The establishment of the photorespiratory CO2 pump is thought to be an early and essential step in the evolution of C4 photosynthesis. This article provides experimental evidence that the establishment of this pump in the genus Flaveria was facilitated by the differential expression of Gly decarboxylase P-protein genes.
Abstract
C4 photosynthesis is nature’s most efficient answer to the dual activity of ribulose-1,5-bisphosphate carboxylase/oxygenase and the resulting loss of CO2 by photorespiration. Gly decarboxylase (GDC) is the key component of photorespiratory CO2 release in plants and is active in all photosynthetic tissues of C3 plants, but only in the bundle sheath cells of C4 plants. The restriction of GDC to the bundle sheath is assumed to be an essential and early step in the evolution of C4 photosynthesis, leading to a photorespiratory CO2 concentrating mechanism. In this study, we analyzed how the P-protein of GDC (GLDP) became restricted to the bundle sheath during the transition from C3 to C4 photosynthesis in the genus Flaveria. We found that C3 Flaveria species already contain a bundle sheath–expressed GLDP gene in addition to a ubiquitously expressed second gene, which became a pseudogene in C4 Flaveria species. Analyses of C3-C4 intermediate Flaveria species revealed that the photorespiratory CO2 pump was not established in one single step, but gradually. The knowledge gained by this study sheds light on the early steps in C4 evolution.
INTRODUCTION
Ribulose-1,5-bisphosphate carboxylase/oxygenase (Rubisco), the key enzyme of CO2 fixation in plants, is a bispecific enzyme. It not only operates as a carboxylase but also as an oxygenase. The product of the oxygenase reaction is the two-carbon compound 2-phosphoglycolate that has to be recycled in a process called photorespiration (Ogren and Bowes, 1971; Ogren, 1984). During photorespiration, CO2 is released, leading to a net loss of photoassimilated CO2. The loss of CO2 becomes problematic especially under hot and arid conditions when stomata have to close to avoid water loss (reviewed in Sage, 2004). Under these conditions, CO2 uptake is drastically reduced, and the relation between photosynthetic CO2 fixation and photorespiratory CO2 release becomes unfavorable.
The release of CO2 is catalyzed by Gly decarboxylase (GDC) in the mitochondria of plant cells (Oliver and Raman, 1995). GDC is a multiprotein system comprising the four proteins P-, L-, T-, and H-protein (gene designations GLDP, GLDL, GLDT, and GLDH, respectively) with the P-protein being the actual decarboxylase (Oliver and Raman, 1995). GDC is not only essential in photorespiration but also necessary for C1 metabolism that presumably takes place in all cells of a plant and provides one-carbon compounds for a number of biosynthetic pathways (Hanson and Roje, 2001). This was experimentally shown with a GLDP double knockout mutant of Arabidopsis thaliana that possesses no active GDC and cannot survive even under elevated CO2 (i.e., nonphotorespiratory conditions) (Engel et al., 2007).
C4 photosynthesis is one of nature’s answers to cope with the oxygenase activity of Rubisco. It is essentially a CO2 pump that concentrates CO2 at the site of Rubisco. In the vast majority of C4 species, the CO2-concentrating mechanism requires the close metabolic interaction of two different cells: mesophyll and bundle sheath cells. The bundle sheath cells typically form a wreath-like layer around the vasculature and harbor Rubisco and the other Calvin-Benson cycle enzymes. Bundle sheath cells are surrounded by the mesophyll cells, which are devoid of Rubisco but contain phosphoenolpyruvate carboxylase, an oxygen-insensitive carboxylase (Hattersley, 1984; Dengler and Nelson, 1999). The atmospheric CO2, after conversion to bicarbonate, is initially fixed by phosphoenolpyruvate carboxylase in the mesophyll, resulting in a four-carbon compound, malate and/or Asp, after which this photosynthetic pathway is named C4 photosynthesis. The C4 compound diffuses along its concentration gradient via the plasmodesmata into the bundle sheath cells where it becomes decarboxylated by NADP/NAD malic enzymes or phosphoenolpyruvate carboxykinase (Hatch et al., 1975). The released CO2 is finally channeled through Rubisco into the Calvin-Benson cycle. Due to the elevated CO2 concentration at the site of Rubisco, its oxygenase reaction is largely abolished, and photorespiration is drastically reduced in C4 plants (Hatch, 1987; Sage, 2004). This includes lower activities of enzymes of the photorespiratory pathway, most of which are restricted to the bundle sheath cells (Li et al., 2010).
