Abstract
The prefrontal cortex (PFC) is involved in working memory, self-regulatory and goal-directed behaviors and displays remarkable structural and functional plasticity over the life course. Neural circuitry, molecular profiles and neurochemistry can be changed by experiences, which influences behavior as well as neuroendocrine and autonomic function. Such effects have a particular impact during infancy and in adolescence. Behavioral stress affects both the structure and function of PFC, though such effects are not necessarily permanent, as young animals show remarkable neuronal resilience if the stress is discontinued. During aging, neurons within the PFC become less resilient to stress. There are also sex differences in the PFC response to stressors. While such stress- and sex-hormone related alterations occur in regions mediating the highest levels of cognitive function and self regulatory control, the fact that they are not necessarily permanent has implications for future behavior-based therapies that harness neural plasticity for recovery.
Introduction
Brain circuitry can be remodeled by experience (Bennett et al., 1964), and stressful experiences have functionally-relevant effects on dendritic arbor and spine/synapse number in many brain regions, including the hippocampus, amygdala and the prefrontal cortex (PFC), with effects not only on cognitive function but also on emotional regulation and other self-regulatory behaviors and upon neuroendocrine and autonomic function (McEwen and Gianaros, 2011). This review focuses primarily on stress-related effects upon the PFC because of its importance in working memory, self regulatory and goal-directed behaviors, and also because the structural and functional plasticity in this brain region illustrates the profound capacity of behavioral experiences to change neural circuitry in a manner that will alter brain function, with particular impact during early childhood and adolescence. There are also sex differences that reflect both developmental programming, as well as the actions of circulating sex hormones in the mature brain via genomic and non-genomic receptors. Aging is also an important factor and loss of resilience to stressful experiences is evident in animal models, with indications that this occurs in the aging human brain. Likewise, in mood disorders that are often precipitated by stressful experiences, the loss of resilience is an indication that external behavioral and pharmacological intervention is needed. Indeed, evidence is mounting that the mature brain has greater capacity for plasticity than previously imagined and this points to future behavioral- as well as pharmacological-based therapies that harness neural plasticity for recovery.
Role of Prefrontal Cortex in Cognition
When we refer to memory, particularly declarative memory as mediated by the medial temporal lobe, there is a strong intuitive sense of what we mean, namely, an integrated record of events, places, and timing that represents our experiences. However, it is more difficult to grasp the concept of cognition as mediated by a region such as the dorsolateral prefrontal cortex (dlPFC) in humans and nonhuman primates (NHPs). Understanding the function of the dlPFC has become increasingly important in light of its vulnerability to stress and aging, and its critically important role in multiple brain disorders. The dlPFC has been characterized as possessing an internal construct of reality that is neither directly dependent on sensory perception of the outside world nor directly controlling actions through motor commands, though it is highly interconnected with both sensory and motor association regions (Funahashi et al., 1989). The dlPFC is responsible for planning approaches and sequences of behavior that are required for goal-directed behavior. This process is critical to the broad realm of executive function, and requires both learning and implementing the rules of behavior that lead to success, as well as modifying those rules as necessary (Miller, 2000).
Timing and establishing the appropriate sequence of actions is critically important for executive function, and the dlPFC is responsible for the “formation of coherent behavioral sequences toward the attainment of goals” (Fuster, 2008). Such processes are highly dependent on attention and shifting attention, along with the capacity to inhibit a response that is counter-productive to the planning and execution of successful goal-directed behavior. These elements of dlPFC function, particularly attention, involve “top-down control” of sensory processing through dlPFC inputs to sensory association cortex that influence perception and focus attention (Gazzaley and Nobre, 2012).
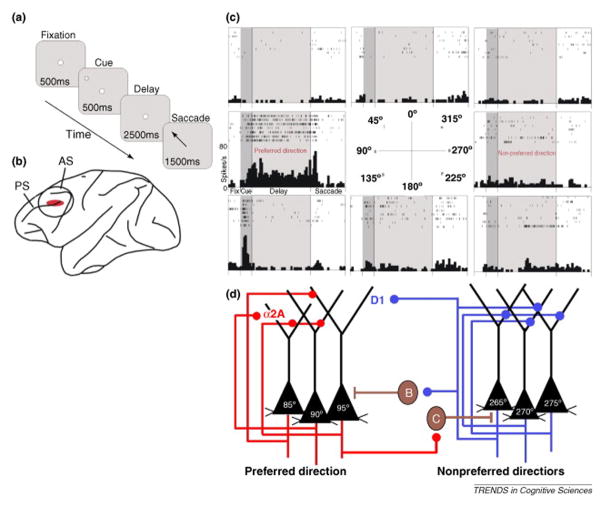
The functions attributed to dlPFC above are largely long-term functions, but they are highly dependent on a process that is central to dlPFC function, yet occurs over seconds to minutes, i.e., working memory. Working memory refers to “the ability to keep events in mind” (Goldman-Rakic, 1995), and the information held in working memory changes as the demands and goals shift from moment to moment (See Figure 1). In this respect, it has been referred to as the “mental sketch pad” (Arnsten et al., 2012). Given the constantly changing nature of what is being held in working memory, it is not surprising that it is thought to be highly dependent on recurrent collaterals of pyramidal cells that reside in dlPFC and GABAergic inputs (Arnsten et al., 2010) rather than long distance projections transmitting specific sensory or motor information from association areas. Electrophysiological studies in dlPFC of awake, behaving NHPs have been particularly informative with respect to working memory (Arnsten et al., 2012; Fuster, 2008; Goldman-Rakic, 1988; Miller, 2000; Wang et al., 2011). Neurons have been identified in area 46 of macaque monkey that respond preferentially during the delay period imposed between the salient cue and the response generating a reward (See Figure 1), effectively holding the relevant information in working memory until the appropriate response is warranted (Arnsten et al., 2010; Funahashi et al., 1989; Fuster, 2008). It has been proposed that such neurons require extensive capacity for synaptic plasticity, given the constantly shifting demands and information content being held in working memory (Arnsten et al., 2010; Morrison and Baxter, 2012).
Figure 1.
The neuronal response properties and neural circuitry underlying spatial working memory task as envisioned by Goldman-Rakic and colleagues (Arnsten et al, 2010; Goldman-Rakic, 1995) (a) The oculomotor delayed response (ODR) task, which is a test of spatial working memory as mediated by Brodmann area 46 in the dlPFC of the monkey. (b) Area 46, delineated in red surrounding the principal sulcus. The electrophysiological response properties depicted in c are generated from recordings of pyramidal neurons within this region. PS=principal sulcus; AS=arcuate sulcus. (c) Recordings from a representative neuron in area 46 with spatially tuned firing during the delay period of the ODR task. For details, see (Wang et al 2007). (d) The PFC microcircuits subserving spatially tuned firing during the delay period in a spatial working memory task. Brown neurons designated by B and C represent GABAergic neurons innervating pyramidal neurons mediating working memory. The red circuit on the left represents the noradrenergic inputs modulating α2A receptors on the pyramidal neurons, and the blue circuit on the right depicts the dopaminergic inputs acting through D1 receptors. From Arnsten (Arnsten et al 2010) with permission.
The degree to which the rat neocortex contains structural and functional homologues of areas in primate dlPFC, such as area 46, remains controversial (Wise, 2008). In fact, it has been argued that rats lack the “granular” prefrontal cortex characteristic of primate dlPFC (Preuss, 1995; Wise, 2008). However, clearly there are areas of PFC in rat cortex that subserve cognitive functions similar to primate dlPFC, with the medial PFC (mPFC)- consisting of anterior cingulate (AC), prelimbic (PL), and Infralimbic (IL) cortices- likely to be the key regions responsible for such functions (Kesner and Churchwell, 2011). These regions mediate such cognitive functions as set shifting and selective attention (Barense et al., 2002; Birrell and Brown, 2000a), which are required for the kinds of goal-directed behaviors that have been linked to dlPFC in monkeys and humans. Thus, as is apparent below, studies of the effects of stress on PFC in rodent have focused on mPFC.
While the PFC is highly evolved in NHPs and humans and mediates particularly complex cognitive processes, it is also highly vulnerable. The PFC has been implicated in multiple brain disorders such as attention deficit disorder, schizophrenia, depression, and PTSD (Arnsten, 2009a; Drevets et al., 1997b; Gamo and Arnsten, 2011; Tan et al., 2007), and it is also vulnerable to stress (McEwen and Gianaros, 2011) and normal aging (Morrison and Baxter, 2012), as well as Alzheimer’s Disease (Hof and Morrison, 2004; Morrison and Hof, 1997) in humans. The PFC has also been identified as a cortical region that is affected by decreased estrogen levels in women (Shanmugan and Epperson, 2012). Monkey studies have highlighted the vulnerability of dorsolateral PFC (dlPFC) to stress (Arnsten, 2009b), aging (Morrison and Baxter, 2012; Wang et al., 2011), and estrogen depletion (Hao et al., 2007; Hao et al., 2006; Rapp et al., 2003). As will be discussed in detail in this review, the homologous mPFC is highly vulnerable to stress (Cook and Wellman, 2004; Holmes and Wellman, 2009; Radley et al., 2004), aging (Bloss et al., 2011), and estrogen depletion (Shansky et al., 2010) in rats. Thus, while PFC clearly is an important target for intervention regarding multiple devastating brain disorders in humans, the animal models faithfully reflect several of its vulnerabilities and can thus provide important mechanistic insights into the unique capacities and vulnerabilities of this neocortical region that plays such a crucial role in higher cognitive processes.
The mPFC has extensive downstream projections to regions as diverse as the amygdala and the brainstem (Sesack et al., 1989), providing a substrate for downstream regulation of autonomic and neuroendocrine balance (Thayer and Brosschot, 2005), with influences on parasympathetic (Thayer and Sternberg, 2006) and hypothalamo-pituitary adrenal (HPA) activity (Diorio et al., 1993). For HPA activity and autonomic control in rat, dorsal and ventral mPFC have different effects, based on experiments showing that lesions to the dorsal mPFC enhanced restraint stress-induced cFos and corticotropin-releasing factor (CRF) mRNA expression in the neurosecretory region of the paraventricular hypothalamus (PVH), whereas ablation of the ventral mPFC decreased stress-induced cFos protein and CRF mRNA expression in this compartment, but increased cFos induction in PVH regions involved in central autonomic control. (Radley et al., 2006). In the monkey prefrontal cortex, descending pathways from orbitofrontal and medial prefrontal cortices, which are also linked with the amygdala, provide both stimulatory and inhibitory influences on the autonomic system related to emotional regulation (Barbas et al., 2003).
Effects of Stress on Prefrontal Cortex
Stressful experiences exert biphasic, time dependent effects upon the prefrontal cortex, as shown in animal models. In 3–4 week old rats, diverse acute stressors (forced swim, restraint, elevated platform) facilitate both PFC-dependent behavior, as well as LTP, tested 4h after stress exposure. Adrenal steroids mediate these effects and facilitate LTP, as well as behaviors known to depend on mPFC via mechanisms dependent not only on glucocorticoid receptors (GR), but also on signaling pathways involving serum- and glucocorticoid-inducible kinase (SGK) and Rab4-mediated recycling of NMDA and AMPA receptors (Yuen et al., 2009; Yuen et al., 2011a). Yet, at this same age, chronic unpredictable stress or restraint stress for 7d impaired temporal order recognition memory in rats, a cognitive process controlled by the mPFC and caused reduced AMPAR- and NMDAR-mediated synaptic transmission and glutamate receptor expression in mPFC (Yuen et al., 2012). All these effects relied on activation of glucocorticoid receptors and the subsequent enhancement of ubiquitin/proteasome-mediated degradation of GluR1 and NR1 subunits, which was controlled by the E3 ubiquitin ligase Nedd4-1 and Fbx2, respectively. Inhibition of proteasomes or knockdown of Nedd4-1 and Fbx2 in PFC prevented the loss of glutamatergic responses and recognition memory in stressed animals. Thus, repeated stress dampens PFC glutamatergic transmission by facilitating glutamate receptor turnover. Indeed the effects of chronic stress carry over to older ages since, in adult rats, 21d of chronic restraint stress impaired working memory and caused spine loss and debranching of dendrites on mPFC neurons (Hains et al., 2009), as will be discussed further below.
