An efficient liquid medium gave high plating efficiencies of protoplasts. Transcription profiles of plantlets and protoplast-derived cells during the first week of culture were used to track major molecular processes of the reentry in the cell cycle as the plant cells transitioned toward a totipotent state. Candidate genes for plant cell reprogramming are highlighted.
Abstract
The molecular mechanisms underlying plant cell totipotency are largely unknown. Here, we present a protocol for the efficient regeneration of plants from Arabidopsis thaliana protoplasts. The specific liquid medium used in our study leads to a high rate of reentry into the cell cycle of most cell types, providing a powerful system to study dedifferentiation/regeneration processes in independent somatic cells. To identify the early events in the establishment of totipotency, we monitored the genome-wide transcript profiles of plantlets and protoplast-derived cells (PdCs) during the first week of culture. Plant cells rapidly dedifferentiated. Then, we observed the reinitiation and reorientation of protein synthesis, accompanied by the reinitiation of cell division and de novo cell wall synthesis. Marked changes in the expression of chromatin-associated genes, especially of those in the histone variant family, were observed during protoplast culture. Surprisingly, the epigenetic status of PdCs and well-established cell cultures differed, with PdCs exhibiting rare reactivated transposons and epigenetic changes. The differentially expressed genes identified in this study are interesting candidates for investigating the molecular mechanisms underlying plant cell plasticity and totipotency. One of these genes, the plant-specific transcription factor ABERRANT LATERAL ROOT FORMATION4, is required for the initiation of protoplast division.
INTRODUCTION
Multiple regeneration mechanisms, based on various progenitor cell sources, exist in plants. Most of these were identified through the development of in vitro tissue culture techniques and were subsequently used in plant biotechnology applications (Vasil, 2008). For over 50 years, it has been known that applications of auxin and cytokinin phytohormones can chemically induce plant cell reprogramming (Skoog and Miller, 1957). However, the underlying mechanisms are poorly understood. Recently, based on a study using in vitro Arabidopsis thaliana root segments, it was proposed that meristem formation arises from trans-differentiation of a specific population of starting cells that are equivalent to adult stem cells and that regeneration occurs by differentiation of these progenitor cells (Atta et al., 2009; Sugimoto et al., 2010, 2011). In root segments, the controlled asymmetric division of specific pericycle cells (De Smet et al., 2008) is closely linked to cell fate respecification in lateral root founder cells (Vanneste et al., 2005). One of the major limits to understanding the initial molecular reprogramming events that lead to totipotency arises from the complexity of the model explants.
Besides trans-differentiation, dedifferentiation is another developmental switch that can provide progenitor cell sources for the regeneration of multicellular organisms. Dedifferentiation is observed after protoplasting, the enzymatic removal of the plant cell wall. Indeed, in appropriate culture conditions, protoplast-derived cells (PdCs) proliferate and form microcalli, which can undergo regeneration (Nitsch and Oyama, 1971; Takebe et al., 1971). Once it was established that regeneration could occur from single somatic dedifferentiated cells, this regeneration process was applied to a large range of plant species (Davey et al., 2005). The plasticity of plant protoplasts is reminiscent of the totipotency of animal stem cells (González et al., 2011; Jopling et al., 2011). In some species, such as tobacco (Nicotiana tabacum), almost 100% of leaf protoplasts yielded cell colonies and ultimately plants (Bourgin et al., 1979), suggesting that dividing protoplasts can be obtained from various differentiated cells. To attain high regeneration rates, it was recommended that young and nonstressed tissues be used for protoplast isolation (Chupeau et al., 1974) and that plant growth conditions be adjusted to avoid premature cell death (Horii and Marubashi, 2005).
The early developmental stages initiated in protoplasts are accompanied by large-scale chromatin remodeling (Zhao et al., 2001; Tessadori et al., 2007) and by major transcriptional changes (Xiao et al., 2012). Thus, plant protoplasts offer an alternative model system to decipher the molecular basis underlying dedifferentiation and cell reprogramming prior to regeneration.
The model plant Arabidopsis offers various large-scale and genome-wide analysis tools (Atias et al., 2009). However, Arabidopsis protoplasts have been mainly used in short-term studies based on transient expression experiments (Yoo et al., 2007; Zhai et al., 2009). Indeed, Arabidopsis protoplast culture is known to be technically challenging. Although plants can regenerate from calli derived from Arabidopsis protoplasts embedded in gelified medium (Damm and Willmitzer, 1988), the regeneration rate is low, with only 1 to 10% forming cell colonies (Masson and Paszkowski, 1992; Dovzhenko et al., 2003). Furthermore, the use of gelified medium prevented the easy collection of PdCs for biochemical analyses and other studies aimed at deciphering the basic web of genes that regulates cell reprogramming.
Here, we report a robust protocol for the isolation of large populations of highly viable and dividing protoplasts from in vitro–grown Arabidopsis plantlets. We established a liquid medium that supports a high rate of protoplast division (up to 50% of the protoplasts). This protocol allowed us to characterize the changes in the transcript profile during the early steps of dedifferentiation and reentry into the cell division process (i.e., from plantlets to 1-week-old PdC colonies). We present a spreadsheet that can be used for gene filtering of our large data set, enabling cross-comparisons with other studies. The protoplasts underwent rapid dedifferentiation and major changes in organelle metabolism, followed by reinitiation and reorientation of protein synthesis, striking changes in the expression of chromatin-associated genes, and reinitiation of cell division with cell wall rebuilding. Comparisons between PdCs and cells of a well-established cell suspension revealed epigenetic differences that suggest that PdCs are more closely related to plant tissues than to cells in suspension. Finally, our study identified an array of molecular factors that function in the early steps of reprogramming. By testing the functional roles of two of these factors, we show that the ALF4 plant-specific factor is crucial for protoplast division. Thus, our data will serve as a valuable source of candidate genes for further investigations of plant cell plasticity and totipotency.
RESULTS
From Efficient Protoplast Culture in Liquid Medium to Plantlet Regeneration
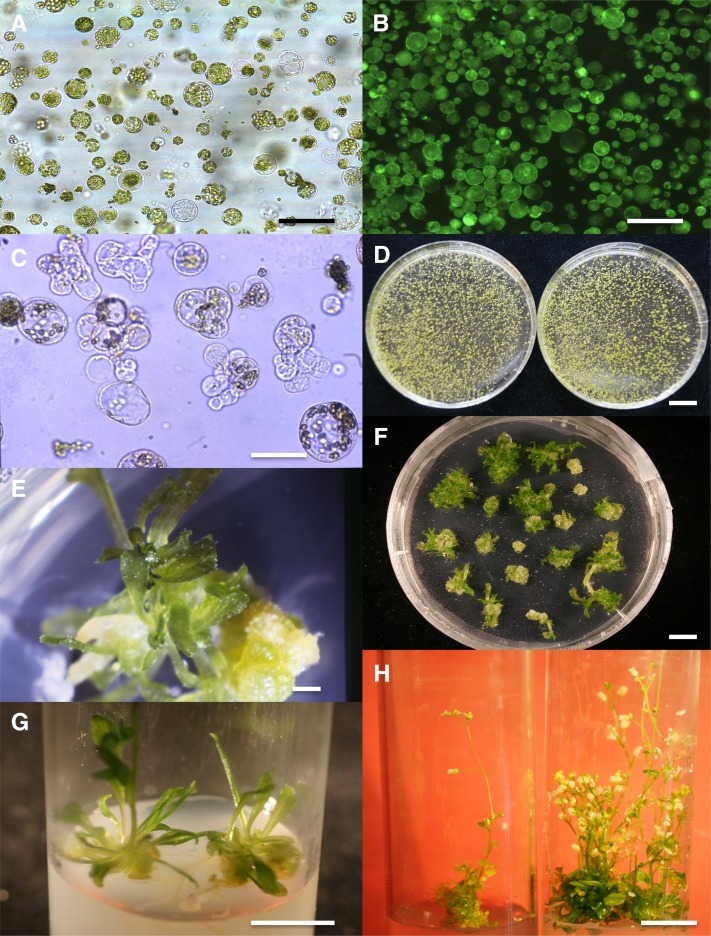
A stable source of axenic plant material devoid of stresses (light, heat, and drought) is crucial for successful cell culture in liquid medium over extended periods of time. Therefore, we first optimized the in vitro culture conditions (i.e., climate, vessels, and media) for the production of Arabidopsis plantlets suitable for protoplast and plant regeneration. Our best results were obtained when plantlets were cultured in a Green Box container on germination medium (GM) (see Supplemental Table 1 online), placed in a growth chamber with 75% controlled relative humidity, short-day conditions, and a constant temperature of 20°C. For optimal yields of viable and dividing protoplasts, plantlets were collected 18 to 21 d after sowing. This narrow developmental window probably depended on complex environmental factors that influence the osmotic potential of seedlings, such as the progressive drying of the culture medium, as well as on developmental factors. We next established maceration conditions that allowed the treated cells to adapt progressively to the osmotic pressure of the maceration Gly Glc medium (MGG) (see Supplemental Table 1 online) and resulted in a moderate level of plasmolysis. Cell walls were slowly degraded by overnight exposure to low levels of cellulolytic enzymes. Under the conditions used here, only the aerial parts of seedlings were efficiently digested. Routinely, ∼3 × 106 protoplasts were released per gram of seedlings. More than 90% were viable, based on a fluorescein diacetate assay (Widholm, 1972) (Figures 1A and 1B).
Figure 1.
Regeneration of Plantlets from Arabidopsis Protoplasts.
(A) Different cell types of freshly isolated Col-0 protoplasts in maceration medium. Bar = 100 μm.
(B) Viable protoplasts (Col-0) fluoresce in fluoroscein diacetate under UV light. Bar = 100 μm.
(C) Representative example of small cell colonies (Col-0) formed in a liquid culture dish after 6 d in culture. Approximately 25% of cells were dead, 25% survived, but were nondividing, and 50% had divided. Bar = 50 μm.
(D) Small cell colonies (Col-0) in liquid culture, 1 month after the second dilution in 10-cm dishes (two replicates). Bar = 1 cm.
(E) A rare example of a Col-0 colony differentiating buds on gelified SIM. Bar = 1 mm.
(F) Regenerating Ws colonies 20 d after transplantation onto gelified SIM. Bar = 0.5 cm.
(G) Development of Ws buds 1 week after transplantation onto rooting medium in culture tubes. Bar = 1 cm.
(H) Numerous flowering stems after 3 weeks of culture on rooting medium. Bar = 1 cm.
To optimize the culture medium for high plating efficiency, we systematically estimated the influence of all of the components in the medium, as well as the environmental conditions, based on our previous successful in vitro cultures of protoplasts from various species, particularly hybrid poplars (Populus tremula × Populus alba) (Chupeau et al., 1993). Using this empirical approach, we established that 2,4-D is the least toxic synthetic auxin in our experimental setup, that thidiazuron (TZ) is the only cytokinin that permits the development of healthy protoplasts, and that the combined use of 2,4-D and TZ results in the highest division rates. We also established that low levels of ammonium and nitrate are essential for high division rates of Arabidopsis protoplasts in liquid culture. A low level of microelements (Heller, 1953) was added to the liquid protoplast culture to increase the division rate. The addition of folic acid to the formulation of vitamins (Morel and Wetmore, 1951) promoted Arabidopsis cell survival and division. Although a 9% (w/v) solution of Glc permitted cell division, a mixture of Glc (4% w/v) and mannitol (6% w/v) in the initial protoplast induction medium (PIM) was more effective at promoting division (see Supplemental Table 1 online).
Using our established PIM, 30 to 50% of the initially plated protoplasts plated at 8 × 104 protoplasts/mL routinely underwent cell division. The first division occurred 3 d after plating for a small population of nonchlorophyllous cells, mainly composed of protoplasts derived from companion cells of the phloem (the less differentiated cells in leaves; Buchanan-Wollaston et al., 2005). Other cell types first divided between 3 and 6 d after plating (Figure 1C). Considering the large range of cell type diversity in the isolated protoplasts, we assumed that such a plating efficiency was a good indication that the overall process, including the preparation and formulation of the media, was well adapted for Arabidopsis protoplast survival and division. During the first week of culture, PdCs progressively formed microcolonies (Figure 1C).
If left in the 2,4-D–containing PIM medium, Arabidopsis colonies became necrotic. Since a low level of auxin is known to favor the growth of cell colonies (Caboche, 1980), the necrotic response that we observed might be due to an excess of 2,4-D or to the absence of its conjugation by indole-3-acetic acid–amido synthases (GH3). Thus, at day 11, we diluted the medium twofold with colony induction medium 1, which lacks auxin (see Supplemental Table 1 online) to decrease the auxin concentration and thus improve the viability of colonies and promote regeneration. One month later, we diluted the PdC suspension with medium enriched in nitrogen and containing TZ, but no auxin (2 mL suspension in 8 mL colony induction medium 2; see Supplemental Table 1 online), to ensure further growth of the microcalli (Figure 1D). Since we never observed bud formation in liquid medium, regardless of the phytohormones present, we transplanted colonies onto semi-solid medium for the subsequent regeneration steps. By comparing the effects of various cytokinins at different concentrations, we found that meta-topolin [6-(3-hydroxybenzylamino) purine] (Aremu et al., 2012) was the most effective at promoting regeneration. We therefore included this compound in the shoot induction medium (SIM; see Supplemental Table 1 online). Approximately 3% of Columbia-0 (Col-0) microcalli formed buds on the SIM medium, whereas up to 90% of Wassilewskija (Ws) calli differentiated buds (Figures 1E and 1F). After the buds were transplanted onto plant development medium (see Supplemental Table 1 online), young plantlets developed for both accessions, generating inflorescences (Figures 1F to 1H). Therefore, the Ws accession was better able to regenerate from protoplasts than was the Col-0 accession, confirming previous results obtained with root explants and leaf calli (Candela and Velazquez, 2001; Zhao et al., 2013). Thus, Ws and Col-0 protoplasts derived from various cell types of the aerial parts of plantlets were able to reenter the cell cycle and, ultimately, regenerate plants.
