Abstract
Objective
We aim to analysis the relationship between HFOs-generating regions and the seizure onset zone (SOZ) in epileptic patients without a visible lesion on MRI.
Methods
Intracerebral EEGs were recorded in 17 patients with intractable focal seizures and normal MRIs. The rates of interictal HFOs and spikes inside and outside the SOZ were analyzed as well as the specificity, sensitivity and accuracy of HFOs and spikes to determine the SOZ.
Results
The mean rate of spikes, ripples and fast ripples (FR) was higher in the SOZ than in the non-SOZ channels. In regard to the identification of the SOZ the sensitivity was 91% for spikes, 91% for ripples and 66% for FR, the specificity was 30% for spikes, 42% for ripples and 80% for FR, and the accuracy was 44% for spikes, 54% for ripples and 76% for FR.
Conclusions
The rates of spikes and HFOs were higher inside than outside the SOZ. However, HFOs are also more specific and accurate than spikes to delineate the SOZ.
Significance
Analysis of interictal HFOs during 5–10 min of sleep recording is a good tool to localize the SOZ in patients with epilepsy and normal MRI, and could potentially reduce the duration of chronic intra-cerebral EEG recordings.
Keywords: High frequency oscillations, Non-lesional epilepsy, Focal epilepsy, Normal MRI, Depth EEG
1. Introduction
Patients with intractable focal epilepsy and normal MRI represent 20–40% of patients undergoing presurgical evaluation (Carne et al., 2004; Hong et al., 2002; Kutsy, 1999). These patients usually have a less favorable surgical outcome compared to patients with focal epileptogenic lesions seen on MRI (Blume et al., 2004; Tonini et al., 2004; Chapman et al., 2005; Tellez-Zenteno et al., 2010). This is explained by the lack of a reliable marker of the epileptic tissue. Although intracranial electroencephalography (iEEG) can provide in selected cases accurate information about the seizure onset zone (SOZ) (Isnard, 2004; Cossu et al., 2005; McGonigal et al., 2007; Nobili et al., 2007; Wetjen et al., 2009) in most patients with epilepsy and normal MRI no single test or combination of tests predict postoperative seizure outcome (Chapman et al., 2005).
Studies over the last decade suggest that localized high frequency oscillations (HFOs) detected during iEEG recordings are linked to the region generating seizures (Bragin et al., 1999a,b; Staba et al., 2002; Jacobs et al., 2008, 2010) and HFO-generating regions identify SOZ with greater sensitivity and specificity compared to spiking-generating regions (Jacobs et al., 2008). HFOs were also found to be a marker for the SOZ independent of the underlying lesion and the highest rates of HFOs were in areas in which the lesion and SOZ overlap, although they were infrequent in lesional regions not related with the SOZ (Jacobs et al., 2009).
Removal of the SOZ alone does not always predict a good surgical outcome (Boling et al., 2009; Prasad et al., 2003). Recently, Jacobs and colleagues (2010) showed good surgical outcome is better correlated with the removal of HFO-generating tissue than with the removal of SOZ or spike-generating region (Jacobs et al., 2010). It was also demonstrated that better surgical outcome was achieved in children when the HFO-generating region detected by electrocorticography was completely removed (Wu et al., 2010).
To the best of our knowledge, previous studies regarding HFOs were done mostly in patients with lesional epilepsy. For the first time a group of patients with focal epilepsy and normal MRI were analyzed with respect to the relationship between HFO-generating regions and the SOZ.
Since HFOs seem to be a reliable biomarker of epileptogenesis, we wondered if the localization of interictal HFOs during intracerebral EEG recording also corresponds to the SOZ in patients without visible lesions on MRI, as was demonstrated in patients with lesional focal epilepsy.
2. Methods
2.1. Selection of patients
The study was carried out retrospectively in patients with pharmacologically intractable seizures who underwent intracranial electrode implantation in the epilepsy unit of the Montreal Neurological Institute and Hospital (MNIH) between 2004 and 2009. This study was approved by the MNIH Research Ethics Committee, and all patients signed an informed consent. The decision for iEEG studies was made when no clear area of seizure onset could be determined with extensive non-invasive evaluation.
The only patient inclusion criterion was absence of focal or diffuse brain lesion considered directly relevant for the epileptogenic process, such as cortical dysplasia, neoplasia, hippocampal sclerosis, or vascular malformations. Patients were not excluded if the only finding was brain atrophy. MRI images were acquired on a 1.5 T Gyroscan (Philips Medical System, Best, The Netherlands) using a T1-fast field echo sequence (TR = 18; TE = 10; NEX = 1; flip angle = 30; matrix size = 256 × 256; FOV = 256; slice thickness = 1 mm). A multiplanar and multisequential MRI of the brain was also performed according to MNI seizure protocol (Global T1 with gadolinium, T1 sagittal, T2 axial and coronal, and FLAIR coronal). All patients underwent more than one MRI, including three patients who had a 3T MRI, on which the absence of lesion was confirmed. The MRIs were analyzed by at least two neuroradiologists experienced in epilepsy.