However, in adult rats, acute mild stress impairs working memory during and immediately after stress exposures and does so via excessive stimulation of dopaminergic and noradrenergic receptors (Arnsten, 2009b). This acute stress effect on working memory and working memory-related activity in dlPFC monitored by fMRI is reported in volunteer subjects viewing movie clips with extremely aversive material (Qin et al., 2009). Intracellular signaling pathways activated by stress exposure have feed forward interactions that rapidly impair PFC-dependent cognitive function. High levels of dopamine (DA) D1 receptor stimulation and noradrenaline (NA) β1-receptor stimulation activate adenylyl cyclases (ACs) to produce cyclic AMP; cAMP opens hyperpolarization-activated cyclic nucleotide-gated cation channels (HCN channels) on dendritic spines to produce the h current (Ih), which weakens network inputs and decreases delay-related firing. High levels of NA also stimulate α1-receptors, which activate phosphatidylinositol biphosphate (PIP2)–protein kinase C (PKC) signaling (Arnsten, 2009b). It is, therefore, noteworthy that blockade of PKC signaling can prevent effects of chronic stress on PFC function (Hains et al., 2009). There may be a connection between PKC signaling and the Nedd4-1 regulation of glutamatergic activity, in that PKC-promoted endocytosis of glutamate transporter GLT-1 requires ubiquitin ligase Nedd4-2-dependent ubiquitination (Garcia-Tardon et al., 2012).
The differences between the outcome of studies described in the two paragraphs above reflect both timing of stress exposure in relation to testing, along with the qualitative nature of the stressors used, and they reveal the biphasic nature of stress responses by the PFC. Since the neurochemical responses, such as the release of dopamine, during stress exposure are transient the timing of cognitive testing is a critical factor, in which 4–24h may be a time of compensatory reactions. This will be essential to clarify in future studies both in terms of age-dependency of both positive and negative effects of stressors as well as timing after stress exposure and the intensity and duration of the stressor.
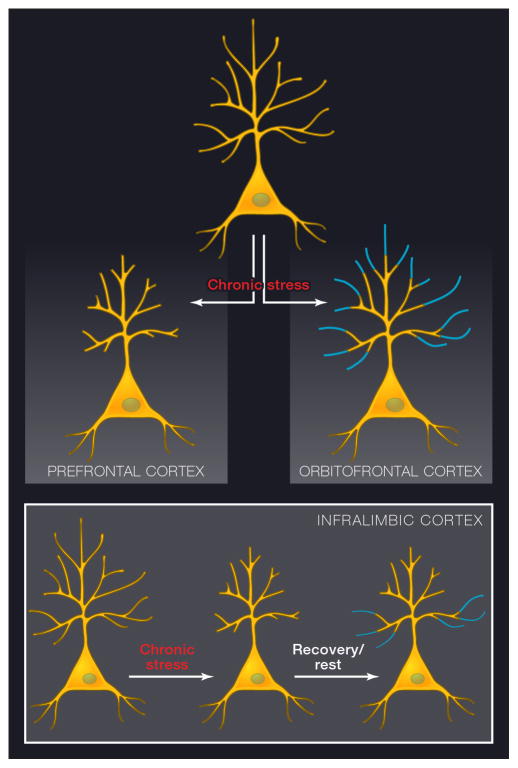
Repeated stress, such as 21days of chronic restraint stress (CRS), causes functional and structural changes in the prefrontal cortex and amygdala, as well as the hippocampus (McEwen and Gianaros, 2011), though these effects exhibit regional specificity (See Figure 2A). For example, CRS and chronic immobilization caused dendritic shortening in medial prefrontal cortex (Cerqueira et al., 2007; Cook and Wellman, 2004; Liston et al., 2006; Radley et al., 2004), but produced dendritic growth in neurons in basolateral amygdala (Vyas et al., 2002), as well as in orbitofrontal cortex (Liston et al., 2006). These actions of stress are reminiscent of recent work on experimenter versus self-administered morphine and amphetamine, in which different, and sometimes opposite, effects were seen on dendritic spine density in orbitofrontal cortex, medial prefrontal cortex and hippocampus CA1 (Crombag et al., 2005; Robinson et al., 2001; Robinson et al., 2002). Indeed, there are clear indications that, besides substance abuse, many other aspects of brain function are subject to structural plasticity, including respiratory and motor control regions during exercise training (Nelson and Iwamoto, 2006; Nelson et al., 2005), the nucleus accumbens after repeated sodium depletion causing increased salt appetite and enhanced amphetamine self-administration (Roitman et al., 2002), and the hippocampus during hibernation (Magarinos et al., 2006; Popov and Bocharova, 1992).
Figure 2.
Schematic diagrams depicting dendritic shrinkage and expansion in response to chronic stress and recovery. A) Chronic stress leads to dendritic shrinkage in layer 3 pyramidal neurons in the prelimbic and anterior cingulate cortex, whereas it causes dendritic expansion in the corresponding neurons within orbitofrontal cortex. Both effects are seen primarily in the distal apical dendritic tree. B) While shrinkage and recovery both affect distal dendrites in neurons depicted in A, layer 5 neurons in infralimbic cortex lose distal dendritic branches in response to stress, yet recovery occurs primarily in proximal dendrites, shifting the dendritic architecture (see text for details).
Pyramidal neurons in layer 3 of all three regions of mPFC (AcG, PL, and IL) in male rats are affected by chronic stress, yet as noted below, there are important sex differences in some of these responses. Apical dendritic length shrinks by 20% in male rats, and this shrinkage is most pronounced in the distal apical dendritic branches, whereas the basal dendritic tree is unaffected (Bloss et al., 2010; Bloss et al., 2011; Cook and Wellman, 2004; Radley et al., 2008; Radley et al., 2004). Importantly, the dendritic shrinkage is accompanied by spine loss, leading to an estimated total loss of axospinous synapses of over 30 % following chronic stress (Bloss et al., 2011; Radley et al., 2008), with the most extensive spine loss occurring in the distal portion of the dendritic arbor. The spines that are most vulnerable to stress are the thin spines, and this selective vulnerability of thin spines has implications for plasticity and cognitive performance, discussed below. While these morphologic effects are quite dramatic, perhaps even more surprising is that the neurons recover in the absence of stress, i.e., with a rest period of three weeks (Bloss et al., 2011; Radley et al., 2005). In young animals, the dendritic arbor fully recovers and spine density partially recovers in the absence of stress (Bloss et al., 2011). It appears that such structural recovery is accompanied by functional recovery, at least in the case of layer 5 neurons in IL. As with layer 3 neurons, chronic stress induced dendritic shrinkage in layer 5 neurons and they recovered with a rest period. However, the recovery occurred primarily in the proximal dendrites, such that the stress/recovery sequence shifted the overall geometry of the neurons to a distal arbor reduced/ proximal arbor expanded configuration (See Figure 2B). However, this shift in geometry did not preclude functional recovery as reflected by D1R-mediated modulation of LTP on layer 5 neurons. The capacity of D1R activation to increase the amplitude of potentiation was decreased by chronic stress, yet fully restored with a post-stress recovery period (Goldwater et al., 2009). It is particularly interesting that such functional recovery occurred against the background of an altered overall dendritic geometry in neurons that have undergone a stress/recovery sequence (Goldwater et al., 2009). The degree to which the altered morphology affects other functional attributes, synaptic connectivity or future capacity for recovery needs to be fully investigated.
Mechanisms for chronic stress effects on PFC structural plasticity
Along with many other brain regions, the amygdala and prefrontal cortex also contain adrenal steroid receptors (Ahima et al., 1991; Ahima and Harlan, 1990) and excitatory amino acids appear to play a role in stress-induced dendritic retraction (Martin and Wellman, 2011). Furthermore, effects of 21d of chronic restraint stress on working memory and dendritic shrinkage and spine loss were prevented by inhibition of protein kinase c (PKC) (Hains et al., 2009).
As to the role of glucocorticoids, three weeks of chronic corticosterone treatment was shown to produce retraction of dendrites in medial prefrontal cortex (Cerqueira et al., 2005; Wellman, 2001), although with subtle differences in the qualitative nature of the effect from what has been described after chronic restraint stress. Other studies confirm a role of adrenal steroids in the mPFC using adrenalectomy and steroid administration. Dexamethasone treatment at a dose that may have been high enough to enter the brain (Meijer et al., 1998), caused a loss of neurons in Layer II of the infralimbic, prelimbic and cingulate cortex, whereas corticosterone treatment reduced the volume, but not the neuron number of these cortical regions (Cerqueira et al., 2005). The dexamethasone treatment was particularly effective in impairing working memory and cognitive flexibility (Cerqueira et al., 2005).
Indeed glucocorticoid actions promote biphasic effects on PFC function by acting via the glutamatergic, GABAergic and noradrenergic systems, in which endocannabinoids (eCB) play an important regulatory role involving interactions between the prefrontal cortex, amygdala and hippocampus. The basolateral amygdala interacts with the medial prefrontal cortex in regulating glucocorticoid effects on working memory impairment (Roozendaal et al., 2004). Yet, endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory (Campolongo et al., 2009; Hill and McEwen, 2009). This works via eCB inhibition of GABA release that disinhibits NA release (Hill and McEwen, 2009). Moreover, glucocorticoid actions in the prefrontal cortex enhance memory consolidation and, at the same time, can impair working memory by a common neural mechanism involving activation of a membrane-bound steroid receptor dependent on noradrenergic activity within the mPFC to increase levels of cAMPdependent protein kinase that may or may not involve eCB signalling (Barsegyan et al., 2010). At the same time, glucocorticoids also interact with the hippocampal eCB system in impairing retrieval of contextual fear memory (Atsak et al., 2012).
The differences between chronic stress and chronic glucocorticoid treatment must be kept in mind. Indeed, in a study in which both a subchronic restraint stress and corticosterone produced mPFC dendritic retraction, stress-induced apical dendritic atrophy resulted in diminished responses to apically targeted excitatory inputs by 5HT and hypocretin, whereas corticosterone played a greater role in stress-induced reductions in EPSCs evoked by 5-HT, as compared with hypocretin, possibly reflecting the different pathways activated by the two transmitters (Liu and Aghajanian, 2008).
This shrinkage has functional consequences in that mPFC-dependent cognitive tasks (i.e., set-shifting) are impaired by stress, and the degree of impairment correlates with the extent of dendritic shrinkage (Liston et al., 2006). Attention set shifting is a task in which a rat first learns that either odor or the digging medium in a pair of bowls predicts where food reward is to be found; then new cues are introduced and the rat needs to learn which ones predict the location of food (Birrell and Brown, 2000b). It has also been demonstrated that chronic stress impairs working memory performance, and the degree of impairment correlates with the extent of spine loss (Hains et al., 2009). There is also a report that chronic restraint stress impairs extinction of a fear conditioning task (Miracle et al., 2006). This is an important lead since the prefrontal cortex is involved in extinction, a type of learning (Santini et al., 2004), but more research is needed to explore the complex relationship between stress, fear conditioning, extinction and possible morphological remodeling that may well accompany each of these experiences.