Experimental Transcript Profiling Scheme
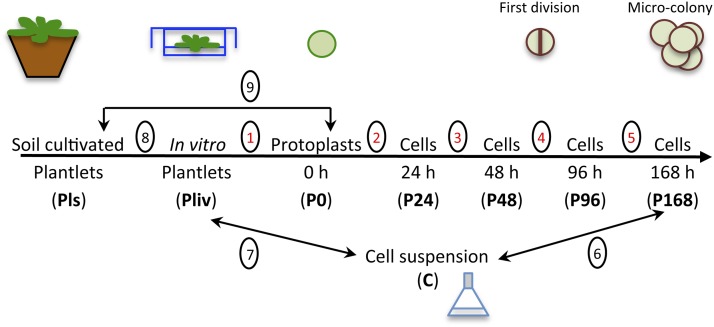
To investigate the early steps of regeneration (i.e., reentry into the cell cycle from differentiated cells), we compared the transcript profiles of 3-week-old plantlets grown in soil (Pls) or in vitro (Pliv), of freshly isolated protoplasts (P0), and of PdCs during the first week of culture (after 24, 48, 96, and 168 h of culture; hitherto referred to as P24, P48, P96, and P168). To assess the effect of prolonged in vitro culture, we also examined the transcript profile of well-established Arabidopsis cell suspensions (C) (Figure 2). We used Complete Arabidopsis Transcriptome MicroArrays (CATMA v2.1) for this study (Crowe et al., 2003; Hilson et al., 2004). Because Col-0 and Ws protoplasts underwent similar changes during the first week of culture (i.e., they both reentered the cell cycle and formed microcolonies) and because CATMA microarrays are based on the Col-0 genome, we performed our transcript analyses using Col-0 material. Massive (8984) transcriptomic changes took place during the first week of protoplast culture, corresponding to 5276 different genes being differentially expressed (Table 1). There was a remarkable balance between the number of genes that were up- and downregulated in each comparison, except that there was a bias toward downregulation in the Pliv/Pls comparison (Table 1).
Figure 2.
Transcript Profiling Experimental Design.
The transcript profiles of eight different samples were compared, and the first five comparisons used in the hierarchical clustering analysis are numbered in red.
Table 1. The Nine Transcript Profile Comparisons and the Number of DEGs for Each Comparison.
| Transcriptomes |
All Deregulated Genes | Genes Upregulated | Genes Downregulated | |
|---|---|---|---|---|
| 1 | P0/Pliv | 3507 | 1728 | 1779 |
| 2 | P24/P0 | 2635 | 1284 | 1351 |
| 3 | P48/P24 | 538 | 308 | 230 |
| 4 | P96/P48 | 1209 | 648 | 561 |
| 5 | P168/P96 | 1095 | 541 | 554 |
| 6 | C/P168 | 2796 | 1378 | 1418 |
| 7 | C/Pliv | 4187 | 2182 | 2005 |
| 8 | Pliv/Pls | 355 | 30 | 325 |
| 9 | P0/Pls | 4661 | 2273 | 2388 |
These data are presented in a searchable format to facilitate analysis and sorting of profiles and information (see Supplemental Data Set 1 online). Supplemental Data Set 1 online includes a user-friendly spreadsheet (gene selector) that was designed with advanced Excel functions to extract expression profiles using lists of genes sorted by AGI annotations. The expression of a small subset of genes was monitored by quantitative RT-PCR to verify our CATMA data (see Supplemental Table 2 online).
Transcriptome Comparison between Plants Grown in Soil and in Vitro
To identify changes induced by in vitro culture itself, we used the same growth conditions (i.e., photoperiod, light intensity, and nutrition) for plantlets grown in soil and in vitro. As expected, few differences were detected in the transcriptomes of plants grown in vitro and those grown in soil (Pliv/Pls; Table 1). The bias between the number of up- and downregulated genes in this comparison prompted us to analyze the Gene Ontology (GO) annotations of the 325 downregulated genes using the Bio-Array Resource for Plant Functional Genomics (BAR) classification superviewer program. The analysis revealed a strong enrichment for genes involved in electron transport or energy pathways and in the response to abiotic or biotic stimuli (3.27 and 3.23 normed frequencies, respectively) and in genes encoding plastid components (4.23 normed frequency). The Pliv/Pls transcript profile comparison reflected adaptations of the photosynthetic apparatus, cell walls, and chromatin composition in plantlets cultured in vitro. Among the downregulated genes, we identified genes encoding two CELLULOSE SYNTHASE genes (CESA1 and CESA3), one chitinase (AT2G43620), the fasciclin-like protein FLA9, a pectinacetylesterase (AT2G46930), and the HTR12 centromeric histone. Among the 30 upregulated genes, we noticed an enrichment in genes involved in other biological processes and in the response to stress (3.52 and 2.99 normed BAR frequencies, respectively) and in several transcription regulators (i.e., LATE EMBRYOGENESIS ABUNDANT3 and MULTIPROTEIN BRIDGING FACTOR1C), which may be interesting candidates for genes involved in the adaptation to in vitro culture conditions (see Supplemental Data Set 1 online, column AM). Thus, our data highlighted a small set of candidate genes likely to be involved in in vitro adaptation.
Eight Gene Clusters Reflect the Transition from in Vitro–Grown Plantlets to 1-Week-Old PdCs
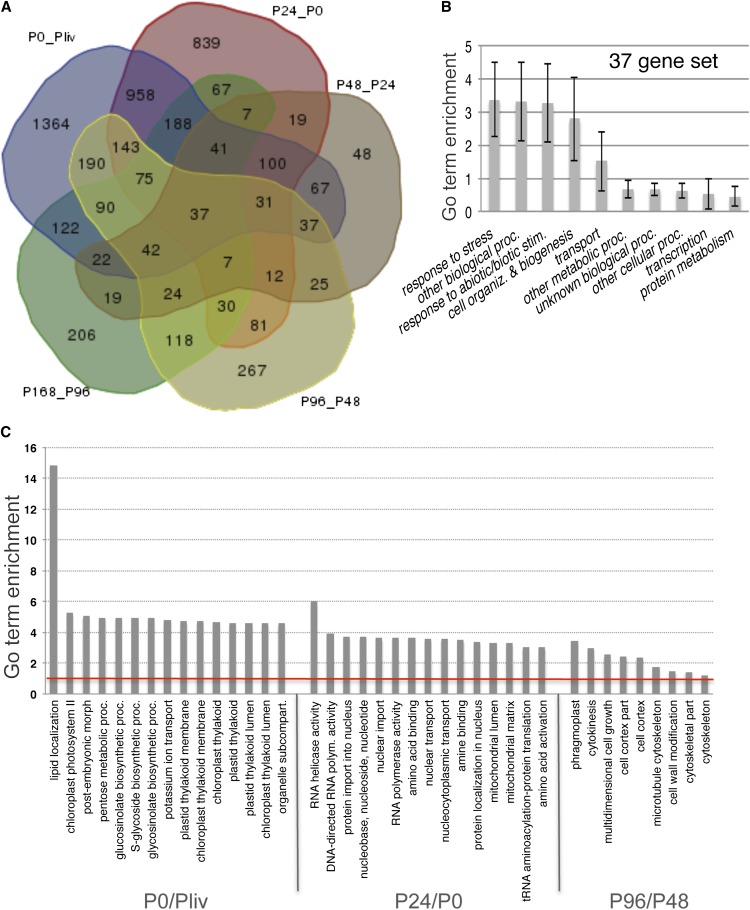
Of the five successive transcriptome variations present from in vitro–grown plantlets to and P168, we identified 8984 variations in transcription, concerning 5276 differentially expressed genes (DEGs; Table 1, Figure 3A), with complex profiles (this set of 5276 DEGs was annotated as DE5). The largest number of DEGs was identified in fresh protoplasts (P0/Pliv: 3507) and at P24 (P24/P0: 2635), suggesting that most dedifferentiation and reprogramming events occur within the first day of culture. Surprisingly, the first five sets of DEGs shared a common set of 37 genes that was enriched in response to stress, suggesting that a permanent, but partial, wounding state is maintained throughout the process (Figure 3B). In parallel with the activation of the basal stress response, dramatic global changes occurred in the expression of genes involved in various processes, which we detected in an analysis of enriched GO terms using the agriGO toolkit. For instance, DE5 genes involved in lipid transfer and in the synthesis of the photosynthesis apparatus were downregulated in freshly isolated protoplasts, those involved in nuclear and RNA processes were reactivated from 24 h (P24), and those that activate cell division were mostly upregulated at 96 h (P96; Figure 3C).
Figure 3.
Genes Differentially Expressed during the First Week of Culture.
(A) Venn diagram representing the five first transcriptome comparisons (i.e., from in vitro plantlets to P168).
(B) GO term enrichment analysis of the set of 37 genes present in all of the first five conditions using the BAR program. The bootstrap sd was calculated using the BAR SuperViewer tool (http://bar.utoronto.ca/ntools/cgi-bin/ntools_classification_superviewer.cgi).
(C) GO term enrichment analysis of P0/Pliv, P24/P0, and P96/P48 using the agriGO tool.
The top 15 GO terms from the analysis are presented for the first two conditions (PO/Pliv and P24/P0), and GO terms with a ratio of >1 are shown for the third condition (P96/P48). P value < 0.001.
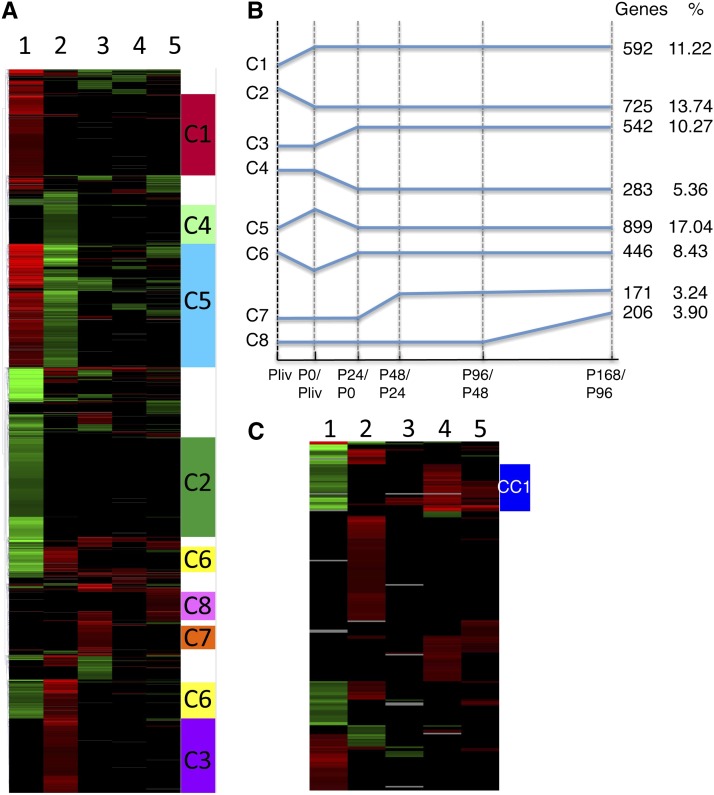
Clustering analysis of the 5276 DEGs using the Multiexperiment Viewer tool revealed eight main clusters (C1-8; Figure 4A; see Supplemental Figure 1 online) comprising 3873 genes (73%). The entire list of genes in each cluster is presented in Supplemental Data Set 1 online (columns AR-AY), and a diagram representing the changes in transcript levels over time is shown in Figure 4B. The expression of most genes (C1-6) changed from 0 to 24 h, suggesting that the major events underlying changes in cell fate occur in the 0 to 24 h developmental window. Interestingly, at least 592 of the genes that were upregulated in freshly isolated protoplasts remained upregulated throughout the entire culture period (C1; Figure 4B).
Figure 4.
Clustering Analysis of DEGs during the Transition from in Vitro–Grown Plantlets to 1-Week-Old PdCs.
(A) Hierarchical clustering using Multiexperiment Viewer of the transcript profiles from in vitro–grown Arabidopsis plantlets to P168. The 3864 genes (73% of DEGs in the DE5 set) were distributed into eight main clusters (see Supplemental Data Set 1 online).
(B) Schematic representation of the eight clusters. The number of genes in each cluster and the percentage of DEGs (out of 5276 total) represented in each cluster are given.
(C) Cell cycle and cell division DEGs in Arabidopsis protoplasts during culture. Hierarchical clustering highlighting a cell cycle–specific cluster (CC1, in blue). The genes used are listed in Supplemental Data Set 1 online.
To better characterize the clusters, we performed GO analysis (Table 2; see Supplemental Table 3 online). This approach highlighted functional specificities for each cluster according to the main GOs present in the clusters and a progression during protoplast culture from stress response to reprogramming processes. The C1 and C2 clusters mainly contained genes involved in stress responses, whose expression seems to be required during the week of culture. However, whereas C1 also contained upregulated genes related to electron transport and the energy pathway, C2 included downregulated genes involved in cell organization, photosynthesis, and biogenesis. In the C3 cluster, various genes involved in DNA and RNA metabolism pathways were upregulated, as were genes related to electron transport and the energy pathway, suggesting the reorientation of cellular fate. In parallel, genes associated with transport and communication were downregulated (C4). Interestingly, a set of genes associated with stress responses were transiently up- and downregulated (C5), suggesting that they are related to protoplasting reactions. The C7 and C8 clusters contained genes required for DNA or RNA metabolism, signal transduction, or developmental processes. Interestingly, the postembryonic development GO annotation (GO:0009791) was significantly represented in clusters C2, C3, and C8, suggesting that three specific and distinct sets of genes involved in this process were successively downregulated at 0 h and then progressively activated at 24 h and 96 h.
