At the turn of the century, microRNAs (miRNAs) were almost unheard of. Although discovered in the early 1990s in Caenorhabditis elegans (Lee et al., 1993; Wightman et al., 1993), they were not recognized as a large class of regulatory molecule with highly conserved functions in eukaryotes— and were not reported in plants—for nearly 10 years. The last decade has seen an explosion of research on the identification, function, and mechanism of action of miRNAs and other small regulatory RNAs in plants and other organisms. For example, the Web of Science reports ∼6500 articles published in 2012 alone on the topics of microRNA and small interfering RNA (siRNA), whereas these topics return just 18 articles published in the year 2000. As of June 2013, miRBase version 20 (http://www.mirbase.org/index.shtml) contains over 6000 MIR genes from 72 plant species including 53 dicots, 12 monocots, four conifers, the bryophyte Physcomitrella, the lycopod Selaginella, and the chlorophyte Chlamydomonas. Two new review articles in The Plant Cell offer a solid grounding in the biogenesis, turnover, and mode of action of plant miRNAs (Rogers and Chen, pages 2383–2399) and a fresh perspective on phased, secondary siRNAs in posttranscriptional regulatory networks (Fei et al., pages 2400–2415).
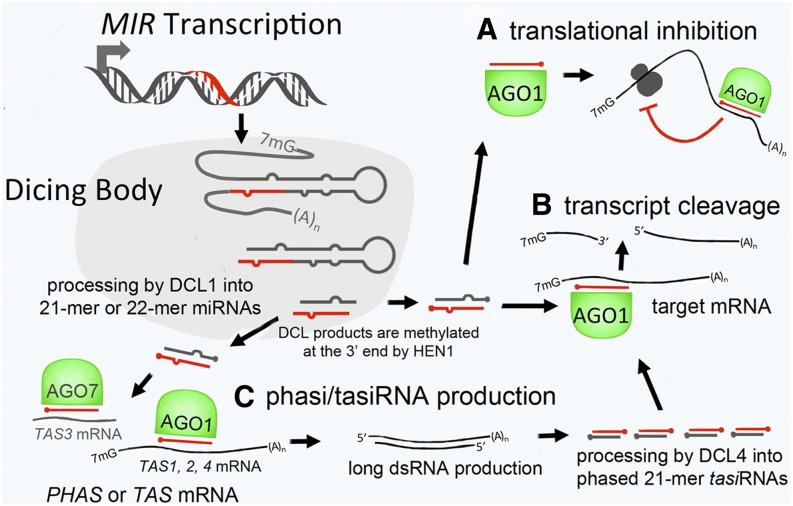
Transcription of MIR genes produces pri-miRNAs that are recruited to dicing bodies and cleaved into miRNAs by the Dicer enzyme (mainly DCL1 in Arabidopsis). Depending on the MIR gene involved (and a host of other factors), the mature miRNAs then go on to participate in translational inhibition (A), transcript cleavage (B), or phasi/tasiRNA production (C). TasiRNAs go on to associate with AGO1 and participate in cleavage of other mRNAs.
Rogers and Chen review the molecular events underlying miRNA biogenesis and degradation and miRNA-mediated gene silencing. They summarize the different mechanisms by which miRNAs affect the repression of target messenger RNAs (mRNAs), including target cleavage, target destabilization, translational inhibition, and DNA methylation. In addition, they discuss the cellular compartments implicated in repression, including cytoplasmic processing bodies (called P-bodies), where translationally repressed mRNAs accumulate; the endoplasmic reticulum, where a portion of the cellular AGO1 resides and miRNA-mediated translation repression occurs; and the cytoskeleton, where microtubule organization is tied to miRNA function. This discussion highlights the importance of considering miRNA function in a cellular context, which is often missing from other reviews on this topic.
Fei et al. review the importance of phased, secondary siRNAs (called phasiRNAs) in posttranscriptional regulatory networks in plants. The designation phasiRNA includes trans-acting tasiRNAs (for which suppression of target transcript levels in trans has been demonstrated) and other small RNAs that require miRNA-mediated cleavage for their biogenesis and have the same characteristic pattern of biogenesis initiated at a miRNA cleavage site. Although the Arabidopsis thaliana genome has relatively few phasiRNA-generating loci, other plant genomes contain hundreds or thousands of them. PhasiRNA targets in dicots include several large or conserved families of genes, one family in particular being the nucleotide binding leucine-rich repeat (NB-LRR) genes encoding disease resistance proteins. Fei et al. review plant phasiRNAs and the possible mechanistic significance of phasiRNA-based regulation of the NB-LRRs. PhasiRNA-mediated regulation of NB-LRRs appears to be lacking in the Poaceae and greatly reduced in the Brassicaceae but is widespread in other plants, such as members of the Solanaceae, Rosaceae, Pinaceae, and the basal group Amborellaceae. The authors outline several hypotheses for the biological significance of this phenomenon, such as possible importance in beneficial plant-microbial interactions, plant defense responses, or more fundamental genomic processes unrelated to the role of NB-LRRs in plant responses to microbes. These two reviews offer a valuable overview and new insights on these important topics in plant gene regulation.
References
- Fei Q., Xia R., Meyers B.C. (2013). Phased, secondary siRNAs in post-transcriptional regulatory networks. Plant Cell 25: 2400–2415 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee R.C., Feinbaum R.L., Ambros V. (1993). The C. elegans heterochronic gene lin-4 encodes small RNAs with antisense complementarity to lin-14. Cell 75: 843–854 [DOI] [PubMed] [Google Scholar]
- Rogers K., Chen X. (2013). Biogenesis, turnover, and mode of action of plant microRNAs. Plant Cell 25: 2383–2399 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wightman B., Ha I., Ruvkun G. (1993). Posttranscriptional regulation of the heterochronic gene lin-14 by lin-4 mediates temporal pattern formation in C. elegans. Cell 75: 855–862 [DOI] [PubMed] [Google Scholar]