Abstract
Objective
High frequency oscillations (HFOs) have been implicated in ictogenesis and epileptogenesis. The effect of contact size (in the clinical range: 1–10 mm2) on HFO detection has not been determined. This study assesses the feasibility of HFO detection in a rat epilepsy model using macrocontacts and clinical amplifiers, and the effect of contact size on HFO detection within the macrocontact range.
Methods
Eight epileptic rats were implanted with intracerebral electrodes containing three adjacent contacts of different sizes (0.02, 0.05 and 0.09 mm2). HFOs were manually marked on 5 min interictal EEG segments. HFO rates and durations were compared between the different contacts.
Results
10,966 ripples and 1475 fast ripples were identified in the recordings from 30 contacts. There were no significant differences in spike or HFO rates between the different contact sizes, nor was there a significant difference in HFO duration.
Conclusions
HFOs can be detected in a rat epilepsy model using macrocontacts. Within the studied range, size did not significantly influence HFO detection.
Significance
Using comparative anatomy of rat and human limbic structures, these findings suggest that reducing the size of macrocontacts (compared to those commercially available) would not improve HFO detection rates.
Keywords: High frequency oscillation, HFO, Ripple, Fast ripple, Intracerebral recording, Depth electrode, SEEG
1. Introduction
High frequency oscillations (HFOs) are transient, low-amplitude electroencephalographic events that are thought to play a role in physiological and pathological neural processes. They have commonly been divided in two subtypes based on frequency: ripples (80–250 Hz) and fast ripples (FRs, 250–500 Hz) (Ylinen et al., 1995; Bragin et al., 1999a,b, Staba et al., 2002). Ripples and FRs have been implicated in ictogenesis (Akiyama et al., 2005; Jirsch et al., 2006; Ochi et al., 2007; Ramachandrannair et al., 2008) and epileptogenesis (Bragin et al., 2004).
However, the neuronal networks responsible for HFO generation are not well understood. Microwire recordings of rat and human non-epileptogenic hippocampi revealed the presence of physiological ripples (Buzsaki, 1986; Suzuki and Smith, 1988; Ylinen et al., 1995; Chrobak and Buzsaki, 1996; Draguhn et al., 2000), thought to be involved in the process of memory consolidation (Buzsaki, 1998; Draguhn et al., 2000; Ponomarenko et al., 2003) and likely the result of synchronous IPSPs on pyramidal neurons (Buzsaki, 1998; Le Van Quyen et al., 2008). FRs were also recorded in non-epileptogenic somatosensory areas and suspected of playing a role in temporal processing of sensory stimuli (Curio et al., 1994; Kandel and Buzsaki, 1997; Curio, 1999, 2000; Jones and Barth, 1999; Barth, 2003). FRs are likely the result of population spikes (Curio, 2000). It is speculated that similar (or the same) networks are responsible for the generation of pathological and physiological HFOs.
Initial animal and human HFO recordings were performed with microwires (40–60 μm diameter, surface area 0.001 mm2) (Buzsaki et al., 1992; Bragin et al., 1999b, 2002b, Staba et al., 2002). Microwire recordings have the advantage of sampling a very localized brain volume (single neurons or local field potentials) but require specialized recording equipment due to their high impedance (typical amplifier input impedance: 1000 MΩ). Furthermore, they do not allow for clinical EEG recordings and must therefore be used in conjunction with clinical contacts in human intracranial recording studies.
More recently, HFOs have been recorded using small clinical contacts (0.8 mm2) and clinical amplifiers (sampling rate 2000 Hz) (Jirsch et al., 2006; Urrestarazu et al., 2006; Jacobs et al., 2008). These recordings allow the correlation of clinical EEG events with the HFOs recorded from the same contact. However, HFO detection rates were much lower in commercial clinical contacts (4–8 mm2) than in microcontacts (Worrell et al., 2008).
The use of animal models in the study of HFOs has several advantages. These include a more homogeneous study group, the ability to implant electrodes without the restriction of clinical relevance and the ability to systematically obtain pathological specimens. As noted above, HFOs have been recorded in humans using macrocontacts but, to the authors’ knowledge, intracranial HFO recordings in rats using macrocontacts and clinical amplifiers have not been documented. Furthermore, the effect of contact size of clinical (macro) contacts on HFO detection is not known.