C4 photosynthesis has independently evolved up to 66 times within the angiosperms (Sage et al., 2012). This polyphyletic origin of C4 photosynthesis suggests that the evolution of a C3 into a C4 species must have been relatively easy in genetic terms. The genus Flaveria (Powell, 1978) is an attractive model in which to study the transition from C3 to C4 photosynthesis. The genus includes not only true C3 and C4 species but also a large number of C3-C4 intermediate species with a differing degree of “C4-ness” (Edwards and Ku, 1987; McKown et al., 2005). The evolutionary analysis of the kinetic and regulatory characteristics of C4 phosphoenolpyruvate carboxylase and of the determinants for the mesophyll-specific transcription of its gene may serve as an example of how this genus can be exploited for dissecting the evolutionary trajectory from C3 to C4 photosynthesis (Stockhaus et al., 1997; Gowik et al., 2004; Akyildiz et al., 2007).
The compartmentation of GDC in the bundle sheath cells and, hence, its virtual absence in mesophyll cells is assumed to constitute a very early and essential step in the evolution toward C4 photosynthesis (Sage, 2004; Bauwe, 2011; Sage et al., 2012). Restriction of GDC to the bundle sheath cells results in the photorespiratory CO2 only being released in the bundle sheath. This results in the establishment of a photorespiratory CO2 pump creating a CO2-enriched environment for the Rubisco of the bundle sheath, but not of the mesophyll cells. Since Gly, a two-carbon compound, serves as a transport metabolite, this photorespiratory CO2 concentrating mechanism is also termed C2 photosynthesis. Immunolocalization experiments with the C3-C4 intermediate Moricandia arvensis indicated that the compartmentation of GDC activity in the bundle sheath cells was caused by the cell-specific restriction of only one of its components, the P-protein (Rawsthorne et al., 1988; Morgan et al., 1993).
This study seeks to answer the question of how the photorespiratory CO2 pump was established during the evolution of C4 photosynthesis in the genus Flaveria. From previous work, we knew that C4 Flaveria species contain only one functional GLDP gene (named GLDPA) that appears to be active only in the bundle sheath (Engelmann et al., 2008). We wanted to know how this expression specificity evolved, bearing in mind that C3 Flaveria species contain several GLDP genes (Bauwe et al., 1995), which should be active in all photosynthetic tissues. In recent work, we showed that the regulation of GLDP expression is complex, involving transcription from two promoters oriented in tandem and most probably also posttranscriptional control via differential RNA stability (Wiludda et al., 2012). The data presented here demonstrate that the photorespiratory CO2 pump in the genus Flaveria was established step by step and that it involved pseudogenization of one, and in addition, a relaxation of the bundle sheath specificity of another already existing GLDP gene.