The prefrontal cortex, amygdala and hippocampus are interconnected and influence each other via direct and indirect neural activity (Akirav and Richter-Levin, 1999; Ghashghaei and Barbas, 2002; McDonald, 1987; McDonald et al., 1996; Petrovich et al., 2001). For example, inactivation of the amygdala blocks stress-induced impairment of hippocampal LTP and spatial memory (Kim et al., 2005) and stimulation of basolateral amygdala enhances dentate gyrus field potentials (Ikegaya et al., 1996), while stimulation of medial prefrontal cortex decreases responsiveness of central amygdala output neurons (Quirk et al., 2003). The processing of emotional memories with contextual information requires amygdala – hippocampal interactions (Phillips and LeDoux, 1992; Richardson et al., 2004), whereas the prefrontal cortex, with its powerful influence on amygdala activity (Quirk et al., 2003) plays an important role in fear extinction (Milad and Quirk, 2002; Morgan and LeDoux, 1995). Because of these interactions, future studies need to address their possible role in the morphological and functional changes produced by single and repeated stress.
Interactions between Aging and Stress in PFC
As reviewed above, pyramidal neurons in mPFC display profound behaviorally induced plasticity (i.e., shrinkage and loss of spines with stress), as well as the capacity to recover from stress (i.e., neuronal resilience). In addition, performance on tasks that require PFC is highly vulnerable to decline with age in humans, non-human primates, and rodents (reviewed in (Gallagher and Rapp, 1997), and recent data from NHPs suggest that age-related decline in cognitive performance reliant on PFC may result from loss of a particular class of axospinous synapses on PFC pyramidal neurons (Dumitriu et al., 2010a). More specifically, the NHP data suggest a model where large, stable synapses remain unaffected by age while thin, highly plastic spines are selectively lost from pyramidal neurons within layer III of mPFC (Dumitriu et al., 2010a).
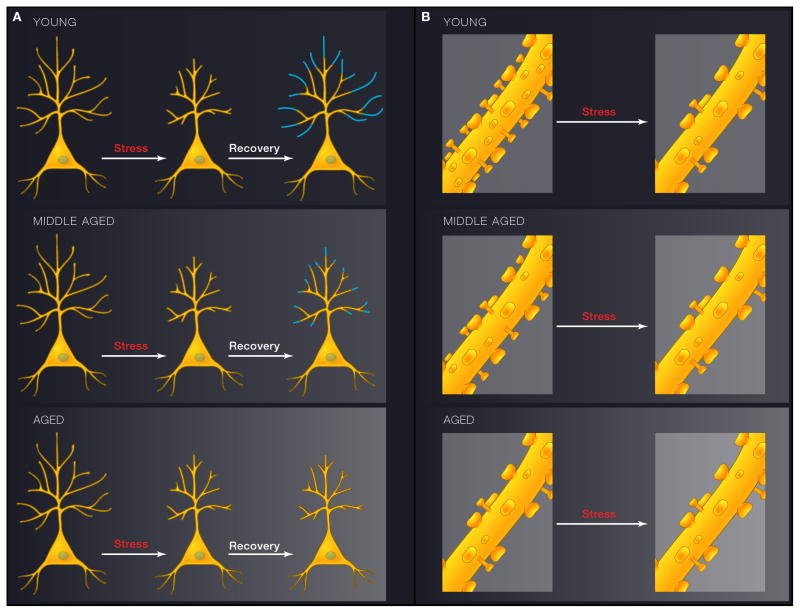
The rat model of chronic stress has proven to be a highly valuable model for the analysis of the potential interactive effects of stress and aging on the vulnerable pyramidal neurons in mPFC. For example, is either the behaviorally induced plasticity, i.e., the response to chronic stress, or the capacity to recover from stress affected by aging? These questions were addressed through exposing young, middle-aged, and aged male rats to stress and recovery followed by detailed morphologic analyses of layer III pyramidal neurons in PL (See Figure 3). As with previous studies, distal apical dendrites shrank with stress and recovered with rest in young rats. However, while the stress-induced shrinkage of apical dendrites also occurred in middle-aged and aged rats, the neurons failed to recover with rest in both groups (Bloss et al., 2010), demonstrating a loss of neuronal resilience that is apparent by middle age (i.e., 12 months old) (See Figure 3A). Spines were also investigated on the same neurons analyzed for dendritic arbor measurements (See Figure 3B). We were particularly interested in whether or not the same spine class(es) were vulnerable to both age and stress. In young animals, as previously reported, stress lead to a loss of spines on distal dendrites, with a partial recovery of spines following rest (Bloss et al., 2011). Spine measurements determined that the spine class most vulnerable to stress was the thin spines(See Figure 3B), the same spine class shown to be vulnerable to aging in PFC of NHPs. However, there was no effect of stress or rest on spine density or size in middle aged or aged animals, i.e., the experience dependent plasticity apparent in young animals was lost with age. Analyses of the control animals provided the insight required to understand the failure of behaviorally-induced plasticity in the middle-aged and aged animals. Middle-Aged and Aged rats lose 30% of their spines in the absence of stress, and this loss is driven primarily by the loss of thin spines, particularly in the aged rats. Taken together, these studies provide evidence that mPFC pyramidal neurons from aged rats suffer losses of plasticity at multiple levels: first, neurons from aging animals lose a certain population of thin spines that may be critical for proper functioning within PFC circuitry; second, the remaining spines are less capable of rewiring in response to experience; and lastly, neuronal dendrites from aging animals lack recovery-related plasticity mechanisms. Importantly, all three of these age-related changes in plasticity were observed in both middle-aged and aged animals, suggesting that preventative measures against such plasticity deficits may be optimally effective when implemented during middle-age. While the “experience” was chronic stress in this case, we suggest that the age-related loss of plasticity reflects a general inability to adapt that would negatively impact cognitive tasks that require a high degree of synaptic flexibility.
Figure 3.
Schematic diagrams depicting the interactive effects between stress and aging on layer 3 pyramidal neurons in the prelimbic area of mPFC. 3A (left) shows the effects on dendritic arbor and 3B (right), shows the effects on spines. In both cases the upper panel represents young male rats, middle panel represents middle-aged rats, and the bottom panel represents aged rats. A) In young animals, chronic stress leads to shrinkage of distal apical dendrites. After cessation of chronic stress, dendritic trees regrow. Such recovery after stress cessation is blunted by middle age and gone in the aged animals. B) Spines are also lost in young animals exposed to chronic stress, and it is primarily the thin spines that are affected. No further spine loss is induced by stress in middle-aged or aged rats, and this is likely due to the fact that age on its own leads to a loss of the thin spine class (See text for details)
Circadian Disruption
Circadian disruption has sometimes been overlooked as a separate yet related phenomenon to sleep deprivation, which alters cognitive function, mood and metabolism (McEwen, 2006). In modern industrialized societies, circadian disruption can be induced in numerous ways, the most common of which are shift work and jet lag. A longitudinal study in a cohort of nurses in night shift work found that exposure to night work can contribute to weight gain and obesity (Niedhammer et al., 1996). Moreover, alternating shift work is an independent risk factor for the development of obesity in a large longitudinal study of male Japanese shift workers (Suwazono et al., 2008). Numerous mouse models have also contributed to our understanding of the relationship between circadian disruption and metabolism, with CLOCK mutant mice showing altered basal metabolism and a tendency towards obesity and metabolic dysregulation, while normal C57Bl/6 mice housed in a disrupted 10hr light:10hr dark cycle show accelerated weight gain and disruptions in metabolic hormones (Karatsoreos et al., 2011; Turek et al., 2005). Behaviorally, circadian disruption can contribute to cognitive impairments. In a study of long recovery vs. short recovery flight crews, it was found that short recovery crews had impaired performance in a psychomotor task, reacting more slowly, and with more errors when compared to a long recovery crew (Cho, 2001). Furthermore, the above-mentioned mouse model of circadian disruption using a 10:10 L:D cycle shows cognitive inflexibility and shrinkage of dendrites in the medial prefrontal cortex (Karatsoreos et al., 2011).
Individual differences and developmental effects
Basal differences in the brain architecture may account for why some individuals are more vulnerable to stress than others. Although trait anxiety behavior varies greatly in human populations, most animal models of anxiety disorders tend to focus on the development of anxiety after a stressful experience. Yet, when viewed in terms of individual differences, naive adult male Sprague-Dawley and Lewis rats both displayed large variations in baseline anxiety-like behavior in the open field, measured by time spent and distance traveled in the center (Miller et al., 2012). In both strains, in spite of the differences in genetic background that exist between them, individuals that fell one standard deviation above (high anxiety) and below (low anxiety) the mean, approximately the top and bottom 15%, had differences in dendritic length and branching in pyramidal neurons from layer II/III of the prelimbic region of the medial prefrontal cortex. In both rat strains, animals in the High Anxiety group had smaller apical dendrites than those in the Low Anxiety group, but there was no difference in basal dendrites (Miller et al., 2012).
As to the possible origin of these individual differences, it is possible that differences in the early life experience of animals in the breeding facility may be involved. Indeed, studies in animal models show that early life experiences can have a powerful influence on brain development and behavior and the role of maternal care in terms of consistency and quantity and maternal self regulation can be considerable (Akers et al., 2008; Meaney and Szyf, 2005; Moriceau and Sullivan, 2006; Parker et al., 2006; Tang et al., 2012). Prenatal stress, as well as postnatal maternal separation stress, are both known to influence prefrontal cortex development and related behavioral responses, particularly after stress in adult life. For example, rats exposed to prenatal stress of immobilization of the mother during the last week of pregnancy, and then exposed to a combined chronic plus acute stress regimen as adults, showed attenuated extinction of cue-conditioned fear (Green et al., 2011). These results are reminiscent of findings that maternal separation from postnatal days 2–12 in rats sensitizes the offspring to show increased anxiety in response to chronic restraint as adults (Eiland and McEwen, 2012). Moreover, fear extinction is known to involve the prefrontal cortex (Quirk et al., 2006), and adolescent rodents and humans show a deficit in fear extinction that is not present before or after the adolescent phase (Pattwell et al., 2012).
The PFC develops at a slower and more prolonged pace than other brain structures, and prenatal stress consisting of exposure of the pregnant dam to an elevated plus maze in bright light increased dendritic branching, length and spine density in the nucleus accumbens and in subregions of the PFC (Muhammad et al., 2012). The prenatal stress experience increased dendritic branching and length in the mPFC in both apical and basilar dendrites; in contrast, a prenatal stress-associated decrease in dendritic branching and length was observed in the basilar branches of neurons of the orbitofrontal cortex. Moreover, maternal separation resulted in an increase in dendritic growth and spine density in the PFC (Muhammad et al., 2012).
Adolescence is a period of remodeling of brain architecture in which hormones play a role along with experience (Sisk and Zehr, 2005). During adolescence, chronic juvenile stress consisting of 6h daily restraint from postnatal day 20 to 41, produced depressive-like behavior and significant neuronal remodeling of brain regions likely involved in these behavioral alterations, namely, the hippocampus, prefrontal cortex and amygdala. Chronically stressed males and females exhibited anhedonia, increased locomotion when exposed to novelty, and altered coping strategies when exposed to acute stress. Coincident with these behavioral changes, there was stress-induced shrinkage of dendrites in the hippocampus and prefrontal cortex and concurrent hypertrophy of dendrites in the amygdala (Eiland et al., 2012).
The human prefrontal cortex undergoes a prolonged course of maturation that continues well after puberty and parallels a slowly emerging ability for flexible social behavior (Casey et al., 2000; Nelson and Guyer, 2011). Interestingly, there are differences within the cerebral cortex in heritability in which primary sensory and motor cortex, which develop earlier show relatively greater genetic effects earlier in childhood, whereas the later developing dorsal prefrontal cortex and temporal lobes show increasingly prominent genetic effects with maturation (Lenroot et al., 2009).
