Abstract
Faster-than-expected post-glacial migration rates of trees have puzzled ecologists for a long time. In Europe, post-glacial migration is assumed to have started from the three southern European peninsulas (southern refugia), where large areas remained free of permafrost and ice at the peak of the last glaciation. However, increasing palaeobotanical evidence for the presence of isolated tree populations in more northerly microrefugia has started to change this perception. Here we use the Northern Eurasian Plant Macrofossil Database and palaeoecological literature to show that post-glacial migration rates for trees may have been substantially lower (60–260 m yr–1) than those estimated by assuming migration from southern refugia only (115–550 m yr–1), and that early-successional trees migrated faster than mid- and late-successional trees. Post-glacial migration rates are in good agreement with those recently projected for the future with a population dynamical forest succession and dispersal model, mainly for early-successional trees and under optimal conditions. Although migration estimates presented here may be conservative because of our assumption of uniform dispersal, tree migration-rates clearly need reconsideration. We suggest that small outlier populations may be a key factor in understanding past migration rates and in predicting potential future range-shifts. The importance of outlier populations in the past may have an analogy in the future, as many tree species have been planted beyond their natural ranges, with a more beneficial microclimate than their regional surroundings. Therefore, climate-change-induced range-shifts in the future might well be influenced by such microrefugia.
Introduction
Estimating rates of tree migration is critical for understanding how species range distributions are shaped by past expansion and contraction, and how species might respond in the future to climate and land-use changes. Plant range-shifts are primarily determined by climate, but life-history traits (rate of establishment, growth, survival, dispersal ability, etc.) are also important [1], [2]. Migration rates of European tree species in response to past climate changes have generally been estimated by assuming that these species persisted during the last glacial maximum (LGM) in southern Europe (southern refugia) with their northernmost distributions at approximately 40–45°N latitude [3], [4], [5], [6]. As a consequence, it has often been assumed that trees dispersed rapidly (100–1000 m yr–1) via long-distance dispersal in response to climate warming during the early post-glacial [3], [7]. The apparent mismatch between observed seed dispersal distances and estimates based on ecological and seed dispersal processes during the Holocene (post-glacial) has often been referred to as Reid's paradox of rapid plant migration [8]. Although palaeoecological, theoretical, and modelling studies have shown that long-distance dispersal could explain rapid migration rates [7], [9], [10], the possibility of such a rapid migration-capacity has been challenged [11].
A growing body of paleoecological, genetic, and climate-modelling literature, particularly from previously less-studied regions, such as eastern Europe and northern Asia, suggests a more northerly glacial survival for both early- and mid-successional tree species [12]–[28]. Genetic studies confirm the importance of advancing leading-edge populations for colonization [16], [29] but it is still unclear how widespread this phenomenon was. However, it is now increasingly acknowledged that these northerly populations might have acted as source populations for post-glacial expansion, in addition to the populations in more southerly locations. This has led to a paradigm shift from colonization via long-distance dispersal to rapid colonization via dispersal from local scattered populations [21], [26], [30]. Considering migration from northern refugia at the end of LGM implies that populations were closer to their present range-limits than estimated under the assumption of dispersal solely from the south, and therefore species migration rates are likely to have been lower than previously assumed [21].
Here we use fossil palaeoecological data to estimate post-glacial migration rates for eight tree taxa (including both shade-intolerant and shade-tolerant trees) taking into account re-population from northern refugia and thus assuming re-colonization via local scattered populations. These eight taxa have wide geographical ranges today in Europe and thus can be presumed to be relatively hardy and to have wide ecological tolerances. We compare these rates to estimates that assume re-colonization only from southern refugia (south of 40–45°N), and show how migration speeds are over-estimated by assuming southern refugia as the only population source. Finally, our post-glacial estimates are compared to maximum migration rates and those projected for the future with a process-based forest succession and dispersal model [31].
We find that post-glacial migration rates for trees may have been substantially lower than those estimated by assuming migration from southern refugia only. We suggest that small outlier populations may be a key factor in understanding past migration rates and in predicting potential future range-shifts.
