Abstract
The metabolism, excretion, and pharmacokinetics of caspofungin (Cancidas; Merck & Co., Inc.) were investigated after administration of a single intravenous dose to mice, rats, rabbits, and monkeys. Caspofungin had a low plasma clearance (0.29 to 1.05 ml/min/kg) and a long terminal elimination half-life (11.7 h to 59.7 h) in all preclinical species. The elimination kinetics of caspofungin were multiphasic and displayed an initial distribution phase followed by a dominant β-elimination phase. The presence of low levels of prolonged radioactivity in plasma was observed and was partially attributable to the chemical degradation product M0. Excretion studies with [3H]caspofungin indicated that the hepatic and renal routes play an important role in the elimination of caspofungin, as a large percentage of the radiolabeled dose was recovered in urine and feces. Excretion of radioactivity in all species studied was slow, and low levels of radioactivity were detected in daily urine and fecal samples throughout a prolonged collection period. Although urinary profiles indicated the presence of several metabolites (M0, M1, M2, M3, M4, M5, and M6), the majority of the total radioactivity was associated with the polar metabolites M1 [4(S)-hydroxy-4-(4-hydroxyphenyl)-l-threonine] and M2 [N-acetyl-4(S)-hydroxy-4-(4-hydroxyphenyl)-l-threonine]. Caspofungin was thus primarily eliminated by metabolic transformation; however, the rate of metabolism was slow. These results suggest that distribution plays a prominent role in determining the plasma pharmacokinetics and disposition of caspofungin, as very little excretion or biotransformation occurred during the early days after dose administration, a period during which concentrations in plasma fell substantially. The disposition of caspofungin in preclinical species was similar to that reported previously in humans.
Caspofungin is a semisynthetic lipopeptide agent of the novel echinocandin class of compounds for the treatment of life-threatening fungal infections. Its antifungal activity is mediated by inhibition of the synthesis of 1,3-β-d-glucan, an essential component in the cell wall of target organisms (5). Caspofungin has demonstrated potent antifungal activity in vitro against Candida and Aspergillus isolates (4) and in vivo efficacy in mouse models of disseminated aspergillosis and candidiasis (1) as well as in a persistently neutropenic rabbit model of pulmonary aspergillosis (14). Caspofungin has been used successfully in the clinical arena for the treatment of candidiases as well as candidemia (2, 7, 12, 13). Caspofungin has also been shown to be efficacious in the treatment of Aspergillus infections (11, 15, 17) and invasive aspergillosis in patients refractory or intolerant to amphotericin B (J. Maertens, I. Raad, C. A. Sable, A. Ngai, R. Berman, T. F. Patterson, D. Denning, and T. Walsh, Abstr. 40th Intersci. Conf. Antimicrob. Agents Chemother., abstr. 1103, 2000). It is generally well tolerated by patients (16) and has an excellent safety profile (6, 10).
Although limited pharmacokinetics of caspofungin in preclinical species have been described previously (8, 9), the disposition and metabolism have not been reported. The long terminal half-life has also not been fully characterized in mice, rats, and monkeys. The specific objectives of this study were to extensively characterize the pharmacokinetics, including the long terminal half-life, compare exposure to total radioactivity versus parent levels in plasma, examine mass balance, study the routes of elimination, and investigate the metabolism of caspofungin in mice, rats, rabbits, and monkeys.
MATERIALS AND METHODS
Chemicals.
[3H]caspofungin and tritiated synthetic standards of metabolites M0, M1, and M2 were prepared by the Labeled Compound Synthesis Group (Drug Metabolism, Merck Research Laboratories, Rahway, N.J.). The radiochemical purity of all materials was >94%. The position of the tritium label on caspofungin and the synthetic standards is depicted in Fig. 3. Unlabeled caspofungin was synthesized by the Department of Process Research (Merck Research Laboratories). All other solvents and reagents were of either high-performance liquid chromatography (HPLC) or analytical grade. All caspofungin stock solutions were prepared based on the molecular weight of the free base.
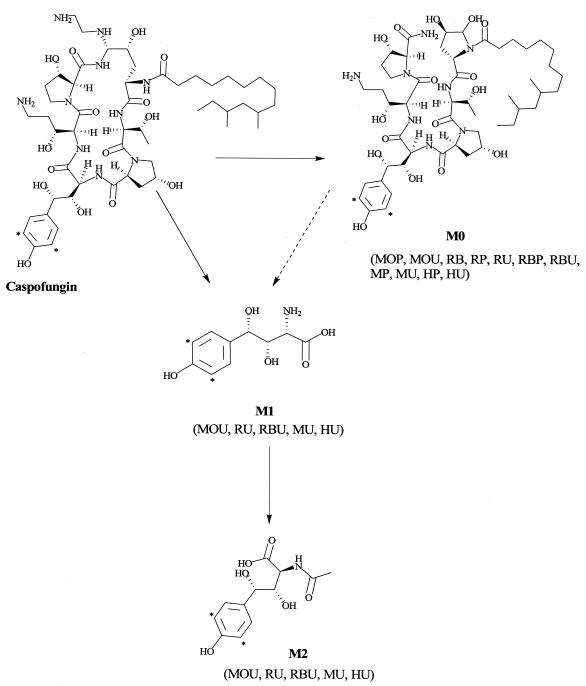
FIG. 3.
Proposed metabolic pathways of caspofungin in samples from mice, rats, rabbits, and monkeys. MOP, mouse plasma; MOU, mouse urine; RB, rat bile; RP, rat plasma; RU, rat urine; RBP, rabbit plasma; RBU, rabbit urine; MP, monkey plasma; MU, monkey urine; HP, human plasma; HU, human urine. The asterisk marks the position of the 3H label.