Table 2. GO Analysis of the Eight Main Clusters of DEGs.
| Cluster | GO Biological Process (BAR Analysis) | GO Molecular Function | GO Cellular Comp. |
GO Biological Process (AgriGO Analysis) |
FDR | |
|---|---|---|---|---|---|---|
| C1 | Electron transport or energy pathways | Kinase activity | Cytosol | GO:0050896 | Response to stimulus | 4.4e-06 |
| Response to abiotic or biotic stimulus | Nucleotide binding | ER | GO:0042221 | Response to chemical stimulus | 4.4e-06 | |
| GO:0044248 | Cellular catabolic process | 9.4e-05 | ||||
| GO:0044265 | Cellular macromolecule catabolic proc. | 0.0006 | ||||
| C2 | Electron transport or energy pathways | Other enzyme activity | Plastid | GO:0010876 | Lipid localization | 0.00031 |
| Cell organization and biogenesis | Other molecular functions | Cell wall | GO:0009791 | Postembryonic development | 0.00035 | |
| GO:0015979 | Photosynthesis | 0.007 | ||||
| GO:0016144 | S-glycoside biosynthetic process | 0.027 | ||||
| C3 | DNA or RNA metabolism | Structural molecule activity | Cytosol | GO:0006396 | RNA processing | 2.7e-07 |
| Electron transport or energy pathways | Nucleotide binding | Ribosome | GO:0016070 | RNA metabolic process | 3.4e-07 | |
| GO:0006139 | Nucleobase, nucleoside, nucleotide, and nucleic acid metabolic proc. | 3.4e-07 | ||||
| GO:0009791 | Postembryonic development | 6.1e-07 | ||||
| C4 | Transport | Receptor binding or activity | ER | NS | ||
| Other biological processes | Transporter activity | Plastid | NS | |||
| C5 | Response to stress | Receptor binding or activity | Cytosol | GO:0006950 | Response to stress | 7.2e-22 |
| Response to abiotic or biotic stimulus | Other enzyme activity | Other cytoplasmic comp. | GO:0050896 | Response to stimulus | 1.0e-17 | |
| GO:0042221 | Response to chemical stimulus | 1.0e-17 | ||||
| GO:0010033 | Response to organic substance | 1.5e-12 | ||||
| C6 | Protein metabolism | Structural molecule activity | Ribosome | GO:0006412 | Translation | 3.2e-62 |
| Electron transport or energy pathways | DNA or RNA binding | Cytosol | GO:0044267 | Cellular protein metabolic proc. | 7.3e-35 | |
| GO:0019538 | Protein metabolic process | 4.7e-30 | ||||
| GO:0009058 | Biosynthetic process | 8.4e-30 | ||||
| C7 | DNA or RNA metabolism | Other molecular functions | Golgi apparatus | NS | ||
| Signal transduction | Kinase activity | Cytosol | NS | |||
| C8 | Other biological processes | Kinase activity | Cell wall | |||
| Developmental processes | Hydrolase activity | Plasma membrane | GO:0009791 | Postembryonic development | 0.029 | |
ER, endoplasmic reticulum; FDR, false discovery rate; NS, no significant enrichment in a GO could be identified.
The C5 and C6 clusters presented a rapid reversal of expression profiles between protoplasts and P24 and contained the largest set of DEGs (1345). In a study of the Arabidopsis apical meristem, 300 genes were identified as responding to protoplasting treatment (Yadav et al., 2009). This discrepancy may be due to differences in maceration times and starting material in the previous study and ours, which identified a larger set of genes corresponding to the C5 and C6 clusters. Furthermore, the C5 and C6 clusters together contained fewer than the 6323 genes identified as being involved in natural leaf senescence (Breeze et al., 2011). This observation indicates that the dedifferentiation process in protoplasts differs from that in senescence. Overall, cross-analysis of stress genes (GO:0006950) and DEGs in senescence (Breeze et al., 2011) revealed that the 5276 DE5 genes during the whole process had 2358 genes in common with senescence and 1313 in common with the stress response (see Supplemental Figure 2 online). The largest number of genes related to the stress response were found in cluster C5, partially confirming that C5 is associated with mostly reversible stress reactions that occur during protoplasting.
A comparison of our data set with transcriptome data of Arabidopsis protoplasts freshly isolated from Landsberg erecta plantlets (Damri et al., 2009; Grafi et al., 2011) or from meristematic tissues (Yadav et al., 2009) revealed a set of common deregulated genes that might be markers of the protoplasting process. Among the 576 transcription factors (TFs) identified by Grafi et al. (2011) and Damri et al. (2009), 179 TFs were deregulated in our experiments, and 90 out of the 261 genes identified as being deregulated upon exposure of meristematic tissues to protoplasting conditions (Yadav et al., 2009) were also present in our list of DE5 genes (see Supplemental Table 4, Supplemental Figure 3, and Supplemental References 1 online). Six TFs and two heat shock factors were found to be deregulated in all three studies (see Supplemental Table 4 online). A few DE5 genes identified in our study were also among the list of genes associated with meristematic activities (Yadav et al., 2009), and most of these were downregulated (see Supplemental Table 5 online).
Previously, the transcriptomes of Physcomitrella patens protoplasts were established at 0, 24, 48, and 72 h after isolation (Xiao et al., 2012). Among the DE5 set of genes identified in our study, 73 and 39 genes were similarly deregulated in moss at the P0/P24 and P24/P48 transitions, respectively. The small number of genes in common may result from the evolutionary distance between the two species and from differences in culture conditions. For instance, the moss protoplasts were cultured in the absence of exogenously added growth substance and under different photoperiod conditions. Most of these DEGs encode proteins involved in RNA metabolism, protein translation (ribosomal protein genes), and in the synthesis of the photosynthesis apparatus. Arabidopsis transcription regulators (e.g., ERF1, BZIP63, TIFY10B, and NFXL1) and their moss homologs that were deregulated in both of these species (see Supplemental Table 6 online) appeared mostly in C5. Since genes present in C5 were transiently deregulated, these DEGs that are common to Arabidopsis and moss represent protoplast markers of cell reprogramming that are independent of protoplasting conditions and species.
Responses of Freshly Isolated Protoplasts
In freshly isolated protoplasts, we detected changes in metabolism that resembled those linked to senescence. For instance, antioxidant pathways were stimulated and various organic metabolites and macromolecules were remobilized through the action of hydrolases (e.g., lipases, proteases, and cellulolytic enzymes) and the proteasome pathway. However, only 2358 of the 6326 genes deregulated during natural senescence (Breeze et al., 2011) were also among the 5276 DE5 genes (see Supplemental Figure 2 online). In protoplasts, this remobilization pathway is activated dramatically faster than in a senescing leaf, where the process lasts for over 20 d (Breeze et al., 2011). However, PdCs could continue to dedifferentiate while already engaged in division, as ∼50% of genes deregulated at each time point were also deregulated during senescence. Noticeably, only 10% of the genes that were stably activated from 24 h (C3) were involved in senescence, a first indication that most C3 genes may have specific roles in reprogramming.
The main difference between senescence and dedifferentiation was the rapidity with which genes involved in photosynthesis were downregulated in protoplasts. Whereas the downregulation of these genes occurred mostly within 24 h in protoplasts, it occurred later in the senescence process (from day 31 after germination; Breeze et al., 2011). A strong response to dehydration was observed in the freshly isolated protoplasts, as exemplified by the net activation of EARLY RESPONSIVE TO DEHYDRATION1, which promotes protein hydrolysis in chloroplasts (Nakashima et al., 1997). The chlorophyll synthase CHLG and most of the genes encoding chlorophyll a/b binding proteins and proteins of the photosystems were also downregulated during protoplast isolation. The GLK2 TF, which coordinates photosynthesis; HEMA1, the glutamyl-tRNA reductase gene involved in tetrapyrrole synthesis; and genes that regulate the stability of chloroplast-encoded transcripts (HCF152 and 173) were downregulated in protoplasts (see Supplemental Data Set 1 online). However, chloroplast degradation was not complete in protoplasts, and some of their essential cellular roles were preserved.
Although six senescence-associated genes (SAG1, 3, 13, 20, 24, and 29) were strongly upregulated in P0, SAG12, which encodes a Cys protease active in chloroplast degradation during late leaf senescence, was not activated in protoplasts. The upregulation of SPERMINE SYNTHASE in P0 could be related to the response to oxidative stress, as the homolog in mouse (Mus musculus) was found to be involved in this process (Eisenberg et al., 2009).
Concurrent with metabolic shifts occurring in the protoplasts, the cells exhibited a hypoxic stress response, as indicated by the activation of key genes involved in glycolysis (e.g., phosphofructokinases [PFK3 and PFK7] and pyruvate kinase). The upregulation of a hemoglobin gene (GLB3) and of genes encoding alcohol dehydrogenases (ADH and ATA1) was also indicative of hypoxia, which represented an additional stress to protoplasts. Furthermore, the stable upregulation of the hypoxia responsive gene (AT5G27760) revealed that hypoxia persisted throughout culture in Petri dishes. Hypoxic conditions probably developed because the cultures were grown without agitation, and protoplasts tend to sink despite the small volume of liquid medium used in our study (depth of medium ∼1.5 mm of liquid in a Petri dish). Similarly, out of the 1728 genes activated in P0 (Table 1), 592 genes (C1; Figure 4B) were permanently activated during the first week of culture, among which 168 were stress-related genes. These observations confirmed that PdCs maintain a permanent but partial wounding state.
Interestingly, two phytosulfokine genes (PSK2 and PSK4) encoding intercellular signal peptides involved in cell proliferation and a receptor kinase gene (PSKR1) (Matsubayashi et al., 2002) were upregulated in P0 (see Supplemental Data Set 1 online). At the onset of culture, the composition of the medium changed rapidly due to exchanges between cells and the medium and the transmission of signals, such as phytosulfokines, between cells. Thus, an analysis of the extracellular proteins and receptors involved in protoplast preparation and culture may shed light on the conditioning effect that cells have on the medium, which results in a certain starting cell density (8 × 104 per mL in our case) having optimal rates of plant cell division in vitro.
Therefore, even in our moderate maceration conditions, the response to injury was part of a fast and specific dedifferentiation process that yielded a fairly homogeneous population of cells by coordinating various regulatory pathways that led to cell division competence. The homogeneous and high rate of protoplast division in response to auxin and cytokinin confirmed that most protoplasts reached a similar cell state.
Protein Biosynthesis Reorientation during Protoplast Culture
As key components of the protein biosynthesis machinery, ribosomes play crucial roles in regulating growth. The expression of ribosomal protein genes (RPs) is tightly developmentally and environmentally regulated (Chantha et al., 2007). Using an RP gene list (Barakat et al., 2001), we observed major changes in the transcript abundance of 130 genes encoding RPs, corresponding to ∼60% of the cytoplasmic RP genes present in the CATMA microarray (total 220 genes on the microarray; Table 3; see Supplemental Data Set 1 online, columns BQ and BR). Most RP DEGs clustered into C6 (Table 3). Among the RP genes upregulated at 24 h, a small fraction was downregulated later during the protoplast culture. In total, 26 out of the 32 RP genes of the small subunit family (RPS) present in the Arabidopsis genome were affected, whereas only 25 of the 48 RP large subunit (RPL) family members present were affected, suggesting that ribosome composition is predominantly regulated by modulating RPS composition. Interestingly, two homologs of the Drosophila melanogaster NOTCHLESS gene involved in cell fate decision and developmental processes through the Notch pathway and ribosomal biogenesis were also upregulated from 24 h (NLE and NLE1). NOTCHLESS plant homologs have been shown to regulate cell growth and proliferation (Chantha et al., 2006). Another gene that regulates ribosomal biogenesis (RRS1) also clustered in C3. NUCLEOLIN genes (NUC-L1 and NUC-L2) involved in pre-rRNA processing (Pontvianne et al., 2010) were also upregulated at 24 h. Therefore, protein biosynthesis resumed rapidly during protoplast culture by the direct modulation of RP transcription and of several nonribosomal regulatory factors. These data suggest that four combinatorial sets of RPs (C2, C3, C5, and C6; Table 3) are important throughout the process and that the ribosomal equipment needs to be precisely regulated during reentry into the cell cycle. Further investigation of the expression of ribosome transacting factors, such as SWA1 (Shi et al., 2005) (absent from the CATMA microarray), that regulate division would facilitate the characterization of RP regulation in our system.
Table 3. Distribution of Ribosomal Protein Genes in the Eight Clusters of DEGs Identified in Arabidopsis Protoplasts.
| Cluster | RP Number | 1 | 2 | 3 | 4 | 5 | AGI Identifier/Name |
|---|---|---|---|---|---|---|---|
| C1 | 0 | Up | nc | nc | nc | nc | |
| C2 | 6 | Dn | nc | nc | nc | nc | RPL22A, RPP2C, RPS15aE, RPS15F, RPS17C, RPS19B |
| C3 | 6 | nc | Up | nc | nc | nc | RPL10B, RPL39B, RPL7D, RPL9D, RPP0C, RPSaB |
| C4 | 0 | nc | Dn | nc | nc | nc | |
| C5 | 2 | Up | Dn | nc | nc | nc | RPL18aA, RPL10C (SAG24) |
| C6 | 101 | Dn | Up | nc | nc | nc | RPS18A (PFL1), (RPS13A (PFL2),(RPL24 (STV1), L10aP (PIGGYBACK1), RP1 (EMB2207), AT3G04400 (EMB2171), AT3G48930 (EMB1080), RPS6B (EMB3010), AT1G58380 (XW6) (see Supplemental Table 1 online, columns AW and BQ, for a complete list) |
| C7 | 0 | nc | nc | Up | Up | nc | |
| C8 | 0 | nc | nc | nc | nc | Up | |
| Others | 15 | nc | nc | nc | nc | nc | |
| Total DEGs | 130 | nc | nc | nc | nc | nc |
Up, upregulation; Dn, downregulation; PFL, pointed first leaf; RPL, RP large subunit; RPS, RP small subunit; SAG, senescence-associated gene; STV, short valve; EMB, embryo defective; nc, no change in expression.
Regulation of protein translation also modulates the protein content of a cell during various adaptive responses, with the initiation of translation being the main target of such regulatory mechanisms. In our data set, four translation initiation factor genes were upregulated (C1 and C3; see Supplemental Data Set 1 online). Furthermore, RACK1A (for Receptor for Activated C Protein Kinase1), a component of the plant 40S ribosome subunit that regulates translation (Chang et al., 2002; Giavalisco et al., 2005; Guo et al., 2011), clustered in C6, and accordingly was also upregulated at 24 h. Another means of regulating protein composition during protoplast culture is protein degradation. Indeed, genes encoding 26S/ubiquitin were sequentially activated and repressed during the transition from plants to protoplasts and then between different time points of the protoplast culture. These drastic events affecting protein metabolism represent indicators of dedifferentiation and reprogramming during the first 24 h of culture.
Progression into the Cell Cycle
Since auxin and cytokinin are key regulators of plant cell division, we investigated the expression of genes involved in their biosynthesis and regulation. In the cytokinin regulatory pathway, only a few genes were deregulated: IPT8, which is involved in cytokinin synthesis, three response regulators (ARR4, ARR6, and ARR7), and two cytokinin response factors (CFR1 and 3) were activated at 24 h. ARR16 was upregulated from 96 h onwards.