2.2. Recording methods
Electrode placement was tailored for each patient and based on clinical history, seizure semiology and careful review of surface video-EEG recordings. Depth electrodes were implanted stereotactically using an image-guidance system (SNN Neuronavigation System, Mississauga, Canada), (Olivier et al., 1994). Electrodes are manufactured onsite (9 contacts per electrode; contact surface 0.8 mm2; 5 mm separation between contacts); contact 1 is deep and mesial and contact 9 is lateral and neocortical. Typically, electrodes are inserted orthogonally in the temporal lobe and orthogonally or obliquely in the extratemporal lobe structures as described previously (Urrestarazu et al., 2006; Jacobs et al., 2008). iEEGs were recorded using the Harmonie monitoring system (Stellate, Montreal, Canada). The iEEG was low-pass filtered at 500 Hz and sampled at 2000 Hz. We also recorded the electrooculogram (EOG) and electromyogram (EMG) for sleep staging. The recording was performed with an epidural reference electrode placed over the parietal region of the hemisphere contralateral to the main epileptic focus.
2.3. iEEG sampling and marking of spikes and HFOs
We analyzed 5–10 min interictal samples of slow-wave sleep. Sleep stages were selected using iEEG, EOG and EMG. The Harmonie software was used to compute spectral trends in the delta, alpha and beta bands in the iEEG channels selected for having no or minimal epileptic activity and low EMG power. EEG sections with high delta and low EMG power were visually reviewed to confirm that they were stage 3 or 4. Slow wave sleep was defined when at least 25% delta activity was found by a visual inspection in 30 s epochs. Additionally, segments were selected only if they were recorded at least 2 h before and after a seizure. Analyses were performed on bipolar montages joining adjacent contacts of the same electrode.
Spikes and HFOs were visually marked independently of each other following a procedure described in previous studies (Jacobs et al., 2008; Zijlmans et al., 2009) and briefly summarized here. Spikes were marked using a time scale of 10 s/page. The spike markers were then made invisible to ensure that the marking of HFOs was not biased by the knowledge of spike localization. To visualize the HFOs, channels were display with the maximum time resolution, which corresponded to approximately 0.6 s across the computer monitor (1200 samples of a signal sampled at 2000 Hz). The computer display was split vertically and hence two versions of the same EEG were displayed side by side, one with a high-pass filter at 80 Hz and another with a high-pass filter at 250 Hz using finite impulse response (FIR) filters to eliminate ringing. Ripples (80–250 Hz) were marked on the side using the 80-Hz high pass filter and fast ripples (FR, >250 Hz) on the other side with the 250-Hz high-pass filter. A ripple was marked if an event was clearly visible on the left and not on the right, and, vice versa an event was regarded as an FR if it was visible on the right. Only events containing at least four consecutive oscillations were regarded as HFOs, and two events were considered distinct when separated by at least two non-HFO oscillations.
The SOZ was defined as the area showing the earliest EEG change (ictal discharge) from baseline prior to or concomitant with clinical onset. All channels involved at the beginning of the ictal electrographic discharge, usually the first 5 s, were considered as the SOZ. Seizure onset was determined visually from the unfiltered iEEG by an experienced electroencephalographer. Some patients had seizures originating from more than one area, independently. In those cases, all contacts within the different SOZs were regarded as SOZ contacts. The SOZ leading (SOZ 1) was the SOZ with the higher number of clinical seizures or more relevant for the expression of the habitual clinical pattern. When surgery was indicated the patient was operated on the SOZ 1.
2.4. Data analysis
After marking all events, a MATLAB (The Mathworks Inc., Natick, Massachusetts, USA) program calculated for each channel the rates of spikes, ripples and FR per minute (computed for every 1-min interval in the data). All the channels studied, with or without fast oscillations or spikes, were classified as inside or outside the SOZ.
We analyzed the rates of spikes and HFOs and the number of channels with spikes and HFOs inside and outside the SOZ, as well as the specificity, sensitivity and accuracy of spikes, ripples, and FRs to determine the SOZ.