Pharmacokinetic studies.
All animal studies described in this paper were approved by the Merck Research Laboratories Institutional Animal Care and Use Committee.
(i) Mice.
Male CD-1 mice (three per time point) from Charles River Laboratories (Raleigh, N.C.), weighing approximately 33 g, were used for the study. [3H]caspofungin (specific activity, 84 μCi/mg) was administered as a bolus intravenously via the tail vein at 5 mg/kg (1 ml/kg). Three mice were sacrificed per time point, and blood samples were collected from the vena cava before the dose, at 0.083, 0.25, 0.50, 1, 2, 4, 8, and 24 h after the dose, and thereafter once daily at 24-h intervals for up to 12 days.
(ii) Rats.
Male Sprague-Dawley rats (n = 3) from Taconic Farms (Germantown, N.Y.), weighing approximately 200 to 350 g were used for the pharmacokinetic and mass balance studies. For the pharmacokinetic studies, a cannula was implanted in the right jugular vein for dose administration and blood sampling. The surgical procedure was performed under pentobarbital anesthesia (40 to 50 mg/kg, intraperitoneally), and the animals were allowed to recover prior to initiation of study. [3H]caspofungin (specific activity, 15.73 μCi/mg) was administered as a bolus intravenously via the jugular vein at 2 mg/kg (1 ml/kg). Blood samples were serially collected before the dose, at 0.083, 0.25, 0.50, 1, 2, 4, 8, 24, 36, and 48 h after the dose, and at days 3, 4, 5, 7, and 10 after dose administration. The cannula was flushed with 0.5 ml of saline after dosing and with 0.2 ml of heparinized saline after sample collection.
(iii) Rabbits.
Female New Zealand White rabbits (n = 4) from Covance (Denver, Pa.) weighing 3.2 to 3.5 kg were used for the pharmacokinetic and mass balance studies. [3H]caspofungin (specific activity, 2.36 μCi/mg) was administered intravenously as a slow bolus over 2 min via a temporary percutaneous catheter placed in a marginal ear vein at 5 mg/kg (1 ml/kg). After dosing, the catheter was flushed with 1 ml of saline. Blood samples were collected from a central auricular artery or a marginal ear vein via venipuncture into tubes containing heparin before the dose, at 0.083, 0.25, 0.50, 1, 2, 4, 8, and 24 h after the dose, and at days 2, 3, 4, 5, 6, 7, 9, 11, 13, 15, 17, 19, 21, 23, 25, 27, and 28 after dose administration.
(iv) Monkeys.
Male Rhesus monkeys (n = 3, from New Iberia, La.) weighing 3.3 to 4.3 kg were used for the pharmacokinetic and mass balance studies. [3H]caspofungin (specific activity, 3.03 μCi/mg) was administered via the cephalic vein as a slow bolus over 2 min at 5 mg/kg (0.1 ml/kg). Blood samples were collected from the iliac artery via a chronically implanted vascular access port before the dose, at 0.083, 0.33, 1, 2, 4, 8, and 24 h after the dose, and at days 2, 3, 5, 7, 10, and 13 after dose administration. The vascular access port was flushed with 3 ml of saline followed by 1 ml of heparinized saline after each sample collection. Dose solutions were prepared in saline. All blood samples were collected in heparinized tubes, and plasma was harvested by centrifugation and stored at −20°C or −70°C until analysis.
Analytical methods for plasma analysis.
A radioimmunoassay with an extremely small plasma volume (2.5 μl) was set up as described by Yuan et al. (20) for determining the concentration of caspofungin in plasma samples from mice, rats, and monkeys. Briefly, the immunogen was prepared by coupling caspofungin to bovine serum albumin through a two-step reaction with difluorodinitrobenzene. An antiserum specific to caspofungin was selected for radioimmunoassay. The assay was based on the competitive immunoassay principle in which the drug competes with iodinated drug for a limited quantity of specific antibody. The bound tracer was separated via goat anti-rabbit globulin. The specificity of the radioimmunoassay method was determined by cross-validation against an established HPLC method. The validated assay demonstrated good linearity and reproducibility for caspofungin in the concentration range of 10 to 1,000 ng/ml (r2 > 0.98). The values for assay accuracy and precision (coefficient of variance) were 94.9 to 106.8% and 3.9 to 15.9%, respectively. Plasma samples exceeding the upper limit of quantitation were diluted with control plasma prior to analysis.
Analysis of rabbit plasma samples was performed by an HPLC method as described by Schwartz et al. (18) with minor modifications. Briefly, plasma (100 μl) spiked with an internal standard (1,000 ng of an aminoethyl ether analog of caspofungin per ml) was diluted with water (300 μl) and mixed with organic solvent (acetonitrile-methanol, 3:2, vol/vol). After addition of buffer and centrifugation, the supernatant was poured into a conditioned diol solid-phase extraction column and washed with water and methanol. Caspofungin and the internal standard were eluted with a solution containing trifluoroacetic acid and ammonium hydroxide in methanol. Separation was carried out isocratically on a Zorbax 330SB-C8 column (4.6 mm by 15 cm, 5 μm inner diameter) at 35°C and a flow rate of 1.5 ml/min over a run time of 9 min. Detection was done by fluorescence at an excitation wavelength of 220 nm and an emission wavelength of 304 nm. The validated assay demonstrated good linearity and reproducibility for caspofungin in the concentration range of 125 to 10,000 ng/ml (r2 > 0.99). The values for assay accuracy and precision (coefficient of variance) were 94.2 to 106.8% and 3.7 to 6.5%, respectively. Plasma samples exceeding the upper limit of quantitation were diluted with control rabbit plasma prior to extraction.
