Abstract
Triclosan MIC determination showed that recent Staphylococcus aureus clinical isolates (n = 100) were highly susceptible to triclosan, with a 50% minimal inhibitory concentration (MIC50) of 0.12 μg/ml and a MIC90 of 0.25 μg/ml. Staphylococcus epidermidis isolates (n = 96) were less susceptible, with a MIC50 of 0.12 μg/ml and a MIC90 of 8 μg/ml. Decreased susceptibility to triclosan was more prevalent among methicillin-resistant S. epidermidis than among methicillin-sensitive S. epidermidis isolates.
Triclosan (2,4,4′-trichloro-2′-hydroxydiphenyl ether) is a widely used antimicrobial agent that is found in a variety of consumer goods and in antiseptic products that are designed for use in the clinical setting (4). Triclosan has been recommended for the control of methicillin-resistant Staphylococcus aureus (MRSA) infections in hospital settings after being used successfully to control in-hospital MRSA outbreaks (2), and it is an additive biocidal agent used in some biomedical devices (9).
Recently, triclosan was determined to have a target-based mechanism, inhibiting the action of one of the enzymes of bacterial fatty acid biosynthesis, enoyl-acyl carrier protein reductase, the product of the fabI gene (11, 12). This finding has led to recent concern about the ability of microbes to become resistant to triclosan through both target-based (8, 14) and efflux-based (5, 6) mechanisms.
Typically, many bacterial species, including the staphylococci, are quite susceptible to triclosan. For S. aureus, a MIC at which 90% of the strains are inhibited (MIC90) of 0.06 μg/ml was determined in a recent study of 232 clinical isolates (1). MRSA strains with reduced susceptibility to triclosan have appeared in clinical settings, with MICs of triclosan ranging from 1 to 4 μg/ml (1, 3). It has been suggested that hospital treatments may contribute to decreased susceptibility to triclosan. Cookson et al. isolated MRSA strains for which triclosan MICs were 2 to 4 μg/ml from patients who had been treated with daily triclosan baths (7). Others have also found clinical isolates of S. aureus with reduced triclosan susceptibility (3, 15), but the resistance levels and frequencies of such isolates remain low. The importance of the reduced triclosan susceptibility in the clinic is unclear.
Reduced susceptibility to triclosan has not been associated with reduced susceptibility to other antibiotic agents. In a previous laboratory study, up to 40-fold increases in the MICs of triclosan, from 0.025 to 1 μg/ml, but the isolates did not show altered susceptibility to vancomycin, beta-lactams, aminoglycosides, or tetracycline (15).
The use of triclosan to eradicate MRSA from patients by reducing skin colonization, and the increasing use of triclosan as an antimicrobial additive in medical devices (9), motivates continued monitoring of triclosan susceptibility among hospital isolates. We report the triclosan MICs for 100 recent clinical isolates of S. aureus and 96 recent clinical isolates of Staphylococcus epidermidis, and we found that more than 20% of the S. epidermidis isolates showed decreased susceptibility to triclosan. To our knowledge, this is the first report of triclosan MICs for a population of clinical S. epidermidis isolates.
A panel of S. aureus and S. epidermidis strains was assembled from the extensive microbe collection of Focus Technologies (Herndon, Va.). The strains were identified to the species level by using standard identification algorithms. The S. aureus strains included 50 MRSA and 50 methicillin-sensitive S. aureus strains, and the S. epidermidis strains included 47 methicillin-resistant S. epidermidis (MRSE) and 49 methicillin-sensitive S. epidermidis (MSSE) strains. The methicillin resistance phenotype was identified by determining oxacillin MICs according to NCCLS guidelines (13). All strains were collected between January 2001 and August 2002 and represent unique, nonconsecutive isolates. To obtain a representative and diverse panel, we collected strains from 27 different states in the United States, from 13 different clinical specimen sources, and from male and female patients ranging from 1 to 94 years of age with an approximately equal distribution between inpatients and outpatients.
Antimicrobial susceptibility testing was conducted at Focus Technologies by using broth microdilution methodology in accordance with NCCLS guidelines (13). Triclosan serial doubling dilutions covered the concentration range between 0.03 and 32 μg/ml.
The MICs for 100 S. aureus and 96 S. epidermidis recent clinical isolates were determined, and the results of these tests are summarized in Table 1. The majority of the S. aureus clinical isolates were highly susceptible to triclosan, with the MIC50 being 0.12 μg/ml and the MIC90 being 0.25 μg/ml. In contrast, the MIC50 for the S. epidermidis isolates was 0.12 μg/ml and the MIC90 was 8 μg/ml, indicating substantial heterogeneity in the S. epidermidis population.