Materials and Methods
We determined from the Northern Eurasian Plant Macrofossil Database [24] and relevant palaeoecological literature, the northernmost geographical distributions of eight tree taxa at the end of the LGM (approximately 18,000 years ago) and the point in time when these trees reached their modern northern limits (Fig. 1; Table 1). Five out of eight tree taxa, however, are only identified to the generic level and could thus involve species with different geographical distributions, climate requirements, and dispersal modes. Compared to previous attempts at determining range shifts, we increased the taxonomic and spatial resolution by mainly using plant macrofossil remains for our migration-rate estimates. This is because pollen alone does not provide unambiguous evidence for the local presence of a species due to the problems of long-distance pollen dispersal.
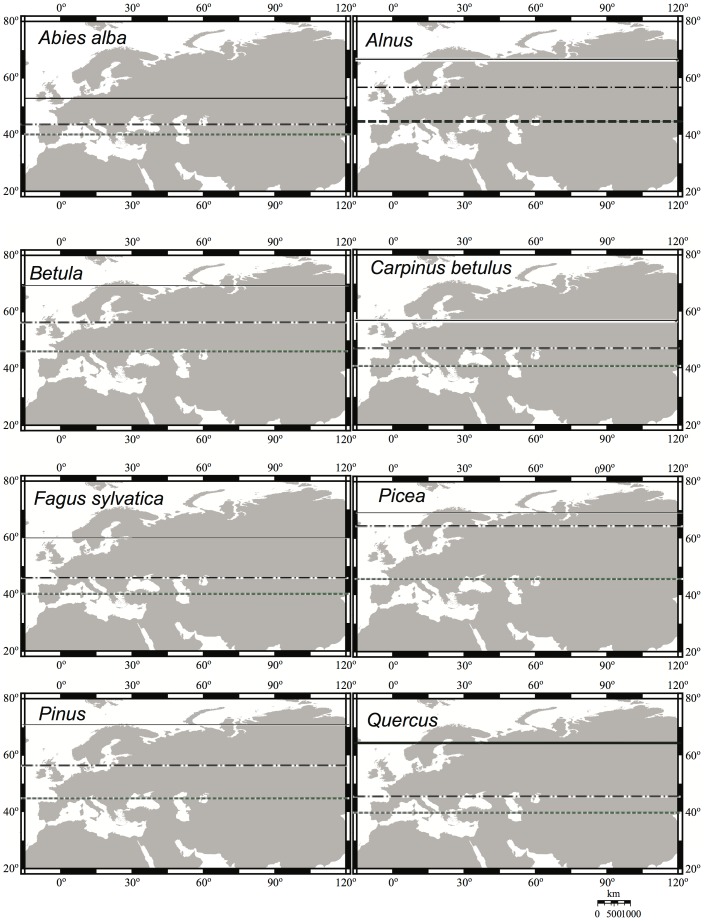
Figure 1. The northern range limit that a tree taxon has reached (line) either from southern refugia (dotted line) located 40–45°N or from northern refugia (dashed line) (see details in Table 1).
Table 1. List of tree taxa for which fossil evidence (pollen, plant macrofossils, charcoal) exists for their survival at 18,000 cal yr BP north of 40°N.
| Species | Distance from southern refugia (km) to the present day limit | Distance northern refugia (km) to the present day limit | Time of arrival at northern limit (cal yr BP) | References |
| Abies alba | 1340 | 1100 | 11,500 | [14] |
| Alnus (tree) | 2450 | 1100 | 7000 | [24] |
| Betula (tree) | 2700 | 1300 | 13,000 | [24] |
| Carpinus betulus | 1850 | 1000 | 2000 | [15] |
| Fagus sylvatica | 2250 | 1500 | 1000 | [16], [47] |
| Picea | 2700 | 500 | 11,000 | [24] |
| Pinus (tree) | 2900 | 1550 | 10,000 | [24] |
| Quercus (temperate) | 2650 | 2000 | 6000 | [5] |
The distance (in km) from the perceived southern location and northern locations, respectively, and the time (calibrated years BP) when each species reached the present-day northern range limit is also given.
Cal yr BP = calibrated years before present (AD 1950).
