Study Outcomes
Adenoma occurrence was determined by large bowel endoscopy and pathology review. In the AFPPS, a central review was conducted; the ukCAP and NHS/HPFS relied on review of pathology reports obtained from local pathologists. In all trials, records for all large bowel procedures (endoscopy or surgery) were also obtained. Lesions were classified as neoplastic (adenomatous, including sessile serrated adenomas in the AFPPS) or non-neoplastic.
The primary outcome was the occurrence of one or more colorectal adenomas detected during at least 6 months and up to 42 months post-randomization. This interval included almost all of the treatment and follow-up in the ukCAP trial, and approximated the initially intended treatment period for the AFPPS and the NHS/HPFS trials. Follow-up examinations prior to 6 months of follow-up were excluded to omit from analysis lesions likely to have been present (but overlooked) at randomization; a maximum follow-up of 3.5 years included a 6-month window for scheduling appointments for an intended 3-year treatment period. Longer-term follow-up is ongoing in the AFPPS and planned for the NHS/HPFS study.
A secondary outcome was advanced lesions, defined as invasive carcinoma or adenomas with any villous component, high-grade dysplasia, or estimated adenoma size of 1 centimeter or greater (as determined by the endoscopist or pathology report).
We also considered the size of the adenomas detected, the number of adenomas detected in follow-up, and location of adenomas (rectal, distal (sigmoid colon or descending colon), and proximal (splenic flexure, transverse colon, hepatic flexure, ascending colon or cecum).
All important medical events reported by participants were verified with medical record review in the AFPPS and NHS/HPFS trials. The ukCAP trial relied on patient reporting of all adverse events; medical records were used to verify all reports of stroke. All adverse events were reported from the time of randomization up to 42 months after randomization.
Statistical Methods
The statistical analysis of the combined datasets followed standard random-effects meta-analysis methods25–27 in a two-stage approach. In the first stage, each clinical trial was analyzed separately to obtain trial-specific unadjusted estimates of the risk ratio of one or more adenomas or advanced lesions for the subjects randomized to folic acid versus those allocated to placebo. Subsequently, the trial-specific relative risk ratios were combined using standard methods for random-effects meta-analysis.25
All p-values were derived from two-sided tests, and we considered a p-value less than 0.05 to be statistically significant. Between-study heterogeneity was assessed using the Q statistic and the I2 statistic. An I2 value of greater than 50%, or a P value less than 0.05 for the Q statistic, was taken to indicate heterogeneity.28, 29 All analyses of study folic acid treatment were conducted according to the principle of intention to treat. In this analysis of randomized studies, effect estimates were unadjusted for covariates.
The main analyses focused on the risk of one or more recurrent (metachronous) adenomas or one or more advanced lesions. A few individuals in these trials (105, 5.2% of those with exams) had more than one endoscopy during the follow-up period, and any occurrence of an adenoma at any examination was considered an event. Analyses, performed as above, were conducted to evaluate the consistency of the folic acid treatment effect across specific subgroups of patients defined according to their baseline characteristics. Interaction tests were performed using Wald tests based on the estimated subgroup-specific log relative risk estimates and their standard errors. Trend tests were based on the appropriate linear contrasts of the estimated subgroup-specific log relative risk estimates. Eleven factors were defined: age (≤57, 58–65, ≥66 years, based on tertiles of the data); sex (male, female), body mass index (<25, 25–29.9, ≥30.0 kg/m2); cigarette smoking status (never, former, current); family history of colorectal cancer, defined as a first-degree relative diagnosed with the disease (present, absent); number of adenomas at baseline (1, ≥2, to divide the sample into two groups of similar size); presence of advanced lesions at baseline exam (present, absent); aspirin treatment assignment (placebo, aspirin 81 mg or 325 mg); alcohol use at baseline (non-drinker, up to 1 drink/day, 1–2 drink(s)/day and more than 2 drinks/day); dietary folate at baseline (≤232, 233–304, 305–378, >378 mcg, based on quartiles of the data); and plasma folate at baseline (<=11, 11.1–18, 18.1–29, >29 nmol/L, based on quartiles of the data). For stratification by aspirin treatment group, baseline adenomas and plasma folate, the NHS/HPFS and ukCAP were excluded respectively, since the NHS/HPFS trial did not examine the effects of aspirin or collect data on baseline adenomas, and the ukCAP trial did not collect serum or plasma samples.
Results
Baseline characteristics and follow-up
A total of 2,632 individuals from three clinical trials were included in this analysis (Table 2); 1,324 randomized to folic acid (AFPPS: 516; Harvard: 338; ukCAP: 470) and 1,308 to placebo (AFPPS: 505, Harvard: 334; uKCAP: 469). The majority of participants in the ukCAP trial (n=747, 80%) had advanced lesions at baseline, but the proportion was smaller in the AFPPS trial (n=290, 31%). NHS/HPFS did not have a designated baseline exam, so these data are not available for this trial. Dietary folate intake was similar in the AFPPS (322 ± 155 mcg/day) and NHS/HPFS (322 ± 87 mcg/day) trials, and higher than in the ukCAP trial (307 ± 112 mcg/day; p=0.04). Plasma folate levels were slightly higher in the AFPPS (23.7 ± 17.4 nmol/L) compared to the NHS/HPFS study (21.5 ± 14.7 nmol/L, p=0.01).
Table 2.