The expression profiles of auxin-related genes were more complex. In protoplasts, the auxin biosynthesis pathway was activated, as suggested by the upregulation of Trp synthases, nitrilases, and specialized cytochrome P450 genes. This early activation resulted from the wounding response of protoplasts, since most of these were deactivated later in the process (see Supplemental Table 7 online). In addition to the early indole-3-acetic acid burst, 2,4-D (i.e., synthetic auxin) in the culture medium may regulate the auxin biosynthesis pathway. Genes encoding conjugating enzymes (GH3-2 and GH3-3) were also activated early in the process, and their expression was maintained throughout the culture period. The auxin signaling pathway was also modified, with auxin efflux carrier genes being deregulated. Two main subsets of efflux carriers could be distinguished based on their expression profiles, suggesting different functions: One class (containing PBP1) was upregulated at P0 and downregulated later, the other (containing PIN1, PIN6, and MEE21) was upregulated after P0, at different time points. Interestingly, from 24 h onwards, two auxin signaling F-box (AFB) genes encoding auxin receptors, AFB2 and AFB5, were upregulated.
Noticeably, IAA14, ARF7, and ARF19, which are involved in the auxin pathway and are activated during lateral root initiation (Vanneste et al., 2005), were not deregulated, whereas IAA7, IAA8, IAA9, IAA20, IAA29, ARF4, ARF5, and ARF6 seemed to be involved in the early steps of protoplast-based regeneration (see Supplemental Table 7 online). The array of genes deregulated in protoplasts was also different from that deregulated during leaf callus initiation (He et al., 2012). The calossin-like BIG gene, which participates in the vesicular targeting of auxin transporters and is required for pericycle cell activation in lateral root primordia (Gil et al., 2001; López-Bucio et al., 2005), was upregulated, as were other genes known to be associated with the pericycle (Parizot et al., 2010) (see Supplemental Table 7 online). These data suggest that a specific auxin-mediated pathway is activated during protoplast culture.
We established a list of 384 genes related to the cell division cycle and cytokinesis based on GO annotations (GO:0007049, GO:0051301, GO:0000910, and GO:0000280) and on reports in the literature (Gutierrez, 2009) (see Supplemental Data Set 1 online, columns BE-BJ tagged as cell cycle). Among them, 373 genes were deregulated in the DE5 set, and a specific cell cycle cluster (CC1) of 31 genes expressed in plantlets was downregulated in the transition to protoplasts and then reactivated at 96 h. The deregulation of genes in CC1 suggests a reversion to a pluricellular organization and cell division control in the microcolonies (Figure 4C).
During the first week of culture, a wave of cell cycle–related gene activation was observed, concomitant with resumption of cell division, which peaked at 96 h and was followed by the formation of microcolonies (see Supplemental Figure 4 online). Downregulation of cell cycle–related genes was largely limited to the protoplasting step (see Supplemental Figure 4 online). Our data highlight a specific role for two cyclin-dependent kinase inhibitor genes (KPR1 and KRP6) in cell cycle arrest during protoplast isolation. At P24, CUL1 and AXR1, which regulate protein degradation activity, may promote cell cycle progression by degrading KRPs. Furthermore, the activation of CYCH1-1, which has a role in cyclin-dependent kinase–mediated activation, and of REPLICATION PROTEIN A1 seems to mark entry into the S phase during early protoplast development.
Between 48 and 96 h, PCNA1 and PCNA2, two key factors of the DNA replication machinery, were upregulated, along with DNA replicating factors (MCM3, 4, and 10). At 96 h, CYCA1-1 and CYCA3-2, which are specific to the G2 phase, were upregulated, along with CYCB1;4 and CDKB2;1 and two AURORA genes (AUR1 and AUR2) (Demidov et al., 2005). These last four genes were not clustered because they were also upregulated at 168 h, thus confirming that a first round of mitosis occurred at around 96 h of culture for most PdCs. At 168 h, CDKB2;1, CYCB1;4, CYCB2;2, and CYC3B, which are also involved in the G2/M transition and mitosis (Dewitte and Murray, 2003), along with TANGLED, which plays a role in cytokinesis and phragmoplast guidance (Walker et al., 2007), were reactivated, marking active cell divisions and the formation of microcolonies. POLTERGEIST (POL), which acts downstream of the CLAVATA signaling pathway in meristem development and is required for stem cell maintenance, was also reactivated in the microcolony stage, which may suggest the onset of functional cellular organization and the formation of cellular mass. In roots, cell divisions are arrested by KRP2 and stimulated by auxin signals and specific cyclins (CYCD3;2, CYCA2;4, and CYCB2;5) (Vanneste et al., 2005). Thus, protoplast culture seems to require a different and specific set of cell cycle–related genes. The protoplast cell cycle progression gene module sequentially activates KRP1/KRP6/CDKC1, CYCH1, CYCA3;2, CYCA1/SIM/PCNA, and CYCB/CDKB2;1/POL. Until now, CDKCs were thought to regulate transcription without directly regulating the cell cycle (Cui et al., 2007). The activation of CDKC1 after 24 h of culture suggests that this protein has a specific role in reactivating protoplast division.
Cell Wall Reestablishment during the First Week of Culture
Cell wall reorganization is essential for protoplast survival and adaptation to the external medium. This complex process is regulated by numerous enzymes and relies on interactions with the cytoskeleton, which determines microfibril orientation (Szymanski and Cosgrove, 2009) and facilitates the extracellular delivery of polysaccharide precursors via Golgi-derived vesicle trafficking (Parsons et al., 2012). As the complete set of genes involved in the elaboration of the cell wall has not yet been established, we selected 285 genes involved in cell wall synthesis based on GO annotations (GO:0071554 and GO:0009664) and the literature (see Supplemental Data Set 1 online, columns BA to BD).
Briefly, clustering analysis of 217 cell wall–tagged genes, such as those encoding pectin lyases and plant invertases, revealed that 42% of the genes were excluded from the eight main clusters identified in this study and showed complex profiles (see Supplemental Data Set 1 online). Most of these genes (112 out of 217) were downregulated in fresh protoplasts and progressively reactivated during culture with kinetics specific for each gene. Two closely related EXPANSIN genes (Kende et al., 2004), EXPAI and EXPA10, were strongly upregulated from 24 h onwards, and six others were upregulated later during culture. This could indicate additional functions for this protein family besides roles in cell wall loosening and abscission (Sampedro and Cosgrove, 2005). A large group of cell wall–related genes was upregulated from 96 h of culture onwards, concomitant to the reorganization of the cytoskeleton and phragmoplast after the first round of PdC division. Consistent with this finding, CSLC04 and CSLC06, two Golgi-located glucan synthases (Parsons et al., 2012), were activated from 96 h onwards.
Rather surprisingly, only two cellulose synthase genes, CESA1 and CESA3, were upregulated early and late during the culture period. This suggests that cellulose synthesis was regulated at the posttranscriptional level in protoplasts. Alternatively, cellulose organization may be a continuous process. This possibility is supported by the almost immediate reappearance of microfibrils in freshly isolated and washed protoplasts (Kwon et al., 2005). Our profiling could provide additional data for deciphering this complex cell wall rebuilding process and the mechanisms that regulate it (Kwon et al., 2005; Yang et al., 2008).
Putative Roles of Organelles in the Reentry into the Cell Cycle
Many nuclear genes encoding proteins targeted to mitochondria and chloroplasts were deregulated in our study; thus, organelles appear to have an important role in the early changes in cell machinery and possibly also in the dedifferentiation process. Nuclear genes that regulate chloroplast division, which precedes cell division, were upregulated by as early as 24 h (i.e., FtsZ, a DNAJ gene, and ARC6, which encodes a factor promoting the plastid-dividing FtsZ ring). Surprisingly, though protoplasts were cultured in the dark, a small number of photosynthetic genes were also activated (i.e., four magnesium chelatase genes and two chlorophyll a/b binding genes). Pentatricopeptide repeat (PPR) proteins are involved in many aspects of RNA processing in organelles. Mutations in PPR genes generally have pronounced effects, and most are embryo lethal (Schmitz-Linneweber and Small, 2008). PPR proteins form one of the largest families in the Arabidopsis genome, with 450 members (Lurin et al., 2004; Fujii and Small, 2011). Twenty PPR genes were deregulated in our study, five of which were specifically activated in C3 and one of which was downregulated in Pliv/Pls.
WHIRLY2, encoding a DNA binding protein involved in gene regulation and essential for proper mitochondrial function (Maréchal et al., 2008), was activated from 24 h onwards. Posttranscriptional control is important for the proper regulation of mitochondrial gene expression, since mitochondrial genes are generally not regulated at the transcriptional level (Holec et al., 2006). The mitochondrial exoribonuclease, which belongs to the polynucleotide phosphorylase family (AT5G14580) and is involved in mRNA metabolism, was also strongly activated from 24 h. This gene product is vital, as downregulation of the corresponding gene causes unprocessed RNAs to accumulate (Perrin et al., 2004). The importance of organelle RNA metabolism in PdCs is reminiscent of the early events in mitochondrial biogenesis during germination (Law et al., 2012).
Interestingly, three prohibitin genes (PROHIBITIN1, 3, and 6) were activated from 24 h onwards. PROHIBITIN proteins play crucial stress protective roles in mitochondria, but also regulate cell proliferation (Merkwirth and Langer, 2009), and as such are needed for planarian regeneration (Reddien et al., 2005). Genes involved in metabolism were also specifically activated early in the process; for example, GOGAT2, a chloroplast gene essential for amino acid biosynthesis, and two genes encoding mitochondrial ATP synthase subunits were upregulated at 24 h.
Progression of Transcriptional Control
Based on knowledge of the role of TFs in animal stem cells (Takahashi and Yamanaka, 2006), we analyzed TF expression in the protoplast cultures. We extracted lists of TFs and their family annotations from previous reviews (Mitsuda and Ohme-Takagi, 2009; Lu et al., 2012). Of the 5276 DEGs in protoplast culture, 500 TFs were deregulated and assigned to different clusters (see Supplemental Data Set 1 online, columns C to J), a high number (193) of which were deregulated in freshly isolated protoplasts (P0). Our data provide an interesting catalog of putative crucial regulators to be tested for functional roles in cell reprogramming. The 61 TFs stably deregulated in C1 and the 41 TFs in C3 may be essential regulators of cell reprogramming. Most TF families were represented. Interestingly, two families related to the stress response, HFS and TIFY (Vanholme et al., 2007), were only present in C5 (see Supplemental Table 8 online).
The expression profiles of a selection of key genes associated with meristem activity or described as being involved in stem cell maintenance (Yadav et al., 2009; Aichinger et al., 2012) were analyzed (see Supplemental Table 9 online). Among them, we identified WOUND INDUCED DEDIFFERENTIATION1, which promotes cell dedifferentiation in Arabidopsis (Iwase et al., 2011). Members of the Wuschel-related homeobox (WOX) gene family, which are key genes in cell division and prevent premature differentiation (van der Graaff et al., 2009), and of the GRAS gene family (SHR and SCARECROW), known to be required for the specification and maintenance of the root stem cell niche, were also upregulated. We further compared the DEGs in protoplasts with genes involved in lateral root initiation (Parizot et al., 2010) (see Supplemental Data Set 1, columns BK to BP, and Supplemental Table 10 online). We identified 18 TFs that were deregulated in both our data set and during lateral root initiation, suggesting that there is a partial overlap between these two processes. Among them, IAA19 and an ERF member (CRF3) were activated early in protoplasts. Surprisingly, most of the genes were known to be associated with wounding and stress responses. The finding that their expression is maintained during PdC culture might indicate that they have adjacent roles in promoting competence for reentry into the cell cycle and dedifferentiation.
To narrow down the list of key candidate genes in the process, we searched for TF targets of Polycomb proteins and especially of LHP1, a subunit of PRC1 (Zhang et al., 2007; Latrasse et al., 2011). Polycomb proteins are key developmental regulators that repress major developmental genes. We identified 63 TF targets of LHP1, some of which are activated at specific time points and may be key regulators (see Supplemental Table 11 online).
Epigenetic Landscapes of Protoplasts and PdCs in Culture
Since nucleosomes, as substrates for epigenetic modifications and remodelling, are at the heart of gene expression regulation, we analyzed the expression of histone genes during protoplast culture (Talbert et al., 2012) (Table 4). Interestingly, we distinguished sets of histone genes with similar expression. Group A contains genes downregulated at the protoplast stage and activated during the culture with different kinetics. Surprisingly, six H4 genes were expressed during protoplast culture (Table 4). The switch to protoplasts is associated with the transient upregulation of an H3.3 variant encoded by HTR8. These data suggest that protoplast and PdC chromatin have specific properties due to the incorporation of distinct sets of histone variants in nucleosomes.
