Abstract
Background
Sponges have long been known to be ecologically important members of the benthic fauna on coral reefs. Recently, it has been shown that sponges are also important contributors to the nitrogen biogeochemistry of coral reefs. The studies that have been done show that most sponges are net sources of dissolved inorganic nitrogen (DIN; NH4 + and NO3 −) and that nitrification, mediated by their symbiotic prokaryotes, is the primary process involved in supplying DIN to adjacent reefs.
Methodology/Principal Findings
A natural experiment was conducted with the Caribbean sponge Xestospongia muta from three different locations (Florida Keys, USA; Lee Stocking Island, Bahamas and Little Cayman, Cayman Islands). The DIN fluxes of sponges were studied using nutrient analysis, stable isotope ratios, and isotope tracer experiments. Results showed that the fluxes of DIN were variable between locations and that X. muta can be either a source or sink of DIN. Stable isotope values of sponge and symbiotic bacterial fractions indicate that the prokaryotic community is capable of taking up both NH4 + and NO3 − while the differences in δ 15N between the sponge and bacterial fractions from the NH4 + tracer experiment suggest that there is translocation of labeled N from the symbiotic bacteria to the host.
Conclusions/Significance
Nitrogen cycling in X. muta appears to be more complex than previous studies have shown and our results suggest that anaerobic processes such as denitrification or anammox occur in these sponges in addition to aerobic nitrification. Furthermore, the metabolism of this sponge and its prokaryotic symbionts may have a significant impact on the nitrogen biogeochemistry on Caribbean coral reefs by releasing large amounts of DIN, including higher NH4 + concentrations that previously reported.
Introduction
Sponges are an ecologically dominant component in many marine ecosystems, including coral reefs, where they contribute to the consolidation of reefs, prevent erosion, filter large quantities of seawater and provide habitat and food for many invertebrates and fishes [1]–[3]. Because of their ability to efficiently filter picoplankton, sponges can also contribute significantly to the coupling of productivity in the overlying water column to the benthos [1], [4], [5]. More recently, sponges and their prokaryotic symbionts have become an important area of research to quantify the fluxes of DIN by sponges and the biogeochemical cycling of nutrients on coral reefs [4], [6]–[8].
Nitrogen cycling on tropical coral reefs is particularly important, as nitrogen is a limiting nutrient and the success of several coral reef taxa (e.g., corals) is dependent on their symbiotic partners and the efficient re-cycling of nitrogen between host and symbionts [9]. Compared to ambient seawater the excurrent water of actively pumping sponges is often enriched in DIN such as NO3 − (or NO2 −+NO3 −) as a result of nitrification [10]. This has been documented for sponges from coral reefs, mangroves, seagrass beds [7], [8], [11]–[13], as well as sponges from temperate and cold-water environments [14]–[16]. In fact, coral reef sponges have been documented to have rates of nitrification that are significantly higher (5.8–16.0 mmol m−2 d−1 NO3 −) [7], [13] than what has been reported for benthic habitats such as microbial mats (up to 1.4 mmol m−2 d−1 NO3 − [17]) or coral reef sediment (1.7 mmol m−2 d−1 NO3 − [18]).
All pathways of nitrogen biogeochemistry have been reported to occur in sponges [10] including nitrogen fixation which was initially measured using the acetylene reduction method [19]–[21]. Stable isotope tracer studies (i.e., 15N2) later confirmed the presence of nitrogen fixation, albeit at low rates, in several species of sponges from coral reefs [21], [22]. Recently, the first sponge-derived nifH gene sequences and transcripts, which encode for the iron protein component of the nitrogenase enzyme responsible for nitrogen fixation, were documented in two sponge species from the Florida Keys [23]. nifH genes have also been recovered from Xestospongia muta from multiple locations in the Caribbean [Fiore and Lesser unpublished] and hypoxic/anoxic conditions, known to occur in sponges [10], would be required for activity since nitrogenase is inactivated by molecular oxygen and only fixes nitrogen under anaerobic or micro-aerobic conditions [10]. With the presence of anaerobic microhabitats in sponges [10], [24], other anaerobic nitrogen transformations including sulfate reduction, denitrification and anaerobic ammonium oxidation (anammox) have been observed and quantified using stable isotopic tracer methods, radiolabeled isotopes and recovery of gene specific sequences for key enzymes [16], [24], [25]. Interestingly, genomic analysis of the candidate phylum Poribacteria, which is found in sponges from numerous marine habitats [26], [27], suggests that Poribacteria may also be capable of denitrification [28].
Nitrification in sponges, which produces the bulk of the DIN released [8], [13], has been documented using collection and incubation methods combined with nutrient analyses [12], [13], [16]. Southwell et al. [7], [8] was the first to use an in situ method to identify actively nitrifying sponges and estimate the flux of DIN onto the adjacent coral reef, and found no significant difference in flux of DIN between the incubation and in situ methods. The interest in nutrient fluxes mediated by sponges and their symbionts as well as the nutrient biogeochemistry of coral reefs has resulted in a surge of research into the prokaryotic community composition of sponges and the processes they mediate. Recently, this has included the use of high throughput sequencing methods, such as 454 pyrosequencing of the 16S rRNA gene [[27], [29], [30],Fiore and Lesser unpublished] which have increased our understanding of the prokaryotic composition of many sponge species from different marine habitats. This genetic information has provided useful complementary insight for characterizing prokaryotic mediated nutrient cycling in these sponges.
On Caribbean coral reefs Xestospongia muta is an ecologically dominant member of the benthic community, and on Conch Reef, FL (USA) the number of X. muta has been shown to be significantly increasing over time [31]. Xestospongia muta is also characterized as a high microbial abundance sponge [32] but little was known about the composition of this community other than it contained Cyanobacteria [33] until recent studies documented a diverse prokaryotic community in Xestospongia muta and other members of this genus [[34], [35],Fiore and Lesser unpublished]. Additionally, X. muta outside of the Florida Keys have not been as well studied generally with quantitative 454 pyrosequencing comparing the prokaryotic symbionts of X. muta from the Florida Keys, Cayman Islands and Bahamas having been recently completed [Fiore and Lesser unpublished].
The primary goal of this study was to quantify DIN fluxes in X. muta from the same three populations where our 16S rRNA 454 pyrosequencing study was done. We ask whether sponges from these same populations in the Caribbean have different fluxes of DIN, and potentially how any differences in DIN fluxes, may be related to the taxonomy of their symbiotic prokaryotes using a comparative approach and a natural experiment [36].
Materials and Methods
Sample Locations
Replicate sponges (n = 6) were sampled at approximately 15 m depth between 9 and 10 AM and again between 4 and 5 PM when indicated from each of three locations: Rock Bottom Reef, Little Cayman, Cayman Islands (LC) (19°42′7.36″ N, 80°3′24.94″ W), North Perry Reef, Lee Stocking Island, Bahamas (LSI) (23°47′0.03″ N, 76°6′5.14″ W), and Conch Reef, Key Largo, FL (FL) (24°57′0.03″ N, 80°27′11.16″ W). All populations were sampled during the late spring and early summer of 2011 where the maximum irradiance of photosynthetically active radiation (PAR; 400–700 nm) at noon for all three locations is ∼500–600 µmol quanta m−2 s−1 [Lesser unpublished]. Necessary permits were obtained for all three locations: the Marine Conservation Board, Cayman Islands; Department of Marine Resources, Bahamas; NOAA ONMS permit number FKNMS-2011-066 for Conch Reef, Florida Keys.