RESULTS
The GLDP Gene Family of the Genus Flaveria
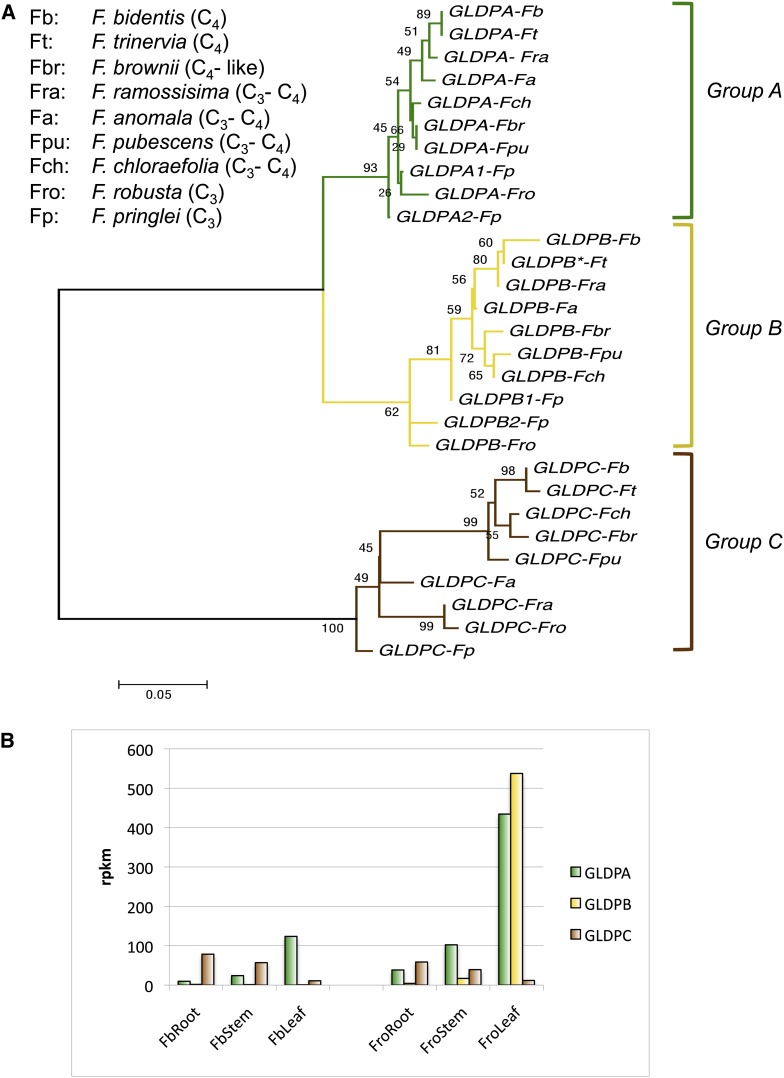
To get an overview of the structure of the GLDP gene family in C3, C4, and C3-C4 intermediate Flaveria species, we conducted a phylogenetic analysis. We used published sequences of cDNA or genomic clones (Kopriva and Bauwe, 1994; Bauwe et al., 1995; Bauwe and Kopriva, 1995; Chu, 1996) and de novo assembled sequences derived from RNaseq of different Flaveria species using 454 (Gowik et al., 2011) or Illumina (J. Mallmann, U. Gowik, and P. Westhoff, unpublished data) sequencing, respectively. The sequences were aligned using ClustalX (see Supplemental Data Set 1 online), and gene trees were constructed using the maximum likelihood method as described in Methods. Figure 1A shows that the GLDP genes of the various Flaveria species group into three clusters, A, B, and C. All nine analyzed Flaveria species contained one gene of each group with the exception of Flaveria pringlei in which two group A and two group B genes were found (Figure 1A). It is known that F. pringlei is a tetraploid (Cameron et al., 1989), probably arisen by allopolyploidization with the C3-C4 intermediate species Flaveria angustifolia (Kopriva et al., 1996; McKown et al., 2005). Additionally, GLDP cDNA sequences from Flaveria were compared with those from eudicot species with fully sequenced genomes. It turned out that the Flaveria GLDPs were more closely related to each other than to any other GLDP sequence (see Supplemental Figure 1 and Supplemental Data Set 2 online).
Figure 1.
Molecular Phylogenetic Analysis of GLDP Genes of the Genus Flaveria and GLDP Transcript Abundance in Organs.
(A) Maximum likelihood tree of GLDP sequences in Flaveria. The tree was constructed with MEGA5 (Tamura et al., 2011) using the Tamura 3 parameter model. The tree is drawn to scale, with branch length measured in the number of substitutes per site. The tree is based on 203 nucleotide positions, starting at the ATG, which were aligned using ClustalX 2.0.8 (Higgins and Sharp, 1988, 1989; Thompson et al., 1997; Larkin et al., 2007) (alignment is shown in Supplemental Data Set 1 online). Bootstrap values (1000 replicates) are shown next to the branches (Felsenstein, 1985). Branches corresponding to partitions reproduced in less than 50% of bootstrap replicates are collapsed.
(B) Abundance of GLDPA, GLDPB, and GLDPC transcripts in stems, roots, and leaves of F. bidentis (Fb; C4) and F. robusta (Fro; C3) as measured by mapping RNaseq Illumina reads on the respective cDNAs and expressed in reads per kilobase per million (rpkm).