Baseline Characteristics of Participants in all Clinical Trials
| Characteristic | Placebo | Folic Acid |
|---|---|---|
| Number of participants | 1308 | 1324 |
|
| ||
| Trial, no. (%) | ||
| AFPPS | 505 (39) | 516 (39) |
| NHS/HPFS | 334 (26) | 338 (26) |
| ukCAP | 469 (36) | 470 (36) |
|
| ||
| Age, yr | 59.7 ± 9.5 | 59.3 ± 9.4 |
| ≤57, no. (%) | 524 (40) | 533 (40) |
| 58–65, no. (%) | 381 (29) | 411 (31) |
| ≥66, no. (%) | 403 (31) | 380 (29) |
|
| ||
| Male sex, no. (%) | 717 (55) | 726 (55) |
|
| ||
| Body mass index (kg/m2) | 26.7 ± 4.3 | 26.7 ± 4.4 |
| <25.0, no. (%) | 417 (32) | 442 (33) |
| 25.0–29.9, no. (%) | 502 (38) | 497 (38) |
| ≥30, no. (%) | 212 (16) | 217 (16) |
| Unknown, no. (%) | 177 (14) | 168 (13) |
|
| ||
| Race or ethnic group, no. (%) | ||
| White | 1040 (80) | 1073 (81) |
| Black | 39 (3) | 31 (2) |
| Hispanic | 24 (2) | 31 (2) |
| Other | 29 (2) | 25 (2) |
| Unknown | 176 (13) | 164 (12) |
|
| ||
| Tobacco smoking status, no (%) | ||
| Never smoker | 443 (34) | 449 (34) |
| Former smoker | 515 (39) | 529 (40) |
| Current smoker | 171 (13) | 176 (13) |
| Unknown | 179 (14) | 170 (13) |
|
| ||
| Family history of colorectal cancer, no. (%) | ||
| No known history | 708 (54) | 747 (56) |
| First-degree relative | 326 (25) | 310 (23) |
| Unknown | 274 (21) | 267 (20) |
|
| ||
| Number of adenomas at baseline exam, no. (%) | ||
| 1 | 533 (41) | 575 (43) |
| ≥2 | 398 (30) | 378 (29) |
| Unknown | 43 (3) | 33 (2) |
| Not collected (NHS/HPFS) | 334 (26) | 338 (26) |
|
| ||
| Advanced adenoma* at baseline exam, no. (%) | ||
| No | 413 (32) | 420 (32) |
| Yes | 515 (39) | 531 (51) |
| Unknown | 46 (4) | 35 (3) |
|
| ||
| Not collected (NHS/HPFS) | 334 (26) | 338 (26) |
| Randomized aspirin treatment, no. (%) | ||
| Placebo | 402 (31) | 404 (31) |
| 81 mg | 169 (13) | 175 (13) |
| 300 mg | 236 (18) | 236 (18) |
| 325 mg | 167 (13) | 171 (13) |
| Not randomized (NHS/HPFS) | 334 (26) | 338 (26) |
|
| ||
| Alcohol intake (drinks/day) | 0.85 ± 1.32 | 0.85 ± 1.29 |
| non-drinker, no. (%) | 308 (24) | 313 (24) |
| ≤1, no. (%) | 571 (44) | 557 (42) |
| >1 and <2, no. (%) | 133 (10) | 159 (12) |
| ≥2, no. (%) | 158 (12) | 165 (12) |
| Unknown, no. (%) | 138 (11) | 130 (10) |
|
| ||
| Dietary Folate (mcg/day) | 316 ± 133 | 317 ± 128 |
| ≤ 232, no. (%) | 264 (20) | 268 (20) |
| 233–304, no. (%) | 264 (20) | 263 (20) |
| 305–378 | 251 (19) | 275 (21) |
| > 378, no. (%) | 258 (20) | 267 (20) |
| unknown, no. (%) | 271 (21) | 251 (19) |
|
| ||
| Baseline Plasma Folate (nmol/L) | 22.5 ± 15.8 | 23.0 ± 16.9 |
| ≤ 11, no. (%) | 197 (15) | 197 (15) |
| 11.1–18, no. (%) | 188 (14) | 200 (15) |
| 18.1–29, no. (%) | 198 (15) | 195 (15) |
| > 29, no. (%) | 196 (15) | 203 (15) |
| unknown, no. (%) | 60 (5) | 59 (6) |
| not measured (ukCAP) | 469 (36) | 470 (35) |
Plus-minus values are mean ± SD
defined as invasive carcinoma or adenomas with any mention of villous component, high grade dysplasia, or estimated size of 1 centimeter or greater.
Among participants who had one or more large bowel endoscopy during the follow-up period, there was no difference in the number of examinations between the placebo and folic acid treatment groups (Kruskal-Wallis p-value=0.93). Overall, the mean follow-up included in the analysis was 30.6 (SD=8.1) months. The AFPPS (32.3 ± 3.1 months) and the ukCAP trial (32.2 ± 9.3 months) had a longer mean follow-up compared to the NHS/HPFS (24.0 ± 10.5 months, p-value<0.0001).
Adenoma and advanced lesion occurrence
The analyses of adenoma occurrence after randomization were restricted to 1957 (74.3%) individuals who had at least one endoscopic follow-up at least 6 months after randomization up to 42 months later, of whom 984 were allocated to folic acid and 973 to placebo.
One or more adenomas were diagnosed in 339 participants (34.8%) in the placebo groups and 343 participants (34.9%) in the folic acid groups (RR, 0.98; 95% CI=0.82–1.17, P=.81, Figure 1). Advanced lesions were found in 97 (10.1%) participants randomized to placebo and 105 (10.9%) randomized to folic acid (RR, 1.06; 95% CI=0.81–1.39, P=.65). There was no evidence of between-study heterogeneity in the relative risk for any adenoma (Q=3.34, P=0.19, I2=70.0) or advanced lesions (Q=2.04, P=0.36, I2=2.0).
Figure 1.
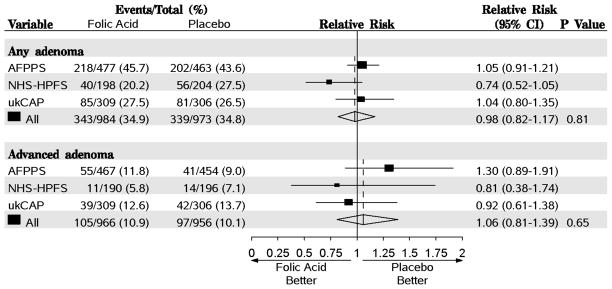
Random-effects meta-analysis comparing folic acid versus placebo after a follow-up of 42 months. Estimates are unadjusted. Tests for heterogeneity are as follows: For any adenoma, Q=3.34 (P=0.19) and I2=70.0. For advanced lesion, Q=2.04 (P=0.36) and I2=2.0. AFPPS, Aspirin/Folate Polyp Prevention Study; NHS/HPFS, Nurses’ Health Study/Health Professional Follow-up Study; ukCAP, United Kingdom Colorectal Adenoma Prevention.