The sensitivity was defined as [SOZ channels with HFOs/(SOZ channels with HFOs + SOZ channels without HFOs)] × 100, the specificity as [non-SOZ channels without HFOs/(non-SOZ channels without HFOs + non-SOZ channels with HFOs)] × 100, and accuracy [(SOZ channels with HFOs + non-SOZ channels without HFOs)/total channels] × 100. The same analyses were done for spikes. Even when spikes or HFOs occurred only once in a channel; we regarded it as a channel with spike or HFO.
We could not analyze the relationship between outcome post surgery and resection of HFOs generating region, since we do not have post operative MRI in a significant number of patients, to quantify the amount of SOZ and HFOs generating region which were surgically removed.
We applied the Kolmogorov–Smirnov test to define the type of distribution of the variables. We used a parametric test when the variables had a normal distribution (Student’s paired t-test) or a non-parametric test in case of non-normal distribution (Wilcoxon rank paired test). For categorical variables, we applied the X2 test according to the expected frequency in the cell. The level of significance was set at 0.05. The data are presented as mean ± standard deviation.
3. Results
3.1. Clinical and iEEG data
Between 2004 and 2009, 51 patients had iEEG investigations and recordings allowing the identification of HFOs (2000 Hz sampling). We selected the 17 patients from this database who were considered to have a normal MRI and were studied using the same type of depth electrode (MNI manufactured). This group consisted of 13 males, had a mean age at epilepsy onset of 13.7 ± 8.7 years and mean age at iEEG evaluation of 31 ± 10.2 years.
The SOZ was located in the temporal lobe structures (mesial or neocortical) in 12/17 (71%) patients, in the temporo-occipital regions in 3/17 (18%), and over the fronto-temporal region in one (6%). The SOZ could not be clearly identified in one patient. More than one SOZ were found in seven patients (41%). Out of a total of 24 SOZ, 10 were temporal or extratemporal neocortical (with or without mesial temporal involvement) and 14 were mesial temporal.
Thirteen patients (71%) underwent surgery, the majority (11 patients), had amygdalo-hippocampectomy with or without anterior neocortical temporal resection and two had resections outside the temporal lobe. Six (46%) patients had Engel class I and II and seven (54%) had Engel class III and IV.
Histopathological findings were gliosis and cell loss in nine patients, among them eight underwent mesial temporal resections, and one, an occipital resection. Two patients had nonspecific abnormality and in two others the surgical specimens were not suited for histopathological analysis. Clinical and iEEG findings are described in Table 1.
Table 1.
Clinical and electrophysiological data of 17 patients.
| Patient | Age (years)/gender | Implantation site | SOZ site | Surgery | Follow up (months) | Outcome-Engel’s class |
|---|---|---|---|---|---|---|
| 1 | 45/M | LTP, LA, LH, LPH, LOF | LA1–3, LH1–3, LP1–3 | Not operated | ||
| 2 | 34/F | RA, RH, RPH, ROF, RC | RA1–4, RH1–3, RP1–3 | R ATL | 53 | I |
| 3 | 46/F | RA, RH, RPH, RO | RO1–4 | Not operated | ||
| 4 | 43/M | LOF, LTP, LA, LH, LPH | LA1–3, LH1–3 | L SeAH | 44 | IV |
| 5 | 44/F | LTP, LA, LH, LPH, RTP, RA, RH, RPH | SOZ1 (LA1–3, LH1–3, LPH2–4), SOZ 2 (RA1–2, RH1–3, RPH2–4) | L SeAH | 40 | III |
| 6 | 29/M | LA, LH, LOF, LC, LSMA, RA, RH, ROF, RC, RSMA | SOZ1 (RA4–8, RH4–8), SOZ2 (LA1–2, LA6–8, LH1–3, LH7–9) | R SeAH | 24 | II |
| 7 | 23/M | LA, LH, LAC, LSMA, LOF, RA, RH, RAC, ROF | Not defined | Not operated | ||
| 8 | 18/M | LA, LH, LPH, RA, RH, RPH | SOZ1 (RA1–3, RH1–3, RPH1–3), SOZ2 (LH1–3, LPH1–3) | R SeAH | 25 | IV |
