Abstract
The activities of primaquine in combination with quinine or artesunate against asexual- and sexual-stage parasites were assessed in 176 adult Thai patients with uncomplicated Plasmodium falciparum malaria. Patients were randomized to one of the six following 7-day oral treatment regimens: (i) quinine alone, (ii) quinine with tetracycline, (iii) quinine with primaquine at 15 mg/day, (iv) quinine with primaquine at 30 mg/day, (v) artesunate alone, or (vi) artesunate with primaquine. Clinical recovery occurred in all patients. There were no significant differences in fever clearance times, rates of P. falciparum reappearance, or recurrent vivax malaria between the six treatment groups. Patients treated with artesunate alone or in combination with primaquine had significantly shorter parasite clearance times (mean ± standard deviation = 65 ± 18 versus 79 ± 21 h) and lower gametocyte carriage rates (40 versus 62.7%) than those treated with quinine (P ≤ 0.007). Primaquine did not affect the therapeutic response (P > 0.2). Gametocytemia was detected in 98 patients (56% [22% before treatment and 34% after treatment]). Artesunate reduced the appearance of gametocytemia (relative risk [95% confidence interval] = 0.34 [0.17 to 0.70]), whereas combinations containing primaquine resulted in shorter gametocyte clearance times (medians of 66 versus 271 h for quinine groups and 73 versus 137 h for artesunate groups; P ≤ 0.038). These results suggest that artesunate predominantly inhibits gametocyte development whereas primaquine accelerates gametocyte clearance in P. falciparum malaria.
Multidrug-resistant Plasmodium falciparum malaria is of increasing public health concern in tropical countries. Combination regimens of two antimalarial drugs with different targets of action have been shown to delay the development of drug resistance and to improve cure rates in falciparum malaria (12, 23). Combination treatments with quinine-tetracycline, artesunate-mefloquine, or artemether-lumefantrine are efficacious worldwide, providing cure rates of 90 to 100% (9, 17, 23). In children and pregnant women, for whom tetracyclines are contraindicated, quinine-clindamycin is an effective alternative to quinine-tetracycline (10, 16), although adherence to the 7-day quinine regimens is often poor. Combination treatments which include an artemisinin derivative are efficacious in 3-day regimens and have the additional benefit of reducing gametocyte carriage and thus reducing transmission potential (14, 13, 21, 22).
Primaquine, the only generally available 8-aminoquinoline antimalarial drug, has been used for half a century as a hypnozoitocidal drug against Plasmodium vivax malaria, as a causal prophylactic against all malaria species, and as a gametocytocidal drug against P. falciparum malaria (3, 6). The World Health Organization has recommended for some areas that primaquine, in a single dose, be added to treatment regimens for falciparum malaria to reduce the transmissibility of the infection (25). Primaquine at hypnozoitocidal doses is also effective against asexual stages of P. vivax malaria (15, 18). The aim of the present study was to assess the value of adding primaquine to artesunate or quinine compared with the standard 7-day oral quinine-tetracycline regimen.
MATERIALS AND METHODS
Patients.
This prospective study was conducted with adult male patients with acute uncomplicated P. falciparum malaria admitted to the Bangkok Hospital for Tropical Diseases, Bangkok, Thailand. Fully informed consent was obtained from each subject. Exclusion criteria were patients with severe malaria (24) or patients with primary mixed malaria infections. Patients who gave a history of drug hypersensitivity, who had taken any antimalarial drugs within the previous 48 h, or whose urine was positive in screening tests for sulfonamides (lignin test) or 4-aminoquinolines (Wilson-Edeson test) were also excluded. Patients with G6PD deficiency were excluded from treatment with primaquine. The study was approved by the Ethics Committee of the Faculty of Tropical Medicine, Mahidol University, Bangkok, Thailand.
Management.
