Abstract
The molecular characterization of five clinical isolates of vanA-containing vancomycin-resistant enterococci with altered resistance to glycopeptides was examined. One strain represented an IS1216V insertion accompanied by partial deletion of the reading frame of vanX following a transposition event. The other four strains represented IS1216V within the vanX-vanY intergenic region associated with deletion of vanY or vanZ.
VanA glycopeptide resistance is characterized by acquired inducible resistance to both vancomycin and teicoplanin, whereas the VanB phenotype is characterized by variable levels of resistance to vancomycin but with susceptibility to teicoplanin (2, 7). Recently, vancomycin-resistant enterococci (VRE) with the vanA genotype that are susceptible to teicoplanin, and hence have the VanB phenotype and the vanA genotype, have been detected in Japan and Taiwan (8, 9). Hashimoto et al. (8) suggested that three point mutations in the putative sensor domain of vanS could be responsible for the impaired resistance to teicoplanin among VRE isolates possessing the vanA gene cluster. Another explanation for the VanB phenotype-vanA genotype VRE is impairment of accessory proteins VanY and VanZ (3, 12). In particular, the VanY d,d-carboxypeptidase has been known to contribute to vancomycin resistance by cleaving the C-terminal d-Ala residue of this precursor. The vanZ gene has also been shown to confer low-level resistance to teicoplanin, and deletion of vanZ could result in loss of teicoplanin resistance (3, 12).
To understand the spread of the van gene among enterococci in Korea, we collected 30 isolates of vanA-containing Enterococcus faecium from eight different university hospitals. Of these, four isolates (13%) were resistant to vancomycin but susceptible to teicoplanin. In contrast, one isolate was susceptible to both vancomycin and teicoplanin, like vancomycin-susceptible enterococci. The aim of present study was therefore to find out the molecular mechanisms responsible for the impaired glycopeptide resistance of VanB phenotype-vanA genotype VRE in Korea.
Five clinical isolates of vanA-containing VRE, AJ01, CA20, JC03, H23, and AS03, were obtained from five university hospitals in Korea during 2002. Previously well-characterized VRE strain E. faecium BM4147 was used as the control (6). The MICs of vancomycin and teicoplanin for all of the isolates are presented in Fig. 1.
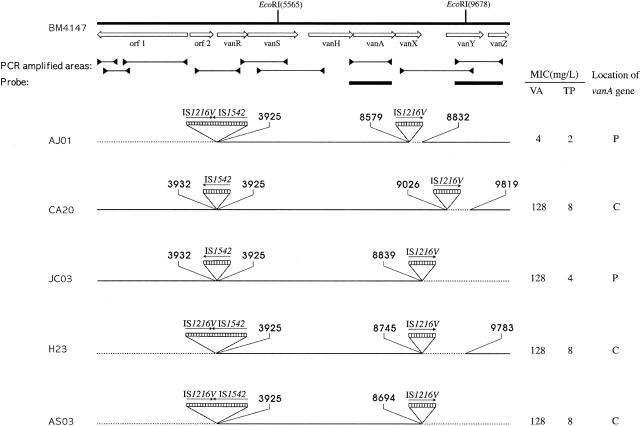
FIG. 1.
Genetic maps of Tn1546 with integration of IS1216V and IS1542 in five clinical isolates including the MICs of vancomycin and teicoplanin and the location of the vanA gene. The positions of genes and open reading frames (orf1 and orf2) and the direction of transcription are marked by open arrows at the top. The positions of the EcoRI sites are shown. Black horizontal bars indicate the positions of the internal Tn1546 fragments used as probes. Dotted boxes represent IS elements. The positions of the first nucleotide upstream and the first nucleotide downstream from the IS insertion sites are depicted. Solid arrows indicate the transcriptional orientation of the inserted IS elements. Deletions are indicated by dotted lines. L, liter; VA, vancomycin; TP, teicoplanin; P, plasmid; C, chromosome.
Pulsed-field gel electrophoresis (PFGE) was performed with SmaI (Gibco BRL, Gaithersburg, Md.) as described by Murray et al. (10). Plasmid analysis was performed as previously described (13). The SmaI-restricted DNA obtained by PFGE and plasmid DNA were Southern blotted and hybridized with a vanA-specific probe. All isolates were genetically unrelated as determined by PFGE typing (data not shown). Hybridization with a vanA probe demonstrated that two isolates had plasmid-borne vanA elements, whereas the other three isolates carried vanA on the chromosome (Fig. 1). These data suggest that the location of the vanA gene cluster may not affect the level of resistance to glycopeptides.
