Abstract
MicroRNAs (miRNAs) are small RNAs that control gene expression through silencing of target mRNAs. Mature miRNAs are processed from primary miRNA transcripts by the endonuclease activity of the DICER-LIKE1 (DCL1) protein complex. Mechanisms exist that allow the DCL1 complex to precisely excise the miRNA from its precursor. Our understanding of miRNA biogenesis, particularly its intersection with transcription and other aspects of RNA metabolism such as splicing, is still evolving. Mature miRNAs are incorporated into an ARGONAUTE (AGO) effector complex competent for target gene silencing but are also subjected to turnover through a degradation mechanism that is beginning to be understood. The mechanisms of miRNA target silencing in plants are no longer limited to AGO-catalyzed slicing, and the contribution of translational inhibition is increasingly appreciated. Here, we review the mechanisms underlying the biogenesis, turnover, and activities of plant miRNAs.
INTRODUCTION
MicroRNAs (miRNAs) are a class of 20- to 24-nucleotide endogenous small RNAs that repress gene expression. In plants, miRNAs control the expression of genes encoding transcription factors, stress response proteins, and other proteins that impact the development, growth, and physiology of plants. The past decade has witnessed an explosion in our knowledge of the roles of miRNAs in various biological processes in plants. However, the diversity of predicted miRNA/target interactions suggests that our understanding of the biological functions of miRNAs will continue to evolve (Jha and Shankar, 2011; Ding et al., 2012). In parallel to understanding the biological roles of miRNAs, intensive research in the past decade has unveiled some of the mysteries behind the miRNAs themselves, such as how they are made and how they regulate target genes. Early studies focused on identifying proteins responsible for the catalysis of miRNA processing, modification, and target cleavage. miRNAs are excised from stem-loop structures within larger primary miRNA (pri-miRNA) transcripts by DICER-LIKE1 (DCL1), 2′-O-methylated by HUA ENHANCER1 (HEN1), and loaded into the ARGONAUTE (AGO) component of an RNA-induced silencing complex (RISC). miRNA/target complementarity directs RISC to recognize and repress specific transcripts by several mechanisms, including target mRNA slicing through AGO endonuclease activity. The focus of research has now turned to studying the regulation of miRNA biogenesis, understanding the mechanisms of miRNA turnover, and dissecting the molecular basis for the translational repression activity of miRNAs. Here, we review our current understanding of the molecular events underlying miRNA biogenesis and degradation and miRNA-mediated gene silencing.
BIOGENESIS
MIR Transcription
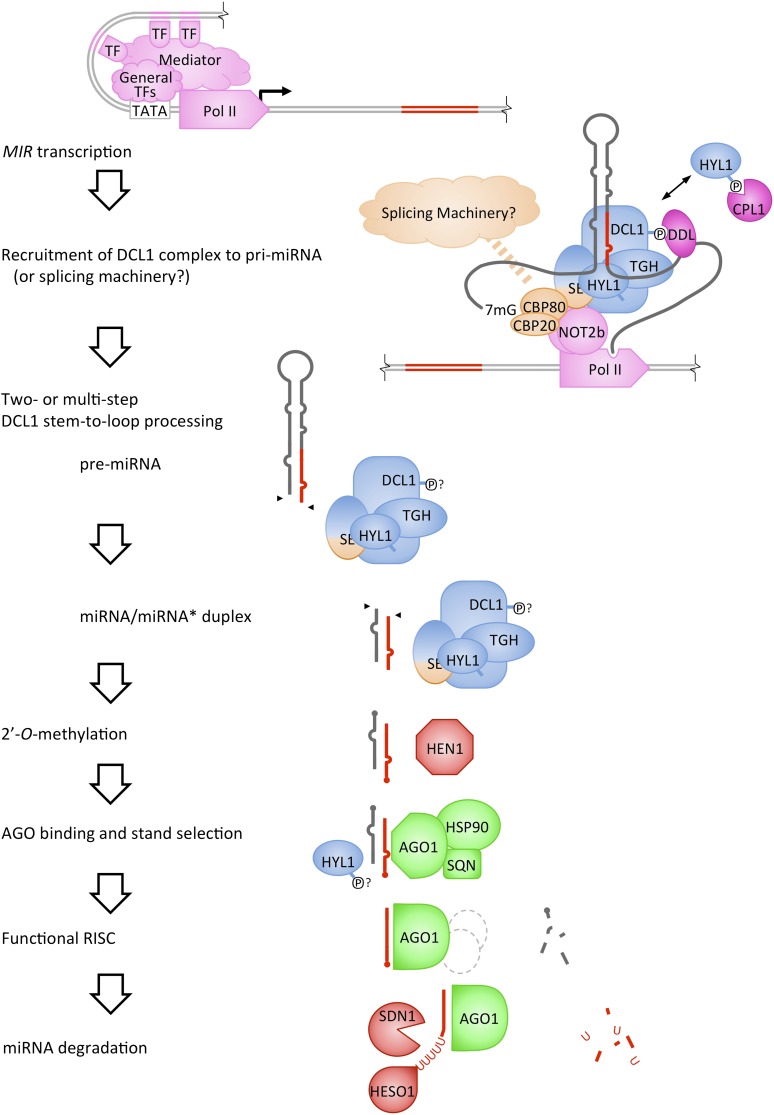
Most plants possess over 100 miRNA genes (MIR) (Nozawa et al., 2012), located mainly in intergenic regions throughout the genome (Reinhart et al., 2002). Most MIR genes possess their own transcriptional unit (Griffiths-Jones et al., 2008); polycistronic pri-miRNA transcripts with multiple miRNA-generating hairpins have been detected but are rare in plants (Merchan et al., 2009; Zhang et al., 2009). MIR genes are transcribed by RNA polymerase II (Pol II) (Xie et al., 2005a; Kim et al., 2011), and pri-miRNAs are stabilized by the addition of a 5′ 7-methylguanosine cap (Xie et al., 2005a) and a 3′ polyadenylate tail (Jones-Rhoades and Bartel, 2004; Zhang et al., 2005).
Transcriptional Regulation
Pol II is recruited to MIR promoters through the general transcriptional coactivator Mediator (Kim et al., 2011; Figure 1). The interaction of transcriptional activators with Mediator triggers Pol II recruitment in eukaryotes (reviewed in Ansari and Morse, 2013). The TATA box core promoter element (Xie et al., 2005a) and at least 21 cis-regulatory motifs (Megraw et al., 2006; Zhao et al., 2013) are overrepresented in MIR promoter sequences, implicating the regulation of MIR transcription by many trans-acting factors. On average, MIR families consist of several genes (Li and Mao, 2007). The diversification of paralogs within a MIR family may allow distinct regulatory mechanisms to impact the expression of the gene family. Mechanisms that regulate MIR transcription, including those that differentially affect the expression of individual MIR family members, are beginning to be characterized. Some MIR172 family members are regulated by POWERDRESS-dependent Pol II recruitment (Yumul et al., 2013). The FUSCA3 transcription factor is recruited to MIR156 loci and is differentially required for the accumulation of some MIR156 family members (Wang and Perry, 2013). APETALA2 is recruited to individual MIR156 and MIR172 loci promoting MIR156 expression and repressing MIR172 expression (Yant et al., 2010). Diverse stresses are known to trigger widespread changes in miRNA levels in part through altered MIR transcription (Liu et al., 2008; Jeong et al., 2011; Hajdarpašić and Ruggenthaler, 2012; Liang et al., 2012). Transcription of MIR398b and c in response to low copper conditions is regulated by SQUAMOSA PROMOTER BINDING PROTEIN-LIKE7 (Yamasaki et al., 2009). SHORT VEGATATIVE PHASE acts as a temperature-sensitive transcriptional repressor of MIR172 (Cho et al., 2012). The MYB2 transcription factor activates MIR399f in response to phosphate starvation (Baek et al., 2013). The spatio-temporal accumulation of specific miRNAs (Válóczi et al., 2006; Jeong et al., 2011; Breakfield et al., 2012) is also mediated in part by transcriptional control. A subset of MIR172 family members is regulated by the transcriptional corepressors LEUNIG and SEUSS in the outer floral whorls (Grigorova et al., 2011).
Figure 1.
Summary of the Major Steps in miRNA Biogenesis and Turnover.
Multiple transcription factors (TFs) control the transcription of MIR genes. The formation of the DCL1 complex and its recruitment to pri-miRNA are mediated by protein–protein interactions and structural features of the pri-miRNA. Some proteins may have dual roles in the recruitment of either the DCL1 complex or the spliceosome to pri-miRNA. As described in the text, protein phosphorylation status may affect the DCL1–DDL interaction and the enhancement of DCL1 processing accuracy by HYL1. Predicted protein phosphorylation (indicated by ℗) may affect distinct steps in pri-miRNA processing, but the role of phosphorylation in other steps (question marks) remains unknown. Functional RISC may include additional unknown AGO interacting proteins (dashed outline). Proteins are color coded according to known functions in MIR transcription (pink), splicing (orange), DCL processing (light blue), phospho-regulation (purple), RISC assembly (green), and miRNA stabilization and turnover (red).
Pri-miRNA Processing by DCL Endonucleases
In plants, pri-miRNA stem-loops are processed into short double-stranded RNAs (dsRNAs) consisting of mature miRNA guide and passenger (miRNA*) strands with 2-nucleotide 3′ overhangs by a family of four DCL RNase III endonucleases (Margis et al., 2006; Figure 1). Structural features of the pri-miRNA stem-loop direct an initial DCL cleavage event near the base of the stem (Bologna et al., 2009; Cuperus et al., 2010b; Mateos et al., 2010; Song et al., 2010; Werner et al., 2010). After excision of the precursor miRNA (pre-miRNA) stem-loop, subsequent cleavage events may occur processively (Liu et al., 2012a), at ∼21-nucleotide intervals along the stem. The precise recognition of the miRNA from the pri-miRNA depends on precursor structure-encoded processing signals, such as a 14- to 15-bp paired stem proximal to the miRNA/miRNA* duplex, a certain length of paired stem distal to the miRNA/miRNA* duplex, and a terminal loop (Mateos et al., 2010; Werner et al., 2010). Additional modes of processing exist, including loop-to-stem processing of pri-miRNAs with highly self-complementary stem structures (Bologna et al., 2009). In animals, the ends of some intronic pre-miRNA hairpins are generated by intron splicing and debranching (mirtrons), bypassing the need for one RNase III cleavage event; similar mechanisms may be possible in plants (Zhu et al., 2008; Meng and Shao, 2012).
Plant miRNAs are predominately 21 nucleotides in length (Chen et al., 2010; Cuperus et al., 2010a). DCL family members generate small RNAs with distinct sizes: 21 nucleotides for DCL1 and DCL4, 22 nucleotides for DCL2, and 24 nucleotides for DCL3 (Xie et al., 2004, 2005b; Akbergenov et al., 2006; Deleris et al., 2006; Cuperus et al., 2010a). DCL1 is responsible for miRNA biogenesis from most MIR genes (Park et al., 2002; Reinhart et al., 2002). The intramolecular spacing of the RNase III active site and the 3′ overhang binding pocket of the PAZ domain is thought to act as a molecular ruler determining the length of the dsRNA product helix generated by Dicer family enzymes (Macrae et al., 2006). DCL1 generates alternative product sizes from specific pre-miRNAs, possibly through accommodation of bulged substrate helices (Kurihara and Watanabe, 2004; Chen et al., 2010; Cuperus et al., 2010a; Manavella et al., 2012b). The remaining DCLs may produce a limited number of species or only a small fraction of total miRNAs (Rajagopalan et al., 2006; Vazquez et al., 2008; Ben Amor et al., 2009). However, multiple DCL proteins may act sequentially to process a single pri-miRNA, adding an additional layer of complexity to miRNA processing (Wu et al., 2010). Differences in miRNA size may make them functionally distinct, for example, 22-nucleotide miRNAs have the ability to trigger the production of secondary small interfering RNAs (siRNAs) from target mRNAs (Chen et al., 2010; Cuperus et al., 2010a; Manavella et al., 2012b). In the absence of individual DCL enzymes, alternative miRNA size classes are generated. The functional hierarchy among DCL enzymes coupled with differences in DCL gene expression in different tissues suggests that competition between DCLs may play a role in the spatio-temporal control of miRNA biogenesis and activity (Vazquez et al., 2008).
REGULATION OF PROCESSING
RNA Binding Proteins
The G-patch domain protein TOUGH (TGH) (Ren et al., 2012b), the zinc finger protein SERRATE (SE) (Grigg et al., 2005; Lobbes et al., 2006; Yang et al., 2006a), and the dsRNA binding domain (DRB) protein HYPONASTIC LEAVES1 (HYL1/DRB1) (Han et al., 2004; Vazquez et al., 2004; Kurihara et al., 2006) are required for pri-miRNA processing and miRNA accumulation (Figure 1). All three proteins bind RNA: TGH binds single-stranded RNA (Ren et al., 2012b), SE binds pri-miRNA (Machida et al., 2011), possibly at single-stranded RNA/dsRNA junctions, and HYL1 binds dsRNA (Hiraguri et al., 2005; Rasia et al., 2010; Yang et al., 2010). This suggests a model in which multiple RNA binding proteins may associate with distinct regions of the pri-miRNA during processing to recruit DCL1 or maintain the structural determinants directing DCL1 activity. A network of physical interactions connects DCL1, HYL1, SE, and TGH (Kurihara et al., 2006; Lobbes et al., 2006; Yang et al., 2006a; Qin et al., 2010; Machida et al., 2011; Ren et al., 2012b), but it is not known if these interactions represent a stable plant microprocessor complex. HYL1 and SE promote the accuracy of pri-miRNA processing by DCL1 (Kurihara et al., 2006; Dong et al., 2008; Manavella et al., 2012a). While TGH is not required for DCL1 processing accuracy, it enhances DCL1 activity in pri-miRNA processing (Ren et al., 2012b).
