Abstract
The azole antifungal drugs that target lanosterol 14-α-demethylase, encoded by the ERG11 gene, are used to treat a variety of infections caused by Candida albicans. Azoles are known to induce expression of ERG11 mRNA. The ERG11 promoter was cloned 5′ of the luciferase-coding region, and the induction of ERG11 expression by azoles was monitored by luciferase assays. Maximal induction of the ERG11 promoter by azoles occurs not during logarithmic growth but after the diauxic shift and requires azoles to be present throughout logarithmic growth. The effects of pH, carbon source, and aerobic or anaerobic growth on induction of the ERG11 promoter by azoles were analyzed. Treatment with terbinafine and fenpropimorph, which target other enzymes in the ergosterol biosynthetic pathway, also resulted in a delayed induction of ERG11 promoter activity. Nascent sterol synthesis was shown to parallel ERG11 promoter activity, and total sterols were reduced coincident with the timing of ERG11 promoter activation. These results as a whole suggest that expression of the ERG11 promoter is regulated in response to sterol depletion.
Candida albicans is a pathogenic yeast that causes oral, vaginal, and systemic infections (26). Oral candidiasis is one of the earliest and most common opportunistic infections detected in human immunodeficiency virus-infected patients (11). C. albicans can also cause potentially life-threatening systemic mycoses in neutropenic patients (37). The drugs used at present to treat Candida infections include the polyenes and azoles, both of which target ergosterol or its biosynthesis. Azole-resistant C. albicans and intrinsically azole-resistant species such as Candida glabrata and Candida krusei have emerged as serious problems in patients receiving antifungal therapy (30).
The activities of the azoles, including the imidazoles (clotrimazole, miconazole, and ketoconazole) and the triazoles (fluconazole, itraconazole, and voriconazole), are directed against lanosterol 14-α-demethylase (Erg11p), which is encoded by the ERG11 gene (for a review, see reference 44). Erg11p is a cytochrome P450 enzyme involved in the biosynthesis of ergosterol and is essential in Saccharomyces cerevisiae under aerobic conditions (5). Two additional classes of antifungal drugs target other enzymes in the ergosterol pathway. The allylamines, such as terbinafine, inhibit Erg1p, upstream of Erg11p; and the morpholines, such as fenpropimorph, target Erg24p and Erg2p, both of which are downstream of Erg11p (for a review, see reference 44).
Several molecular mechanisms of drug resistance have been identified in C. albicans. Resistant clinical isolates have increased levels of mRNA for genes encoding the ATP binding cassette efflux pumps CDR1 and CDR2 (2, 25, 33, 41; M. Niimi, F. J. Fischer, J. Piper, H. F. Jenkinson, M. Arisawa, and R. D. Cannon, 13th Congr. Int. Soc. Hum. Anim. Mycol., abstr. P456, 1997), the major facilitator efflux pump MDR1 (33, 41; Niimi et al., 13th Congr. Int. Soc. Hum. Animal Mycol., 1997), and ERG11 (32, 41). In addition, point mutations and the loss of heterozygosity of ERG11 may contribute to azole resistance (9, 24, 42). The resistant isolates from among a series of 17 isolates from a human immunodeficiency virus-infected patient have been shown to use all of these resistance mechanisms (44).
Increased mRNA levels, as observed in the clinical isolates described above, may be the result of altered RNA transcription, processing, nuclear transport, or degradation. Gene expression is usually regulated at the transcriptional level (1). Nuclear run-on assays have been used to show that the increased mRNA levels observed in fluconazole-resistant clinical isolates correlate with increased levels of transcription of the MDR1, CDR1, and CDR2 genes (22). However, the sensitivity of the nuclear run-on assay was not sufficient to detect a change in ERG11 gene transcription.
Previous studies have shown that the amounts of ERG11 transcripts vary depending on the growth state of the culture in S. cerevisiae and C. albicans (12, 22, 39). Yeast cells undergo several phases of growth in vitro (for a review, see reference 40). When yeast cells have exhausted the glucose in the medium, growth temporarily arrests and the cells switch from fermentative to respiratory-based growth, referred to as the diauxic shift. In both S. cerevisiae and C. albicans, the levels of the ACT1 mRNA signal and other basic housekeeping transcripts decrease dramatically at the diauxic shift and are not detectable in the postdiauxic growth phase (22, 40), which makes Northern blots difficult to normalize and interpret at later time points of growth. The levels of mRNA for the efflux pumps and ERG11 have been found to vary depending on the growth state of the culture (16, 22).
This study investigated the transcriptional regulation of ERG11 by using a luciferase reporter gene system. The luciferase gene (RLUC) from Renilla neoformis has successfully been used for analysis of the promoters of the CDR1, CDR2, OP4, and WH11 genes in C. albicans (7, 17, 20, 28, 36). In this study, the effects of azoles and other sterol biosynthesis inhibitors on ERG11 promoter activity were examined under various environmental conditions. The results have implications for the azole susceptibilities of Candida isolates colonizing and infecting humans. Our overall results suggest that induction of the ERG11 promoter by azoles may be a result of the transcriptional regulation mediated by sterol depletion after prolonged growth of cultures in the presence of drug.
MATERIALS AND METHODS
Maintenance and growth of cultures.
C. albicans strain CAI8 (ade2::hisG/ade2::hisG, ura3::imm434/ura3::imm434) was generously provided by W. A. Fonzi (8). Cultures were routinely grown at 30°C in YEPD/U medium (10 g of yeast extract, 20 g of peptone, and 20 g of dextrose per liter supplemented with 50 μg of uridine ml−1) or on YEPD/U plates (YEPD/U medium with 10 g of Bacto Agar per 500 ml). To maintain the plasmid integrated in the ERG1200 oligonucleotide, cultures were grown on CSM-Ade (Bio 101, Inc., Vista, Calif.) agar plates and in CSM-Ade medium (0.75 g of CSM-Ade, 5 g of ammonium sulfate, 1.7 g of yeast nitrogen base without amino acids or ammonium sulfate, and 100 ml of 20% glucose per liter supplemented with 50 μg of uridine ml−1). CSM-Ade medium was used in all of the studies for which data are presented unless otherwise noted. Cells were subcultured routinely or were stored at −80°C in CSM-Ade or YEPD/U medium containing 10% glycerol.
Overnight cultures were inoculated with single colonies of each strain and grown at 30°C at 180 rpm. For standard growth, the overnight cultures were diluted to 4 × 106 cells ml−1 and grown in the appropriate medium with a carbon source (2% glucose unless otherwise specified) in the absence or presence of various drugs.
Construction of the Renilla reporter plasmid containing the ERG11 promoter.
The Renilla luciferase reporter plasmid pCRW3 was generously provided by David Soll (36). Plasmid pCRW3 contains a functional ADE2 gene, the ampicillin resistance (AMP) gene, the C. albicans autonomous replication site, and the R. reniformis luciferase (RLUC) gene with a multiple-cloning site (MCS) upstream of the RLUC ATG. For cloning, plasmid pCRW3 was digested with PstI and KpnI within the MCS upstream of RLUC and was purified as described previously (4, 23).
Oligonucleotides (Gibco BRL, Rockville, Md.) (Table 1) were synthesized to clone the ERG11 promoter into the MCS of pCRW3 at the PstI and KpnI sites. The PstERG-3 oligonucleotide is located at the ERG11 start codon. The ERG1200 oligonucleotide is located 1,200 bp upstream of the ATG codon immediately downstream of the adjacent coding region. The PstERG-3 and ERG1200 oligonucleotides were used to generate a full-length ERG11 promoter linked to RLUC. The PCR fragment was amplified from genomic DNA with Pfu polymerase (Stratagene, La Jolla, Calif.) according to the instructions of the manufacturer. The PCR fragment was purified with Centricon-100 columns (Millipore Corporation, Bedford, Mass.) according to the instructions of the manufacturer, digested with PstI and KpnI, and repurified. These digested PCR fragments were ligated to digested plasmid pCRW3 and transformed into competent DH5α cells (Gibco-BRL Life Technologies, Rockville, Md.). The plasmids were isolated from the cells by using a Plasmid Maxi kit (Qiagen Inc., Valencia, Calif.).