Adenoma size, multiple adenomas and location in the colorectum
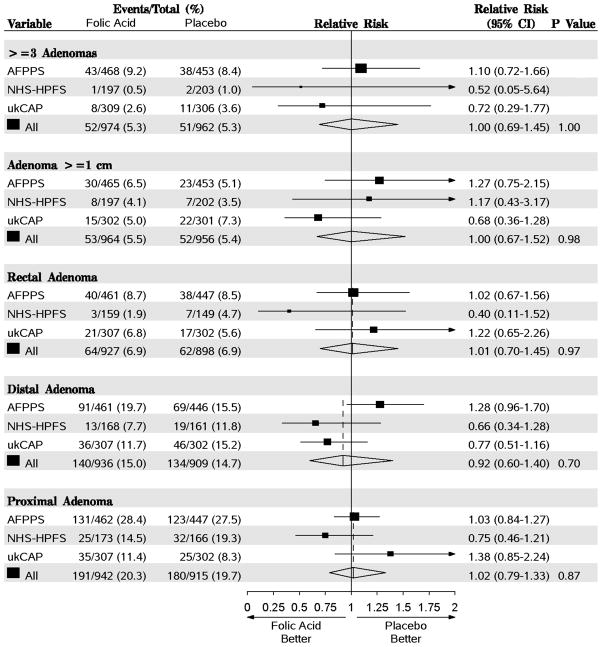
There was no association between folic acid treatment and the risk for three or more adenomas detected at follow-up (RR=1.00, 95% CI=0.69–1.45, Figure 2). Few adenomas 1.0 cm or greater in estimated diameter were found after randomization, and we found no association with risk of these lesions (RR=1.00; 95% CI=0.67–1.52, Figure 2). When we examined the effect of folic acid supplementation by location in the colorectum, we found no indication of significance differences (rectum: RR=1.01, 95% CI=0.70–1.45; distal colon: RR=0.92; 95% CI=0.60–1.40; proximal colon: RR=1.023, 95% CI=0.79–1.33, Figure 2).
Figure 2.
Random-effects meta-analysis comparing folic acid versus placebo for the endpoints of 3 or more adenomas, adenomas greater than or equal to 1 cm in estimated diameter and location in the colorectum. Estimates are unadjusted. Tests for heterogeneity are as follows: For >=3 adenomas, Q=0.99 (P=0.61) and I2=0.0; adenomas >= 1cm, Q=2.30 (P=0.32) and I2=13.0; rectal adenoma Q=2.19 (P=0.33) and I2=8.7; distal adenoma Q=5.32 (P=0.70) and I2=62.4 proximal adenoma Q=3.09 (P=0.21) and I2=35.3. Proximal adenomas include those in the transverse colon, hepatic flexure, ascending colon and cecum; distal adenomas include those in the sigmoid colon, descending colon and splenic flexure. AFPPS, Aspirin/Folate Polyp Prevention Study; NHS/HPFS, Nurses’ Health Study/Health Professional Follow-up Study; ukCAP, United Kingdom Colorectal Adenoma Prevention.
Subgroup analyses
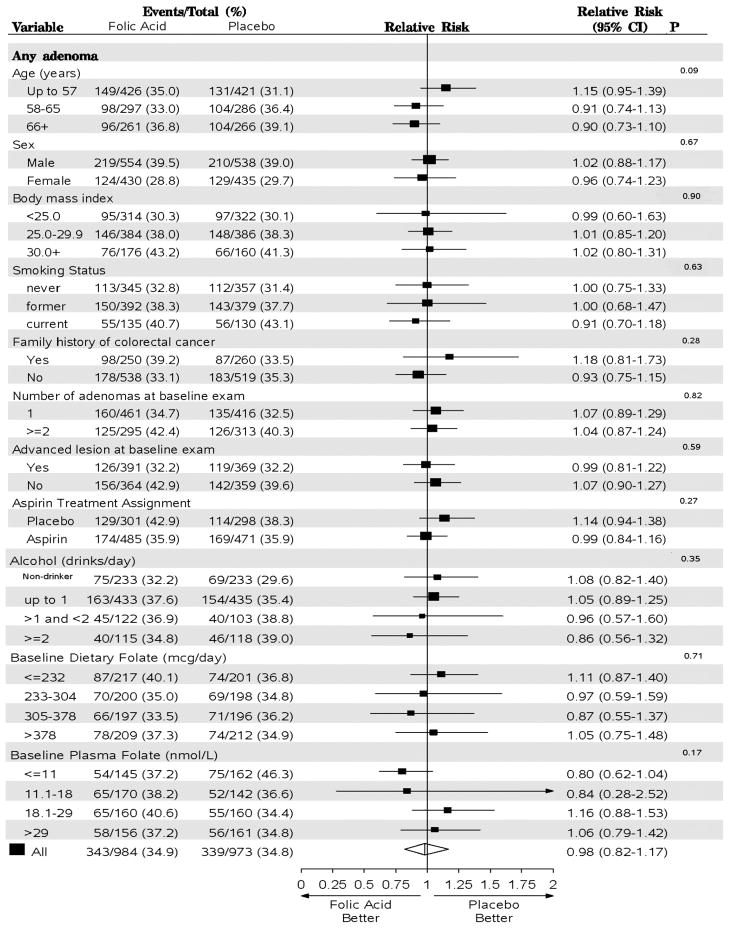
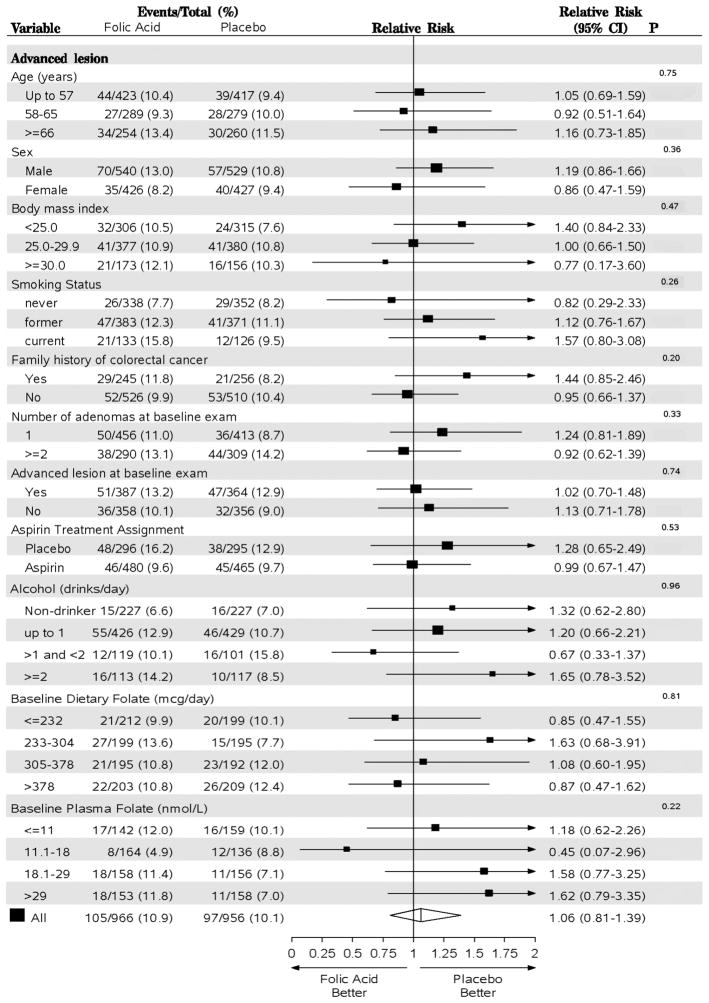
Figures 3 and 4 show the results of the random-effects meta-analysis for all adenomas and advanced lesions, respectively, by the subgroups examined. There was a non-significant adverse effect of folic acid supplementation on the risk of any adenoma among individuals in the lowest age group (under age 58 years: RR = 1.15; 95% CI=0.95–1.39) and no effect among those aged 58–65 years or those older than 65 years at the time of recruitment (p-trend=0.09). An apparent trend of decreasing RR for advanced lesions with increasing BMI group was compatible with chance, as was a pattern of increasing RRs across never-, former- and current-smoking groups (Figure 4).
Figure 3.