Adolescents have a propensity for risk-taking that is related to the capacity to exert self control, as can be assessed by tests of delayed gratification, such as the “marshmallow test” (Mischel et al., 1972) that, in turn, has had considerable predictive power for social, cognitive and mental health outcomes over the lifecourse (Mischel et al., 2011). The neural basis of self regulation involves frontal-striatal circuitries that integrate motivational and control processes and appear to be stable for a lifetime, based upon studies of the same individuals over 4 decades (Casey et al., 2011). A key feature is an exaggerated ventral striatal representation of appetitive cues in adolescents relative to the ability to exert control, and the connectivity within a ventral fronto-striatal circuit, including the inferior frontal gyrus and dorsal striatum, is particularly important to the ability to exert self regulation (Somerville et al., 2011).
In adolescents, the ventral mPFC undergoes a progressive increase in activation during self-evaluations compared to other evaluations from ages 10 to 13, particularly in the social domain. This neurodevelopmental pattern is consistent with the heightened importance adolescents place on peer relationships and social standing (Pfeifer et al., 2013).
It is also noteworthy that the PFC to amygdala connectivity changes from positive to negative between early childhood and adolescence and young adulthood (Gee et al., 2013). Indeed, young children are wary of strangers as secure attachment to the mother develops, and one index of this sensitive period is that, early in life, ambiguous facial expressions are perceived as conveying negative meaning (Tottenham et al., 2013). Then, during adolescence, there is a restriction on extinction of fear learning, suggesting that negative experiences may have greater impact during that developmental period (Pattwell et al., 2012), although it is not yet known whether fearful events during adolescence may be more difficult to extinguish later in adult life.
Finally, it is important to note that early life adversity in rhesus monkeys and humans impairs development of the prefrontal cortex, among other effects in the brain and body (Anda et al., 2010; Felitti et al., 1998). In rhesus, peer rearing causes changes in 5HT1A receptor density in a number of brain regions including prefrontal cortex (Spinelli et al., 2010) and is associated with an enlarged vermis, dorsomedial prefrontal cortex, and dorsal anterior cingulate cortex without any apparent differences in the corpus callosum and hippocampus (Spinelli et al., 2009). In fact, the size of the social network for group housed monkeys affected prefrontal circuitry, with larger groups leading to increased gray matter size and increased connectivity with the temporal lobe (Sallet et al., 2011). In humans, adverse childhood experiences were associated with smaller volume of the prefrontal cortex, greater activation of the HPA axis, and elevation in inflammation levels compared to non-maltreated children, while adults with a history of childhood maltreatment showed smaller volume of the prefrontal cortex and hippocampus, greater activation of the HPA axis, and elevation in inflammation levels compared to non-maltreated individuals (Danese and McEwen, 2012). Furthermore, cumulative adversity is associated with smaller gray matter volume in medial prefrontal, anterior cingulate and insula (Ansell et al., 2012). Moreover, chaos in the family and living environment is associated with impaired self regulatory behaviors along with elevated blood pressure and signs of obesity in childhood (Evans et al., 2005; Evans and Wachs, 2010) and major life events in early adolescence are linked to impaired self control that reflects, at least in part, impaired prefrontal cortical development (Duckworth et al., 2012).
Moreover, in a study using a Childhood Trauma Questionnaire and MRI imaging of the brain (Edmiston et al., 2011), adverse childhood experiences correlated negatively with gray matter (GM) volume in prefrontal cortex, striatum, amygdala, sensory association cortices, and cerebellum. In particular, physical abuse, physical neglect, and emotional neglect were associated with rostral prefrontal gray matter reductions, and decreases in dorsolateral and orbitofrontal cortices, insula, and ventral striatum were associated with physical abuse, while decreases in cerebellum were associated with physical neglect and decreases in dorsolateral, orbitofrontal, and subgenual prefrontal cortices, striatum, amygdala, hippocampus, and cerebellum were associated with emotional neglect (Edmiston et al., 2011). There were sex differences, in that decreases in the emotional regulation regions, including prefrontal cortex, were associated with childhood trauma in girls, while reductions in caudate GM volume, a brain region related to impulse control were seen in boys (Edmiston et al., 2011).
Sex differences
There are important sex differences both in how early life stressors affect the prefrontal cortex development and in connectivity with other brain regions involved in cognitive function and emotional regulation. Prenatal stress caused sexually dimorphic, opposite changes in synaptic connectivity in response to the same experience, and both male and female offspring demonstrated a loss of neuron number and estimated synapse number in the hippocampus despite exhibiting increased spine density (Mychasiuk et al., 2012). Prenatal stress also led to a sex-specific pattern of dendrite structure that was manifested during adolescence in prenatally stressed males, but not females, which became evident later, in adulthood (Markham et al., 2012). Yet, in studies of chronic juvenile stress (Eiland et al., 2012), the absence of qualitative sex differences in morphological and behavioral responses to chronic stress from postnatal days 20–41 speaks to the important role of the onset of puberty and the role of circulating gonadal hormones in conferring sex differences in response to stressors.
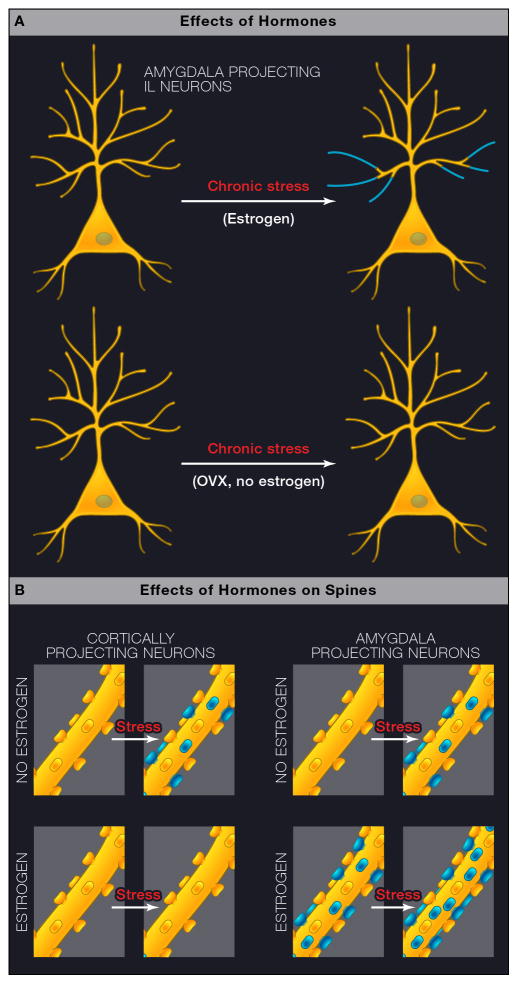
Indeed, in adult life, there are sex differences in the effects of stress on the prefrontal cortex, in that the ability of stress to cause shortening of dendrites is evident in males, but not in females (Garrett and Wellman, 2009). Indeed, in male rats, mPFC neurons that project to the basolateral nucleus of the amygdala (BLA) are resilient to stress-induced dendritic remodeling, whereas those neurons projecting elsewhere showed stress-induced retraction of apical dendrites, as described above (Shansky et al., 2009). In female rats, stress induced remodeling of dendrites in mPFC neurons projecting to the amygdala showed increased length and branching as long as the females were estrogen treated, but not in ovariectomized animals without E treatment (See Figure 4). mPFC neurons projecting elsewhere failed to show any dendritic changes after chronic stress with or without E treatment in females (Shansky et al., 2010). Chronic stress also caused an increase in spine density in all neurons in OVX animals, including a spine density increase in BLA-projecting neurons in E treated OVX females. Estrogen also increased spine density on BLA-projecting neurons in unstressed animals. Given these sex differences in two regions of the brain subserving cognitive functions, one must wonder how many other subtle sex differences exist throughout the brain, since gonadal steroid receptors and actions via genomic and non-genomic mechanisms are widespread (McEwen and Milner, 2007) and sexual differentiation early in life affects many aspects of brain function (Cahill, 2006; McCarthy, 2008).
Figure 4.
Interactive effects of stress and estrogen on neurons within layer 3 of infralimbic cortex. - Schematic depicting a three-way interaction between stress, estrogen, and circuit-specificity in female rats. A) Chronic stress increased dendritic arbor in layer 3 neurons in IL neurons that project to amygdala, whereas this is not the case with the general population of layer 3 pyramidal neurons (not shown). Furthermore, in OVX females, this effect is only seen if the rat receives estrogen treatment. B) Schematic representation of the different effects of estrogen on stress-induced spine formation in the female rat PFC in cortically-projecting and amygdala-projecting neurons. On left, for cortically-projecting neurons, chronic stress induces spine formation in OVX females but fails to do so in OVX females treated with estrogen; on right, for amygdala-projecting neurons, chronic stress induces spine formation in OVX females and estrogen treatment increases spine density in non-stressed animals and promotes further spinogenesis in chronically stressed estrogen-treated OVX females. These dendritic and spine effects differ from those seen in males.
The ability of estrogens to potentiate stress-induced plasticity may help explain the finding that 1h of restraint, as well as a pharmacological stressor, the benzodiazepine inverse agonist, FG7142, impaired working memory only in females in proestrus (high estradiol (E)), while 120 minutes of restraint produced significant impairments in females in estrus (low E) and in males, as well as in females in proestrus (Shansky et al., 2004; Shansky et al., 2006).
Together, these findings demonstrate both independent effects of estrogen on pyramidal cell morphology and effects in which ovarian hormones are interactive with stress, with the BLA-projecting neurons being sensitive to both kinds of effects. Indeed, mPFC neurons show E induction of spines and, based on similar spine-inducing effects of E in the hippocampus, these appear to be mediated by a complex action of estrogens on signaling pathways that lead to actin polymerization among other effects (Dumitriu et al., 2010b; Yuen et al., 2011b). Studies have also shown that post-pubertal female rats are resistant to the stress-induced shrinkage of apical dendrites of hippocampal CA3 neurons (Galea et al., 1997).
Relevance to human vulnerabilities and potential for brain plasticity
Studies of the human brain by functional and structural imaging and neuropsychological testing, along with investigations of autopsy brain tissue, have begun to establish connections between findings in animal models and the human brain in disease, as well as in health, emphasizing the plasticity of neural architecture and the reciprocal connections between brain and body systems, such as the cardiovascular system.
For example, in tissue samples from brains of depressed individuals, frontal cortex and hippocampus showed evidence of glial cell loss and smaller neuron cell body size but not neuronal loss, implying dendritic shrinkage (Rajkowska, 2000; Stockmeier et al., 2004). Indeed, imaging studies on brains of depressed individuals revealed smaller prefrontal volume with structural MRI, while at the same time indicating increased functional activity in the same area (Drevets et al., 1997a).
Yet healthy brains show plasticity and undergo experience-related alterations in prefrontal cortical structure and function. In studies on medical students during the school year, perceived stress scores predicted performance on a cognitive flexibility test, as well as reduced functional connectivity in fMRI imaging during that test; these effects largely disappeared after the students had a summer vacation (Liston et al., 2009). These findings are consistent with a parallel rat model study involving chronic stress, a cognitive flexibility decrement and dendritic shrinkage in the mPFC (Liston et al., 2006). Moreover, regular aerobic exercise in sedentary older adults improves executive function (Kramer et al., 1999) and fMRI signals of increased blood flow in prefrontal and parietal cortex (Colcombe et al., 2004).