The present-day northern limits for each species were based on Atlas Florae Europaeae. Two sets of values were computed for each tree taxon: (1) northern migration rate, taking into account that the tree was present in northern refugia (>45°N) and spread to its post-glacial northern limit from these locations, and (2) southern migration rate, assuming that the tree only spread from southern refugia located between 40 and 45°N latitude to its post-glacial northern limit (Figs. 1, 2, Table 1). In the case of southern refugia, the maximum northern limit for cold-tolerant deciduous and coniferous tree species was estimated at 45°N, whereas for temperate deciduous trees the limit is 40°N [4], [5], [6]. The distances (in km) between the start of the migration and the northern range-limit locations were estimated linearly assuming a uniform spread from 18,000 yr BP until the time when a taxon reached its northern distribution (Figs. 1. 2, Table 1). Over-estimation (as percentages) of the migration rates was calculated as the ratio between the southern (2) and northern migration rates (1) multiplied by 100 (Table 2). In addition, we have estimated rates of migration assuming that superimposed on this long-term expansion of tree populations, tree movements could have been halted during the two major cold periods: Heinrich Event1, HE1 (lasting ∼4000 years) and the Younger Dryas, YD (∼1000 years) (Table 2)).
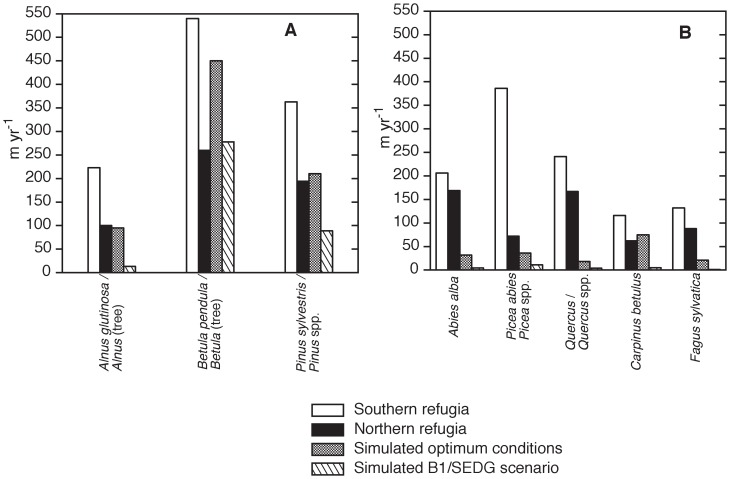
Figure 2. Post-glacial migration-rate estimates for (A) early-successional trees and (B) mid- to late-successional trees assuming colonization from southern and northern refugia, and comparisons with projected mean migration rates from a process-based model [from 31].
When no exact species name is available in the fossil record the genus name is used. Picea spp. includes P. abies and P. obovata, Pinus spp. includes P. sylvestris and P. sibirica, and Quercus spp. could include several species although we expect mainly temperate species such as Q. petraea, Q. robur, and Q. pubescens.
Table 2. Southern post-glacial migration-rate estimates (m yr–1) assuming that species spread to their present-day northern limit from the south (40–45°N latitude), and northern migration rates assuming that species spread to their present-day northern limit from their northernmost refugia.
| Species | Southern fossil estimates | Northern fossil estimates | Over-estimates (fossil) | Projected mean rates (CC-Scenario B1/SEDG) | Projected max. rates (under optimal conditions) |
| Early succesional | |||||
| Alnus glutinosa/Alnus (tree) | 225 (410) | 100 (185) | 225 | 13.6±15.3 | 95 |
| Betula pendula/Betula (tree) | 540 (0) | 260 (0) | 210 | 278.2±103.8 | 450 |
| Pinus sylvestris/Pinus (tree) | 360 (970) | 195 (515) | 185 | 89±35.4 | 210 |
| Mid- to late succesional | |||||
| Abies alba | 205 (895) | 170 (755) | 120 | 4.5±6.1 | 32 |
| Carpinus betulus | 115 (170) | 60 (90) | 185 | 5.0±6.5 | 75 |
| Fagus sylvatica | 130 (190) | 90 (125) | 150 | 1.1±1.7 | 21 |
| Picea abies | 385 (1350) | 70 (250) | 540 | 11.2±6.1 | 36 |
| Quercus petraea/ Quercus spp. | 220 (380) | 165 (285) | 130 | 3.6±4.5 | 18 |
Estimated rates of migration assuming no movement of taxa during the two major cold periods: Heinrich Event1 (lasting ∼4000 years) and the Younger Dryas (lasting ∼1000 years) are given in italics. Projected mean migration rates (m yr–1) for several tree species for 2100 using B1/SEDG greenhouse gas emission/land-use change scenarios, as well as the maximum rates derived from weak competition and optimum temperature conditions [31] are also given. When no exact species name is available in the fossil record the genus name is used.