Random-effects meta-analysis comparing folic versus placebo by subgroups (all exams) for any adenoma. Estimates are unadjusted. P values are from tests for heterogeneity between groups for categorical variables (sex, family history of colorectal cancer, number of adenomas at baseline, advanced lesion at baseline, and aspirin treatment) and tests for trend for ordinal variables (age, BMI, smoking status, alcohol, dietary folate and plasma folate). Analysis of aspirin treatment group was restricted to the AFPPS and ukCAP trials and the analysis of plasma folate was restricted to the AFPPS and NHS/HPFS trials. AFPPS, Aspirin/Folate Polyp Prevention Study; NHS/HPFS, Nurses’ Health Study/Health Professional Follow-up Study; ukCAP, United Kingdom Colorectal Adenoma Prevention.
Figure 4.
Random-effects meta-analysis comparing folic acid versus placebo by subgroups (all exams) for advanced adenoma. Estimates are unadjusted. P values are from tests for heterogeneity between groups for categorical variables (sex, family history of colorectal cancer, number of adenomas at baseline, advanced lesion at baseline, and aspirin treatment) and tests for trend for ordinal variables (age, BMI, smoking status, alcohol, dietary folate and plasma folate). Analysis of aspirin treatment group was restricted to the AFPPS and ukCAP trials and the analysis of plasma folate was restricted to the AFPPS and NHS/HPFS trials. AFPPS, Aspirin/Folate Polyp Prevention Study; NHS/HPFS, Nurses’ Health Study/Health Professional Follow-up Study; ukCAP, United Kingdom Colorectal Adenoma Prevention.
There were inconsistent suggestions that the effects of folic acid differed according to baseline folate status. Folic acid supplementation was associated with a non-significantly lower risk among individuals in the lowest quartile of baseline plasma folate (<=11 nmol/L) (RR, 0.80; 95% CI=0.62–1.04) and no effect for those in the highest quartile (>29 nmol/L) (RR, 1.06; 95% CI=0.79–1.42, p-trend=0.17) (Figure 3). There was a corresponding non-significant trend towards an increased relative risk for advanced lesions: the RR in the highest quartile was 1.62 (95% CI=0.79–3.35) versus 1.18 (95% CI=0.62–2.26, p-trend=0.22) in the lowest quartile (Figure 4). However, this pattern was not observed for baseline dietary folate intake (Figures 3 & 4). The effect of folic acid on the risk of any adenomas decreased non-significantly with increasing number of alcoholic drinks (p-trend=0.35, Figure 3), but there was no similar pattern for advanced lesions (p-trend=0.96, Figure 4). When we considered individuals who do not drink and had a baseline plasma folate higher than 18 nmol/L, the RR for any adenoma was 1.32 (95% CI =0.69–2.50); among those who consumed any alcohol and had a lower than 18nmol/L folate level at baseline the RR was 0.78 (95% CI =0.33–1.83; p for interaction=0.71).
The estimated effect of folic acid supplementation on the risk of any adenoma or advanced lesions did not differ by the number of baseline adenomas or aspirin treatment group (Figure 3 & 4).