TABLE 1.
Oligonucleotide sequences used for ERG11 deletion constructs, screening by PCR, and Southern and Northern blot analyses
| Oligonucleotide name | Gene | GenBank accession no. | Nucleotide positionsa | Reference | Restriction enzyme | Sequenceb |
|---|---|---|---|---|---|---|
| ACT50 | ACT1 | X16377 | 2478-2527 | 21 | GATTTAGGTTTGGAAGCTGCTGGTATTGACC AAACCACTTTCAACTCC | |
| ADE2-11487R | ADE2 | 34 | CGTTTACTTGTTTAATATGC | |||
| ADE2-11545R | ADE2 | 34 | CAGTTAAATAGTCTTCATATC | |||
| ERG1200 | ERG11 | U67192 | 85-102 | 42 | KpnI | GGCGGTACCAGGAAAATGAAAGGGAC |
| pSP73-2358 | pSP73 | X65333 | 2358-2376 | 15 | CAGATTGTACTGAGAGTG | |
| PstERG-3 | ERG11 | U67192 | 1285-1266 | 42 | PstI | ATACTGCAGATTGAGTTATGATCTTCTTG |
| RLUC | RERLUC | M63501 | 62-41 | 36 | CACCACTGCGGACCAGTTATCATCCGTTTCC | |
| SP6 | pSP73 | X65333 | 1-18 | 15 | ATTAGGTGACACTATAG |
Nucleotide positions are according to the indicated references.
The underlined nucleotides in the sequences are restriction enzyme cutting sites.
Sequencing of ERG11 promoter construct.
The resulting ERG11 promoter construct was purified through G-50 columns (Amersham Pharmacia Biotech Inc., Piscataway, N.J.). The plasmid construct was sequenced with an ABI automated DNA sequencer with Taq dye-primer and dye-terminator chemistries (Applied Biosystems, Foster City, Calif.) by using oligonucleotides spanning the ERG11 promoter and oligonucleotides on the pCRW3 backbone, including SP6 and RLUC (Table 1). Constructs that had been amplified from either of the two alleles of the ERG11 promoter in strain SC5314 (contigs 6-1855 and 6-2172 [34]) were identified. Constructs from both alleles were tested in preliminary experiments and found to exhibit the same phenomena. A single construct containing allele 2 of ERG11 was selected for complete analysis. In addition, chimeras containing sequences from both alleles were identified and were not analyzed further (data not shown).
Electroporation transformation.
Competent C. albicans cells were transformed by electroporation by previously described methods (6, 38). Approximately 5 μg of plasmid DNA that had been linearized with NsiI was transformed into 40-μl aliquots of competent CAI8 cells. The electroporation conditions were set at 1.6 kV, 25 μF, and 200 Ω; and electroporation was performed with a Gene Pulser (Bio-Rad, Hercules, Calif.). After electroporation, 1 ml of YEPD (yeast extract-peptone-dextrose) was added to the cells and the mixture was transferred to 1.5-ml Eppendorf tubes. The cells were incubated at 30°C at 180 rpm for 60 to 90 min. The cells were pelleted, resuspended in 500 μl of sterile double-distilled H2O, plated onto CSM-Ade plates with 250 μl of culture per plate, and grown at 30°C for 3 to 5 days. Transformed colonies were serially streaked from single colonies onto CSM-Ade plates three times to ensure a homogeneous population.
PCR and Southern blot analysis of transformants.
Genomic DNA of the CAI8 cell transformants was prepared as described previously (13). The transformants were analyzed by PCR with oligonucleotides 1 to 4 (Fig. 1). The PCR conditions were as described above, and an annealing temperature of 49°C and 40 cycles were used. PCR-positive transformants were further analyzed by Southern blot analysis of genomic DNA digested with PstI, EcoRV, or XhoI and hybridized with radiolabeled oligonucleotide 1 to ensure integration of a single copy of the plasmid at the ADE2 locus of CAI8. The confirmed CAI8 transformant with the full-length ERG11 promoter region upstream of the ERG11 ATG codon fused to RLUC is designated CaErg1200.
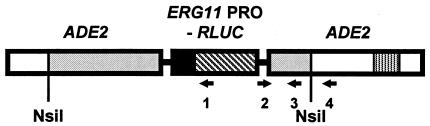
FIG. 1.
Site of integration for ERG11 promoter fusion. The ERG11 promoter (black box) fused to the RLUC reporter gene (box with diagonal lines) was flanked by the backbone from plasmid pCRW3 (thin lines). PCRW3 also contains a functional ADE2 gene (shaded boxes). The plasmid containing the promoter was cut at the NsiI site for integration into the ADE2 genomic locus (white boxes) containing a deletion of part of the coding region (box with vertical stripes) that makes the CAI8 parental strain Ade−. The correct single integration is shown. Oligonucleotides were used in PCRs to screen for correct integrations of the construct. Oligonucleotides 2 (pSP73-2358) and 3 (ADE2-11487R) generate a DNA fragment that is a part of the plasmid pCRW3 backbone, which is a test for the presence of the construct in the transformants. Oligonucleotides 2 and 4 (ADE2-11545R) test for the correct integration of the ERG11 promoter construct into the ADE2 locus of CAI8. Oligonucleotide 1 (RLUC) was used as a probe for Southern blot analyses to make sure that a single copy of the ERG11 promoter construct was integrated into the ADE2 locus. The figure shows the linear order of the genes and the relative positions of the oligonucleotides but is not drawn to scale.
Northern blot analysis and radiolabeled probes.
CaErg1200 was grown for 6 h to reach logarithmic growth (2 × 107 to 3 × 107 cells ml−1). Total RNA was prepared, and Northern blotting was performed as described previously (4, 23, 31). The DNA probes used included a plasmid containing the ERG11 gene (positions 184 to 1589; GenBank accession number X13296 [18]) and ACT50 (Table 1). mRNA signals were quantitated by using Storm Phosphorimager and ImageQuaNT software (Molecular Dynamics, Sunnyvale, Calif.). The ERG11 mRNA signals were normalized to the ACT1 mRNA signals. Relative intensities were calculated by comparing the normalized ERG11 mRNA signals to the lowest normalized ERG11 mRNA signal.
When the oligonucleotides were used as probes, they were end labeled with [γ-32P]ATP and polynucleotide kinase (23, 31). Plasmids were labeled by random priming with [α-32P]dATP and the large subunit of DNA polymerase I (Klenow fragment) (23, 31).
Drugs and chemicals in ERG11 induction experiments.
The drugs used for this study included fluconazole dissolved in water (stock concentration, 3.33 mg ml−1; final concentration, 1 to 100 μg ml−1; Pfizer, New York, N.Y.), miconazole dissolved in dimethyl sulfoxide (DMSO; stock concentration, 10 mg ml−1; final concentration, 0.1 to 10 μg ml−1; Sigma-Aldrich, St. Louis, Mo.), clotrimazole dissolved in DMSO (stock concentration, 10 mg ml−1; final concentration, 0.1 to 10 μg ml−1; Sigma-Aldrich), terbinafine dissolved in DMSO (stock concentration, 10 mg ml−1; final concentration, 1 to 100 μg ml−1; Novartis, Vienna, Austria), and fenpropimorph dissolved in DMSO (stock concentration, 10 mg ml−1; final concentration, 1 to 100 μg ml−1; Sigma-Aldrich). For the pH studies, the CSM-Ade media were buffered to pH 7.0 with 100 mM sodium citrate.