There are four additional HYL1 paralogs in Arabidopsis thaliana (Hiraguri et al., 2005). In addition to roles in other small RNA pathways, these DRB proteins have minor roles in miRNA processing (i.e., they are required for the accumulation of only a few miRNAs) (Eamens et al., 2012). The roles of HYL1 (Szarzynska et al., 2009; Laubinger et al., 2010) and TGH (Ren et al., 2012b) in miRNA accumulation vary even among MIR family members, suggesting that these proteins can modulate the accumulation of specific miRNAs despite their general roles in miRNA biogenesis. The restricted expression of RNA binding proteins, such as SE and several DRBs, may spatially confine their functions during development (Prigge and Wagner, 2001; Eamens et al., 2011), allowing them to act as general but tissue-specific regulators of miRNA processing.
Phospho-Regulation
C-TERMINAL DOMAIN PHOSPHATASE-LIKE1 (CPL1) is required for accurate miRNA processing but not for DCL1 activity (Manavella et al., 2012a). HYL1 is a phospho-protein, and CPL1 is required to maintain the hypophosphorylated state of HYL1 (Manavella et al., 2012a; Figure 1). The phosphorylation status of HYL1 is also affected by a mutation in SE (Manavella et al., 2012a). CPL1 interacts with SE (Manavella et al., 2012a), suggesting that CPL1 is recruited to the DCL1 complex by SE, where it regulates HYL1 function through dephosphorylation. Although CPL1 possesses DRB domains, it is not known if these direct CPL1 to pri-miRNAs. There are two CPL paralogs in Arabidopsis, and the stronger phenotype of the cpl1 cpl2 double mutant relative to each single mutant suggests functional redundancy or phospho-regulation of additional processing factors.
The phosphothreonine binding forkhead-associated domain protein DAWDLE (DDL) is required for miRNA biogenesis (Yu et al., 2008). DDL binds RNA and associates with DCL1 (Yu et al., 2008). DCL1 is phosphorylated in vivo (Engelsberger and Schulze, 2012), and an intact phosphothreonine binding cleft in DDL is required to mediate a direct interaction with DCL1 fragments expected to be phosphorylated in vivo (Yu et al., 2008; Machida and Yuan, 2013). While phosphothreonine peptides have been identified from DCL2 and 4 (Sugiyama et al., 2008; Engelsberger and Schulze, 2012), it is not known if DDL interacts with other DCL enzymes.
Dicing Bodies
Protein complexes containing DCL1, HYL1, SE, TGH, and CPL1 have been detected in subnuclear foci by bimolecular fluorescence complementation (Fang and Spector, 2007; Fujioka et al., 2007; Song et al., 2007; Ren et al., 2012b; Manavella et al., 2012a). Additionally, the Pro-rich protein SICKLE (SIC) is required for the accumulation of mature miRNAs (Zhan et al., 2012) and colocalizes with HYL1 foci (Zhan et al., 2012). These subnuclear foci are proposed to be sites of miRNA metabolism (M.-H. Han et al., 2004). The presence of DCL1 and pri-miRNAs suggested that these sites have a role in pri-miRNA processing and led to their designation as dicing bodies (Fang and Spector, 2007; Fujioka et al., 2007).
Dicing bodies are present in several miRNA processing mutants (Fang and Spector, 2007) but may vary in number and composition. MODIFIER OF SNC1, 2 (MOS2) is required for efficient pri-miRNA processing and for the association of pri-miRNAs with HYL1 (Wu et al., 2013). While MOS2 is not necessary for the maintenance of interactions among DCL1, HYL1, and SE, the number of HYL1 foci is decreased in mos2 plants. This suggests that processing is regulated by the recruitment of assembled DCL1 processing complex to qualified pri-miRNA substrates. A hyperphosphorylation mimic form of HYL1 also does not accumulate in nuclear foci (Manavella et al., 2012a), suggesting a possible relationship between dephosphorylation of HYL1 and HYL1 substrate recruitment. TGH, which also influences the ability of HYL1 to associate with RNA (Ren et al., 2012b), may similarly affect the formation of HYL1-containing bodies.
OVERLAP BETWEEN miRNA AND mRNA BIOGENESIS
Transcription
Interactions between the transcriptional machinery and pri-miRNA processing proteins have been detected in plants. Arabidopsis possesses two homologs (NOT2a and NOT2b) of the animal CCR4-NOT complex component NOT (Wang et al., 2013). NOT2b interacts with the Pol II C-terminal domain and is required for efficient transcription of both MIR and protein coding genes (Wang et al., 2013). NOT2b also interacts with several pri-miRNA processing factors, including DCL1 and SE, and could act as a scaffold for the assembly of larger transcription/splicing/processing complexes (Wang et al., 2013). The number of subnuclear DCL1 foci is increased in not2 plants, while that of HYL1 foci is unaffected (Wang et al., 2013), suggesting that DCL1 is mistargeted in not2 plants. Additionally, TGH interacts with the general transcription factor TATA box binding protein 2 (Calderon-Villalobos et al., 2005). In animals, the DCL1 homolog Drosha similarly associates with chromatin at MIR loci (Morlando et al., 2008). An association between the transcriptional machinery and miRNA processing components suggests that pri-miRNAs may be processed cotranscriptionally.
Splicing
Pri-miRNAs contain introns, and widespread alternative splicing of pri-miRNA transcripts has been detected (Mica et al., 2009; Szarzynska et al., 2009; Kruszka et al., 2013). In Arabidopsis, alternative splicing of pri-miR162a (Hirsch et al., 2006) or the pri-miR842-miR846 dicistron (Jia and Rock, 2013) disrupts miRNA-containing stem-loops and reduces the accumulation of processed miRNAs. In rice (Oryza sativa), intron removal from pri-miRNA stem-loop structures is necessary for natural antisense miRNA generation (Liu et al., 2012b). Pri-miRNA processing is dependent on stem-loop structural features, and variation of the pri-miRNA transcript sequence through alternative splicing in combination with alternative transcription initiation and polyadenylation sites (Xie et al., 2005a; Szarzynska et al., 2009) may directly affect structures required for processing.
In addition to this basic relationship, several lines of evidence point to a more intimate association between the splicing machinery and pri-miRNA processing. First, spliceosomal markers SmD3, SmB (Fujioka et al., 2007), the Ser/Arg splicing factor SR33 (Fang and Spector, 2007), and the alternative splicing factor SRp34 colocalize with DCL1 body components in subnuclear foci (Calderon-Villalobos et al., 2005). Second, splicing may directly affect the processing of intronic miRNAs. In Arabidopsis, 17 intronic miRNAs have been identified (Brown et al., 2008). Heat stress–induced alternative splicing of the MIR400 host gene leads to the retention of the pri-miR400 hairpin sequence within the host mRNA and a coincidental reduction in mature miR400 levels (Yan et al., 2012). This may be due to the retained miR400 intron adopting a conformation inhibitory to processing. In animals, the DCL1 homolog Drosha may associate with the spliceosome during the cropping of intronic miRNAs (Kataoka et al., 2009), and in some cases splicing and pre-miRNA cropping may be mutually cooperative processes (Janas et al., 2011). Third, genes (described below) with functional roles in both pre-mRNA splicing and pri-miRNA processing have been identified in plants. While the animal pri-miRNA microprocessor may be minimally defined as Drosha and DGCR8 (Denli et al., 2004; J. Han et al., 2004), microprocessor interactions include the spliceosome-associated components p68 and p72 (Gregory et al., 2004), FUS (Shiohama et al., 2007), and small nuclear RNAs (Janas et al., 2011). Additionally, p68 (Fukuda et al., 2007) and alternative splicing factors (Guil and Cáceres, 2007) play roles in miRNA biogenesis as well as splicing.
Cap Binding Complex
The cap structure of Pol II transcripts is bound by a complex of CAP BINDING PROTEINs (CBP20 and CBP80). The cap binding complex (CBC) is required for the correct splicing of the first intron both in plants (Raczynska et al., 2010) and in animals (Lewis et al., 1996). In Arabidopsis, cbp20 and cbp80/abh1 mutants are partially compromised in pri-miRNA processing (Gregory et al., 2008; S. Kim et al., 2008; Laubinger et al., 2008). CBP20 interacts with SE (Wang et al., 2013), and these proteins may have similar functions in miRNA biogenesis and splicing. cbp20, abh1/cbp80, and se plants exhibit overlapping populations of inefficiently processed miRNAs and pre-mRNAs with splicing defects (Laubinger et al., 2008). cbc20, cbc80, and se mutants accumulate elevated levels of pri-miRNAs from MIR loci with or without introns (Laubinger et al., 2008), demonstrating their roles in miRNA biogenesis independent of splicing catalysis. It is not known if the CBC affects the accuracy of pri-miRNA processing as does SE; however, recruitment of SE to either pre-mRNA or pri-miRNA by the CBC could be a common step prior to splicing or processing.
STABILIZED1
STABILIZED1 (STA1) regulates pre-mRNA splicing (Lee et al., 2006; Ben Chaabane et al., 2013). Altered splicing of the first intron is a common feature of sta1 (Lee et al., 2006), se, cbc20, and cbc80 mutants (Laubinger et al., 2008). However, it has not been established whether STA1 interacts with the CBC. sta1 plants also exhibit reduced levels of most detected miRNAs (Ben Chaabane et al., 2013). This may be due to the combined effects of defective pri-miRNA splicing and reduced DCL1 expression (Ben Chaabane et al., 2013).
pri-miRNA–Specific Splicing?
Loss of function in genes required for pri-miRNA processing also leads to the accumulation of unspliced pri-miRNAs. While general pre-mRNA splicing defects have not been detected in hyl1 or dcl1 plants, both spliced and unspliced forms of some pri-miRNAs accumulate in these mutants (Song et al., 2007; Laubinger et al., 2008; Szarzynska et al., 2009; Ben Chaabane et al., 2013). While it is unknown if SIC, a Pro-rich protein, has a general role in pre-mRNA splicing, unspliced pri-miRNAs accumulate in sic plants and the accumulation of mature miRNAs is reduced (Zhan et al., 2012). The nature of the relationship between pri-miRNA splicing and processing is not yet clear. As has been proposed in animals (Guil and Cáceres, 2007), regulation of miRNA biogenesis by splicing factors may be through the modulation of pri-miRNA structures. If not required for specific processing events, splicing may recruit a subset of RNA binding proteins with distinct functions in subsequent pri-miRNA processing.
NUCLEAR EXPORT, RISC ASSEMBLY, AND miRNA TURNOVER
Nuclear Export
In animals, Exportin 5 binds pre-miRNAs and transports them to the cytoplasm prior to their being processed into mature miRNAs (Zeng and Cullen, 2004). In Arabidopsis, the Exportin 5 homolog HASTY (HST) is required for the accumulation of some miRNAs. HST could be responsible for the nuclear export of miRNAs in plants, although the nucleo-cytoplasmic partitioning of miRNAs is unchanged in hst mutants (Park et al., 2005). Alternatively, HST might affect miRNA stability, another role of Exportin 5 (Zeng and Cullen, 2004). The dependence on HST for miRNA accumulation varies among miRNAs and between tissue types (Park et al., 2005). The incomplete requirement for HST in terms of miRNA accumulation is consistent with the existence of as yet unidentified miRNA export mechanisms.
AGO Family
A PAZ and PIWI domain–containing AGO protein serves as a central component of the RISC complex. The mature miRNA guide strand is separated from the passenger strand and loaded into an AGO protein to form the RISC. The miRNA 5′-nucleotide is the primary feature directing the sorting of miRNAs to the different AGO proteins (Mi et al., 2008; Takeda et al., 2008). The majority of miRNAs possess a 5′ uridine and are sorted into AGO1; however, some miRNAs are bound by other AGOs (Mi et al., 2008; Montgomery et al., 2008; Takeda et al., 2008; Ji et al., 2011; Maunoury and Vaucheret, 2011; Zhu et al., 2011).
In Arabidopsis, a family of 10 AGO proteins directs small RNA–mediated gene silencing (Mourrain et al., 2000). Arabidopsis AGO paralogs are differentially expressed in various tissues Mallory and Vaucheret, 2010; Thieme et al., 2012). Additionally, AGO1 protein levels are reduced by the F-BOX WITH WD-40 2 protein in a proteasome-independent fashion (Earley et al., 2010) and by the autophagy pathway (Derrien et al., 2012). Selection based on the 5′ nucleotide cannot direct exclusive sorting among the 10 AGOs. Tissue-specific accumulation of AGOs is likely to affect the competition between AGO proteins during RISC assembly.