Mass balance and urine profiling studies.
(i) Mouse.
Radiolabeled and unlabeled caspofungin were dissolved in saline to give a final specific activity of 84 μCi/mg. A dose of 5 mg/kg was administered to mice (four to six per time point) via the tail vein. Urine was collected at 24-h intervals on days 1, 3, 5, and 11.
(ii) Rat.
Radiolabeled and unlabeled caspofungin was dissolved in saline to give a final specific activity of 53.7 μCi/mg. A dose of 2 mg/kg was administered to rats (n = 3) as described earlier for the pharmacokinetic studies. The animals were housed in metabolism cages for collection of urine and feces. Urine and fecal samples were collected at 24-h intervals daily for up to 12 days. Urine samples were collected over dry ice.
In a separate study, biliary excretion was investigated in rats (n = 3) after intravenous administration of a 2-mg/kg dose. In this study, radiolabeled or unlabeled caspofungin was dissolved in saline to give a final specific activity of 50 μCi/mg. The rats were anesthetized with pentobarbital (50 mg/kg, intraperitoneally), and a cannula was inserted in the right jugular vein. After a 1-day recovery period, the bile ducts were cannulated. The animals were allowed to recover prior to initiation of the study. Bile samples were collected for 24 h over dry ice.
(iii) Rabbit and monkey.
Radiolabeled caspofungin was diluted with saline to give a final specific activity of 2.36 μCi/mg (rabbit) or 3.03 μCi/mg (monkey). A dose of 5 mg/kg was administered to rabbits (n = 4) and monkeys (n = 3) as described earlier for the pharmacokinetic studies. Urine and fecal samples were collected at 24-h intervals daily for up to 28 days.
Measurement of total radioactivity.
Total radioactivity was determined in urine and plasma by adding aliquots of urine (0.1 to 1.0 ml) or plasma (0.025 to 0.2 ml) to Packard Ultima Gold scintillation cocktail (10 to 20 ml) for liquid scintillation counting on a Packard (Meriden, Conn.) TriCarb 2900TR instrument. Quench correction was done with the tSIE/AEC (transformed spectral index of external standard/automatic efficiency correction) method (Ultima Gold-based quench curve). Fecal samples were homogenized in water (two- to fivefold dilution). Total radioactivity in feces was determined by adding aliquots of the fecal homogenate (0.2 to 0.5 ml) for combustion on Combusto-cones. The cones were allowed to dry overnight in a fume hood. The samples were combusted in a Packard Oximate 80 model 307 sample oxidizer. The resulting 3H2O was trapped and mixed in 15 ml of Monophase S scintillation cocktail for counting. A toluene-based quench curve for 3H was used for quench correction.
Conditions for radiochromatography of plasma and urine samples for metabolite profile analysis.
Separate HPLC methods were developed for profiling urine and plasma samples due to the various polarities of the metabolites present in these matrices.
(i) Plasma.
The procedure used for analysis and profiling of plasma and urine samples was similar to the one described by Balani et al. (3). Separation was carried out on a Zorbax RX C8 column (4.6 mm by 25 cm, 5 μm inner diameter) with a mobile phase consisting of acetonitrile (solvent A) and 0.1% trifluoroacetic acid-water (solvent B) at a flow rate of 1.0 ml/min. The gradient began with an isocratic stage of 18% A for 4 min, followed by a linear increase to 50% A over a 36-min interval. The HPLC effluent was fractionated at 1-min intervals, mixed with scintillation cocktail, and assayed by scintillation spectrophotometry. The major metabolite (M0) in plasma was identified by HPLC comparison with a synthetic standard in a flowthrough radioactivity detector. For each of the four species studied, plasma samples were profiled from earlier (8 or 24 h) as well as later (day 3 to 7) time points. For mouse and rat plasma profiles, samples were sometimes pooled across three to six subjects per time point.
(ii) Urine.
Urine samples (from day 1 or 3) were concentrated either in a Speed-Vac system or under nitrogen and analyzed by HPLC. Separation was carried out at a flow rate of 1.0 ml/min as described by Balani et al. (3). The HPLC effluent was fractionated at 1-min intervals, mixed with scintillation cocktail, and assayed by scintillation spectrophotometry for urine profiles. In the rat and monkey experiments, metabolites M0, M1, and M2 were isolated and characterized by liquid chromatography-tandem mass spectrometry and/or nuclear magnetic resonance analyses as described before (3). In the rabbit and mouse experiments, M0, M1, and M2 were identified by HPLC comparison with synthetic standards. For mouse urine profiles, samples were pooled across four to six subjects for a given time point.
Pharmacokinetic analysis.
Pharmacokinetic parameters were determined with the WinNonlin computer software program (version 3.2; Pharsight Corporation). Plasma concentration-time data were fitted to a biexponential decay model with intravenous bolus input and linear first-order elimination from the central compartment by using iterative weighted nonlinear least squares regression analysis. The regression lines through the plots of observed versus predicted concentrations did not differ from the line of identity, and no bias was observed.
RESULTS
Pharmacokinetics of caspofungin in mice, rats, rabbits, and monkeys.