Aerobic cultures were grown in 40 ml of culture medium in 500-ml baffled flasks at 180 rpm. Semianaerobic cultures were grown as described previously (39). Briefly, 30 ml of culture was grown in 250-ml nonbaffled flasks at 50 rpm. To minimize oxygen exchange, the semianaerobic cultures had an overlay of 15 ml of mineral oil in screw-cap culture flasks. The caps were tightened, and the tops of the flasks were covered with Parafilm.
In vitro luciferase assays.
Luciferase assays were performed as described previously (20, 36), with the following modification. To accurately determine the protein concentrations of the cell extracts, bovine serum albumin (BSA) was omitted from the previously described (36) RLUC buffer (final composition, 0.5 M NaCl, 0.1 M K2HPO4 [pH 6.7], 0.6 mM sodium azide, 1 mM disodium EDTA). Cell extracts were prepared in the presence and absence of BSA in the buffer, and no differences in the activities were detected (data not shown). In addition, extracts were prepared by vortexing the cells at maximum speed for five cycles of 30 s of beating with an intervening 30 s of chilling at 4°C. The bottom of the Eppendorf tube was pierced with a heated 22-gauge needle and placed on top of another Eppendorf tube. These tubes were placed in a 15-ml conical tube and spun at 2,000 × g for 1 min to collect the flowthrough. The cell extracts were spun at 16,000 × g for 15 min at 4°C to remove the cell debris, and the supernatants were transferred to new tubes. These clear supernatants of the cell extracts were immediately used for the luciferase assay.
Luciferase assays were performed with a Monolight 2010 luminometer (Analytical Luminescence, San Diego, Calif.). A total of 20 μl of the cell extract was placed in a luminometer tube (PharMingen, San Diego, Calif.). RLUC buffer supplemented with 0.02% BSA, 1 mM PefablocSC, and 0.5 μM coelentrazine (Molecular Probes, Inc., Eugene, Oreg.) was used to read the luciferase activity. The samples were tested in duplicate. The total amount of protein in each extract was measured by the Bradford assay (Bio-Rad Laboratories), according to the instructions of the manufacturer, by using BSA as a standard. The specific activity of a sample was defined as the luciferase activity (in units) obtained with the luminometer divided by the total protein concentration of the cell extract (in micrograms) (specific activity = units micrograms−1). The fold drug induction was calculated as the specific activity of the samples grown in the presence of drug divided by the specific activity of the samples grown in the absence of drug.
The absolute levels of luciferase can vary from experiment to experiment. Therefore, statistical analysis of independent experiments is generally not feasible. The data presented here represent the results of one typical experiment. In general, the experiments for determination of luciferase activity were repeated at least twice.
Measurement of ergosterol and sterol production over time.
To measure the level of sterol synthesis at several time points, cells were labeled with [14C]acetic acid and the sterols were extracted and analyzed by thin-layer chromatography (TLC). Briefly, overnight cultures of parental strain SC5314 were inoculated in YAD medium (1.7 g of yeast nitrogen base without ammonium sulfate, 5 g of ammonium sulfate, 20 g dextrose per liter). Two cultures at an optical density (OD) at 600 nm (OD600) of 0.1, one with and one without fluconazole (32 μg ml−1), were inoculated and grown with shaking at room temperature. At 3, 21, and 45 h, the OD of each culture was determined; and 3 ml of each culture was transferred to a new tube containing 30 μl of [14C]acetic acid (sodium salt; 0.1 μCi μl−1 and 54 mCi mmol−1 in ethanol; Amersham). The cultures were labeled for 3 h, and the cells were pelleted. The pellets were stored at −80°C until they were processed.
The [14C]acetic acid pulse of 3 h was determined experimentally in preliminary experiments. After addition of the pulse, incorporation into sterols is complete by 3 h.
The cell pellet was resuspended in 1 ml of water and transferred to a glass tube, and 1 ml of KOH in ethanol (2.7 ml of 10 M KOH, 7.3 ml of 100% ethanol) was added. The tube was covered and incubated at 80°C for 1 h. The tube was cooled and extracted with 3 ml of petroleum ether (boiling point, 40 to 60°C). The petroleum ether phase was removed and placed in a new glass vial. The KOH-ethanol was extracted a second time with petroleum ether, and the two ether phases were pooled. The pooled samples were evaporated to dryness by using a gentle stream of N2 and heating at 50°C. Each dried sample was dissolved in a small volume (∼100 μl) of petroleum ether and spotted 1.5 cm from the bottom of a TLC plate (LK6 TLC plate [catalog no. 4865 820]; Whatman). Unlabeled ergosterol (Sigma) was also spotted onto the TLC plate, adjacent to the labeled samples, and used as a standard. The plate was placed in a TLC tank containing toluene-diethyl ether (9:1 [vol/vol]). The plate was removed from the tank when the solvent was ∼1 cm from the top of the plate. After the plate was dried overnight in a fume hood, the plate was wrapped in Saran wrap and exposed to a Molecular Dynamics phosphorimager cassette for 24 to 48 h. The phosphorimager screen was scanned, and the intensities of the regions corresponding to radioactive bands on the TLC plate were measured by using the ImageQuant program. The unlabeled ergosterol standard was detected on the plate by detection of ergosterol absorbance with UV light. Total sterol synthesis was calculated as the sum of the intensities of all bands that migrated from the origin. Ergosterol synthesis was calculated as the intensity of the band that comigrated with the ergosterol standard. Sterol and ergosterol levels were normalized to the number of cells, as determined by measurement of the OD600. The fold induction of sterol or ergosterol synthesis was calculated as the levels in the presence of drug divided by the levels in the absence of drug.
Total ergosterol levels.
Total ergosterol levels were determined as described previously (3). Cells were grown in YAD medium for the appropriate times (6, 24, and 48 h) in the presence and the absence of drug. For the 6-h time points, the culture volumes were increased up to 10-fold to obtain sufficient signals. Equivalent cell numbers (as determined by measurement of the OD) were determined at each time point.
RESULTS
Construction of C. albicans strains expressing luciferase from the ERG11 promoter.
A previous study (12) used dot blots of total RNA to show that the level of endogenous ERG11 mRNA increases two- to fivefold in the presence of azoles during mid-logarithmic growth. However, there are technical difficulties in analyzing promoter activities under a variety of conditions by using total RNA in dot blots and/or Northern blots, especially in promoter deletion analyses (see Discussion). To avoid these problems, the ERG11 promoter was linked to the RLUC luciferase gene and integrated as a single copy in the C. albicans genome, creating strain CaErg1200 (see Materials and Methods for details).
Sequence analysis of ERG11 promoter constructs and allelic differences.
The plasmid construct was sequenced to detect errors that might be introduced during PCR. Two alleles of the ERG11 promoter have been identified in laboratory strain SC5314 (contigs 6-1855 and 6-2172 [34]). These two promoter alleles contain 23 nucleotide differences and an insertion of thymidine in allele 2 between positions −288 and −289 bp upstream of the ATG codon compared to the sequence of allele 1. The first 269 bp of the ERG11 promoter sequence in the two alleles are identical. The allele 2 ERG11 promoter sequence from SC5314 (contig 6-2172) closely resembles the ERG11 promoter in another strain, strain SS (42). Reverse transcription-PCR and restriction fragment length polymorphism analysis of the ERG11-coding region demonstrated that both alleles of ERG11 in SC5314 are expressed during induction by fluconazole (data not shown). Constructs containing both alleles and chimeras were tested in preliminary tests and were shown to exhibit the same trends (data not shown). The CaErg1200 construct encoding allele 2 of SC5314 was selected for further analysis.