For these reasons, intracranial HFO recordings were performed in an intraperitoneal pilocarpine epileptic rat model using 3-contact electrodes and a clinical amplifier. The primary goal of the study was to compare HFO detection rates in adjacent contacts of different sizes. A comparison of HFO duration versus contact size was also performed.
2. Methods
The intraperitoneal pilocarpine rat model was used (Turski et al., 1983). Status epilepticus (SE) was induced in 250–275 g male Sprague–Dawley rats by injection of scopolamine (methyl nitrate, 1 mg/kg IP, stock solution 1 mg/ml; Sigma–Aldrich, Canada), followed 30 min later by pilocarpine (370 mg/kg IP, stock solution 370 mg/ml; Sigma–Aldrich, Canada). If SE was not elicited within 30 min, a second injection of pilocarpine (185 mg/kg IP) was administered. SE was arrested using diazepam (5 mg/kg IP; Sandoz, Canada) and ketamine (50 mg/kg IP: Wyeth, Canada), injected separately. Postictally, the animals were monitored until full recovery.
Three-contact depth electrodes were manufactured using 0.01″ Teflon-insulated stainless steel and 0.006″ resin-insulated copper wires. All three contacts were within 0.2 mm of each other at the distal tip of the electrode. The contacts consisted of the cut edge of the 0.006″ wire (0.018 mm2), the cut edge of the 0.01″ wire (0.051 mm2) and a 1 mm stripped distal segment of the 0.01″ wire (0.849 mm2).
Contact impedances were measured in all electrodes prior to implantation by immersion of the contact in a 0.9% saline solution and with a stainless steel cortical screw as reference. Impedance values were variable and any electrode containing a contact with an impedance greater than 50 kΩ was discarded. Of the implanted electrodes, impedance values varied between 30 and 50 kΩ for the small contacts and 20–40 kΩ for the medium and large contacts.
The rats were given 72 h to recover after induction of SE. Hybrid electrodes were then stereotactically implanted in bilateral limbic structures (CA3, dentate gyrus) following Paxinos (Paxinos and Watson, 1998). Cortical screws were inserted to monitor surface cortical activity and as reference and ground. The electrode and screw wires were inserted into a pin connector with multiple slots. The connector, screws, electrodes and redundant wires were fixed onto the rat’s skull using dental cement. The pin connector was then connected to the amplifier using multi-channel cables and electrical swivels (Slip ring T13EEG, Air Precision, France; or Commutator SL 18C, HRS Scientific, Canada) to allow the rats to move freely in the cage. Animals received a topical application of chloramphenicol (Erfa, Canada) and lidocaine (5%; Odan, Canada); they were injected with Ketoprofen (5 mg/kg s.c.; Merail, Canada), buprenorphine (0.01–0.05 mg/kg s.c.; Schering-Plough, UK), and 2 ml of 0.9% sterile saline (s.c.) repeated every 12 h if necessary.
Pre-determined periods of raw EEG signal were submitted to a 500 Hz low-pass hardware filter to minimize aliasing, then recorded at a 2000 Hz sampling rate using the Harmonie system (Stellate, Montreal, Canada; input impedance 50 MΩ). One to four hours of daytime recording were obtained between the second and tenth day after the observation of the first spontaneous partial complex seizure.
Two channels were selected to evaluate sleep stages. The first channel (a cortical screw) was selected for the delta activity during sleep (confirmation of sleep was done by correlation with the video recording). A second channel was chosen to assess the amount of muscle artefact (usually another cortical screw). Spectral trends in the delta (0.5–3.9 Hz) and muscle activity (30–100 Hz) ranges were calculated using a 30 s epoch size. A 5 min slow wave sleep segment was selected by identifying periods of high delta and minimal muscle activity. Occasionally, a channel of interest that had been identified during the initial EEG screening to have periods of significant artefact was also monitored for high frequency artefact (30–100 Hz) in order to exclude periods with visible artefact during the segment selection process.