Table 4. Transcript Profiles of Histone Genes.
| AGI | ChromDB | Protein | P0/Pliv | P24/P0 | P48/P24 | P96/P48 | P168/P96 | C/P168 | C/Pliv | Pliv/Pls | P0/Pls | Group |
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 1 | 2 | 3 | 4 | 5 | 6 | 7 | 8 | 9 | ||||
| AT5G27670 | HTA7 | Histone H2A.W.7 | nc | nc | nc | nc | 0.76 | nc | nc | nc | −0.90 | A |
| AT5G59870 | HTA6 | Histone H2A.W.6 | −3.53 | nc | 1.44 | 1.52 | 0.69 | 0,71 | nc | nc | −3.49 | A |
| AT5G22880 | HTB2 | Histone HB2.2 | −1.52 | nc | 0.72 | 0.93 | nc | 2.51 | 2.17 | nc | −1.79 | A |
| AT5G10390 | HTR13 | Histone H3.1 | −2.19 | nc | 0.88 | 1.31 | nc | 1.06 | 0.82 | nc | −2.91 | A |
| AT2G28740 | HFO3 | Histone H4 | −2.24 | nc | 1.18 | 1.34 | nc | 1.18 | 1.90 | nc | −1.91 | A |
| AT3G46320 | HFO1 | Histone H4 | −3.09 | −0.82 | 1.16 | 1.40 | nc | 2.55 | 1.69 | nc | −2.86 | A |
| AT5G59690 | HFO2 | Histone H4 | −2.88 | −0.62 | 0.96 | 1.24 | 0.53 | 1.26 | nc | nc | −2.98 | A |
| AT3G54560 | HTA11 | Histone H2A.Z.11 | −1.47 | nc | nc | 0.73 | nc | nc | nc | nc | −3.13 | A |
| AT3G45980 | HTB9 | Histone H2B.9 | −1.15 | −1.03 | nc | 0.80 | nc | 1,17 | nc | nc | −1.18 | A |
| AT1G07820 | HFO4 | Histone H4 | −1.85 | nc | nc | 0.72 | nc | 1.24 | 1.03 | nc | −1.88 | A |
| AT3G53730 | HFO5 | Histone H4 | nc | −1.29 | nc | 0.96 | nc | 1.95 | 1.71 | nc | nc | A |
| AT5G59970 | HFO6 | Histone H4 | −1.34 | nc | nc | 1.01 | nc | 1.27 | nc | nc | −1.76 | A |
| AT2G37470 | HTB5 | Histone H2B.5 | nc | −1.37 | nc | nc | nc | 1.11 | nc | nc | nc | B |
| AT2G28720 | HTB3 | Histone H2B.3 | nc | −1.61 | nc | nc | nc | 2.36 | 1.25 | nc | nc | B |
| AT2G38810 | HTA8 | Histone H2A.Z.8 | nc | nc | nc | nc | nc | 1.29 | 1.88 | nc | −1.39 | B |
| AT5G12910 | HTR15 | Histone H3.15 | −1.03 | nc | nc | nc | nc | 1.18 | 1.54 | nc | nc | B |
| AT1G52740 | HTA9 | Histone H2A.Z.9 | nc | nc | nc | nc | nc | 1.27 | nc | nc | nc | C |
| AT5G54640 | HTA1 | Histone H2A.1 | nc | nc | nc | nc | nc | 1.07 | 1.15 | nc | 0.61 | C |
| AT4G40030 | HTR4 | Histone H3.3 | nc | nc | nc | nc | nc | 1.70 | nc | nc | nc | C |
| AT4G40040 | HTR5 | Histone H3.3 | nc | nc | nc | nc | nc | 0.99 | 0.86 | nc | nc | C |
| AT5G10980 | HTR8 | Histone H3.3 | 1.14 | −0.87 | nc | nc | nc | 1.60 | 2.00 | nc | 1.05 | D |
| AT2G18050 | HON3 | Histone H1.3 | nc | nc | nc | nc | nc | nc | nc | nc | 2.23 | D |
| AT5G02560 | HTA12 | Histone H2A.W.12 | nc | nc | nc | nc | nc | −1.68 | −1.41 | nc | nc | E |
| AT1G07790 | HTB1 | Histone H2B.1 | −1.66 | nc | nc | nc | nc | nc | nc | nc | −1.79 | E |
Values in bold, upregulated genes; values in italics, downregulated genes; nc, no change in expression.
To characterize the chromatin states of protoplasts and PdCs, we analyzed the ChromDB annotated loci in our data set (see Supplemental Data Set 1, columns K to M, and Supplemental Tables 12 and 13 online). Among them, 108 genes were deregulated in the first five profiles, with major downregulation in protoplasts and a global upregulation at 24 h. The genes were distributed into the eight clusters, C3 being the most abundant, with 25 genes. The genes found in the different clusters encoded proteins involved in histone modification, DNA methylation, and chromatin remodelling (Table 5). Thus, early regulation of the epigenome, specifically through the activation of C3 genes, seems to play an important role in the overall reprogramming of plant cells. It is worth noting the striking similarity with the epigenome plasticity in animal stem cells (Barerro and Izpisua Belmonte, 2011).
Table 5. DEGs of the Eight Main Clusters Involved in Chromatin Regulation.
| Cluster | AGI | Selection | ChromDB ID/Formal Name |
|---|---|---|---|
| C1 | AT3G51000 | α/β-Hydrolases superfamily | ABHF10 |
| AT5G64630 | Nucleosome/chromatin assembly complex | NFB1/FAS2 | |
| AT1G08620 | Jumonji domain group | JMJ16 | |
| C2 | AT4G37470 | α/β-Hydrolases superfamily protein | ABHF2 |
| AT5G49160 | DNA methyltransferase | DMT1/DDM2/MET1 | |
| AT3G15790 | Methyl binding domain protein | MBD11 | |
| AT5G41070 | Double stranded RNA binding protein group | DRB5 | |
| AT5G63670 | Transcription elongation-nucleosome displacement protein | GTG1 | |
| AT1G14900 | HMG group family | HMGA3 | |
| AT1G76110 | HMG group family | HMGB9 | |
| C3 | AT3G03990 | α/β-Hydrolases superfamily protein | ABHF1 |
| AT2G17410 | ARID/BRIGHT DNA binding domain-containing protein | ARID3 | |
| AT1G08600 | Chromatin remodeling complex | CHR20 | |
| AT5G19310 | Chromatin remodeling complex | CHR23 | |
| AT4G26110 | Nucleosome/chromatin assembly complexes | NFA1/NAP1 | |
| AT5G58230 | Nucleosome/chromatin assembly complex | NFC1/MSI1 | |
| AT5G67630 | ATPase/helicase | RUVBL2 | |
| AT2G27170 | Structural maintenance of chromosomes family protein | CPC5/TTN7 | |
| AT3G17310 | DNA methyltransferase | DMT10/DRM3 | |
| AT5G46550 | Global TF | GTE12 | |
| AT4G38130 | Histone deacetylase | HDA19/D1 | |
| AT3G44750 | Histone deacetylase | HDT1/HD2A/HDA3 | |
| AT5G03740 | Histone deacetylase | HDT3/HD2C/HDA11 | |
| AT1G55255 | Histone ubiquitination protein | HUPA2/HUB2 | |
| AT3G48430 | Jumonji domain group | JMJ12/REF6 | |
| AT4G20400 | Jumonji domain group | JMJ14 | |
| AT2G38950 | Jumonji domain group | JMJ19 | |
| AT5G04940 | SET domain protein | SDG32/SUVH1 | |
| AT5G13960 | SET domain protein | SDG33/SUVH4 | |
| AT1G01920 | SET domain protein | SDG42/SDG42 | |
| AT4G29510 | Protein Arg methyltransferase | PRMT11/PAM1 | |
| AT1G79730 | PAF1 complex | PAFA1/ELF7 | |
| AT3G49660 | COMPASS complex | SWDC2 | |
| AT1G45000 | Proteasomal ATPase | PATPA2 | |
| AT5G23570 | Suppressor of gene silencing | SGS3 | |
| C4 | AT5G19850 | α/β-Hydrolases superfamily protein | ABHF9 |
| AT1G15340 | Methyl binding domain protein | MBD10 | |
| AT5G03220 | Mediator subunit | MED7SUB2 | |
| AT1G14400 | Histone ubiquitination protein | HUPB1/UBC1 | |
| AT5G09230 | Histone deacetylase | SRT2 | |
| C5 | AT2G02760 | Histone ubiquitination protein | HUPB2/UBC2 |
| AT5G46910 | Jumonji domain group | JMJ13 | |
| AT1G26665 | Mediator subunit | MED10SUB2 | |
| AT1G55080 | Mediator subunit | MED9SUB1 | |
| AT1G17520 | Single myb histone protein group | SMH13 | |
| C6 | AT2G27040 | Argonaute family protein | AGO4 |
| AT3G17590 | Chromatin remodeling complex | CHE1/BSH | |
| AT3G46580 | Methyl binding domain protein | MBD5 | |
| AT2G19520 | NURF complex | NFC4/FVE | |
| AT5G49020 | Protein Arg methyltransferase | PRMT4a | |
| AT5G38110 | Antisilencing function 1b | SGA1 | |
| AT1G49480 | Transcription regulation (VRN1 homolog) | VPGA2 | |
| C7 | AT5G62410 | Condensin complex | CPC3/TTN3 |
| C8 | AT5G43810 | Argonaute family protein | AGO10/PNH |
| AT2G25170 | Chromatin remodeling complex | CHR6/PKL | |
| AT3G47460 | Structural maintenance of chromosomes family protein | CPC4/SMC2 | |
| Various | AT1G58025 | Bromodomain containing protein | BRD5 |
| AT4G11130 | RNA-dependent RNA polymerase | RDR2 | |
| AT1G18800 | Nucleosome/chromatin assembly complex | NFA5/NRP2 | |
| AT5G06550 | Jumonji domain group | JMJ22 | |
| AT5G22650 | Histone deacetylase | HDT2/HD2B | |
| AT2G19640 | SET domain protein | SDG39/ASHR2 | |
| AT2G17900 | SET domain protein | SDG37/ASHR1 |
PdCs Have Epigenetic Differences from Established Cell Suspensions
It is currently accepted that in vitro culture induces somatic variations, probably involving epigenetic mechanisms. We thus compared the epigenetic status of PdCs to that of a well-established cell suspension. Compared with P168 PdCs, cells in suspension culture had a specific set of histone genes that were upregulated (Groups B and C, Table 4; see Supplemental Table 13 online), whereas H2A.W.12, encoding an H2A histone variant, was downregulated. We noticed that numerous other genes associated with chromatin regulation were activated in the cell suspension and identified five new clusters that were specifically deregulated in cell suspension (C10-14; see Supplemental Table 14 and Supplemental Figure 5 online). We also noticed that transposable elements present in the CATMA microarray were reactivated in cell suspension, whereas only two transposable elements were reactivated in PdCs (see Supplemental Table 14 online). Because the microarray used in our analysis was not designed to examine transposable element expression profiles, we expect that many more transposable elements are deregulated in cell suspension.
The floral repressor gene FWA, which is silenced in adult tissues and subjected to imprinting, was also specifically reactivated in this established cell suspension. ROS1, involved in active DNA demethylation, was also upregulated in cell suspension, as was DRM1, which is involved in de novo DNA methylation, suggesting that major changes in methylation status occur due to prolonged cell culture. In addition, key genes in silencing mechanisms (i.e., AGO5, DCL3, and HEN1) were also activated. Thus, small RNA regulation appeared to be differently affected in cell suspension compared with PdCs. Therefore, PdCs were epigenetically closer to plants than to a cell suspension, suggesting that PdCs maintained epigenetic imprinting despite dedifferentiation events and reentry into the cell cycle.
The Roles of AGO4 and ALF4 during Protoplast Culture
Given that the first day in culture is crucial for the development of the protoplast, we tested the functional roles of two genes upregulated at 24 h: ABERRANT LATERAL ROOT FORMATION4 (ALF4) in C3 and ARGONAUTE4 (AGO4) in C6. Since DNA hypermethylation correlates with reprogramming efficiency in animal somatic cells (Barrero et al., 2012), and knowing the essential role of PIWI genes in maintaining germ line cells and stem cell properties (stemness) (Alié et al., 2011), we were intrigued by the early upregulation of AGO4. Protoplasts of homozygous ago4-4 mutant plantlets were able to divide, revealing that AGO4 is not essential for the reentry into cell division. There is functional redundancy between AGO4 and AGO9 (Elmayan et al., 2005; Mallory and Vaucheret, 2010); thus, it remains to be tested whether ago9 and ago4 ago9 protoplasts are able to reenter the cell division cycle.
Initially identified as regulating the formation of lateral roots (Celenza et al., 1995; DiDonato et al., 2004), ALF4 is also essential for callus formation from pericycle cells of Arabidopsis explants (Sugimoto et al., 2010). Under binocular loupe, afl4-1 homozygous plants were strictly selected based on their morphological root phenotype (see Supplemental Figure 6 online). Protoplasts prepared from homozygous alf4-1 plantlets were unable to divide, except for about one in 104 protoplasts, which were probably derived from either rare heterozygous seedlings or wild-type seedlings retarded in lateral root development. Furthermore, nondividing alf4 protoplasts were fully viable and could condition the culture medium, thus supporting colony formation for the rare PdCs of other genotypes. These data demonstrate that ALF4 is necessary for protoplast division. This finding expands the role of ALF4 to every cell type, highlighting its crucial involvement in the reinitiation of cell division in tissues beyond the meristem.
DISCUSSION
Large populations of homogenized plant cells can be obtained overnight and easily handled in liquid culture under conditions determined in this study to yield a large fraction of cells that reenter the cell cycle. The flexibility and efficiency of the protoplast system developed here provides a useful tool for examining the fundamental processes of reentry into the cell cycle and totipotency. Furthermore, the high frequency of bud regeneration may be used to characterize the early molecular events underlying meristem formation.
Transcript profiling at various time points during the preparation and culture of protoplasts revealed 5276 deregulated genes. With next-generation sequencing approaches, this number could certainly increase, which would allow us to investigate the regulatory roles of noncoding RNAs in this process. The deregulated genes were organized into eight main gene clusters with specific GO patterns that suggest sequential transcriptional phases and an apparent synchronization of reentry into the cell cycle for most PdCs in culture. The temporal profiling conducted here has yielded much data that can be used in future studies. Thus, protoplast dedifferentiation provides a simple and homogeneous experimental cell system as an alternative to in planta activation of tissue-specific cell division, which is involved in transdifferentiation or differentiation of precursor cells (Sena and Birnbaum, 2010; Sugimoto et al., 2011).
During protoplast isolation and the early culture steps, dedifferentiation occurred rapidly and affected all cell types originating from the aerial parts of plantlets. Dedifferentiation resulted from the massive degradation of various cellular constituents (e.g., proteins, the cell wall, and the photosynthetic apparatus) by repression of the underlying genes and transcriptional reorientation, and the activation of numerous TFs and posttranscriptional controls.
Protein synthesis pathways were notably repressed by protoplasting, with all of the ribosomal protein genes being downregulated, in agreement with the low number of ribosomes observed in tobacco protoplasts by microscopy analysis (Gigot et al., 1975). Once protoplasts were cultured, protein synthesis was reactivated (Zelcer and Galun, 1976). We show a clear reorientation of protein synthesis toward cell division. Identifying the ribosomal equipment needed at specific stages to regulate reentry into the cell cycle will require further studies; however, the importance of RPs in gene expression and development have been reported (Byrne, 2009; Kondrashov et al., 2011). We identified a specific cell cycle cluster containing genes upregulated at 96 h, consistent with microscopy observations of divisions in culture.