Regulation of ERG11 is at the transcriptional level.
While luciferase assays are preferable to Northern or dot blot analysis for promoter characterization, it is important to demonstrate that the two techniques are comparable. Therefore, the level of induction of ERG11 mRNA by azoles, as measured by Northern blot analysis, was compared to the level of induction of ERG11 promoter activity by azoles, as evaluated by measurement of luciferase activity (Table 2). CaErg1200 was grown in medium containing 2% glucose or 2% galactose in the absence or presence of miconazole for 6 h, starting with 4 × 106 cells ml−1. Miconazole was chosen on the basis of the results presented below. Regulation of ERG11 gene expression may be regulated by carbon source in S. cerevisiae (14). Therefore, both galactose and glucose carbon sources were used. The RNA samples for the Northern blot analysis and the cell extracts for the luciferase assays were simultaneously prepared from the same cultures. Northern blots were probed with ERG11 and ACT1. The ERG11 mRNA signal was normalized to the ACT1 signal, and the relative levels of gene expression were determined by comparing each normalized signal to that of the sample with the lowest level of ERG11 expression (galactose without drug; Table 2). In the absence of drug, the ERG11 mRNA levels from cells grown in glucose were twofold higher than the ERG11 mRNA levels from cells grown in the presence of galactose. Miconazole induced the ERG11 mRNA levels 10-fold when the cells were grown in the presence of galactose and 4.5-fold when the cells were grown in the presence of glucose.
TABLE 2.
Comparison of the results of Northern blot analysis and those of the luciferase assay at 6 h of growth
| Drug | Relative level with the indicated carbon source
|
|||
|---|---|---|---|---|
|
ERG11 mRNA levelsa
|
RLUC sp act
|
|||
| Glucose | Galactose | Glucose | Galactose | |
| None | 2.0 | 1.0 | 2.0 | 1.0 |
| Miconazole | 9.0 | 10.0 | 4.5 | 4.4 |
| Fold inductionb | 4.5 | 10.0 | 2.3 | 4.4 |
ERG11 mRNA signals were quantified and normalized to the ACT1 mRNA signal. Relative intensities were calculated by comparing the normalized ERG11 mRNA signals to the lowest normalized ERG11 signal (galactose without drug).
Fold induction is the ratio of normalized mRNA signals or specific activities for azole-treated cells compared to those for untreated cells.
The results of the luciferase assay correlated with the results of the Northern blot analysis. The relative specific activity of luciferase under each condition was compared to that for the sample with the lowest level of specific activity of luciferase (galactose without drug). In the absence of drug, the ERG11 promoter activity in cells grown in the presence of glucose was twofold higher than that in cells grown in the presence of galactose, which was the same difference observed by Northern blot analysis. Miconazole induced ERG11 promoter activity 2.3-fold in cells grown in the presence of galactose and 2.2-fold in cells grown in the presence of glucose. The difference observed between the luciferase assays and Northern blot analyses may be a result of intrinsic differences between the two assays. These results indicate that the regulation of ERG11 is at least in part at the transcriptional level and that the luciferase assay is a valid method for examination of ERG11 gene regulation.
Prolonged incubation with azoles is required for ERG11 induction.
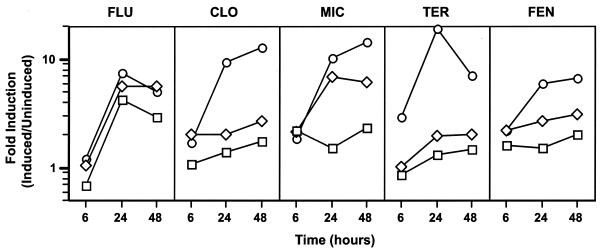
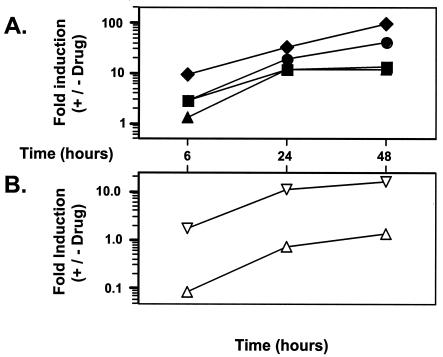
Three different azoles were tested for their effects on ERG11 transcription in CaErg1200. Clotrimazole, fluconazole, and miconazole were tested over a range of concentrations at 6 h (logarithmic growth), 24 h (late logarithmic growth), and 48 h (postdiauxic growth) in the presence of glucose (Fig. 2). The drug concentrations used in this experiment were selected to bracket the clinical breakpoint for each of the drugs. The clinical breakpoint for fluconazole (MIC ≥ 64 μg ml−1) is at least 10-fold greater than the clinical breakpoint for itraconazole (MIC ≥ 1 μg ml−1) (29). The distributions of the MICs for collections of clinical isolates and the serum drug levels are similar for itraconazole, clotrimazole, and miconazole (43), suggesting that the breakpoints for the three drugs are similar, although the breakpoints for clotrimazole and miconazole have not been determined. For the three azoles tested, minimal induction (≤2.2-fold) of the ERG11 promoter was observed at 6 h (Fig. 2). At 24 h, the level of induction was concentration dependent for all three azoles. The levels of induction by clotrimazole and miconazole at the highest drug concentrations tested were higher at 48 h than at 24 h. The level of induction by fluconazole appeared to be maximal at 24 h. Therefore, the level of induction of CaErg1200 by all three azoles was maximal in the late-logarithmic and postdiauxic stages of growth. On the basis of the results of this experiment, 10 μg of clotrimazole and miconazole ml−1 and 100 μg of fluconazole ml−1 were used for subsequent drug studies.
FIG. 2.
Time course of induction by drugs. CaErg1200 was grown in the absence or presence of fluconazole (FLU), clotrimazole (CLO), miconazole (MIC), terbinafine (TER), and fenpropimorph (FEN) at different concentrations. Fluconazole, terbinafine, and fenpropimorph were used at 1, 10, and 100 μg ml−1 (squares, diamonds, and circles, respectively). Clotrimazole and miconazole were used at 0.1, 1.0, and 10 μg ml−1 (squares, diamonds, and circles, respectively). Samples were collected at 6, 24, and 48 h. Fold induction is defined as the specific activity in the presence of drug divided by the specific activity in the absence of drug.
Timing of induction by azoles.
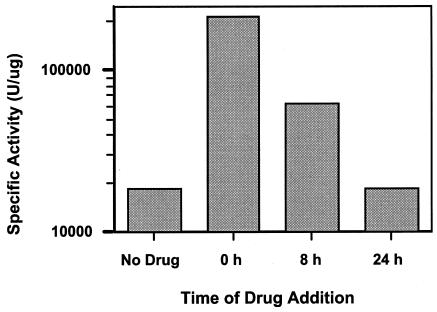
Because maximal induction by the azoles was observed between 24 and 48 h, it was of interest to determine if the induction by the azoles at 24 to 48 h was the result of a specific effect of the azole at that time or if the azole must be present throughout the period of growth. CaErg1200 cultures were grown for 48 h and then tested for their luciferase activities. Miconazole was added to separate cultures at specific times during the 48 h of growth. Miconazole was added at time zero (4 × 106 cells ml−1), 8 h (5.7 × 107 cells ml−1), or 24 h (1.5 × 108 cells ml−1) of growth (Fig. 3). After 48 h of growth the CaErg1200 cultures were tested for their luciferase activities. A 12-fold increase was observed when the drug was added at time zero, a 3.4-fold increase was observed when the drug was added at 8 h, and no induction was observed when the drug was added at 24 h. This lack of induction by the azoles after 24 h in postdiauxic growth (24 to 48 h after inoculation) is in contrast to the induction by the azoles observed after 24 h of logarithmic growth (10-fold induction; Fig. 2). Therefore, the azole must be present for 24 to 48 h throughout the logarithmic stage of growth to maximally upregulate ERG11 transcription.