| 9 | 23/M | RA, RH, ROF, RR, RAC, RSMA | RSMA1–6, RAC1–8, RR1–2, ROF1–3, RH1–3 | Not operated | ||
| 10 | 44/M | RA, RH, RPH, LA, LH, LPH | LA1–3, LH1–3, LPH1–2 | L SeAH | 28 | I |
| 11 | 24/F | RA, RH, RTO, RSC, RIC, RPC | RPC1–9, RTO2–9, RSC1–8, RIC1–8 | R occipital corticectomy | 7 | III |
| 12 | 17/M | LA, LH, LPH, LOF, LMC, LAC | LA1–3, LH1–3, LPH1–3 | L ATL | 37 | III |
| 13 | 23/M | LH, LAC, RA, RH, RPH, ROF, RAC, RSMA | SOZ1 (RA5–8, RH4–5, RH5–9, RPH5–9), SOZ2 (LH1–4) | R ATL | 12 | II |
| 14 | 32/M | RA, RH, RPH, RHG, RE1–7, LA, LH | SOZ1 (RHG2–7 + RE1–4), SOZ2 (RA1–4, RH1–4, RPH1–2), SOZ3 (LA1–4, LH1–4) | R SeAH | 6 | III |
| 15 | 33/M | ROF, RA, RH, RPH, RE1–4, LOF, LA, LH, LPH | LA1–9, LH1–3, LPH1–2 | L SeAH | 4 | IV |
| 16 | 29/M | LA, LH, RA, RH, RPH, RAG | SOZ1 (RA1–2, RH1–2, RPH1–4), SOZ2 (LA1–2, LH1–2) | R SeAH | 29 | I |
| 17 | 19/M | RSC, RAC, ROF, RA, RH, RSC, RIC | SOZ1. (RSC3–5), SOZ2 (RAC5–8, RH5–8) | R occipital | 12 | I |
F, female; M, male; L, left; R, right; A, amygdala; H, hippocampus; PH, parahippocampus, SOZ, seizure onset zone; TP, temporal pole; HG, Heschl’s Gyrus; C, cingulate gyrus, AC, anterior cingulate gyrus; SC, superior cingulate gyrus; MC, mid cingulate gyrus; SMA, supplementary motor area; OF, orbito-frontal, RG, rectus gyrus; AG, angular gyrus; TO, temporo-occipital junction; O, isthmus; PC, precuneus; IC, infra-calcarine; SC, supra-calcarine; E, epidural electrodes; SeAH, selective amygdalo-hippocampectomy; ATL, anterior neocortical temporal resection (plus selective amygdalo-hippocampectomy).
3.2. SOZ vs. non-SOZ: HFOs and spikes rates
We marked spikes and HFOs in all 17 patients, but in one patient the SOZ could not be clearly identified. Since all analyses compare SOZ channels vs. non-SOZ channels, this patient was excluded from the analyses. A total of 726 channels were studied: 551 non-SOZ channels and 175 SOZ channels. Among them 552 channels showed spikes, 477 showed ripples and 224 showed fast ripples.
The proportion of channels showing any of the three types of events was consistently higher inside than outside the SOZ: spikes {160/175 (91%) vs. 392/551 (71%), p < 0.0001, X2, OR 4.3 (95% CI 2.5–7.6)}, ripples {159/175 (91%) vs. 318/551 (58%), p < 0.0001, X2, OR 7.3 (95% CI 4.2–12.5)}, and FRs {113/175 (65%) vs. 111/551 (20%), p < 0.0001, X2, OR 7.2 (95% CI 5.0–10.5)}.
Overall, the mean spiking rate was higher in SOZ channels (16.0 ± 10.5/min) than non-SOZ channels (4.1 ± 3.3), p = 0.0002; paired t test. The mean spiking rate was higher inside the SOZ in 14/16 patients (88%). The channel with the highest spiking rate was inside the SOZ in 9/16 (56%) patients.
The mean rate of ripples was higher in SOZ channels (43.4 ± 32.7/min) than non-SOZ channels (10.8 ± 11.6/min), p = 0.0016, paired t test. The mean rate of ripples was higher in SOZ channels in 15/16 patients (94%). The channel with the highest rate of ripples was inside of SOZ in 12/16 (75%) patients.
The mean rate of FR was higher in SOZ channels (10.20 ± 11.01/min) than non-SOZ channels (1.95 ± 3.5/min), p = 0.0047, Wilcoxon rank paired test. The mean rate of FR was higher in SOZ channels in 14/16 patients (88%). The channel with the highest rate of FR was inside of SOZ in 12/16 (75%) patients.
The sensitivity to identify the SOZ was 91% for spikes, 91% for ripples and 66% for FR; the specificity was 30% for spikes, 42% for ripples and 80% for FR; and the accuracy was 44% for spikes, 54% for ripples and 76% for FR.
In summary, when comparing SOZ to non-SOZ regions, the rates of spikes and HFOs and the number of channels with spikes and HFOs are higher inside than outside the SOZ. However, HFOs are also more specific and accurate than spikes to delineate the SOZ, FRs more so than ripples.