Adverse events
During the early follow-up reported here, more deaths occurred in the placebo group than in the folic acid group (1.7% vs. 0.5%, p=.002). There were no significant differences in the occurrence of myocardial infractions, stroke or invasive cancers. As expected in a population that was under endoscopic surveillance, the numbers of colorectal cancers observed were small (placebo: 0.8% vs. folic acid: 0.6%, P=0.64).
Discussion
In this combined analysis of the three largest randomized clinical trials of folic acid supplementation for the prevention of colorectal adenomas, we found that up to 42 months of treatment and follow-up (mean duration=30.6 months), daily use of folic acid supplements of 0.5–1.0 mg does not prevent the occurrence of new colorectal adenomas in the large bowel among men and women with a previous history of adenomas. There was a non-significant trend towards a beneficial effect of folic acid supplementation among those with lowest plasma levels of folate, and adverse effects among those with the highest. Mortality was significantly lower in the folic acid arms.
Daily use of alcohol, a known folate “antagonist,” 30 did not significantly modify the effect of folic acid supplementation on adenoma risk, but we did observe a non-significant trend of decreasing risk of all adenomas associated with folic acid with increasing number of drinks. Many observational studies of colorectal and other cancers31 have indicated an interaction such that among individuals who drink alcohol, folate intake and blood levels are inversely associated with risk, in contrast to lesser or no benefit among those who abstain.
We also evaluated whether there may be other subgroups who are more susceptible to the effects of folic acid in the large bowel. Unlike observational findings for colorectal cancer from the NHS/HPFS study,32 we did not observe any difference in the effect of folic acid supplementation by family history. We observed no indication that other personal demographic characteristics, lifestyle and dietary factors significantly modified the risk of adenomas or advanced lesions aside from the trend in alcohol intake.
We found no differences in the rates of adverse events comparing folic acid and placebo treatment group in terms of myocardial infarction, stroke, colorectal cancer and all cancer types combined. Interestingly, we observed a potential beneficial effect of folic acid supplements on overall mortality. This statistically significant result is a marked contrast with the lack of mortality benefit seen in the much larger combined analysis of trials conducted to investigate the effect of folic acid supplementation (typically with other B-vitamins) for the possible prevention of cardiovascular outcomes among high-risk individuals.33 The reasons for this difference are not clear, although the cardiovascular trials all studied individuals at high risk of cardiovascular endpoints, in contrast to the generally healthy population in the adenoma trials.
We obtained data from all larger folic acid randomized clinical trials to conduct this combined analysis. Two small clinical trials were not included. A trial in Michigan randomized 137 individuals to placebo or 5 mg of folic acid and reported a significantly lower number of adenomas per patient in the folic acid compared to placebo group after 3 years of follow-up.11 The second small trial, which investigated 60 patients randomized to 1 mg of “folate” (presumably folic acid) or placebo and followed them for 2 years, reported a non-significant reduction in adenoma recurrence associated with folic acid use.12 Results from these trials differ from those reported here, for reasons that are not clear. The small Michigan trial used a 5 mg folic acid dose, and it is possible that this would have a different effect on adenoma occurrence than the lower doses used in the larger studies. In addition to the limited sample size and the high drop out rate, another limitation of this trial was that associations were only assessed in terms of numbers of adenomas per participant, i.e. a few participants with a large number of adenomas could have artificially inflated those observed associations. There was a substantial but non-significant increase in mortality and adenoma numbers in the folic acid group among subjects over 70 years old.
Results from this combined analysis of clinical trials also are not consistent with earlier observational studies that suggested that high intake and blood levels of folate are inversely associated with colorectal adenomas or cancer risk.1, 2 There are several potential explanations. For one thing, the trials have studied folic acid rather than the reduced folates found in natural food sources, and it is possible that the synthetic and natural folates differ in their effects.34 However, the fact that studies of folic acid supplements (largely from multivitamins) have also found apparent protective associations with risk of colorectal cancer argues against this possibility. Even if folic acid and natural folates have similar effects, the folate doses used in the trials represent folate intakes considerably higher than those obtained from diet, and it is a possibility that at these high doses, folate from any source may be detrimental. Of course, it is also possible that the observational studies are affected by confounding by the effects of other nutrients in folate-rich foods and other lifestyle characteristics of those who have high folate intake or use multivitamins. There were also few individuals with deficient levels of folate (<7nmol/L) in these trials, and there is a growing appreciation that while adequate levels of folate are important at some yet undefined threshold of folate, higher levels may not be beneficial.35 Therefore, our results suggest with greatest certainty that most people in fortified or similar populations are unlikely to benefit substantially from folic acid supplements within 3.5 years of follow-up and treatmen. However, it is unclear whether an effect may be observed in alcoholics with poor diets or populations with low folate levels or with a longer follow-up.
A further possible reason for the difference between the observational and clinical trial findings relates to the timing of the folate administration. Evidence from animal models suggests that folate may play a dual role in the colorectum depending on the timing of supplementation. In a Apc+/− Msh2−/− mouse model, modest doses of folate supplementation given after the formation of neoplastic foci appeared to increase the development of new tumors.8 Folic acid supplementation was also shown to incrementally increase the total number of aberrant crypt foci, the earliest precursor for colorectal cancer, and the mean number of crypts per focus.7 Since all the subjects in the trials included in this analysis had previous adenomas, presumably they already had transformed crypts that could be promoted by folic acid supplementation.
Studies of colorectal cancer in populations where the food supply is fortified with folic acid have suggested that folate may increase risk or benefit only those who do not take multivitamins.4, 36–38 Even in a population in which folic acid fortification of foods does not occur, a U-shaped association between folate levels and colorectal cancer risk has been observed in one study.5 Our findings focus on pre-cursor lesions to colorectal cancer, and the effects of folic acid may differ for adenoma development compared to those for invasive colorectal cancer.