Furthermore, the plasticity of the prefrontal cortex has implications for functions in the cardiovascular system and provides a basis for understanding the power of psychosocial factors. For example, there is growing evidence that the perigenual anterior cingulate cortex (pACC) is involved in mediating individual differences in stressor-evoked cardiovascular reactivity, which have long been associated with risk for cardiovascular disease (Krantz and Manuck, 1984; Treiber et al., 2003). For example, greater stressor-evoked pACC activity across individuals has been associated with larger magnitude blood pressure reactions to a variant of a Stroop color-word interference stressor (Gianaros et al., 2007), particularly in interactions with the amygdala (Gianaros et al., 2009). Such a role for the pACC in mediating stressor-evoked cardiovascular reactivity is mediated through its reciprocal circuitry with adjacent areas of the orbital and medial prefrontal cortex, anterior insula, amygdala, and areas in the hypothalamus, periaqueductal gray (PAG), pons, medulla, and the pre-sympathetic intermediolateral (IML) cell column of the spinal cord (Berntson, 2007). As such, the pACC, along with cingulate and prefrontal areas, may provide for an interface between stressor appraisal processes and concurrent dynamic top-down cardiovascular control (Berntson, 2007).
Can behavior change the brain? Besides what we are learning about brain plasticity in relation to stress and exercise, there is a longitudinal study showing that a behavioral intervention in the form of a mindfulness-based stress reduction (MBSR) can change mPFC volume in subjects with chronic fatigue where the intervention resulted in significant improvement in symptoms (de Lange et al., 2008). Similarly, for other brain regions, MBSR has been shown to decrease amygdala volume in subjects who show reduced chronic anxiety (Holzel et al., 2010), and intense learning produces sustained increases in hippocampal volume (Draganski et al., 2006).
Conclusions and future directions
Given the plasticity described above, can experiences that appear to be embedded by early life experience (e.g. adversity) be changed by enhancing plasticity while using a targeted intervention? In addition, can we develop means to retain resilience and plasticity of prefrontal neurons as we age? Along with studies summarized in this article on stress effects on prefrontal cortical plasticity, the pioneering work on reorganization of the adult cerebral cortex (Bezzola et al., 2011; Blake et al., 2006; Jancke, 2009) and pioneering studies of the reversal of developmentally-induced monocular deprivation in visual cortex (Spolidoro et al., 2011; Vetencourt et al., 2008) raises the possibility of interventions that could change brain architecture so as to improve cognitive function and self-regulatory behaviors. Ongoing studies, at the cellular and molecular level, are beginning to reveal mechanisms involving perineuronal nets and excitatory/inhibitory balance and possible intervention strategies (Bavelier et al., 2010). Moreover, the method of optogenetics now allows for studies of connectivity between prefrontal cortex, amygala, hippocampus and the mesolimbic and nigrostriatal systems that can elucidate the functional relationships that are suggested by traditional neuroanatomy. Such studies synergize with advances in imaging functional connectivity of the human and nonhuman primate brain. Thus, the next 5 years should be a period of accelerating understanding of the plasticity and vulnerability of the prefrontal cortex across the life course, and using such knowledge to enhance synaptic properties and circuit characteristics that promote mental and cognitive health.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- Ahima R, Krozowski Z, Harlan R. Type I corticosteroid receptor-like immunoreactivity in the rat CNS: distribution and regulation by corticosteroids. J Comp Neurol. 1991;313:522–538. doi: 10.1002/cne.903130312. [DOI] [PubMed] [Google Scholar]
- Ahima RS, Harlan RE. Charting of type II glucocorticoid receptor-like immunoreactivity in the rat central nervous system. Neuroscience. 1990;39:579–604. doi: 10.1016/0306-4522(90)90244-x. [DOI] [PubMed] [Google Scholar]
- Akers KG, Yang Z, DelVecchio DP, Reeb BC, Romeo RD, McEwen BS, Tang AC. Social competitiveness and plasticity of neuroendocrine function in old age: influence of neonatal novelty exposure and maternal care reliability. PLoS ONE. 2008;3(7):e2840. doi: 10.1371/journal.pone.0002840. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akirav I, Richter-Levin G. Biphasic modulation of hippocampal plasticity by behavioral stress and basolateral amygdala stimulation in the rat. J Neurosci. 1999;19:10530–10535. doi: 10.1523/JNEUROSCI.19-23-10530.1999. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Anda RF, Butchart A, Felitti VJ, Brown DW. Building a framework for global surveillance of the public health implications of adverse childhood experiences. Am J Prev Med. 2010;39:93–98. doi: 10.1016/j.amepre.2010.03.015. [DOI] [PubMed] [Google Scholar]
- Ansell EB, Rando K, Tuit K, Guarnaccia J, Sinha R. Cumulative adversity and smaller gray matter volume in medial prefrontal, anterior cingulate, and insula regions. Biol Psychiatry. 2012;72:57–64. doi: 10.1016/j.biopsych.2011.11.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Ameliorating prefrontal cortical dysfunction in mental illness: inhibition of phosphotidyl inositol-protein kinase C signaling. Psychopharmacology. 2009a;202:445–455. doi: 10.1007/s00213-008-1274-9. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF. Stress signalling pathways that impair prefrontal cortex structure and function. Nat Rev Neurosci. 2009b;10:410–422. doi: 10.1038/nrn2648. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Paspalas CD, Gamo NJ, Yang Y, Wang M. Dynamic Network Connectivity: A new form of neuroplasticity. Trends Cogn Sci. 2010;14:365–375. doi: 10.1016/j.tics.2010.05.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Arnsten AF, Wang MJ, Paspalas CD. Neuromodulation of thought: flexibilities and vulnerabilities in prefrontal cortical network synapses. Neuron. 2012;76:223–239. doi: 10.1016/j.neuron.2012.08.038. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Atsak P, Hauer D, Campolongo P, Schelling G, McGaugh JL, Roozendaal B. Glucocorticoids interact with the hippocampal endocannabinoid system in impairing retrieval of contextual fear memory. Proc Natl Acad Sci U S A. 2012;109:3504–3509. doi: 10.1073/pnas.1200742109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barbas H, Saha S, Rempel-Clower N, Ghashghaei T. Serial pathways from primate prefrontal cortex to autonomic areas may influence emotional expression. BMC Neurosci. 2003;4:25. doi: 10.1186/1471-2202-4-25. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barense MD, Fox MT, Baxter MG. Aged rats are impaired on an attentional set-shifting task sensitive to medial frontal cortex damage in young rats. Learning & memory. 2002;9:191–201. doi: 10.1101/lm.48602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barsegyan A, Mackenzie SM, Kurose BD, McGaugh JL, Roozendaal B. Glucocorticoids in the prefrontal cortex enhance memory consolidation and impair working memory by a common neural mechanism. Proc Natl Acad Sci U S A. 2010;107:16655–16660. doi: 10.1073/pnas.1011975107. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bavelier D, Levi DM, Li RW, Dan Y, Hensch TK. Removing brakes on adult brain plasticity: from molecular to behavioral interventions. J Neurosci. 2010;30:14964–14971. doi: 10.1523/JNEUROSCI.4812-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennett E, Diamond M, Krech D, Rosenzweig M. Chemical and anatomical plasticity of brain. Science. 1964;146:610–619. doi: 10.1126/science.146.3644.610. [DOI] [PubMed] [Google Scholar]
- Berntson GG, Cacioppo JT. Integrative physiology: homeostasis, allostasis and the orchestration of systemic physiology. In: Cacioppo JT, Tassinary LG, Berntson GG, editors. Handbook of Psychophysiology. Cambridge University Press; Cambridge, UK: 2007. [Google Scholar]
- Bezzola L, Merillat S, Gaser C, Jancke L. Training-induced neural plasticity in golf novices. J Neurosci. 2011;31:12444–12448. doi: 10.1523/JNEUROSCI.1996-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000a;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Birrell JM, Brown VJ. Medial frontal cortex mediates perceptual attentional set shifting in the rat. J Neurosci. 2000b;20:4320–4324. doi: 10.1523/JNEUROSCI.20-11-04320.2000. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Blake DT, Heiser MA, Caywood M, Merzenich MM. Experience-dependent adult cortical plasticity requires cognitive association between sensation and reward. Neuron. 2006;52:371–381. doi: 10.1016/j.neuron.2006.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloss EB, Janssen WG, McEwen BS, Morrison JH. Interactive effects of stress and aging on structural plasticity in the prefrontal cortex. J Neurosci. 2010;30:6726–6731. doi: 10.1523/JNEUROSCI.0759-10.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bloss EB, Janssen WG, Ohm DT, Yuk FJ, Wadsworth S, Saardi KM, McEwen BS, Morrison JH. Evidence for reduced experience-dependent dendritic spine plasticity in the aging prefrontal cortex. J Neurosci. 2011;31:7831–7839. doi: 10.1523/JNEUROSCI.0839-11.2011. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cahill L. Why sex matters for neuroscience. Nat Rev Neurosci. 2006;7:477–484. doi: 10.1038/nrn1909. [DOI] [PubMed] [Google Scholar]
- Campolongo P, Roozendaal B, Trezza V, Hauer D, Schelling G, McGaugh JL, Cuomo V. Endocannabinoids in the rat basolateral amygdala enhance memory consolidation and enable glucocorticoid modulation of memory. Proc Natl Acad Sci U S A. 2009;106:4888–4893. doi: 10.1073/pnas.0900835106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Casey BJ, Giedd JN, Thomas KM. Structural and functional brain development and its relation to cognitive development. Biol Psychol. 2000;54:241–257. doi: 10.1016/s0301-0511(00)00058-2. [DOI] [PubMed] [Google Scholar]
- Casey BJ, Somerville LH, Gotlib IH, Ayduk O, Franklin NT, Askren MK, Jonides J, Berman MG, Wilson NL, Teslovich T, et al. Behavioral and neural correlates of delay of gratification 40 years later. Proc Natl Acad Sci U S A. 2011;108:14998–15003. doi: 10.1073/pnas.1108561108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira JJ, Mailliet F, Almeida OFX, Jay TM, Sousa N. The prefrontal cortex as a key target of the maladaptive response to stress. J Neurosci. 2007;27:2781–2787. doi: 10.1523/JNEUROSCI.4372-06.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cerqueira JJ, Pego JM, Taipa R, Bessa JM, Almeida OFX, Sousa N. Morphological correlates of corticosteroid-induced changes in prefrontal cortex-dependent behaviors. J Neurosci. 2005;25:7792–7800. doi: 10.1523/JNEUROSCI.1598-05.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho K. Chronic ‘jet lag’ produces temporal lobe atrophy and spatial cognitive deficits. Nature Neurosci. 2001;4:567–568. doi: 10.1038/88384. [DOI] [PubMed] [Google Scholar]