After clinical assessment and confirmation of the diagnosis from thick and thin blood smears, baseline blood samples were taken for routine hematology and biochemistry. Patients were randomized to 7-day treatment with one of the six following oral regimens: (i) quinine sulfate (Thai Government Pharmaceutical Organization; 300 mg of salt/tablet) at 10 mg of salt/kg of body weight three times a day for 7 days, (ii) quinine sulfate (10 mg of salt/kg three times a day) in combination with tetracycline (Thai Government Pharmaceutical Organization; 250 mg/tablet) at 4 mg/kg four times a day for 7 days, (iii) quinine sulfate (10 mg of salt/kg three times a day) in combination with primaquine (Thai Government Pharmaceutical Organization; 15 mg of base/tablet) at 0.25 mg of base/kg daily (adult dose, 15 mg of base/day) for 7 days, (iv) quinine sulfate (10 mg of salt/kg three times a day) in combination with primaquine at 0.50 mg of base/kg daily for 7 days, (v) artesunate (Guilin No. 1 Factory, Guangxi, People's Republic of China; 50 mg of salt/tablet) at 3.3 mg/kg (adult dose, 200 mg) on the first day and then 1.65 mg/kg (adult dose, 100 mg/day) for a further 6 days, and (vi) artesunate (3.3 mg/kg on the first day and then 1.65 mg/kg for a further 6 days) in combination with primaquine at 0.50 mg of base/kg daily for 7 days.
Oral acetaminophen (0.5 to 1 g every 4 h) was given for fever of >38°C. Vital signs were recorded every 4 h until resolution of fever and thereafter every 6 to 12 h. The fever clearance time (FCT) was defined as the time taken for the body temperature to fall below 37.5°C and remain below this value for >48 h. Patients who were unable subsequently to stay in the hospital until clearance of both fever and parasites were excluded from the study. Reappearance of infection was assessed for patients who remained in Bangkok either in the hospital or at home (i.e., outside the malaria transmission area) for at least 28 days. Patients with recrudescences were retreated with a 7-day course of quinine (10 mg of salt/kg three times a day) combined with tetracycline (4 mg/kg four times a day), and those who had late vivax appearances (relapses) were subsequently treated with the standard doses of chloroquine and primaquine.
Laboratory investigations.
Parasite and gametocyte counts were measured every 12 h in thin films or thick films until clearance and thereafter daily for 28 days. Parasite density was expressed as the number of parasites per microliter of blood, which was derived from number of parasites per 1,000 red blood cells in a thin film stained with Giemsa or Field stain or calculated from the white cell count and the number of parasites per 200 white blood cells in a thick film. The parasite clearance time (PCT) was the interval from the start of antimalarial treatment until the asexual malaria parasite count fell below detectable levels in a peripheral blood smear. The gametocyte clearance time (GCT) was the interval from the first detection to the last detection of gametocytes in a peripheral blood smear. Gametocyte carriage was described as the total number of hours for each patient during which gametocytemia was detectable; this differed slightly from the GCT in that if gametocytemia was intermittent, then intervals without gametocytes were subtracted from the GCT. Routine biochemical and hematological tests were repeated on days 7, 14, 21, and 28 after admission.
Statistical analysis.
The quantitative data from each treatment group were compared by one-way analysis of variance with post hoc adjustment for multiple comparisons using the Bonferroni correction. Nonparametric data were compared by the Kruskal-Wallis test. Categorical data were compared by Fisher's exact test or the chi-square test with Yates' correction. The cumulative cure rates were calculated by Kaplan-Meier survival analysis and compared by using the log rank test. The gametocytemia rates were compared by stratified analysis with the Mantel-Haenszel test. Gametocyte carriage was assessed by two-way analysis of variance after square root transformation. All statistical analyses were performed with the statistical computing package SPSS version 10.1 for Windows (SSPS Inc.).
RESULTS
Patients.