Prior analyses were conducted in this laboratory to determine the length of the EEG segment needing to be marked in order to obtain consistent event rates, as per the following procedure. Events (spikes, ripples and FRs) were marked manually in 10 min EEG segments. Intervals of variable length were compared to the total ten minute segment. An interval was considered representative of the whole EEG segment if marking this interval resulted in the same amount of information as marking the 10 min reference interval. It was found that 5 min provided, in most cases, the same information as a longer interval, in terms of a stable measurement of rates and ranking of channels (Zelmann et al., 2009). This is why 5 min of EEG were selected for final marking in this study. All events were marked manually in referential montage. The reference electrode was always the right frontal cortical screw. Channel names were changed by a third party (EEG technician) to blind the marker to contact location and size.
All events were marked manually by a single reviewer. Spikes were marked on single channels at the following parameters: high-pass filter 0.3 Hz, sensitivity 30 μV/mm, 10 s/page, eight channels/page. In this study, ripples were defined as brief events, clearly different from baseline and composed of at least four complete oscillations in the 80–250 Hz range. EEG was visualized with a high-pass filter at 80 Hz, 1.5 μV/mm, 0.8 s/page, 8 channels/page. FRs were defined as brief events, clearly different from baseline and composed of at least 4 complete oscillations in the 250–500 Hz range. EEG was visualized with a high-pass filter at 250 Hz, 1.5 μV/mm, 0.8 s/page, 8 channels/page.
Event rates per minute were calculated for each channel using a Matlab program (vR2007b; The MathWorks, Natick, USA). A paired non-parametric (Friedman’s) test was used to calculate the main effect of contact size on event rate.
The durations of marked events were computed using Matlab and mean duration per channel was calculated for each event type. Channels containing no events had to be excluded from the statistical test along with their contiguous contact because no comparison could be performed. A Friedman test was performed to determine the main effect of contact size on duration. Non-parametric tests were used for all analyses, given the non-normal distribution of rates and event durations in the different channels. This research protocol was reviewed and approved by the Montreal Neurological Institute animal care committee.
3. Results
Three-contact electrodes were implanted bilaterally in eight rats. Of these 16 electrodes, six were excluded because of lead breakage in at least one contact. Of the remaining ten electrodes (30 contacts), eight had been placed bilaterally in the CA3 hippocampal region of four rats; two had been placed bilaterally in the dentate gyrus of one rat.
A total of 3684 spikes, 10966 ripples and 1475 FRs were identified in the five minute recordings from 30 contacts. 53.6% of spikes contained HFOs. 82% of ripples were outside spikes. 11.5% of ripples contained FRs, and 46.1% of those co-occurring ripple-FRs were associated with a spike. 89.4% of FRs occurred within a ripple. 83.0% of ripples and 65.9% of FRs were co-occurring in all three channels. When ripples and FRs occurred simultaneously, ripple onset preceded FR onset in nearly every case.
The rates of spikes, ripples and FRs were similar between the three contact sizes (Fig. 1). A paired non-parametric two-factor (Friedman’s) test performed on the data revealed no significant main effect for contact size (Fig. 1, p = 0.538).
Fig. 1.
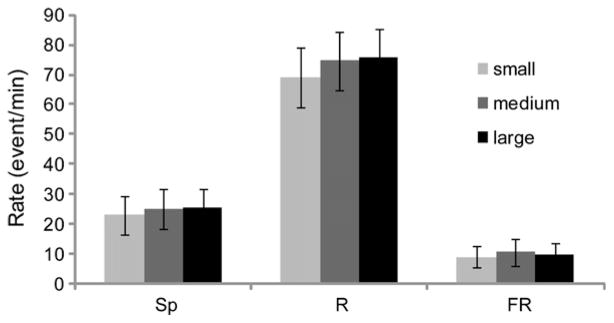
The effect of contact size on HFO rates. Sp: spike, R: ripple, FR: fast ripple. Error bars quantitate the standard error of the mean.