The phylogenetic analysis revealed that group A and B GLDP genes are more closely related to each other than to group C genes. Group A GLDP genes contain GLDPA of Flaveria trinervia (C4; formerly gdcsPA; Cossu and Bauwe, 1998; accession number Z99767) and GLDPA1 and GLDPA2 of F. pringlei (C3; formerly gdcsPB and gdcsPA; Kopriva and Bauwe, 1994; Bauwe et al., 1995; Bauwe and Kopriva, 1995; accession numbers Z36879 and Z54239), all of which have been characterized by sequencing of genomic clones. Group B GLDP genes are exemplified by the GLDPB* pseudogene of F. trinervia (C4; formerly gdcsPB; Cossu and Bauwe, 1998; accession number Z99768) and the GLDPB1 and GLDPB2 genes of F. pringlei (C3; formerly gdcsPE and gdcsPD; Chu, 1996; accession numbers KC545951 and KC545950, respectively). The GLDP group C contains the GLDPC gene of F. pringlei (C3; formerly gdcsPC; Chu, 1996; accession number KC545949).
To determine the spatial expression patterns of the different GLDP genes, we used data sets from Illumina RNaseq experiments of roots, stems, and leaves of Flaveria bidentis (C4) and Flaveria robusta (C3). Figure 1B shows that transcripts of group C GLDP genes could be detected predominantly in roots and stems in both species, while the GLDP group A and group B genes accumulated preferentially in leaves. Accordingly, group A and group B GLDP genes are mainly relevant for photorespiration, whereas group C GLDP genes most likely are not involved in photorespiration but in the maintenance of basal C1 metabolism (Hanson and Roje, 2001) in stems and roots.
The Group A GLDP Genes of the C3 Flaveria Species F. pringlei and F. robusta Are Expressed Specifically in Bundle Sheath Cells
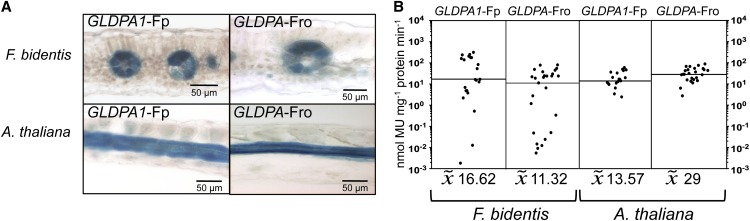
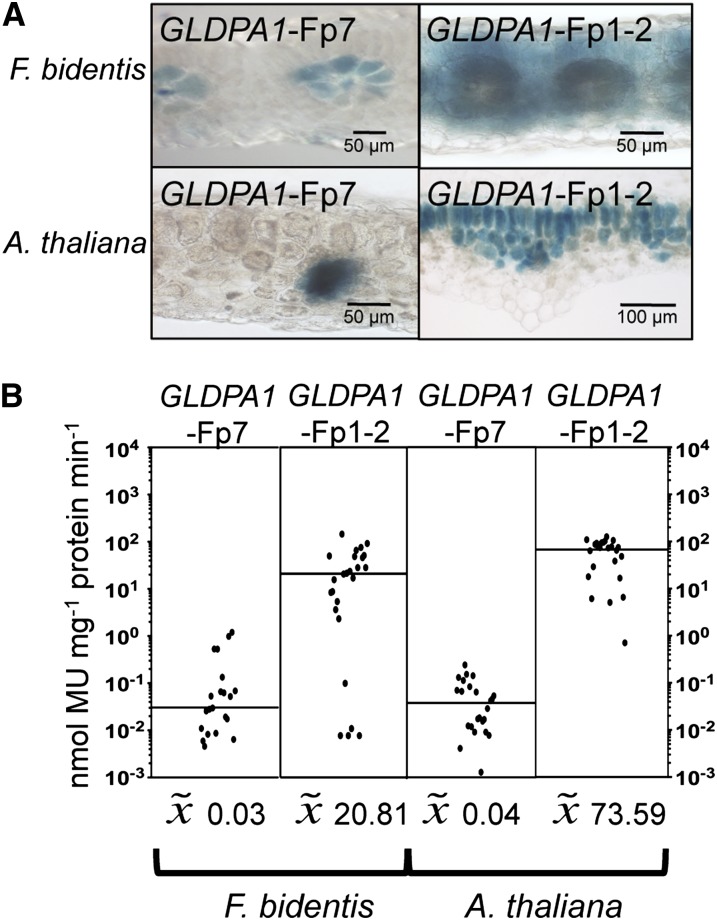
RNA in situ hybridization experiments had shown that GLDP transcripts accumulate in the leaves of F. trinervia and F. bidentis (both C4) only in bundle sheath cells (Engelmann et al., 2008). Promoter-reporter gene studies were in line with these observations and conclusions. When 1571 bp of 5′ flanking sequences of the F. trinervia GLDPA gene (including the 5′ untranslated region upstream of the AUG codon) were fused to the β-glucuronidase (GUS) reporter gene and transformed into F. bidentis (C4), reporter gene activity was only observed in the bundle sheath cells and to a small degree in the vascular bundle, but not in the mesophyll tissue (Engelmann et al., 2008). To gain insight into how bundle sheath–specific GLDP expression evolved in the genus Flaveria, we analyzed the expression specificity of the GLDPA genes of F. pringlei (C3) and F. robusta (C3) in both the C4 plant F. bidentis and the C3 species Arabidopsis.