The study included 176 male patients with P. falciparum malaria, aged between 14 and 62 (mean ± standard deviation [SD] = 24 ± 9) years. Patients were randomized to one of six oral treatment regimens with quinine or artesunate or combined therapy with primaquine or quinine-tetracycline as detailed in Table 1. The majority of patients (n = 143; 81%) came from the western border of Thailand, where the most multidrug-resistant P. falciparum is prevalent. More than half of the patients had a history of previous malaria infection (n = 91; 52%). There were no significant differences in admission parasite counts between the quinine groups (P = 0.33), but patients who received artesunate alone had significantly higher baseline parasitemias than those treated with artesunate-primaquine (P = 0.021) (Table 1). Between the six treatment groups, there were no significant differences in rates of previous malaria infection or other baseline laboratory data. Elevated serum bilirubin (total bilirubin of ≥3 mg/dl) was noted in 30 patients from all groups. None of the studied patients had other complications, and all patients with hyperbilirubinemia had normal bilirubin levels by day 14.
TABLE 1.
Demographic data and immediate therapeutic responses for patients with P. falciparum malaria
| Parameter | Valuea for the following treatment group:
|
|||||
|---|---|---|---|---|---|---|
| Quinine | Quinine + tetracycline | Quinine + primaquineb | Quinine + primaquinec | Artesunate | Artesunate + primaquinec | |
| n | 30 | 30 | 29 | 37 | 23 | 27 |
| Age (yr) | 24 ± 8 | 27 ± 9 | 25 ± 9 | 24 ± 10 | 23 ± 8 | 24 ± 8 |
| Parasite count (/μl) | 9,004 (234-116,054) | 14,066 (630-231,104) | 10,306 (168-229,094) | 17,637 (405-200,458) | 64,449 (321-569,722)* | 26,566 (800-350,173) |
| FCT (h) | 63 (7-152) | 33 (8-117) | 48 (8-152) | 60 (7-154) | 34 (7-180) | 32 (8-164) |
| PCT (h) | 80 ± 26 | 81 ± 19 | 78 ± 23 | 79 ± 19 | 69 ± 19 | 63 ± 18 |
| PRR48d | 35 (<1-3,038) | 176 (<1-8,895) | 139 (1.3-4,270) | 221 (<1-2,052) | 759 (59-5,801) | 1,374 (108-12,521) |
Data are shown as mean ± SD or median (range); the parasite count is the geometric mean. *, value is significantly different from that for the other groups.
Primaquine was at 0.25 mg/day.
Primaquine was at 0.50 mg/day.
PRR48, parasite reduction ratio at 48 h.
Clinical responses.
Clinical recovery following treatment occurred in all patients, and none developed severe malaria (Table 1). The overall median (range) FCT was 46 (7 to 180) h and was not significantly different between patients treated with the artesunate regimens (33 [7 to 180] h) and those treated with the quinine regimens (50 [7 to 154 h]) (P = 0.10). Between the four quinine groups, the combined therapies yielded shorter FCTs than quinine alone, but this was statistically significant only for the quinine-tetracycline group (P < 0.001). Patients treated with artesunate either alone or in combination with primaquine had similar FCTs (P = 0.92). None of the studied patients developed allergic rashes or other serious adverse effects as monitored by clinical symptoms and laboratory data (data not shown).
Parasitological responses.