RISC Assembly
Guide strand selection is directed in part by lower thermodynamic stability of the guide strand 5′-end relative to that of the miRNA* (Eamens et al., 2009). Correct strand selection is also enhanced by HYL1 and the HYL1 regulator CPL1 (Eamens et al., 2009; Manavella et al., 2012a). Therefore, strand selection may be partially determined by the association of pri-miRNAs with RNA binding proteins early in processing. AGO1 forms a complex with HEAT SHOCK PROTEIN90 (HSP90) during its association with the miRNA/miRNA* duplex (Iki et al., 2010). Removal of the miRNA passenger strand does not require AGO1 endonuclease activity (Iki et al., 2010; Carbonell et al., 2012). Instead, disassociation of HSP90, and other AGO1-associated proteins such as SQUINT (SQN), is associated with passenger strand removal (Iki et al., 2012). The chaperone activity of HSP90 may trigger AGO1 conformational changes upon its binding to, or disassociation from, AGO1 (Figure 1). This is consistent with models of passenger strand removal due to AGO conformational changes in animals (Gu et al., 2012; Kwak and Tomari, 2012). AGO1 (Fang and Spector, 2007; Pomeranz et al., 2010; Li et al., 2013) and miRNAs (Park et al., 2005) have been detected in both the nucleus and the cytoplasm, leaving open the possibility of nuclear RISC assembly. However, the requirement for the cytosolic protein SQN (Prunet et al., 2008) and the predicted cytoplasmic protein HSP90 (Krishna and Gloor, 2001) in RISC assembly suggests that AGO1 loading occurs in the cytoplasm (Iki et al., 2010).
miRNA Stabilization by 2′-O-Methylation
The 3′ nucleotides of plant miRNA/miRNA* duplexes are 2′-O-methylated by the methyltransferase HEN1 in diverse plant species (Yu et al., 2005; Molnár et al., 2007; Abe et al., 2010; Figure 1). HEN1’s preference for a dsRNA substrate with features of DCL products (Yu et al., 2005; Yang et al., 2006b) indicates that HEN1 methylates miRNAs before the disassociation of the miRNA and miRNA* strands. HEN1 is present in both the nucleus and cytoplasm (Fang and Spector, 2007), and it is not clear where methylation occurs. HEN1 is necessary for miRNA accumulation (Park et al., 2002), and in the absence of methylation, the remaining miRNAs vary in size due to combined 3′-end truncation and oligouridylation (Li et al., 2005).
miRNA Degradation
A family of SMALL RNA DEGRADING NUCLEASE (SDN) genes limits the accumulation of miRNAs in Arabidopsis (Ramachandran and Chen, 2008; Figure 1). SDN1 possesses 3′-5′ exoribonuclease activity against short, single-stranded RNA substrates (Ramachandran and Chen, 2008; Figure 1). Interestingly, SDN1 is capable of degrading 2′-O-methylated substrates but is inhibited by 3′ oligouridylation (Ramachandran and Chen, 2008). The noncannonical poly(A) polymerase HEN1 SUPPRESSOR1 (HESO1) is a nucleotidyltransferase that adds 3′ oligouridylate tails to unmethylated miRNAs (Ren et al., 2012a; Zhao et al., 2012b; Figure 1). Loss of function in HESO1 partially suppresses both the oligouridylation and the destabilization of miRNAs in hen1 (Ren et al., 2012a; Zhao et al., 2012b), demonstrating a causal relationship between oligouridylation and degradation. The 3′ oligouridylation of miRNAs may be redundantly conducted by the other nine nucleotidyltransferase proteins in Arabidopsis (Zhao et al., 2012a) because oligouridylation is not completely lost in hen1 heso1 (Zhao et al., 2012b). Since SDN1 is inhibited by 3′ oligouridylation (Ramachandran and Chen, 2008), it is possible that other nucleases contribute to the decay of 3′ oligouridylated miRNAs. Although HESO1 activity is completely inhibited by 2′-O-methylation of its substrate miRNA, that of SDN1 is not (Ramachandran and Chen, 2008; Ren et al., 2012a; Zhao et al., 2012b). It can be envisioned that SDN1 and HESO1 cooperate in the degradation of methylated miRNAs, with HESO1 acting on a 3′-truncated miRNA generated by the initial removal of the 2′-O-methylated nucleotide by SDN1. The fact that truncated and uridylated miRNAs are associated with AGO1 in vivo (Zhao et al., 2012a) suggests that SDN1 and HESO1 act on AGO1-bound miRNAs.
REPRESSION OF miRNA TARGETS
Target Cleavage
The PIWI domain of AGO proteins forms an RNaseH-like fold with a slicer endonuclease activity capable of cleaving RNA targets that are complementary to the loaded guide strand (Liu et al., 2004). Slicer activity has been demonstrated for Arabidopsis AGO1, AGO2, AGO4, AGO7, and AGO10 (Mi et al., 2008; Montgomery et al., 2008; Takeda et al., 2008; Ji et al., 2011; Maunoury and Vaucheret, 2011; Zhu et al., 2011). Since the early identification of a small number of sliced targets in plants (Llave et al., 2002), sequencing of the mRNA degradome has indicated that a large number of miRNA targets undergo slicing (Addo-Quaye et al., 2008; German et al., 2008; Gregory et al., 2008; Figure 2). Plant miRNAs are highly complementary to targets throughout their length (Fahlgren and Carrington, 2010), and the high degree of complementarity is a requirement for effective target slicing by AGO proteins (Mallory et al., 2004).
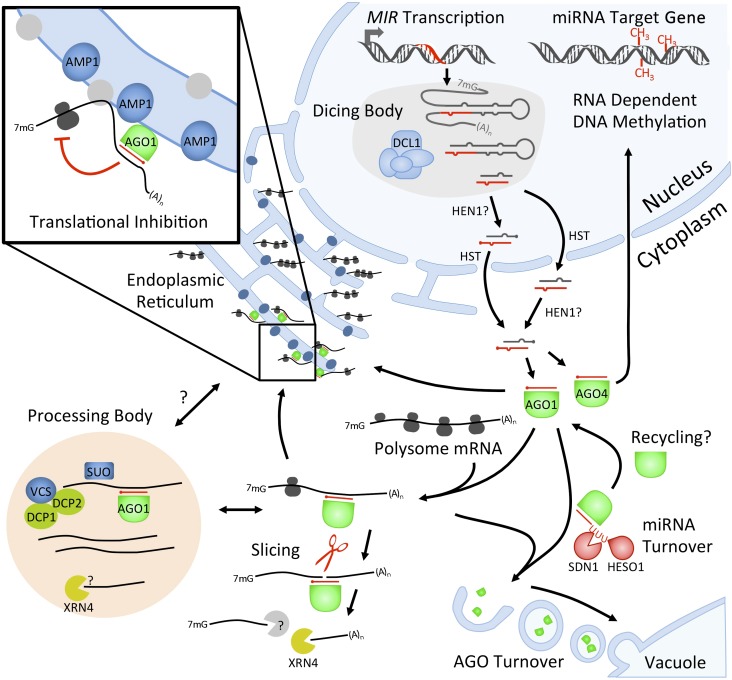
Figure 2.
Overview of miRNA-Mediated Gene Silencing.
Pri-miRNAs are processed in the nucleus and mature miRNAs are exported to the cytoplasm. miRNAs are incorporated into AGO proteins and mediate posttranscriptional gene silencing through slicing or translational inhibition. The cytoplasmic locations of RISC loading or miRNA target slicing are unknown in plants, but a recent study indicated that translational repression occurs on the ER. P-body components required for translational inhibition play additional but uncharacterized roles in this process, and their relationship to the ER is unknown. AGO import into the nucleus directs transcriptional silencing of target genes. Speculative molecular events, or locations of the events, are indicated by a question mark. Proteins are color coded as in Figure1 with additional classification according to known functions in target mRNA degradation (yellow) and non-AGO proteins implicated in translational repression (dark blue).
AGO slicing may bypass the general requirements for 3′ deadenylation or 5′ decapping prior to mRNA degradation by exonucleases. In Arabidopsis, loss of function of the cytoplasmic 5′-3′ exoribonuclease XRN4 (Souret et al., 2004), or the putative XRN regulator FIERY1 (Gy et al., 2007), leads to the accumulation of 3′ cleavage products of miRNA target mRNAs (Figure 2). In Chlamydomonas reinhardtii, the core exosome degrades 5′ cleavage products after their 3′ oligoadenylaton by the terminal nucleotidyltransferase MUT68 (Ibrahim et al., 2006). It is not known if HESO1 and its paralogs similarly modify RISC cleavage products and recruit the exosome in higher plants.
Not all RNA cleavage events lead to immediate decay. During trans-acting small interfering RNA biogenesis, the cleavage of TAS transcripts directed by 22-nucleotide miRNAs precedes the RNA-DEPENDENT RNA POLYMERASE6–dependent generation of dsRNA from the cleavage fragments (Chen et al., 2010; Cuperus et al., 2010a; Manavella et al., 2012b). SUPPRESSOR OF GENE SILENCING3 associates with RISC after recognition of features specific to the 22-nucleotide miRNA/target duplex and stabilizes the 5′ and 3′ cleavage fragments (Yoshikawa et al., 2005, 2013).
Target mRNA Destabilization?
In animals, the complementarity between a miRNA and its target mRNA is generally limited to the 5′ region of the miRNA (Lewis et al., 2003; Brennecke et al., 2005) and slicing does not appear to be common (Karginov et al., 2010; Shin et al., 2010). Instead, miRNA RISC destabilizes its target mRNA in a process that requires both deadenylation and decapping (Behm-Ansmant et al., 2006; Wu et al., 2006). In plants, the possibility of slicing-independent target deadenylation followed by destabilization by RISC has not yet been demonstrated biochemically, and the preponderance of slicing in plants may mask any effects of destabilization in specific cell or tissue types. In addition, potential redundancy within deadenylase families may complicate the genetic analysis to dissect the role of deadenylation in miRNA function (Walley et al., 2010; Wang et al., 2013). Intriguingly, degradome analysis revealed that a minority of miRNA targets do not accumulate slicer cleavage products (German et al., 2008). It remains possible that some miRNA targets may undergo slicing-independent destabilization.
Arguments for either separate mechanisms of translational repression and mRNA destabilization (Wu et al., 2006; Fabian et al., 2009) or sequentially coupled repression and destabilization (Djuranovic et al., 2011) have been put forward and both are plausible. In animals, kinetic analysis has demonstrated that translational repression precedes mRNA deadenylation and destabilization (Fabian et al., 2009; Bazzini et al., 2012; Béthune et al., 2012; Djuranovic et al., 2012) and may be required for mRNA destabilization (Meijer et al., 2013). In animals, miRNA-induced translational repression is reversible (Bhattacharyya et al., 2006; Muddashetty et al., 2011). Reversible repression could be one means of rapidly responding to temporary environmental stresses in plants (Voinnet, 2009). While this hypothesis has not been investigated, it is supported by the observed alteration of miRNA and target mRNA association with ribosomes in response to infection (Reynoso et al., 2012). Rapid reversibility might require limited cleavage or destabilization of target mRNAs.
Translational Inhibition
Several examples of targets regulated at the protein level in the absence of noticeable changes in mRNA level have suggested that plant miRNAs also interfere with target mRNA translation (Aukerman and Sakai, 2003; Chen, 2004; Gandikota et al., 2007; Brodersen et al., 2008; Dugas and Bartel, 2008; Beauclair et al., 2010). The identification of Arabidopsis mutants selectively impaired in miRNA-mediated gene repression at protein but not mRNA levels (Brodersen et al., 2008; Yang et al., 2012; Li et al., 2013), together with the insensitivity of this repression to the inhibition of AGO1 slicing (Lanet et al., 2009), has suggested that translational repression is distinct from slicing and is more widespread in plants than previously thought.
Although the disproportionate effects of plant miRNAs on target gene repression at the protein versus mRNA levels have been attributed to their translational inhibition activity, there has been no experimental evidence showing that plant miRNAs inhibit protein synthesis. In a recent study, ALTERED MERISTEM PROGRAM1 (AMP1) (Helliwell et al., 2001) was found to mediate the disproportionate effects of plant miRNAs in target gene repression at the protein versus mRNA levels (Li et al., 2013). Measurements of protein synthesis through pulse labeling experiments in wild-type and amp1 plants demonstrated that a plant miRNA inhibits protein synthesis of its target gene in an AMP1-dependent manner. Examination of the distribution of miRNA target mRNAs along polysomes demonstrated a role for AMP1 in the exclusion of miRNA target mRNAs from membrane-bound polysomes (Li et al., 2013; Figure 2). Multiple mechanisms of miRNA-mediated translational inhibition, such as inhibition of initiation, ribosome stalling, and ribosome drop-off, have been proposed in animals (reviewed in Fabian et al., 2010). The mechanism of translational repression in plants is still unknown; however, the increased recruitment of miRNA target transcript throughout the polysome fractions in amp1 is consistent with miRNAs acting to inhibit translation initiation (Li et al., 2013).
Translational inhibition by miRNAs is common in animals, where it is generally associated with limited miRNA/target complementarity (Zeng et al., 2003). In plants, translational repression can be directed by miRNAs with almost perfect target complementarity (Aukerman and Sakai, 2003; Chen, 2004; Gandikota et al., 2007; Brodersen et al., 2008). However, the degree of miRNA-target RNA complementarity necessary to support the translational repression activity of plant miRNAs remains unknown. The relationship between the two modes of miRNA action (cleavage versus translational repression) is also unknown. While the population of a given target mRNA has been shown to be simultaneously regulated by both slicing and translational repression (Aukerman and Sakai, 2003; Brodersen et al., 2008; Beauclair et al., 2010), understanding the balance between these two repression mechanisms at the molecular level will require closer investigation.