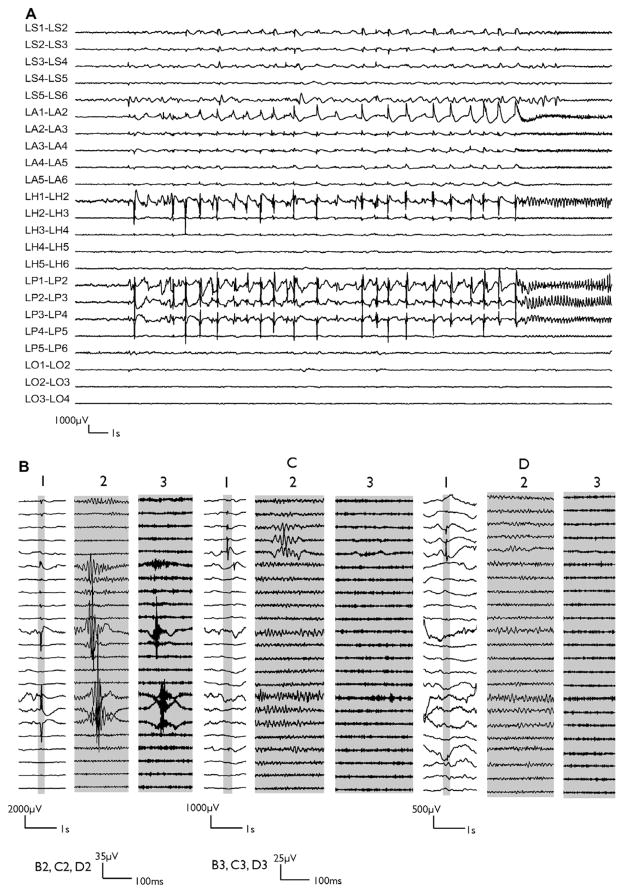
Fig. 1 illustrates an example of ictal discharge and interictal spikes, ripples and FRs on electrodes contacts inside and outside the SOZ in patient 1. Interictal spikes were observed outside the SOZ in this patient and some of them were occasionally associated with ripples, but not with FRs. Only the spikes in the SOZ were accompanied with FRs.
Fig. 1.
Example of ictal discharge (A) recorded in wakefulness and interictal spike samples (B–D) recorded in slow wave sleep from patient 1. The electrodes were implanted orthogonally, aimed at the anterior hippocampus (H), amygdala (A), parahippocampus (P), orbito-frontal region (O) and temporal pole (S) in both hemispheres. We show only electrodes in the left hemisphere (L). In parts B–D, sections labeled (1) show the raw EEG, sections with expanded time (highlighted by gray) labeled (2) show the ripple band (high pass 80 Hz) and sections labeled (3) show the FR band (high pass 250 Hz). Examples of interictal spikes (B1), with ripples (B2) and FR (B3) on electrode contacts inside the seizure onset zone (SOZ). Examples of interictal spikes outside the SOZ (C1, D1). Some of these were associated with ripples (C2) and some were not (D2); neither were associated with FRs (C3, D3). Note different amplitude calibrations. The same channels were represented in ictal and interictal samples. LS, left temporal pole (labeled as LTP in Table 1); LA, left amygdala; LH, left hippocampus; LO, left orbito-frontal (labeled as LOF in Table 1).
3.3. Rates of HFOs: leading SOZ vs. secondary SOZ
We compared the rates of ripples and FR between the leading SOZ and the secondary SOZs in the seven patients with more than one SOZ. We did not find any difference between the rates of ripples (leading SOZ = 31.32 ± 33.2 vs. secondary SOZ = 56.4 ± 49.23; p = 0.15, paired t test) and FR (leading SOZ = 8.69 ± 21.81 vs. secondary SOZ = 7.50 ± 7.61, p = 0.56, Wilcoxon rank paired test).
4. Discussion
In patients with normal MRI, iEEG recordings are often required when non-invasive data are insufficiently concordant or inconclusive (Olivier et al., 1983; Isnard, 2004; Cossu et al., 2005; Alarcon et al., 2006; McGonigal et al., 2007). The conventional analysis of iEEG comprises frequencies below 50 Hz and is strongly based in the recordings of seizures. In addition, the precise localization of the epileptogenic brain tissue depends of the appropriate spatial sampling of the iEEG investigation.
In this scenario the analysis of interictal epileptogenic markers, not based on record of ictal event, could provide useful information regarding the localization of the SOZ, decreasing the requirement of extensive and prolonged iEEG recordings. Although, interictal spikes are closely related to SOZ, their value to outline the extension of resection of the epileptogenic region is debated (Bautista et al., 1999; Hufnagel et al., 2000; Krendl et al., 2008). On the other hand, there is evidence in favor of interictal HFOs as a more reliable marker of epileptogenesis (Jacobs et al., 2010; Wu et al., 2010).
