Abstract
The doripenem MICs at which 90% of the tested strains were inhibited ranged from 0.03 to 1 μg/ml for 10 species of Enterobacteriaceae (n = 351), from 0.03 to 0.12 μg/ml for oxacillin-susceptible staphylococci (n = 119), from 4 to 32 μg/ml for oxacillin-resistant staphylococci (n = 64), from ≤0.008 to 0.06 μg/ml for penicillin-susceptible streptococci (n = 132), and from 1 to 4 μg/ml for penicillin-resistant streptococci (n = 51). Overall, doripenem demonstrated in vitro activity similar to that of meropenem against gram-negative pathogens and to that of imipenem against gram-positive pathogens.
The synthesis of new carbapenems remains an area of intense research because of the broad-spectrum antibacterial activity of this chemical class (6-9, 11, 12). Doripenem (formerly S-4661) is an investigational parenteral 1β-methlycarbapenem, originally discovered by Shionogi & Co., Ltd. (Osaka, Japan) (1), that is currently being developed in the United States by Peninsula Pharmaceuticals, Inc. (Alameda, Calif.) for the treatment of hospitalized patients with serious systemic bacterial infections. Preliminary reports indicate that doripenem has activity against a broad spectrum of bacterial pathogens (6, 9, 11; R. N. Jones, H. Huynh, and D. J. Biedenbach, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-527, 2003), that it has favorable pharmacokinetic properties (K. Shiba, M. Nakashima, H. Tanimura, H. Okada, J. Shimada, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-217, 1997; D. A. Thye, T. Kilfoil, A. Leighton, and M. Wikler, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. A-21, 2003), that it is safe and well tolerated in humans (D. A. Thye et al., 43rd ICAAC), that it is not hydrolyzed by renal dehydropeptidase I like imipenem (2), and that it does not promote the release of endotoxin (10). The objectives of the present study were to investigate the in vitro activity of doripenem against a collection of clinically relevant gram-negative and gram-positive pathogens and to compare the activity of doripenem with the activities of other carbapenems, broad-spectrum cephalosporins, and piperacillin-tazobactam.
(Part of this research was presented at the 43rd Interscience Conference on Antimicrobial Agents and Chemotherapy, Chicago, Ill., 14 to 17 September 2003.)
Isolates tested in the present study were selected from the isolate repository maintained by Focus Technologies (Herndon, Va.) based upon the isolates' species or group identity and their antimicrobial susceptibility testing phenotype; isolates were chosen irrespective of the age of the patient (<1 to 98 years), the specimen source (respiratory, wound, urine, or blood) from which they were isolated, or any other patient demographic parameter. All isolates tested in the present study were collected in U.S. clinical microbiology laboratories from 1999 to 2003. In total, 815 isolates were tested, of which 381 were gram-negative isolates and 434 were gram-positive isolates. Each isolate was taken from frozen stock (−70°C) and subcultured twice onto sheep blood agar; the identity of each isolate was confirmed by using standard clinical laboratory methods applicable to each species or organism group (3).
Antimicrobial susceptibility testing was performed in accordance with the recommended procedures of the National Committee for Clinical Laboratory Standards (NCCLS) (4) by using frozen broth microdilution panels prepared by TREK Diagnostics (Cleveland, Ohio). All isolates were tested against cefepime, ceftriaxone, doripenem, ertapenem, imipenem, meropenem, and piperacillin-tazobactam. Additional antimicrobial agents were tested to confirm antimicrobial susceptibility testing phenotypes and for reference. These agents varied with bacterial species or organism group as follows: gram-negative bacteria were also tested against ampicillin, amoxicillin-clavulanate, aztreonam, ceftazidime, and ciprofloxacin; staphylococci and enterococci were also tested against oxacillin, levofloxacin, and vancomycin; and streptococci were also tested against penicillin, azithromycin, and levofloxacin. Isolates were defined as susceptible, intermediate, or resistant to antimicrobial agents according to NCCLS standard M100-S13 (5). Extended-spectrum β-lactamase (ESBL) confirmatory testing was performed by using the NCCLS recommended method for isolates of Escherichia coli, Klebsiella pneumoniae, and Klebsiella oxytoca (5).
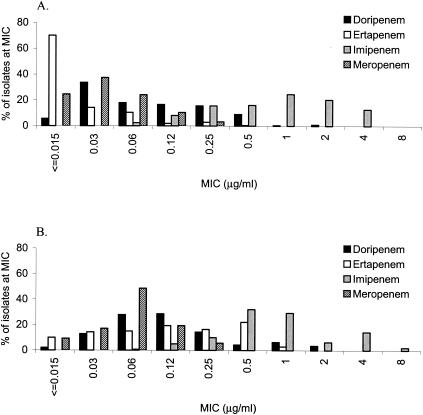
The doripenem MICs at which 90% of the strains were inhibited (MIC90) ranged from 0.03 to 0.5 μg/ml for all Enterobacteriaceae tested, with the exception of Proteus mirabilis (MIC90, 1 μg/ml) (Table 1). The MICs of doripenem for the isolates of Enterobacteriaceae tested were similar to the MICs of ertapenem and meropenem, while imipenem MICs were approximately 2 to 4 doubling dilutions higher than the MICs of the other three carbapenems (Fig. 1). The MICs at which 50% of the strains were inhibited, MIC90s, modal MICs, and MIC ranges of doripenem for ESBL-negative and ESBL-positive E. coli and K. pneumoniae were similar; the MICs of doripenem for ceftazidime-intermediate and -resistant isolates of other Enterobacteriaceae were identical or 1 to 2 doubling dilutions higher than for ceftazidime-susceptible isolates. Generally, the differences in the MICs of ertapenem for ceftazidime-susceptible and ceftazidime-intermediate and -resistant isolates of Enterobacteriaceae were greater (2 to 6 doubling dilutions for all species tested except Providencia spp.) than the differences in those of doripenem, imipenem, and meropenem (Table 1). Against the isolates of Acinetobacter baumannii tested, doripenem demonstrated better activity against ceftazidime-susceptible isolates (MIC90, 1 μg/ml) than against ceftazidime-intermediate and -resistant isolates (MIC90, >16 μg/ml).
TABLE 1.