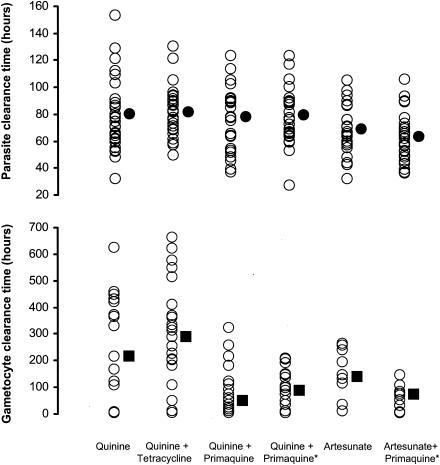
The overall mean PCT ± SD was 75.4 ± 21.5 h and was significantly shorter in the artesunate groups (65 ± 18 h) than in the quinine groups (79 ± 21 h) (P < 0.001) (Fig. 1). There was no significant difference in PCT between the quinine subgroups (P = 0.93) or between the artesunate subgroups (P = 0.26). The parasite reduction ratios at 48 h (parasite count on admission/parasite count at 48 h) were significantly higher in the artesunate groups (median [range] = 798 [59 to 12,521]) than in the quinine groups (136 [0.2 to 8,895]) (P < 0.001). There was no significant difference in the 48-h parasite reduction ratio between the quinine subgroups (P = 0.19) or between the artesunate subgroups (P = 0.55).
FIG. 1.
PCTs and GCTs in the six treatment groups of patients with P. falciparum malaria. The average values are shown as means (circles) and medians (squares). Primaquine was given at 0.25 or 0.50 (*) mg/day.
Clinical course.
Overall, 142 (84%) of the recruited patients completed at least 28 days of follow-up or remained in the hospital until appearance of vivax or falciparum malaria (Table 2). Of these 142 patients, 23 (16%) had subsequent reappearance of P. falciparum malaria and another 22 (16%) had delayed appearance of vivax malaria. Among the six treatment groups, there were no significant differences in recrudescence rates (P = 0.16) or rates of recurrent P. vivax infection (P = 0.33). The overall cure rate (no subsequent appearance of P. falciparum malaria) was 84%, and it ranged from 100% in the quinine-tetracycline group to 72% in the quinine-primaquine group. The cure rate in the quinine-tetracycline group was significantly higher than that in the other three quinine groups combined (P = 0.01). Between the six treatment groups, there were no significant differences in the cumulative cure rates, intervals to onset of recrudescent infection (mean ± SD = 21 ± 3 days), or intervals to onset of P. vivax infection (23 ± 4 days) (P ≥ 0.12).
TABLE 2.
Clinical outcomes for patients monitored up to 28 days after starting treatment
| Treatment group | No. (%) of patients with:
|
||
|---|---|---|---|
| Completed follow-up | Subsequent appearance of:
|
||
| P. falciparum | P. vivax | ||
| Quinine | 25 | 4 (16) | 2 (8) |
| Quinine + tetracycline | 22 | 0 | 4 (18) |
| Quinine + primaquine (0.25 mg/day) | 18 | 5 (28) | 3 (17) |
| Quinine + primaquine (0.50 mg/day) | 31 | 8 (7) | 5 (16) |
| Artesunate | 21 | 2 (9.5) | 5 (23.8) |
| Artesunate + primaquine | 25 | 4 (16.0) | 3 (12.0) |
| Total | 142 | 23 (16.2) | 22 (15.5) |
Gametocytemia.
Circulating gametocytes were detected in 98 patients (56%) from all groups (in 39 patients before treatment and in 59 after initiation of treatment) (Table 3). The overall gametocyte detection rate on admission was 22% (n = 39), and it was not significantly different between the six treatment groups (P = 0.88). Following treatment, the emergence of gametocytes was significantly less frequent in the artesunate group than the quinine groups (14 versus 47%) (relative risk [95% confidence interval] = 0.34 [0.17 to 0.70]; P < 0.001). The gametocyte detection rates after the different treatments were not significantly different between the two artesunate groups (P = 0.80). Among quinine-treated patients, the overall rate of gametocytemia was lower in the high-dose quinine-primaquine group than in the normal-dose quinine-primaquine group or the quinine-tetracycline group (P ≤ 0.045). By stratified analysis, the addition of primaquine to the two other drugs resulted in a significant reduction in gametocyte carriage rates (odds ratio [95% confidence interval] = 0.42 [0.20 to 0.83]; P = 0.009).
TABLE 3.