This study shows that analysis of interictal HFOs during 5–10 min of sleep may also provide useful information in patients with epilepsy and normal MRI, given that the rates of HFOs were significantly higher in SOZ than in non-SOZ channels. We found that FRs were more accurate than ripples with respect to localizing the SOZ, ripples being themselves more accurate than spikes. The study of interictal HFOs during clinical investigation may help the detection of the seizure generating cortex, particularly in patients where there is not a perfect match between the onset of clinical and electrographic seizure. Uncertainty regarding the localization of SOZ is not uncommon in non-lesional focal epilepsy and this is more so in neocortical seizures, which are often poorly localized or widespread from the very onset (Lee et al., 2000; Worrell et al., 2004).
Increased knowledge about HFOs has accumulated in recent years. Interictal HFOs in humans were first reported from micro-electrodes in patients with mesial temporal lobe epilepsy (Bragin et al., 1999a,b). HFOs proved to be a reliable epileptogenic marker since they were closely related to SOZ in ictal (Jirsch et al., 2006; Worrell et al., 2008), and interictal periods (Staba et al., 2002, 2004; Urrestarazu et al., 2007; Jacobs et al., 2008; Crepon et al., 2010), not only in mesial temporal lobe epilepsy but also in patients with neocortical seizures (Urrestarazu et al., 2007; Jacobs et al., 2008), and apparently regardless of type of the underlying epileptogenic brain lesion (Jacobs et al., 2009).
Interestingly, in patients with more than one SOZ, we found no difference regarding the rates of HFOs between the leading and the secondary SOZs. This could explain in part the often poor outcome after epilepsy surgery in patients with non-lesional epilepsy. Since the duration of iEEG monitoring is limited, this study is susceptible to sampling bias regarding the site of clustering of seizure.
The good correlation between HFOs-generating regions and the SOZ in patients without a visible lesion on MRI reinforces the concept that the epileptogenic process is not fundamentally different in this group and in the group with lesional epilepsy. Previous studies, mostly in patients with lesional epilepsy, demonstrated that removal of HFO-generating regions was a good indicator of surgical outcome (Jacobs et al., 2010; Wu et al., 2010). A future study will indicate whether this is also the case in the group of patients with non-lesional epilepsy.
Currently, available EEG systems allow recording of high frequency activity, which undoubtedly will add useful neurophysiological information to the classical interpretation of iEEG.
HIGHLIGHTS.
We demonstrated, for the first time, that interictal high frequency oscillations (HFOs) can be used to localize the seizure onset zone (SOZ) in a group of patients with epilepsy and normal MRI.
Fast ripples and ripples are more specific and accurate than spike to delineate the SOZ in patients with epilepsy and normal MRI.
In non-lesional epileptic patients with more than one SOZ there were no significant differences between the leading and the secondary SOZ regarding the rates of HFOs.
Acknowledgments
We are grateful to Lorraine Allard and Nicole Drouin for technical EEG support and Natalja Zazubovitz for help with the figures. This study was supported by grants MOP-102710 and MOP 10189 from Canadian Institutes of Health Research.
Dr. Andrade-Valenca has received scholarship support from CIHR MOP-102710 and CIHR MOP 10189.
Dr. Dubeau is a co-investigator of CIHR grants MOP 102710 and MOP 10189 and associate editor from Epileptic Disorders.
Dr. Mari has received scholarship support from CIHR MOP-102710 and CIHR MOP 10189.
Dr. Gotman is the PI of CIHR grants MOP 102710 and MOP 10189.
Footnotes
The remaining authors have no conflicts of interest.