In vitro MICs of doripenem and comparative β-lactams for A. baumannii, 10 species of Enterobacteriaeae, enterococci, staphylococci, and streptococcia
| Organism, antimicrobial agent, and isolate phenotype | No. of isolates tested | MIC (μg/ml)
|
|||
|---|---|---|---|---|---|
| Range | Mode | MIC50 | MIC90 | ||
| Acinetobacter baumannii | |||||
| Aztreonam | |||||
| Ceftazidime-susceptible | 20 | 8->32 | 16 | 32 | >32 |
| Ceftazidime-intermediate or resistant | 10 | 16->32 | >32 | >32 | >32 |
| Cefepime | |||||
| Ceftazidime-susceptible | 20 | ≤0.5-16 | 4 | 4 | 8 |
| Ceftazidime-intermediate or resistant | 10 | 8->32 | >32 | >32 | >32 |
| Ceftriaxone | |||||
| Ceftazidime-susceptible | 20 | 4-32 | 16 | 16 | 32 |
| Ceftazidime-intermediate or resistant | 10 | 32->64 | >64 | >64 | >64 |
| Doripenem | |||||
| Ceftazidime-susceptible | 20 | 0.12-1 | 0.12 | 0.25 | 1 |
| Ceftazidime-intermediate or resistant | 10 | 0.25->16 | 1 | 1 | >16 |
| Ertapenem | |||||
| Ceftazidime-susceptible | 20 | 0.5-16 | 2 | 2 | 8 |
| Ceftazidime-intermediate or resistant | 10 | 2->16 | 8 | 8 | >16 |
| Imipenem | |||||
| Ceftazidime-susceptible | 20 | 0.06-0.5 | 0.25 | 0.25 | 0.25 |
| Ceftazidime-intermediate or resistant | 10 | 0.25->16 | 1 | 1 | 8 |
| Meropenem | |||||
| Ceftazidime-susceptible | 20 | 0.12-2 | 0.25 | 0.25 | 1 |
| Ceftazidime-intermediate or resistant | 10 | 0.25->16 | 2 | 2 | 16 |
| Piperacillin-tazobactam | |||||
| Ceftazidime-susceptible | 20 | ≤1-32 | ≤1 | 4 | 32 |
| Ceftazidime-intermediate or resistant | 10 | 32->128 | >128 | 128 | >128 |
| Citrobacter freundii | |||||
| Aztreonam | |||||
| Ceftazidime-susceptible | 21 | ≤1-8 | ≤1 | ≤1 | ≤1 |
| Ceftazidime-intermediate or resistant | 20 | 4->32 | 16 | 16 | >32 |
| Cefepime | |||||
| Ceftazidime-susceptible | 21 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 |
| Ceftazidime-intermediate or resistant | 20 | ≤0.5-16 | ≤0.5 | 1 | 4 |
| Ceftriaxone | |||||
| Ceftazidime-susceptible | 21 | ≤0.5-2 | ≤0.5 | ≤0.5 | ≤0.5 |
| Ceftazidime-intermediate or resistant | 20 | 8->64 | 32 | 32 | >64 |
| Doripenem | |||||
| Ceftazidime-susceptible | 21 | ≤0.015-0.06 | 0.03 | 0.03 | 0.03 |
| Ceftazidime-intermediate or resistant | 20 | 0.03-0.25 | 0.06 | 0.06 | 0.12 |
| Ertapenem | |||||
| Ceftazidime-susceptible | 21 | ≤0.015-0.03 | ≤0.015 | ≤0.015 | ≤0.015 |
| Ceftazidime-intermediate or resistant | 20 | 0.03-0.5 | 0.12 | 0.12 | 0.5 |
| Imipenem | |||||
| Ceftazidime-susceptible | 21 | 0.12-1 | 1 | 1 | 1 |
| Ceftazidime-intermediate or resistant | 20 | 0.5-2 | 0.5 | 0.5 | 1 |
| Meropenem | |||||
| Ceftazidime-susceptible | 21 | ≤0.015-0.03 | ≤0.015 | ≤0.015 | 0.03 |
| Ceftazidime-intermediate or resistant | 20 | ≤0.015-0.12 | 0.06 | 0.06 | 0.06 |
| Piperacillin-tazobactam | |||||
| Ceftazidime-susceptible | 21 | ≤1-8 | ≤1 | ≤1 | 4 |
| Ceftazidime-intermediate or resistant | 20 | 4->128 | 128 | 64 | >128/PICK> |
| Enterobacter aerogenes | |||||
| Aztreonam | |||||
| Ceftazidime-susceptible | 23 | ≤1-8 | ≤1 | ≤1 | 2 |
| Ceftazidime-intermediate or resistant | 20 | 8->32 | 16 | 16 | >32 |
| Cefepime | |||||
| Ceftazidime-susceptible | 23 | ≤0.5-1 | ≤0.5 | ≤0.5 | ≤0.5 |
| Ceftazidime-intermediate or resistant | 20 | ≤0.5->32 | ≤0.5 | ≤0.5 | 4 |
| Ceftriaxone | |||||
| Ceftazidime-susceptible | 23 | ≤0.5-4 | ≤0.5 | ≤0.5 | 1 |
| Ceftazidime-intermediate or resistant | 20 | 4->64 | 32 | 32 | >64 |
| Doripenem | |||||
| Ceftazidime-susceptible | 23 | 0.03-0.12 | 0.06 | 0.06 | 0.12 |
| Ceftazidime-intermediate or resistant | 20 | 0.06-0.12 | 0.12 | 0.12 | 0.12 |
| Ertapenem | |||||
| Ceftazidime-susceptible | 23 | ≤0.015-0.25 | ≤0.015 | ≤0.015 | 0.06 |
| Ceftazidime-intermediate or resistant | 20 | 0.06-1 | 0.12 | 0.25 | 0.5 |
| Imipenem | |||||
| Ceftazidime-susceptible | 23 | 0.5-2 | 2 | 2 | 2 |
| Ceftazidime-intermediate or resistant | 20 | 0.5-2 | 0.5 | 0.5 | 1 |
| Meropenem | |||||
| Ceftazidime-susceptible | 23 | ≤0.015-0.06 | 0.03 | 0.03 | 0.06 |
| Ceftazidime-intermediate or resistant | 20 | 0.03-0.12 | 0.06 | 0.06 | 0.12 |
| Piperacillin-tazobactam | |||||
| Ceftazidime-susceptible | 23 | ≤1-32 | 2 | 2 | 4 |
| Ceftazidime-intermediate or resistant | 20 | 16->128 | 64 | 64 | >128 |
| Enterobacter cloacae | |||||
| Aztreonam | |||||