TABLE 1.
In vitro susceptibilities of clinical staphylococcal isolates to triclosan
| Organism (no. of isolates) | MIC (μg/ml) of triclosan
|
||
|---|---|---|---|
| MIC50 | MIC90 | Range | |
| S. aureus (100) | 0.12 | 0.25 | 0.06-4.0 |
| MSSAa (50) | 0.12 | 0.12 | 0.06-2.0 |
| MRSA (50) | 0.12 | 0.25 | 0.06-4.0 |
| S. epidermidis (96) | 0.12 | 8 | ≤0.03-8.0 |
| MSSE (49) | 0.12 | 1 | ≤0.03-8.0 |
| MRSE (47) | 0.12 | 8 | ≤0.03-8.0 |
MSSA, methicillin-sensitive S. aureus.
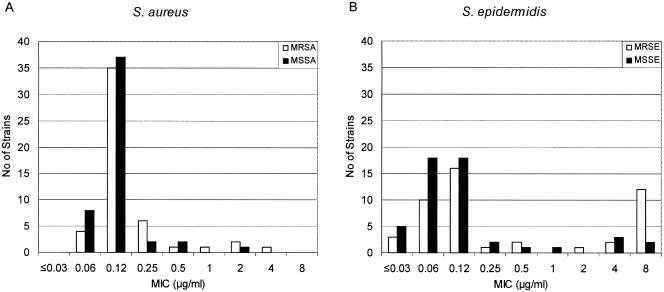
The distribution of MIC results for the S. aureus and S. epidermidis strains is shown in Fig. 1. Of the S. epidermidis strains the, triclosan MICs were above 0.5 μg/ml for 22%. This finding is in contrast to the S. aureus results, in which the triclosan MICs were greater than 0.5 μg/ml for only 5%. For the S. epidermidis isolates, decreased susceptibility to triclosan was more prevalent among the MRSE strains than among the MSSE strains: the triclosan MICs were greater than 0.5 μg/ml for 15 of the 47 MRSE isolates (32%), were only 6 of the 49 MSSE isolates (12%). As with the MRSE strains, the majority of the S. aureus strains with reduced susceptibility to triclosan (4 of 5) possessed the MRSA phenotype.
FIG. 1.
Distribution of triclosan MICs among S. aureus (A) and S. epidermidis (B) strains. Open bars indicate methicillin-resistant strains, and solid bars indicate methicillin-sensitive strains.
Our results confirm a relatively low proportion of S. aureus clinical isolates with reduced susceptibility to triclosan. We found that for 5% of the S. aureus population (n = 100), triclosan MICs were greater than 0.5 μg/ml. These results are very similar to those from other studies of S. aureus clinical isolates, in which triclosan MICs were greater than 0.5 μg/ml for 4.3% (n = 232) and 7.5% (n = 186) (1, 3).
The S. epidermidis isolates include a higher percentage of isolates with reduced triclosan susceptibility than do the S. aureus isolates. The reasons for the larger population of S. epidermidis isolates with decreased triclosan susceptibility may be greater selective pressure and exposure to triclosan (because of frequent contact with triclosan-containing antimicrobial products) of S. epidermidis strains than of S. aureus strains. Alternatively, the mechanisms and frequencies of resistance may be different in S. epidermidis and S. aureus.
The strains selected here were chosen to be diverse and representative, a design which may have introduced unintended biases. Nonetheless, we believe that these results warrant additional follow-up studies aimed to further understand and explore the extent of S. epidermidis strains with decreased susceptibility and the molecular mechanisms by which these strains are reducing their susceptibility to triclosan.
The importance of staphylococci with reduced susceptibility to triclosan in the clinical setting is uncertain. While the amount of triclosan found in products is generally quite high (2.5 mg/ml is typical in soaps [10]), the potency of triclosan appears to be reduced in the formulated product. Levy showed that the potency of triclosan against Escherichia coli was reduced by 10- to 20-fold in a soap formulation (10). Thus, the decreased susceptibility of S. epidermidis to triclosan observed here may pose some increased risk to patients. We conclude that caution should be observed in the use of triclosan-containing agents as antiseptics expected to limit the growth of S. epidermidis strains and that additional studies to understand the association between reduced triclosan susceptibility and methicillin resistance are warranted.