Results
Our analysis shows that: 1) the spreading-rate estimates from northern refugia are substantially lower than those that assume colonization only from the south, and 2) early-successional trees (Betula, Pinus, Alnus) migrated faster than mid- and late-successional ones (Picea, Abies alba, Quercus, Carpinus betulus, Fagus sylvatica) (Fig. 2, Table 2). The early-successional tree migration rates that assume spreading from the north vary from 100 to 260 m yr–1, whereas migration-rates that assume spreading from the south vary from 225 to 540 m yr–1 (Fig. 2A, Table 2). The mid- and late-successional tree migration rates that assume spreading from the north vary between 60 and 170 m yr–1 whereas migration-rates that assume spreading from the south range between 115 and 385 m yr–1 (Fig. 2B, Table 2). The northerly estimates are lower than those obtained by assuming survival in southern Europe only, with over-estimates ranging from 120 to 540 m yr–1 (Table 2). The northerly migration estimates assuming no movement of taxa during the Heinrich Event1 and the Younger Dryas range between 0 and 515 m yr–1 for early-successional trees and between 90 and 735 m yr–1 for mid- and late-successional trees (Table 2).
Discussion
The post-glacial migration-rate estimates assuming colonization from northern populations suggest that the rates for both early- and mid- to late-successional trees are much lower than previously estimated, and that early-successional trees generally migrated faster than mid- and late-successional ones (Fig. 2). Our results also show that many taxa, in particular early- and mid-successional trees, reached their modern northern distribution in the early Holocene, a time of rapid climate changes (Table 1). The ability of early-successional pioneer taxa to persist in the harsh cold and dry LGM climate and to migrate faster than mid- and late-successional taxa would be expected as a result of differences in life history (fast growth, large seed production, far-distance dispersal) and greater stress tolerance to large amplitude temperature change, drought, etc [3], [9], [22], [32]. Generation time might also explain differences in migration rates among taxa with similar dispersal properties. For example, the early-successional trees Pinus, Betula, and Alnus first set seed at an age of 10–20 years, whereas Picea abies first sets seed at 30–40 years or even after 50 years [33]. Thus, although Pinus and Picea have similar seed dispersal properties, Pinus is able to spread much faster than Picea does (Fig. 2, Table 1).
Post-glacial migration rates similar to our fossil estimates have been derived from climate-driven modelled refugia at a spatial resolution of ca 16 km, and range between 35 and 380 m yr–1, with generally higher values for shade-intolerant trees (Betula pendula, B. pubescens, Pinus sylvestris) and for Picea abies than for other shade-tolerant species [2], [19]. These authors also suggest that accessibility from refugia explains to a large extent the post-glacial range shifts for many species, in particular those with a limited dispersal ability. However, they do not use fossil data as evidence of actual refugia [2], [19]. Fossil migration estimates of 250 m yr–1 for Picea abies and 100 m yr–1 for Fagus sylvatica have been recently obtained for southern Scandinavia based on pollen records and model simulation output [34] and are lower than those previously obtained of ca. 500 m yr–1 for the same region [3]. Using a similar approach as above, range displacements (contraction and expansion) between −170 and 270 m yr–1 have been reported from North America [35], which are higher than those previously obtained (<100 m yr–1) in this region based on phylogeographraphic data [29]. It is therefore evident that most species were generally only capable of migration rates less than 260 m yr–1. Some late-successional taxa such as Picea, Abies alba, and Quercus that spread quickly in the early Holocene (Fig. 2B) under conditions of low competition and low human impact should more accurately reflect their intrinsic rates of spread than other late-succession species such as Fagus sylvatica and Carpinus betulus that spread during the late Holocene and were probably dependent, to some degree, on anthropogenic disturbance of the already established forests. Our estimates indeed show fast migration rates for these species, namely Abies alba (170 m yr–1; max. 735 m yr–1) and Quercus (165 m yr–1; max. 285 m yr–1), except for Picea (70 m yr–1; max. 250 m yr–1), suggesting that their northern populations responded rapidly to climate change and were therefore not greatly affected by migrational lags during the post-glacial. Models of post-glacial population expansion indicate that initially tree species spread rapidly as low-density populations or isolated individuals in an advancing wave front reaching their northern limit ahead of mass colonization [34]–[37]. Later, when climate conditions became suitable and more stable, and there were higher population densities, species migrated more slowly due to increased competition from already present populations [19], [33], [35], [36]. A reduction in migration rates is also predicted to have occurred towards the species distributional limits because of less suitable climate [19], [35]–[38]. It should, however, be noted that in the case of Picea, for which our calculations are based on the Eurasian macrofossil data-base and thus on northern locations, migration rates are lower than the estimates for Picea abies [34]–[36], which are based on European pollen and macrofossil data. The migration-rate estimates for Picea involve two potential species, namely Picea abies and P. obovata; the latter tolerates colder temperatures and has a more northern and eastern geographical distribution than P. abies, which could have lowered the overall migration rates for Picea.