Folate deficiency may increase risk of colorectal cancer via alteration in normal methylation patterns, which can affect maintenance of DNA integrity and stability and expression of oncogenes, tumor suppressor genes, and genes associated with changes in the expression of apoptosis and cell cycle genes.10, 39, 40 Folate supplementation might then inhibit carcinogenesis in deficient individuals, but have no particular effect in folate-replete populations.
On the other hand, high levels of folate may promote colorectal carcinogenesis, for example via their role in nucleotide synthesis.41, 42 Neoplastic cells have a relatively high rate of proliferation43 and an up-regulation of folate receptors44 compared to normal tissue. Folate antagonists, such as methotrexate, have been used as chemotherapeutic agents for several cancers including those in the colorectum.45 Furthermore, folic acid is a pharmaceutical fully oxidized, monoglutamyl form of folate. Unmetabolized folic acid may be detected in blood after folic acid supplementation 46 and may be associated with reduced natural killer cell cytotoxicity.34
Our meta-analysis has several limitations. First, only one clinical trial, the ukCAP trial, examined the effect of 0.5 mg of folic acid. This was also the only study conducted in a country that did not fortify food products with folic acid. However, in none of our analyses did we find substantial indications of between-trial heterogeneity. Second, results from this meta-analysis address only the short-term effects of folic acid supplementation, during follow-up of up to 42 months. Longer studies are needed to examine potential differences by duration of use and duration of follow-up. Follow-up in the AFPPS and NHS/HPFS is continuing, though not in the ukCAP trial.
We were also unable to examine potential effects on other cancers and cardiovascular events due to small numbers. All participants in this clinical trial were volunteers who had a previous history of at least one colorectal adenoma, and our findings may therefore not pertain to individuals without a history of such neoplasia. In general, colorectal lesion size was estimated by the endoscopist during the endoscopy at the time of resection, and so is subject to measurement error. Of the three trials, only the AFPPS had a central pathology review.
This meta-analysis included the three large randomized clinical trials that have tested folic acid as a chemopreventive agent against colorectal adenomas. The trials were well conducted, with high compliance. Follow-up rates were high, although in two of the trials only about 2/3 of subjects had an endoscopy during the initially intended window. The sample size in the pooled studies was substantial, providing good statistical power to detect moderate effects. Additional strengths include the systematic collection of risk factor and dietary information. Because of the randomized analysis, confounding is unlikely and the fixed doses of folic acid reduce or eliminate the measurement error associated with dietary assessment. In addition, inclusion only of individuals with a “clean” (“no polyps”) endoscopy in the AFPPS and ukCAP trials allowed them to assess the effect of folic acid on incident rather than prevalent adenomas. However, the NHS/HPFS did not restrict entry to subjects with a clean colon (although 80% had had a endoscopy within 2 years prior to the start of the trial and the rate of recurrence was 24%) and it is still possible polyps were missed during endoscopy from participants in the AFPPS and ukCAP trials. Uniform, blinded follow-up prevented differential ascertainment of endpoints according to folate intake.
In this large-scale collaborative combined analysis, we found no evidence overall that folic acid is beneficial for the prevention of colorectal adenomas in the large bowel after a follow-up of up to 42 months. These data, together with other findings dampen enthusiasm for the potential chemopreventive effects of folic acid. More follow-up is needed to assess the longer-term effects of folic acid use.
Acknowledgments
Funding: This work was supported in part by funding (N01-CO-12400, R01-CA-059005, U54-CA-100971, CA 55075 and CA95589 and R01 CA 67883) from the National Cancer Institute, National Institutes of Health.
Footnotes
Disclosures: Wyeth (now Pfizer) provided folic acid and placebo tablets for the Aspirin/Folate Polyp Prevention Study, one of the trials included in this combined analysis.
Trial Registration: clinicaltrials.gov Identifier: NCT00272324; http://www.controlled-trials.com/ISRCTN22669537 and NCT00512850 (NHS/HPFS).
References
- 1.Giovannucci E. Epidemiologic studies of folate and colorectal neoplasia: a review. J Nutr. 2002;132:2350S–5S. doi: 10.1093/jn/132.8.2350S. [DOI] [PubMed] [Google Scholar]
- 2.Sanjoaquin MA, Allen N, Couto E, Roddam AW, Key TJ. Folate intake and colorectal cancer risk: a meta-analytical approach. Int J Cancer. 2005;113:825–8. doi: 10.1002/ijc.20648. [DOI] [PubMed] [Google Scholar]