| Ceftazidime-susceptible | 23 | ≤1-8 | ≤1 | ≤1 | 2 |
| Ceftazidime-intermediate or resistant | 19 | 16->32 | >32 | >32 | >32 |
| Cefepime | |||||
| Ceftazidime-susceptible | 23 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 |
| Ceftazidime-intermediate or resistant | 19 | ≤0.5-16 | 1 | 2 | 8 |
| Ceftriaxone | |||||
| Ceftazidime-susceptible | 23 | ≤0.5-4 | ≤0.5 | ≤0.5 | 1 |
| Ceftazidime-intermediate or resistant | 19 | 16->64 | >64 | >64 | >64 |
| Doripenem | |||||
| Ceftazidime-susceptible | 23 | 0.03-0.12 | 0.03 | 0.03 | 0.06 |
| Ceftazidime-intermediate or resistant | 19 | 0.06-0.25 | 0.12 | 0.12 | 0.25 |
| Ertapenem | |||||
| Ceftazidime-susceptible | 23 | ≤0.015-0.5 | ≤0.015 | ≤0.015 | 0.06 |
| Ceftazidime-intermediate or resistant | 19 | 0.06-1 | 0.5 | 0.5 | 1 |
| Imipenem | |||||
| Ceftazidime-susceptible | 23 | 0.06-4 | 0.5 | 0.5 | 2 |
| Ceftazidime-intermediate or resistant | 19 | 0.5-2 | 0.5 | 0.5 | 1 |
| Meropenem | |||||
| Ceftazidime-susceptible | 23 | ≤0.015-0.25 | 0.03 | 0.03 | 0.06 |
| Ceftazidime-intermediate or resistant | 19 | 0.06-0.25 | 0.06 | 0.06 | 0.25 |
| Piperacillin-tazobactam | |||||
| Ceftazidime-susceptible | 23 | ≤1-16 | 2 | 2 | 8 |
| Ceftazidime-intermediate or resistant | 19 | 8->128 | 128 | 128 | >128 |
| Escherichia coli | |||||
| Aztreonam | |||||
| ESBL-negative | 22 | ≤1-8 | ≤1 | ≤1 | ≤1 |
| ESBL-positive | 18 | 4->32 | >32 | >32 | >32 |
| Cefepime | |||||
| Ertapenem | |||||
| ESBL-negative | 20 | ≤0.015-0.25 | ≤0.015 | ≤0.015 | ≤0.015 |
| ESBL-positive | 20 | ≤0.015-0.5 | 0.12 | 0.06 | 0.25 |
| Imipenem | |||||
| ESBL-negative | 20 | 0.12-1 | 0.25 | 0.25 | 1 |
| ESBL-positive | 20 | 0.06-1 | 0.5 | 0.5 | 1 |
| Meropenem | |||||
| ESBL-negative | 20 | ≤0.015-0.25 | 0.03 | 0.03 | 0.03 |
| ESBL-positive | 20 | ≤0.015-0.12 | 0.06 | 0.03 | 0.06 |
| Piperacillin-tazobactam | |||||
| ESBL-negative | 20 | ≤1-8 | 2 | 2 | 4 |
| ESBL-positive | 20 | 4->128 | >128 | >128 | >128 |
| Morganella morganii | |||||
| Aztreonam | |||||
| Ceftazidime-susceptible | 20 | ≤1 | ≤1 | ≤1 | ≤1 |
| Ceftazidime-intermediate or resistant | 1 | 2 | |||
| Cefepime | |||||
| Ceftazidime-susceptible | 20 | ≤0.5-1 | ≤0.5 | ≤0.5 | ≤0.5 |
| Ceftazidime-intermediate or resistant | 1 | ≤0.5 | |||
| Ceftriaxone | |||||
| Ceftazidime-susceptible | 20 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 |
| Ceftazidime-intermediate or resistant | 1 | ≤0.5 | |||
| Doripenem | |||||
| Ceftazidime-susceptible | 20 | 0.06-0.5 | 0.25 | 0.25 | 0.5 |
| Ceftazidime-intermediate or resistant | 1 | 0.5 | |||
| Ertapenem | |||||
| Ceftazidime-susceptible | 20 | ≤0.015-0.06 | ≤0.015 | ≤0.015 | 0.03 |
| Ceftazidime-intermediate or resistant | 1 | 0.06 | |||
| Imipenem | |||||
| Ceftazidime-susceptible | 20 | 0.5-4 | 4 | 4 | 4 |
| Ceftazidime-intermediate or resistant | 1 | 4 | |||
| Meropenem | |||||
| Ceftazidime-susceptible | 20 | 0.03-0.25 | 0.12 | 0.06 | 0.12 |
| Ceftazidime-intermediate or resistant | 1 | 0.12 | |||
| Piperacillin-tazobactam | |||||
| Ceftazidime-susceptible | 20 | ≤1-4 | ≤1 | ≤1 | ≤1 |
| Ceftazidime-intermediate or resistant | 1 | 32 | |||
| Proteus mirabilis | |||||
| Aztreonam | |||||
| Ceftazidime-susceptible | 22 | ≤1->32 | ≤1 | ≤1 | ≤1 |
| Ceftazidime-intermediate or resistant | 19 | ≤1->32 | >32 | >32 | >32 |
| Cefepime | |||||
| Ceftazidime-susceptible | 22 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 |
| Ceftazidime-intermediate or resistant | 19 | ≤0.5->32 | >32 | >32 | >32 |
| Ceftriaxone | |||||
| Ceftazidime-susceptible | 22 | ≤0.5 | ≤0.5 | ≤0.5 | ≤0.5 |
| Ceftazidime-intermediate or resistant | 19 | ≤0.5->64 | ≤0.5 | 8 | 64 |
| Doripenem | |||||
| Ceftazidime-susceptible | 22 | 0.12-2 | 0.25 | 0.5 | 1 |
| Ceftazidime-intermediate or resistant | 19 | 0.25-2 | 1 | 1 | 2 |
| Ertapenem | |||||
| Ceftazidime-susceptible | 22 | ≤0.015-0.25 | ≤0.015 | ≤0.015 | 0.06 |
| Ceftazidime-intermediate or resistant | 19 | ≤0.015-0.5 | 0.06 | 0.06 | 0.5 |
| Imipenem | |||||
| Ceftazidime-susceptible | 22 | 1-4 | 4 | 4 | 4 |
| Ceftazidime-intermediate or resistant | 19 | 4-8 | 4 | 4 | 8 |
| Meropenem | |||||
| Ceftazidime-susceptible | 22 | 0.03-0.25 | 0.06 | 0.06 | 0.25 |
| Ceftazidime-intermediate or resistant | 19 | 0.06-0.25 | 0.12 | 0.12 | 0.25 |