Our migration estimates are calculated assuming a uniform spread from 18,000 yr BP until the time when the taxon reached its northern distribution, despite the possibility that superimposed on this long-term expansion of tree populations, tree movements could have been halted by two major cold periods: Heinrich Event 1 and the Younger Dryas [39]–[40]. Nevertheless, our migration estimates that assume no movement during HE1 and YD (5000 years) (Table 2) change little for taxa that reached their modern northern distribution during the late Holocene (Fagus sylvatica, Carpinus betulus). However, our estimates under this scenario (Table 2) show high migration rates for most taxa that had already reached their modern northern distribution during the early Holocene (Pinus, Abies, Betula, Picea), at times of rapid climate changes. This probably reflects tree spread at low-densities in an advancing wave front ahead of mass colonization at the late-glacial/Holocene transition. Overall, we think, that assuming uniform migration rates over the entire time interval, as opposed to no movement for a period of 5000 years, appears to provide more realistic migration-rate estimates as this procedure balances fast northward movements during periods of rapid climate change and slower northward movements during periods of cold or stable climate conditions and at higher population density. Estimates of latitudinal taxa displacement from North America show dynamic changes between 16 and 12 k yr BP (expansion, contraction, stagnation), predominant fast northward expansion between 12 and 7 k yr BP and overall lower migration rates during warm and/or stable conditions occurring between 7 and 1 k yr BP [35]. Like with any fossil estimates, the spatial and temporal scales of fossil data considered for our analysis cannot provide exact locations of all northerly refugia or of the time when a taxon reached its modern northern limit. Our post-glacial migration rates should therefore be regarded as approximate estimates. Migration rates are also integrated over the varying biotic and abiotic conditions along the different migrational paths. The presence of large European mountains chains (Pyrenees, Alps, Carpathians) were previously found to not have acted as geographical barriers in the case of the spread of Fagus sylvatica in Europe during the last glacial–postglacial, but rather to have facilitated its survival and spread [16]. Topography might therefore be less important for many other trees considered here.
We compared our post-glacial migration-rate estimates with simulated migration rates under i) optimal conditions of competition and climate, and ii) conditions of future climate change and land-use scenarios that have been simulated using a tree migration meta-model [31]. This meta-model had been regressed against simulations of the spatio-temporal forest landscape model TreeMig [41] under various climate, competition, and fragmentation conditions and was used to constrain migration distances in an empirical species distribution model at the European scale under future land-use and resulting fragmentation and climate change. This approach thus accounts for climate influence, landscape pattern, habitat suitability, population dynamics, competition, and seed dispersal (for details see 31), unlike other models projecting the impact of 21st century climate and landscape fragmentation on species range-shifts, which commonly use two extreme scenarios, i.e. unlimited or no dispersal [42], [43], [44]. The simulated rates fit better with our estimated “northern” than with the “southern” post-glacial migration rates (Fig. 2, Table 2) or with previous pollen-derived estimates [3]. The fact that the simulated rates for shade-intolerant species (mean value for future rates of 155 m yr–1) are of the same magnitude as those derived for the post-glacial rates implies that this is an important step towards understanding how some trees migrate in response to climate change and habitat fragmentation. Nevertheless, the simulated migration rates for shade-tolerant tree species (mean rate for the future is 15 m yr–1) are still an order of magnitude slower than the post-glacial migration rates, although the optimum simulated values (the maximum rate a species could move without competition or with weak competition only and without habitat fragmentation) are closer to the post-glacial estimates (Fig. 2, Table 2). This might indicate a weak influence of competition and fragmentation during the Holocene compared to the future. In addition, the reason that the simulated migration rates for shade-tolerant tree species are generally lower than the estimated post-glacial migration rates further suggests that glacial refugia for shade-tolerant tree species were farther north than currently known. Interestingly, Picea, whose post-glacial migration rate based on fossil data [this study, 34, 36] and hindcast model results [2], [19] suggests a rather fast migration (70–250 m yr–1), yields much slower rates of 10 m yr–1 in the future and 36 m yr–1 with the optimal dispersal simulations (Fig. 2). One potential reason is that, in the simulation model, Picea abies is assigned to the class of “medium dispersers” instead of “long-distance dispersers”, despite its (particularly at high latitudes) small seeds [45].