The 5′ flanking sequences of the GLDPA genes of F. pringlei (GLDPA1, 2217 bp; GLDPA2, 2040 bp; Bauwe et al., 1995) and F. robusta (1154 bp; accession number KC545947) were fused to the GUS reporter gene and transformed into F. bidentis plants. Figure 2A demonstrates that the 5′ flanking sequences of the GLDPA1 gene of F. pringlei and the GLDPA gene of F. robusta were able to drive expression of the GUS reporter gene predominantly in the bundle sheath cells and to a lesser extent in the vasculature (compared with Engelmann et al., 2008; Wiludda et al., 2012). No GUS activity could be detected in the mesophyll cells (Figure 2A, top panel). The promoter strengths of the two GLDPA 5′ flanking sequences were comparable (Figure 2B) and were in the same range as that of the GLDPA promoter of F. trinervia (compared with Engelmann et al., 2008). The 5′ flanking sequence of GLDPA2 of F. pringlei exhibited a similar expression behavior in F. bidentis as the other two GLDPA 5′ flanking sequences (see Supplemental Figure 2A online, top panel).
Figure 2.
Functional Analysis of the 5′ Flanking Sequences of the GLDPA Genes of the C3 Species F. pringlei and F. robusta.
The 5′ flanking sequences of GLDPA1-Fp (2217 bp) and GLDPA-Fro (1154 bp) were fused to the GUS reporter gene and analyzed in transgenic F. bidentis (C4) and transgenic Arabidopsis (C3).
(A) Histochemical localization of GUS activity in leaf sections of transgenic plants.
(B) GUS activities in leaves of transgenic plants. Each dot indicates an independent transgenic line. The black line represents the median. MU, 4-methylumbelliferone.
Transformation systems for C3 Flaveria species are not available. Since the Brassicacean C3 species Arabidopsis faithfully recapitulates the expression profile of the GLDPA 5′ flanking region of the C4 species F. trinervia (Engelmann et al., 2008), this species was also used for the analysis of the 5′ flanking sequences of the GLDPA genes of F. pringlei and F. robusta. Figure 2A shows that the spatial expression pattern of the GLDPA1 5′ flanking region of the C3 Flaveria species F. pringlei in transgenic Arabidopsis resembles that observed for the C4 plant F. bidentis. Promoter activity could not be detected in the mesophyll cells but only in the bundle sheath and the vasculature. In addition, the promoter strength in Arabidopsis was also comparable to that in F. bidentis (Figure 2B). Thus, the expression profile of the GLDPA1 promoter of the C3 species F. pringlei in Arabidopsis is indistinguishable from that of the GLDPA promoter of F. trinervia in this distantly related C3 species (compared with Engelmann et al., 2008). The 5′ flanking regions of the GLDPA gene of F. robusta and of the GLDPA2 gene of F. pringlei also function essentially as bundle sheath/vasculature-specific promoters in Arabidopsis; however, they additionally exhibit a faint activity in the mesophyll tissue (Figure 2A; see Supplemental Figure 2A online).
Taken together, the expression specificities and quantities encoded by the 5′ flanking sequences of the group A GLDP genes of the two C3 Flaveria species are almost indistinguishable from that of the 5′ flanking region of the GLDPA gene of the C4 species F. trinervia. This suggests that the last common ancestor, leading to extant C3 and C4 Flaveria species, already had a bundle sheath specific GLDP gene.