Nutrient analyses and rates of sponge pumping
Ambient and excurrent water samples for nutrient analysis were collected from individual sponges (n = 6) at each location for nutrient analysis by slowly filling 100 ml syringes and placing all water samples on ice for transport to shore. Ambient water was obtained by filling the syringe adjacent to each sponge (within 20 cm of the sponge body wall) and excurrent water was obtained by placing weighted Tygon ® tubing inside the sponge close to the base of the spongocoel that was attached to a 100 ml syringe and drawing water into the syringe slowly (∼1 ml s−1). Syringes were then purged of approximately 10 ml and then 40 ml was saved and frozen for NH4 + and NO2 −+NO3 − analysis (NOx −). For the nutrient analyses the water samples were thawed and filtered (0.22 µm, Whatman, USA) to remove particulate matter then re-frozen and sent to the Nutrient Analytical Facility at Woods Hole Oceanographic Institute (WHOI, Woods Hole, MA, USA) for analysis using a Lachet QuickChem 8000 (flow injection analysis system) according to standard protocols to determine concentration of NH4 + and NOx −. Instrumental errors associated with the measurements were calculated as relative standard deviation (RSD) and includes: NH4 + −0.6% measured RSD, and NO2 −+NO3 − −0.59% measured RSD. Sponges were marked near their base with labeled flagging tape attached to nails embedded in the substrate to facilitate repeated measurements on the same sponges.
The volume flow or pumping rates for each individual sponge was determined as previously described [37]. A small amount (∼1 ml) of fluorescein dye was injected using a syringe and 16 gauge needle into the sponge just below the base of the spongocoel and the time(s) that the dye front took from its first appearance at the base of the spongocoel to the top of the spongocoel was recorded to obtain the centerline fluid velocity to calculate volume flux or pumping rate. We understand that unlike previous studies on tubular sponges where plug flow can be reasonably assumed (e.g., [37]) the morphology of X. muta likely creates more complicated excurrent plumes where the velocity across the osculum is not uniform [38]. This is easily observed using the timing of multiple dye tracks on X. muta injected in different locations with dye tracks closer to the sponge wall being slower than the centerline flow [Lesser unpublished]. As a result we recognize that our measurements of volume flow or pumping rates are likely to be an overestimate. That said our estimates of volume flow are in agreement with the results of Southwell et al. [8] using similar techniques for X. muta. Additionally, in our hands we have never observed cessation of pumping, or other artifacts, as a result of exposure to fluorescein in both thin walled and thick walled sponges [5], [37]. Both spongocoel and total sponge volume were calculated by measuring sponge height, base circumference, osculum diameter, and spongocoel depth and inner diameter with a measuring tape (to ±1.0 mm) and volume calculated as previously described [31]. The mass (kg) of individual sponges was then calculated by multiplying the individual total sponge volume (l), obtained as described above, by the average density of X. muta sponges (0.617 g cm−3) which was determined from direct measurements of the displacement volume and mass of pieces (n = 5) of sponge (including both mesohyl and pinacoderm). The flux of nutrients was then calculated by multiplying the ΔDIN (the difference in nutrient concentration between the ambient and excurrent water in µmol l−1 by the flow rate (cm s−1) and normalized to both sponge volume and mass for comparisons between sites.
Pumping rates and nutrient flux data were tested for assumptions of ANOVA and if the data failed either normality or homoscedasticity a constant integer to all values was added followed by log transformation. The transformed data passed Bartlett's test [39] for homoscedasticity but often failed the Shapiro-Wilks [40] test for normality. Because Bartlett's test is sensitive to deviations in normality [41] we choose to proceed with ANOVA, which is known to be robust to deviations from normality [42], on the transformed data. To determine if time of day was a significant factor in the flux of DIN, a two-way ANOVA with interaction was performed using the statistical program R [43] with time (AM and PM) and location as fixed factors for the flux of NOx −, NH4 +, total DIN and pumping rate as the response variables. A repeated measures ANOVA was not performed because the general requirement of this approach is three time points. Since the effect of time and interaction of time with location was not significant the flux of NOx −, NH4 +, total DIN and pumping rate, ΔDIN and pumping rates for each location were calculated by averaging the AM and PM values for each individual sponge. Collapsing the design to a single factor analysis to examine differences between locations was then assessed using a one-way ANOVA with location as a fixed factor [41].
Flow Cytometry
Ambient and excurrent water samples were collected as described above for the nutrient analyses for another set of sponges (n = 4) from LSI only. Approximately 3 ml from each collected water sample were fixed in electron microscopy grade paraformaldehyde at a final concentration of 0.5% in filtered (0.22 µm) seawater and frozen at −50°C. Frozen water samples were sent to the Bigelow Laboratory for Ocean Sciences J.J. MacIsaac Aquatic Cytometry Facility where they were stored in liquid nitrogen until analysis. Each sample was analyzed for cell abundances using a Becton Dickinson FACScan flow cytometer with a 30 mW, 488 nm laser. Simultaneous measurements of forward light scattering (FSC, relative size), 90° light scatter (SSC), chlorophyll fluorescence (>650 nm), and phycoerythrin fluorescence (560–590 nm) were made simultaneously on each sample as previously described [5]. Calculations of cyanobacteria, prochlorophyte, and heterotrophic cell concentration and filtering efficiency were performed as previously described [5]. Technical replicates (n = 2) were averaged for each sample and the cell abundance of heterotrophic bacteria was determined using PicoGreen (Molecular Probes), a dsDNA specific dye, which stains all prokaryotes (emission fluorescence 515–525 nm). Subtraction of the chl a containing picoplankton from the total prokaryotes yielded the heterotrophic bacterial component of the community while cyanobacterial and prochlorophyte cells were differentiated by the presence or absence, respectively, of phycoerythrin fluorescence. All filtered cells were converted to carbon and nitrogen equivalents using the following conversions; heterotrophic bacteria: 20 fg C cell−1 [44], Prochlorococcus: 53 fg C cell−1 [45], Synechococcus: 470 fg C cell−1 [46], heterotrophic bacteria: 3.3 fg N cell−1 [47], Prochlorococcus: 9.4 fg N cell−1 [48], Synechococcus: 35 fg N cell−1 [48]. Data were log transformed or arcsin transformed as necessary and an ANOVA followed by Tukey's HSD were performed to test for significant differences in the number of filtered cells between cell types (cyanobacteria, prochlorophytes, heterotrophic bacteria and total cells), filtration efficiency and total particulate carbon (POC) and nitrogen (PON) consumed by sponges.
Stable isotopic analyses and tracer experiments
Sponge samples that were frozen without buffer (n = 3 each location) were later lyophilized, ground to a powder with a mortar and pestle, and then acid treated with 1 M HCl to remove carbonate and rinsed with distilled water and allowed to dry. An analysis of samples from FL, separated into the outer pigmented layers of the sponge and the non-pigmented inner tissues (containing the pinacoderm and outer mesohyl respectively), showed no significant differences in stable isotope signatures [Fiore and Lesser, unpublished data] so whole cross-sections of sponge samples, consisting of both pinacoderm and mesohyl, from all locations were analyzed. Samples were then sent to the Marine Biological Laboratory (MBL) for the analysis of particulate C and N as well as the natural abundance of the stable isotopes δ 15N and δ 13C. Samples were analyzed using a Europa ANCA-SL elemental analyzer-gas chromatograph attached to a continuous-flow Europa 20–20 gas source stable isotope ratio mass spectrometer. The carbon isotope results are reported relative to Vienna Pee Dee Belemnite and nitrogen isotope results are reported relative to atmospheric air and both are expressed using the delta (δ) notation in units per mil (‰). The analytical precision of the instrument is ±0.1‰, and the mean precision of sample replicates for δ 13C was ±0.4‰ and δ 15N was ±0.2‰. A one-way ANOVA was used to test for significant differences between locations for δ 13C, δ 15N and C∶N ratios followed by the post hoc multiple comparison Tukey's HSD test as needed.