- Colcombe SJ, Kramer AF, Erickson KI, Scalf P, McAuley E, Cohen NJ, Webb A, Jerome GJ, Marquez DX, Elavsky S. Cardiovascular fitness, cortical plasticity, and aging. Proc Natl Acad Sci USA. 2004;101:3316–3321. doi: 10.1073/pnas.0400266101. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cook SC, Wellman CL. Chronic stress alters dendritic morphology in rat medial prefrontal cortex. J Neurobiol. 2004;60:236–248. doi: 10.1002/neu.20025. [DOI] [PubMed] [Google Scholar]
- Crombag HS, Gorny G, Li Y, Kolb B, Robinson TE. Opposite effects of amphetamine self-administration experience on dendritic spines in the medial and orbital prefrontal cortex. Cerebral Cortex. 2005;15:341–348. doi: 10.1093/cercor/bhh136. [DOI] [PubMed] [Google Scholar]
- Danese A, McEwen BS. Adverse childhood experiences, allostasis, allostatic load, and age-related disease. Physiol Behav. 2012;106:29–39. doi: 10.1016/j.physbeh.2011.08.019. [DOI] [PubMed] [Google Scholar]
- de Lange FP, Koers A, Kalkman JS, Bleijenberg G, Hagoort P, van der Meer JWM, Toni I. Increase in prefrontal cortical volume following cognitive behavioural therapy in patients with chronic fatigue syndrome. Brain. 2008;131:2172–2180. doi: 10.1093/brain/awn140. [DOI] [PubMed] [Google Scholar]
- Diorio D, Viau V, Meaney MJ. The Role of the Medial Prefrontal Cortex (Cingulate Gyrus) in the Regulation of Hypothalamic-Pituitary-Adrenal Responses to Stress. The Journal of Neuroscience. 1993;13:3839–3847. doi: 10.1523/JNEUROSCI.13-09-03839.1993. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Draganski B, Gaser C, Kempermann G, Kuhn HG, Winkler J, Buchel C, May A. Temporal and spatial dynamics of brain structure changes during extensive learning. J Neurosci. 2006;26:6314–6317. doi: 10.1523/JNEUROSCI.4628-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997a;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Drevets WC, Price JL, Simpson JR, Jr, Todd RD, Reich T, Vannier M, Raichle ME. Subgenual prefrontal cortex abnormalities in mood disorders. Nature. 1997b;386:824–827. doi: 10.1038/386824a0. [DOI] [PubMed] [Google Scholar]
- Duckworth AL, Kim B, Tsukayama E. Life stress impairs self-control in early adolescence. Frontiers in psychology. 2012;3:608. doi: 10.3389/fpsyg.2012.00608. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu D, Hao J, Hara Y, Kaufmann J, Janssen WG, Lou W, Rapp PR, Morrison JH. Selective changes in thin spine density and morphology in monkey prefrontal cortex correlate with aging-related cognitive impairment. J Neurosci. 2010a;30:7507–7515. doi: 10.1523/JNEUROSCI.6410-09.2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dumitriu D, Rapp PR, McEwen BS, Morrison JH. Estrogen and the aging brain: an elixir for the weary cortical network. Ann N Y Acad Sci. 2010b;1204:104–112. doi: 10.1111/j.1749-6632.2010.05529.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edmiston EE, Wang F, Mazure CM, Guiney J, Sinha R, Mayes LC, Blumberg HP. Corticostriatal-limbic gray matter morphology in adolescents with self-reported exposure to childhood maltreatment. Arch Pediatr Adolesc Med. 2011;165:1069–1077. doi: 10.1001/archpediatrics.2011.565. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eiland L, McEwen BS. Early life stress followed by subsequent adult chronic stress potentiates anxiety and blunts hippocampal structural remodeling. Hippocampus. 2012;22:82–91. doi: 10.1002/hipo.20862. [DOI] [PubMed] [Google Scholar]
- Eiland L, Ramroop J, Hill MN, Manley J, McEwen BS. Chronic juvenile stress produces corticolimbic dendritic architectural remodeling and modulates emotional behavior in male and female rats. Psychoneuroendocrinology. 2012;37:39–47. doi: 10.1016/j.psyneuen.2011.04.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Evans GW, Gonnella C, Marcynyszyn LA, Gentile L, Salpekar N. The role of chaos in poverty and children’s socioemotional adjustment. Psychological science. 2005;16:560–565. doi: 10.1111/j.0956-7976.2005.01575.x. [DOI] [PubMed] [Google Scholar]
- Evans GW, Wachs TD. Chaos and its influence on children’s development: an ecological perspective. 1. Washington, DC: American Psychological Association; 2010. [Google Scholar]
- Felitti VJ, Anda RF, Nordenberg D, Williamson DF, Spitz AM, Edwards V, Koss MP, Marks JS. Relationship of childhood abuse and household dysfunction to many of the leading causes of death in adults. The adverse childhood experiences (ACE) study. AmJPrevMed. 1998;14:245–258. doi: 10.1016/s0749-3797(98)00017-8. [DOI] [PubMed] [Google Scholar]
- Funahashi S, Bruce CJ, Goldman-Rakic PS. Mnemonic coding of visual space in the monkey’s dorsolateral prefrontal cortex. Journal of neurophysiology. 1989;61:331–349. doi: 10.1152/jn.1989.61.2.331. [DOI] [PubMed] [Google Scholar]
- Fuster JM. The Prefrontal Cortex. 4. London: Academic Press; 2008. [Google Scholar]
- Galea LAM, McEwen BS, Tanapat P, Deak T, Spencer RL, Dhabhar FS. Sex differences in dendritic atrophy of CA3 pyramidal neurons in response to chronic restraint stress. Neuroscience. 1997;81:689–697. doi: 10.1016/s0306-4522(97)00233-9. [DOI] [PubMed] [Google Scholar]
- Gallagher M, Rapp PR. The use of animal models to study the effects of aging on cognition. Annu Rev Psychol. 1997;48:339–370. doi: 10.1146/annurev.psych.48.1.339. [DOI] [PubMed] [Google Scholar]
- Gamo NJ, Arnsten AF. Molecular modulation of prefrontal cortex: rational development of treatments for psychiatric disorders. Behav Neurosci. 2011;125:282–296. doi: 10.1037/a0023165. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garcia-Tardon N, Gonzalez-Gonzalez IM, Martinez-Villarreal J, Fernandez-Sanchez E, Gimenez C, Zafra F. Protein kinase C (PKC)-promoted endocytosis of glutamate transporter GLT-1 requires ubiquitin ligase Nedd4–2-dependent ubiquitination but not phosphorylation. J Biol Chem. 2012;287:19177–19187. doi: 10.1074/jbc.M112.355909. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Garrett JE, Wellman CL. Chronic stress effects on dendritic morphology in medial prefrontal cortex: sex differences and estrogen dependence. Neuroscience. 2009;162:195–207. doi: 10.1016/j.neuroscience.2009.04.057. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gazzaley A, Nobre AC. Top-down modulation: bridging selective attention and working memory. Trends Cogn Sci. 2012;16:129–135. doi: 10.1016/j.tics.2011.11.014. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gee DG, Humphreys KL, Flannery J, Goff B, Telzer EH, Shapiro M, Hare TA, Bookheimer SY, Tottenham N. A developmental shift from positive to negative connectivity in human amygdala-prefrontal circuitry. J Neurosci. 2013;33:4584–4593. doi: 10.1523/JNEUROSCI.3446-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ghashghaei HT, Barbas H. Pathways for emotion: Interactions of prefrontal and anterior temporal pathways in the amygdala of the Rhesus monkey. Neuroscience. 2002;115:1261–1279. doi: 10.1016/s0306-4522(02)00446-3. [DOI] [PubMed] [Google Scholar]
- Gianaros PJ, Hariri AR, Sheu LK, Muldoon MF, Sutton-Tyrrell K, Manuck SB. Preclinical atherosclerosis covaries with individual differences in reactivity and functional connectivity of the amygdala. Biol Psychiatry. 2009;65:943–950. doi: 10.1016/j.biopsych.2008.10.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gianaros PJ, Jennings JR, Sheu LK, Derbyshire SWG, Matthews KA. Heightened functional neural activation to psychological stress covaries with exaggerated blood pressure. Hypertension. 2007;49:134–140. doi: 10.1161/01.HYP.0000250984.14992.64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Topography of cognition: parallel distributed networks in primate association cortex. Annu Rev Neurosci. 1988;11:137–156. doi: 10.1146/annurev.ne.11.030188.001033. [DOI] [PubMed] [Google Scholar]
- Goldman-Rakic PS. Cellular basis of working memory. Neuron. 1995;14:477–485. doi: 10.1016/0896-6273(95)90304-6. [DOI] [PubMed] [Google Scholar]
- Goldwater DS, Pavlides C, Hunter RG, Bloss EB, Hof PR, McEwen BS, Morrison JH. Structural and functional alterations to rat medial prefrontal cortex following chronic restraint stress and recovery. Neuroscience. 2009;164:798–808. doi: 10.1016/j.neuroscience.2009.08.053. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Green MK, Rani CS, Joshi A, Soto-Pina AE, Martinez PA, Frazer A, Strong R, Morilak DA. Prenatal stress induces long term stress vulnerability, compromising stress response systems in the brain and impairing extinction of conditioned fear after adult stress. Neuroscience. 2011;192:438–451. doi: 10.1016/j.neuroscience.2011.06.041. [DOI] [PubMed] [Google Scholar]
- Hains AB, Vu MA, Maciejewski PK, van Dyck CH, Gottron M, Arnsten AF. Inhibition of protein kinase C signaling protects prefrontal cortex dendritic spines and cognition from the effects of chronic stress. Proceedings of the National Academy of Sciences of the United States of America. 2009;106:17957–17962. doi: 10.1073/pnas.0908563106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Janssen WG, Lou W, Lasley BL, Hof PR, Morrison JH. Interactive effects of age and estrogen on cognition and pyramidal neurons in monkey prefrontal cortex. Proc Natl Acad Sci U S A. 2007;104:11465–11470. doi: 10.1073/pnas.0704757104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hao J, Rapp PR, Leffler AE, Leffler SR, Janssen WG, Lou W, McKay H, Roberts JA, Wearne SL, Hof PR, et al. Estrogen alters spine number and morphology in prefrontal cortex of aged female rhesus monkeys. J Neurosci. 2006;26:2571–2578. doi: 10.1523/JNEUROSCI.3440-05.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hill MN, McEwen BS. Endocannabinoids: The silent partner of glucocorticoids in the synapse. Proc Natl Acad Sci U S A. 2009;106:4579–4580. doi: 10.1073/pnas.0901519106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hof PR, Morrison JH. The aging brain: morphomolecular senescence of cortical circuits. Trends Neurosci. 2004;27:607–613. doi: 10.1016/j.tins.2004.07.013. [DOI] [PubMed] [Google Scholar]
- Holmes A, Wellman CL. Stress-induced prefrontal reorganization and executive dysfunction in rodents. Neurosci & Biobehav Rev. 2009;33:773–783. doi: 10.1016/j.neubiorev.2008.11.005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holzel BK, Carmody J, Evans KC, Hoge EA, Dusek JA, Morgan L, Pitman RK, Lazar SW. Stress reduction correlates with structural changes in the amygdala. Soc Cogn Affect Neurosci. 2010;5:11–17. doi: 10.1093/scan/nsp034. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikegaya Y, Saito H, Abe K. Dentate gyrus field potentials evoked by stimulation of the basolateral amygdaloid nucleus in anesthetized rats. Brain Res. 1996;718:53–60. doi: 10.1016/0006-8993(95)01465-9. [DOI] [PubMed] [Google Scholar]