FIG. 3.
Time course of miconazole addition. CaErg1200 was grown in the absence or presence of miconazole (10 μg ml−1). Cultures were started with 4 × 106 cells ml−1. Miconazole was added to the separate cultures at 0, 8, and 24 h of growth; and all cultures were grown for 48 h, when the luciferase assays were performed.
Lower endogenous ERG11 promoter activity at low pH.
As yeast cultures grow, the pH of the culture decreases, which may have an effect on ERG11 expression. Growth media were prepared with glucose and galactose as carbon sources with or without citrate buffer at pH 7. CaErg1200 was grown in these media for 24 and 48 h to assess the effect of pH on ERG11 promoter activity, with comparable results obtained for both periods of growth. The data obtained for 48 h of growth are shown in Table 3. Although the data obtained for 24 h of growth (not shown) were comparable to those for 48 h of growth, maximum induction by the azoles was not observed until 48 h. At 48 h, the specific activity of the uninduced ERG11 promoter in the presence of buffered (pH 7) medium was higher than the specific activity of the promoter in unbuffered medium, in which the pH was reduced to less than 5. The fold induction by miconazole at 48 h in buffered media was less than half of that in unbuffered media for both carbon sources. In summary, buffering of the growth medium results in less induction by the azoles, but this may be due in part to the higher levels of uninduced ERG11 promoter activity in buffered media.
TABLE 3.
ERG11 promoter activity in buffered media at 48 h
| Drug | Sp act (U μg−1) with the following carbon source and buffered or unbuffered mediuma:
|
|||
|---|---|---|---|---|
| Glucose
|
Galactose
|
|||
| − | + | − | + | |
| None | 5.0 × 103 | 7.2 × 103 | 2.8 × 103 | 4.7 × 103 |
| Miconazole | 5.5 × 104 | 3.0 × 104 | 5.1 × 104 | 3.3 × 104 |
| Fold induction | 11 | 4.2 | 18 | 7.0 |
−, unbuffered medium (pH <5); +, buffered medium (pH 7.0).
Semianaerobic conditions induce ERG11 expression.
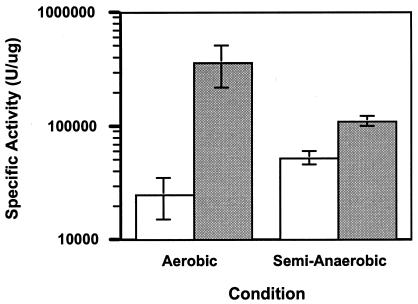
ERG11 expression in Saccharomyces is known to be regulated by aerobic versus anaerobic growth (39). The luciferase activity of CaErg1200 was monitored during aerobic and semianaerobic growth in the presence or absence of miconazole at 48 h (Fig. 4) and 24 h (data not shown; the data for 24 h of growth were very similar to those for 48 h of growth). Miconazole induced ERG11 expression 15-fold under aerobic conditions and 2-fold under semianaerobic conditions. The higher levels of specific activity in the sample in which ERG11 expression was not induced and the lower levels of specific activity in the miconazole-treated sample resulted in an overall lower level of induction by miconazole under semianaerobic conditions compared to that under aerobic conditions.
FIG. 4.
ERG11 promoter activity under aerobic and semianaerobic conditions. CaErg1200 was grown aerobically or semianaerobically as described in Materials and Methods. Cells were grown in the absence (white bars) or presence (gray bars) of miconazole (10 μg ml−1). The average of three independent experiments, with error bars, is shown.
Effects of carbon sources on ERG11 induction by azoles.
As a yeast cell grows, it undergoes a diauxic shift that changes the yeast's metabolism. As cells deplete the glucose in the medium, respiratory carbon sources are used (14). In addition, glucose may repress ERG11 expression in Saccharomyces (14). A time course experiment with the fermentable carbon sources glucose, galactose, and maltose and with the respiratory carbon glycerol was conducted to determine the effects of these carbohydrates on ERG11 promoter regulation (Fig. 5A). CaErg1200 was grown in the presence of these carbon sources and in the presence or absence of an azole for 6, 24, and 48 h. At 6 h, the ERG11 promoter was induced 9-fold with galactose, 2.9-fold with glucose and maltose, and 1.3-fold with glycerol. This suggests that glucose (and maltose) may repress the ERG11 promoter or that galactose may activate the ERG11 promoter.
FIG. 5.
(A) ERG11 promoter activity in the presence of various carbon sources. CaErg1200 was grown in 2% glucose (YAD; closed squares) or in 2% galactose (closed diamonds), 2% maltose (closed circles), or 3% glycerol (closed triangles), where one of the last three carbon sources replaced the dextrose (glucose) in YAD. Luciferase assays were conducted at 6, 24, and 48 h. The y axis represents the ratio of the specific activity of luciferase in the presence of drug (miconazole at 10 μg ml−1) divided by the specific activity of luciferase in the absence of drug. (B) Sterol synthesis during growth. The total levels of sterol synthesis (open inverted triangles) and ergosterol synthesis (open triangles) of cells grown in YAD were measured as described in Materials and Methods. Raw values were normalized to cell number. The y axis represents the ratio of the normalized levels of sterols or ergosterol in the presence of drug (fluconazole at 32 μg/ml−1) to the normalized levels of sterols or ergosterol in the absence of drug. The sterols were labeled for 3 h before measurement (at 3, 21, and 45 h).
At 24 and 48 h, the level of induction of the ERG11 promoter by the azoles increased in the presence of all carbon sources compared to that at 6 h (4.5-, 10-, 14-, and 9.2-fold for glucose, galactose, maltose, and glycerol, respectively). The fold induction for all carbon sources suggests that the fold induction seen at 24 or 48 h is not the result of a shift in carbon source. The results again suggest that glucose has less of an effect than the other carbon sources. The carbon source of the cells affects the specific activity of the ERG11 promoter in both the presence and the absence of drug (data not shown), suggesting that a carbon source shift is not the primary reason for the fold induction over time.
Regulation of ERG11 may be a general response to the amounts of sterols in cells.
The delayed induction of the ERG11 promoter by the azoles may be due to the depletion of ergosterol stores over time in the presence of sterol biosynthesis inhibitors, such as azoles. Therefore, it was of interest to test the effects of two other sterol inhibitors, terbinafine and fenpropimorph, on ERG11 promoter activity. A time course experiment similar to the azole time course experiment described above was performed with CaErg1200 and terbinafine or fenpropimorph (Fig. 2). The concentrations of drugs tested were 1, 10, and 100 μg ml−1; and samples were collected at 6, 24, and 48 h. For both drugs, 100 μg of drug ml−1 induced ERG11 promoter activity to a significant level. Overall, both terbinafine and fenpropimorph were able to induce ERG11 promoter activity over 24 to 48 h, indicating that drugs that inhibit different parts of the ergosterol biosynthetic pathway have similar delayed effects on induction of ERG11 promoter activity.
If the depletion of endogenous ergosterol levels resulted in delayed induction by the azoles, then exogenous ergosterol might reduce the induction effect if the cells took it up. However, previous work with Saccharomyces indicated that ergosterol is not imported into the cell under aerobic conditions (35). Exogenous ergosterol was added to the growth medium for Candida strain CaErg1200, as described previously (39), but had no effect on induction by the azoles (data not shown).
Sterol production parallels promoter activity.