| Piperacillin-tazobactam | |||||
| Ceftazidime-susceptible | 22 | ≤1-2 | ≤1 | ≤1 | ≤1 |
| Ceftazidime-intermediate or resistant | 19 | ≤1-4 | ≤1 | ≤1 | 2 |
| Providencia spp. | |||||
| Aztreonam | |||||
| Ceftazidime-susceptible | 20 | ≤1-2 | ≤1 | ≤1 | ≤1 |
| Ceftazidime-intermediate or resistant | 3 | ≤1-16 | |||
| Cefepime | |||||
| Ceftazidime-susceptible | 20 | ≤0.5-2 | ≤0.5 | ≤0.5 | ≤0.5 |
| Ceftazidime-intermediate or resistant | 3 | ≤0.5-2 | |||
| Ceftriaxone | |||||
| Ceftazidime-susceptible | 20 | ≤0.5-2 | ≤0.5 | ≤0.5 | ≤0.5 |
| Ceftazidime-intermediate or resistant | 3 | ≤0.5-8 | |||
| Doripenem | |||||
| Ceftazidime-susceptible | 20 | 0.12-0.5 | 0.25 | 0.25 | 0.5 |
| Ceftazidime-intermediate or resistant | 3 | 0.25 | |||
| Ertapenem | |||||
| Ceftazidime-susceptible | 20 | ≤0.015-0.12 | ≤0.015 | ≤0.015 | 0.06 |
| Ceftazidime-intermediate or resistant | 3 | ≤0.015-0.03 | |||
| Imipenem | |||||
| Ceftazidime-susceptible | 20 | 0.5-4 | 2 | 2 | 2 |
| Ceftazidime-intermediate or resistant | 3 | 1-4 | |||
| Meropenem | |||||
| Ceftazidime-susceptible | 20 | 0.03-0.12 | 0.06 | 0.06 | 0.12 |
| Ceftazidime-intermediate or resistant | 3 | 0.06-0.12 | |||
| Piperacillin-tazobactam | |||||
| Ceftazidime-susceptible | 20 | ≤1-16 | ≤1 | ≤1 | 4 |
| Ceftazidime-intermediate or resistant | 3 | 2-8 | |||
| Serratia marcescens | |||||
| Aztreonam | |||||
| Ceftazidime-susceptible | 21 | ≤1->32 | ≤1 | ≤1 | ≤1 |
| Ceftazidime-intermediate or resistant | 19 | 2->32 | >32 | >32 | >32 |
| Cefepime | |||||
| Ceftazidime-susceptible | 21 | ≤0.5-2 | ≤0.5 | ≤0.5 | ≤0.5 |
| Ceftazidime-intermediate or resistant | 19 | 1->32 | 32 | 8 | >32 |
| Ceftriaxone | |||||
| Ceftazidime-susceptible | 21 | ≤0.5-64 | ≤0.5 | ≤0.5 | 2 |
| Ceftazidime-intermediate or resistant | 19 | 4->64 | >64 | >64 | >64 |
| Doripenem | |||||
| Ceftazidime-susceptible | 21 | 0.06-0.5 | 0.12 | 0.12 | 0.25 |
| Ceftazidime-intermediate or resistant | 19 | 0.12-0.25 | 0.12 | 0.12 | 0.25 |
| Ertapenem | |||||
| Ceftazidime-susceptible | 21 | ≤0.015-0.25 | ≤0.015 | 0.03 | 0.12 |
| Ceftazidime-intermediate or resistant | 19 | ≤0.015-0.5 | 0.12 | 0.12 | 0.5 |
| Imipenem | |||||
| Ceftazidime-susceptible | 21 | 0.25-4 | 1 | 1 | 2 |
| Ceftazidime-intermediate or resistant | 19 | 0.5-4 | 1 | 1 | 2 |
| Meropenem | |||||
| Ceftazidime-susceptible | 21 | 0.03-0.12 | 0.06 | 0.06 | 0.06 |
| Ceftazidime-intermediate or resistant | 19 | 0.03-0.12 | 0.06 | 0.06 | 0.12 |
| Piperacillin-tazobactam | |||||
| Ceftazidime-susceptible | 21 | ≤1-16 | 2 | 2 | 4 |
| Ceftazidime-intermediate or resistant | 19 | 4->128 | >128 | 16 | >128 |
| Enterococcus faecalis | |||||
| Cefepime | |||||
| Vancomycin-susceptible | 11 | 32->32 | >32 | >32 | >32 |
| Vancomycin-intermediate | 2 | >32 | |||
| Vancomycin-resistant | 7 | 32->32 | |||
| Ceftriaxone | |||||
| Vancomycin-susceptible | 11 | >64 | >64 | >64 | >64 |
| Vancomycin-intermediate | 2 | >64 | |||
| Vancomycin-resistant | 7 | >64 | |||
| Doripenem | |||||
| Vancomycin-susceptible | 11 | 1-8 | 8 | 4 | 8 |
| Vancomycin-intermediate | 2 | 4 | |||
| Vancomycin-resistant | 7 | 2-8 | |||
| Ertapenem | |||||
| Vancomycin-susceptible | 11 | 4-32 | 8 | 8 | 16 |
| Vancomycin-intermediate | 2 | 8-16 | |||
| Vancomycin-resistant | 7 | 4-32 | |||
| Imipenem | |||||
| Vancomycin-susceptible | 11 | 1-2 | 1 | 1 | 2 |
| Vancomycin-intermediate | 2 | 1-2 | |||
| Vancomycin-resistant | 7 | 1-4 | |||
| Meropenem | |||||
| Vancomycin-susceptible | 11 | 2-32 | 8 | 8 | 16 |
| Vancomycin-intermediate | 2 | 8 | |||
| Vancomycin-resistant | 7 | 2-16 | |||
| Piperacillin-tazobactam | |||||
| Vancomycin-susceptible | 11 | 2-4 | 4 | 4 | 4 |
| Vancomycin-intermediate | 2 | 2 | |||
| Vancomycin-resistant | 7 | ≤1-4 | |||
| Enterococcus faecium | |||||
| Cefepime | |||||
| Vancomycin-susceptible | 10 | >32 | >32 | >32 | >32 |
| Vancomycin-resistant | 11 | >32 | >32 | >32 | >32 |
| Ceftriaxone | |||||
| Vancomycin-susceptible | 10 | 16->64 | >64 | >64 | >64 |
| Vancomycin-resistant | 11 | >64 | >64 | >64 | >64 |
| Doripenem | |||||
| Vancomycin-susceptible | 10 | 2->32 | >32 | 32 | >32 |
| Vancomycin-resistant | 11 | >32 | >32 | >32 | >32 |
| Ertapenem | |||||
| Vancomycin-susceptible | 10 | 16->32 | >32 | >32 | >32 |