- Jancke L. Music drives brain plasticity. F1000 biology reports. 2009;1:78. doi: 10.3410/B1-78. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karatsoreos IN, Bhagat S, Bloss EB, Morrison JH, McEwen BS. Disruption of circadian clocks has ramifications for metabolism, brain, and behavior. Proc Natl Acad Sci U S A. 2011;108:1657–1662. doi: 10.1073/pnas.1018375108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kesner RP, Churchwell JC. An analysis of rat prefrontal cortex in mediating executive function. Neurobiol Learn Mem. 2011;96:417–431. doi: 10.1016/j.nlm.2011.07.002. [DOI] [PubMed] [Google Scholar]
- Kim JJ, Koo JW, Lee HJ, Han JS. Amygdalar inactivation blocks stress-induced impairments in hippocampal long-term potentiation and spatial memory. J Neurosci. 2005;25:1532–1539. doi: 10.1523/JNEUROSCI.4623-04.2005. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kramer AF, Hahn S, Cohen NJ, Banich MT, McAuley E, Harrison CR, Chason J, Vakil E, Bardell L, Boileau RA, et al. Ageing, fitness and neurocognitive function. Nature. 1999;400:418–419. doi: 10.1038/22682. [DOI] [PubMed] [Google Scholar]
- Krantz DS, Manuck SB. Acute psychophysiologic reactivity and risk of cardiovascular disease: a review and methodologic critique. Psychological bulletin. 1984;96:435–464. [PubMed] [Google Scholar]
- Lenroot RK, Schmitt JE, Ordaz SJ, Wallace GL, Neale MC, Lerch JP, Kendler KS, Evans AC, Giedd JN. Differences in genetic and environmental influences on the human cerebral cortex associated with development during childhood and adolescence. Human brain mapping. 2009;30:163–174. doi: 10.1002/hbm.20494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, McEwen BS, Casey BJ. Psychosocial stress reversibly disrupts prefrontal processing and attentional control. Proc Natl Acad Sci USA. 2009;106:912–917. doi: 10.1073/pnas.0807041106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liston C, Miller MM, Goldwater DS, Radley JJ, Rocher AB, Hof PR, Morrison JH, McEwen BS. Stress-induced alterations in prefrontal cortical dendritic morphology predict selective impairments in perceptual attentional set-shifting. J Neurosci. 2006;26:7870–7874. doi: 10.1523/JNEUROSCI.1184-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu RJ, Aghajanian GK. Stress blunts serotonin- and hypocretin-evoked EPSCs in prefrontal cortex: Role of corticosterone-mediated apical dendritic atrophy. Proc Natl Acad Sci USA. 2008;105:359–364. doi: 10.1073/pnas.0706679105. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Magarinos AM, McEwen BS, Saboureau M, Pevet P. Rapid and reversible changes in intrahippocampal connectivity during the course of hibernation in European hamsters. Proc Natl Acad Sci USA. 2006;103:18775–18780. doi: 10.1073/pnas.0608785103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Markham JA, Mullins SE, Koenig JI. Peri-adolescent maturation of the prefrontal cortex is sex-specific and disrupted by prenatal stress. J Comp Neurol. 2012 doi: 10.1002/cne.23262. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Martin KP, Wellman CL. NMDA Receptor Blockade Alters Stress-Induced Dendritic Remodeling in Medial Prefrontal Cortex. Cereb Cortex. 2011 doi: 10.1093/cercor/bhr021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McCarthy MM. Estradiol and the developing brain. Physiol Rev. 2008;88:91–124. doi: 10.1152/physrev.00010.2007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McDonald AJ. Organization of amygdaloid projections to the mediodorsal thalamus and prefrontal cortex: a fluorescence retrograde transport study in the rat. J Comp Neurol. 1987;262:46–58. doi: 10.1002/cne.902620105. [DOI] [PubMed] [Google Scholar]
- McDonald AJ, Mascagni F, Guo L. Projections of the medial and lateral prefrontal cortices to the amygdala: a Phaseolus vulgaris leucoagglutinin study in the rat. Neuroscience. 1996;71:55–75. doi: 10.1016/0306-4522(95)00417-3. [DOI] [PubMed] [Google Scholar]
- McEwen BS. Sleep deprivation as a neurobiologic and physiologic stressor: allostasis and allostatic load. Metabolism. 2006;55:S20–S23. doi: 10.1016/j.metabol.2006.07.008. [DOI] [PubMed] [Google Scholar]
- McEwen BS, Gianaros PJ. Stress- and allostasis-induced brain plasticity. Annu Rev Med. 2011;62:431–445. doi: 10.1146/annurev-med-052209-100430. [DOI] [PMC free article] [PubMed] [Google Scholar]
- McEwen BS, Milner TA. Hippocampal formation: Shedding light on the influence of sex and stress on the brain. Brain Res Rev. 2007;55:343–355. doi: 10.1016/j.brainresrev.2007.02.006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meaney MJ, Szyf M. Environmental programming of stress responses through DNA methylation: life at the interface between a dynamic environment and a fixed genome. Dialogues Clin Neurosci. 2005;7:103–123. doi: 10.31887/DCNS.2005.7.2/mmeaney. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer OC, de Lange ECM, Breimer DD, de Boer AG, Workel JO, De Kloet ER. Penetration of dexamethasone into brain glucocorticoid targets is enhanced in mdr1A P-Glycoprotein knockout mice. Endocrinology. 1998;139:1789–1793. doi: 10.1210/endo.139.4.5917. [DOI] [PubMed] [Google Scholar]
- Milad MR, Quirk GJ. Neurons in medial prefrontal cortex signal memory for fear extinction. Nature. 2002;420:70–74. doi: 10.1038/nature01138. [DOI] [PubMed] [Google Scholar]
- Miller EK. The prefrontal cortex and cognitive control. Nat Rev Neurosci. 2000;1:59–65. doi: 10.1038/35036228. [DOI] [PubMed] [Google Scholar]
- Miller MM, Morrison JH, McEwen BS. Basal anxiety-like behavior predicts differences in dendritic morphology in the medial prefrontal cortex in two strains of rats. Behav Brain Res. 2012;229:280–288. doi: 10.1016/j.bbr.2012.01.029. [DOI] [PubMed] [Google Scholar]
- Miracle AD, Brace MF, Huyck KD, Singler SA, Wellman CL. Chronic stress impairs recall of extinction of conditioned fear. Neurobiol Learning & Memory. 2006;85:213–218. doi: 10.1016/j.nlm.2005.10.005. [DOI] [PubMed] [Google Scholar]
- Mischel W, Ayduk O, Berman MG, Casey BJ, Gotlib IH, Jonides J, Kross E, Teslovich T, Wilson NL, Zayas V, et al. ‘Willpower’ over the life span: decomposing self-regulation. Soc Cogn Affect Neurosci. 2011;6:252–256. doi: 10.1093/scan/nsq081. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mischel W, Ebbesen EB, Zeiss AR. Cognitive and attentional mechanisms in delay of gratification. J Pers Soc Psychol. 1972;21:204–218. doi: 10.1037/h0032198. [DOI] [PubMed] [Google Scholar]
- Morgan MA, LeDoux JE. Differential contribution of dorsal and ventral medial prefrontal cortex to the acquisition and extinction of conditioned fear in rats. Behav Neurosci. 1995;109:681–688. doi: 10.1037//0735-7044.109.4.681. [DOI] [PubMed] [Google Scholar]
- Moriceau S, Sullivan R. Maternal presence serves as a switch between learning fear and attraction in infancy. Nature Neurosci. 2006;8:1004–1006. doi: 10.1038/nn1733. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Baxter MG. The ageing cortical synapse: hallmarks and implications for cognitive decline. Nature reviews Neuroscience. 2012;13:240–250. doi: 10.1038/nrn3200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morrison JH, Hof PR. Life and death of neurons in the aging brain. Science. 1997;278:412–419. doi: 10.1126/science.278.5337.412. [DOI] [PubMed] [Google Scholar]
- Muhammad A, Carroll C, Kolb B. Stress during development alters dendritic morphology in the nucleus accumbens and prefrontal cortex. Neuroscience. 2012;216:103–109. doi: 10.1016/j.neuroscience.2012.04.041. [DOI] [PubMed] [Google Scholar]
- Mychasiuk R, Gibb R, Kolb B. Prenatal stress alters dendritic morphology and synaptic connectivity in the prefrontal cortex and hippocampus of developing offspring. Synapse. 2012;66:308–314. doi: 10.1002/syn.21512. [DOI] [PubMed] [Google Scholar]
- Nelson AJ, Iwamoto GA. Reversibility of exercise-induced dendritic attenuation in brain cardiorespiratory and locomotor areas following exercise detraining. J Appl Physiol. 2006;101:1243–1251. doi: 10.1152/japplphysiol.00483.2006. [DOI] [PubMed] [Google Scholar]
- Nelson AJ, Juraska JM, Musch TI, Iwamoto GA. Neuroplastic adaptations to exercise: neuronal remodeling in cardiorespiratory and locomotor areas. J Appl Physiol. 2005;99:2312–2322. doi: 10.1152/japplphysiol.00693.2005. [DOI] [PubMed] [Google Scholar]
- Nelson EE, Guyer AE. The development of the ventral prefrontal cortex and social flexibility. Developmental cognitive neuroscience. 2011;1:233–245. doi: 10.1016/j.dcn.2011.01.002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Niedhammer I, Lert F, Marne MJ. Prevalence of overweight and weight gain in relation to night work in a nurses’ cohort. Int J Obes Relat Metab Disord. 1996;20:625–633. [PubMed] [Google Scholar]
- Parker KJ, Buckmaster CL, Sundlass K, Schatzberg AF, Lyons DM. Maternal mediation, stress inoculation, and the development of neuroendocrine stress resistance in primates. Proc Natl Acad Sci USA. 2006;103:3000–3005. doi: 10.1073/pnas.0506571103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattwell SS, Duhoux S, Hartley CA, Johnson DC, Jing D, Elliott MD, Ruberry EJ, Powers A, Mehta N, Yang RR, et al. Altered fear learning across development in both mouse and human. Proc Natl Acad Sci U S A. 2012;109:16318–16323. doi: 10.1073/pnas.1206834109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Petrovich GD, Canteras NS, Swanson LW. Combinatorial amygdalar inputs to hippocampal domains and hypothalamic behavior systems. Brain Res Rev. 2001;38:247–289. doi: 10.1016/s0165-0173(01)00080-7. [DOI] [PubMed] [Google Scholar]
- Pfeifer JH, Kahn LE, Merchant JS, Peake SJ, Veroude K, Masten CL, Lieberman MD, Mazziotta JC, Dapretto M. Longitudinal Change in the Neural Bases of Adolescent Social Self-Evaluations: Effects of Age and Pubertal Development. J Neurosci. 2013;33:7415–7419. doi: 10.1523/JNEUROSCI.4074-12.2013. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Phillips RG, LeDoux JE. Differential contribution of amygdala and hippocampus to cued and contextual fear conditioning. BehavNeurosci. 1992;106:274–285. doi: 10.1037//0735-7044.106.2.274. [DOI] [PubMed] [Google Scholar]
- Popov VI, Bocharova LS. Hibernation-induced structural changes in synaptic contacts between mossy fibres and hippocampal pyramidal neurons. Neuroscience. 1992;48:53–62. doi: 10.1016/0306-4522(92)90337-2. [DOI] [PubMed] [Google Scholar]
- Preuss T. Do Rats Have Prefrontal Cortex? J Cogn Neurosci. 1995;7:1–24. doi: 10.1162/jocn.1995.7.1.1. [DOI] [PubMed] [Google Scholar]
- Qin S, Hermans EJ, van Marle HJ, Luo J, Fernandez G. Acute psychological stress reduces working memory-related activity in the dorsolateral prefrontal cortex. Biol Psychiatry. 2009;66:25–32. doi: 10.1016/j.biopsych.2009.03.006. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Garcia R, Gonzalez-Lima F. Prefrontal mechanisms in extinction of conditioned fear. Biol Psychiat. 2006;60:337–343. doi: 10.1016/j.biopsych.2006.03.010. [DOI] [PubMed] [Google Scholar]