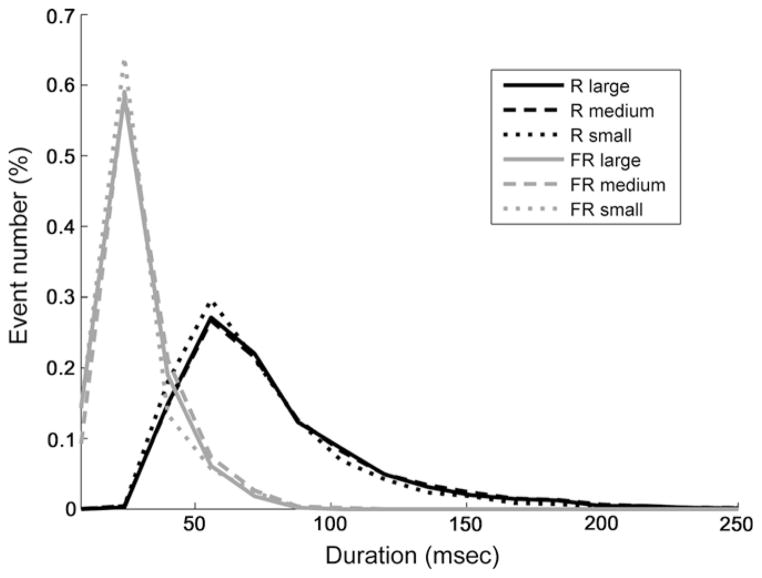
The distribution of event durations was very similar between contiguous contacts of different sizes (Fig. 2). Most ripples lasted between 30 and 120 ms; most FRs lasted 10–50 ms. Ripple durations were more broadly distributed than FRs (Fig. 2). A Friedman’s test revealed no significant main effect of contact size on event duration (p = 0.221).
Fig. 2.
Outline of a distribution histogram of HFO durations for the different contact sizes. Very similar distribution patterns were seen for the different contact sizes.
4. Discussion
The role of HFOs in epileptogenesis and ictogenesis is still unclear and is likely to generate further research in this field. Because it has been demonstrated that HFOs can be recorded using (clinical) macrocontacts (Akiyama et al., 2005; Jirsch et al., 2006; Urrestarazu et al., 2007), clinical investigators are increasingly likely to use macrocontacts for HFO studies. The reasons for this are twofold. Firstly, data from epileptic patients is readily available (from clinical recordings) and directly applicable to the ultimate target population of this line of research (epileptic patients). Secondly, if HFO patterns are detectable using clinical contacts and clinical amplifiers, it is more likely that they will become clinically relevant in epilepsy diagnosis, seizure focus localization or seizure prediction.
This study demonstrates the feasibility of recording HFOs using macroelectrodes and clinical amplifiers in a rat epilepsy model. Furthermore, there were no significant differences in detection rates or HFO duration within the size range studied (0.018–0.849 mm2). These findings confirm that HFO detection can be achieved in animal models without the need for microelectrodes and high input impedance amplifiers, as it has already been demonstrated in humans (Akiyama et al., 2005; Jirsch et al., 2006; Urrestarazu et al., 2007). This finding does not undermine the relevance of microelectrode HFO studies, which remain crucial in understanding HFO pathophysiology. Microelectrode electrical characteristics allow researchers to study the firing characteristics of just a few adjacent neurons. These very local recordings will likely be necessary to understand the cellular circuitry underlying HFO generation. On the other hand, studies that require only the detection (presence and rate) of HFOs, for example the determination of the use of HFOs in seizure onset zone delimitation and postoperative success, can readily be performed using macrocontacts and clinical amplifiers.
Contact size may influence HFO recording ability by affecting impedance or sampling volume. Impedance is inversely correlated to contact size. Smaller contacts therefore have a higher impedance. In this context, it is the ratio of electrode impedance to amplifier input impedance that determines the signal attenuation related to a change in impedance. As long as this ratio remains small (typically below 1%), the signal attenuation is negligible. Within the clinical contact range (electrode impedance <50 kΩ: 10–20 kΩ for MNI electrodes, 1–5 kΩ for standard intracerebral electrodes) and considering an amplifier input impedance of 50 MΩ, the attenuation effect is insignificant: in this range, contact size does not play a role in signal attenuation as a result of impedance. Conversely, the attenuation effect is clear when comparing the amplitude of events recorded in this study to similar events recorded using microelectrodes (Table 1) (Bragin et al., 1999b).
Table 1.