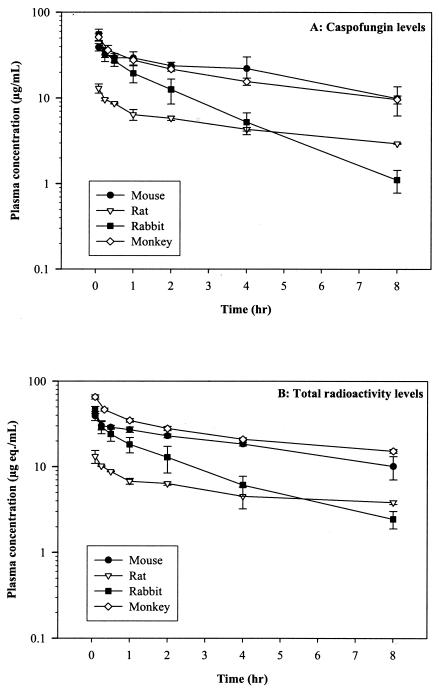
The pharmacokinetics of caspofungin were examined in several preclinical species after intravenous administration. An earlier study in which rats were dosed at 50 mg/kg orally indicated that the oral bioavailability of caspofungin was poor (<1%; data not shown). A follow-up study demonstrated that the absorption of caspofungin was very low upon direct injection of the compound into the rat jejunal lumen. As a result, all subsequent studies were performed after intravenous administration of the compound. Plasma concentration-versus-time profiles for caspofungin after a single intravenous bolus dose are presented in Fig. 1A, and the mean pharmacokinetic parameters are summarized in Table 1. Caspofungin had low plasma clearance properties (mean range, 0.29 to 1.05 ml/min/kg) and a long terminal elimination half-life (t1/2β) (≈12 h in rabbits; mean range, 44.7 to 59.7 h in mice, rats, and monkeys) in all species. The elimination kinetics of caspofungin were multiphasic and displayed an initial distribution phase (t1/2α) followed by a dominant β-elimination phase. The concentrations in plasma at 24 h were substantial and ranged from 0.20 μg/ml in rabbits to 2.29 μg/ml in monkeys. The volume of distribution at steady state was small in rabbits and monkeys (0.28 to 0.30 liters/kg) and slightly less than total body water in mice and rats (0.45 to 0.50 liters/kg).
FIG. 1.
Early plasma concentration-time profile of (A) caspofungin (micrograms per milliliter) and (B) total radioactivity (microgram equivalents per milliliter) after intravenous administration of [3H]caspofungin to mice (5 mg/kg, ≈14 μCi), rats (2 mg/kg, ≈6 to 11 μCi), rabbits (5 mg/kg, ≈40 μCi), and monkeys (5 mg/kg, ≈50 to 65 μCi). Each point represents the mean concentration ± standard deviation (for three mice, rats, and monkeys and four rabbits).
TABLE 1.
Pharmacokinetic parameters of caspofungin and total radioactivity in mice, rats, rabbits, and monkeys after an intravenous dosea
| Species | Dose (mg/kg) | Parent compound
|
Total radioactivity
|
AUC ratio, parent/total | Caspofungin concn in plasma 24 h after a single intravenous dose (μg/ml) | |||||
|---|---|---|---|---|---|---|---|---|---|---|
| Clearance from plasma (ml/min/kg) | Vss (liters/kg) | t1/2α (h) | t1/2β (h) | AUC0-∞ (μg · h/ml) | t1/2β (h) | AUC0-∞ (μg eq · h/ml) | ||||
| Mouse | 5 | 0.29 ± 0.07 | 0.45 ± 0.17 | 4.4 ± 1.2 | 46.9 ± 2.8 | 296.4 ± 67.8 | 54.8 ± 2.8 | 407.1 ± 48.5 | 0.73 ± 0.09 | 1.76 ± 0.67 |
| Rat | 2 | 0.43 ± 0.02 | 0.50 ± 0.10 | 5.9 ± 0.4 | 59.7 ± 14.5 | 77.1 ± 4.2 | 62.8 ± 4.2 | 157.9 ± 13.8 | 0.49 ± 0.05 | 0.46 ± 0.09 |
| Rabbit | 5 | 1.05 ± 0.25 | 0.28 ± 0.05 | 1.2 ± 0.2 | 11.7 ± 8.5 | 82.9 ± 21.3 | 151.4 ± 10.0 | 647.6 ± 66.3 | 0.13 ± 0.02 | 0.20 ± 0.06 |
| Monkey | 5 | 0.30 ± 0.03 | 0.30 ± 0.02 | 5.5 ± 0.6 | 44.7 ± 7.2 | 278.3 ± 26.1 | 199.7 ± 14.6 | 1,658.9 ± 119.7 | 0.17 ± 0.03 | 2.29 ± 0.33 |
Data are means ± standard deviations for three mice, rats, and monkeys and four rabbits.
Parent compound versus total radioactivity levels in plasma.
The plasma concentration-versus-time profiles of total radioactivity versus caspofungin after a single intravenous bolus dose are presented in Fig. 1B and 2, and the mean pharmacokinetic parameters are summarized in Table 1. Total radioactivity was comparable to caspofungin concentrations on day 1 (Fig. 2) in each of the four species, but at the later time points (day 2 onwards), a prolonged terminal phase consisting of low levels of radioactivity was observed throughout the study. The mean terminal t1/2 of total radioactivity was slightly longer than the half-life of the parent compound in mice (46.9 versus 54.8 h) and rats (59.7 versus 62.8 h). It was significantly longer in rabbits (11.7 versus 151.4 h) and monkeys (44.7 versus 199.7 h), suggesting the presence of circulating metabolites. This was further supported by the lower mean area under the curve (AUC0-∞) ratio of parent compound to total radioactivity for rabbits (0.13) and monkeys (0.17) relative to mice (0.73) and rats (0.49).