Two stable isotope tracer experiments were conducted during the summer of 2011 at LSI: the first used Na15NO3 (5 mg l−1 final concentration) plus H13CO3 (50 mg l−1) and the second used 15NH4 (0.31 mg l−1) as tracers (Sigma-Aldrich, USA). The method was the same for each experiment: 11 individual X. muta (average volume 172±77 ml or mass 0.106±0.048 kg; mean ±SD) were collected by cutting through the bottom of the sponge but keeping the tissue from the base of the spongocoel intact, from approximately 12 m at North and South Perry reefs at LSI and held in a large holding tank with flow through seawater for 5 d to recover from being removed from the reef. Care was taken to ensure that sponges were never exposed to air and that light levels were maintained at the same levels as found at ∼12 m using neutral density screens over the outdoor flowing seawater tanks. Sponges were checked for pumping activity using fluorescein dye and incubated statically with the tracer compound(s) for 4 h. Subsequently, T0 sponges (n = 3) were then removed and stored for analysis.
The remaining sponges were placed in individual aquaria with flow through seawater and sponges were sampled at 3 h (n = 2), 6 h (n = 3) and at 12 h (n = 3) for each fraction. Frozen samples were initially processed by separating the bacteria and sponge fractions following the methods of Freeman and Thacker [49] and Freeman et al. [50] except for two steps: an initial centrifugation was performed at 520× g for 4 min, and the resulting sponge pellet was rinsed an additional two times. The purity of the sponge and bacterial fractions were assessed using light and epifluorescence light microscopy as described by Freeman and Thacker [49]. The sponge fractions always contained large cells (8–10 µm diameter) consisting of at least 85% per microscopic field and exhibiting low natural fluorescence, whereas the bacterial fractions contained only small cells (<1–2 µm diameter) and high natural fluorescence. While efforts were made to separate and purify the sponge fraction as much as possible from all prokaryotic cells, it is possible that some prokaryotes that were located intracellularly were not detected (non-fluorescent) in the sponge fraction. These methods have been shown to be effective for other sponge species [49] and were optimized for use with X. muta. Additionally, a one-way ANOVA of the C∶N ratios for the two fractions yielded significant differences (F1,13 = 8.38, p = 0.01 (NH4 + tracer experiment); F1,13 = 24.6, p<0.01 (NO3 −+HCO3 − tracer experiment) indicating that good separation of these fractions occurred. It is likely, however, that some contamination occurred and was considered when interpreting the results of these experiments. Samples were then lyophilized and ∼1.0 mg was weighed and placed into silver capsules (Costech, CA, USA) and acidified three times with 20 µl of 12 M HCl. Samples were allowed to dry in between acidifications, then oven dried at 50°C for 48 h. Samples were combusted in a Carlo-Erba NC2500 elemental analyzer, and the resulting gas was analyzed in a Thermo Delta V isotope ratio mass spectrometer via a Conflo III open-split interface. The analytical precision of the instrument was ±0.2‰, and the mean standard deviation of sample replicates for δ 13C was ±0.4‰ and for δ 15N it was ±0.8‰ for enriched samples and ±0.1‰ and ±0.1‰ for natural abundance samples, respectively. For the tracer experiments the data were log transformed as necessary to meet the assumptions of parametric statistics and a two-way ANOVA with interaction, with fraction and time as fixed factors, was used to assess treatment effects.
Results
Stable Isotopic Signatures of Sponges
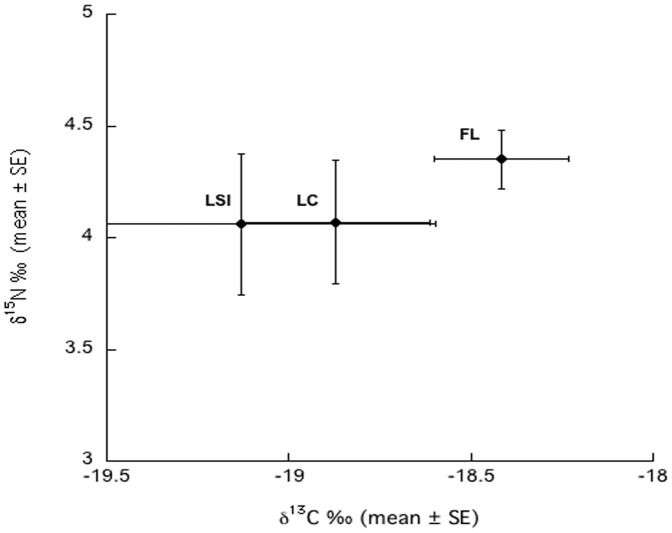
The values of δ 13C from each location were not significantly different from each other (ANOVA, F2,6 = 1.16, p = 0.38). The δ 13C of sponge samples ranged from −19.1 to −18.4‰ (Fig. 1). The δ 15N of sponge samples ranged from 4.0–4.4‰ (Fig. 1), and were not significantly different between locations (ANOVA, F2,6 = 0.52, p = 0.62). The ratios of C∶N were significantly different between locations (ANOVA, F2,6 = 22.57, p = 0.002), with post hoc pairwise comparisons showing that FL sponges had significantly higher C∶N ratios than LSI (Tukey's HSD, p<0.05), and that LC sponges significantly higher than LSI (Tukey's HSD, p<0.05). There was no significant difference between LC and FL (Tukey's HSD, p>0.05). Despite the significant results for C∶N ratios the mean values did not vary greatly, with a range of 4.33–4.83.
Figure 1. δ15N and δ13C values (mean ±SE) for Xestospongia muta (n = 3) for each location.
FL = Florida Keys LC = Little Cayman, LSI = Lee Stocking Island, Bahamas.
Inorganic Nitrogen Fluxes in Xestospongia muta
The difference in nutrient concentration between the ambient and excurrent of NH4 +, NO3 −+NO2 − (NOx −) and total DIN for X. muta varied considerably between individual sponges as expected for sponges over a large size range (Table 1). The ΔNH4 + values were not significantly different between locations (ANOVA, F2,15 = 3.19, p = 0.07) as were the ΔNOx − values (ANOVA, F2,15 = 2.58, p = 0.11). ΔDIN values, however, were significantly different between sites (ANOVA, F2,15 = 6.82, p = 0.008) with post hoc pairwise comparisons showing that FL sponges were significantly lower than both LSI and LC sponges LSI (Tukey's HSD, p<0.05) which were not significantly different than each other (Tukey's HSD, p>0.05). No measurements of ambient NOx − exceeded 4 µM eliminating the potential for ambient nutrient concentrations to be confounded by oceanographic features such as internal waves [51], [52]. Sponge pumping rates varied with size (Table 1) and did not differ significantly with location (ANOVA, F2, 15 = 1.61, p = 0.23).
Table 1. Calculated volume, mass, and flux parameters for samples of Xestospongia muta at each location.