In summary, deriving insights on how species range distributions were shaped by expansions during the post-glacial represents a contribution to our understanding of tree-species migration rates and their likely response to future climate and land-use changes. We demonstrate that post-glacial migration estimates assuming colonization from local northern populations are much lower than previously estimated assuming colonization from the south only. Thought migration estimates presented here may be conservative because of our assumption of uniform dispersal, tree migration-rates clearly need reconsideration (Figs. 1, 2, Table 2). Although these post-glacial migration-rate estimates might change in the future when more fossil evidence becomes available, palaeobotanical data suggest that populations persisting in northern Europe during the LGM are less likely to be limited in their distribution by migrational lag. We suggest that allowing for small outlier populations is crucial for understanding past tree migration-rates and for simulating potential future changes, but this has rarely been done. The importance of outlier populations in the past may have an analogy in the future, as many tree species have been planted in small populations beyond their natural ranges, for example in parks [46], usually with a slightly more beneficial micro-climate than their surroundings. Therefore, climate-change induced range-shifts in the future might well be influenced by such microrefugia.
Acknowledgments
H.J.B.B. acknowledges help from Cathy Jenks. T. Giesecke and D. Magri are gratefully acknowledged for providing constructive comments on early draft of the paper.
Funding Statement
The macrofossil database was funded through National Environment Research Council grant number NE/D001578/1, awarded to MEE and KJW. AF and TH acknowledge funding from the German Research Foundation (grant FE-1096/2-1), TH from the Biodiversity and Climate Research Center, and HL from the Swiss National Science foundation (grant 315230_122434). The funders had no role in study design, data collection and analysis, decision to publish, or preparation of the manuscript.
References
- 1. Higgins SI, Clark JS, Nathan R, Hovestadt T, Schurr F, et al. (2003) Forecasting plant migration rates: managing uncertainty for risk assessment. J Ecol 91: 341–347. [Google Scholar]
- 2. Normand S, Ricklefs RE, Skov F, Bladt J, Tackenberg O, et al. (2011) Postglacial migration supplements climate in determining plant species ranges in Europe. Proc R Soc Lond B 278: 3644–3653. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Huntley B, Birks HJB (1983) An Atlas of Past and Present Pollen Maps for Europe: 0–13000years ago. Cambridge University Press, Cambridge.