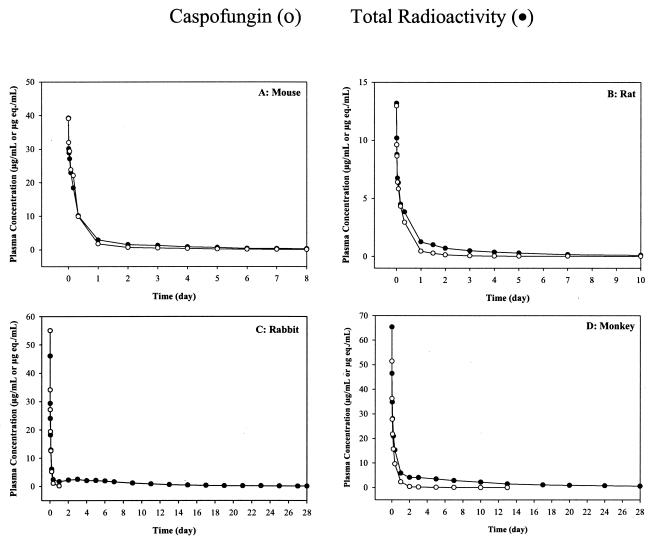
FIG. 2.
Comparison of plasma concentration-time profile of caspofungin (micrograms per milliliter) and total radioactivity (microgram equivalents per milliliter) over several days after intravenous administration of [3H]caspofungin to (A) mice (5 mg/kg, ≈14 μCi), (B) rats (2 mg/kg, ≈6 to 11 μCi), (C) rabbits (5 mg/kg, ≈40 μCi), and (D) monkeys (5 mg/kg, ≈50 to 65 μCi). Each point represents the mean concentration ± standard deviation for three mice, rats, and monkeys and four rabbits.
Excretion of [3H]caspofungin in rats, rabbits, and monkeys.
The mean total recovery of radioactivity after a single intravenous bolus dose of [3H]caspofungin to rats, rabbits, and monkeys is presented in Table 2. A large percentage of the dose was recovered in the urine (42.1, 36.0, and 45.4%) and feces (29.4, 50.8, and 36.2%) for rats, rabbits, and monkeys, respectively, over a collection period of 12 days (rats) or 28 days (rabbits and monkeys). In rats, approximately 3% of the dose was excreted into the bile over 0 to 24 h. Excretion of radioactivity in all species studied was slow, and low levels of radioactivity were detected in urine and feces throughout the collection period. The highest daily excretion of radioactivity occurred between days 1 and 2, 2 and 4, and 3 and 4 postdose for rats, rabbits, and monkeys, respectively.
TABLE 2.
Cumulative radioactivity excretion in urine and feces of caspofungin in rats, rabbits, and monkeys after a single intravenous dosea
| Species | Dose (mg/kg) | Collection period (days) | % of dose excreted in feces | % of dose excreted in urine | Total % of dose excreted |
|---|---|---|---|---|---|
| Rat | 2 | 12 | 29.4 ± 2.5 | 42.1 ± 1.3 | 71.5 ± 1.8 |
| Rabbit | 5 | 28 | 50.8 ± 3.0 | 36.0 ± 4.4 | 86.8 ± 2.9 |
| Monkey | 5 | 28 | 36.2 ± 0.7 | 45.4 ± 0.3 | 81.6 ± 1.1 |
Data are presented as means ± standard deviation for three rats and monkeys and four rabbits.
Metabolism of [3H]caspofungin in mice, rats, rabbits and monkeys.
The proposed known metabolic pathways of caspofungin in the mouse, rat, rabbit, and monkey are depicted in Fig. 3.
Plasma.
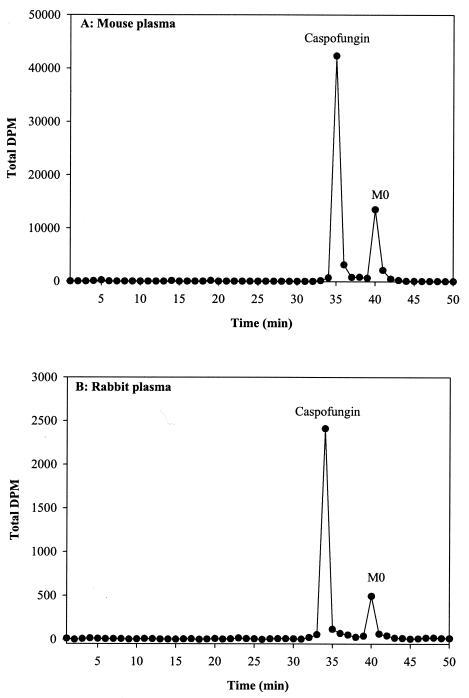
Plasma profiles of mouse, rat, rabbit, and monkey samples after a single intravenous dose demonstrated that the prolonged radioactivity observed (see section on parent versus total radioactivity levels in plasma) was partly attributable to the hydrolytic product, M0, formed via chemical degradation. A significant amount of the radioactivity was not extractable and appeared to be irreversibly bound to plasma proteins. At the earlier time points (day 1), caspofungin was the major component in plasma, with M0 as the only other detectable but minor component (Fig. 4). At the later time points (beyond day 3), M0 was a major component of the extractable radioactivity.
FIG. 4.
Representative radiochromatographic profiles of extracts from (A) 24-h mouse plasma samples and (B) 8-h rabbit plasma samples after intravenous administration of [3H]caspofungin at 5 mg/kg. The metabolite peak was identified as M0. The y axis represents disintegrations per minute.
Bile.
Biliary profiles of rat samples after a single intravenous dose showed the presence of two components, parent and M0. At 24 h, approximately 70% of the radioactivity was associated with the parent compound and the remaining 30% with M0 (radiochromatogram not shown). These data thus indicate that the metabolic profile in rat bile is similar to that observed in rat plasma.
Urine.