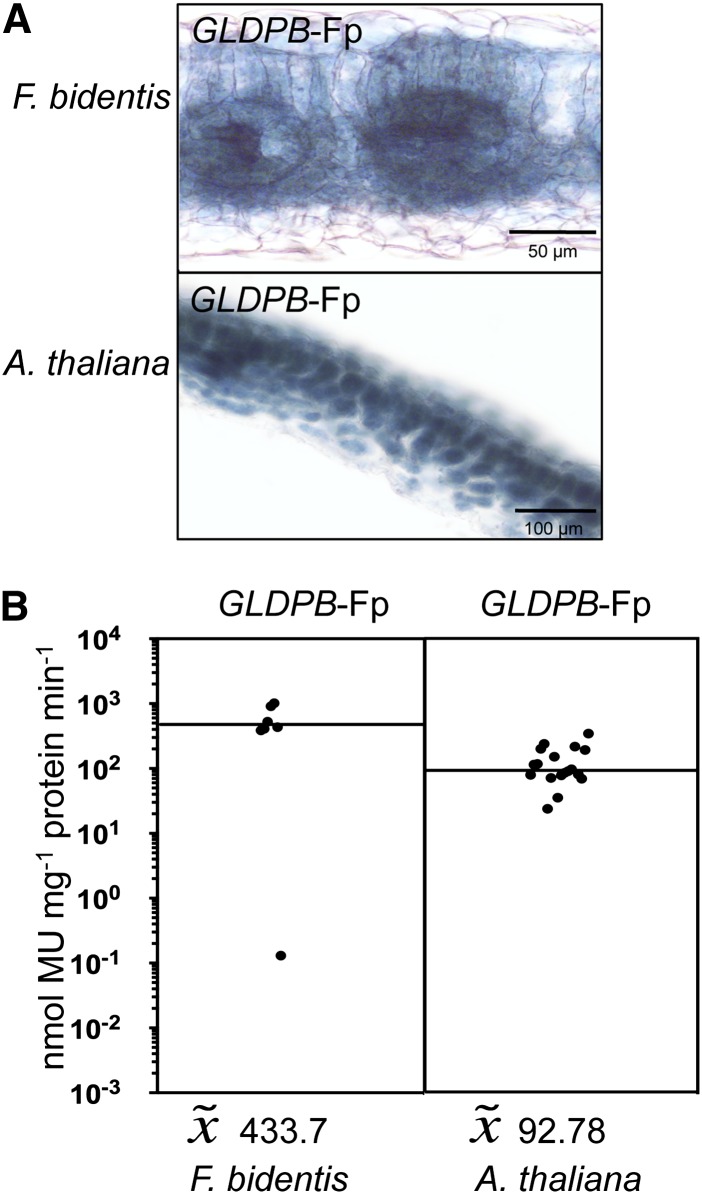
The GLDPB2 Gene of the C3 Species F. pringlei Is Active in All Photosynthetic Tissues of Both F. bidentis (C4) and Arabidopsis (C3)
The group B gene GLDPB* of F. trinervia (C4) is known to be a pseudogene due to an insertion into the first exon leading to an interruption of the GLDPB reading frame (Cossu and Bauwe, 1998). Moreover, the 5′ flanking region (1981 bp) of GLDPB* did not show any promoter activity when analyzed in Arabidopsis (see Supplemental Figure 3 online). To assess the expression specificity of the 5′ flanking sequences of group B GLDP genes of C3 Flaveria species, 2733 bp of the 5′ flanking region of the GLDPB2 gene of F. pringlei were fused to the GUS reporter gene and analyzed in transgenic F. bidentis (C4) and Arabidopsis (C3). Figure 3 shows that this 5′ flanking region drives the expression of the reporter gene in all photosynthetic leaf tissues in both species.
Figure 3.
Functional Analysis of the 5′ Flanking Sequence of a Group B GLDP Gene of the C3 Species F. pringlei.
The 5′ flanking sequence of GLDPB2-Fp (2733 bp) as a representative of group B GLDP genes of C3 Flaveria was fused to the GUS reporter gene and analyzed in transgenic F. bidentis (C4) and transgenic Arabidopsis (C3).
(A) Histochemical localization of GUS activity in leaf sections of transgenic plants.