| Vancomycin-resistant | 11 | >32 | >32 | >32 | >32 |
| Imipenem | |||||
| Vancomycin-susceptible | 10 | 2->32 | >32 | 16 | >32 |
| Vancomycin-resistant | 11 | >32 | >32 | >32 | >32 |
| Meropenem | |||||
| Vancomycin-susceptible | 10 | 4->32 | >32 | >32 | >32 |
| Vancomycin-resistant | 11 | >32 | >32 | >32 | >32 |
| Piperacillin-tazobactam | |||||
| Vancomycin-susceptible | 10 | 4->64 | >64 | >64 | >64 |
| Vancomycin-resistant | 11 | 64->64 | >64 | >64 | >64 |
| Staphylococcus aureus | |||||
| Cefepime | |||||
| Oxacillin-susceptible | 42 | 2-4 | 2 | 2 | 4 |
| Oxacillin-resistant | 23 | 8->32 | >32 | >32 | >32 |
| Ceftriaxone | |||||
| Oxacillin-susceptible | 42 | ≤1-4 | 2 | 2 | 4 |
| Oxacillin-resistant | 23 | 16->64 | >64 | >64 | >64 |
| Doripenem | |||||
| Oxacillin-susceptible | 42 | ≤0.015-0.06 | 0.03 | 0.03 | 0.06 |
| Oxacillin-resistant | 23 | 0.25->32 | 32 | 4 | 32 |
| Ertapenem | |||||
| Oxacillin-susceptible | 42 | 0.06-0.25 | 0.12 | 0.12 | 0.25 |
| Oxacillin-resistant | 23 | 0.5->32 | >32 | 8 | >32 |
| Imipenem | |||||
| Oxacillin-susceptible | 42 | ≤0.015-0.03 | 0.03 | ≤0.015 | 0.03 |
| Oxacillin-resistant | 23 | 0.06->32 | >32 | 1 | >32 |
| Meropenem | |||||
| Oxacillin-susceptible | 42 | 0.03-0.25 | 0.06 | 0.06 | 0.12 |
| Oxacillin-resistant | 23 | 0.5->32 | >32 | 4 | >32 |
| Piperacillin-tazobactam | |||||
| Oxacillin-susceptible | 42 | ≤1-2 | ≤1 | ≤1 | 2 |
| Oxacillin-resistant | 23 | 4->64 | >64 | 32 | >64 |
| Staphylococcus epidermidis | |||||
| Cefepime | |||||
| Oxacillin-susceptible | 39 | ≤1 | ≤1 | ≤1 | ≤1 |
| Oxacillin-resistant | 17 | ≤1-16 | 4 | 4 | 16 |
| Ceftriaxone | |||||
| Oxacillin-susceptible | 39 | ≤1-2 | ≤1 | ≤1 | 2 |
| Oxacillin-resistant | 17 | 2-32 | 4 | 4 | 32 |
| Doripenem | |||||
| Oxacillin-susceptible | 39 | ≤0.015-0.06 | 0.03 | 0.03 | 0.03 |
| Oxacillin-resistant | 17 | ≤0.015-4 | 0.5 | 1 | 4 |
| Ertapenem | |||||
| Oxacillin-susceptible | 39 | 0.12-0.5 | 0.25 | 0.25 | 0.25 |
| Oxacillin-resistant | 17 | 0.25-32 | 2 | 2 | 16 |
| Imipenem | |||||
| Oxacillin-susceptible | 39 | ≤0.015 | ≤0.015 | ≤0.015 | ≤0.015 |
| Oxacillin-resistant | 17 | 0.03-4 | 0.06 | 0.12 | 2 |
| Meropenem | |||||
| Oxacillin-susceptible | 39 | 0.03-0.12 | 0.06 | 0.06 | 0.06 |
| Oxacillin-resistant | 17 | 0.06-16 | 1 | 1 | 8 |
| Piperacillin-tazobactam | |||||
| Oxacillin-susceptible | 39 | ≤1 | ≤1 | ≤1 | ≤1 |
| Oxacillin-resistant | 17 | ≤1-4 | ≤1 | ≤1 | 4 |
| Coagulase-negative staphylococci (non-S. epidermidis) | |||||
| Cefepime | |||||
| Oxacillin-susceptible | 38 | ≤1-4 | ≤1 | ≤1 | 2 |
| Oxacillin-resistant | 24 | ≤1->32 | 2 | 4 | >32 |
| Ceftriaxone | |||||
| Oxacillin-susceptible | 38 | ≤1-16 | ≤1 | 2 | 4 |
| Oxacillin-resistant | 24 | 2->64 | 2 | 8 | >64 |
| Doripenem | |||||
| Oxacillin-susceptible | 38 | ≤0.015-0.5 | 0.03 | 0.03 | 0.12 |
| Oxacillin-resistant | 24 | 0.03-32 | 0.06 | 0.5 | 16 |
| Ertapenem | |||||
| Oxacillin-susceptible | 38 | 0.06-2 | 0.25 | 0.25 | 0.5 |
| Oxacillin-resistant | 24 | 0.12->32 | >32 | 2 | >32 |
| Imipenem | |||||
| Oxacillin-susceptible | 38 | ≤0.015-0.06 | ≤0.015 | ≤0.015 | 0.03 |
| Oxacillin-resistant | 24 | ≤0.015-32 | 0.03 | 0.06 | 8 |
| Meropenem | |||||
| Oxacillin-susceptible | 38 | ≤0.015-1 | 0.06 | 0.06 | 0.25 |
| Oxacillin-resistant | 24 | 0.06->32 | 0.5 | 1 | 32 |
| Piperacillin-tazobactam | |||||
| Oxacillin-susceptible | 38 | ≤1-2 | ≤1 | ≤1 | ≤1 |
| Oxacillin-resistant | 24 | ≤1->64 | ≤1 | 2 | 64 |
| Streptococcus pyogenes | |||||
| Penicillin | |||||
| Macrolide-susceptible | 10 | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06 |
| Cefepime | |||||
| Macrolide-susceptible | 10 | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06 |
| Ceftriaxone | |||||
| Macrolide-susceptible | 10 | ≤0.03 | ≤0.03 | ≤0.03 | ≤0.03 |
| Doripenem | |||||
| Macrolide-susceptible | 10 | ≤0.008 | ≤0.008 | ≤0.008 | ≤0.008 |
| Ertapenem | |||||
| Macrolide-susceptible | 10 | ≤0.008-0.015 | ≤0.008 | ≤0.008 | ≤0.008 |
| Imipenem | |||||
| Macrolide-susceptible | 10 | ≤0.008 | ≤0.008 | ≤0.008 | ≤0.008 |
| Meropenem | |||||
| Macrolide-susceptible | 10 | ≤0.008 | ≤0.008 | ≤0.008 | ≤0.008 |
| Piperacillin-tazobactam | |||||
| Macrolide-susceptible | 10 | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06 |