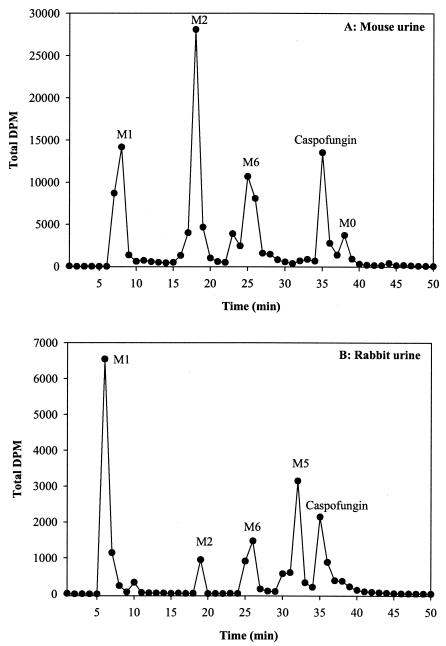
In all the species examined, after a single intravenous dose, unchanged drug was detected in the initial urine samples, whereas metabolites (M0, M1, M2, M3, M4, M5, and/or M6) were the predominant components in the later (day 3) urine samples. In rabbit urine, polar metabolites were observed in the earlier samples. A representative radiochromatograph of the mouse (48 to 72 h) and rabbit (0 to 24 h) urine samples is presented in Fig. 5. In mice, the presence of at least three polar metabolites was evident. At 72 h, approximately two-thirds of the radioactivity was associated with the parent compound (14%) and the major metabolites M1 (20%) and M2 (32%). The two minor metabolites, M0 and M6, accounted for 3 and 15% of the urinary radioactivity, respectively. In rabbits, the presence of at least four polar metabolites was evident. At 24 h, more than half the radioactivity was associated with the parent compound (16%) and the major metabolite M1 (37%). The three minor metabolites M2, M5, and M6 accounted for 4, 15, and 11% of the urinary radioactivity, respectively. Trace amounts of M0 were detected in rabbit as well as rat and monkey urine samples. Metabolites M3, M4, M5, and M6 have not been identified yet.
FIG. 5.
Representative radiochromatographic profiles of extracts from (A) 48- to 72-h mouse urine samples and (B) 0- to 24-h rabbit urine samples after intravenous administration of [3H]caspofungin at 5 mg/kg. Metabolite peaks were identified as M0, M1 [4(S)-hydroxy-4-(4-hydroxyphenyl)-l-threonine], and M2 [N-acetyl-4(S)-hydroxy-4-(4-hydroxyphenyl)-l-threonine]. Metabolites M5 and M6 have not been identified. The y axis represents disintegrations per minute.
The majority of the total urinary radioactivity in preclinical species was associated with metabolites M1 and M2, which were identified as 4(S)-hydroxy-4-(4-hydroxyphenyl)-l-threonine and N-acetyl-4(S)-hydroxy-4-(4-hydroxyphenyl)-l-threonine, respectively. The urinary profile in rats and monkeys (radiochromatograms not shown) was simpler than that observed for mice and rabbits, as M1 and M2 accounted for 81 and 82% of the urinary radioactivity in rats and monkeys, respectively, over a 6- to 7-day collection period. Another polar metabolite, M4, accounted for 4 and 10% of the urinary radioactivity in rats and monkeys, respectively. Trace amounts of another metabolite (M3) were also detected in both of these species.
DISCUSSION
The objective of the studies described above was to conduct definitive experiments to investigate the pharmacokinetics, metabolism, and mass balance of caspofungin in various preclinical species, mice, rats, rabbits, and monkeys. Caspofungin displayed slow elimination kinetics across the four species studied. It had low plasma clearance (0.29 to 1.05 ml/min/kg) and a long terminal half-life (11.7 to 59.7 h) across species after intravenous administration. The concentrations in plasma in all species declined in a multiphasic manner. In rabbits and monkeys, the volume of distribution at steady state was smaller than that in mice and rats, suggesting that the extent of tissue distribution was lower in the higher species. It is likely that the volume of distribution at steady state values presented is an underestimation of the true volume of distribution at steady state because, for caspofungin, drug taken up into the tissues does not appear to equilibrate freely with plasma.
The overall pharmacokinetic properties of caspofungin reported in this study are in concurrence with those of a preliminary investigation conducted earlier by our colleagues (9) in which plasma samples were monitored for 24 h in mice, rats, and monkeys. In the present study, it was observed that levels of caspofungin in plasma were significant until day 8 or beyond postdose for mice, rats, and monkeys, and therefore, the terminal half-life was better characterized and included all time points above the limit of quantitation. Pharmacokinetics in the rabbit were also comparable to those reported by Groll et al. (8) in an earlier study in which the pharmacokinetics of caspofungin was examined after single and multiple intravenous doses. Our data indicate that most of the area under the curve in each of the four species was described by the β-phase and that caspofungin appears to display similar pharmacokinetic properties in humans and in preclinical species. After a single 1-h intravenous infusion in human subjects, clearance from plasma was slow, with a relatively long half-life (19). As in the preclinical species, concentrations of caspofungin in plasma in humans also declined in a polyphasic manner, with a dominant β-phase characterizing much of the elimination profile.