Both AGO1 and miRNAs associate with polysomes, and the polysome association of miRNAs is AGO1 dependent (Lanet et al., 2009; Reynoso et al., 2012; Figure 2). AGO1 and AGO10, the two AGO proteins examined to date with respect to the translational inhibition activity of plant miRNAs, have been shown to be required for this activity of miRNAs (Brodersen et al., 2008; Beauclair et al., 2010). At least one endogenous target was similarly dysregulated in both ago1 and ago10 mutants (Brodersen et al., 2008). The extent of redundancy or distinction between different AGOs in translational inhibition is unknown.
DNA Methylation
In addition to posttranscriptional gene silencing, miRNAs both in plants (Wu et al., 2010) and in animals (D.H. Kim et al., 2008; Khraiwesh et al., 2010) are capable of transcriptional gene silencing (Figure 2). In rice, DCL3-dependent long miRNAs of 24 nucleotides are sorted to AGO4 and trigger cytosine DNA methylation at both MIR and target loci (Wu et al., 2010). In Arabidopsis, DCL3 similarly generates miRNAs 23 to 25 nucleotides in size (Vazquez et al., 2008). An additional class of DCL3-dependent small RNAs, heterochromatic small interfering RNA, are generated in the nucleus and loaded into AGO4 in the cytoplasm prior to import into the nucleus (Ye et al., 2012), where they act in RNA-directed DNA methylation (reviewed in Law and Jacobsen, 2010). AGO4-loaded miRNAs are likely to trigger cytosine methylation by a similar mechanism. The partial redundancy of AGO6 and AGO4 in RNA-directed DNA methylation (Zheng et al., 2007; Eun et al., 2011) suggests a functional specialization of the AGO4/AGO6/AGO9 clade. Some 24-nucleotide miRNAs, such as the DCL1-dependent miR163, are primarily incorporated into AGO1 (Wu et al., 2010) and may not trigger DNA methylation.
ADDITIONAL SILENCING EFFECTORS
GW-Repeat Proteins
GW-repeat domains are known to interact directly with the PIWI domains of AGO proteins within the RISC (Behm-Ansmant et al., 2006; El-Shami et al., 2007; Till et al., 2007). Homologs of the GW-repeat protein GW182 are involved in promoting the decay or translational repression of miRNA target mRNAs in animals (Behm-Ansmant et al., 2006; Zekri et al., 2009). Several GW-repeat domain proteins have been identified in the Arabidopsis genome; however, the majority of these genes remain uncharacterized and no clear GW182 orthologs have been identified (Karlowski et al., 2010). Structural analysis of AGO proteins suggests that GW-repeat binding may be less stable in plant AGOs, which might limit the biochemical identification of such AGO interactors (Poulsen et al., 2013).
The GW-repeat-containing protein SUO is conserved in plants but not in metazoans. SUO facilitates miRNA-mediated translational repression (Yang et al., 2012) and therefore may act as a plant-specific GW182 family equivalent. GW182 may facilitate translational repression through the direct recruitment of the CCR4-NOT deadenylase complex to mRNA to result in the disassociation of cytosolic poly(A) binding proteins from the mRNA (Zekri et al., 2013). It will be interesting to learn if SUO recapitulates GW182’s interactions with AGO proteins and deadenylase complexes.
Decapping Complex
Removal of the 5′ cap from RNAs is catalyzed by the DECAPPING2 (DCP2) component of a conserved decapping complex containing DCP1 and VARICOSE (VCS) (Xu et al., 2006). DCP1 and VCS may be required for the degradation of some miRNA targets, suggesting a possible role in target destabilization; however, the decapping complex also has a broader role in the destabilization of many nontarget mRNAs (Xu et al., 2006; Goeres et al., 2007; Motomura et al., 2012). A relationship between decapping and slicing has not yet been established; therefore, a role for VCS in slicing-independent target destabilization may be an alternative explanation for target mRNA accumulation in vcs mutants. Alternatively, secondary effects of decapping mutants on miRNA levels may contribute to the observed target upregulation (Motomura et al., 2012). VCS also mediates translational repression as some targets are upregulated only at the protein level in vcs mutants (Brodersen et al., 2008). Decapping of mRNA occurs subsequent to removal of the decapping-inhibitive poly(A) tract (Muhlrad and Parker, 1994; Couttet et al., 1997) and renders mRNA accessible to degradation by 5′-3′ exonucleases (Decker and Parker, 1993; Kastenmayer and Green, 2000; Gy et al., 2007). While decapping is known to block translation, it is also a presumed irreversible step in the mRNA decay pathway and it is unclear how the decapping complex might inhibit translation in the absence of mRNA destabilization.
COMPARTMENTS IMPLICATED IN REPRESSION
Cytoplasmic Processing Body
Processing (P)-bodies are cytoplasmic ribonucleoprotein aggregates where translationally repressed mRNAs, including miRNA targets, accumulate (Teixeira et al., 2005; Liu et al., 2005), prior to either degradation or reinitiation of translation (reviewed in Eulalio et al., 2007a). While P-body formation results from miRNA repression, the loss of components required for visible P-body assembly in animals has no effect on miRNA-mediated silencing (Chu and Rana, 2006; Eulalio et al., 2007b). Known components of plant P-bodies include decapping complex proteins (Xu et al., 2006; Iwasaki et al., 2007; Xu and Chua, 2009), the 5′-3′ exonuclease XRN4 (Weber et al., 2008), AGO1 (Pomeranz et al., 2010), and SUO (Yang et al., 2012; Figure 2). The P-body localization of VCS, AGO1, and SUO proteins required for the translational repression activity of plant miRNAs suggests that plant P-bodies may also accumulate translationally repressed mRNAs in response to miRNA-mediated repression. However, while repressed targets are known to accumulate in animal P-bodies, only a small fraction of cellular Ago is present in P-bodies (Leung and Sharp, 2013), and some miRNA target mRNA foci only partially overlap with P-bodies (Pillai et al., 2005). Translational repression may occur at other sites.
Endoplasmic Reticulum
In Arabidopsis, a portion of the cellular AGO1 pool associates with the endomembrane system as a peripheral membrane protein and cofractionates with the endoplasmic reticulum (ER) (Brodersen et al., 2012; Li et al., 2013; Figure 2). The integral ER membrane protein AMP1 is specifically required for miRNA-mediated translational inhibition but not transcript slicing (Li et al., 2013). AMP1 is necessary for the efficient exclusion of miRNA target mRNAs from membrane-bound polysomes, demonstrating a functional role for the ER in miRNA-mediated translational inhibition. mRNAs encoding both cytosolic and secreted proteins associate with the ER. The mechanisms that govern the recruitment of both AGO1 and miRNA target mRNAs to the ER are unknown but do not require AMP1 (Li et al., 2013). Some AGO1 subcellular foci reside at points along the ER network (Li et al., 2013) and may represent sites where translationally repressed mRNAs accumulate. Localization of specific mRNAs to subdomains of the ER has been observed in plants (Li et al., 1993; Washida et al., 2012), and compartmentalization of miRNA target mRNAs within AMP1 compartments might provide a mechanistic basis for the separation of slicing and translational repression.
A link between miRNA function and the endomembrane system has also been described in animals. In animals, Ago2 exhibits partial ER and Golgi membrane localization (Cikaluk et al., 1999), and miRNA-loaded RISC is enriched on the cytosolic leaflet of rough ER membranes (Stalder et al., 2013). The siRNA passenger strand and siRNA RISC-directed mRNA cleavage products cosediment with the rough ER, suggesting that the ER may be a site of siRNA RISC loading and target slicing and could play similar roles in miRNA function (Stalder et al., 2013). This led to a model in which AGO loading at the ER membrane might increase the efficiency of silencing by allowing RISC to sample translationally active mRNA populations during their transient interactions with the ER (Stalder et al., 2013). The animal multivesicular body is also proposed to be a site of dynamic RISC loading and disassembly. Impaired trafficking to and from this compartment results in perturbation of miRNA function (Gibbings et al., 2009; Lee et al., 2009). It is not yet clear if the ER represents an initial site of RISC assembly to be followed by RISC turnover at the multivesicular body or if molecular events such as RISC loading may vary in their dependence on specific membranes in different cell types or model systems. The diversity of endomembrane compartments currently implicated in various aspects of miRNA function in plants and animals may indicate a more general involvement of endomembrane compartments in both systems.
Isoprenoid Synthesis
3-HYDROXY-3-METHYLGLUTARYL CoA REDUCTASE (HMG1) catalyzes a step in the biosynthesis of isoprenoids, which feed into a variety of pathways, including the production of membrane sterols, several plant hormones, and dolichol that is required for the N-linked glycosylation of proteins. The sterol C-8 isomerase HYDRA1 (HYD1) catalyzes a subsequent step in the synthesis of sterols. Mutations in either HMG1 or HYD1 derepress the expression of miRNA target genes at both the mRNA and protein levels (Brodersen et al., 2008, 2012). The decreased percentage of sliced target mRNA in hydra and hmg1 seedlings indicates that sterol biosynthesis, and therefore presumably membrane function, may be required for effective slicing (Brodersen et al., 2008). However, HMG1 is not required for the production of tasiR255 (Brodersen et al., 2012), a trans-acting siRNA whose biogenesis entails miRNA RISC-mediated cleavage of TAS1 transcripts (Allen et al., 2005; Yoshikawa et al., 2005). This may reflect tissue- or miRNA-specific functions of HMG1. Examined miRNAs do not exhibit altered association with AGO1 in hmg1 inflorescences, suggesting that the defects in miRNA activity in hmg1 are downstream of AGO loading (Brodersen et al., 2012). The isoprenoid biosynthetic enzyme HMGS-1 is also involved in miRNA function in Caenorhabditis elegans (Shi and Ruvkun, 2012). However, a central role for posttranslational protein N-linked glycosylation, rather than sterol biosynthesis, was implicated in C. elegans. The membrane association of miRNA function and the known mislocalization of some integral membrane proteins associated with defective sterol biosynthesis (Men et al., 2008) suggests that sterols may be required for the organization of specific membrane compartments involved in miRNA function.
Cytoskeleton
In Arabidopsis, the single microtubule severing enzyme KATANIN (KTN) catalyzes the severing of microtubules in an ATP-dependent manner and is required for the proper organization of the cortical microtubule array (Stoppin-Mellet et al., 2006). Mutations in KTN, including disruption of the predicted ATP binding pocket, impair miRNA-mediated translational repression (Brodersen et al., 2008), indicating that miRNA function requires KTN catalysis. Microtubule organization affects diverse cellular processes, and several examples of microtubules intersecting with miRNA-related features suggest possible roles of KTN in translational repression. In animals, the ER and microtubules are closely associated (Terasaki et al., 1986), and reorganization of microtubules can affect the organization of the ER (Waterman-Storer and Salmon, 1998). Additionally, microtubules are involved in P-body dynamics (Sweet et al., 2007; Aizer et al., 2008). A limited association between microtubules and the ER, P-bodies (Hamada et al., 2012), and HSP90 has also been reported in plants (Krtková et al., 2012). Alteration of the microtubule network may affect the trafficking or assembly of cellular compartments required for translational inhibition.
CONCLUSION
The identification of interactions between transcription, splicing, and pri-miRNA processing machineries illustrates that DCL1-mediated processing is linked to additional aspects of pri-miRNA maturation. The challenge lies in understanding the functional consequences of these relationships. The transcriptional regulation of individual MIR loci, phospho-protein binding within the DCL1 complex, and association of processing with transcription and splicing elements all have the potential to integrate miRNA biogenesis into cellular responses to stress and development. The small RNA world has long been divided into plants/animals or miRNA/siRNA. A primary basis for the plant and animal divide was the difference in repression mechanisms in these two systems. It is increasingly clear that plants also commonly use translational inhibition to repress miRNA target gene expression. Protein levels were often not considered in early studies, perhaps due to the necessity for epitope tagged transgenes, and even now the full extent of translational repression by plant miRNAs is not clear. Similarly, degradome sequencing in animals indicates that although occurring at a low level, miRNA-dependent target cleavage is widespread (Bracken et al., 2011). While differences do exist between plant and animal miRNAs, they are not as categorical as once thought. Additionally, the involvement of miRNAs in RNA-dependent DNA methylation begins to blur the line between miRNA and siRNA function.
Acknowledgments
Research in the Chen lab is supported by grants from the National Institutes of Health (GM061146) and the National Science Foundation (MCB-1021465) and by the Howard Hughes Medical Institute and Gordon and Betty Moore Foundation (through Grant GBMF3046).
AUTHOR CONTRIBUTIONS
Both authors contributed to writing the article.