Our study revealed a complex array of 500 TF profiles during the early steps of establishing totipotency. Some TF families, such as the Tify and HFS families, were associated with specific clusters and therefore are good markers of particular time points, whereas the members of other families were activated at different time points in the process. Because the early dedifferentiation events are critical for reentry into the cell cycle, an analysis of TFs activated in protoplasts after 0 and 24 h in culture (C1 and C3, respectively) will help to elucidate the transcriptional network underlying totipotency. For example, of all the TFs activated in C1, only 26 were not expressed during senescence. These 26 TFs may be crucial for dedifferentiation and the acquisition of basal competence for cell division. The 34 TFs of C3 not deregulated during senescence may be involved in the core transcriptional regulatory network underlying totipotency. Further comparisons with studies conducted in other species will help identify which TFs are crucial for establishing totipotency.
Numerous TFs with known meristematic or stem cell functions, such as the three members of the WOX family, were deregulated during the establishment of totipotency. WOX5 is an auxin-inducible gene expressed in the quiescent center of the root meristem and in its direct precursor cells during the early globular stage of embryogenesis (Haecker et al., 2004; Gonzali et al., 2005). WOX8 is expressed in the egg cell and zygote, whereas WOX13 is expressed during primary and lateral root initiation and development, in the gynecium, and during embryo development (Deveaux et al., 2008; Romera-Branchat et al., 2012). The sequential reactivation of WOX13, WOX5, and then WOX8 suggests that these genes are also regulators of cell division during PdC culture. Surprisingly, genes encoding TFs specific to embryo development (LEC1), leaf development (PHB), and floral development (PI and SVP) were transiently activated, also suggesting that these genes have broader functions than reported previously. We found that ALF4, in addition to its well-characterized role in lateral root initiation (Celenza et al., 1995; DiDonato et al., 2004) and callus formation (Sugimoto et al., 2010), is crucial for the reentry of protoplasts into the cell cycle.
We observed striking similarities between animal cell reprogramming and the early developmental events of Arabidopsis protoplasts in culture, highlighting some degree of conservation of reprogramming processes. The dedifferentiation of protoplasts, which is accompanied by stress responses (Grafi et al., 2011), is reminiscent of inflammatory reactions in animal cells (Barrero and Izpisua Belmonte, 2011; Lee et al., 2012). PdCs reacted to hypoxia by shifting to glycolysis. In animal cells, a similar shift toward energy production by glycolysis is a distinctive trait of the transition from differentiated cells to stem cells (Zhang et al., 2011; Zhou et al., 2012). We highlighted major changes in chromatin-related gene expression, suggesting that chromatin has a specific composition in protoplasts and PdCs, with specific sets of histone variants and histone posttranslational modifications. Thus, in Arabidopsis, dedifferentiation and further steps toward PdC development require extensive epigenetic reprogramming reminiscent of animal stem cell reprogramming (Barrero and Izpisua Belmonte, 2011; Jullien et al., 2012; Solana et al., 2012). The genome-wide transcriptional reorientation we observed in this study confirmed the large-scale chromatin rearrangements we previously described at the microscopic and cytological levels (Tessadori et al., 2007). A number of LHP1 targets were deregulated throughout the entire process, suggesting that Polycomb complexes are also involved in the early regeneration steps from isolated somatic cells and expanding their roles to include the regulation of cell fate and differentiation (Köhler and Hennig, 2010). Interestingly, the nature of epigenetic reprogramming differed from the epigenetic modifications observed in a well-established cell suspension not undergoing organogenesis. The maintenance of imprinted genes, such as FWA, and of transposons in a silent state in PdCs is consistent with the absence of activation of silent transgenes in protoplasts, despite chromatin decondensation (Tessadori et al., 2007). Our data suggest that silencing is maintained in PdCs, but not in cell suspension.
In our study, only a few genes specific to particular developmental stages (e.g., root, meristem, and embryo development) were upregulated during protoplast culture. These observations suggest that these genes have broader functions than previously thought. Furthermore, they show that totipotent protoplasts and the derived cells develop in response to unique and complex combinations of molecular and metabolic signatures. The assumption that animal stem cell identity is more a product of the transient, specific molecular and metabolic status of the cell than of cellular identity (Zipori, 2004) seems to apply well to the totipotency of plant cells.
METHODS
Plant Materials and Growth Conditions
Seeds of Arabidopsis thaliana Col-0 and Ws accessions were obtained from the Institut National de la Recherche Agronomique Versailles Genetics and Plant Breeding Laboratory (Arabidopsis Resource Centre). Disinfectant solution was prepared by dissolving a pill of sodium dichloroisocyanurate (Bayrol) in 40 mL water and adding 160 mL ethanol. Seeds (20 mg) were disinfected in 1 mL of disinfectant solution in an Eppendorf tube for 10 min, rinsed twice with ethanol, and left to dry overnight. Approximately 250 seeds were sown on 75 mL GM (see Supplemental Table 1, online) in green boxes (Kalys), and placed at 4°C for 2 to 3 d. To cultivate plantlets in soil, seeds were sown directly on compost covered with a thin vermiculite layer and watered with GM as for in vitro culture, but in the absence of Suc. Plantlets were cultivated in soil or in vitro on plates of medium under 10 h light, 75% relative air humidity, at 20°C. A mix of Biolux and plain white light tubes were used, giving an average light intensity of 50 μEs−1 s−1 m2. The light intensity at the level of the leaves of plants grown in vitro was around 30 μEs−1 s−1 m2. PSB-L Arabidopsis cells (May and Leaver, 1993) were cultured according to De Sutter et al. (2005).
Seeds from the heterozygous afl4-1 mutant in the Col-0 accession background (a gift from John Celenza) were sown in large Petri dishes (diameter, 145 mm), at a low density (40 seeds per dish) on GM. Based on the developmental phenotype, homozygous plantlets without secondary roots were selected for protoplast isolation (see Supplemental Figure 6 online). Plantlets were selected under binocular loupe. Of 964 seedlings, 139 plantlets without lateral roots were selected (far fewer than the expected 241 homozygous mutants) and used to isolate 5 × 106 alf4-1−/− protoplasts.
Protoplast Isolation and Culture
Optimal protoplast yield and viability were obtained using maceration medium that contained Gly and Glc as osmotic agents (MGG; see Supplemental Table 1 online). Approximately 0.6 g of the aerial parts of 3-week-old sterile plantlets (at the four to six leaf stage) was soaked in 5 mL of maceration medium in a Petri dish (see Supplemental Table 1 online) to prevent drying and rapidly chopped. Five milliliters of MGG was added, bringing the total volume to 10 mL. Maceration was performed in the dark, at 24°C, overnight. Due to the toxicity of ammonium ions in culture, the mineral composition of the maceration medium was adjusted to 0.2 mM ammonium (MGG; see Supplemental Table 1 online). After cell wall digestion, 20 mL protoplast suspension in MGG was filtered through an autoclaved 80-μm mesh filter, over 10 mL washing solution (2.5% KCl and 0.2% CaCl2) already added to a 30-mL glass tube. After centrifugation (70g, 6 min), protoplast pellets were gently resuspended in 25 mL washing solution and centrifuged again (70g, 6 min). Washing was performed two more times. This procedure allowed for the collection of protoplasts isolated from most cell types. Protoplast numbers were estimated on a Malassez slide from an aliquot of the resuspended suspension before the last centrifugation. Approximately 4.5 × 107 viable protoplasts were routinely isolated from plantlets cultured in six green boxes. After a 1-h incubation at 4°C in tubes in a volume of washing medium just enough to cover the pellet, the protoplast suspension was diluted in PIM to a concentration of 8 × 105 protoplasts per milliliter. One milliliter of protoplast suspension in PIM was then added to 9 mL PIM already present in Petri dishes to reach the starting density of 8 × 104/mL. Liquid media (PIM, colony induction medium 1, and colony induction medium 2; see Supplemental Table 1 online) were supplemented with 10 μL/L Tween 80 to facilitate the wetting of plastic dishes and thus prevent the bursting of plated protoplasts. All media were autoclaved for 20 min at 115°C, and growth substances and FeCitrateNH4 were added in a sterile manner after autoclaving. The plates were subsequently placed in large plastic boxes to limit evaporation, and cell suspensions were cultured in the dark at 20°C. The suspensions were diluted as described in Results to regenerate plantlets from the PdC suspensions. The cell division rate was estimated by counting the number of dividing protoplasts on a Malassez slide under a light microscope.
RNA Extraction and Microarray Data
RNAs from 3-week-old seedlings, protoplasts, and PdCs after 24, 48, 96, and 168 h of culture were extracted using the RNAeasy mini kit (Qiagen). RNA integrity was tested with an Agilent Bioanalyzer. For each biological replicate, 4.5 × 107 protoplasts were isolated: 107 of freshly prepared protoplasts were used for time point 0 h, and 3 × 107 protoplasts were dispatched and cultured in 40 Petri dishes (8 × 105 per dish). Cells from nine Petri dishes were pooled for each culture time point. Hybridization microarray analysis and statistical analyses based on two independent biological replicates, and two dye-swaps were performed as previously described using the 25 K CATMA_v2.1 microarray bearing 24 576 gene-specific tags (Lurin et al., 2004; Gagnot et al., 2008) (see Supplemental Methods 1 online).
After statistical validation, variations in transcript abundance were expressed as the log2 of the ratio of hybridization intensities between biological materials and/or successive steps. The microarray data sets were deposited into the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/; accession number GSE7984) and CATdb (http://urgv.evry.inra.fr/cgi-bin/projects/CATdb/consult_project.pl?project_id=28.), according to Minimum Information About a Microarray Experiment standards.
Transcriptome Analysis
Supplemental Data Set 1 online lists the 8206 genes differentially expressed in at least one of the nine conditions tested in our experimental design. The various publicly available data sets used in this study, such as the chromatin-related genes (ChromDB; http://chromdb.org/), the lists of TFs (Mitsuda and Ohme-Takagi, 2009; Lu et al., 2012), and the list of genes from Visual LRTC (Parizot et al., 2010), were merged with our transcript profiles, our lists of genes associated with selected GO information related to the cell wall and cell cycle (GO website), and the identity of the clusters. A specific spreadsheet in Supplemental Data Set 1 online, called gene selector, was designed in Excel using advanced functions, to extract expression profiles and information related to small user lists of sorted AGIs. The function was updated with the latest version available on TAIR (TAIR10_functional_descriptions, 23/08/2011 version, Columnumn Short_description).
BAR (http://www.bar.utoronto.ca/) and its Classification SuperViewer Tool (Provart et Zhu, 2003), based on the GO functional classifications (January 5, 2010; file ATH_GO_GOSLIM.20100105), was used to calculate normed frequencies of the classes, bootstrap standard deviations, and P values. EasyGO (GO enrichment analysis tool, http://bioinformatics.cau.edu.cn/easygo/) and agriGO (Du et al., 2010) were also used for detailed analysis of GO term enrichment. Genes related to the cell cycle (GO:0007049, GO:0051301, GO:0000910, and GO:0000280) and cell wall elaboration (GO:0071554 and GO:0009664) were extracted from AmiGO (http://amigo.geneontology.org/cgi-bin/amigo/go.cgi; all-association files: gene products annotated to the GO term or any of its children). Clustering and visualization of the gene expression differences were performed using the Multiexperiment Viewer tool. Hierarchical clustering was done using the Euclidean distance metric and average linkage clustering as the linkage method (Saeed et al., 2003). Venn diagrams were constructed using the tool Venn diagrams from Gent University (http://bioinformatics.psb.ugent.be/webtools/Venn/).
Accession Numbers
Sequence data from this article can be found in the Arabidopsis Genome Initiative under the accession numbers presented in Supplemental Data Set 1 online. The microarray data sets were deposited into the Gene Expression Omnibus (http://www.ncbi.nlm.nih.gov/geo/) under accession number GSE7984.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Clustering of the Transcript Profiles.
Supplemental Figure 2. Venn Diagram Showing the Distribution of DE5 Genes Isolated in Our Study and Known to Be Expressed during Senescence and to Be Involved in Stress Responses.
Supplemental Figure 3. Venn Diagram Showing Common Genes Deregulated in Protoplast Transcriptomes Established in Our Study and in Two Previous Studies.
Supplemental Figure 4. The Expression of A. thaliana Cell Cycle Genes at Various Time Points during Protoplast Culture.
Supplemental Figure 5. Venn Diagram Highlighting Genes Specific to Cell Suspension (Common in C/P168 and C/Pliv) or to the Protoplast Stage (Only Deregulated in P0/Pliv).
Supplemental Figure 6. Phenotype of the alf4-1 Mutant.
Supplemental Table 1. Media Compositions for A. thaliana Protoplast Culture (mg/L).
Supplemental Table 2. Validation of Microarray Data.
Supplemental Table 3. Complete GO Descriptions of the Eight Main Clusters Identified in This Study.
Supplemental Table 4. List of DEGs Common to Our Study (P0/Pliv) and Two Previous Studies (Damri et al., 2009; Yadav et al., 2009).
Supplemental Table 5. List of DEGs Deregulated in DE5 (5276 Total Genes) and Present among the 70 Genes Involved in Meristem (Yadav et al., 2009).
Supplemental Table 6. List DEGs Deregulated in Pliv/P0 (This Work), Meristem Protoplasts (Yadav et al., 2009), and Moss Protoplasts (Xiao et al., 2012).
Supplemental Table 7. Expression Profiles of Auxin-Related Genes at Various Time Points in A. thaliana Protoplast Culture.
Supplemental Table 8. Selected TF Families and Their Distribution in the Eight Clusters Identified in This Study.
Supplemental Table 9. Expression Profiles of Selected TFs Involved in Developmental Processes.
Supplemental Table 10. Transcription Factors Involved in Lateral Root Initiation (Parizot et al., 2010) and Deregulated in Our Study.
Supplemental Table 11. Transcription Factor Targets of LHP1 That Were Deregulated in Our Study.
Supplemental Table 12. Distribution of the 559 ChromDB Genes Deregulated in Our Study and in the Eight Main Clusters.
Supplemental Table 13. The ChromDB Genes Deregulated in A. thaliana Cell Suspension Form Five New Clusters.