For structural analysis of Tn1546, PCR amplification of overlapping internal regions of Tn1546 was performed. The purified PCR products were directly sequenced with an ABI Prism 3100 DNA sequencer (Applied Biosystems, Foster City, Calif.). All isolates were analyzed for detection of the point mutations in vanR and vanS and the genetic rearrangement at the left and right ends of Tn1546. Genomic DNAs from all isolates were digested with EcoRI (Gibco BRL) and hybridized with internal Tn1546 PCR fragments (Fig. 1).
Activities of d,d-dipeptidase and d,d-carboxypeptidase were determined in crude extracts from all of the isolates as described by Arthur et al. (4). Vancomycin-resistant E. faecium BM4147 and vancomycin-susceptible E. faecalis JH2-2 were used as the controls (5, 6). To estimate d,d-dipeptidase activity, the amount of d-Ala released from d-Ala-d-Ala was determined by using d-amino acid oxidase coupled to peroxidase as the indicator reaction. For estimation of d,d-carboxypeptidase activity, d-Ala-d-Ala was replaced with the pentapeptide l-Ala-d-γ-Glu-l-Lys-d-Ala-d-Ala.
No polymorphism or size variation was found among PCR products from the central regions of Tn1546, i.e., vanR, vanS, vanH, vanA, and vanX. However, the remaining parts of Tn1546, including the right and left ends, were heterogeneous. The major rearrangements investigated among all of the isolates were the insertions of IS1542 and IS1216V, accompanied by deletions adjacent to the insertion site (Fig. 1). All isolates were characterized by IS1542 or IS1216V-IS1542 insertions in the orf2-vanR region and IS1216V insertions within or downstream of vanX. It has therefore been suggested that no association was found between the resistant phenotype and the genetic rearrangement in the left end of Tn1546. In contrast, the genetic rearrangement of the right part of the transposon including vanX, vanY, and vanZ was associated with the resistant phenotype, consistent with previous studies (3, 11, 12).
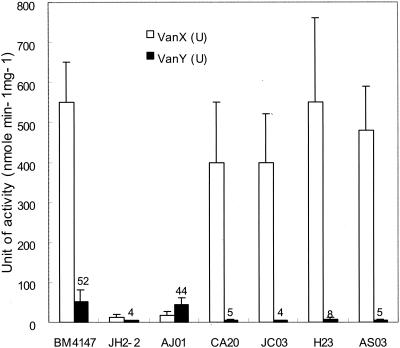
vanX encodes d,d-dipeptidase, which breaks the d-Ala-d-Ala dipeptide that is a product of the chromosomal ligase gene ddl. The essential role of vanX in the expression of glycopeptide resistance has been determined in several reports (1, 11). Strain AJ01 represented IS1216V in the vanX region with a 252-bp deletion of the right end of the vanX gene (corresponding to Tn1546 nucleotides 8580 to 8831). Insertion at this position would be predicted to cause the loss of 14 amino acid (DEPYPNSYFDFPVK) of the VanX peptide. Moreover, according to enzyme assay, strain AJ01 demonstrated extremely low d,d-dipeptidase activity (Fig. 2), indicating that the expression of vanX was significantly altered in AJ01. Therefore, partial deletion of the reading frame of vanX following a transposition event produced susceptibility to both vancomycin and teicoplanin.
FIG. 2.
VanX d,d-dipeptidase and VanY d,d-carboxypeptidase activities in VRE isolates. Induction was performed with 50 μ g of vancomycin ml−1. Results are means ± standard deviations obtained from a minimum of three independent extracts.
In contrast, four isolates (JC03, AS03, CA20, and H23) were resistant to vancomycin but susceptible to teicoplanin. Strains JC03 and AS03 were characterized by IS1216V insertions in the vanX-vanY intergenic region accompanied by deletion of the region downstream of the vanX-vanY intergenic region including vanY and vanZ (Fig. 1). A probe specific for the vanY and vanZ genes did not hybridize with these two strains lacking the vanY and vanZ genes (data not shown). Strains CA20 and H23 revealed insertions of IS1216V in the vanX-vanY intergenic region associated with deletions of the vanX-vanY intergenic region and most of vanY (corresponding to Tn1546 nucleotides 9027 to 9818 and 8746 to 9782, respectively). As shown in Fig. 2, low levels of d,d-carboxypeptidase activity were present in these four strains, indicating that expression of vanY was significantly altered. Consistent with this, earlier data also suggested that deletion of vanY or vanZ could play a role in the loss of teicoplanin resistance (3, 12). It is conceivable that deletion of vanY affects the transcription of vanZ, resulting in a lower MIC of teicoplanin, since vanZ has been shown to be involved in teicoplanin resistance.