- 4. Bennett KD, Tzedakis PC, Willis KJ (1991) Quaternary refugia of North European trees. J. Biogeogr 18: 103–115. [Google Scholar]
- 5. Petit RJ, Brewer S, Bordács S, Burg K, Cheddadi R, et al. (2002) Identification of refugia and post-glacial colonisation routes of European white oaks based on chloroplast DNA and fossil pollen evidence. For Ecol Manag 156: 49–74. [Google Scholar]
- 6. Tzedakis PC, Lawson IT, Frogley MR, Hewitt GM, Preece RC (2002) Buffered tree population changes in a Quaternary refugium: evolutionary implications. Science 297: 2044–2047. [DOI] [PubMed] [Google Scholar]
- 7. Ritchie JC, MacDonald GM (1996) The patterns of post-glacial spread of white spruce. J Biogeogr 13: 527–540. [Google Scholar]
- 8. Clark JS, Fastie C, Hurtt G, Jackson ST, Johnson C, et al. (1998) Reid's paradox of rapid plant migration. BioScience 48: 13–24. [Google Scholar]
- 9. Clark JS (1998) Why trees migrate so fast: Confronting theory with dispersal biology and the paleo record. Am Nat 152: 204–224. [DOI] [PubMed] [Google Scholar]
- 10. Nathan R, Katul G, Horn HS, Thomas SM, Oren R, et al. (2002) Mechanisms of long-distance dispersal of seeds by wind. Nature 418: 409–413. [DOI] [PubMed] [Google Scholar]
- 11. Clark JS, Lewis M, Horvath L (2001) Invasion by extremes: population spread with variation in dispersal and reproduction. Am Nat 157: 537–554. [DOI] [PubMed] [Google Scholar]
- 12. Willis KJ, Rudner E, Sumegi P (2000) The full-glacial forests of central and southeastern Europe. Quat Res 53: 203–213. [Google Scholar]
- 13. Kullman L (2002) Rapid recent range-margin rise of tree and shrub species in the Swedish Scandes. J Ecol 90: 68–77. [Google Scholar]
- 14. Terhurne-Berson R, Litt T, Cheddadi R (2004) The spread of Abies throughout Europe since the last glacial period: combined macrofossil and pollen data. Veg Hist & Archaeobot 13: 257–268. [Google Scholar]
- 15. Willis KJ, van Andel TH (2004) Trees or no trees? The environments of central and eastern Europe during the Last Glaciation. Quat Sci Rev 23: 2369–2387. [Google Scholar]
- 16. Magri D, Vendramin GG, Comps B, Dupanloup I, Geburek T, et al. (2006) A new scenario for the Quaternary history of European beech populations: palaeobotanical evidence and genetic consequences. New Phytol 117: 199–221. [DOI] [PubMed] [Google Scholar]
- 17. Feurdean A, Wohlfarth B, Björkman L, Tantau I, Bennike O, et al. (2007) The influence of refugial populations on lateglacial and early vegetational changes in Romania. Rev Palaeobot & Palynol 145: 305–320. [Google Scholar]
- 18. Svenning JC, Skov F (2007a) Ice age legacies in the geographical distribution of tree species richness in Europe. Global Ecol & Biogeogr 16: 234–245. [Google Scholar]
- 19. Svenning JC, Skov F (2007b) Could the tree diversity pattern in Europe be generated by postglacial dispersal limitation? Ecol Lett 10: 453–460. [DOI] [PubMed] [Google Scholar]
- 20. Svenning JC, Normand S, Kageyama M (2008) Glacial refugia of temperate trees in Europe: insights from species distribution modelling. J Ecol 96: 1117–1127. [Google Scholar]
- 21. Birks HJB, Willis KJ (2008) Alpines, trees, and refugia in Europe. Plant Ecol & Divers 1: 147–160. [Google Scholar]
- 22. Bhagwat SA, Willis KJ (2008) Species persistence in northerly glacial refugia of Europe: a matter of chance or biogeographical traits? J Biogeogr 35: 464–482. [Google Scholar]
- 23. Kuneš P, Pelánková B, Chytrý M, Jankovská V, Pokorný P, et al. (2008) Interpretation of the last-glacial vegetation of eastern-central Europe using modern analogues from southern Siberia. J Biogeogr 35: 2223–2236. [Google Scholar]
- 24. Binney HA, Willis KJ, Edwards ME, Bhagwat SA, Anderson PA, et al. (2009) The distribution of late-Quaternary woody taxa in northern Eurasia: evidence from a new macrofossil database. Quat Sci Rev 28: 2445–2464. [Google Scholar]
- 25. Allen JRM, Hickler T, Singarayer JS, Sykes MT, Valdes PJ, et al. (2010) Last glacial vegetation of northern Eurasia. Quat Sci Rev 29: 2604–2618. [Google Scholar]