| Streptococcus agalactiae | |||||
| Penicillin | |||||
| Macrolide-susceptible | 20 | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06 |
| Macrolide-resistant | 20 | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06 |
| Cefepime | |||||
| Macrolide-susceptible | 20 | ≤0.06-0.12 | ≤0.06 | ≤0.06 | ≤0.06 |
| Macrolide-resistant | 20 | ≤0.06-0.12 | ≤0.06 | ≤0.06 | 0.12 |
| Ceftriaxone | |||||
| Macrolide-susceptible | 20 | ≤0.03-0.06 | 0.06 | 0.06 | 0.06 |
| Macrolide-resistant | 20 | ≤0.03-0.12 | 0.06 | 0.06 | 0.06 |
| Doripenem | |||||
| Macrolide-susceptible | 20 | ≤0.008-0.015 | 0.015 | 0.015 | 0.015 |
| Macrolide-resistant | 20 | 0.015-0.03 | 0.015 | 0.015 | 0.015 |
| Ertapenem | |||||
| Macrolide-susceptible | 20 | 0.03-0.06 | 0.03 | 0.03 | 0.06 |
| Macrolide-resistant | 20 | 0.03-0.06 | 0.06 | 0.06 | 0.06 |
| Imipenem | |||||
| Macrolide-susceptible | 20 | ≤0.008-0.015 | 0.015 | 0.015 | 0.015 |
| Macrolide-resistant | 20 | ≤0.008-0.03 | 0.015 | 0.015 | 0.015 |
| Meropenem | |||||
| Macrolide-susceptible | 20 | 0.03 | 0.03 | 0.03 | 0.03 |
| Macrolide-resistant | 20 | 0.03-0.06 | 0.03 | 0.03 | 0.06 |
| Piperacillin-tazobactam | |||||
| Macrolide-susceptible | 20 | 0.25-0.5 | 0.25 | 0.25 | 0.25 |
| Macrolide-resistant | 20 | 0.25-0.5 | 0.25 | 0.25 | 0.5 |
| Streptococcus pneumoniae | |||||
| Cefepime | |||||
| Penicillin-susceptible | 44 | ≤0.06 | ≤0.06 | ≤0.06 | ≤0.06 |
| Penicillin-intermediate | 23 | ≤0.06-1 | 0.5 | 0.25 | 1 |
| Penicillin-resistant | 33 | 0.5-4 | 1 | 1 | 2 |
| Ceftriaxone | |||||
| Penicillin-susceptible | 44 | ≤0.03 | ≤0.03 | ≤0.03 | ≤0.03 |
| Penicillin-intermediate | 23 | ≤0.03-0.5 | 0.5 | 0.25 | 0.5 |
| Penicillin-resistant | 33 | 0.5-8 | 1 | 1 | 4 |
| Doripenem | |||||
| Penicillin-susceptible | 44 | ≤0.008-0.015 | ≤0.008 | ≤0.008 | ≤0.008 |
| Penicillin-intermediate | 23 | 0.015-0.5 | 0.25 | 0.12 | 0.25 |
| Penicillin-resistant | 33 | 0.25-1 | 1 | 0.5 | 1 |
| Ertapenem | |||||
| Penicillin-susceptible | 44 | ≤0.008-0.03 | 0.015 | 0.015 | 0.015 |
| Penicillin-intermediate | 23 | 0.03-1 | 0.03 | 0.25 | 1 |
| Penicillin-resistant | 33 | 0.5-4 | 0.5 | 1 | 2 |
| Imipenem | |||||
| Penicillin-susceptible | 44 | ≤0.008-0.015 | ≤0.008 | ≤0.008 | ≤0.008 |
| Penicillin-intermediate | 23 | ≤0.008-0.25 | 0.12 | 0.06 | 0.12 |
| Penicillin-resistant | 33 | 0.12-1 | 0.5 | 0.5 | 1 |
| Meropenem | |||||
| Penicillin-susceptible | 44 | ≤0.008-0.03 | ≤0.008 | ≤0.008 | ≤0.008 |
| Penicillin-intermediate | 23 | 0.015-0.5 | 0.25 | 0.12 | 0.5 |
| Penicillin-resistant | 33 | 0.25-2 | 0.5 | 0.5 | 1 |
| Piperacillin-tazobactam | |||||
| Penicillin-susceptible | 44 | ≤0.06-0.12 | ≤0.06 | ≤0.06 | ≤0.06 |
| Penicillin-intermediate | 23 | ≤0.06-4 | 2 | 1 | 4 |
| Penicillin-resistant | 33 | 2->8 | 4 | 4 | 8 |
| Viridans group streptococci | |||||
| Cefepime | |||||
| Penicillin-susceptible | 38 | ≤0.06-0.5 | ≤0.06 | 0.12 | 0.5 |
| Penicillin-intermediate | 4 | 0.25-1 | |||
| Penicillin-resistant | 18 | 0.5->4 | 2 | 2 | >4 |
| Ceftriaxone | |||||
| Penicillin-susceptible | 38 | ≤0.03-0.5 | 0.06 | 0.06 | 0.25 |
| Penicillin-intermediate | 4 | 0.25-1 | |||
| Penicillin-resistant | 18 | 1->8 | 4 | 4 | 4 |
| Doripenem | |||||
| Penicillin-susceptible | 38 | ≤0.008-0.06 | 0.03 | 0.03 | 0.06 |
| Penicillin-intermediate | 4 | 0.03-1 | |||
| Penicillin-resistant | 18 | 0.25-4 | 1 | 1 | 4 |
| Ertapenem | |||||
| Penicillin-susceptible | 38 | 0.03-0.5 | 0.06 | 0.06 | 0.25 |
| Penicillin-intermediate | 4 | 0.12-2 | |||
| Penicillin-resistant | 18 | 1->8 | 4 | 4 | 8 |
| Imipenem | |||||
| Penicillin-susceptible | 38 | ≤0.008-0.06 | 0.015 | 0.015 | 0.06 |
| Penicillin-intermediate | 4 | 0.03-1 | |||
| Penicillin-resistant | 18 | 0.12-4 | 2 | 2 | 2 |
| Meropenem | |||||
| Penicillin-susceptible | 38 | ≤0.008-0.12 | 0.03 | 0.03 | 0.06 |
| Penicillin-intermediate | 4 | 0.06-1 | |||
| Penicillin-resistant | 18 | 0.25-4 | 2 | 2 | 4 |
| Piperacillin-tazobactam | |||||
| Penicillin-susceptible | 38 | ≤0.06-2 | 0.12 | 0.12 | 1 |
| Penicillin-intermediate | 4 | 0.25-8 | |||
| Penicillin-resistant | 18 | 0.5->8 | >8 | 8 | >8 |
MIC50, the MIC at which 50% of the isolates are inhibited by an antimicrobial agent. The mode, MIC50, and MIC90 were not calculated for phenotype groups of <10 isolates.