The plasma concentration-versus-time profiles of total radioactivity versus caspofungin after a single intravenous dose indicated that total radioactivity was comparable to caspofungin concentrations on day 1 (Fig. 2) in mice, rats, rabbits, and monkeys, but at the later time points (day 2 onwards), a prolonged terminal phase consisting of low levels of radioactivity was observed. The mean terminal t1/2β of total radioactivity was slightly longer than the half-life of the parent compound in mice and rats. It was, however, significantly longer in rabbits and monkeys, suggesting the presence of circulating metabolites. This was further supported by the mean area under the curve (AUC0-∞) ratio of parent compound to total radioactivity, which was lower for rabbits and monkeys than for mice and rats. The plasma profiles of these samples demonstrated that the prolonged radioactivity was partly attributable to M0. A significant amount of radioactivity was not extractable and appeared to be irreversibly bound to plasma proteins. At the earlier time points, caspofungin was the major component in plasma, with M0 as a minor component (Fig. 4). At the later time points, however, M0 was a major component. These results are consistent with observations made in human subjects, in whom the hydrolysis product, M0, was also detected as a circulating metabolite in plasma at later time points (3).
Excretion studies after intravenous dosing showed that both the hepatic and renal routes play an important role in the elimination of caspofungin and its metabolites. A large percentage of the dose was recovered in the urine and feces of rats, rabbits, and monkeys. Excretion of radioactivity in all species studied was slow, and low levels of radioactivity were detected throughout the collection period. Less than 20% of the administered dose was excreted over a 24-h period in rats, rabbits, and monkeys. These data suggest that the initial rapid decline of radioactivity in plasma was probably due to distribution of radioactivity to tissues rather than to excretion. This was further supported by in vivo rat and in situ rat liver perfusion experiments conducted to assess the uptake of caspofungin in rat liver (X. Xu, F. DeLuna, M. Cartwright, C. Huber, J. Frank, M. Chiba, and J. H. Lin, Abstr. 10th Annu. Meet. Am. Assoc. Pharm. Sci., Pharm. Res. 13:S454, 1996). These studies demonstrated that the initial hepatic uptake of radioactivity was slow but ultimately extensive, with radioactivity levels peaking at 24 to 48 h postdosing and approximately 35 to 39% of the dose present in the liver at these time points. Elimination of radioactivity from the liver also occurred slowly, with approximately 14.2 and 2.8% of the dose still in the liver at 120 h (day 5) and 288 h (day 12), respectively.
Data from the in situ studies demonstrated that the hepatic uptake of caspofungin was a two-step process, involving an initial rapid binding of the drug to the cell surface followed by a slow mechanism of transport into the cell. Collectively, these results suggested that hepatic uptake and elimination of caspofungin were very slow processes and that equilibration between blood and liver tissue was not established rapidly. The liver therefore appears to play an important role in the overall disposition of caspofungin. A similar observation was made in mice, in which levels in plasma dropped rapidly after a single intraperitoneal dose, while levels in the liver continued to rise for 8 h and were significant at 16 to 24 h postdose (9).
The metabolism of caspofungin was qualitatively similar across all species examined. Urine profiles from mouse, rat, rabbit, and monkey samples indicated the presence of several metabolites (M0, M1, M2, M3, M4, M5, and M6), but the majority of the total radioactivity was associated with metabolites M1 [4(S)-hydroxy-4-(4-hydroxyphenyl)-l-threonine] and M2 [N-acetyl-4(S)-hydroxy-4-(4-hydroxyphenyl)-l-threonine], suggesting that hydrolytic pathways, as opposed to oxidative reactions, dominate the biotransformation of caspofungin in preclinical species. A similar metabolic profile was observed in human urine samples, in which M1 and M2 were the predominant metabolites (3). In both preclinical and clinical urine extracts, unchanged drug was detected in the initial urine samples, whereas polar metabolites were the predominant components in the later urine samples. These observations suggest that the rate of formation of metabolites is slow, but the ultimate conversion is quite extensive, as little parent drug is detected in later samples.
In summary, the results from the current set of studies investigated the metabolism, disposition, and single-dose pharmacokinetics of caspofungin in mice, rats, rabbits, and monkeys. Caspofungin had low plasma clearance and long half-life properties in all species examined. The plasma profile was dominated by a β-phase, and most of the area under the curve was defined by this phase. Elimination of caspofungin was slow, and the prolonged presence of a circulating metabolite was observed. Metabolism of caspofungin in vivo was initially slow but ultimately rather extensive. These results suggest that distribution plays a prominent role in the disposition of caspofungin, as very little excretion or biotransformation occurred during the early days after administration of a single intravenous dose. The slow decline in radioactivity in plasma can thus be attributed to slow release from the tissues. In humans as well, the plasma profile of caspofungin was governed by the rate of distribution of compound to the tissues. In conclusion, caspofungin displays similar disposition and metabolism properties in preclinical species and in humans.
Acknowledgments
We thank D. Cylc, F. DeLuna, J. Lin, M. Luu, I. McIntosh, J. Stone, A. Yuan, and members of the laboratory animal research group, Department of Drug Metabolism, Merck Research Laboratories, for their contributions.