References
- Alarcon G, Valentin A, Watt C, Selway RP, Lacruz ME, Elwes RDC, et al. Is it worth pursuing surgery for epilepsy in patients with normal neuroimaging? J Neurol Neurosurg Psychiatry. 2006;77:474–80. doi: 10.1136/jnnp.2005.077289. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bautista RE, Cobbs MA, Spencer DD, Spencer SS. Prediction of surgical outcome by interictal epileptiform abnormalities during intracranial EEG monitoring in patients with extra hippocampal seizure. Epilepsia. 1999;40:880–90. doi: 10.1111/j.1528-1157.1999.tb00794.x. [DOI] [PubMed] [Google Scholar]
- Blume WT, Ganapathy GR, Munoz D, Lee DH. Indices of resective surgery effectiveness for intractable nonlesional focal epilepsy. Epilepsia. 2004;45:46–53. doi: 10.1111/j.0013-9580.2004.11203.x. [DOI] [PubMed] [Google Scholar]
- Boling W, Aghakhani Y, Andermann F, Sziklas V, Olivier A. Surgical treatment of independent bitemporal lobe epilepsy defined by invasive recordings. J Neurol Neurosurg Psychiatry. 2009;80:533–8. doi: 10.1136/jnnp.2008.155291. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Buzsaki G. High-frequency oscillations in human brain. Hippocampus. 1999a;9:137–42. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid – treated rats with chronic seizures. Epilepsia. 1999b;40:127–37. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- Carne RP, O’Brien TJ, Kilpatrick CJ, MacGregor LR, Hicks RJ, Murphy MA, et al. MRI-negative PET-positive temporal lobe epilepsy: a distinct surgically remediable syndrome. Brain. 2004;127:2276–85. doi: 10.1093/brain/awh257. [DOI] [PubMed] [Google Scholar]
- Chapman K, Wyllie E, Najm I, Ruggieri P, Bingaman W, Luders J, et al. Seizure outcome after epilepsy surgery in patients with normal preoperative MRI. J Neurol Neurosurg Psychiatry. 2005;76:710–3. doi: 10.1136/jnnp.2003.026757. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cossu M, Cardinale F, Castana L, Citterio A, Francione S, Tassi L, et al. Stereoelectroencephalography in the presurgical evaluation of focal epilepsy: a retrospective analysis of 215 procedures. Neurosurgery. 2005;57:706–18. [PubMed] [Google Scholar]
- Crepon B, Navarro V, Hasboun D, Clemenceau S, Martinerie J, Baulac M, et al. Mapping interictal oscillations greater than 200 Hz recorded with intracranial macroelectrodes in human epilepsy. Brain. 2010;133:33–45. doi: 10.1093/brain/awp277. [DOI] [PubMed] [Google Scholar]
- Hong KS, Lee SK, Kim JY, Lee DS, Chung CK. Pre-surgical evaluation and surgical outcome of 41 patients with non-lesional neocortical epilepsy. Seizure. 2002;11:184–92. doi: 10.1053/seiz.2001.0616. [DOI] [PubMed] [Google Scholar]
- Hufnagel A, Dumpelmann M, Zentner J, Schijns O, Elger CE. Clinical relevance of quantified intracranial interictal spike activity in presurgical evaluation of epilepsy. Epilepsia. 2000;41:467–78. doi: 10.1111/j.1528-1157.2000.tb00191.x. [DOI] [PubMed] [Google Scholar]
- Isnard J. Drug-resistant partial epilepsy. Invasive electrophysiological explorations. Rev Neurol. 2004;160(Spec No 1):5S138–143. [PubMed] [Google Scholar]
- Jacobs J, LeVan P, Chander R, Hall J, Dubeau F, Gotman J. Interictal high-frequency oscillations (80–500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia. 2008;49:1893–907. doi: 10.1111/j.1528-1167.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Levan P, Chatillon C-E, Olivier A, Dubeau F, Gotman J. High frequency oscillations in intracranial EEGs mark epileptogenicity rather than lesion type. Brain. 2009;132:1022–37. doi: 10.1093/brain/awn351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jacobs J, Zijlmans M, Zelmann R, Chatillon CE, Hall J, Olivier A, et al. High-frequency electroencephalographic oscillations correlate with outcome of epilepsy surgery. Ann Neurol. 2010;67:209–20. doi: 10.1002/ana.21847. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirsch JD, Urrestarazu E, LeVan P, Olivier A, Dubeau F, Gotman J. High-frequency oscillations during human focal seizures. Brain. 2006;129:1593–608. doi: 10.1093/brain/awl085. [DOI] [PubMed] [Google Scholar]
- Krendl R, Lurger S, Baumgartner C. Absolute spike frequency predicts surgical outcome in TLE with unilateral hippocampal atrophy. Neurology. 2008;71:413–8. doi: 10.1212/01.wnl.0000310775.87331.90. [DOI] [PubMed] [Google Scholar]