Gametocytemia rates and GCTs in patients with P. falciparum malaria
| Treatment group | n | No. (%) of patients with gametocyte appearances:
|
Median GCT (h) (range) | ||
|---|---|---|---|---|---|
| On admission | After treatment | Total | |||
| Quinine | 30 | 7 (23.3) | 10 (33.3) | 17 (56.7) | 216 (6-624) |
| Quinine + tetracycline | 30 | 7 (23.3) | 16 (53.3) | 23 (76.7) | 288 (6-662) |
| Quinine + primaquine (0.25 mg/day) | 29 | 4 (13.8) | 17 (58.6) | 21 (72) | 48 (6-324) |
| Quinine + primaquine (0.50 mg/day) | 37 | 8 (21.6) | 9 (24.3) | 17 (45.9) | 87 (5-207) |
| Artesunate | 23 | 6 (26.1) | 4 (17.4) | 10 (43.5) | 138 (12-264) |
| Artesunate + primaquine (0.50 mg/day) | 27 | 7 (25.9) | 3 (11.1) | 10 (37.0) | 73 (6-145) |
| Total | 176 | 39 (22.2) | 59 (33.5) | 98 (55.7) | 112 (5-662) |
Duration of gametocyte carriage.
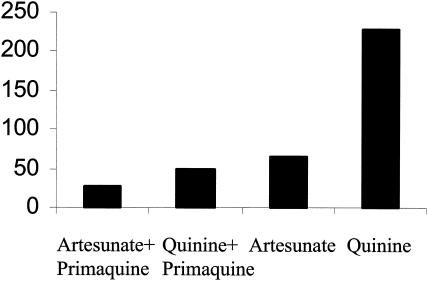
Transmission potential is related to the duration of gametocyte carriage. The average gametocyte carriage of all studied patients was 92 person-hours. The combined primaquine regimens were associated with shorter overall gametocyte carriage times than the corresponding regimens without primaquine (Fig. 2) (50 versus 229 person-hours for the quinine regimens [P = 0.004] and 27 versus 65 person-hours for the artesunate regimens [P = 0.12]). The overall median (range) of GCTs of the 98 patients with gametocytemia was 112 h (5 to 662 h). Combinations containing primaquine resulted in significantly shorter GCTs (median [range] = 66 [5 to 324] h versus 271 [6 to 662] h for quinine groups and 73 [6 to 145] h versus 137 [12 to 264] h for artesunate groups; P ≤ 0.038). There were no significant differences in GCTs between the two quinine-primaquine regimens (normal- and high-dose primaquine) (median [range] = 48 [6 to 324] h versus 87 [5 to 207] h; P = 0.45) or between the nonprimaquine artesunate and quinine regimens (median [range] = 138 [12 to 264] h versus 271 [6 to 662] h; P = 0.14). When all of the groups were pooled (and considering the quinine and quinine-tetracycline groups as one and the two quinine-primaquine groups as one) in a two-way analysis of variance, primaquine was associated with a significant shortening of GCTs (P < 0.001) but artesunate was not (P = 0.25). There were no significant correlations between GCT and PCT either within the quinine or artesunate groups or within the three combined primaquine regimens (r ≤ 0.20; P ≥ 0.27).
FIG. 2.
Mean gametocyte carriage (in person-hours) in patients with P. falciparum malaria treated with artesunate or quinine in the presence and absence of primaquine.