| Location | Spongocoel (L) | Volume (L) | Mass (kg) | Flow rate (L h-1) | ΔDIN NH4 + (µmol L−1) | ΔDIN NOx (µmol L−1) | Flux NH4 + (µmol h−1 L−1) | Flux NOx (µmol h−1 L−1) | Flux DIN (µmol h−1 L−1) | Flux NH4 + (µmol h−1 kg−1) | Flux NOx (µmol h−1 kg−1) | Flux DIN (µmol h−1 kg−1) |
| FL | 4 | 43 | 26.8 | 4050 | −0.15 | −0.99 | −13 | −103 | −116 | −21 | −167 | −188 |
| FL | 20 | 101 | 62.2 | 18090 | 0.00 | 0.65 | 0 | 117 | 117 | 0 | 189 | 189 |
| FL | 39 | 36 | 22.4 | 62370 | 0.35 | −0.30 | 668 | −496 | 172 | 904 | −804 | 100 |
| FL | 33 | 111 | 68.2 | 21938 | 0.10 | −0.09 | 26 | −26 | 1 | 43 | −42 | 1 |
| FL | 14 | 76 | 47.0 | 15377 | 0.90 | 0.26 | −188 | 51 | −137 | −387 | 82 | −305 |
| FL | 7 | 37 | 22.9 | 6480 | −0.05 | −0.01 | −9 | −2 | −9 | −5 | −3 | −7 |
| LC | 12 | 64 | 39.3 | 4656 | 5.10 | 2.60 | 328 | 116 | 444 | 1653 | 1037 | 2690 |
| LC | 5 | 12 | 7.4 | 2649 | 1.80 | −0.30 | 2665 | −181 | 2484 | 1313 | −122 | 1191 |
| LC | 4 | 50 | 31.1 | 5423 | 0.35 | 0.90 | 38 | 92 | 130 | 681 | 1887 | 2567 |
| LC | 6 | 49 | 30.3 | 7109 | 0.40 | 0.35 | 81 | 71 | 151 | 333 | 1021 | 1354 |
| LC | 4 | 17 | 10.5 | 2145 | 3.30 | 0.25 | 213 | 41 | 254 | 4279 | 324 | 4603 |
| LC | 14 | 63 | 38.9 | 8463 | 0.45 | 0.20 | 60 | 31 | 90 | 350 | 272 | 622 |
| LSI | 5 | 22 | 13.6 | 6912 | 0.36 | 1.23 | −180 | 438 | 258 | −292 | 710 | 418 |
| LSI | 10 | 45 | 27.6 | 16640 | 0.11 | 0.53 | 40 | 66 | 106 | 64 | 107 | 172 |
| LSI | 29 | 81 | 50.2 | 28800 | 0.50 | 0.63 | 213 | 159 | 372 | 344 | 258 | 603 |
| LSI | 2 | 18 | 11.0 | 11040 | −0.12 | 0.87 | −162 | 1202 | 1040 | −262 | 1948 | 1686 |
| LSI | 3 | 23 | 14.0 | 2687 | −0.19 | 0.30 | 12 | 15 | 27 | 19 | 24 | 43 |
| LSI | 2 | 14 | 8.6 | 11220 | 2.53 | 0.67 | 4313 | 793 | 5106 | 6990 | 1286 | 8276 |
The volume and mass normalized fluxes of NH4 + (Table 1, Fig. 2 a) were not significantly different between locations (ANOVA, F2, 15 = 0.45, p = 0.65 (volume); F2, 15 = 0.85, p = 0.45 (mass)). The fluxes of NOx − normalized to sponge volume (Table 1, Fig. 2 b) were significantly different between locations (ANOVA, F2, 15 = 4.89, p = 0.02) with FL sponges significantly lower than LSI and LC not significantly different than either FL or LSI (Fig. 2A) but when normalized to mass did not show a significant effect of location (ANOVA, F2, 15 = 3.56, p = 0.054). The flux of total DIN (NOx −+NH4 +) normalized to volume and to mass were not significantly different among locations (ANOVA, F2, 15 = 1.24, p = 0.32 (volume); F2, 15 = 2.09, p = 0.16 (mass)).
Figure 2. The average flux (mean ±SE) of NH4 +, NOx − and DIN.
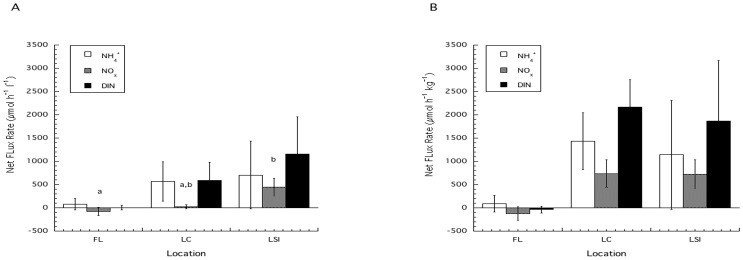
The average flux is shown for each location normalized to sponge volume (A) and mass (B). Treatment groups with similar superscripts are not statistically different from each other.
Feeding Study
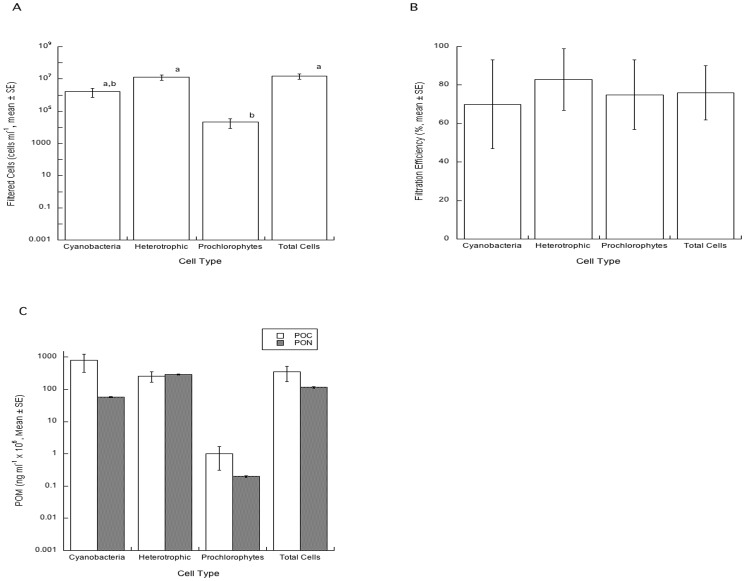
Xestospongia muta (n = 4) from LSI instantaneously filtered an average of 1.5×107 cells ml−1 and there was a significant effect of cell type (ANOVA, F3, 12 = 4.45, p = 0.03) (Fig. 3A). The number of both total cells and heterotrophic bacteria filtered was significantly higher than that of prochlorophytes (Tukey's HSD, p<0.05) but not cyanobacteria (Tukey's HSD, p>0.05), while the number of cyanobacterial cells filtered was indistinguishable (Tukey's HSD, p>0.05) from the prochlorophyte or total cell and heterotrophic cell groupings (Fig. 3A). For the filtration efficiency of each cell type there were no significant differences (ANOVA, F3, 12 = 0.45, p = 0.72) (Fig. 3B). The total amount of POC for each retained cell type was greatest for cyanoacteria, but there was no significant difference between cell types (ANOVA, F3, 12 = 2.77, p = 0.09). Differences between the amount of PON for each retained cell type was significant (ANOVA, F3, 12 = 4.68, p = 0.02) and greatest for total cells and heterotrophic bacteria compared to prochlorophytes (Tukey's HSD, p = 0.03) but not cyanobacteria (Tukey's HSD, p>0.05), while the PON of cyanobacterial cells was indistinguishable (Tukey's HSD, p>0.05) from the prochlorophyte or total cell and heterotrophic cell groupings (Fig. 3C).
Figure 3. Filtration of bacterioplankton from the water column by X. muta.
Number of filtered cells (A), filtration efficiency (B), and particulate organic matter as carbon and nitrogen (C) available from filtered cells for X. muta from LSI (n = 4). Treatment groups (mean ±SE) with similar superscripts are not statistically different from each other.