Comparison of HFO characteristics with previous reports. Values in the current study (mean +/− standard deviation) compared to those in reported in a previous study (Bragin et al., 1999b) (range, mean in parentheses). The amplitude drop (imputable to the much larger size of clinical contacts compared to microelectrodes) is evident. Nonetheless the duration and frequency of the events were comparable to previous studies, suggesting that similar events were being recorded.
| Ripples
|
FRs
|
|||
|---|---|---|---|---|
| Chatillon et al. | Bragin et al. | Chatillon et al. | Bragin et al. | |
| Rate (event/min) | 73.1 +/− 31.2 | 0.1–36 (6) | 9.8 +/− 12.3 | 1–5 (1.6) |
| Duration (ms) | 74.8 +/− 12.6 | 30–140 (73) | 23.9 +/− 5.1 | 10–65 (28) |
| Peak frequency (Hz) | 109.4 +/− 4.4 | 130–180 (148) | 314.6 +/− 19.6 | 250–500 (357) |
| Amplitude (μV) | 0.8 +/− 0.8 | 500–2500 (1100) | 1.3 +/− 0.8 | 200–1500 (720) |
Contact size also affects the volume of brain tissue being sampled (recorded). In theory, very localized events of low amplitude will be best recorded by an electrode of a small size (sampling a small volume of tissue) placed in close proximity. As contact size increases, the volume of sampled tissue will also increase. Therefore, the signal from individual, localized, low-amplitude events will more likely be “drowned” in background electrical activity. On the other hand, a larger sampling volume may contain a greater number of HFO “foci”, allowing the potential detection of a greater number of events. The optimal size of a recording electrode will therefore vary in function of the size and distribution of the sources, the amplitude and frequency of the signal that is being sought.
Previous studies had suggested that HFO generators (and especially FR generators) were less than 1 mm3 (Bragin et al., 2000, 2002a), suggesting that smaller contacts should be better able to detect them. Furthermore, a previous report comparing contact sizes and HFO detection rates had demonstrated a significant advantage of microwires in HFO detection (Worrell et al., 2008). Admittedly, this report was comparing rates of HFOs between clinical depth electrodes with a surface area of 9.4 mm2 (impedances 200–500 Ω) and microcontacts with a surface area more than 7000 times smaller (wire diameter: 40 μm, estimated surface area: 0.0012 mm2, impedances 0.5–1 MΩ). Also, macro and microelectrode recordings were collected using a 9000 Hz low-pass filter and a sampling rate at 32 kHz. In comparison, the smallest contacts used in this experiment (0.018 mm2) were 47 times smaller than the largest contacts (0.849 mm2), with impedances 1.3 times larger on average, and the EEG was collected with a clinical amplifier with a 500 Hz low-pass filter and a 2000 Hz sampling rate. It is likely that the different sizes compared in the current study were not different enough to demonstrate a detection advantage of smaller contacts. Indeed, it is possible that brief low amplitude events were not distinguishable from background in all macrocontact recordings, but would have been detected using microelectrodes. However, the rates and duration of events recorded in this study appear greater than in previous reports where HFOs were recorded using microelectrodes (Table 1) (Bragin et al., 1999b). Although many factors differ between the two experiments (method of induction of SE, timing of recordings after SE, duration of analyzed EEG segment, HFO detection method), it is possible that large (clinical) electrodes detect more HFOs because they sample a larger volume of brain parenchyma.
The ultimate goal of animal studies in medicine is the transposition of the findings to humans. In this context, the optimal contact size of a recording electrode is bound to be dependent on the size of the target. In the hippocampus, histological layers are well conserved from rat to human, although the overall size and shape of the limbic structure are different. As discussed earlier, HFOs are hypothesized to be population IPSPs (ripples) or action potentials (FRs) from discrete cortical layers. We speculated that the ratio of hippocampal layer thicknesses between rats and humans is an adequate tool to correlate optimal contact size between the two species (for hippocampal recordings). The thickness of the different histological layers were measured from the dorsal hippocampus of the rat (Paxinos et al., 1999) and human hippocampal body (Duvernoy, 1998). The overall layer thickness ratio from rat to human was found to be 0.46 (Table 2). If this ratio indeed reflects the difference in HFO generator size between rats and humans, it would suggest that contacts of a given size in rats should produce equivalent recordings to contacts twice as large in humans (placed in an equivalent anatomical location). It also suggests that there should be no difference in HFO detection in human recordings using contacts from 0.036 mm2 (0.018 mm2 × 2) to 1.698 mm2 (0.849 mm2 × 2).