FIG. 1.
MIC distributions of doripenem, ertapenem, imipenem, and meropenem against 211 isolates of ESBL-negative or ceftazidime-susceptible Enterobacteriaceae (A) and 140 isolates of ESBL-positive or ceftazidime-intermediate or ceftazidime-resistant Enterobacteriaceae (B).
As demonstrated for ertapenem, imipenem, and meropenem, doripenem showed limited activity against enterococci (Table 1). Doripenem was more active against both vancomycin-resistant and vancomycin-susceptible isolates of Enterococcus faecalis (MIC90, 8 μg/ml) than against Enterococcus faecium (MIC90, >32 μg/ml).
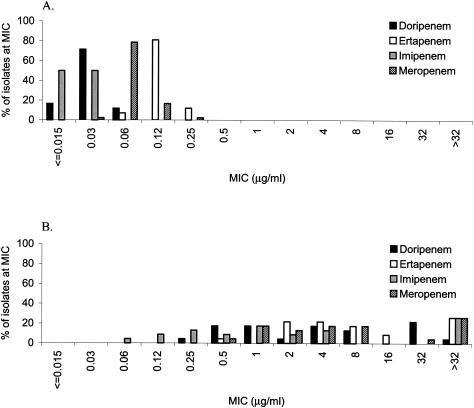
Doripenem was more active against oxacillin-susceptible isolates (MIC90s, 0.03 to 0.12 μg/ml) than against oxacillin-resistant isolates (MIC90s, 4 to 32 μg/ml) of Staphylococcus aureus, Staphylococcus epidermidis, and coagulase-negative staphylococci other than S. epidermidis (Table 1). The MICs of doripenem for staphylococci were similar to those of imipenem and lower than those of ertapenem and meropenem. The MICs of all of the carbapenems including doripenem (Fig. 2) and the other β-lactams tested were higher for oxacillin-resistant staphylococci than for oxacillin-susceptible staphylococci.
FIG. 2.
MIC distributions of doripenem, ertapenem, imipenem, and meropenem against 42 isolates of oxacillin-susceptible S. aureus (A) and 23 isolates of oxacillin-resistant S. aureus (B).
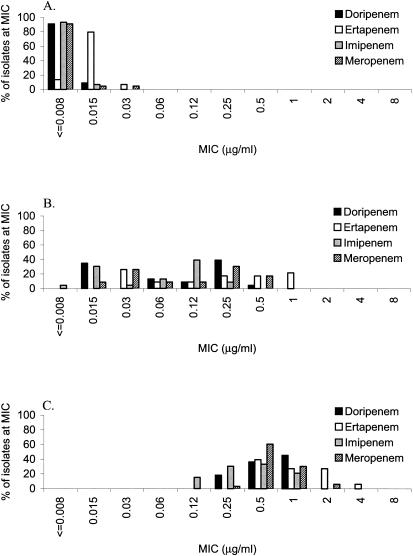
Doripenem, ertapenem, imipenem, and meropenem MIC90s were all ≤0.008 μg/ml for Streptococcus pyogenes (Table 1). For Streptococcus agalactiae, the MIC90s of doripenem (0.015 μg/ml) and imipenem (0.015 μg/ml) were lower than those of ertapenem (0.06 μg/ml) and meropenem (0.03 to 0.06 μg/ml). For penicillin-susceptible Streptococcus pneumoniae, the MIC90s of doripenem, meropenem, imipenem, and ertapenem were ≤0.015 μg/ml; for penicillin-resistant S. pneumoniae, the MIC90s of all four carbapenems were 1 to 2 μg/ml (Table 1). The MIC distributions of doripenem, ertapenem, and imipenem were similar for penicillin-susceptible, penicillin-intermediate, and penicillin-resistant S. pneumoniae (Fig. 3); however, the MICs of ertapenem and meropenem were higher for all three subgroups of S. pneumoniae. For penicillin-susceptible and penicillin-resistant isolates belonging to the viridans group streptococci, the MIC90s of doripenem (penicillin-susceptible, 0.06 μg/ml; penicillin-resistant, 4 μg/ml), imipenem (0.06 μg/ml; 2 μg/ml), and meropenem (0.06 μg/ml; 4 μg/ml) were lower than those of ertapenem (0.25 μg/ml; 8 μg/ml). Expanded-spectrum cephalosporins and piperacillin-tazobactam were not as potent as doripenem, imipenem, and meropenem against both penicillin-susceptible and penicillin-resistant isolates.
FIG. 3.
MIC distributions of doripenem, ertapenem, imipenem, and meropenem against 44 isolates of penicillin-susceptible S. pneumoniae (A), 23 isolates of penicillin-intermediate S. pneumoniae (B), and 33 isolates of penicillin-resistant S. pneumoniae (C).