References
- Abe M., Yoshikawa T., Nosaka M., Sakakibara H., Sato Y., Nagato Y., Itoh J. (2010). WAVY LEAF1, an ortholog of Arabidopsis HEN1, regulates shoot development by maintaining microRNA and trans-acting small interfering RNA accumulation in rice. Plant Physiol. 154: 1335–1346 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Addo-Quaye C., Eshoo T.W., Bartel D.P., Axtell M.J. (2008). Endogenous siRNA and miRNA targets identified by sequencing of the Arabidopsis degradome. Curr. Biol. 18: 758–762 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aizer A., Brody Y., Ler L.W., Sonenberg N., Singer R.H., Shav-Tal Y. (2008). The dynamics of mammalian P body transport, assembly, and disassembly in vivo. Mol. Biol. Cell 19: 4154–4166 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Akbergenov R., Si-Ammour A., Blevins T., Amin I., Kutter C., Vanderschuren H., Zhang P., Gruissem W., Meins F., Jr, Hohn T., Pooggin M.M. (2006). Molecular characterization of geminivirus-derived small RNAs in different plant species. Nucleic Acids Res. 34: 462–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Allen E., Xie Z., Gustafson A.M., Carrington J.C. (2005). MicroRNA-directed phasing during trans-acting siRNA biogenesis in plants. Cell 121: 207–221 [DOI] [PubMed] [Google Scholar]
- Ansari S.A., Morse R.H. (2013). Mechanisms of Mediator complex action in transcriptional activation. Cell. Mol. Life Sci. 70: 2743–2756 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Aukerman M.J., Sakai H. (2003). Regulation of flowering time and floral organ identity by a microRNA and its APETALA2-like target genes. Plant Cell 15: 2730–2741 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Baek D., Park H.C., Kim M.C., Yun D.-J. (2013). The role of Arabidopsis MYB2 in miR399f-mediated phosphate-starvation response. Plant Signal. Behav. 8: e23488. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bazzini A.A., Lee M.T., Giraldez A.J. (2012). Ribosome profiling shows that miR-430 reduces translation before causing mRNA decay in zebrafish. Science 336: 233–237 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Beauclair L., Yu A., Bouché N. (2010). MicroRNA-directed cleavage and translational repression of the copper chaperone for superoxide dismutase mRNA in Arabidopsis. Plant J. 62: 454–462 [DOI] [PubMed] [Google Scholar]
- Behm-Ansmant I., Rehwinkel J., Doerks T., Stark A., Bork P., Izaurralde E. (2006). mRNA degradation by miRNAs and GW182 requires both CCR4:NOT deadenylase and DCP1:DCP2 decapping complexes. Genes Dev. 20: 1885–1898 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Amor B., et al. (2009). Novel long non-protein coding RNAs involved in Arabidopsis differentiation and stress responses. Genome Res. 19: 57–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben Chaabane S., Liu R., Chinnusamy V., Kwon Y., Park J.-H., Kim S.Y., Zhu J.-K., Yang S.W., Lee B.-H. (2013). STA1, an Arabidopsis pre-mRNA processing factor 6 homolog, is a new player involved in miRNA biogenesis. Nucleic Acids Res. 41: 1984–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Béthune J., Artus-Revel C.G., Filipowicz W. (2012). Kinetic analysis reveals successive steps leading to miRNA-mediated silencing in mammalian cells. EMBO Rep. 13: 716–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bhattacharyya S.N., Habermacher R., Martine U., Closs E.I., Filipowicz W. (2006). Relief of microRNA-mediated translational repression in human cells subjected to stress. Cell 125: 1111–1124 [DOI] [PubMed] [Google Scholar]
- Bologna N.G., Mateos J.L., Bresso E.G., Palatnik J.F. (2009). A loop-to-base processing mechanism underlies the biogenesis of plant microRNAs miR319 and miR159. EMBO J. 28: 3646–3656 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bracken C.P., Szubert J.M., Mercer T.R., Dinger M.E., Thomson D.W., Mattick J.S., Michael M.Z., Goodall G.J. (2011). Global analysis of the mammalian RNA degradome reveals widespread miRNA-dependent and miRNA-independent endonucleolytic cleavage. Nucleic Acids Res. 39: 5658–5668 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Breakfield N.W., Corcoran D.L., Petricka J.J., Shen J., Sae-Seaw J., Rubio-Somoza I., Weigel D., Ohler U., Benfey P.N. (2012). High-resolution experimental and computational profiling of tissue-specific known and novel miRNAs in Arabidopsis. Genome Res. 22: 163–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brennecke J., Stark A., Russell R.B., Cohen S.M. (2005). Principles of microRNA-target recognition. PLoS Biol. 3: e85. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brodersen P., Sakvarelidze-Achard L., Bruun-Rasmussen M., Dunoyer P., Yamamoto Y.Y., Sieburth L., Voinnet O. (2008). Widespread translational inhibition by plant miRNAs and siRNAs. Science 320: 1185–1190 [DOI] [PubMed] [Google Scholar]
- Brodersen P., Sakvarelidze-Achard L., Schaller H., Khafif M., Schott G., Bendahmane A., Voinnet O. (2012). Isoprenoid biosynthesis is required for miRNA function and affects membrane association of ARGONAUTE 1 in Arabidopsis. Proc. Natl. Acad. Sci. USA 109: 1778–1783 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brown J.W.S., Marshall D.F., Echeverria M. (2008). Intronic noncoding RNAs and splicing. Trends Plant Sci. 13: 335–342 [DOI] [PubMed] [Google Scholar]
- Calderon-Villalobos L.I.A., Kuhnle C., Dohmann E.M.N., Li H., Bevan M., Schwechheimer C. (2005). The evolutionarily conserved TOUGH protein is required for proper development of Arabidopsis thaliana. Plant Cell 17: 2473–2485 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carbonell A., Fahlgren N., Garcia-Ruiz H., Gilbert K.B., Montgomery T.A., Nguyen T., Cuperus J.T., Carrington J.C. (2012). Functional analysis of three Arabidopsis ARGONAUTES using slicer-defective mutants. Plant Cell 24: 3613–3629 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen H.-M., Chen L.-T., Patel K., Li Y.-H., Baulcombe D.C., Wu S.-H. (2010). 22-Nucleotide RNAs trigger secondary siRNA biogenesis in plants. Proc. Natl. Acad. Sci. USA 107: 15269–15274 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen X. (2004). A microRNA as a translational repressor of APETALA2 in Arabidopsis flower development. Science 303: 2022–2025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cho H.J., Kim J.J., Lee J.H., Kim W., Jung J.-H., Park C.-M., Ahn J.H. (2012). SHORT VEGETATIVE PHASE (SVP) protein negatively regulates miR172 transcription via direct binding to the pri-miR172a promoter in Arabidopsis. FEBS Lett. 586: 2332–2337 [DOI] [PubMed] [Google Scholar]
- Chu C.Y., Rana T.M. (2006). Translation repression in human cells by microRNA-induced gene silencing requires RCK/p54. PLoS Biol. 4: e210. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cikaluk D.E., Tahbaz N., Hendricks L.C., DiMattia G.E., Hansen D., Pilgrim D., Hobman T.C. (1999). GERp95, a membrane-associated protein that belongs to a family of proteins involved in stem cell differentiation. Mol. Biol. Cell 10: 3357–3372 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Couttet P., Fromont-Racine M., Steel D., Pictet R., Grange T. (1997). Messenger RNA deadenylylation precedes decapping in mammalian cells. Proc. Natl. Acad. Sci. USA 94: 5628–5633 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuperus J.T., Carbonell A., Fahlgren N., Garcia-Ruiz H., Burke R.T., Takeda A., Sullivan C.M., Gilbert S.D., Montgomery T.A., Carrington J.C. (2010a). Unique functionality of 22-nt miRNAs in triggering RDR6-dependent siRNA biogenesis from target transcripts in Arabidopsis. Nat. Struct. Mol. Biol. 17: 997–1003 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cuperus J.T., Montgomery T.A., Fahlgren N., Burke R.T., Townsend T., Sullivan C.M., Carrington J.C. (2010b). Identification of MIR390a precursor processing-defective mutants in Arabidopsis by direct genome sequencing. Proc. Natl. Acad. Sci. USA 107: 466–471 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Decker C.J., Parker R. (1993). A turnover pathway for both stable and unstable mRNAs in yeast: Evidence for a requirement for deadenylation. Genes Dev. 7: 1632–1643 [DOI] [PubMed] [Google Scholar]
- Deleris A., Gallego-Bartolome J., Bao J., Kasschau K.D., Carrington J.C., Voinnet O. (2006). Hierarchical action and inhibition of plant Dicer-like proteins in antiviral defense. Science 313: 68–71 [DOI] [PubMed] [Google Scholar]
- Denli A.M., Tops B.B.J., Plasterk R.H.A., Ketting R.F., Hannon G.J. (2004). Processing of primary microRNAs by the microprocessor complex. Nature 432: 231–235 [DOI] [PubMed] [Google Scholar]
- Derrien B., Baumberger N., Schepetilnikov M., Viotti C., De Cillia J., Ziegler-Graff V., Isono E., Schumacher K., Genschik P. (2012). Degradation of the antiviral component ARGONAUTE1 by the autophagy pathway. Proc. Natl. Acad. Sci. USA 109: 15942–15946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ding J., Li D., Ohler U., Guan J., Zhou S. (2012). Genome-wide search for miRNA-target interactions in Arabidopsis thaliana with an integrated approach. BMC Genomics 13 (Suppl 3): S3. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djuranovic S., Nahvi A., Green R. (2011). A parsimonious model for gene regulation by miRNAs. Science 331: 550–553 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Djuranovic S., Nahvi A., Green R. (2012). miRNA-mediated gene silencing by translational repression followed by mRNA deadenylation and decay. Science 336: 237–240 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dong Z., Han M.-H., Fedoroff N. (2008). The RNA-binding proteins HYL1 and SE promote accurate in vitro processing of pri-miRNA by DCL1. Proc. Natl. Acad. Sci. USA 105: 9970–9975 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dugas D.V., Bartel B. (2008). Sucrose induction of Arabidopsis miR398 represses two Cu/Zn superoxide dismutases. Plant Mol. Biol. 67: 403–417 [DOI] [PubMed] [Google Scholar]
- Eamens, A.L., Curtin, S.J., and Waterhouse, P.M. (2011). The Arabidopsis thaliana double-stranded RNA binding (DRB) domain protein family. In Non Coding RNAs in Plants, V.A. Erdmann and J. Barciszewski, eds (Berlin, Heidelberg: Springer), pp. 385–406. [Google Scholar]
- Eamens A.L., Smith N.A., Curtin S.J., Wang M.-B., Waterhouse P.M. (2009). The Arabidopsis thaliana double-stranded RNA binding protein DRB1 directs guide strand selection from microRNA duplexes. RNA 15: 2219–2235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eamens A.L., Wook Kim K., Waterhouse P.M. (2012). DRB2, DRB3 and DRB5 function in a non-canonical microRNA pathway in Arabidopsis thaliana. Plant Signal. Behav. 7: 1224–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Earley K., Smith M., Weber R., Gregory B., Poethig R. (2010). An endogenous F-box protein regulates ARGONAUTE1 in Arabidopsis thaliana. Silence 1: 15. [DOI] [PMC free article] [PubMed] [Google Scholar]
- El-Shami M., Pontier D., Lahmy S., Braun L., Picart C., Vega D., Hakimi M.-A., Jacobsen S.E., Cooke R., Lagrange T. (2007). Reiterated WG/GW motifs form functionally and evolutionarily conserved ARGONAUTE-binding platforms in RNAi-related components. Genes Dev. 21: 2539–2544 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelsberger W.R., Schulze W.X. (2012). Nitrate and ammonium lead to distinct global dynamic phosphorylation patterns when resupplied to nitrogen-starved Arabidopsis seedlings. Plant J. 69: 978–995 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eulalio A., Behm-Ansmant I., Izaurralde E. (2007a). P bodies: At the crossroads of post-transcriptional pathways. Nat. Rev. Mol. Cell Biol. 8: 9–22 [DOI] [PubMed] [Google Scholar]
- Eulalio A., Behm-Ansmant I., Schweizer D., Izaurralde E. (2007b). P-body formation is a consequence, not the cause, of RNA-mediated gene silencing. Mol. Cell. Biol. 27: 3970–3981 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eun C., Lorkovic Z.J., Naumann U., Long Q., Havecker E.R., Simon S.A., Meyers B.C., Matzke A.J.M., Matzke M. (2011). AGO6 functions in RNA-mediated transcriptional gene silencing in shoot and root meristems in Arabidopsis thaliana. PLoS ONE 6: e25730. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian M.R., et al. (2009). Mammalian miRNA RISC recruits CAF1 and PABP to affect PABP-dependent deadenylation. Mol. Cell 35: 868–880 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fabian M.R., Sonenberg N., Filipowicz W. (2010). Regulation of mRNA translation and stability by microRNAs. Annu. Rev. Biochem. 79: 351–379 [DOI] [PubMed] [Google Scholar]
- Fahlgren N., Carrington J.C. (2010). miRNA target prediction in plants. Methods Mol. Biol. 592: 51–57 [DOI] [PubMed] [Google Scholar]
- Fang Y., Spector D.L. (2007). Identification of nuclear dicing bodies containing proteins for microRNA biogenesis in living Arabidopsis plants. Curr. Biol. 17: 818–823 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fujioka Y., Utsumi M., Ohba Y., Watanabe Y. (2007). Location of a possible miRNA processing site in SmD3/SmB nuclear bodies in Arabidopsis. Plant Cell Physiol. 48: 1243–1253 [DOI] [PubMed] [Google Scholar]