Table 2.
Comparison of layer thickness (μm) between rat and human hippocampi.
| CA1
|
CA2
|
CA3
|
Average Ratio | |||||||
|---|---|---|---|---|---|---|---|---|---|---|
| Rat | Human | Ratio | Rat | Human | Ratio | Rat | Human | Ratio | ||
| SO | 130 | 250 | 0.52 | 153 | 285 | 0.54 | 191 | 330 | 0.58 | 0.55 |
| SPy | 56 | 175 | 0.32 | 67 | 179 | 0.37 | 74 | 225 | 0.33 | 0.34 |
| SR | 287 | 504 | 0.57 | 304 | 445 | 0.68 | 200 | 415 | 0.48 | 0.58 |
| SL-M | 165 | 607 | 0.27 | 126 | 397 | 0.32 | 150 | 362 | 0.41 | 0.33 |
| Total | 638 | 1536 | 0.42 | 650 | 1306 | 0.5 | 615 | 1332 | 0.46 | 0.46 |
SO: stratum oriens, SPy: stratum pyramidale, SR: stratum radiatum, SL-M: strata lacunosum and moleculare. Similar results were obtained when comparing dentate gyrus cell layers. Rat measurements taken from a coronal hippocampal section of the dorsal hippocampus in the Chemoarchitectonic Atlas of the Rat Forebrain (Paxinos et al., 1999), human measurements taken from the Human Hippocampus, p.17 (Duvernoy, 1998).
In summary, this study demonstrates that macrocontacts and clinical amplifiers can be used to detect HFOs in rat epilepsy models. Furthermore, contact size does not significantly influence HFO detection within the range of contact sizes (and impedances) that can be used with clinical amplifiers.
HIGHLIGHTS.
HFOs can be recorded in a rat model of epilepsy using macrocontacts.
Contact size does not influence HFO detection in the macrocontact range.
Results suggest that using smaller macrocontacts will not improve HFO detection in human recordings.
Acknowledgments
This study was funded by the Canadian Institute of Health Research (CIHR) Grant MOP-10189 and 8109, as well as of the Savoy foundation.
References
- Akiyama T, Otsubo H, Ochi A, Ishiguro T, Kadokura G, Ramachandrannair R, et al. Focal cortical high-frequency oscillations trigger epileptic spasms: confirmation by digital video subdural EEG. Clin Neurophysiol. 2005;116:2819–25. doi: 10.1016/j.clinph.2005.08.029. [DOI] [PubMed] [Google Scholar]
- Barth DS. Submillisecond synchronization of fast electrical oscillations in neocortex. J Neurosci. 2003;23:2502–10. doi: 10.1523/JNEUROSCI.23-06-02502.2003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Buzsaki G. High-frequency oscillations in human brain. Hippocampus. 1999a;9:137–42. doi: 10.1002/(SICI)1098-1063(1999)9:2<137::AID-HIPO5>3.0.CO;2-0. [DOI] [PubMed] [Google Scholar]
- Bragin A, Engel J, Jr, Wilson CL, Fried I, Mathern GW. Hippocampal and entorhinal cortex high-frequency oscillations (100–500 Hz) in human epileptic brain and in kainic acid–treated rats with chronic seizures. Epilepsia. 1999b;40:127–37. doi: 10.1111/j.1528-1157.1999.tb02065.x. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Staba R, Fried I, Reddick M, Engel J. Ripples and fast ripples in the human epileptic brain. Depth profiles and unit correlates. Epilepsia. 2000;41:8. [Google Scholar]
- Bragin A, Mody I, Wilson CL, Engel J., Jr Local generation of fast ripples in epileptic brain. J Neurosci. 2002a;22:2012–21. doi: 10.1523/JNEUROSCI.22-05-02012.2002. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Staba RJ, Reddick M, Fried I, Engel J., Jr Interictal high-frequency oscillations (80–500 Hz) in the human epileptic brain: entorhinal cortex. Ann Neurol. 2002b;52:407–15. doi: 10.1002/ana.10291. [DOI] [PubMed] [Google Scholar]