Nitrogen tracer experiment: Nitrate and Bicarbonate
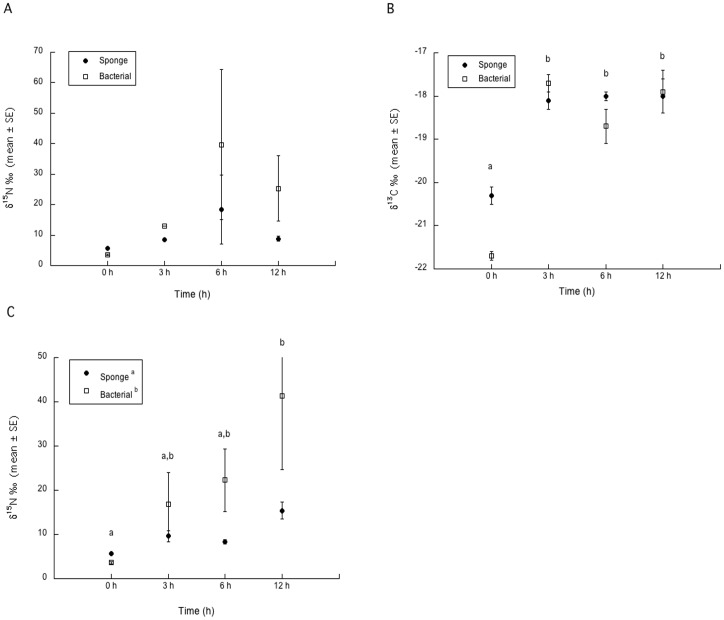
While sponge and bacterial fractions became more enriched from 3 to 6 hours there was no significant effect of enrichment of 15N from the NO3 − tracer in those fractions (ANOVA, F7, 14 = 0.84, p = 0.57). There was also no significant difference between sponge and bacterial fractions or over time or the interaction of fraction and time (fraction, F1,3 = 0.89, p = 0.36; time, F1,3 = 1.16, p = 0.36; interaction term, F1,3 = 0.28, p = 0.84) (Fig. 4A). Additionally, the enrichment of 13C from the bicarbonate tracer experiment was significant (ANOVA, F7, 14 = 20.9, p<0.0001) with fraction being non-significant (F1,3 = 0.13, p = 0.13) and time being significant (F1,3 = 44.3, p<0.001) with a non-significant interaction term, (F1,3 = 3.2, p = 0.056) (Fig. 4B). As a result post-hoc multiple comparison tests were only performed for time. Both the sponge and bacterial fractions became more enriched in13C then the sponge fraction over time with all sampling periods being significantly different than T0 (Tukey's HSD p<0.05) and not significantly different (Tukeys' HSD p>0.05) from each other (Fig. 4B).
Figure 4. δ 15N and δ 13C isotopic values from uptake experiments with X. muta.
δ 15N over time (A) for 15N nitrate and δ 13C over time (B) for 13C bicarbonate enriched sponge and bacterial fractions, and δ 15N over time for 15N ammonium enriched sponge and bacterial fractions (C). Samples collected at 3 h were under low irradiances while samples collected at 6 h had been exposed to sunlight (Under neutral density screens representative of irradiances at the depth of collection) and samples from 12 h were collected at night (for both experiments). Treatment groups (mean ±SE) with similar superscripts are not statistically different from each other.
Nitrogen tracer experiment: Ammonium
There was significant enrichment of 15N from the NH4 + tracer in the experimental sponges (ANOVA, F7, 13 = 3.38, p = 0.03) with the effects tests for fraction (F1,3 = 6.4, p = 0.03) and time (F1,3 = 5.0, p = 0.02) being significant and the interaction term non-significant (F1,3 = 1.8, p = 0.19) (Fig. 4C). Post-hoc multiple comparison testing for time revealed a significant (Tukey's HSD p<0.05) increase in enrichment over time with the bacterial fraction exhibiting greater enrichment (Fig. 4C).
Discussion
This study of the inorganic nitrogen fluxes in Xestospongia muta is the first to show differences in net fluxes of DIN both within and between populations of this ecologically dominant sponge on Caribbean coral reefs. Xestospongia muta densities are increasing [31], so quantifying both the fluxes of DIN and understanding the underlying processes driving the nitrogen biogeochemistry in this sponge is important for understanding the DIN availability on reefs in the Caribbean.
In a survey of nitrification in sponges on Conch Reef, Florida Southwell et al. [7], [8] reported evidence of nitrification in nine out of twelve sponge species, including X. muta. The rates of nitrification measured in these sponges varied, but overall they were at least two orders of magnitude higher than other habitats (e.g., benthos, coral rubble). In the current study, we observed similar rates of X. muta pumping activity reported for Conch Reef sponges by Southwell et al. [8]. However, unlike Southwell et al. [7], [8], X. muta from Conch Reef exhibited a negative flux of NOx −, indicating that either denitrification or anammox processes were taking place or possibly dissimilatory nitrate reduction. Our results from LSI and LC are consistent with Southwell et al. [7], [8] and for LSI the fluxes of NOx − are significantly higher than fluxes of NOx − from FL compared to Southwell et al. [8].
Xestopongia muta were actively pumping for all measurements taken during this study, which was not significantly different over time of day or between locations. While pumping rates were not significantly different, the observed variability in the unidirectional pumping of sponges has the potential to create microhabitats where both anaerobic nitrogen transformations (e.g., denitrification) and aerobic nitrogen transformations (e.g., nitrification) could occur [10], [16], [24], [53]. Additionally, recent studies of the bacterial communities of X. muta using16S rRNA sequencing [35,Fiore and Lesser unpublished] have reported many bacterial groups that are capable of denitrification and anammox (i.e., Burkholderiales, Pseudoalteromonadaceae, Poribacteria, Planctomycetes).
Interestingly, Southwell et al. [8] found that NOx − made up the majority of the DIN pool from X. muta and that NOx − was almost entirely NO3 −. In this study we observed, in addition to positive net NO3 − fluxes, a greater net efflux of NH4 + for all samples of X. muta. For some X. muta populations (i.e., LSI and LC) the flux of NH4 + had a significant impact on total DIN fluxes. These differences in the fluxes of NH4 +, probably generated from the utilization of nitrogen rich POM by the sponge host, are unusual given there is an active nitrifying community [7], [8], and a prokaryotic photosynthetic community [22] that could readily utilize NH4 + in this sponge [54].
The fluxes of DIN from sponges such as X. muta can have a significant impact on the availability and composition of DIN on coral reefs [7], [8]. Results of the current study indicate that fluxes of DIN from X. muta, the primary contributor to DIN on Caribbean coral reefs [7], [8], is more complex than previously thought and is significantly different between locations. Additionally, these fluxes vary over time as a previous study of the same population of X. muta from LSI in 2010 showed both positive and negative fluxes of NOx − with a net ΔNOx − of −0.27 µM±0.06 (mean ±SE) [Fiore and Lesser unpublished].
Natural abundance stable isotope values have been commonly used to trace sources of C and N through the food chain [55]. Nitrogen fixation yields an average δ 15N signature of approximately 0.0‰ [56], [57], and trophic enrichment typically results in a +2.2 to +3.5‰ increase per trophic level for δ 15N [58], [59]. Therefore, several studies have used a cutoff of ≤2.0‰ to indicate N from a fixed source [23], [60], [61]. Additionally, carbon fixation by marine phytoplankton typically results in δ 13C values of about −19 to −24‰ [55], with an average of +0.5 to +1.0‰ enrichment per trophic level [62]. Based on previous studies that have used stable isotope analysis to investigate the relationship between sponges and their symbionts [6], [7], [49], a cutoff for δ 13C of −18‰ or lower was used as an indication of photoautotrophic carbon fixation for X. muta. The bulk stable isotopic values measured for both C and N in X. muta tissue, comprising both the host tissue and prokaryotic biomass, were not significantly different between sites and similar to those documented in previous studies on X. muta [22], [23].
Using the cutoff values described above, there is no stable isotopic evidence that nitrogen fixation was occurring in X. muta (Fig. 1) although nifH genes have been sequenced from X. muta [Fiore and Lesser unpublished]. The δ13C values of the sponge samples show evidence of photoautotrophy, although we cannot say definitively that there is transfer of C from symbionts to the sponge. Freeman and Thacker [49] demonstrated that high microbial abundance (HMA) sponges, such as X. muta, can obtain either C or N, or both, from their symbionts. Any interpretation of tissue stable isotopic signatures must, however, include heterotrophy on picoplankton for sponges. Based on the feeding study of LSI sponges, and despite the high abundance of resident bacteria, sponges are actively and non-selectively filtering most of the bacteria from the ambient water, which would supply significant amounts of POC and PON that could be potentially used by the host which is consistent with studies on other sponge species from the Caribbean [5], [37]. Taken together, the results of this study suggest some site related differences with sponges from the more open ocean sites of LSI and LC being more dependent on photoautotrophic sources of C and coastal FL sponges being more dependent on POM for their C requirements (Fig. 1).