- Fukuda T., et al. (2007). DEAD-box RNA helicase subunits of the Drosha complex are required for processing of rRNA and a subset of microRNAs. Nat. Cell Biol. 9: 604–611 [DOI] [PubMed] [Google Scholar]
- Gandikota M., Birkenbihl R.P., Höhmann S., Cardon G.H., Saedler H., Huijser P. (2007). The miRNA156/157 recognition element in the 3′ UTR of the Arabidopsis SBP box gene SPL3 prevents early flowering by translational inhibition in seedlings. Plant J. 49: 683–693 [DOI] [PubMed] [Google Scholar]
- German M.A., et al. (2008). Global identification of microRNA-target RNA pairs by parallel analysis of RNA ends. Nat. Biotechnol. 26: 941–946 [DOI] [PubMed] [Google Scholar]
- Gibbings D.J., Ciaudo C., Erhardt M., Voinnet O. (2009). Multivesicular bodies associate with components of miRNA effector complexes and modulate miRNA activity. Nat. Cell Biol. 11: 1143–1149 [DOI] [PubMed] [Google Scholar]
- Goeres D.C., Van Norman J.M., Zhang W., Fauver N.A., Spencer M.L., Sieburth L.E. (2007). Components of the Arabidopsis mRNA decapping complex are required for early seedling development. Plant Cell 19: 1549–1564 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gregory B.D., O’Malley R.C., Lister R., Urich M.A., Tonti-Filippini J., Chen H., Millar A.H., Ecker J.R. (2008). A link between RNA metabolism and silencing affecting Arabidopsis development. Dev. Cell 14: 854–866 [DOI] [PubMed] [Google Scholar]
- Gregory R.I., Yan K.-P., Amuthan G., Chendrimada T., Doratotaj B., Cooch N., Shiekhattar R. (2004). The Microprocessor complex mediates the genesis of microRNAs. Nature 432: 235–240 [DOI] [PubMed] [Google Scholar]
- Griffiths-Jones S., Saini H.K., van Dongen S., Enright A.J. (2008). miRBase: Tools for microRNA genomics. Nucleic Acids Res. 36 (Database issue): D154–D158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grigg S.P., Canales C., Hay A., Tsiantis M. (2005). SERRATE coordinates shoot meristem function and leaf axial patterning in Arabidopsis. Nature 437: 1022–1026 [DOI] [PubMed] [Google Scholar]
- Grigorova B., Mara C., Hollender C., Sijacic P., Chen X., Liu Z. (2011). LEUNIG and SEUSS co-repressors regulate miR172 expression in Arabidopsis flowers. Development 138: 2451–2456 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gu S., Jin L., Huang Y., Zhang F., Kay M.A. (2012). Slicing-independent RISC activation requires the argonaute PAZ domain. Curr. Biol. 22: 1536–1542 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Guil S., Cáceres J.F. (2007). The multifunctional RNA-binding protein hnRNP A1 is required for processing of miR-18a. Nat. Struct. Mol. Biol. 14: 591–596 [DOI] [PubMed] [Google Scholar]
- Gy I., Gasciolli V., Lauressergues D., Morel J.-B., Gombert J., Proux F., Proux C., Vaucheret H., Mallory A.C. (2007). Arabidopsis FIERY1, XRN2, and XRN3 are endogenous RNA silencing suppressors. Plant Cell 19: 3451–3461 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hajdarpašić A., Ruggenthaler P. (2012). Analysis of miRNA expression under stress in Arabidopsis thaliana. Bosn. J. Basic Med. Sci. 12: 169–176 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hamada T., Tominaga M., Fukaya T., Nakamura M., Nakano A., Watanabe Y., Hashimoto T., Baskin T.I. (2012). RNA processing bodies, peroxisomes, Golgi bodies, mitochondria, and endoplasmic reticulum tubule junctions frequently pause at cortical microtubules. Plant Cell Physiol. 53: 699–708 [DOI] [PubMed] [Google Scholar]
- Han J., Lee Y., Yeom K.-H., Kim Y.-K., Jin H., Kim V.N. (2004). The Drosha-DGCR8 complex in primary microRNA processing. Genes Dev. 18: 3016–3027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Han M.-H., Goud S., Song L., Fedoroff N. (2004). The Arabidopsis double-stranded RNA-binding protein HYL1 plays a role in microRNA-mediated gene regulation. Proc. Natl. Acad. Sci. USA 101: 1093–1098 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Helliwell C.A., Chin-Atkins A.N., Wilson I.W., Chapple R., Dennis E.S., Chaudhury A. (2001). The Arabidopsis AMP1 gene encodes a putative glutamate carboxypeptidase. Plant Cell 13: 2115–2125 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hiraguri A., Itoh R., Kondo N., Nomura Y., Aizawa D., Murai Y., Koiwa H., Seki M., Shinozaki K., Fukuhara T. (2005). Specific interactions between Dicer-like proteins and HYL1/DRB-family dsRNA-binding proteins in Arabidopsis thaliana. Plant Mol. Biol. 57: 173–188 [DOI] [PubMed] [Google Scholar]
- Hirsch J., Lefort V., Vankersschaver M., Boualem A., Lucas A., Thermes C., d’Aubenton-Carafa Y., Crespi M. (2006). Characterization of 43 non-protein-coding mRNA genes in Arabidopsis, including the MIR162a-derived transcripts. Plant Physiol. 140: 1192–1204 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ibrahim F., Rohr J., Jeong W.-J., Hesson J., Cerutti H. (2006). Untemplated oligoadenylation promotes degradation of RISC-cleaved transcripts. Science 314: 1893. [DOI] [PubMed] [Google Scholar]
- Iki T., Yoshikawa M., Meshi T., Ishikawa M. (2012). Cyclophilin 40 facilitates HSP90-mediated RISC assembly in plants. EMBO J. 31: 267–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iki T., Yoshikawa M., Nishikiori M., Jaudal M.C., Matsumoto-Yokoyama E., Mitsuhara I., Meshi T., Ishikawa M. (2010). In vitro assembly of plant RNA-induced silencing complexes facilitated by molecular chaperone HSP90. Mol. Cell 39: 282–291 [DOI] [PubMed] [Google Scholar]
- Iwasaki S., Takeda A., Motose H., Watanabe Y. (2007). Characterization of Arabidopsis decapping proteins AtDCP1 and AtDCP2, which are essential for post-embryonic development. FEBS Lett. 581: 2455–2459 [DOI] [PubMed] [Google Scholar]
- Janas M.M., Khaled M., Schubert S., Bernstein J.G., Golan D., Veguilla R.A., Fisher D.E., Shomron N., Levy C., Novina C.D. (2011). Feed-forward microprocessing and splicing activities at a microRNA-containing intron. PLoS Genet. 7: e1002330. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jeong D.-H., Park S., Zhai J., Gurazada S.G.R., De Paoli E., Meyers B.C., Green P.J. (2011). Massive analysis of rice small RNAs: Mechanistic implications of regulated microRNAs and variants for differential target RNA cleavage. Plant Cell 23: 4185–4207 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jha A., Shankar R. (2011). Employing machine learning for reliable miRNA target identification in plants. BMC Genomics 12: 636. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ji L., et al. (2011). ARGONAUTE10 and ARGONAUTE1 regulate the termination of floral stem cells through two microRNAs in Arabidopsis. PLoS Genet. 7: e1001358. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jia F., Rock C.D. (2013). MIR846 and MIR842 comprise a cistronic MIRNA pair that is regulated by abscisic acid by alternative splicing in roots of Arabidopsis. Plant Mol. Biol. 81: 447–460 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jones-Rhoades M.W., Bartel D.P. (2004). Computational identification of plant microRNAs and their targets, including a stress-induced miRNA. Mol. Cell 14: 787–799 [DOI] [PubMed] [Google Scholar]
- Karginov F.V., Cheloufi S., Chong M.M.W., Stark A., Smith A.D., Hannon G.J. (2010). Diverse endonucleolytic cleavage sites in the mammalian transcriptome depend upon microRNAs, Drosha, and additional nucleases. Mol. Cell 38: 781–788 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Karlowski W.M., Zielezinski A., Carrère J., Pontier D., Lagrange T., Cooke R. (2010). Genome-wide computational identification of WG/GW Argonaute-binding proteins in Arabidopsis. Nucleic Acids Res. 38: 4231–4245 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kastenmayer J.P., Green P.J. (2000). Novel features of the XRN-family in Arabidopsis: Evidence that AtXRN4, one of several orthologs of nuclear Xrn2p/Rat1p, functions in the cytoplasm. Proc. Natl. Acad. Sci. USA 97: 13985–13990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kataoka N., Fujita M., Ohno M. (2009). Functional association of the Microprocessor complex with the spliceosome. Mol. Cell. Biol. 29: 3243–3254 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Khraiwesh B., Arif M.A., Seumel G.I., Ossowski S., Weigel D., Reski R., Frank W. (2010). Transcriptional control of gene expression by microRNAs. Cell 140: 111–122 [DOI] [PubMed] [Google Scholar]
- Kim D.H., Saetrom P., Snøve O., Jr, Rossi J.J. (2008). MicroRNA-directed transcriptional gene silencing in mammalian cells. Proc. Natl. Acad. Sci. USA 105: 16230–16235 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim S., Yang J.-Y., Xu J., Jang I.-C., Prigge M.J., Chua N.-H. (2008). Two cap-binding proteins CBP20 and CBP80 are involved in processing primary microRNAs. Plant Cell Physiol. 49: 1634–1644 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kim Y.J., Zheng B., Yu Y., Won S.Y., Mo B., Chen X. (2011). The role of Mediator in small and long noncoding RNA production in Arabidopsis thaliana. EMBO J. 30: 814–822 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krishna P., Gloor G. (2001). The Hsp90 family of proteins in Arabidopsis thaliana. Cell Stress Chaperones 6: 238–246 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Krtková J., Zimmermann A., Schwarzerová K., Nick P. (2012). Hsp90 binds microtubules and is involved in the reorganization of the microtubular network in angiosperms. J. Plant Physiol. 169: 1329–1339 [DOI] [PubMed] [Google Scholar]
- Kruszka K., Pacak A., Swida-Barteczka A., Stefaniak A.K., Kaja E., Sierocka I., Karlowski W., Jarmolowski A., Szweykowska-Kulinska Z. (2013). Developmentally regulated expression and complex processing of barley pri-microRNAs. BMC Genomics 14: 34. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara Y., Takashi Y., Watanabe Y. (2006). The interaction between DCL1 and HYL1 is important for efficient and precise processing of pri-miRNA in plant microRNA biogenesis. RNA 12: 206–212 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kurihara Y., Watanabe Y. (2004). Arabidopsis micro-RNA biogenesis through Dicer-like 1 protein functions. Proc. Natl. Acad. Sci. USA 101: 12753–12758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwak P.B., Tomari Y. (2012). The N domain of Argonaute drives duplex unwinding during RISC assembly. Nat. Struct. Mol. Biol. 19: 145–151 [DOI] [PubMed] [Google Scholar]
- Lanet E., Delannoy E., Sormani R., Floris M., Brodersen P., Crété P., Voinnet O., Robaglia C. (2009). Biochemical evidence for translational repression by Arabidopsis microRNAs. Plant Cell 21: 1762–1768 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubinger S., Sachsenberg T., Zeller G., Busch W., Lohmann J.U., Rätsch G., Weigel D. (2008). Dual roles of the nuclear cap-binding complex and SERRATE in pre-mRNA splicing and microRNA processing in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 105: 8795–8800 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Laubinger S., Zeller G., Henz S.R., Buechel S., Sachsenberg T., Wang J.-W., Rätsch G., Weigel D. (2010). Global effects of the small RNA biogenesis machinery on the Arabidopsis thaliana transcriptome. Proc. Natl. Acad. Sci. USA 107: 17466–17473 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law J.A., Jacobsen S.E. (2010). Establishing, maintaining and modifying DNA methylation patterns in plants and animals. Nat. Rev. Genet. 11: 204–220 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee B.H., Kapoor A., Zhu J., Zhu J.-K. (2006). STABILIZED1, a stress-upregulated nuclear protein, is required for pre-mRNA splicing, mRNA turnover, and stress tolerance in Arabidopsis. Plant Cell 18: 1736–1749 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee Y.S., et al. (2009). Silencing by small RNAs is linked to endosomal trafficking. Nat. Cell Biol. 11: 1150–1156 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Leung A.K.L., Sharp P.A. (2013). Quantifying Argonaute proteins in and out of GW/P-bodies: Implications in microRNA activities. Adv. Exp. Med. Biol. 768: 165–182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lewis B.P., Shih I.H., Jones-Rhoades M.W., Bartel D.P., Burge C.B. (2003). Prediction of mammalian microRNA targets. Cell 115: 787–798 [DOI] [PubMed] [Google Scholar]
- Lewis J.D., Izaurralde E., Jarmolowski A., McGuigan C., Mattaj I.W. (1996). A nuclear cap-binding complex facilitates association of U1 snRNP with the cap-proximal 5′ splice site. Genes Dev. 10: 1683–1698 [DOI] [PubMed] [Google Scholar]
- Li A., Mao L. (2007). Evolution of plant microRNA gene families. Cell Res. 17: 212–218 [DOI] [PubMed] [Google Scholar]