DISCUSSION
The primary objective of antimalarial treatment is to cure the infection, but an important secondary objective is to prevent transmission. Primaquine has unique multiple-stage activity against malaria parasites. In 1951, primaquine was selected as the most active and least toxic hypnozoitocidal drug among the 8-aminoquinoline series (5). The causal prophylactic and gametocytocidal effects of primaquine in P. falciparum were characterized later (6). Since then primaquine has been recommended and used as a transmission-blocking agent in falciparum malaria, albeit with little evidence that this policy has a significant effect on the incidence of malaria at the community level. Primaquine is considered to have insignificant activity against asexual stages of P. falciparum (20), although there have been uncertainties as to whether or not this results from resistance. Primaquine does have significant activity against asexual blood stages in P. vivax malaria, but the activity is weaker than those of other major antimalarial drugs (15). A new long-acting 8-aminoquinoline, tafenoquine (19), has been shown in vitro to be more effective than primaquine against asexual stages of malaria parasites and is still under investigation for the treatment and prophylaxis of falciparum malaria infection.
In the present study, primaquine in combination with quinine or artesunate had no additional significant effects on the activity of either drug against asexual blood stages as assessed by FCT and PCT. This confirms earlier in vivo studies indicating a lack of activity against blood stages in falciparum malaria (1). Only the quinine-tetracycline regimen gave a 100% cure rate, which is significantly better than those of the other quinine-containing regimens. In areas where malaria is endemic, mixed infection with P. falciparum and P. vivax is common, and mixed infection is found in over 30% of patients coming from the border areas of Thailand where malaria transmission is high (8, 15, 18). However, 7-day regimens of primaquine as used here are probably not sufficient to eradicate the hypnozoites of P. vivax (2).
Artemisinin derivatives are the most active of the antimalarial drugs against the asexual blood stages of malaria parasites. They also reduce gametocyte carriage in P. falciparum infections (4, 13, 14, 22). In recent field studies in Thailand, this effect has been greater than that with primaquine (21). In the present study, artesunate with or without primaquine was more rapidly acting than the quinine regimens as assessed by PCTs, although recrudescence rates and rates of cryptic vivax infections in the artesunate groups were not significantly different from those in the quinine groups. Patients treated with artesunate alone had significantly lower gametocyte carrier rates (44%) than those treated with the other nonprimaquine regimens (57% for quinine and 77% for quinine-tetracycline). Artesunate effectively prevented the appearance of gametocytemia. The average GCT with artesunate alone (median = 138 h) was also shorter than that with quinine or quinine-tetracycline (216 and 288 h, respectively), but there was considerable variation and these differences were not statistically significant. The mechanism whereby artemisinins reduce P. falciparum gametocytemia in vivo (4, 13, 14, 21, 22) and in vitro (7, 11) has not been fully elucidated. The artemisinin derivatives could act in three linked ways: they may possess gametocytocidal effects against more mature sexual stages, as in the case of primaquine; they may directly inhibit gametocyte development by preventing development of the younger sequestered stages (stages I to III); or they may simply prevent gametocytogenesis through rapid elimination of the asexual stages. These results confirm that artesunate is more effective than quinine in the prevention of gametocyte development.
Primaquine at all studied doses showed significant gametocytocidal effects, in that gametocyte clearance was accelerated, but primaquine did not prevent gametocyte development, and there was no evidence of synergy with quinine or artesunate against asexual stages of P. falciparum. The incidences of gametocytemia in patients treated with combined primaquine regimens were not significantly different from those for the nonprimaquine regimens in either the quinine or artesunate groups, but combinations including primaquine shortened the duration of gametocyte carriage two- to sixfold compared to the corresponding regimens without primaquine (P ≤ 0.038). This corresponds with earlier studies and indicates that primaquine is a potent gametocytocidal drug. In the present study, the overall GCTs did not correlate with PCTs, supporting the unrelated activities of primaquine against asexual and mature gametocyte stages of P. falciparum. In summary, the results of the present study indicate that artesunate is a potent inhibitor of gametocytogenesis but is inferior to primaquine in terms of gametocyte clearance. In reducing transmission potential, primaquine had a greater effect when added to quinine than to artesunate. Of all the studied regimens, the artesunate-primaquine combination gave the lowest rate of gametocyte detection and the shortest duration of gametocytemia.