- 3.Le Marchand L, White KK, Nomura AM, Wilkens LR, Selhub JS, Tiirikainen M, Goodman MT, Murphy SP, Henderson BE, Kolonel LN. Plasma levels of B vitamins and colorectal cancer risk: the multiethnic cohort study. Cancer Epidemiol Biomarkers Prev. 2009;18:2195–201. doi: 10.1158/1055-9965.EPI-09-0141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Mason JB, Dickstein A, Jacques PF, Haggarty P, Selhub J, Dallal G, Rosenberg IH. A temporal association between folic acid fortification and an increase in colorectal cancer rates may be illuminating important biological principles: a hypothesis. Cancer Epidemiol Biomarkers Prev. 2007;16:1325–9. doi: 10.1158/1055-9965.EPI-07-0329. [DOI] [PubMed] [Google Scholar]
- 5.Van Guelpen B, Hultdin J, Johansson I, Hallmans G, Stenling R, Riboli E, Winkvist A, Palmqvist R. Low folate levels may protect against colorectal cancer. Gut. 2006;55:1461–6. doi: 10.1136/gut.2005.085480. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Ulrich CM. Folate and cancer prevention: a closer look at a complex picture. Am J Clin Nutr. 2007;86:271–3. doi: 10.1093/ajcn/86.2.271. [DOI] [PubMed] [Google Scholar]
- 7.Lindzon GM, Medline A, Sohn KJ, Depeint F, Croxford R, Kim YI. Effect of folic acid supplementation on the progression of colorectal aberrant crypt foci. Carcinogenesis. 2009 doi: 10.1093/carcin/bgp152. [DOI] [PubMed] [Google Scholar]
- 8.Song J, Sohn KJ, Medline A, Ash C, Gallinger S, Kim YI. Chemopreventive effects of dietary folate on intestinal polyps in Apc+/−Msh2−/− mice. Cancer Res. 2000;60:3191–9. [PubMed] [Google Scholar]
- 9.Song J, Medline A, Mason JB, Gallinger S, Kim YI. Effects of dietary folate on intestinal tumorigenesis in the apcMin mouse. Cancer Res. 2000;60:5434–40. [PubMed] [Google Scholar]
- 10.Kim YI. Folate, colorectal carcinogenesis, and DNA methylation: lessons from animal studies. Environ Mol Mutagen. 2004;44:10–25. doi: 10.1002/em.20025. [DOI] [PubMed] [Google Scholar]
- 11.Jaszewski R, Misra S, Tobi M, Ullah N, Naumoff JA, Kucuk O, Levi E, Axelrod BN, Patel BB, Majumdar AP. Folic acid supplementation inhibits recurrence of colorectal adenomas: a randomized chemoprevention trial. World J Gastroenterol. 2008;14:4492–8. doi: 10.3748/wjg.14.4492. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Paspatis GA, Karamanolis DG. Folate supplementation and adenomatous colonic polyps. Dis Colon Rectum. 1994;37:1340–1. doi: 10.1007/BF02257810. [DOI] [PubMed] [Google Scholar]
- 13.Cole BF, Baron JA, Sandler RS, Haile RW, Ahnen DJ, Bresalier RS, McKeown-Eyssen G, Summers RW, Rothstein RI, Burke CA, Snover DC, Church TR, et al. Folic acid for the prevention of colorectal adenomas: a randomized clinical trial. JAMA. 2007;297:2351–9. doi: 10.1001/jama.297.21.2351. [DOI] [PubMed] [Google Scholar]
- 14.Logan RF, Grainge MJ, Shepherd VC, Armitage NC, Muir KR. Aspirin and folic acid for the prevention of recurrent colorectal adenomas. Gastroenterology. 2008;134:29–38. doi: 10.1053/j.gastro.2007.10.014. [DOI] [PubMed] [Google Scholar]
- 15.Wu K, Platz EA, Willett WC, Fuchs CS, Selhub J, Rosner BA, Hunter DJ, Giovannucci E. A randomized trial on folic acid supplementation and risk of recurrent colorectal adenoma. Am J Clin Nutr. 2009;90:1623–31. doi: 10.3945/ajcn.2009.28319. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Baron JA, Cole BF, Sandler RS, Haile RW, Ahnen D, Bresalier R, McKeown-Eyssen G, Summers RW, Rothstein R, Burke CA, Snover DC, Church TR, et al. A randomized trial of aspirin to prevent colorectal adenomas. N Engl J Med. 2003;348:891–9. doi: 10.1056/NEJMoa021735. [DOI] [PubMed] [Google Scholar]
- 17.Giovannucci E, Stampfer MJ, Colditz GA, Hunter DJ, Fuchs C, Rosner BA, Speizer FE, Willett WC. Multivitamin use, folate, and colon cancer in women in the Nurses’ Health Study. Ann Intern Med. 1998;129:517–24. doi: 10.7326/0003-4819-129-7-199810010-00002. [DOI] [PubMed] [Google Scholar]
- 18.Wu K, Platz EA, Willett WC, Fuchs C, Selhub J, Rosner B, Hunter DJ, Giovannucci E. A Randomized Trial on Folic Acid Supplementation and Risk of Recurrent Colorectal Adenoma. submitted. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Willett WC, Stampfer MJ, Colditz GA, Rosner BA, Hennekens CH, Speizer FE. Dietary fat and the risk of breast cancer. N Engl J Med. 1987;316:22–8. doi: 10.1056/NEJM198701013160105. [DOI] [PubMed] [Google Scholar]
- 20.Rimm EB, Giovannucci EL, Willett WC, Colditz GA, Ascherio A, Rosner B, Stampfer MJ. Prospective study of alcohol consumption and risk of coronary disease in men. Lancet. 1991;338:464–8. doi: 10.1016/0140-6736(91)90542-w. [DOI] [PubMed] [Google Scholar]
- 21.Colditz GA, Hankinson SE. The Nurses’ Health Study: lifestyle and health among women. Nat Rev Cancer. 2005;5:388–96. doi: 10.1038/nrc1608. [DOI] [PubMed] [Google Scholar]
- 22.Final report on the aspirin component of the ongoing Physicians’ Health Study. Steering Committee of the Physicians’ Health Study Research Group. N Engl J Med. 1989;321:129–35. doi: 10.1056/NEJM198907203210301. [DOI] [PubMed] [Google Scholar]