Supplemental Table 14. Expression Profiles of Transposable Elements during A. thaliana Protoplast Culture Compared with Cell Suspension (C) Culture.
Supplemental Methods 1. Transcriptome Hybridization, Data Analysis and qRT-PCR.
Supplemental References 1. References for Supplemental Figures 2 and 3 and Supplemental Tables 1, 4, 5, and 6.
Supplemental Data Set 1. Excel File Containing Expression Profiles, Annotations, and the Gene Selector Tool, for Extraction from These Data Sets of a Given Gene or Set of Genes in the Process of Cell Reprogramming.
Acknowledgments
This work was supported by the Institut National de la Recherche Agronomique, France. We thank John Celenza for afl4-1 seeds, Hervé Vaucheret for ago4-4 seeds and fruitful discussions, and all technicians in charge of the Institut Jean-Pierre Bourgin greenhouses, with special mention to Bruno Letarnec. We thank Carolyn Napoli and the Chromatin Database for providing the Arabidopsis chromatin gene list, Boris Parizot for providing the VisuaLRTC Excel module, and Chi Zhang for the list of TFs and their families. We also thank Margaret Platt-Hommel, Herman Höfte, and Loïc Lepiniec for critical reading of the article.
AUTHOR CONTRIBUTIONS
M.-C.C., Y.C., and V.G. conceived and designed the experiments. M.-C.C., V.G., and O.P. performed the experiments. O.P., J.-P.R., F.G., V.G., and Y.C. analyzed the data. V.G. and Y.C. wrote the article.
Glossary
- PdC
protoplast-derived cell
- GM
germination medium
- MGG
maceration Gly Glc medium
- TZ
thidiazuron
- PIM
protoplast induction medium
- SIM
shoot induction medium
- Col-0
Columbia-0
- Ws
Wassilewskija
- GO
Gene Ontology
- BAR
Bio-Array Resource for Plant Functional Genomics
- DEG
differentially expressed gene
References
- Aichinger E., Kornet N., Friedrich T., Laux T. (2012). Plant stem cell niches. Annu. Rev. Plant Biol. 63: 615–636 [DOI] [PubMed] [Google Scholar]
- Alié A., Leclère L., Jager M., Dayraud C., Chang P., Le Guyader H., Quéinnec E., Manuel M. (2011). Somatic stem cells express Piwi and Vasa genes in an adult ctenophore: Ancient association of “germline genes” with stemness. Dev. Biol. 350: 183–197 [DOI] [PubMed] [Google Scholar]
- Aremu A.O., Bairu M.W., Doležal K., Finnie J.F., Staden J. (2012). Topolins: A panacea to plant tissue culture challenges? Plant Cell Tissue Organ Cult. 108: 1–16 [Google Scholar]
- Atta R., Laurens L., Boucheron-Dubuisson E., Guivarc’h A., Carnero E., Giraudat-Pautot V., Rech P., Chriqui D. (2009). Pluripotency of Arabidopsis xylem pericycle underlies shoot regeneration from root and hypocotyl explants grown in vitro. Plant J. 57: 626–644 [DOI] [PubMed] [Google Scholar]
- Atias O., Chor B., Chamovitz D.A. (2009). Large-scale analysis of Arabidopsis transcription reveals a basal co-regulation network. BMC Syst. Biol. 3: 86. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Barakat A., Szick-Miranda K., Chang I.F., Guyot R., Blanc G., Cooke R., Delseny M., Bailey-Serres J. (2001). The organization of cytoplasmic ribosomal protein genes in the Arabidopsis genome. Plant Physiol. 127: 398–415 [PMC free article] [PubMed] [Google Scholar]
- Barrero M.J., Berdasco M., Paramonov I., Bilic J., Vitaloni M., Esteller M., Izpisua Belmonte J.C. (2012). DNA hypermethylation in somatic cells correlates with higher reprogramming efficiency. Stem Cells 30: 1696–1702 [DOI] [PubMed] [Google Scholar]
- Barrero M.J., Izpisua Belmonte J.C. (2011). Regenerating the epigenome. EMBO Rep. 12: 208–215 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bourgin J.P., Chupeau Y., Missonier C. (1979). Plant regeneration from mesophyll protoplasts of several Nicotiana species. Physiol. Plant. 45: 288–292 [Google Scholar]
- Breeze E., et al. (2011). High-resolution temporal profiling of transcripts during Arabidopsis leaf senescence reveals a distinct chronology of processes and regulation. Plant Cell 23: 873–894 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Byrne M.E. (2009). A role for the ribosome in development. Trends Plant Sci. 14: 512–519 [DOI] [PubMed] [Google Scholar]
- Buchanan-Wollaston V., Page T., Harrison E., Breeze E., Lim P.O., Nam H.G., Lin J.F., Wu S.H., Swidzinski J., Ishizaki K., Leaver C.J. (2005). Comparative transcriptome analysis reveals significant differences in gene expression and signalling pathways between developmental and dark/starvation-induced senescence in Arabidopsis. Plant J. 42: 567–585 [DOI] [PubMed] [Google Scholar]
- Caboche M. (1980). Nutritional requirements of protoplast derived, haploid tobacco cell grown at low cell densities in liquid medium. Planta 149: 7–18 [DOI] [PubMed] [Google Scholar]
- Candela M., Velazquez I. (2001). Differences in plant regeneration in vitro among four Arabidopsis ecotypes. In Vitro Cell. Dev. Biol. Plant 37: 638–643 [Google Scholar]
- Celenza J.L., Jr, Grisafi P.L., Fink G.R. (1995). A pathway for lateral root formation in Arabidopsis thaliana. Genes Dev. 9: 2131–2142 [DOI] [PubMed] [Google Scholar]
- Chang B.Y., Harte R.A., Cartwright C.A. (2002). RACK1: A novel substrate for the Src protein-tyrosine kinase. Oncogene 21: 7619–7629 [DOI] [PubMed] [Google Scholar]
- Chantha S.-C., Emerald B.S., Matton D.P. (2006). Characterization of the plant Notchless homolog, a WD repeat protein involved in seed development. Plant Mol. Biol. 62: 897–912 [DOI] [PubMed] [Google Scholar]
- Chantha S.C., Tebbji F., Matton D.P. (2007). From the notch signaling pathway to ribosome biogenesis. Plant Signal. Behav. 2: 168–170 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chupeau M.C., Lemoine M., Chupeau Y. (1993). Requirement of thidiazuron for healthy protoplast development to efficient tree regeneration of a hybrid Poplar (P. tremula x P. alba). J. Plant Physiol. 141: 601–609 [Google Scholar]
- Chupeau, Y., Bourgin, J.P., Missonier, C., Dorion, N. and Morel, G. (1974). Préparation et culture de protoplastes de divers Nicotiana C. R. Acad. Sci. Paris D 278: 1565–1568.
- Crowe M.L., et al. (2003). CATMA: A complete Arabidopsis GST database. Nucleic Acids Res. 31: 156–158 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cui X., Fan B., Scholz J., Chen Z. (2007). Roles of Arabidopsis cyclin-dependent kinase C complexes in cauliflower mosaic virus infection, plant growth, and development. Plant Cell 19: 1388–1402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Damm B., Willmitzer L. (1988). Regeneration of fertile plants from protoplasts of different Arabidopsis thaliana genotypes. Mol. Gen. Genet. 213: 15–20 [Google Scholar]
- Damri M., Granot G., Ben-Meir H., Avivi Y., Plaschkes I., Chalifa-Caspi V., Wolfson M., Fraifeld V., Grafi G. (2009). Senescing cells share common features with dedifferentiating cells. Rejuvenation Res. 12: 435–443 [DOI] [PubMed] [Google Scholar]
- Davey M.R., Anthony P., Power J.B., Lowe K.C. (2005). Plant protoplasts: Status and biotechnological perspectives. Biotechnol. Adv. 23: 131–171 [DOI] [PubMed] [Google Scholar]
- Demidov D., Van Damme D., Geelen D., Blattner F.R., Houben A. (2005). Identification and dynamics of two classes of aurora-like kinases in Arabidopsis and other plants. Plant Cell 17: 836–848 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Smet I., et al. (2008). Receptor-like kinase ACR4 restricts formative cell divisions in the Arabidopsis root. Science 322: 594–597 [DOI] [PubMed] [Google Scholar]
- De Sutter V., Vanderhaeghen R., Tilleman S., Lammertyn F., Vanhoutte I., Karimi M., Inze D., Goossens A., Hilson P. (2005). Exploration of jasmonate signalling via automated and standardized transient expression assays in tobacco cells. Plant J. 44: 1065–1076 [DOI] [PubMed] [Google Scholar]
- Deveaux Y., Toffano-Nioche C., Claisse G., Thareau V., Morin H., Laufs P., Moreau H., Kreis M., Lecharny A. (2008). Genes of the most conserved WOX clade in plants affect root and flower development in Arabidopsis. BMC Evol. Biol. 8: 291. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dewitte W., Murray J.A. (2003). The plant cell cycle. Annu. Rev. Plant Biol. 54: 235–264 [DOI] [PubMed] [Google Scholar]
- DiDonato R.J., Arbuckle E., Buker S., Sheets J., Tobar J., Totong R., Grisafi P., Fink G.R., Celenza J.L. (2004). Arabidopsis ALF4 encodes a nuclear-localized protein required for lateral root formation. Plant J. 37: 340–353 [DOI] [PubMed] [Google Scholar]
- Dovzhenko A., Dal Bosco C., Meurer J., Koop H.U. (2003). Efficient regeneration from cotyledon protoplasts in Arabidopsis thaliana. Protoplasma 222: 107–111 [DOI] [PubMed] [Google Scholar]
- Du Z., Zhou X., Ling Y., Zhang Z., Su Z. (2010). agriGO: A GO analysis toolkit for the agricultural community. Nucleic Acids Res. 38 (Web Server issue): W64–W70 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Elmayan T., Proux F., Vaucheret H. (2005). Arabidopsis RPA2: A genetic link among transcriptional gene silencing, DNA repair, and DNA replication. Curr. Biol. 15: 1919–1925 [DOI] [PubMed] [Google Scholar]
- Eisenberg T., et al. (2009). Induction of autophagy by spermidine promotes longevity. Nat. Cell Biol. 11: 1305–1314 [DOI] [PubMed] [Google Scholar]
- Fujii S., Small I. (2011). The evolution of RNA editing and pentatricopeptide repeat genes. New Phytol. 191: 37–47 [DOI] [PubMed] [Google Scholar]
- Gagnot S., Tamby J.-P., Martin-Magniette M.-L., Bitton F., Taconnat L., Balzergue S., Aubourg S., Renou J.-P., Lecharny A., Brunaud V. (2008). CATdb: A public access to Arabidopsis transcriptome data from the URGV-CATMA platform. Nucleic Acids Res. 36 (Database issue): D986–D990 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Giavalisco P., Wilson D., Kreitler T., Lehrach H., Klose J., Gobom J., Fucini P. (2005). High heterogeneity within the ribosomal proteins of the Arabidopsis thaliana 80S ribosome. Plant Mol. Biol. 57: 577–591 [DOI] [PubMed] [Google Scholar]
- Gil P., Dewey E., Friml J., Zhao Y., Snowden K.C., Putterill J., Palme K., Estelle M., Chory J. (2001). BIG: A calossin-like protein required for polar auxin transport in Arabidopsis. Genes Dev. 15: 1985–1997 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gigot C., Kopp M., Schmitt C., Milne R. (1975). Subcellular changes during isolation and culture of tobacco mesophyll protoplasts. Protoplasma 84: 31–41 [Google Scholar]
- González F., Boué S., Izpisúa Belmonte J.C. (2011). Methods for making induced pluripotent stem cells: Reprogramming à la carte. Nat. Rev. Genet. 12: 231–242 [DOI] [PubMed] [Google Scholar]
- Grafi G., Chalifa-Caspi V., Nagar T., Plaschkes I., Barak S., Ransbotyn V. (2011). Plant response to stress meets dedifferentiation. Planta 233: 433–438 [DOI] [PubMed] [Google Scholar]
- Gonzali S., Novi G., Loreti E., Paolicchi F., Poggi A., Alpi A., Perata P. (2005). A turanose insensitive mutant suggests a role for WOX5 in auxin homeostasis in Arabidopsis thaliana. Plant J. 44: 633–645 [DOI] [PubMed] [Google Scholar]
- Guo J., Wang S., Valerius O., Hall H., Zeng Q., Li J.F., Weston D.J., Ellis B.E., Chen J.G. (2011). Involvement of Arabidopsis RACK1 in protein translation and its regulation by abscisic acid. Plant Physiol. 155: 370–383 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gutierrez, C. (2009). The Arabidopsis cell division cycle. In The Arabidopsis Book 7:e0120, /10.1199/tab.0120. [DOI] [PMC free article] [PubMed]
- Haecker A., Gross-Hardt R., Geiges B., Sarkar A., Breuninger H., Herrmann M., Laux T. (2004). Expression dynamics of WOX genes mark cell fate decisions during early embryonic patterning in Arabidopsis thaliana. Development 131: 657–668 [DOI] [PubMed] [Google Scholar]
- He C., Chen X., Huang H., Xu L. (2012). Reprogramming of H3K27me3 is critical for acquisition of pluripotency from cultured Arabidopsis tissues. PLoS Genet. 8: e1002911. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Heller R. (1953). Recherches sur la nutrition minérale des tissus végétaux cultivés in vitro. Ann. Sci. Nat. Bot. Biol. Veg. 14: 1–223 [Google Scholar]
- Hilson P.J., et al. (2004). Versatile gene-specific sequence tags for Arabidopsis functional genomics: Transcript profiling and reverse genetics applications. Genome Res. 14 (10B): 2176–2189 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Holec S., Lange H., Kühn K., Alioua M., Börner T., Gagliardi D. (2006). Relaxed transcription in Arabidopsis mitochondria is counterbalanced by RNA stability control mediated by polyadenylation and polynucleotide phosphorylase. Mol. Cell. Biol. 26: 2869–2876 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Horii M., Marubashi W. (2005). Even juvenile leaves of tobacco exhibit programmed cell death. Plant Biotechnol. 22: 339–344 [Google Scholar]
- Iwase A., Mitsuda N., Koyama T., Hiratsu K., Kojima M., Arai T., Inoue Y., Seki M., Sakakibara H., Sugimoto K., Ohme-Takagi M. (2011). The AP2/ERF transcription factor WIND1 controls cell dedifferentiation in Arabidopsis. Curr. Biol. 21: 508–514 [DOI] [PubMed] [Google Scholar]
- Jopling C., Boue S., Izpisua Belmonte J.C. (2011). Dedifferentiation, transdifferentiation and reprogramming: Three routes to regeneration. Nat. Rev. Mol. Cell Biol. 12: 79–89 [DOI] [PubMed] [Google Scholar]