- Bragin A, Wilson CL, Almajano J, Mody I, Engel J., Jr High-frequency oscillations after status epilepticus: epileptogenesis and seizure genesis. Epilepsia. 2004;45:1017–23. doi: 10.1111/j.0013-9580.2004.17004.x. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Hippocampal sharp waves: their origin and significance. Brain Res. 1986;398:242–52. doi: 10.1016/0006-8993(86)91483-6. [DOI] [PubMed] [Google Scholar]
- Buzsaki G, Horvath Z, Urioste R, Hetke J, Wise K. High-frequency network oscillation in the hippocampus. Science. 1992;256:1025–7. doi: 10.1126/science.1589772. [DOI] [PubMed] [Google Scholar]
- Buzsaki G. Memory consolidation during sleep: a neurophysiological perspective. J Sleep Res. 1998;7:17–23. doi: 10.1046/j.1365-2869.7.s1.3.x. [DOI] [PubMed] [Google Scholar]
- Chrobak JJ, Buzsaki G. High-frequency oscillations in the output networks of the hippocampalentorhinal axis of the freely behaving rat. J Neurosci. 1996;16:3056–66. doi: 10.1523/JNEUROSCI.16-09-03056.1996. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Curio G, Mackert BM, Burghoff M, Koetitz R, Abraham-Fuchs K, Harer W. Localization of evoked neuromagnetic 600 Hz activity in the cerebral somatosensory system. Electroencephalogr Clin Neurophysiol. 1994;91:483–7. doi: 10.1016/0013-4694(94)90169-4. [DOI] [PubMed] [Google Scholar]
- Curio G. High frequency (600 Hz) bursts of spike-like activities generated in the human cerebral somatosensory system. Electroencephalogr Clin Neurophysiol Suppl. 1999;49:56–61. [PubMed] [Google Scholar]
- Curio G. Linking 600-Hz « spikelike » EEG/MEG wavelets (« sigma-bursts ») to cellular substrates: concepts and caveats. J Clin Neurophysiol. 2000;17:377–96. doi: 10.1097/00004691-200007000-00004. [DOI] [PubMed] [Google Scholar]
- Draguhn A, Traub RD, Bibbig A, Schmitz D. Ripple (approximately 200-Hz) oscillations in temporal structures. J Clin Neurophysiol. 2000;17:361–76. doi: 10.1097/00004691-200007000-00003. [DOI] [PubMed] [Google Scholar]
- Duvernoy H. The Human Hippocampus, functional anatomy, vascularization and serial sections with MRI. New York: Springer–Verlag; 1998. p. 213. [Google Scholar]
- Jacobs J, LeVan P, Chander R, Hall J, Dubeau F, Gotman J. Interictal high-frequency oscillations (80–500 Hz) are an indicator of seizure onset areas independent of spikes in the human epileptic brain. Epilepsia. 2008;49:1893–907. doi: 10.1111/j.1528-1167.2008.01656.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jirsch JD, Urrestarazu E, LeVan P, Olivier A, Dubeau F, Gotman J. High-frequency oscillations during human focal seizures. Brain. 2006;129:1593–608. doi: 10.1093/brain/awl085. [DOI] [PubMed] [Google Scholar]
- Jones MS, Barth DS. Spatiotemporal organization of fast (>200 Hz) electrical oscillations in rat Vibrissa/Barrel cortex. J Neurophysiol. 1999;82:1599–609. doi: 10.1152/jn.1999.82.3.1599. [DOI] [PubMed] [Google Scholar]
- Kandel A, Buzsaki G. Cellular-synaptic generation of sleep spindles, spike-and-wave discharges, and evoked thalamocortical responses in the neocortex of the rat. J Neurosci. 1997;17:6783–97. doi: 10.1523/JNEUROSCI.17-17-06783.1997. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Le Van Quyen M, Bragin A, Staba R, Crepon B, Wilson CL, Engel J., Jr Cell type-specific firing during ripple oscillations in the hippocampal formation of humans. J Neurosci. 2008;28:6104–10. doi: 10.1523/JNEUROSCI.0437-08.2008. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ochi A, Otsubo H, Donner EJ, Elliott I, Iwata R, Funaki T, et al. Dynamic changes of ictal high-frequency oscillations in neocortical epilepsy: using multiple band frequency analysis. Epilepsia. 2007;48:286–96. doi: 10.1111/j.1528-1167.2007.00923.x. [DOI] [PubMed] [Google Scholar]