- 26. Stewart JR, Lister AM, Barnes J, Dalen L (2010) Refugia revisited: individualistic responses of species in space and time. Proc R Soc Lond B 277: 661–671. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27. Väliranta M, Kaakinen A, Kuhry P, Kultti S, Salonen JS, et al. (2011) Scattered late-glacial and early-Holocene tree populations as dispersal nuclei for forest development in NE European Russia. J Biogeogr 38: 922–932. [Google Scholar]
- 28. Ohlemuller R, Huntley B, Normand S, Svenning JC (2012) Potential source and sink locations for climate-driven species range shifts in Europe since the Last Glacial Maximum. Global Ecol & Biogeogr 21: 152–163. [Google Scholar]
- 29. McLachlan JS, Clark JS, Manos PS (2005) Molecular indicators of tree migration capacity under rapid climate change. Ecology 86: 2088–2098. [Google Scholar]
- 30. Stewart JR, Lister AM (2001) Cryptic northern refugia and the origins of the modern biota. Trends Ecol Evol 16: 608–613. [Google Scholar]
- 31. Meier ES, Lischke H, Schmatz DR, Zimmermann NE (2012) Climate, competition and connectivity affect future migration and ranges of European trees. Global Ecol Biogeogr 21: 164–178. [Google Scholar]
- 32. Feurdean A, Tămaş T, Tanţău I, Fărcaş S (2012) Elevational variation in regional vegetation responses to late-glacial climate changes in the Carpathians. J Biogeogr 29: 258–271. [Google Scholar]
- 33. Lischke H, Löffler T (2006) Intra-specific density dependence is required to maintain diversity in spatio-temporal forest simulations with reproduction. Ecol Model 198: 341–361. [Google Scholar]
- 34. Bialozyt R, Bradley LR, Bradshaw RHW (2012) Modelling the spread of Fagus sylvatica and Picea abies in southern Scandinavia during the late Holocene. J Biogeogr 39: 665–675. [Google Scholar]
- 35. Ordonez A, Williams JW (2013) Climatic and biotic velocities for woody taxa distribution over the last 16 000 years in eastern North America. Ecol Lett 16: 773–781. [DOI] [PubMed] [Google Scholar]
- 36. Giesecke T (2005) Moving front or population expansion: How did Picea abies (L.) Karst. become frequent in central Sweden? Quat Sci Rev 24: 2495–2509. [Google Scholar]
- 37. Giesecke T, Bennett KD (2004) The Holocene spread of Picea abies (L.) Karst. In Fennoscandia and adjacent areas. J Biogeogr 31: 1523–1548. [Google Scholar]
- 38. Birks HJB (1989) Holocene isochrone maps and patterns of tree-spreading in the British Isles. J. Biogeogr 16: 503–540. [Google Scholar]
- 39. Naughton F, Sánchez Gofii MF, Kageyama M, Bard E, Cortijo E, et al. (2009) Wet to dry climatic trend in north western Iberia within Heinrich events. Earth Planet Sci Lett 284: 329–342. [Google Scholar]
- 40. Blockley SPE, Lane CS, Hardiman M, Rasmussen SO, Seierstad IK, et al. (2012) Synchronisation of palaeoenvironmental records over the last 60,000 years, and an extended INTIMATE1 event stratigraphy to 48,000 b2k. Quat Sci Rev 36: 2–10. [Google Scholar]
- 41. Lischke H, Zimmermann NE, Bolliger J, Rickebusch S, Loffler TJ (2006) TreeMig: A forest-landscape model for simulating spatio-temporal patterns from stand to landscape scale. Ecol Model 199: 409–420. [Google Scholar]
- 42. Thomas CD, Cameron A, Green RE, Bakkenes M, Beaumont LJ, et al. (2004) Extinction risk from climate change. Nature 427: 145–148. [DOI] [PubMed] [Google Scholar]
- 43. Thuiller W, Lavorel S, Araujo MB, Sykes MT, Prentice IC (2005) Climate change threats to plant diversity in Europe. Proc Nat Acad Sci USA 102: 8245–8250. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Hickler T, Vohland K, Feehan J, Miller P, Fronzek S, et al. (2012) Projecting tree species – based climate- driven changes in European potential natural vegetation with a generalized dynamic vegetation model. Global Ecol & Biogeogr 21: 50–63. [Google Scholar]
- 45.Helmisaari H, Nedialko N (1989) Survey of ecololgical characteristics of boreal tree species in Fennoscandia and the USSR. IIASA Working Papers, vol 89–65. IIASA, Laxenburg.
- 46. Van der Veken S, Hermy M, Vellend M, Knapen A, Verheyen K (2008) Garden plants get a head start on climate change. Front Ecol & Environ 6: 212–216. [Google Scholar]
- 47. Giesecke T, Hickler T, Kunkel T, Sykes MT, Bradshaw RHW (2007) Towards an understanding of the Holocene distribution of Fagus sylvatica L. J Biogeogr. 34: 118–131. [Google Scholar]