- Kutsy RL. Focal extratemporal epilepsy: clinical features, EEG patterns, and surgical approach. J Neurol Sci. 1999;166:1–15. doi: 10.1016/s0022-510x(99)00107-0. [DOI] [PubMed] [Google Scholar]
- Lee SA, Spencer DD, Spencer SS. Intracranial EEG seizure-onset patterns in neocortical epilepsy. Epilepsia. 2000;41:297–307. doi: 10.1111/j.1528-1157.2000.tb00159.x. [DOI] [PubMed] [Google Scholar]
- McGonigal A, Bartolomei F, Regis J, Guye M, Gavaret M, Trebuchon-Da Fonseca A, et al. Stereoelectroencephalography in presurgical assessment of MRI-negative epilepsy. Brain. 2007;130:3169–83. doi: 10.1093/brain/awm218. [DOI] [PubMed] [Google Scholar]
- Nobili L, Francione S, Mai R, Cardinale F, Castana L, Tassi L, et al. Surgical treatment of drug-resistant nocturnal frontal lobe epilepsy. Brain. 2007;130:561–73. doi: 10.1093/brain/awl322. [DOI] [PubMed] [Google Scholar]
- Olivier A, Gloor P, Quesney LF, Andermann F. The indications for and the role of depth electrode recording in epilepsy. Appl Neurophysiol. 1983;46:33–6. doi: 10.1159/000101238. [DOI] [PubMed] [Google Scholar]
- Olivier A, Germano IM, Cukiert A, Peters T. Frameless stereotaxy for surgery of the epilepsies: preliminary experience. Technical note. J Neurosurg. 1994;81:629–33. doi: 10.3171/jns.1994.81.4.0629. [DOI] [PubMed] [Google Scholar]
- Prasad A, Pacia SV, Vazquez B, Doyle WK, Devinsky O. Extent of ictal origin in mesial temporal sclerosis patients monitored with subdural intracranial electrodes predicts outcome. J Clin Neurophysiol. 2003;20:243–8. doi: 10.1097/00004691-200307000-00003. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Fried I, Engel J., Jr Quantitative analysis of high-frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol. 2002;88:1743–52. doi: 10.1152/jn.2002.88.4.1743. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Jhung D, Fried I, Engel J., Jr High-frequency oscillations recorded in human medial temporal lobe during sleep. Ann Neurol. 2004;56:108–15. doi: 10.1002/ana.20164. [DOI] [PubMed] [Google Scholar]
- Tellez-Zenteno JF, Hernandez Ronquillo L, Moien-Afshari F, Wiebe S. Surgical outcomes in lesional and non-lesional epilepsy: a systematic review and meta-analysis. Epilepsy Res. 2010;89:310–8. doi: 10.1016/j.eplepsyres.2010.02.007. [DOI] [PubMed] [Google Scholar]
- Tonini C, Beghi E, Berg AT, Bogliun G, Giordano L, Newton RW, et al. Predictors of epilepsy surgery outcome: a meta-analysis. Epilepsy Res. 2004;62:75–87. doi: 10.1016/j.eplepsyres.2004.08.006. [DOI] [PubMed] [Google Scholar]
- Urrestarazu E, Jirsch JD, LeVan P, Hall J, Avoli M, Dubeau F, et al. High-frequency intracerebral EEG activity (100–500 Hz) following interictal spikes. Epilepsia. 2006;47:1465–76. doi: 10.1111/j.1528-1167.2006.00618.x. [DOI] [PubMed] [Google Scholar]
- Urrestarazu E, Chander R, Dubeau F, Gotman J. Interictal high-frequency oscillations (100–500 Hz) in the intracerebral EEG of epileptic patients. Brain. 2007;130:2354–66. doi: 10.1093/brain/awm149. [DOI] [PubMed] [Google Scholar]
- Wetjen NM, Marsh WR, Meyer FB, Cascino GD, So E, Britton JW, et al. Intracranial electroencephalography seizure onset patterns and surgical outcomes in nonlesional extratemporal epilepsy. J Neurosurg. 2009;110:1147–52. doi: 10.3171/2008.8.JNS17643. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Worrell GA, Parish L, Cranstoun SD, Jonas R, Baltuch G, Litt B. High-frequency oscillations and seizure generation in neocortical epilepsy. Brain. 2004;127:1496–506. doi: 10.1093/brain/awh149. [DOI] [PubMed] [Google Scholar]
- Worrell GA, Gardner AB, Stead SM, Hu S, Goerss S, Cascino GJ, et al. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain. 2008;131:928–37. doi: 10.1093/brain/awn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu JY, Sankar R, Lerner JT, Matsumoto JH, Vinters HV, Mathern GW. Removing interictal fast ripples on electrocorticography linked with seizure freedom in children. Neurology. 2010 doi: 10.1212/WNL.0b013e3181fc27d0. Published online: 06-oct-2010. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zijlmans M, Jacobs J, Zelmann R, Dubeau F, Gotman J. High-frequency oscillations mirror disease activity in patients with epilepsy. Neurology. 2009;72:979–86. doi: 10.1212/01.wnl.0000344402.20334.81. [DOI] [PMC free article] [PubMed] [Google Scholar]