- Paxinos G, Watson C. The Rat Brain in Stereotactic Coordinates. San Diego: Academic Press; 1998. p. 237. [Google Scholar]
- Paxinos G, Kus L, Ashwell K, Watson C. Chemoarchitectonic Atlas of the Rat Forebrain. San Diego: Academic Press; 1999. pp. 1–248. [Google Scholar]
- Ponomarenko AA, Lin JS, Selbach O, Haas HL. Temporal pattern of hippocampal high-frequency oscillations during sleep after stimulant-evoked waking. Neuroscience. 2003;121:759–69. doi: 10.1016/s0306-4522(03)00524-4. [DOI] [PubMed] [Google Scholar]
- Ramachandrannair R, Ochi A, Imai K, Benifla M, Akiyama T, Holowka S, et al. Epileptic spasms in older pediatric patients: MEG and ictal high-frequency oscillations suggest focal-onset seizures in a subset of epileptic spasms. Epilepsy Res. 2008;78:216–24. doi: 10.1016/j.eplepsyres.2007.12.007. [DOI] [PubMed] [Google Scholar]
- Staba RJ, Wilson CL, Bragin A, Fried I, Engel J., Jr Quantitative analysis of high-frequency oscillations (80–500 Hz) recorded in human epileptic hippocampus and entorhinal cortex. J Neurophysiol. 2002;88:1743–52. doi: 10.1152/jn.2002.88.4.1743. [DOI] [PubMed] [Google Scholar]
- Suzuki SS, Smith GK. Spontaneous EEG spikes in the normal hippocampus. II. Relations to synchronous burst discharges. Electroencephalogr Clin Neurophysiol. 1988;69:532–40. doi: 10.1016/0013-4694(88)90165-4. [DOI] [PubMed] [Google Scholar]
- Turski WA, Cavalheiro EA, Schwarz M, Czuczwar SJ, Kleinrok Z, Turski L. Limbic seizures produced by pilocarpine in rats: behavioural, electroencephalographic and neuropathological study. Behav Brain Res. 1983;9:315–35. doi: 10.1016/0166-4328(83)90136-5. [DOI] [PubMed] [Google Scholar]
- Urrestarazu E, Jirsch JD, LeVan P, Hall J, Avoli M, Dubeau F, et al. High-frequency intracerebral EEG activity (100–500 Hz) following interictal spikes. [erratum appears in Epilepsia. 2006 Nov; 47(11):1979 Note: Avoli, Massimo [added]; Dubeau, Francois [added]; Gotman, Jean [added]] Epilepsia. 2006;47:1465–76. doi: 10.1111/j.1528-1167.2006.00618.x. [DOI] [PubMed] [Google Scholar]
- Urrestarazu E, Chander R, Dubeau F, Gotman J. Interictal high-frequency oscillations (100–500 Hz) in the intracerebral EEG of epileptic patients. Brain. 2007;130:2354–66. doi: 10.1093/brain/awm149. [DOI] [PubMed] [Google Scholar]
- Worrell GA, Gardner AB, Stead SM, Hu S, Goerss S, Cascino GJ, et al. High-frequency oscillations in human temporal lobe: simultaneous microwire and clinical macroelectrode recordings. Brain. 2008;131:928–37. doi: 10.1093/brain/awn006. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ylinen A, Bragin A, Nadasdy Z, Jando G, Szabo I, Sik A, et al. Sharp wave-associated high-frequency oscillation (200 Hz) in the intact hippocampus: network and intracellular mechanisms. J Neurosci. 1995;15:30–46. doi: 10.1523/JNEUROSCI.15-01-00030.1995. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zelmann R, Zijlmans M, Jacobs J, Chatillon CE, Gotman J. Improving the identification of High Frequency Oscillations. Clin Neurophysiol. 2009;120:1457–64. doi: 10.1016/j.clinph.2009.05.029. [DOI] [PMC free article] [PubMed] [Google Scholar]