Acknowledgments
We thank Daniel Sahm, Renee Blosser, and James Karlowsky of Focus Technologies for performing these studies and providing excellent advice and counsel during our interpretation of the results.
REFERENCES
- 1.Al-Doori, Z., D. Morrison, G. Edwards, and C. Gemmell. 2003. Susceptibility of MRSA to triclosan. J. Antimicrob. Chemother. 51:185-186. [DOI] [PubMed] [Google Scholar]
- 2.Anonymous. 1998. Revised guidelines for the control of methicillin-resistant Staphylococcus aureus infection in hospitals. British Society for Antimicrobial Chemotherapy, Hospital Infection Society and the Infection Control Nurses Association. J. Hosp. Infect. 39:253-290. [DOI] [PubMed] [Google Scholar]
- 3.Bamber, A. I., and T. J. Neal. 1999. An assessment of triclosan susceptibility in methicillin-resistant and methicillin-sensitive Staphylococcus aureus. J. Hosp. Infect. 41:107-109. [DOI] [PubMed] [Google Scholar]
- 4.Boyce, J. M., and D. Pittet. 2002. Guideline for hand hygiene in health-care settings: recommendations of the Healthcare Infection Control Practices Advisory Committee and the HICPAC/SHEA/APIC/IDSA Hand Hygiene Task Force. Infect. Control Hosp. Epidemiol. 23:S3-S40. [DOI] [PubMed] [Google Scholar]
- 5.Chuanchuen, R., K. Beinlich, T. T. Hoang, A. Becher, R. R. Karkhoff-Schweizer, and H. P. Schweizer. 2001. Cross-resistance between triclosan and antibiotics in Pseudomonas aeruginosa is mediated by multidrug efflux pumps: exposure of a susceptible mutant strain to triclosan selects nfxB mutants overexpressing MexCD-OprJ. Antimicrob. Agents Chemother. 45:428-432. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Chuanchuen, R., C. T. Narasaki, and H. P. Schweizer. 2002. The MexJK efflux pump of Pseudomonas aeruginosa requires OprM for antibiotic efflux but not for efflux of triclosan. J. Bacteriol. 184:5036-5044. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Cookson, B. D., H. Farrelly, P. Stapleton, R. P. Garvey, and M. R. Price. 1991. Transferable resistance to triclosan in MRSA. Lancet 337:1548-1549. [DOI] [PubMed] [Google Scholar]
- 8.Fan, F., K. Yan, N. G. Wallis, S. Reed, T. D. Moore, S. F. Rittenhouse, W. E. DeWolf, Jr., J. Huang, D. McDevitt, W. H. Miller, M. A. Seefeld, K. A. Newlander, D. R. Jakas, M. S. Head, and D. J. Payne. 2002. Defining and combating the mechanisms of triclosan resistance in clinical isolates of Staphylococcus aureus. Antimicrob. Agents Chemother. 46:3343-3347. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Gilbert, P., and A. J. McBain. 2002. Literature-based evaluation of the potential risks associated with impregnation of medical devices and implants with triclosan. Surg. Infect. (Larchmt) 3(Suppl. 1):55-63. [DOI] [PubMed] [Google Scholar]
- 10.Levy, S. B. 2001. Antibacterial household products: cause for concern. Emerg. Infect. Dis. 7:512-515. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.McMurry, L. M., P. F. McDermott, and S. B. Levy. 1999. Genetic evidence that InhA of Mycobacterium smegmatis is a target for triclosan. Antimicrob. Agents Chemother. 43:711-713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.McMurry, L. M., M. Oethinger, and S. B. Levy. 1998. Triclosan targets lipid synthesis. Nature 394:531-532. [DOI] [PubMed] [Google Scholar]
- 13.National Committee for Clinical Laboratory Standards. 2000. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically—fifth edition. Approved standard M7-A5. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 14.Slater-Radosti, C., G. Van Aller, R. Greenwood, R. Nicholas, P. M. Keller, W. E. DeWolf, Jr., F. Fan, D. J. Payne, and D. D. Jaworski. 2001. Biochemical and genetic characterization of the action of triclosan on Staphylococcus aureus. J. Antimicrob. Chemother. 48:1-6. [DOI] [PubMed] [Google Scholar]
- 15.Suller, M. T., and A. D. Russell. 2000. Triclosan and antibiotic resistance in Staphylococcus aureus. J. Antimicrob. Chemother. 46:11-18. [DOI] [PubMed] [Google Scholar]