Overall, doripenem demonstrated in vitro activity similar to that of meropenem against gram-negative isolates and similar to that of imipenem against gram-positive isolates, confirming results reported by other investigators (6, 9, 11) (Table 1). Isolates of Enterobacteriaceae harboring ESBLs and/or inducible or derepressed AmpC β-lactamases are not associated with increased MICs of doripenem, imipenem, and meropenem relative to β-lactamase-free control isolates (S. Mushtaq, Y. Ge, and D. M. Livermore, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-529, 2003; H. Huynh, P. R. Rhomberg, and R. N. Jones, Abstr. 43rd Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-528, 2003) (Table 1). Doripenem, like all known carbapenems, is inactive against isolates of Enterobacteriaceae containing carbapenemases (S. Mushtaq et al., 43rd ICAAC; H. Huynh et al., 43rd ICAAC). Other preliminary studies have reported that doripenem has potent in vitro activity against Haemophilus influenzae, Moraxella catarrhalis, Aeromonas spp., Bacillus spp., Bordetella pertussis, and common gram-positive and gram-negative anaerobic bacteria and less or no activity in vitro against Cornynebacterium spp. and Stenotrophomonas maltophilia (6, 9, 11; R. N. Jones et al., 43rd ICAAC). Doripenem has also been reported to demonstrate activity against P. aeruginosa that is as potent as, or slightly more potent than, that of meropenem and imipenem (6, 9, 11; R. N. Jones et al., 43rd ICAAC).
Very limited animal study and clinical trial data have been published on doripenem (9; A. Saito, T. Inamatsu, and J. Shimada, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-219, 1997; D. A. Thye et al., 43rd ICAAC). In humans, approximately 60 to 75% of doripenem administered intravenously was recovered in the urine within 24 h (11; K. Shiba et al., 37th ICAAC; D. A. Thye et al., 43rd ICAAC) and maximum concentrations in serum of approximately 50 μg/ml were achieved following a 1,000-mg dose of doripenem given as an infusion over 1 h (D. A. Thye et al., 43rd ICAAC). Doripenem has not demonstrated the adverse effects associated with other carbapenems (renal toxicity and neurotoxicity) in two animal models (9) and in humans (D. A. Thye et al., 43rd ICAAC). Doripenem has also been reported to have a weaker neurological side-effect profile than imipenem and meropenem in an animal model (S. Hori, J. Sato, M. Kawamura, and J. Shimada, Abstr. 37th Intersci. Conf. Antimicrob. Agents Chemother., abstr. F-220, 1997).
Based upon the data generated in the present study, as well as on published data in other preliminary studies (6, 9, 11), further investigation of doripenem as a broad-spectrum parenteral agent is warranted.
Acknowledgments
This study was funded by Peninsula Pharmaceuticals, Inc., Alameda, Calif.
REFERENCES
- 1.Iso, Y., T. Irie, Y. Nishino, K. Motokawa, and Y. Nishitani. 1996. A novel 1β-methylcarbapenem antibiotic, S-4661: synthesis and structure-activity relationships of 2-(5 substituted pyrrolidin-3-ylthio)-1β-methylcarbapenems. J. Antibiot. (Tokyo) 49:199-209. [DOI] [PubMed] [Google Scholar]
- 2.Mori, M., M. Hikida, T. Nishihara, T. Nasu, and S. Mitsuhashi. 1996. Comparative stability of carbapenem and penem antibiotics to human recombinant dehydropeptidase-I. J. Antimicrob. Chemother. 37:1034-1036. [DOI] [PubMed] [Google Scholar]
- 3.Murray, P. R., E. J. Baron, J. H. Jorgensen, M. A. Pfaller, and R. H. Yolken (ed.). 2003. Manual of clinical microbiology, 8th ed. ASM Press, Washington, D.C.
- 4.National Committee for Clinical Laboratory Standards. 2003. Methods for dilution antimicrobial susceptibility tests for bacteria that grow aerobically, 6th ed. Approved standard M7-A6. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 5.National Committee for Clinical Laboratory Standards. 2003. Performance standards for antimicrobial susceptibility testing; thirteenth informational supplement, vol. 23. M100-S13. National Committee for Clinical Laboratory Standards, Wayne, Pa.
- 6.Nomura, S., and A. Nagayama. 2002. In vitro antibacterial activity of S-4661, a new parenteral carbapenem, against urological pathogens isolated from patients with complicated urinary tract infections. J. Chemother. 14:155-160. [DOI] [PubMed] [Google Scholar]
- 7.Ohiba, F., M. Nakamura-Kamigo, N. Watanabe, and K. Katsu. 1997. In vitro and in vivo antibacterial activities of ER-35786, a new antipseudomonal carbapenem. Antimicrob. Agents Chemother. 41:298-307. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.Peterson, P. J., N. V. Jacobus, W. J. Weiss, and R. J. Testa. 1991. In vitro and in vivo activities of LJC10,627, a new carbapenem with stability to dehydropeptidase I. Antimicrob. Agents Chemother. 35:203-207. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Tsuji, M., Y. Ishii, A. Ohno, S. Miyazaki, and K. Yamaguchi. 1998. In vitro and in vivo antibacterial activities of S-4661, a new carbapenem. Antimicrob. Agents Chemother. 42:94-99. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Tsuji, M., H. Matsuda, H. Miwa, and S. Miyazaki. 2003. Antimicrobial-induced release of endotoxin from Pseudomonas aeruginosa: comparison of in vitro and animal models. J. Antimicrob. Chemother. 51:353-359. [DOI] [PubMed] [Google Scholar]
- 11.Watanabe, A., H. Takahashi, T. Kikuchi, T. Kobayashi, K. Gomi, S. Fujimura, Y. Tokue, and T. Nukiwa. 2000. Comparative in vitro activity of S-4661, a new parenteral carbapenem, and other antimicrobial agents against respiratory pathogens. Chemotherapy 46:184-187. [DOI] [PubMed] [Google Scholar]
- 12.Yamaguchi, K., H. Domon, S. Miyazaki, K. Tateda, A. Ohno, K. Ihii, T. Matsumoto, and N. Furuya. 1998. In vitro and in vivo antibacterial activities of CS-834, a new oral carbapenem. Antimicrob. Agents Chemother. 42:555-563. [DOI] [PMC free article] [PubMed] [Google Scholar]