Acknowledgments
This study was part of the Wellcome Trust-Mahidol University Oxford Tropical Medicine Research Programme funded by the Wellcome Trust of Great Britain.
REFERENCES
- 1.Arnold, J., A. S. Alving, R. S. Hockwald, C. B. Clayman, R. J. Dern, E. Beutler, C. L. Flanagan, and G. M. Jeffery. 1955. The antimalarial action of primaquine against the blood and tissue stages of falciparum malaria (Panama, P-F-6 strain). J. Lab. Clin. Med. 46:391-397. [PubMed] [Google Scholar]
- 2.Baird, J. K., and K. H. Rieckmann. 2003. Can primaquine therapy for vivax malaria be improved? Trends Parasitol. 19:115-120. [DOI] [PubMed] [Google Scholar]
- 3.Bunnag, D., T. Harinasuta, S. Pinichpongse, and P. Suntharasamai. 1980. Effect of primaquine on gametocytes of Plasmodium falciparum in Thailand. Lancet ii:91.12. [DOI] [PubMed] [Google Scholar]
- 4.Chen, P. Q., G. Q. Li, X. B. Guo, K. R. He, Y. X. Fu, L. C. Fu, and Y. Z. Song. 1994. The infectivity of gametocytes of Plasmodium falciparum from patients treated with artemisinin. Chin. Med. J. 107:709-711. [PubMed] [Google Scholar]
- 5.Cooper, W. C., A. V. Myatt, Z. T. Hernande, G. M. Jeffery, and G. R. Coatney. 1953. Studies in human malaria. XXXI. Comparison of primaquine, isopentaquine, SN-3883, and pamaquine as curative agent against Chesson strain vivax malaria. Am. J. Trop. Med. Hyg. 2:949-957. [PubMed] [Google Scholar]
- 6.Grewal, R. S. 1981. Pharmacology of 8-aminoquinolines. Bull. W. H. O. 59:397-406. [PMC free article] [PubMed] [Google Scholar]
- 7.Kumar, N., and H. Zheng. 1990. Stage-specific gametocytocidal effect in vitro of the antimalaria drug qinghaosu on Plasmodium falciparum. Parasitol. Res. 76:214-218. [DOI] [PubMed] [Google Scholar]
- 8.Looareesuwan, S., N. J. White, S. Chittamas, D. Bunnag, and T. Harinasuta. 1987. High rate of Plasmodium vivax relapse following treatment of falciparum malaria in Thailand. Lancet ii:1052-1055. [DOI] [PubMed] [Google Scholar]
- 9.Looareesuwan, S., P. Wilairatana, S. Vanijanonta, D. Kyle, and K. Webster. 1992. Efficacy of quinine-tetracycline for acute uncomplicated falciparum malaria in Thailand. Lancet i:367-370. [DOI] [PubMed] [Google Scholar]
- 10.McGready, R., T. Cho, Samuel, L. Villegas, A. Brockman, M. van Vugt, S. Looareesuwan, N. J. White, and F. Nosten. 2001. Randomized comparison of quinine-clindamycin versus artesunate in the treatment of falciparum malaria in pregnancy. Trans. R. Soc. Trop. Med. Hyg. 95:651-656. [DOI] [PubMed] [Google Scholar]
- 11.Mehra, N., and V. K. Bhasin. 1993. In vitro gametocytocidal activity of artemisinin and its derivatives on Plasmodium falciparum. Jpn. J. Med. Sci. Biol. 46:37-43. [DOI] [PubMed] [Google Scholar]
- 12.Nosten, F., M. van Vugt, R. Price, C. Luxemburger, K. L. Thway, A. Brockman, R. McGready, F. ter Kuile, S. Looareesuwan, and N. J. White. 2000. Effects of artesunate-mefloquine combination on incidence of Plasmodium falciparum malaria and mefloquine resistance in western Thailand: a prospective study. Lancet 356:297-302. [DOI] [PubMed] [Google Scholar]