No isolates had point mutations in the vanS regulatory gene, and this finding suggested that the VanB phenotype-vanA genotype of the VRE isolates in the present study was not due to the point mutations of vanS observed in previous studies (8, 9). All of the isolates investigated were collected from geographically different areas of Korea and were genetically unrelated. Therefore, genetic rearrangement of vanY or vanZ or partial or complete deletion of both genes following insertion of IS1216V may be a common cause of VanB phenotype-vanA genotype VRE in Korea. Recently, the prevalence of this transposon type is increasing and these strains may be mistaken for VanB VRE by phenotypic testing.
Acknowledgments
This work was supported by grant R05-2002-000-00649-0 from the Basic Research Program of the Korea Science and Engineering Foundation.
REFERENCES
- 1.Araoz, R., E. Anhalt, L. Rene, M. A. Badet-Denisot, P. Courvalin, and B. Badet. 2000. Mechanism-based inactivation of VanX, a d-alanyl-d-alanine dipeptidase necessary for vancomycin resistance. Biochemistry 39:15971-15979. [DOI] [PubMed] [Google Scholar]
- 2.Arthur, M., and P. Courvalin. 1993. Genetics and mechanisms of glycopeptide resistance in enterococci. Antimicrob. Agents Chemother. 37:1563-1571. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Arthur, M., F. Depardieu, C. Molinas, P. Reynolds, and P. Courvalin. 1995. The vanZ gene of Tn1546 from Enterococcus faecium BM4147 confers resistance to teicoplanin. Gene 154:87-92. [DOI] [PubMed] [Google Scholar]
- 4.Arthur, M., F. Depardieu, P. Reynolds, and P. Courvalin. 1996. Quantitative analysis of the metabolism of soluble cytoplasmic peptidoglycan precursors of glycopeptide-resistant enterococci. Mol. Microbiol. 21:33-44. [DOI] [PubMed] [Google Scholar]
- 5.Arthur, M., C. Molinas, and P. Courvalin. 1992. The VanS-VanR two-component regulatory system controls synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J. Bacteriol. 174:2582-2591. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Arthur, M., C. Molinas, F. Depardieu, and P. Courvalin. 1993. Characterization of Tn1546, a Tn3-related transposon conferring glycopeptide resistance by synthesis of depsipeptide peptidoglycan precursors in Enterococcus faecium BM4147. J. Bacteriol. 175:117-127. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Clark, N. C., R. C. Cooksey, B. C. Hill, J. M. Swenson, and F. C. Tenover. 1993. Characterization of glycopeptide-resistant enterococci from U.S. hospitals. Antimicrob. Agents Chemother. 37:2311-2317. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Hashimoto, Y., K. Tanimoto, Y. Ozawa, T. Murata, and Y. Ike. 2000. Amino acid substitutions in the VanS sensor of the VanA-type vancomycin-resistant Enterococcus strains result in high-level vancomycin resistance and low level teicoplanin resistance. FEMS Microbiol. Lett. 185:247-254. [DOI] [PubMed] [Google Scholar]
- 9.Lauderdale, T.-L., L. C. McDonald, Y. R. Shiau, P. C. Chen, H. Y. Wang, J. F. Lai, and M. Ho. 2002. Vancomycin-resistant enterococci from humans and retail chickens in Taiwan with unique VanB phenotype-vanA genotype incongruence. Antimicrob. Agents Chemother. 46:525-527. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Murray, B. E., K. V. Singh, J. D. Heath, B. R. Sharma, and G. M. Weinstock. 1990. Comparison of genomic DNAs of different enterococcal isolates using restriction endonucleases with infrequent recognition sites. J. Clin. Microbiol. 28:2059-2063. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 11.Reynolds, P. E., F. Depardieu, S. Dutka-Malen, M. Arhtur, and P. Courvalin. 1994. Glycopeptide resistance mediated by enterococcal transposon Tn1546 requires production of VanX for hydrolysis of d-alanyl-d-alanine. Mol. Microbiol. 13:1065-1070. [DOI] [PubMed] [Google Scholar]
- 12.Simonsen, G. S., M. R. M. Myhre, K. H. Dahl, Ø. Olsvik, and A. Sundsfjord. 2000. Typeability of Tn1546-like elements in vancomycin-resistance enterococci using long-range PCRs and specific analysis of polymorphic regions. Microb. Drug Resist. 6:49-57. [DOI] [PubMed] [Google Scholar]
- 13.Woodford, N., D. Morrison, B. Cookson, and R. C. George. 1993. Comparison of high-level gentamicin-resistant Enterococcus faecium isolates from different continents. Antimicrob. Agents Chemother. 37:681-684. [DOI] [PMC free article] [PubMed] [Google Scholar]