- Jullien J., Astrand C., Szenker E., Garrett N., Almouzni G., Gurdon J.B. (2012). HIRA dependent H3.3 deposition is required for transcriptional reprogramming following nuclear transfer to Xenopus oocytes. Epigenetics Chromatin 5: 17. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kende H., Bradford K., Brummell D., Cho H.-T., Cosgrove D., Fleming A., Gehring C., Lee Y., McQueen-Mason S., Rose J., Voesenek L.A.C.J. (2004). Nomenclature for members of the expansin superfamily of genes and proteins. Plant Mol. Biol. 55: 311–314 [DOI] [PubMed] [Google Scholar]
- Köhler C., Hennig L. (2010). Regulation of cell identity by plant Polycomb and trithorax group proteins. Curr. Opin. Genet. Dev. 20: 541–547 [DOI] [PubMed] [Google Scholar]
- Kondrashov N., Pusic A., Stumpf C.R., Shimizu K., Hsieh A.C., Xue S., Ishijima J., Shiroishi T., Barna M. (2011). Ribosome-mediated specificity in Hox mRNA translation and vertebrate tissue patterning. Cell 145: 383–397 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kwon H.K., Yokoyama R., Nishitani K. (2005). A proteomic approach to apoplastic proteins involved in cell wall regeneration in protoplasts of Arabidopsis suspension-cultured cells. Plant Cell Physiol. 46: 843–857 [DOI] [PubMed] [Google Scholar]
- Latrasse D., et al. (2011). Control of flowering and cell fate by LIF2, an RNA binding partner of the polycomb complex component LHP1. PLoS ONE 6: e16592. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Law S.R., Narsai R., Taylor N.L., Delannoy E., Carrie C., Giraud E., Millar A.H., Small I., Whelan J. (2012). Nucleotide and RNA metabolism prime translational initiation in the earliest events of mitochondrial biogenesis during Arabidopsis germination. Plant Physiol. 158: 1610–1627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lee J., Sayed N., Hunter A., Au K.F., Wong W.H., Mocarski E.S., Pera R.R., Yakubov E., Cooke J.P. (2012). Activation of innate immunity is required for efficient nuclear reprogramming. Cell 151: 547–558 [DOI] [PMC free article] [PubMed] [Google Scholar]
- López-Bucio J., Hernández-Abreu E., Sánchez-Calderón L., Pérez-Torres A., Rampey R.A., Bartel B., Herrera-Estrella L. (2005). An auxin transport independent pathway is involved in phosphate stress-induced root architectural alterations in Arabidopsis. Identification of BIG as a mediator of auxin in pericycle cell activation. Plant Physiol. 137: 681–691 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lu T., Yang Y., Yao B., Liu S., Zhou Y., Zhang C. (2012). Template-based structure prediction and classification of transcription factors in Arabidopsis thaliana. Protein Sci. 21: 828–838 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Lurin C., et al. (2004). Genome-wide analysis of Arabidopsis pentatricopeptide repeat proteins reveals their essential role in organelle biogenesis. Plant Cell 16: 2089–2103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mallory A., Vaucheret H. (2010). Form, function, and regulation of ARGONAUTE proteins. Plant Cell 22: 3879–3889 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Maréchal A., Parent J.S., Sabar M., Véronneau-Lafortune F., Abou-Rached C., Brisson N. (2008). Overexpression of mtDNA-associated AtWhy2 compromises mitochondrial function. BMC Plant Biol. 8: 42. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Masson J., Paszkowski J. (1992). The culture response of Arabidopsis thaliana protoplasts is determined by the growth-conditions of donor plants. Plant J. 2: 829–833 [Google Scholar]
- Matsubayashi Y., Ogawa M., Morita A., Sakagami Y. (2002). An LRR receptor kinase involved in perception of a peptide plant hormone, phytosulfokine. Science 296: 1470–1472 [DOI] [PubMed] [Google Scholar]
- May M.J., Leaver C.J. (1993). Oxidative stimulation of glutathione synthesis in Arabidopsis thaliana suspension cultures. Plant Physiol. 103: 621–627 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Merkwirth C., Langer T. (2009). Prohibitin function within mitochondria: Essential roles for cell proliferation and cristae morphogenesis. Biochim. Biophys. Acta 1793: 27–32 [DOI] [PubMed] [Google Scholar]
- Mitsuda N., Ohme-Takagi M. (2009). Functional analysis of transcription factors in Arabidopsis. Plant Cell Physiol. 50: 1232–1248 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morel G., Wetmore R.H. (1951). Fern callus tissue culture. Am. J. Bot. 38: 141–143 [Google Scholar]
- Nakashima K., Kiyosue T., Yamaguchi-Shinozaki K., Shinozaki K. (1997). A nuclear gene, erd1, encoding a chloroplast-targeted Clp protease regulatory subunit homolog is not only induced by water stress but also developmentally up-regulated during senescence in Arabidopsis thaliana. Plant J. 12: 851–861 [DOI] [PubMed] [Google Scholar]
- Nitsch J.P., Oyama K. (1971). Obtention de plantes à partir de protoplastes haploïdes cultivés in vitro. C. R. Acad. Sci. Paris 273: 801–804 [Google Scholar]
- Parizot B., De Rybel B., Beeckman T. (2010). VisuaLRTC: A new view on lateral root initiation by combining specific transcriptome data sets. Plant Physiol. 153: 34–40 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Parsons H.T., et al. (2012). Isolation and proteomic characterization of the Arabidopsis Golgi defines functional and novel components involved in plant cell wall biosynthesis. Plant Physiol. 159: 12–26 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Perrin R., Lange H., Grienenberger J.M., Gagliardi D. (2004). AtmtPNPase is required for multiple aspects of the 18S rRNA metabolism in Arabidopsis thaliana mitochondria. Nucleic Acids Res. 32: 5174–5182 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pontvianne F., et al. (2010). Nucleolin is required for DNA methylation state and the expression of rRNA gene variants in Arabidopsis thaliana. PLoS Genet. 6: e1001225. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Provart N., Zhu T. (2003). A browser-based functional classification SuperViewer for Arabidopsis genomics. Currents Comp. Mol. Biol. 271: 2 [Google Scholar]
- Reddien P.W., Bermange A.L., Murfitt K.J., Jennings J.R., Sánchez Alvarado A. (2005). Identification of genes needed for regeneration, stem cell function, and tissue homeostasis by systematic gene perturbation in planaria. Dev. Cell 8: 635–649 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Romera-Branchat M., Ripoll J.J., Yanofsky M.F., Pelaz S. (September 4, 2012). The WOX13 homeobox gene promotes replum formation in the Arabidopsis thaliana fruit. Plant J.http//dx..org/. [DOI] [PubMed] [Google Scholar]
- Saeed A.I., et al. (2003). TM4: A free, open-source system for microarray data management and analysis. Biotechniques 34: 374–378 [DOI] [PubMed] [Google Scholar]
- Sampedro J., Cosgrove D.J. (2005). The expansin superfamily. Genome Biol. 6: 242. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schmitz-Linneweber C., Small I. (2008). Pentatricopeptide repeat proteins: A socket set for organelle gene expression. Trends Plant Sci. 13: 663–670 [DOI] [PubMed] [Google Scholar]
- Sena G., Birnbaum K.D. (2010). Built to rebuild: in search of organizing principles in plant regeneration. Curr. Opin. Genet. Dev. 20: 460–465 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Shi D.Q., Liu J., Xiang Y.H., Ye D., Sundaresan V., Yang W.C. (2005). SLOW WALKER1, essential for gametogenesis in Arabidopsis, encodes a WD40 protein involved in 18S ribosomal RNA biogenesis. Plant Cell 17: 2340–2354 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Skoog F., Miller C.O. (1957). Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp. Soc. Exp. Biol. 11: 118–130 [PubMed] [Google Scholar]
- Solana J., Kao D., Mihaylova Y., Jaber-Hijazi F., Malla S., Wilson R., Aboobaker A. (2012). Defining the molecular profile of planarian pluripotent stem cells using a combinatorial RNAseq, RNA interference and irradiation approach. Genome Biol. 13: R19. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sugimoto K., Gordon S.P., Meyerowitz E.M. (2011). Regeneration in plants and animals: Dedifferentiation, transdifferentiation, or just differentiation? Trends Cell Biol. 21: 212–218 [DOI] [PubMed] [Google Scholar]
- Sugimoto K., Jiao Y., Meyerowitz E.M. (2010). Arabidopsis regeneration from multiple tissues occurs via a root development pathway. Dev. Cell 18: 463–471 [DOI] [PubMed] [Google Scholar]
- Szymanski D.B., Cosgrove D.J. (2009). Dynamic coordination of cytoskeletal and cell wall systems during plant cell morphogenesis. Curr. Biol. 19: R800–R811 [DOI] [PubMed] [Google Scholar]
- Takahashi K., Yamanaka S. (2006). Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell 126: 663–676 [DOI] [PubMed] [Google Scholar]
- Takebe I., Labib G., Melchers G. (1971). Regeneration of whole plants from isolated mesophyll protoplasts of tobacco. Naturwiss. 58: 318–320 [Google Scholar]
- Talbert P.B., et al. (2012). A unified phylogeny-based nomenclature for histone variants. Epigenetics Chromatin 5: 7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tessadori F., Chupeau M.C., Chupeau Y., Knip M., Germann S., van Driel R., Fransz P., Gaudin V. (2007). Large-scale dissociation and sequential reassembly of pericentric heterochromatin in dedifferentiated Arabidopsis cells. J. Cell Sci. 120: 1200–1208 [DOI] [PubMed] [Google Scholar]
- van der Graaff E., Laux T., Rensing S.A. (2009). The WUS homeobox-containing (WOX) protein family. Genome Biol. 10: 248. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vanholme B., Grunewald W., Bateman A., Kohchi T., Gheysen G. (2007). The tify family previously known as ZIM. Trends Plant Sci. 12: 239–244 [DOI] [PubMed] [Google Scholar]
- Vanneste S., et al. (2005). Cell cycle progression in the pericycle is not sufficient for SOLITARY ROOT/IAA14-mediated lateral root initiation in Arabidopsis thaliana. Plant Cell 17: 3035–3050 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vasil I.K. (2008). A history of plant biotechnology: From the Cell Theory of Schleiden and Schwann to biotech crops. Plant Cell Rep. 27: 1423–1440 [DOI] [PubMed] [Google Scholar]
- Walker, K.L., Muller, S., Moss, D., Ehrhardt, D.W., and Smith, L.G. (2007). Arabidopsis TANGLED identifies the division plane throughout mitosis and cytokinesis. Curr. Biol. 17: 1827–1836. [DOI] [PMC free article] [PubMed]
- Widholm J.M. (1972). The use of fluorescein diacetate and phenosafranine for determining viability of cultured plant cells. Stain Technol. 47: 189–194 [DOI] [PubMed] [Google Scholar]
- Xiao L., Zhang L., Yang G., Zhu H., He Y. (2012). Transcriptome of protoplasts reprogrammed into stem cells in Physcomitrella patens. PLoS ONE 7: e35961. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yadav R.K., Girke T., Pasala S., Xie M., Reddy G.V. (2009). Gene expression map of the Arabidopsis shoot apical meristem stem cell niche. Proc. Natl. Acad. Sci. USA 106: 4941–4946 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yang X., Tu L., Zhu L., Fu L., Min L., Zhang X. (2008). Expression profile analysis of genes involved in cell wall regeneration during protoplast culture in cotton by suppression subtractive hybridization and macroarray. J. Exp. Bot. 59: 3661–3674 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoo S.D., Cho Y.H., Sheen J. (2007). Arabidopsis mesophyll protoplasts: A versatile cell system for transient gene expression analysis. Nat. Protoc. 2: 1565–1572 [DOI] [PubMed] [Google Scholar]
- Zelcer A., Galun E. (1976). Culture of newly isolated tobacco protoplasts: Precursor incorporation into protein, RNA and DNA. Plant Sci. Lett. 7: 331–336 [Google Scholar]
- Zhai Z., Sooksa-nguan T., Vatamaniuk O.K. (2009). Establishing RNA interference as a reverse-genetic approach for gene functional analysis in protoplasts. Plant Physiol. 149: 642–652 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhao J., Morozova N., Williams L., Libs L., Avivi Y., Grafi G. (2001). Two phases of chromatin decondensation during dedifferentiation of plant cells: Distinction between competence for cell fate switch and a commitment for S phase. J. Biol. Chem. 276: 22772–22778 [DOI] [PubMed] [Google Scholar]
- Zhao X.Y., Su Y.H., Zhang C.L., Wang L., Li X.G., Zhang X.S. (2013). Differences in capacities of in vitro organ regeneration between two Arabidopsis ecotypes Wassilewskija and Columbia. Plant Cell Tissue Organ Cult. 112: 65–74 [Google Scholar]
- Zhang J., et al. (2011). UCP2 regulates energy metabolism and differentiation potential of human pluripotent stem cells. EMBO J. 30: 4860–4873 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang X., Germann S., Blus B.J., Khorasanizadeh S., Gaudin V., Jacobsen S.E. (2007). The Arabidopsis LHP1 protein colocalizes with histone H3 Lys27 trimethylation. Nat. Struct. Mol. Biol. 14: 869–871 [DOI] [PubMed] [Google Scholar]
- Zhou W., Choi M., Margineantu D., Margaretha L., Hesson J., Cavanaugh C., Blau C.A., Horwitz M.S., Hockenbery D., Ware C., Ruohola-Baker H. (2012). HIF1α induced switch from bivalent to exclusively glycolytic metabolism during ESC-to-EpiSC/hESC transition. EMBO J. 31: 2103–2116 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zipori D. (2004). The nature of stem cells: State rather than entity. Nat. Rev. Genet. 5: 873–878 [DOI] [PubMed] [Google Scholar]