- Li J., Yang Z., Yu B., Liu J., Chen X. (2005). Methylation protects miRNAs and siRNAs from a 3′-end uridylation activity in Arabidopsis. Curr. Biol. 15: 1501–1507 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li S., et al. (2013). MicroRNAs inhibit the translation of target mRNAs on the endoplasmic reticulum in Arabidopsis. Cell 153: 562–574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li X., Franceschi V.R., Okita T.W. (1993). Segregation of storage protein mRNAs on the rough endoplasmic reticulum membranes of rice endosperm cells. Cell 72: 869–879 [DOI] [PubMed] [Google Scholar]
- Liang G., He H., Yu D. (2012). Identification of nitrogen starvation-responsive microRNAs in Arabidopsis thaliana. PLoS ONE 7: e48951. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu C., Axtell M.J., Fedoroff N.V. (2012a). The helicase and RNaseIIIa domains of Arabidopsis Dicer-Like1 modulate catalytic parameters during microRNA biogenesis. Plant Physiol. 159: 748–758 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu H.-H., Tian X., Li Y.-J., Wu C.-A., Zheng C.-C. (2008). Microarray-based analysis of stress-regulated microRNAs in Arabidopsis thaliana. RNA 14: 836–843 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Carmell M.A., Rivas F.V., Marsden C.G., Thomson J.M., Song J.-J., Hammond S.M., Joshua-Tor L., Hannon G.J. (2004). Argonaute2 is the catalytic engine of mammalian RNAi. Science 305: 1437–1441 [DOI] [PubMed] [Google Scholar]
- Liu J., Jung C., Xu J., Wang H., Deng S., Bernad L., Arenas-Huertero C., Chua N.-H. (2012b). Genome-wide analysis uncovers regulation of long intergenic noncoding RNAs in Arabidopsis. Plant Cell 24: 4333–4345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Valencia-Sanchez M.A., Hannon G.J., Parker R. (2005). MicroRNA-dependent localization of targeted mRNAs to mammalian P-bodies. Nat. Cell Biol. 7: 719–723 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Llave C., Xie Z., Kasschau K.D., Carrington J.C. (2002). Cleavage of Scarecrow-like mRNA targets directed by a class of Arabidopsis miRNA. Science 297: 2053–2056 [DOI] [PubMed] [Google Scholar]
- Lobbes D., Rallapalli G., Schmidt D.D., Martin C., Clarke J. (2006). SERRATE: A new player on the plant microRNA scene. EMBO Rep. 7: 1052–1058 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida S., Chen H.-Y., Adam Yuan Y. (2011). Molecular insights into miRNA processing by Arabidopsis thaliana SERRATE. Nucleic Acids Res. 39: 7828–7836 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Machida S., Yuan Y.A. (2013). Crystal Structure of Arabidopsis thaliana Dawdle Forkhead-Associated Domain reveals a conserved phospho-threonine recognition cleft for Dicer-like1 binding. Mol. Plant. 6: 1290–1300 [DOI] [PubMed] [Google Scholar]
- Macrae I.J., Zhou K., Li F., Repic A., Brooks A.N., Cande W.Z., Adams P.D., Doudna J.A. (2006). Structural basis for double-stranded RNA processing by Dicer. Science 311: 195–198 [DOI] [PubMed] [Google Scholar]
- Mallory A., Vaucheret H. (2010). Form, function, and regulation of ARGONAUTE proteins. Plant Cell 22: 3879–3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory A.C., Reinhart B.J., Jones-Rhoades M.W., Tang G., Zamore P.D., Barton M.K., Bartel D.P. (2004). MicroRNA control of PHABULOSA in leaf development: Importance of pairing to the microRNA 5′ region. EMBO J. 23: 3356–3364 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Manavella P.A., Hagmann J., Ott F., Laubinger S., Franz M., Macek B., Weigel D. (2012a). Fast-forward genetics identifies plant CPL phosphatases as regulators of miRNA processing factor HYL1. Cell 151: 859–870 [DOI] [PubMed] [Google Scholar]
- Manavella P.A., Koenig D., Weigel D. (2012b). Plant secondary siRNA production determined by microRNA-duplex structure. Proc. Natl. Acad. Sci. USA 109: 2461–2466 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Margis R., Fusaro A.F., Smith N.A., Curtin S.J., Watson J.M., Finnegan E.J., Waterhouse P.M. (2006). The evolution and diversification of Dicers in plants. FEBS Lett. 580: 2442–2450 [DOI] [PubMed] [Google Scholar]
- Mateos J.L., Bologna N.G., Chorostecki U., Palatnik J.F. (2010). Identification of microRNA processing determinants by random mutagenesis of Arabidopsis MIR172a precursor. Curr. Biol. 20: 49–54 [DOI] [PubMed] [Google Scholar]
- Maunoury N., Vaucheret H. (2011). AGO1 and AGO2 act redundantly in miR408-mediated Plantacyanin regulation. PLoS ONE 6: e28729. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Megraw M., Baev V., Rusinov V., Jensen S.T., Kalantidis K., Hatzigeorgiou A.G. (2006). MicroRNA promoter element discovery in Arabidopsis. RNA 12: 1612–1619 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Meijer H.A., Kong Y.W., Lu W.T., Wilczynska A., Spriggs R.V., Robinson S.W., Godfrey J.D., Willis A.E., Bushell M. (2013). Translational repression and eIF4A2 activity are critical for microRNA-mediated gene regulation. Science 340: 82–85 [DOI] [PubMed] [Google Scholar]
- Men S., Boutté Y., Ikeda Y., Li X., Palme K., Stierhof Y.-D., Hartmann M.-A., Moritz T., Grebe M. (2008). Sterol-dependent endocytosis mediates post-cytokinetic acquisition of PIN2 auxin efflux carrier polarity. Nat. Cell Biol. 10: 237–244 [DOI] [PubMed] [Google Scholar]
- Meng Y., Shao C. (2012). Large-scale identification of mirtrons in Arabidopsis and rice. PLoS ONE 7: e31163. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merchan F., Boualem A., Crespi M., Frugier F. (2009). Plant polycistronic precursors containing non-homologous microRNAs target transcripts encoding functionally related proteins. Genome Biol. 10: R136. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mi S., et al. (2008). Sorting of small RNAs into Arabidopsis argonaute complexes is directed by the 5′ terminal nucleotide. Cell 133: 116–127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mica E., et al. (2009). High throughput approaches reveal splicing of primary microRNA transcripts and tissue specific expression of mature microRNAs in Vitis vinifera. BMC Genomics 10: 558. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Molnár A., Schwach F., Studholme D.J., Thuenemann E.C., Baulcombe D.C. (2007). miRNAs control gene expression in the single-cell alga Chlamydomonas reinhardtii. Nature 447: 1126–1129 [DOI] [PubMed] [Google Scholar]
- Montgomery T.A., Howell M.D., Cuperus J.T., Li D., Hansen J.E., Alexander A.L., Chapman E.J., Fahlgren N., Allen E., Carrington J.C. (2008). Specificity of ARGONAUTE7-miR390 interaction and dual functionality in TAS3 trans-acting siRNA formation. Cell 133: 128–141 [DOI] [PubMed] [Google Scholar]
- Morlando M., Ballarino M., Gromak N., Pagano F., Bozzoni I., Proudfoot N.J. (2008). Primary microRNA transcripts are processed co-transcriptionally. Nat. Struct. Mol. Biol. 15: 902–909 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Motomura K., Le Q.T., Kumakura N., Fukaya T., Takeda A., Watanabe Y. (2012). The role of decapping proteins in the miRNA accumulation in Arabidopsis thaliana. RNA Biol. 9: 644–652 [DOI] [PubMed] [Google Scholar]
- Mourrain P., et al. (2000). Arabidopsis SGS2 and SGS3 genes are required for posttranscriptional gene silencing and natural virus resistance. Cell 101: 533–542 [DOI] [PubMed] [Google Scholar]
- Muddashetty R.S., Nalavadi V.C., Gross C., Yao X., Xing L., Laur O., Warren S.T., Bassell G.J. (2011). Reversible inhibition of PSD-95 mRNA translation by miR-125a, FMRP phosphorylation, and mGluR signaling. Mol. Cell 42: 673–688 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Muhlrad D., Parker R. (1994). Premature translational termination triggers mRNA decapping. Nature 370: 578–581 [DOI] [PubMed] [Google Scholar]
- Nozawa M., Miura S., Nei M. (2012). Origins and evolution of microRNA genes in plant species. Genome Biol. Evol. 4: 230–239 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park M.Y., Wu G., Gonzalez-Sulser A., Vaucheret H., Poethig R.S. (2005). Nuclear processing and export of microRNAs in Arabidopsis. Proc. Natl. Acad. Sci. USA 102: 3691–3696 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Park W., Li J., Song R., Messing J., Chen X. (2002). CARPEL FACTORY, a Dicer homolog, and HEN1, a novel protein, act in microRNA metabolism in Arabidopsis thaliana. Curr. Biol. 12: 1484–1495 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pillai R.S., Bhattacharyya S.N., Artus C.G., Zoller T., Cougot N., Basyuk E., Bertrand E., Filipowicz W. (2005). Inhibition of translational initiation by Let-7 microRNA in human cells. Science 309: 1573–1576 [DOI] [PubMed] [Google Scholar]
- Pomeranz M.C., Hah C., Lin P.-C., Kang S.G., Finer J.J., Blackshear P.J., Jang J.-C. (2010). The Arabidopsis tandem zinc finger protein AtTZF1 traffics between the nucleus and cytoplasmic foci and binds both DNA and RNA. Plant Physiol. 152: 151–165 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Poulsen C., Vaucheret H., Brodersen P. (2013). Lessons on RNA silencing mechanisms in plants from eukaryotic argonaute structures. Plant Cell 25: 22–37 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prigge M.J., Wagner D.R. (2001). The Arabidopsis serrate gene encodes a zinc-finger protein required for normal shoot development. Plant Cell 13: 1263–1279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Prunet N., Morel P., Thierry A.-M., Eshed Y., Bowman J.L., Negrutiu I., Trehin C. (2008). REBELOTE, SQUINT, and ULTRAPETALA1 function redundantly in the temporal regulation of floral meristem termination in Arabidopsis thaliana. Plant Cell 20: 901–919 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Qin H., Chen F., Huan X., Machida S., Song J., Yuan Y.A. (2010). Structure of the Arabidopsis thaliana DCL4 DUF283 domain reveals a noncanonical double-stranded RNA-binding fold for protein-protein interaction. RNA 16: 474–481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Raczynska K.D., Simpson C.G., Ciesiolka A., Szewc L., Lewandowska D., McNicol J., Szweykowska-Kulinska Z., Brown J.W.S., Jarmolowski A. (2010). Involvement of the nuclear cap-binding protein complex in alternative splicing in Arabidopsis thaliana. Nucleic Acids Res. 38: 265–278 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rajagopalan R., Vaucheret H., Trejo J., Bartel D.P. (2006). A diverse and evolutionarily fluid set of microRNAs in Arabidopsis thaliana. Genes Dev. 20: 3407–3425 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ramachandran V., Chen X. (2008). Degradation of microRNAs by a family of exoribonucleases in Arabidopsis. Science 321: 1490–1492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Rasia R.M., Mateos J., Bologna N.G., Burdisso P., Imbert L., Palatnik J.F., Boisbouvier J. (2010). Structure and RNA interactions of the plant MicroRNA processing-associated protein HYL1. Biochemistry 49: 8237–8239 [DOI] [PubMed] [Google Scholar]
- Reinhart B.J., Weinstein E.G., Rhoades M.W., Bartel B., Bartel D.P. (2002). MicroRNAs in plants. Genes Dev. 16: 1616–1626 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G., Chen X., Yu B. (2012a). Uridylation of miRNAs by hen1 suppressor1 in Arabidopsis. Curr. Biol. 22: 695–700 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren G., Xie M., Dou Y., Zhang S., Zhang C., Yu B. (2012b). Regulation of miRNA abundance by RNA binding protein TOUGH in Arabidopsis. Proc. Natl. Acad. Sci. USA 109: 12817–12821 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reynoso M.A., Blanco F.A., Bailey-Serres J., Crespi M., Zanetti M.E. (2012). Selective recruitment of mRNAs and miRNAs to polyribosomes in response to rhizobia infection in Medicago truncatula. Plant J. 73: 289–301 [DOI] [PubMed] [Google Scholar]
- Shi Z., Ruvkun G. (2012). The mevalonate pathway regulates microRNA activity in Caenorhabditis elegans. Proc. Natl. Acad. Sci. USA 109: 4568–4573 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shin C., Nam J.-W., Farh K.K.-H., Chiang H.R., Shkumatava A., Bartel D.P. (2010). Expanding the microRNA targeting code: functional sites with centered pairing. Mol. Cell 38: 789–802 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shiohama A., Sasaki T., Noda S., Minoshima S., Shimizu N. (2007). Nucleolar localization of DGCR8 and identification of eleven DGCR8-associated proteins. Exp. Cell Res. 313: 4196–4207 [DOI] [PubMed] [Google Scholar]
- Song L., Axtell M.J., Fedoroff N.V. (2010). RNA secondary structural determinants of miRNA precursor processing in Arabidopsis. Curr. Biol. 20: 37–41 [DOI] [PubMed] [Google Scholar]
- Song L., Han M.-H., Lesicka J., Fedoroff N. (2007). Arabidopsis primary microRNA processing proteins HYL1 and DCL1 define a nuclear body distinct from the Cajal body. Proc. Natl. Acad. Sci. USA 104: 5437–5442 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Souret F.F., Kastenmayer J.P., Green P.J. (2004). AtXRN4 degrades mRNA in Arabidopsis and its substrates include selected miRNA targets. Mol. Cell 15: 173–183 [DOI] [PubMed] [Google Scholar]