- 13.Nosten, F., T. T. Hien, and N. J. White. 1998. Use of artemisinin derivatives for the control of malaria. Med. Trop. 58(Suppl. 3):45-49. [PubMed] [Google Scholar]
- 14.Price, R. N., F. Nosten, C. Luxemburger, F. O. ter Kuile, L. Paiphun, T. Chongsuphajaisiddhi, and N. J. White. 1996. Effects of artemisinin derivatives on malaria transmissibility. Lancet 347:1654-1658. [DOI] [PubMed] [Google Scholar]
- 15.Pukrittayakamee, S., A. Chantra, J. A. Simpson, S. Vanijanonta, R. Clemens, S. Looareesuwan, and N. J. White. 2000. Therapeutic responses to different antimalarial drugs in vivax malaria. Antimicrob. Agents Chemother. 44:1680-1685. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Pukrittayakamee, S., A. Chantra, S. Vanijanonta, R. Clemens, S. Looareesuwan, and N. J. White. 2000. Therapeutic responses to quinine and clindamycin in multidrug-resistant falciparum malaria. Antimicrob. Agents Chemother. 44:2395-2398. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Pukrittayakamee, S., and N. J. White. 2001. Combination therapy: making the best use of existing drugs. Pharm. News 8:21-25. [Google Scholar]
- 18.Pukrittayakamee, S., S. Vanijanonta, A. Chantra, R. Clemens, and N. J. White. 1994. Blood stage antimalarial efficacy of primaquine in Plasmodium vivax malaria. J. Infect. Dis. 169:932-935. [DOI] [PubMed] [Google Scholar]
- 19.Ramharter, M., H. Noedl, K. Thimasarn, G. Wiedermann, G. Wernsdorfer, and W. H. Wernsdorfer. 2002. In vitro activity of tafenoquine alone and in combination with artemisinin against Plasmodium falciparum. Am. J. Trop. Med. Hyg. 67:39-43. [DOI] [PubMed] [Google Scholar]
- 20.Schmidt, L. H. 1969. Chemotherapy of the drug-resistant malarias. Annu. Rev. Microbiol. 23:427-454. [DOI] [PubMed] [Google Scholar]
- 21.Suputtamongkol, Y., S. Chindarat, S. Silpasakorn, S. Chaikachonpatd, K. Lim, K. Chanthapakajee, N. Kaewkaukul, and V. Thamlikitkul. 2003. The efficacy of combined mefloquine-artesunate versus mefloquine-primaquine on subsequent development of Plasmodium falciparum gametocytemia. Am. J. Trop. Med. Hyg. 68:620-623. [DOI] [PubMed] [Google Scholar]
- 22.Targett, G., C. Drakeley, M. Jawara, L. von Seidlein, R. Coleman, J. Deen, M. Pinder, T. Doherty, C. Sutherland, G. Walraven, and P. Milligan. 2001. Artesunate reduces but does not prevent posttreatment transmission of Plasmodium falciparum to Anopheles gambiae. J. Infect. Dis. 183:1254-1259. [DOI] [PubMed] [Google Scholar]
- 23.White, N. J., F. Nosten, S. Looareesuwan, W. M. Watkins, K. Marsh, R. W. Snow, G. Kokwaro, J. Ouima, T. T. Hien, M. E. Molyneux, T. E. Taylor, C. I. Newbold, T. K. Ruebush, M. Danis, B. M. Greenwood, R. M. Anderson, and P. Olliaro. 1999. Averting a malaria disaster. Lancet 353:1965-1967. [DOI] [PubMed] [Google Scholar]
- 24.World Health Organization. 1990. Severe and complicated malaria. Trans. R. Soc. Trop. Med. Hyg. 84(Suppl. 2):1-65. [PubMed] [Google Scholar]
- 25.World Health Organization. 1994. W. H. O./MAL/94.1070. World Health Organization, Geneva, Switzerland.