The results from the ERG11 promoter analyses indicate that ERG11 promoter activity is increased at 24 to 48 h in the presence of azole drugs. To study the potential consequence of this induction, the level of sterol synthesis at different times was measured by radiolabeling sterols for 3-h intervals at 3, 21, and 45 h of cell growth in the presence and absence of azoles (see Materials and Methods for details). The level of synthesis of total sterols was not significantly affected at 6 h but was affected at 24 and 48 h (Fig. 5B), in a manner similar to the activities of the drugs on the ERG11 promoter (Fig. 5A).
Synthesis of ergosterol was also monitored at these time points (Fig. 5B). Azole treatment significantly reduced the level of ergosterol synthesis (greater than 10-fold) by 6 h, as expected for a sterol biosynthesis inhibitor. However, the levels of ergosterol synthesis were increased at 24 and 48 h, consistent with the increased level of ERG11 promoter activity described above. The levels of sterol and ergosterol production (Fig. 5B) are measures of the levels of synthesis at the time of radiolabel addition and not the total levels of sterols or ergosterol at these times.
Total ergosterol levels are eliminated in the presence of azole drugs.
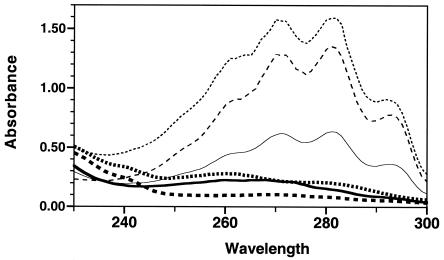
The level of induction of the ERG11 promoter by the azoles may be the result of the depletion of ergosterol levels. A previously described (3) quantitative spectrophotometric test was used to directly measure ergosterol levels. Measurements were taken at 6, 24, and 48 h. As seen in Fig. 6, ergosterol levels were clearly detectable at 6 h and were substantially increased at 24 and 48 h in the absence of azoles. Essentially no ergosterol signal was detectable at 6, 24, or 48 h in the presence of azoles. Therefore, the lack of ergosterol in the presence of drug becomes more significant at 24 to 48 h, when sterol levels are normally increasing in the absence of drug. The increasingly significant lack of ergosterol may trigger a feedback mechanism that increases the level of ERG11 expression and the levels of expression of other enzymes involved in the ergosterol biosynthetic pathway.
FIG. 6.
Total ergosterol levels. Parental strain SC5314 was analyzed for its ergosterol content as described previously (3). The absorbance scans detected only ergosterol and 24(28)-dehydroergosterol. Cells were grown in YAD medium for 6 h (solid lines), 24 h (lines with short dashes), or 48 h (lines with long dashes). The cells were grown in the absence (thin lines) or presence (thick lines) of fluconazole (32 μg/ml). For normalization, equal amounts of cells were processed and analyzed for each time point (amount of culture used = 300/OD of culture).
DISCUSSION
This study analyzed the expression of the C. albicans ERG11 gene under a variety of conditions by using the ERG11 promoter fused to the R. reniformis RLUC reporter system. The results of both the luciferase assays and the Northern blot analyses indicate that azole drug treatment affects the transcriptional regulation of the ERG11 promoter. The level of induction by azoles is maximal during postdiauxic growth. Other sterol biosynthesis inhibitors have a similar effect. This induction of the ERG11 promoter by azoles is affected by the carbon source, pH, and anaerobic conditions. The level of sterol synthesis parallels the level of ERG11 promoter activity, and total sterols are depleted as the ERG11 promoter is induced. All of these results are consistent with the hypothesis that the transcriptional regulation of ERG11 is a response to sterol depletion within the cells.
The induction of ERG11 by the azoles was examined by Northern blot analysis for determination of the level of mRNA and by luciferase assays for determination of the level of promoter activity (Fig. 2 and Table 2). The lower relative intensity and fold induction detected by luciferase assays compared to those detected by Northern blot analysis may be due to the intrinsic differences between the two assays and/or the differences in sample processing times. A recent study of the CDR1 promoter with the RLUC reporter system also showed two- to threefold lower levels of activity by the luciferase in comparison to that obtained by Northern blot analysis (7). The results showed that the two assays gave comparable results when ERG11 promoter activity was measured at 6 h. In total RNA prepared from 24- and 48-h cultures, the levels of ERG11 and RLUC RNA were too low to be detected by Northern blot analyses (data not shown) but were measurable by the luciferase assays. Therefore, luciferase assays can be used to directly monitor ERG11 promoter activity. The results suggest that the mechanism of induction of ERG11 by azoles is at least in part at the level of transcriptional regulation.
Repeated attempts to determine the half-life of the luciferase protein have been confounded by Candida's intrinsically high level of resistance to drugs that inhibit cellular transcription and translation, including cycloheximide (J. B. Harry and T. C. White, unpublished data). However, preliminary data suggest that the half-life of the luciferase protein is less than 3 h (J. B. Harry, J. L. Song, and T. C. White, unpublished data). Therefore, the induction by the azoles observed at 24 and 48 h was not the result of the accumulation of luciferase protein over a long period of time.
Increased ERG11 promoter activity may be the result of either the accumulation of lanosterol or the depletion of ergosterol. To study these possibilities, drugs that target other enzymes in the ergosterol biosynthetic pathway both upstream and downstream of Erg11p were analyzed by luciferase assays. High terbinafine and fenpropimorph concentrations significantly induced ERG11 promoter activity after 24 to 48 h of treatment (Fig. 2). The drop in the level of induction of ERG11 promoter activity by terbinafine at 48 h may be due to decreased drug stability or removal of the drug by efflux pumps. Recently, terbinafine has been found to induce CDR1 expression, suggesting that it might be a substrate for CDR1 (7). These results suggest that ERG11 may be subject to feedback regulation by the depletion of ergosterol and argue against regulation due to the accumulation of lanosterol alone.
On the basis of the results of the present study and previous research, we hypothesize that the induction of ERG11 at 24 to 48 h is a delayed transcriptional upregulation of ERG11 in response to azoles and is a response to gradual sterol and ergosterol depletion within the cells. Previous studies with mammalian cells and S. cerevisiae indicate that transcriptional regulation of several genes involved in sterol metabolism is dependent on the level of intracellular sterols (10, 19). Two factors determine the level of induction of ERG11 by the azoles in C. albicans: the length of time that cells are grown in the presence of azoles and the growth state of the cells when the azoles are introduced into the culture. Maximal induction of the ERG11 promoter by the azoles in C. albicans requires the presence of azoles throughout logarithmic growth (Fig. 2 and 3), which would gradually reduce sterol levels. Cells require ergosterol when they are actively dividing during the logarithmic phase of growth; therefore, ergosterol is both being used by the cells and being inhibited by the azoles. In addition, the level of induction of ERG11 by the azoles was maximal from 24 to 48 h, suggesting that cells have depleted their pools of sterols over time (Fig. 6) and have upregulated sterol synthesis (Fig. 2) and increased their levels of sterol synthesis (Fig. 5B). This is consistent with previous research in which fluconazole and other azoles were observed to increase total ERG11 RNA levels two- to fivefold when azoles were introduced in the mid-logarithmic phase of growth with 3 × 107 to 5 × 107 cells ml−1 (12). During the late-logarithmic phase of growth, cells are more quiescent and have fewer requirements for ergosterol. Therefore, when azoles are introduced into the cells during the late-logarithmic phase of growth for the same length of time, induction of ERG11 does not occur (Fig. 2 and 3). In these cells, even though azoles inhibit the synthesis of ergosterol in the late-logarithmic phase of growth, they have sufficient sterols for their growth state. Results from this study suggest that the transcriptional regulation of the ERG11 promoter in C. albicans may respond to ergosterol feedback regulation, similar to genes involved in sterol synthesis in mammalian cells and S. cerevisiae. Attempts to abolish induction of ERG11 by the azoles with exogenous ergosterol have been unsuccessful (data not shown), most likely because of the inability of Candida cells to import ergosterol, similar to what is observed in S. cerevisiae (27).