- Stalder L., et al. (2013). The rough endoplasmatic reticulum is a central nucleation site of siRNA-mediated RNA silencing. EMBO J. 32: 1115–1127 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stoppin-Mellet V., Gaillard J., Vantard M. (2006). Katanin’s severing activity favors bundling of cortical microtubules in plants. Plant J. 46: 1009–1017 [DOI] [PubMed] [Google Scholar]
- Sugiyama N., Nakagami H., Mochida K., Daudi A., Tomita M., Shirasu K., Ishihama Y. (2008). Large-scale phosphorylation mapping reveals the extent of tyrosine phosphorylation in Arabidopsis. Mol. Syst. Biol. 4: 193. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sweet T.J., Boyer B., Hu W., Baker K.E., Coller J. (2007). Microtubule disruption stimulates P-body formation. RNA 13: 493–502 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Szarzynska B., Sobkowiak L., Pant B.D., Balazadeh S., Scheible W.-R., Mueller-Roeber B., Jarmolowski A., Szweykowska-Kulinska Z. (2009). Gene structures and processing of Arabidopsis thaliana HYL1-dependent pri-miRNAs. Nucleic Acids Res. 37: 3083–3093 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Takeda A., Iwasaki S., Watanabe T., Utsumi M., Watanabe Y. (2008). The mechanism selecting the guide strand from small RNA duplexes is different among argonaute proteins. Plant Cell Physiol. 49: 493–500 [DOI] [PubMed] [Google Scholar]
- Teixeira D., Sheth U., Valencia-Sanchez M.A., Brengues M., Parker R. (2005). Processing bodies require RNA for assembly and contain nontranslating mRNAs. RNA 11: 371–382 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Terasaki M., Chen L.B., Fujiwara K. (1986). Microtubules and the endoplasmic reticulum are highly interdependent structures. J. Cell Biol. 103: 1557–1568 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thieme C.J., Schudoma C., May P., Walther D. (2012). Give It AGO: The search for miRNA-Argonaute sorting signals in Arabidopsis thaliana indicates a relevance of sequence positions other than the 5′-position alone. Front. Plant Sci. 3: 272. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Till S., Lejeune E., Thermann R., Bortfeld M., Hothorn M., Enderle D., Heinrich C., Hentze M.W., Ladurner A.G. (2007). A conserved motif in Argonaute-interacting proteins mediates functional interactions through the Argonaute PIWI domain. Nat. Struct. Mol. Biol. 14: 897–903 [DOI] [PubMed] [Google Scholar]
- Válóczi A., Várallyay E., Kauppinen S., Burgyán J., Havelda Z. (2006). Spatio-temporal accumulation of microRNAs is highly coordinated in developing plant tissues. Plant J. 47: 140–151 [DOI] [PubMed] [Google Scholar]
- Vazquez F., Blevins T., Ailhas J., Boller T., Meins F., Jr (2008). Evolution of Arabidopsis MIR genes generates novel microRNA classes. Nucleic Acids Res. 36: 6429–6438 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vazquez F., Gasciolli V., Crété P., Vaucheret H. (2004). The nuclear dsRNA binding protein HYL1 is required for microRNA accumulation and plant development, but not posttranscriptional transgene silencing. Curr. Biol. 14: 346–351 [DOI] [PubMed] [Google Scholar]
- Voinnet O. (2009). Origin, biogenesis, and activity of plant microRNAs. Cell 136: 669–687 [DOI] [PubMed] [Google Scholar]
- Walley J.W., Kelley D.R., Savchenko T., Dehesh K. (2010). Investigating the function of CAF1 deadenylases during plant stress responses. Plant Signal. Behav. 5: 802–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang F., Perry S.E. (2013). Identification of direct targets of FUSCA3, a key regulator of Arabidopsis seed development. Plant Physiol. 161: 1251–1264 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang L., Song X., Gu L., Li X., Cao S., Chu C., Cui X., Chen X., Cao X. (2013). NOT2 proteins promote polymerase II-dependent transcription and interact with multiple microRNA biogenesis factors in Arabidopsis. Plant Cell 25: 715–727 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Washida H., Sugino A., Doroshenk K.A., Satoh-Cruz M., Nagamine A., Katsube-Tanaka T., Ogawa M., Kumamaru T., Satoh H., Okita T.W. (2012). RNA targeting to a specific ER sub-domain is required for efficient transport and packaging of α-globulins to the protein storage vacuole in developing rice endosperm. Plant J. 70: 471–479 [DOI] [PubMed] [Google Scholar]
- Waterman-Storer C.M., Salmon E.D. (1998). Endoplasmic reticulum membrane tubules are distributed by microtubules in living cells using three distinct mechanisms. Curr. Biol. 8: 798–806 [DOI] [PubMed] [Google Scholar]
- Weber C., Nover L., Fauth M. (2008). Plant stress granules and mRNA processing bodies are distinct from heat stress granules. Plant J. 56: 517–530 [DOI] [PubMed] [Google Scholar]
- Werner S., Wollmann H., Schneeberger K., Weigel D. (2010). Structure determinants for accurate processing of miR172a in Arabidopsis thaliana. Curr. Biol. 20: 42–48 [DOI] [PubMed] [Google Scholar]
- Wu L., Fan J., Belasco J.G. (2006). MicroRNAs direct rapid deadenylation of mRNA. Proc. Natl. Acad. Sci. USA 103: 4034–4039 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wu L., Zhou H., Zhang Q., Zhang J., Ni F., Liu C., Qi Y. (2010). DNA methylation mediated by a microRNA pathway. Mol. Cell 38: 465–475 [DOI] [PubMed] [Google Scholar]
- Wu X., Shi Y., Li J., Xu L., Fang Y., Li X., Qi Y. (2013). A role for the RNA-binding protein MOS2 in microRNA maturation in Arabidopsis. Cell Res. 23: 645–657 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z., Allen E., Fahlgren N., Calamar A., Givan S.A., Carrington J.C. (2005a). Expression of Arabidopsis MIRNA genes. Plant Physiol. 138: 2145–2154 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z., Allen E., Wilken A., Carrington J.C. (2005b). DICER-LIKE 4 functions in trans-acting small interfering RNA biogenesis and vegetative phase change in Arabidopsis thaliana. Proc. Natl. Acad. Sci. USA 102: 12984–12989 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xie Z., Johansen L.K., Gustafson A.M., Kasschau K.D., Lellis A.D., Zilberman D., Jacobsen S.E., Carrington J.C. (2004). Genetic and functional diversification of small RNA pathways in plants. PLoS Biol. 2: E104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Chua N.-H. (2009). Arabidopsis decapping 5 is required for mRNA decapping, P-body formation, and translational repression during postembryonic development. Plant Cell 21: 3270–3279 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Xu J., Yang J.-Y., Niu Q.-W., Chua N.-H. (2006). Arabidopsis DCP2, DCP1, and VARICOSE form a decapping complex required for postembryonic development. Plant Cell 18: 3386–3398 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yamasaki H., Hayashi M., Fukazawa M., Kobayashi Y., Shikanai T. (2009). SQUAMOSA Promoter Binding Protein-Like7 is a central regulator for copper homeostasis in Arabidopsis. Plant Cell 21: 347–361 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yan K., Liu P., Wu C.-A., Yang G.-D., Xu R., Guo Q.-H., Huang J.-G., Zheng C.-C. (2012). Stress-induced alternative splicing provides a mechanism for the regulation of microRNA processing in Arabidopsis thaliana. Mol. Cell 48: 521–531 [DOI] [PubMed] [Google Scholar]
- Yang L., Liu Z., Lu F., Dong A., Huang H. (2006a). SERRATE is a novel nuclear regulator in primary microRNA processing in Arabidopsis. Plant J. 47: 841–850 [DOI] [PubMed] [Google Scholar]
- Yang L., Wu G., Poethig R.S. (2012). Mutations in the GW-repeat protein SUO reveal a developmental function for microRNA-mediated translational repression in Arabidopsis. Proc. Natl. Acad. Sci. USA 109: 315–320 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang S.W., Chen H.-Y., Yang J., Machida S., Chua N.-H., Yuan Y.A. (2010). Structure of Arabidopsis HYPONASTIC LEAVES1 and its molecular implications for miRNA processing. Structure 18: 594–605 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang Z., Ebright Y.W., Yu B., Chen X. (2006b). HEN1 recognizes 21-24 nt small RNA duplexes and deposits a methyl group onto the 2′ OH of the 3′ terminal nucleotide. Nucleic Acids Res. 34: 667–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yant L., Mathieu J., Dinh T.T., Ott F., Lanz C., Wollmann H., Chen X., Schmid M. (2010). Orchestration of the floral transition and floral development in Arabidopsis by the bifunctional transcription factor APETALA2. Plant Cell 22: 2156–2170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ye R., Wang W., Iki T., Liu C., Wu Y., Ishikawa M., Zhou X., Qi Y. (2012). Cytoplasmic assembly and selective nuclear import of Arabidopsis Argonaute4/siRNA complexes. Mol. Cell 46: 859–870 [DOI] [PubMed] [Google Scholar]
- Yoshikawa M., Iki T., Tsutsui Y., Miyashita K., Poethig R.S., Habu Y., Ishikawa M. (2013). 3′ Fragment of miR173-programmed RISC-cleaved RNA is protected from degradation in a complex with RISC and SGS3. Proc. Natl. Acad. Sci. USA 110: 4117–4122 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshikawa M., Peragine A., Park M.Y., Poethig R.S. (2005). A pathway for the biogenesis of trans-acting siRNAs in Arabidopsis. Genes Dev. 19: 2164–2175 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B., Bi L., Zheng B., Ji L., Chevalier D., Agarwal M., Ramachandran V., Li W., Lagrange T., Walker J.C., Chen X. (2008). The FHA domain proteins DAWDLE in Arabidopsis and SNIP1 in humans act in small RNA biogenesis. Proc. Natl. Acad. Sci. USA 105: 10073–10078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yu B., Yang Z., Li J., Minakhina S., Yang M., Padgett R.W., Steward R., Chen X. (2005). Methylation as a crucial step in plant microRNA biogenesis. Science 307: 932–935 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yumul R.E., Kim Y.J., Liu X., Wang R., Ding J., Xiao L., Chen X. (2013). POWERDRESS and diversified expression of the MIR172 gene family bolster the floral stem cell network. PLoS Genet. 9: e1003218. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zekri L., Huntzinger E., Heimstädt S., Izaurralde E. (2009). The silencing domain of GW182 interacts with PABPC1 to promote translational repression and degradation of microRNA targets and is required for target release. Mol. Cell. Biol. 29: 6220–6231 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zekri L., Kuzuoğlu-Öztürk D., Izaurralde E. (2013). GW182 proteins cause PABP dissociation from silenced miRNA targets in the absence of deadenylation. EMBO J. 32: 1052–1065 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y., Cullen B.R. (2004). Structural requirements for pre-microRNA binding and nuclear export by Exportin 5. Nucleic Acids Res. 32: 4776–4785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zeng Y., Yi R., Cullen B.R. (2003). MicroRNAs and small interfering RNAs can inhibit mRNA expression by similar mechanisms. Proc. Natl. Acad. Sci. USA 100: 9779–9784 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhan X., Wang B., Li H., Liu R., Kalia R.K., Zhu J.-K., Chinnusamy V. (2012). Arabidopsis proline-rich protein important for development and abiotic stress tolerance is involved in microRNA biogenesis. Proc. Natl. Acad. Sci. USA 109: 18198–18203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang B.H., Pan X.P., Wang Q.L., Cobb G.P., Anderson T.A. (2005). Identification and characterization of new plant microRNAs using EST analysis. Cell Res. 15: 336–360 [DOI] [PubMed] [Google Scholar]
- Zhang L., Chia J.-M., Kumari S., Stein J.C., Liu Z., Narechania A., Maher C.A., Guill K., McMullen M.D., Ware D. (2009). A genome-wide characterization of microRNA genes in maize. PLoS Genet 5: e1000716. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao X., Zhang H., Li L. (2013). Identification and analysis of the proximal promoters of microRNA genes in Arabidopsis. Genomics 101: 187–194 [DOI] [PubMed] [Google Scholar]
- Zhao Y., Mo B., Chen X. (2012a). Mechanisms that impact microRNA stability in plants. RNA Biol. 9: 1218–1223 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao Y., Yu Y., Zhai J., Ramachandran V., Dinh T.T., Meyers B.C., Mo B., Chen X. (2012b). The Arabidopsis nucleotidyl transferase HESO1 uridylates unmethylated small RNAs to trigger their degradation. Curr. Biol. 22: 689–694 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zheng X., Zhu J., Kapoor A., Zhu J.-K. (2007). Role of Arabidopsis AGO6 in siRNA accumulation, DNA methylation and transcriptional gene silencing. EMBO J. 26: 1691–1701 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu H., Hu F., Wang R., Zhou X., Sze S.-H., Liou L.W., Barefoot A., Dickman M., Zhang X. (2011). Arabidopsis Argonaute10 specifically sequesters miR166/165 to regulate shoot apical meristem development. Cell 145: 242–256 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhu Q.-H., Spriggs A., Matthew L., Fan L., Kennedy G., Gubler F., Helliwell C. (2008). A diverse set of microRNAs and microRNA-like small RNAs in developing rice grains. Genome Res. 18: 1456–1465 [DOI] [PMC free article] [PubMed] [Google Scholar]