- Quirk GJ, Likhtik E, Pelletier JG, Pare D. Stimulation of medial prefrontal cortex decreases the responsiveness of central amygdala output neurons. J Neurosci. 2003;23:8800–8807. doi: 10.1523/JNEUROSCI.23-25-08800.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Arias CM, Sawchenko PE. Regional differentiation of the medial prefrontal cortex in regulating adaptive responses to acute emotional stress. J Neurosci. 2006;26:12967–12976. doi: 10.1523/JNEUROSCI.4297-06.2006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Janssen WGM, Hof PR, McEwen BS, Morrison JH. Reversibility of apical dendritic retraction in the rat medial prefrontal cortex following repeated stress. Exper Neurol. 2005;196:199–203. doi: 10.1016/j.expneurol.2005.07.008. [DOI] [PubMed] [Google Scholar]
- Radley JJ, Rocher AB, Rodriguez A, Ehlenberger DB, Dammann M, McEwen BS, Morrison JH, Wearne SL, Hof PR. Repeated stress alters dendritic spine morphology in the rat medial prefrontal cortex. J Comp Neurol. 2008;507:1141–1150. doi: 10.1002/cne.21588. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Radley JJ, Sisti HM, Hao J, Rocher AB, McCall T, Hof PR, McEwen BS, Morrison JH. Chronic behavioral stress induces apical dendritic reorganization in pyramidal neurons of the medial prefrontal cortex. Neuroscience. 2004;125:1–6. doi: 10.1016/j.neuroscience.2004.01.006. [DOI] [PubMed] [Google Scholar]
- Rajkowska G. Postmortem studies in mood disorders indicate altered numbers of neurons and glial cells. Biol Psychiat. 2000;48:766–777. doi: 10.1016/s0006-3223(00)00950-1. [DOI] [PubMed] [Google Scholar]
- Rapp PR, Morrison JH, Roberts JA. Cyclic estrogen replacement improves cognitive function in aged ovariectomized rhesus monkeys. J Neurosci. 2003;23:5708–5714. doi: 10.1523/JNEUROSCI.23-13-05708.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richardson MP, Strange BA, Dolan RJ. Encoding of emotional memories depends on amygdala and hippocampus and their interactions. Nature Neurosci. 2004;7:278–285. doi: 10.1038/nn1190. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Mitton E, Kolb B. Cocaine self-administration alters the morphology of dendrites and dendritic spines in the nucleus accumbens and neocortex. Synapse. 2001;39:257–266. doi: 10.1002/1098-2396(20010301)39:3<257::AID-SYN1007>3.0.CO;2-1. [DOI] [PubMed] [Google Scholar]
- Robinson TE, Gorny G, Savage VR, Kolb B. Widespread but regionally specific effects of experimenter- versus self-administered morphine on dendritic spines in the nucleus accumbens, hippocampus, and neocortex of adult rats. Synapse. 2002;46:271–279. doi: 10.1002/syn.10146. [DOI] [PubMed] [Google Scholar]
- Roitman MF, Na E, Anderson G, Jones TA, Bernstein IL. Induction of a salt appetite alters dendritic morphology in nucleus accumbens and sensitizes rats to amphetamine. J Neurosci. 2002;22:RC225. doi: 10.1523/JNEUROSCI.22-11-j0001.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Roozendaal B, McReynolds JR, McGaugh JL. The basolateral amygdala interacts with the medial prefrontal cortex in regulating glucocorticoid effects on working memory impairment. J Neurosci. 2004;24:1385–1392. doi: 10.1523/JNEUROSCI.4664-03.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sallet J, Mars RB, Noonan MP, Andersson JL, O’Reilly JX, Jbabdi S, Croxson PL, Jenkinson M, Miller KL, Rushworth MF. Social network size affects neural circuits in macaques. Science. 2011;334:697–700. doi: 10.1126/science.1210027. [DOI] [PubMed] [Google Scholar]
- Santini E, Ge H, Ren K, Pena de Ortiz S, Quirk GJ. Consolidation of fear extinction requires protein synthesis in the medial prefrontal cortex. J Neurosci. 2004;24:5704–5710. doi: 10.1523/JNEUROSCI.0786-04.2004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sesack SR, Deutch AY, Roth RH, Bunney BS. Topographical organization of the efferent projections of the medial prefrontal cortex in the rat: an anterograde tract-tracing study with Phaseolus vulgaris leucoagglutinin. The Journal of comparative neurology. 1989;290:213–242. doi: 10.1002/cne.902900205. [DOI] [PubMed] [Google Scholar]
- Shanmugan S, Epperson CN. Estrogen and the prefrontal cortex: Towards a new understanding of estrogen’s effects on executive functions in the menopause transition. Human brain mapping. 2012 doi: 10.1002/hbm.22218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Glavis-Bloom C, Lerman D, McRae P, Benson C, Miller K, Cosand L, Horvath TL, Arnsten AFT. Estrogen mediates sex differences in stress-induced prefrontal cortex dysfunction. Mol Psychiat. 2004;9:531–538. doi: 10.1038/sj.mp.4001435. [DOI] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, Lou W, McEwen BS, Morrison JH. Estrogen promotes stress sensitivity in a prefrontal cortex-amygdala pathway. Cereb Cortex. 2010;20:2560–2567. doi: 10.1093/cercor/bhq003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Hamo C, Hof PR, McEwen BS, Morrison JH. Stress-induced dendritic remodeling in the prefrontal cortex is circuit specific. Cerebral Cortex. 2009 doi: 10.1093/cercor/bhp003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shansky RM, Rubinow K, Brennan A, Arnsten AF. The effects of sex and hormonal status on restraint-stress-induced working memory impairment. Behav Brain Funct. 2006;2:8. doi: 10.1186/1744-9081-2-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sisk CL, Zehr JL. Pubertal hormones organize the adolescent brain and behavior. Front Neuroendocrin. 2005;26:163–174. doi: 10.1016/j.yfrne.2005.10.003. [DOI] [PubMed] [Google Scholar]
- Somerville LH, Hare T, Casey BJ. Frontostriatal maturation predicts cognitive control failure to appetitive cues in adolescents. Journal of cognitive neuroscience. 2011;23:2123–2134. doi: 10.1162/jocn.2010.21572. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli S, Chefer S, Carson RE, Jagoda E, Lang L, Heilig M, Barr CS, Suomi SJ, Higley JD, Stein EA. Effects of early-life stress on serotonin(1A) receptors in juvenile Rhesus monkeys measured by positron emission tomography. Biol Psychiatry. 2010;67:1146–1153. doi: 10.1016/j.biopsych.2009.12.030. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spinelli S, Chefer S, Suomi SJ, Higley JD, Barr CS, Stein E. Early-life stress induces long-term morphologic changes in primate brain. Arch Gen Psychiatry. 2009;66:658–665. doi: 10.1001/archgenpsychiatry.2009.52. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Spolidoro M, Baroncelli L, Putignano E, Maya-Vetencourt JF, Viegi A, Maffei L. Food restriction enhances visual cortex plasticity in adulthood. Nature communications. 2011;2:320. doi: 10.1038/ncomms1323. [DOI] [PubMed] [Google Scholar]
- Stockmeier CA, Mahajan GJ, Konick LC, Overholser JC, Jurjus GJ, Meltzer HY, Uylings HBM, Friedman L, Rajkowska G. Cellular changes in the postmortem hippocampus in major depression. Biol Psychiat. 2004;56:640–650. doi: 10.1016/j.biopsych.2004.08.022. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Suwazono Y, Dochi M, Sakata K, Okubo Y, Oishi M, Tanaka K, Kobayashi E, Kido T, Nogawa K. A longitudinal study on the effect of shift work on weight gain in male Japanese workers. Obesity (Silver Spring) 2008;16:1887–1893. doi: 10.1038/oby.2008.298. [DOI] [PubMed] [Google Scholar]
- Tan HY, Callicott JH, Weinberger DR. Dysfunctional and compensatory prefrontal cortical systems, genes and the pathogenesis of schizophrenia. Cereb Cortex. 2007;17(Suppl 1):i171–181. doi: 10.1093/cercor/bhm069. [DOI] [PubMed] [Google Scholar]
- Tang AC, Yang Z, Reeb-Sutherland BC, Romeo RD, McEwen BS. Maternal modulation of novelty effects on physical development. Proc Natl Acad Sci U S A. 2012;109:2120–2125. doi: 10.1073/pnas.1121056109. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thayer JF, Brosschot JF. Psychosomatics and psychopathology: looking up and down from the brain. Psychoneuroendocrinology. 2005;30:1050–1058. doi: 10.1016/j.psyneuen.2005.04.014. [DOI] [PubMed] [Google Scholar]
- Thayer JF, Sternberg E. Beyond heart rate variability: Vagal regulation of allostatic systems. Ann NY Acad Sci. 2006;1088:361–372. doi: 10.1196/annals.1366.014. [DOI] [PubMed] [Google Scholar]
- Tottenham N, Phuong J, Flannery J, Gabard-Durnam L, Goff B. A negativity bias for ambiguous facial-expression valence during childhood: Converging evidence from behavior and facial corrugator muscle responses. Emotion. 2013;13:92–103. doi: 10.1037/a0029431. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Treiber FA, Kamarck T, Schneiderman N, Sheffield D, Kapuku G, Taylor T. Cardiovascular reactivity and development of preclinical and clinical disease states. Psychosom Med. 2003;65:46–62. doi: 10.1097/00006842-200301000-00007. [DOI] [PubMed] [Google Scholar]
- Turek FW, Joshu C, Kohsaka A, Lin E, Ivanova G, McDearmon E, Laposky A, Losee-Olson S, Easton A, Jensen DR, et al. Obesity and metabolic syndrome in circadian clock mutant mice. Science. 2005;308:1043–1045. doi: 10.1126/science.1108750. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vetencourt JFM, Sale A, Viegi A, Baroncelli L, De Pasquale R, O’Leary OF, Castren E, Maffei L. The antidepressant fluoxetine restores plasticity in the adult visual cortex. Science. 2008;320:385–388. doi: 10.1126/science.1150516. [DOI] [PubMed] [Google Scholar]
- Vyas A, Mitra R, Rao BSS, Chattarji S. Chronic stress induces contrasting patterns of dendritic remodeling in hippocampal and amygdaloid neurons. J Neurosci. 2002;22:6810–6818. doi: 10.1523/JNEUROSCI.22-15-06810.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang M, Gamo NJ, Yang Y, Jin LE, Wang XJ, Laubach M, Mazer JA, Lee D, Arnsten AF. Neuronal basis of age-related working memory decline. Nature. 2011;476:210–213. doi: 10.1038/nature10243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wellman CL. Dendritic reorganization in pyramidal neurons in medial prefrontal cortex after chronic corticosterone administration. J Neurobiol. 2001;49:245–253. doi: 10.1002/neu.1079. [DOI] [PubMed] [Google Scholar]
- Wise SP. Forward frontal fields: phylogeny and fundamental function. Trends Neurosci. 2008;31:599–608. doi: 10.1016/j.tins.2008.08.008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Feng J, McEwen BS, Yan Z. Acute stress enhances glutamatergic transmission in prefrontal cortex and facilitates working memory. Proc Natl Acad Sci USA. 2009;106:14075–14079. doi: 10.1073/pnas.0906791106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Liu W, Karatsoreos IN, Ren Y, Feng J, McEwen BS, Yan Z. Mechanisms for acute stress-induced enhancement of glutamatergic transmission and working memory. Mol Psychiatry. 2011a;16:156–170. doi: 10.1038/mp.2010.50. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen EY, Wei J, Liu W, Zhong P, Li X, Yan Z. Repeated stress causes cognitive impairment by suppressing glutamate receptor expression and function in prefrontal cortex. Neuron. 2012;73:962–977. doi: 10.1016/j.neuron.2011.12.033. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yuen GS, McEwen BS, Akama KT. LIM kinase mediates estrogen action on the actin depolymerization factor Cofilin. Brain Res. 2011b;1379:44–52. doi: 10.1016/j.brainres.2010.07.067. [DOI] [PMC free article] [PubMed] [Google Scholar]