The tracer experiments provide additional insight into what may be occurring in terms of the dynamics of C and N uptake by the prokaryotic community. Results of the HCO3 − tracer experiment indicate that there is uptake by prokaryotes and then immediate equilibrium of C with the sponge tissue. These results show that there is an active autotrophic community present in X. muta and provides evidence for transfer of C from symbiont to host. Uptake of HCO3 − is not limited to autotrophs, and heterotrophic bacteria may have also taken up HCO3 − for use in aneplurotic pathways [63].
The NH4 + tracer experiment indicates that the symbiotic prokaryotic community of X. muta actively assimilated the NH4 + and the host sponge is the likely source of NH4 +. The small increase in sponge host δ 15N during the NH4 + experiment may also indicate direct uptake of NH4 + by the sponge, as has been demonstrated for corals [64]. Alternatively, as in the NO3 − tracer experiment, the accumulation of tracer in the sponge fraction may also be explained by transfer of N from the bacteria to the host.
NO3 − can also be utilized by the prokaryotic community, as demonstrated by the increased δ 15N of the bacterial fractions incubated with 15NO3 − (Fig 4 A). Photosynthetically driven NO3 − uptake has been demonstrated in planktonic communities [65], and may explain the increase in NO3 − uptake when the sponges were exposed to natural solar radiation. However, it should be noted that heterotrophic bacteria could take up NO3 − as well [66]. As the symbiotic nitrifiers in the sponge produce NO3 − it would provide a source of NO3 − for uptake by the photosynthetic community as well as a substrate for denitrification. Similarly, NH4 + is also a likely substrate for aerobic ammonia oxidation by the crenarcheote community, which is supported by the recovery of crenarchaeal amoA genes in X. muta [34], expression of amoA genes [34] and tracer studies [8]. If assimilatory and dissimilatory processes are competing for NO3 −, NO2 −, and NH4 +, which have been documented in other communities [67], then this may have a significant role in nitrogen cycling in the sponge holobiont. Lastly, the anaerobic oxidation of NH4 + (anammox) is another process that may utilize both NH4 + and the NO2 − generated from nitrification. The rates, however, of anammox are relatively low in the water column [68], as is the only documented rate for anammox in sponges [16]. It is possible that anammox may occur within anoxic microhabitats of X. muta, and support for this is provided by the presence of planctomycete bacteria in X. muta [35,Fiore and Lesser unpublished] but the nutrient flux data clearly show that a net efflux of NH4 + is still occurring in all sponges suggesting an abundance of this substrate for either nitrification or anammox. Other factors that may be important in explaining the observed variability in net fluxes of DIN include the variability in tissue O2 concentration as a result of pumping activity [10] and the concentration of other compounds that are known to influence N cycling such as H2S. Variable O2 concentrations within the sponge tissue will determine the relative rates of nitrification and denitrification [10], [53], while H2S is known to inhibit nitrification and denitrification [69], [70]. H2S may be present in X. muta, as bacteria involved in sulfur cycling have been recovered from this sponge (Chromatiales, Syntrophobacteraceae, Fiore and Lesser unpublished). If nitrification and denitrification are tightly coupled, then variations in H2S or O2 concentrations may indeed influence the rates of these processes and the net fluxes of various species of DIN.
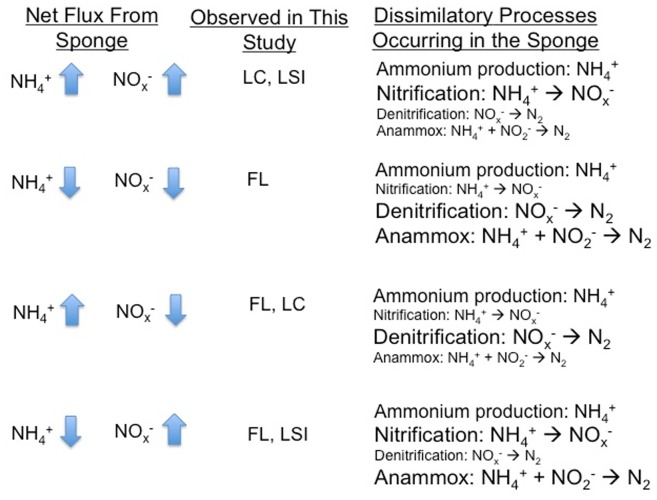
If we consider each outcome in terms of net flux of DIN from X. muta from the current study, we can model which dissimilatory processes are likely occurring that can then be used to formulate testable hypotheses (Fig. 5) for future studies. The model also allows us ask questions on the broader ecological impacts of sponge-derived DIN; for example, LC sponges, which generally had a net positive flux of both NH4 + and NOx − (Fig. 5) and often NH4 + was a significant component to total DIN (Fig. 2), may differentially influence N cycling on the surrounding coral reef relative to FL or LSI sponges. We do not know the extent to which sponge-derived DIN influences the biogeochemistry and ecology of the surrounding habitat, but studies on multi-species sponge assemblages, and coral reef communities dominated by active suspension feeding sponges, have shown the significant role of active suspension feeding and the coupling of POC and PON from the water column to the benthos [71], [72]. The composition of DIN released into the water column by sponges would influence how it might be utilized, and who utilizes it in the surrounding environment, as NH4 + is more readily incorporated into biomass than NO3 − which can then potentially support local increases in planktonic community production [73]. As discussed by Southwell et al. [8], excess inorganic nutrients, such as release of DIN by sponges, may have detrimental effects on coral reef ecosystems by stimulating an increase in the growth of fleshy algae in the absence of herbivores [8]. It is important that further research be done to determine the ecosystem level effects of DIN release by sponges, and particularly from X. muta in regards to Caribbean coral reefs as it is believed to be a primary contributor of DIN released by sponges [8].
Figure 5. Potential dissimilatory N transformations occurring in X. muta based on the observed net flux of NH4 + and NOx − from the sponge.
Font size for a given process indicates the relative importance of that process. Locations may appear more than once due to differences in individual sponges at each location.
We have shown that the flux of DIN from three populations of X. muta is highly variable which may have a significant impact on the availability of DIN on coral reefs given the high abundance of these sponges. Nitrification had been previously demonstrated to occur in X. muta, and we show here that other nitrogen transformations including denitrification and/or anammox may occur in these sponges as well as the importance of active suspension feeding on the nitrogen rich pool of picoplankton. Further work is needed to better characterize the flux of DIN from X. muta and other sponges on Caribbean coral reefs including whether sponges can fix N2. This will require additional investigations on the functional activity of the symbiotic prokaryotic community of sponges using a combination of experimental and molecular approaches (i.e., transcriptomics) that will yield insight into the taxonomy and function of this community, and how this impacts nutrient fluxes and biogeochemical cycling on Caribbean coral reefs.
Acknowledgments
We thank Marc Slattery, Deborah Gochfeld, Erica Hunkin, Cole Easson, Sylvester Lee, Julia Stevens, Christopher Freeman, Julie Olson, Mauritius Bell, and the Aquarius team for help in the field. Marshall Otter at the stable isotope laboratory at the Marine Biological Laboratory performed the stable isotope analyses. Marilyn Fogel and Roxane Bowden at the Carnegie Institution of Washington for additional support and assistance with stable isotope analyses. Paul Henderson at the Nutrient Analytical Facility at Woods Hole Oceanographic Institute conducted the nutrient analyses and the flow cytometry analyses were performed at the J.J. MacIsaac Facility for Aquatic Cytometry at the Bigelow Laboratory for Ocean Sciences.