The growth state of C. albicans in the human host during infection is unknown. Therefore, it is important to examine the transcriptional regulation of ERG11 under various environmental conditions that might mimic in vivo conditions. Hap1p regulates ERG11 promoter activity in S. cerevisiae, and its function may be repressed by glucose (14). The results in Fig. 5A do suggest that in the presence of glucose the level of induction by the azoles is lowest at 6 h.
The effect of pH on ERG11 promoter activity was examined, as mucosal surfaces and the bloodstream have different pHs in the human host. The results indicated that ERG11 promoter activity was higher when the pH was buffered to pH 7. The twofold lower level of induction by the azoles observed in the buffered media may be a result of the pH or the presence of the sodium citrate component of the buffer.
Under semianaerobic conditions in vitro, miconazole had a reduced effect on ERG11 promoter activity (Fig. 4). This is consistent with the function of lanosterol 14-α-demethylase, which is to catalyze the oxidative removal of the C-32 methyl group of lanosterol. Since Erg11p cannot function maximally under semianaerobic conditions, miconazole had a minimal effect on the transcriptional regulation of ERG11. The level of ERG11 promoter activity in the absence of miconazole was increased when oxygen levels were limited, indicating that ERG11 of C. albicans may be regulated by a repressor that is orthologous to Rox1p in S. cerevisiae. In an environment with limited oxygen levels, such a repressor may be removed, which would result in the upregulation of ERG11. These results may be of clinical importance, as aerobic and semianaerobic conditions may mimic the conditions of cell growth in the bloodstream and in the oral and vaginal cavities.
The observation that the level of sterol synthesis parallels the level of ERG11 promoter activity (Fig. 5A and B) suggests that the entire ergosterol biosynthetic pathway is upregulated at the same time as ERG11 promoter activity. Alternately, although it is unlikely, Erg11p could be the limiting enzyme in the pathway.
The measurement of total ergosterol levels (Fig. 6) demonstrates that azole drugs eliminate detectable ergosterol within 6 h. It also indicates that in the absence of drug, total ergosterol levels are increased substantially between 6 and 24 h. These increased ergosterol levels are likely to include the excess ergosterol stored as sterol esters in the cell as lipid particles (45). The increased level of ERG11 transcription between 6 and 24 h in the presence of drug may be a programmed cellular response to the lack of increased ergosterol levels during that time.
The results of this study strongly suggest that the transcriptional regulation of ERG11 in C. albicans responds to the sterol levels within the cells. ERG11 promoter activity is affected by the growth state, carbon source, external pH, and semianaerobic growth of the cells. The level of ERG11 promoter activity parallels the rates of sterol and ergosterol synthesis and appears to be a response to lower total sterol levels. This study contributes to the basic knowledge of the ERG11 promoter that is paramount to the understanding of the interactions of C. albicans with the azoles and azole resistance in C. albicans.
Acknowledgments
We thank William Fonzi (Georgetown University, Washington, D.C.) for providing us with strain CAI8 and David Soll (University of Iowa, Iowa City) for Renilla luciferase reporter plasmid pCRW3. We thank members of the laboratory of T.C.W. for valuable comments and support.
This research was funded by NIH NIDCR grants R01 DE11367 and R01 DE14161. J.L.S. was supported by NIH pathobiology training grant T32 AI 07509. J.B.H. was supported by NIAID NRSA grant 1F32 AI10497-01. T.C.W. was the recipient of a New Investigator Award in Molecular Pathogenic Mycology from the Burroughs Wellcome Fund.
REFERENCES
- 1.Alberts, B., D. Bray, J. Lewis., M. Raff, K. Roberts, and J. D. Watson. 1994. Molecular biology of the cell, 3rd ed. Garland Publishing, Inc., New York, N.Y.
- 2.Albertson, G. D., M. Niimi, R. D. Cannon, and H. F. Jenkinson. 1996. Multiple efflux mechanisms are involved in Candida albicans fluconazole resistance. Antimicrob. Agents Chemother. 40:2835-2841. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arthington Skaggs, B. A., H. Jradi, T. Desai, and C. J. Morrison. 1999. Quantitation of ergosterol content: novel method for determination of fluconazole susceptibility of Candida albicans. J. Clin. Microbiol. 37:3332-3337. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Ausubel, F. M., R. Brent, R. E. Kingston, D. D. Moore, J. G. Seidman, J. A. Smith, and K. Struhl. 1995. Current protocols in molecular biology. John Wiley & Sons, Inc., New York, N.Y.
- 5.Bard, M., N. D. Lees, T. Turi, D. Craft, L. Cofrin, R. Barbuch, C. Koegel, and J. C. Loper. 1993. Sterol synthesis and viability of erg11 (cytochrome P450 lanosterol demethylase) mutations in Saccharomyces cerevisiae and Candida albicans. Lipids 28:963-967. [DOI] [PubMed] [Google Scholar]
- 6.De Backer, M. D., D. Maes, S. Vandoninck, M. Logghe, R. Contreras, and W. Luyten. 1999. Transformation of Candida albicans by electroporation. Yeast 15:1609-1618. [DOI] [PubMed] [Google Scholar]
- 7.de Micheli, M., J. Bille, C. Schueller, and D. Sanglard. 2002. A common drug-responsive element mediates the upregulation of the Candida albicans ABC transporters CDR1 and CDR2, two genes involved in antifungal drug resistance. Mol. Microbiol. 43:1197-1214. [DOI] [PubMed] [Google Scholar]
- 8.Fonzi, W. A., and M. Y. Irwin. 1993. Isogenic strain construction and gene mapping in Candida albicans. Genetics 134:717-728. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Franz, R., S. L. Kelly, D. C. Lamb, D. E. Kelly, M. Ruhnke, and J. Morschhauser. 1998. Multiple molecular mechanisms contribute to a stepwise development of fluconazole resistance in clinical Candida albicans strains. Antimicrob. Agents Chemother. 42:3065-3072. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Goldstein, J. L., and M. S. Brown. 1990. Regulation of the mevalonate pathway. Nature 343:425-430. [DOI] [PubMed] [Google Scholar]
- 11.Greenspan, D., J. Greenspan, M. Schiodt, and J. Pindborg. 1990. AIDS and the mouth, p. 91-102. Munksgaard, Copenhagen, Denmark.