- 23.Figueiredo JC, Levine AJ, Grau MV, Barry EL, Ueland PM, Ahnen DJ, Byers T, Bresalier RS, Summers RW, Bond J, McKeown-Eyssen GE, Sandler RS, et al. Colorectal adenomas in a randomized folate trial: the role of baseline dietary and circulating folate levels. Cancer Epidemiol Biomarkers Prev. 2008;17:2625–31. doi: 10.1158/1055-9965.EPI-08-0382. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Wolpin BM, Wei EK, Ng K, Meyerhardt JA, Chan JA, Selhub J, Giovannucci EL, Fuchs CS. Prediagnostic plasma folate and the risk of death in patients with colorectal cancer. J Clin Oncol. 2008;26:3222–8. doi: 10.1200/JCO.2008.16.1943. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.DerSimonian R, Laird N. Meta-analysis in clinical trials. Control Clin Trials. 1986;7:177–88. doi: 10.1016/0197-2456(86)90046-2. [DOI] [PubMed] [Google Scholar]
- 26.Egger M, Smith GD, Phillips AN. Meta-analysis: principles and procedures. BMJ. 1997;315:1533–7. doi: 10.1136/bmj.315.7121.1533. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Friedman LM, Furberg CD, DeMets DL. Fundamentals of Clinical Trials. New York: Springer; 1988. [Google Scholar]
- 28.Deeks JJ. Issues in the selection of a summary statistic for meta-analysis of clinical trials with binary outcomes. Stat Med. 2002;21:1575–600. doi: 10.1002/sim.1188. [DOI] [PubMed] [Google Scholar]
- 29.Higgins JP, Thompson SG, Deeks JJ, Altman DG. Measuring inconsistency in meta-analyses. BMJ. 2003;327:557–60. doi: 10.1136/bmj.327.7414.557. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Halsted CH, Villanueva JA, Devlin AM, Chandler CJ. Metabolic interactions of alcohol and folate. J Nutr. 2002;132:2367S–72S. doi: 10.1093/jn/132.8.2367S. [DOI] [PubMed] [Google Scholar]
- 31.Mason JB, Choi SW. Effects of alcohol on folate metabolism: implications for carcinogenesis. Alcohol. 2005;35:235–41. doi: 10.1016/j.alcohol.2005.03.012. [DOI] [PubMed] [Google Scholar]
- 32.Fuchs CS, Willett WC, Colditz GA, Hunter DJ, Stampfer MJ, Speizer FE, Giovannucci EL. The influence of folate and multivitamin use on the familial risk of colon cancer in women. Cancer Epidemiol Biomarkers Prev. 2002;11:227–34. [PubMed] [Google Scholar]
- 33.Armitage J, Collins R, Bowman L, Parish S. SEARCH (Study of the Effectiveness of Additional Reductions in Cholesterol and Homocysteine): Randomized Comparison of Folic Acid 2 mg Plus Vitamin B12 1 mg Daily versus Placebo for 7 years in 12,064 Myocardial Infarction Survivors. American Heart Association; 2008. [Google Scholar]
- 34.Troen AM, Mitchell B, Sorensen B, Wener MH, Johnston A, Wood B, Selhub J, McTiernan A, Yasui Y, Oral E, Potter JD, Ulrich CM. Unmetabolized folic acid in plasma is associated with reduced natural killer cell cytotoxicity among postmenopausal women. J Nutr. 2006;136:189–94. doi: 10.1093/jn/136.1.189. [DOI] [PubMed] [Google Scholar]
- 35.Ulrich CM, Potter JD. Folate supplementation: too much of a good thing? Cancer Epidemiol Biomarkers Prev. 2006;15:189–93. doi: 10.1158/1055-9965.EPI-152CO. [DOI] [PubMed] [Google Scholar]
- 36.Luebeck EG, Moolgavkar SH, Liu AY, Boynton A, Ulrich CM. Does folic acid supplementation prevent or promote colorectal cancer? Results from model-based predictions. Cancer Epidemiol Biomarkers Prev. 2008;17:1360–7. doi: 10.1158/1055-9965.EPI-07-2878. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Martinez ME, Giovannucci E, Jiang R, Henning SM, Jacobs ET, Thompson P, Smith-Warner SA, Alberts DS. Folate fortification, plasma folate, homocysteine and colorectal adenoma recurrence. Int J Cancer. 2006;119:1440–6. doi: 10.1002/ijc.21978. [DOI] [PubMed] [Google Scholar]
- 38.Zhang SM, Moore SC, Lin J, Cook NR, Manson JE, Lee IM, Buring JE. Folate, vitamin B6, multivitamin supplements, and colorectal cancer risk in women. Am J Epidemiol. 2006;163:108–15. doi: 10.1093/aje/kwj016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39.Kim YI. Folate and DNA methylation: a mechanistic link between folate deficiency and colorectal cancer? Cancer Epidemiol Biomarkers Prev. 2004;13:511–9. [PubMed] [Google Scholar]
- 40.Novakovic P, Stempak JM, Sohn KJ, Kim YI. Effects of folate deficiency on gene expression in the apoptosis and cancer pathways in colon cancer cells. Carcinogenesis. 2006;27:916–24. doi: 10.1093/carcin/bgi312. [DOI] [PubMed] [Google Scholar]
- 41.Kim YI. Role of folate in colon cancer development and progression. J Nutr. 2003;133:3731S–9S. doi: 10.1093/jn/133.11.3731S. [DOI] [PubMed] [Google Scholar]
- 42.Choi SW, Mason JB. Folate status: effects on pathways of colorectal carcinogenesis. J Nutr. 2002;132:2413S–8S. doi: 10.1093/jn/132.8.2413S. [DOI] [PubMed] [Google Scholar]
- 43.Shpitz B, Bomstein Y, Mekori Y, Cohen R, Kaufman Z, Grankin M, Bernheim J. Proliferating cell nuclear antigen as a marker of cell kinetics in aberrant crypt foci, hyperplastic polyps, adenomas, and adenocarcinomas of the human colon. Am J Surg. 1997;174:425–30. doi: 10.1016/s0002-9610(97)00122-0. [DOI] [PubMed] [Google Scholar]
- 44.Kelemen LE. The role of folate receptor alpha in cancer development, progression and treatment: cause, consequence or innocent bystander? Int J Cancer. 2006;119:243–50. doi: 10.1002/ijc.21712. [DOI] [PubMed] [Google Scholar]
- 45.Ulrich CM, Robien K, Sparks R. Pharmacogenetics and folate metabolism -- a promising direction. Pharmacogenomics. 2002;3:299–313. doi: 10.1517/14622416.3.3.299. [DOI] [PubMed] [Google Scholar]
- 46.Kelly P, McPartlin J, Goggins M, Weir DG, Scott JM. Unmetabolized folic acid in serum: acute studies in subjects consuming fortified food and supplements. Am J Clin Nutr. 1997;65:1790–5. doi: 10.1093/ajcn/65.6.1790. [DOI] [PubMed] [Google Scholar]