Funding Statement
This project was funded by grants from the National Oceanic and Atmospheric Administration Ocean Exploration Program, Undersea Research Program, National Institute for Undersea Science and Technology as well as the National Science Foundation. The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Reiswig H (1971) In situ pumping activities of tropical Demospongiae. Mar Biol 9: 38–50. [Google Scholar]
- 2. Diaz CM, Rützler K (2001) Sponges: an essential component of Caribbean coral reefs. Bull Mar Sci 69: 535–546. [Google Scholar]
- 3. Ribeiro SM, Omena EP, Muricy G (2003) Macrofauna associated to Mycale microsigmatosa (Porifera, Demospongiae) in Rio de Janeiro State, SE Brazil. Estuar Coast Shelf Sci 57: 951–959. [Google Scholar]
- 4. Ribes M, Coma R, Atkinson MJ, Kinzie RA (2005) Sponges and ascidians control removal of particulate organic nitrogen form coral reef water. Limnol Oceangr 50: 1480–1489. [Google Scholar]
- 5. Lesser MP (2006) Benthic-pelagic coupling on coral reefs: Feeding and growth of Caribbean sponges. J Exp Mar Biol Ecol 328: 277–288. [Google Scholar]
- 6. Weisz JB, Hentschel U, Lindquist N, Martens CS (2007) Linking abundance and diversity of sponge-associated microbial communities to metabolic differences in host sponges. Mar Biol 152: 475–483. [Google Scholar]
- 7. Southwell MW, Popp BN, Martens CS (2008 a) Nitrification controls on fluxes and isotopic composition of nitrate form Florida Keys sponges. Mar Chem 108: 96–108. [Google Scholar]
- 8. Southwell MW, Weisz JB, Martens CS, Lindquist N (2008 b) In situ fluxes of dissolved inorganic nitrogen from the sponge community on Conch Reef, Key Largo, Florida. Limnol Oceanogr 53: 986–996. [Google Scholar]
- 9. Muscatine L, Porter JW (1977) Reef corals: mutualistic symbioses adapted to nutrient-poor environments. Bioscience 27: 454–460. [Google Scholar]
- 10. Fiore CL, Jarett JK, Olson ND, Lesser MP (2010) Nitrogen fixation and nitrogen transformations in marine symbioses. Trends Microbiol 18: 455–463. [DOI] [PubMed] [Google Scholar]
- 11. Reiswig H (1981) Partial carbon and energy budgets of the bacteriosponge Verongia fistularis (Porifera: Demospongiae) in Barbados. Mar Ecol 2: 273–293. [Google Scholar]
- 12. Corredor JE, Wilkinson CR, Vicente VP, Morell JM, Otero E (1988) Nitrate release by Caribbean reef sponges. Limnol Oceangr 33: 114–129. [Google Scholar]
- 13. Diaz M, Ward B (1997) Sponge-mediated nitrification in tropical benthic communities. Mar Ecol Prog Ser 156: 97–107. [Google Scholar]
- 14. Eroteida J, Ribes M (2007) Sponges as a source of dissolved inorganic nitrogen: nitrification mediated by temperate sponges. Limnol Oceangr 52: 948–958. [Google Scholar]
- 15. Bayer K, Schmitt S, Hentschel U (2008) Physiology, phylogeny and in situ evidence for bacterial and archaeal nitrifiers in the marine sponge Aplysina aerophoba . Environ Microbiol 10: 2942–2955. [DOI] [PubMed] [Google Scholar]
- 16. Hoffmann F, Radax R, Woebken D, Holtappels M, Lavik G, et al. (2009) Complex nitrogen cycling in the sponge Geodia barretti . Environ Microbiol 11: 2228–2243. [DOI] [PubMed] [Google Scholar]
- 17. Bonin PC, Michetoy VD (2006) Nitrogen budget in a microbial mat in the Camargue (southern France). Mar Ecol Prog Ser 322: 75–84. [Google Scholar]
- 18. Capone DG, Dunham SE, Horrigan SG, Duguay LE (1992) Microbial nitrogen transformations in unconsolidated coral-reef sediments. Mar Ecol Prog Ser 80: 75–88. [Google Scholar]
- 19. Wilkinson C, Fay P (1979) Nitrogen fixation in coral reef sponges with symbiotic cyanobacteria. Nature 279: 527–529. [Google Scholar]
- 20. Shieh WY, Lin YM (1994) Association of heterotrophic nitrogen-fixing bacteria with a marine sponge of Halichondria sp. Bull Mar Sci 54: 557–564. [Google Scholar]
- 21. Wilkinson CR, Summons RE, Evans E (1999) Nitrogen fixation in symbiotic marine sponges: ecological significance and difficulties in detection. Mem Queensland Mus 44: 667–673. [Google Scholar]
- 22.Southwell MW (2007) Sponges impacts on coral reef nitrogen cycling, Key Largo, Florida. Dissertation, University of North Carolina at Chapel Hill.
- 23. Mohamed NM, Colman AS, Tai Y, Hill RT (2008) Diversity and expression of nitrogen fixation genes in bacterial symbionts of marine sponges. Environ Microbiol 10: 2910–2921. [DOI] [PubMed] [Google Scholar]
- 24. Hoffmann F, Larsen O, Thiel V, Rapp HT, Pape T, et al. (2005a) An anaerobic world in sponges. Geomicrobiol J 22: 1–10. [Google Scholar]
- 25. Mohamed NM, Saito K, Tal Y, Hill RT (2009) Diversity of aerobic and anaerobic ammonia-oxidizing bacteria in marine sponges. ISME J 4: 38–48. [DOI] [PubMed] [Google Scholar]
- 26. Taylor MW, Radax R, Stegor D, Wagner M (2007) Sponge-associated microorganisms: evolution, ecology, and biotechnological potential. Microbiol Mol Biol Rev 71: 1–53. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Schmitt S, Tsai P, Bell J, Fromont, Ilan JM, et al. (2011) Assessing the complex sponge microbiota: core, variable and species-specific bacterial communities in marine sponges. ISME J 6: 564–576. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28. Siegl A, Kamke J, Hochmuth T, Piel JOR, Richter M, et al. (2010) Single-cell genomics reveals the lifestyle of Poribacteria, a candidate phylum symbiotically associated with marine sponges. ISME J 5: 61–70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29. Webster NS, Taylor MW, Behnam F, Lücker S, Rattei T, et al. (2010) Deep sequencing reveals exceptional diversity and modes of transmission for bacterial sponge symbionts. Environ Microbiol 12: 2070–2082. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30. Lee OO, Wang Y, Yang J, Lafi FF, Al-Suwailem A, Qian PY (2010) Pyrosequencing reveals highly diverse and species-specific microbial communities in sponges from the Red Sea. ISME J 5: 650–664. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31. McMurray SE, Blum JE, Pawlik JR (2008) Redwood of the reef: growth and age of the giant barrel sponge Xestospongia muta in the Florida Keys. Mar Biol 155: 159–171. [Google Scholar]
- 32. Hentschel U, Usher KM, Taylor MW (2006) Marine sponges as microbial fermenters. FEMS Microbiol Ecol 55: 167–177. [DOI] [PubMed] [Google Scholar]
- 33. Steindler L, Huchon D, Avni A, Ilan M (2005) 16S rRNA Phylogeny of Sponge-Associated Cyanobacteria. Appl Environ Microbiol 71: 4127–4131. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34. Lopez-Legentil S, Erwin OM, Pawlik JR, Song B (2010) Effects of sponge bleaching on ammonia-oxidizing Archaea: distribution and relative expression of ammonia monooxygenase genes associated with the barrel sponge Xestospongia muta . Microbial Ecol 60: 561–571. [DOI] [PubMed] [Google Scholar]
- 35. Montalvo NF, Hill RT (2011) Sponge-Associated Bacteria Are Strictly Maintained in Two Closely Related but Geographically Distant Sponge Hosts. Appl Environ Microbiol 77: 7207–7216. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Diamond J (1986) Overview: laboratory experiments, field experiments, and natural experiments. In: Community Ecology. Diamond J,Case TJ, New York: Harper and Row, 3–22.