- 12.Henry, K. W., J. T. Nickels, and T. D. Edlind. 2000. Upregulation of ERG genes in Candida species by azoles and other sterol biosynthesis inhibitors. Antimicrob. Agents Chemother. 44:2693-2700. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Hoffman, C. S., and F. Winston. 1987. A ten-minute DNA preparation from yeast efficiently releases autonomous plasmids for transformation of Escherichia coli. Gene 57:267-272. [DOI] [PubMed] [Google Scholar]
- 14.Johnston, M., and M. Carlson. 1992. Regulation of carbon and phosphate utilization, p. 193-281. In E. W. Jones, J. R. Pringle, and J. R. Broach (ed.), The molecular and cellular biology of the yeast Saccharomyces, vol. 2. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y. [Google Scholar]
- 15.Krieg, P. A., and D. A. Melton. 1987. In vitro RNA synthesis with SP6 RNA polymerase. Methods Enzymol. 155:397-415. [DOI] [PubMed] [Google Scholar]
- 16.Krishnamurthy, S., V. Gupta, R. Prasad, S. L. Panwar, and R. Prasad. 1998. Expression of CDR1, a multidrug resistance gene of Candida albicans: transcriptional activation by heat shock, drugs and human steroid hormones. FEMS Microbiol. Lett. 160:191-197. [DOI] [PubMed] [Google Scholar]
- 17.Kvaal, C. A., T. Srikantha, and D. R. Soll. 1997. Misexpression of the white-phase-specific gene WH11 in the opaque phase of Candida albicans affects switching and virulence. Infect. Immun. 65:4468-4475. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18.Lai, M. H., and D. R. Kirsch. 1989. Nucleotide sequence of cytochrome P450 L1A1 (lanosterol 14 alpha-demethylase) from Candida albicans. Nucleic Acids Res. 17:804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Leber, R., E. Zinser, C. Hrastnik, F. Paltauf, and G. Daum. 1995. Export of steryl esters from lipid particles and release of free sterols in the yeast, Saccharomyces cerevisiae. Biochim. Biophys. Acta 1234:119-126. [DOI] [PubMed] [Google Scholar]
- 20.Lockhart, S. R., M. Nguyen, T. Srikantha, and D. R. Soll. 1998. A MADS box protein consensus binding site is necessary and sufficient for activation of the opaque-phase-specific gene OP4 of Candida albicans. J. Bacteriol. 180:6607-6616. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Losberger, C., and J. F. Ernst. 1989. Sequence of the Candida albicans gene encoding actin. Nucleic Acids Res. 17:9488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Lyons, C. N., and T. C. White. 2000. Transcriptional analyses of antifungal drug resistance in Candida albicans. Antimicrob. Agents Chemother. 44:2296-2303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Maniatis, T., E. F. Fritsch, and J. Sambrook. 1982. Molecular cloning: a laboratory manual. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 24.Marichal, P., L. Koymans, S. Willemsens, D. Bellens, P. Verhasselt, W. Luyten, M. Borgers, F. C. S. Ramaekers, F. C. Odds, and H. Vanden Bossche. 1999. Contribution of mutations in the cytochrome P450 14 alpha-demethylase (Erg11p, Cyp51p) to azole resistance in Candida albicans. Microbiology 10:2701-2713. [DOI] [PubMed] [Google Scholar]
- 25.Marr, K. A., C. N. Lyons, T. R. Rustad, R. A. Bowden, and T. C. White. 1998. Rapid, transient fluconazole resistance in Candida albicans is associated with increased mRNA levels of CDR. Antimicrob. Agents Chemother. 42:2584-2589. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Odds, F. C. 1988. Candida and candidosis: a review and bibliography. Bailliere Tindall, London, United Kingdom.
- 27.Parks, L. W., and W. M. Casey. 1995. Physiological implications of sterol biosynthesis in yeast. Annu. Rev. Microbiol. 49:95-116. [DOI] [PubMed] [Google Scholar]
- 28.Puri, N., S. Krishnamurthy, S. Habib, S. E. Hasnain, S. K. Goswami, and K. Prasad. 1999. CDR1, a multidrug resistance gene from Candida albicans, contains multiple regulatory domains in its promoter and the distal AP-1 element mediates its induction by miconazole. FEMS Microbiol. Lett. 180:213-219. [DOI] [PubMed] [Google Scholar]
- 29.Rex, J. H., M. A. Pfaller, J. N. Galgiani, M. S. Bartlett, A. Espinel-Ingroff, M. A. Ghannoum, M. Lancaster, F. C. Odds, M. G. Rinaldi, T. J. Walsh, and A. L. Barry. 1997. Development of interpretive breakpoints for antifungal susceptibility testing: conceptual framework and analysis of in vitro/in vivo correlation data for fluconazole, itraconazole, and Candida infections. Clin. Infect. Dis. 24:235-247. [DOI] [PubMed] [Google Scholar]
- 30.Rex, J. H., M. G. Rinaldi, and M. A. Pfaller. 1995. Resistance of Candida species to fluconazole. Antimicrob. Agents Chemother. 39:1-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 31.Sambrook, J., E. F. Fritsch, and T. Maniatis. 1989. Molecular cloning: a laboratory manual, 2nd ed. Cold Spring Harbor Laboratory Press, Cold Spring Harbor, N.Y.
- 32.Sanglard, D., F. Ischer, L. Koymans, and J. Bille. 1998. Amino acid substitutions in the cytochrome P-450 lanosterol 14 α-demethylase (CYP51A1) from azole-resistant Candida albicans clinical isolates contribute to resistance to azole antifungal agents. Antimicrob. Agents Chemother. 42:241-253. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33.Sanglard, D., K. Kuchler, F. Ischer, J. L. Pagani, M. Monod, and J. Bille. 1995. Mechanisms of resistance to azole antifungal agents in Candida albicans isolates from AIDS patients involve specific multidrug transporters. Antimicrob. Agents Chemother. 39:2378-2386. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 34.Scherer, S., and Y. Ran. 1996. Candida albicans information. [Online.] http://alces.med.umn.edu/Candida.html.
- 35.Shianna, K. V., W. D. Dotson, S. Tove, and L. W. Parks. 2001. Identification of a UPC2 homolog in Saccharomyces cerevisiae and its involvement in aerobic sterol uptake. J. Bacteriol. 183:830-834. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 36.Srikantha, T., A. Klapach, W. W. Lorenz, L. K. Tsai, L. A. Laughlin, J. A. Gorman, and D. R. Soll. 1996. The sea pansy Renilla reniformis luciferase serves as a sensitive bioluminescent reporter for differential gene expression in Candida albicans. J. Bacteriol. 178:121-129. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 37.Swerdloff, J. N., S. G. Filler, and J. E. Edwards, Jr. 1993. Severe candidal infections in neutropenic patients. Clin. Infect. Dis. 2:S457-S467. [DOI] [PubMed] [Google Scholar]
- 38.Thompson, J. R., E. Register, J. Curotto, M. Kurtz, and R. Kelly. 1998. An improved protocol for the preparation of yeast cells for transformation by electroporation. Yeast 14:565-571. [DOI] [PubMed] [Google Scholar]
- 39.Turi, T. G., and J. C. Loper. 1992. Multiple regulatory elements control expression of the gene encoding the Saccharomyces cerevisiae cytochrome P450, lanosterol 14 alpha-demethylase (ERG11). J. Biol. Chem. 267:2046-2056. [PubMed] [Google Scholar]
- 40.Werner-Washburne, M., E. Braun, G. C. Johnston, and R. A. Singer. 1993. Stationary phase in the yeast Saccharomyces cerevisiae. Microbiol. Rev. 57:383-401. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41.White, T. C. 1997. Increased mRNA levels of ERG16, CDR, and MDR1 correlate with increases in azole resistance in Candida albicans isolates from a patient infected with human immunodeficiency virus. Antimicrob. Agents Chemother. 41:1482-1487. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.White, T. C. 1997. The presence of an R467K amino acid substitution and loss of allelic variation correlate with an azole-resistant lanosterol 14 alpha demethylase in Candida albicans. Antimicrob. Agents Chemother. 41:1488-1494. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.White, T. C., S. Holleman, F. Dy, L. F. Mirels, and D. A. Stevens. 2002. Resistance mechanisms in clinical isolates of Candida albicans. Antimicrob. Agents Chemother. 46:1704-1713. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.White, T. C., K. A. Marr, and R. A. Bowden. 1998. Clinical, cellular, and molecular factors that contribute to antifungal drug resistance. Clin. Microbiol. Rev. 11:382-402. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Zinser, E., F. Paltauf, and G. Daum. 1993. Sterol composition of yeast organelle membranes and subcellular distribution of enzymes involved in sterol metabolism. J. Bacteriol. 175:2853-2858. [DOI] [PMC free article] [PubMed] [Google Scholar]