REFERENCES
- 1.Abruzzo, G. K., A. M. Flattery, C. J. Gill, L. Kong, J. G. Smith, V. B. Pikounis, J. M. Balkovec, A. F. Bouffard, J. F. Dropinski, H. Rosen, H. Kropp, and K. Bartizal. 1997. Evaluation of the echinocandin antifungal MK-0991 (L-743,872): efficacies in mouse models of disseminated aspergillosis, candidiasis, and cryptococcosis. Antimicrob. Agents Chemother. 41:2333-2338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Arathoon, E. G., E. Gotuzzo, L. M. Noriega, R. S. Berman, M. J. DiNubile, and C. A. Sable. 2002. Randomized, double-blind multicenter study of caspofungin versus amphotericin B for treatment of oropharyngeal and esophageal candidiases. Antimicrob. Agents Chemother. 46:451-457. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Balani, S. K., X. Xu, B. H. Arison, M. V. Silva, A. Gries, F. A. DeLuna, D. Cui, P. H. Kari, T. Ly, C. E. C. A. Hop, R. Singh, M. A. Wallace, D. C. Dean, J. H. Lin, P. G. Pearson, and T. A. Baillie. 2000. Metabolites of caspofungin acetate, a potent antifungal agent, in human plasma and urine. Drug Metab. Dispos. 28:1274-1278. [PubMed] [Google Scholar]
- 4.Bartizal, K., C. J. Gill, G. K. Abruzzo, A. M. Flattery, L. Kong, P. M. Scott, J. G. Smith, C. E. Leighton, A. Bouffard, J. F. Dropinski, and J. Balkovec. 1997. In vitro preclinical evaluation studies with the echinocandin antifungal MK-0991 (L-743,872). Antimicrob. Agents Chemother. 41:2326-2332. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Chandrasekar, P. H., and E. K. Manavathu. 2002. Caspofungin. Drugs Today 38:829-846, [DOI] [PubMed]
- 6.Deresinski, S. C., and D. A. Stevens. 2003. Caspofungin. Clin. Infect. Dis. 36:1445-1457. [DOI] [PubMed] [Google Scholar]
- 7.Garbino, J., D. Lew, B. Hirschel, and P. Rohner. 2003. Caspofungin in the treatment of oropharyngeal candidiasis. Int. J. Clin. Pract. 57:143-144. [PubMed] [Google Scholar]
- 8.Groll, A. H., B. M. Gullick, R. Petraitiene, V. Petraitis, M. Candelario, S. C. Piscitelli, and T. J. Walsh. 2001. Compartmental pharmacokinetics of the antifungal echinocandin caspofungin (MK-0991) in rabbits. Antimicrob. Agents Chemother. 45:596-600. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Hajdu, R., R. Thompson, J. G. Sundelof, B. A. Pelak, F. A. Bouffard, J. F. Dropinski, and H. Kropp. 1997. Preliminary animal pharmacokinetics of the parenteral antifungal agent MK-0991 (L-743,872). Antimicrob. Agents Chemother. 41:2339-2344. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Johnson, M. D., and J. R. Perfect. 2003. Caspofungin: first approved agent in a new class of antifungals. Expert Opin. Pharmacother. 4:807-823. [DOI] [PubMed] [Google Scholar]
- 11.Koss, T., B. Bagheri, C. Zeana, M. F. Romagnoli, and M. E. Grossman. 2002. Amphotericin B-resistant Aspergillus flavus infection successfully treated with caspofungin, a novel antifungal agent. J. Am. Acad. Dermatol. 46:945-947. [DOI] [PubMed] [Google Scholar]
- 12.McGee, W. T., and G. J. Tereso. 2003. Successful treatment of Candida krusei infection with caspofungin acetate: a new antifungal agent. Crit. Care Med. 31:1577-1578. [DOI] [PubMed] [Google Scholar]
- 13.Mora-Duarte, J., R. Betts, C. Rotstein, A. L. Colombo, L. Thompson-Moya, J. Smietana, R. Lupinacci, C. Sable, N. Kartsonis, and J. Perfect. 2002. Comparison of caspofungin and amphotericin B for invasive candidiasis. N. Engl. J. Med. 347:2020-2029. [DOI] [PubMed] [Google Scholar]
- 14.Petraitiene, R., V. Petraitis, A. H. Groll, T. Sein, R. L. Schaufele, A. Francesconi, J. Bacher, N. A. Avila, and T. J. Walsh. 2002. Antifungal efficacy of caspofungin (MK-0991) in experimental pulmonary aspergillosis in persistently neutropenic rabbits: pharmacokinetics, drug disposition, and relationship to galactomannan antigenemia. Antimicrob. Agents Chemother. 46:12-23. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Rubin, M. A., K. C. Carroll, and B. C. Cahill. 2002. Caspofungin in combination with itraconazole for the treatment of invasive aspergillosis in humans. Clin. Infect. Dis. 34:1160-1161. [DOI] [PubMed] [Google Scholar]
- 16.Sable, C. A., B.-Y. T. Nguyen, J. A. Chodakewitz, and M. J. DiNubile. 2002. Safety and tolerability of caspofungin acetate in the treatment of fungal infections. Transplant. Infect. Dis. 4:25-30. [DOI] [PubMed] [Google Scholar]
- 17.Sallmann, S., A. Heilmann, F. Heinke, M.-L. Kerkmann, M. Schuppler, G. Hahn, M. Gahr, A. Rosen-Wolff, and J. Roesler. 2003. Caspofungin therapy for Aspergillus lung infection in a boy with chronic granulomatous disease. Pediatr. Infect. Dis. J. 22:199-200. [PubMed] [Google Scholar]
- 18.Schwartz, M., W. Kline, and B. Matuszewski. 1997. Determination of a cyclic hexapeptide (L-743872), a novel pneumocandin antifungal agent in human plasma and urine by high-performance liquid chromatography with fluorescence detection. Anal. Chim. Acta 352:299-307. [Google Scholar]
- 19.Stone, J. A., S. D. Holland, P. J. Wickersham, A. Sterrett, M. Schwartz, C. Bonfiglio, M. Hesney, G. A. Winchell, P. J. Deutsch, H. Greenberg, T. L. Hunt, and S. A. Waldman. 2002. Single- and multiple-dose pharmacokinetics of caspofungin in healthy men. Antimicrob. Agents Chemother. 46:739-745. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Yuan, A. S., D. Cylc, J. Y.-K. Hsieh, and B. K. Matuszewski. 2001. Determination of an echinocandin, MK-0991, in mammalian plasma by radioimmunoassay. J. Pharm. Biomed. Anal. 25:811-820. [DOI] [PubMed] [Google Scholar]