- 37. Trussel GC, Lesser MP, Patterson MR, Genovese SJ (2006) Depth-specific differences in growth of the reef sponge Callyspongia vaginalis: role of bottom-up effects. Mar Ecol Prog Ser 323: 149–158. [Google Scholar]
- 38. Weisz JB, Lindquist UN, Martens CS (2008) Do associated microbial abundances impact demosponge pumping rates and tissue densities? Oecologia 155: 367–376. [DOI] [PubMed] [Google Scholar]
- 39. Bartlett MS (1937) Properties of sufficiency and statistical tests. Proc Royal Soc London Ser 160: 268–282. [Google Scholar]
- 40. Royston P (1995) A remark on Algorithm AS 181: the W test for normality. Appl Stat 44: 547–551. [Google Scholar]
- 41.Sokal RR, Rohlf JF (1995) Biometry, 3rd ed. W. H. Freeman and Company. 880.
- 42. Schmider E, Ziegler M, Danay E, Beyer L, Bühner M (2010) Is it really robust? Reinvestigating the robustness of ANOVA against violations of the normal distribution assumption. Methodology: Euro J Res Meth Behav Social Sci 6: 147–151. [Google Scholar]
- 43.R Core Team (2012) R: A language and environment for statistical computing. Vienna, Austria, R Foundation for Statistical Computing. ISBN 3-900051-07-0, R project website. Available: http://www.R-project.org/. Accessed 2012 Feb 19.
- 44. Ducklow HW, Kirchman DL, Quinby HL, Carlson CA, Dam HG (1993) Stocks and dynamics of bacterioplankton carbon during the spring bloom in the eastern North Atlantic Ocean. Deep Sea Res 40: 245–263. [Google Scholar]
- 45. Morel A, Ahn YH, Partensky F, Vaulot D, Claustre H (1993) Prochlorococcus and Synechococcus: a comparative study of their optical properties in relation to their size and pigmentation. J Mar Res 51: 617–649. [Google Scholar]
- 46. Campbell L, Nolla HA, Vaulot D (1994) The importance of Prochlorococcus to community structure in the central North Pacific Ocean. Limnol Oceanogr 39: 954–960. [Google Scholar]
- 47. FaggerBakke KM, Heldal M, Norland S (1996) Content of carbon, nitrogen, oxygen, sulfur and phosphorus in native aquatic and cultured bacteria. Mar Ecol Prog Ser 10: 15–27. [Google Scholar]
- 48. Bertilsson S, Berglund O, Karl DM, Chisholm SW (2003) Elemental composition of marine Prochlorococcus and Synechococcus: implications for the ecological stoichiometry of the sea. Limnol Oceanogr 48: 1721–1731. [Google Scholar]
- 49. Freeman CJ, Thacker RW (2011) Complex interactions between marine sponges and their symbiotic microbial communities. Limnol Oceangr 56: 1577–1586. [Google Scholar]
- 50.Freeman CJ, Thacker RW, Baker DM, Fogel ML (2013) Quality or quantity: is nutrient transfer driven more by symbiont identity and productivity than by symbiont abundance? J ISME doi:10.1038/ismej.2013.7 [DOI] [PMC free article] [PubMed]
- 51. Leichter JJ, Wing SR, Miller SL, Denny MW (1996) Pulsed delivery of subthermocline water to Conch Reef (Florida Keys) by internal tidal bores. Limnol Oceanogr 41: 1490–1501. [Google Scholar]
- 52. Leichter JJ, Stewart HL, Miller SL (2003) Episodic nutrient transport to Florida coral reefs. Limnol Oceanogr 48: 1394–1407. [Google Scholar]
- 53. Schläppy M-L, Schottner SL, Lavik G, Kuypers MMM, de Beer D, et al. (2010a) Evidence of nitrification and denitrification in high and low microbial abundance sponges. Mar Biol 157: 593–602. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 54. Muro-Pastor MI, Reyes JC, Florencio FJ (2005) Ammonium assimilation in cyanobacteria. Photosynth Res 83: 135–150. [DOI] [PubMed] [Google Scholar]
- 55.Fry B (2006) Stable Isotope Ecology. New York: Springer. 308.
- 56. Mariotti A (1983) Atmospheric nitrogen is a reliable standard for natural 15N abundance measurements. Nature 303: 685–687. [Google Scholar]
- 57. Peterson BJ, Fry B (1987) Stable isotopes in ecosystem studies. Ann. Rev. Ecol. Syst 18: 293–320. [Google Scholar]
- 58. Vander Zanden JM, Rasmussen JB (2001) Variation in δ15N and δ13C trophic fractionation: Implications for aquatic food web studies. Limnol Oceanogr 46: 2061–2066. [Google Scholar]
- 59. McCutchan JH, Lewis WM, Kendall C, McGrath CC (2003) Variation in trophic shift for stable isotope ratios of carbon, nitrogen, and sulfur. Oikos 102: 378–390. [Google Scholar]
- 60. Carpenter EJ, Harvey HR, Fry B, Capone DG (1997) Biogeochemical tracers of the marine cyanobacterium Trichodesmium . Deep-Sea Res 44: 27–38. [Google Scholar]
- 61. Montoya JP, Carpenter EJ, Capone DG (2002) Nitrogen fixation and nitrogen isotope abundances in zooplankton of the oligotrophic North Atlantic. Limnol Oceanogr 47: 1617–1628. [Google Scholar]
- 62.Michener RH, Schell DM (1994) Stable isotope ratios as tracers in marine aquatic food webs. In: Lajtha K, Michener RH, Stable isotopes in ecology and environmental science. Blackwell Scientific. 138–157.
- 63. DeLorenzo S, Brauer SL, Edgmont CA, Herfort L, Tebo BM, et al. (2012) Ubiquitous dissolved inorganic carbon assimilation by marine bacteria in the Pacific Northwest coastal ocean as determined by stable isotope probing. PLOS ONE 7: e46695. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64. Yellowlees D, Rees TAV, Fitt WK (1994) Effect of ammonium-supplemented seawater on glutamine synthetase and glutamate dehydrogenase activities in the host tissue and zooxanthellae of Pocillopora damicornis and on ammonium uptake rates of the zooxanthellae. Pac Sci 48: 291–295. [Google Scholar]
- 65. Maguer J-F, L'Helguen S, Caradec J, Klein C (2011) Size-dependent uptake of nitrate and ammonium as a function of light in well-mixed temperate coastal waters. Cont Shelf Res 31: 1620–1631. [Google Scholar]
- 66. Kirchman DL (1994) The uptake of inorganic nutrients by heterotrophic bacteria. Microb Ecol 28: 255–271. [DOI] [PubMed] [Google Scholar]
- 67. Mackey KRM, Bristow L, Parks DR, Altabet MA, Post AF, et al. (2011) The influence of light on nitrogen cycling and the primary nitrite maximum in a seasonally stratified sea. Prog Oceanogr 91: 545–560. [Google Scholar]
- 68. Kuypers MMM, Sliekers AO, Lavik G, Schmid M, Jorgensen BB, et al. (2003) Anaerobic ammonium oxidation by anammox bacteria in the Black Sea. Nature 422: 608–611. [DOI] [PubMed] [Google Scholar]
- 69. Caffey JM, Sloth NP, Kaspar H, Blackburn TH (1993) Effect of organic loading on nitrification and denitrification in marine sediment microcosms. FEMS Microbiol Ecol 12: 159–167. [Google Scholar]
- 70. Purubsky WP, Weston NB, Joye SB (2009) Benthic metabolism and the fate of dissolved inorganic nitrogen in intertidal sediments. Estuar Coast Mar Sci 83: 392–402. [Google Scholar]
- 71. Ribes M, Coma R, Atkinson MJ, Kinzie III RA (2003) Particle removal by coral reef communities: picoplankton is a major source of nitrogen. Mar Ecol Prog Ser 257: 13–23. [Google Scholar]
- 72. Perea-Blásquez A, Davy SK, Bell JJ (2012) Estimates of particulate organic carbon flowing from the pelagic environment to the benthos through sponge assemblages. Hydrobiologia 687: 237–250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 73.O'Neil JM, Capone DG (2008) Nitrogen Cycling in Coral Reef Environments. In: Capone DG, Bronk DA, Mullholland MR, Carpenter EJ, Nitrogen in the Marine Environment. Burlington: Academic Press. 949–989.