(B) GUS activities in leaves of transgenic plants. Each dot indicates an independent transgenic line. The black line represents the median. MU, 4-methylumbelliferone.
It follows that C3 Flaveria species contain at least two GLDP genes, one each from groups A and B, which differ in their expression patterns in leaves. While group A GLDP genes are specifically/preferentially expressed in the bundle sheath, group B GLDP genes are active in all photosynthetic tissues. During evolution of C4, the ubiquitously expressed group B GLDP gene was converted into a pseudogene; hence, GLDP expression in leaves became bundle sheath specific.
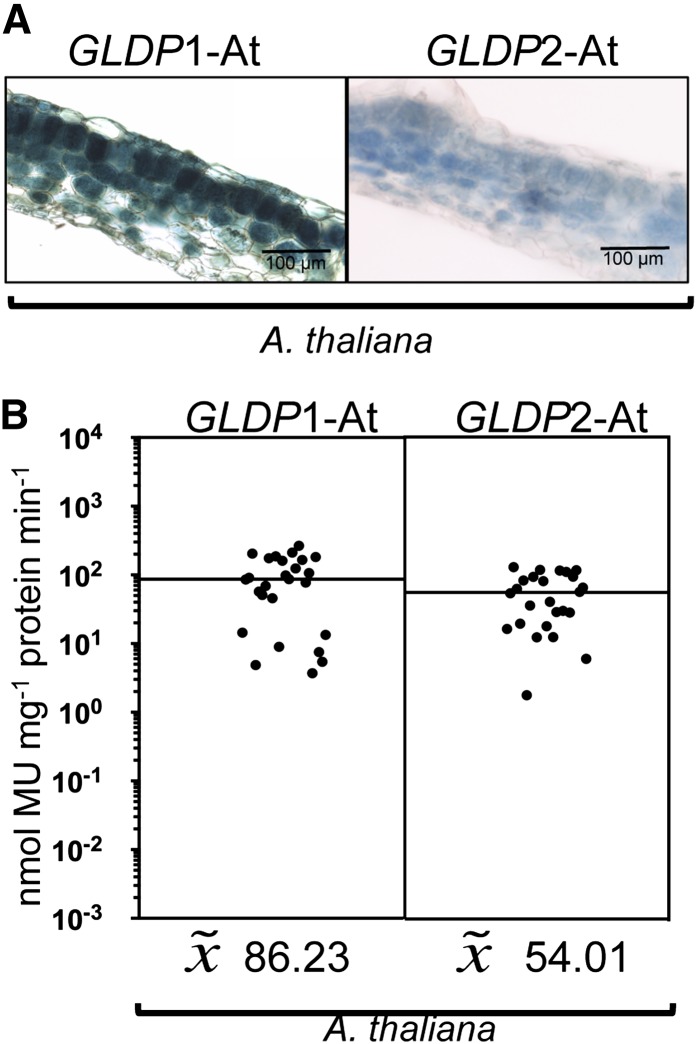
The 5′ Flanking Sequences of the Two GLDP Genes of Arabidopsis Do Not Direct Any Tissue Specificity in Leaves
To investigate whether a bundle sheath–specific GLDP gene might be a common feature of C3 plants, we analyzed the promoters of the two GLDP genes of Arabidopsis. We fused the 1852- and 1451-bp 5′ flanking sequences of both genes, GLDP1 (AT4G33010) and GLDP2 (AT2G26080), to the GUS reporter gene and transformed these constructs into Arabidopsis. Figure 4 demonstrates that both 5′ flanking regions drive GUS expression in all photosynthetic leaf tissues, supporting the genetic findings that the two GLDP genes act redundantly (Engel et al., 2007). A bundle sheath–specific GLDP gene is therefore not a common feature of dicotyledonous C3 plants.
Figure 4.
Functional Analysis of the 5′ Flanking Sequences of GLDP1 and GLDP2 Genes of Arabidopsis.
The 5′ flanking sequences of GLDP1 (1852 bp) and GLDP2 (1451 bp) of Arabidopsis (C3) were fused to the GUS reporter gene and analyzed in transgenic Arabidopsis (C3).
(A) Histochemical localization of GUS activity in leaf sections of transgenic plants.
(B) GUS activities in leaves of transgenic plants. Each dot indicates an independent transgenic line. The black line represents the median. MU, 4-methylumbelliferone.
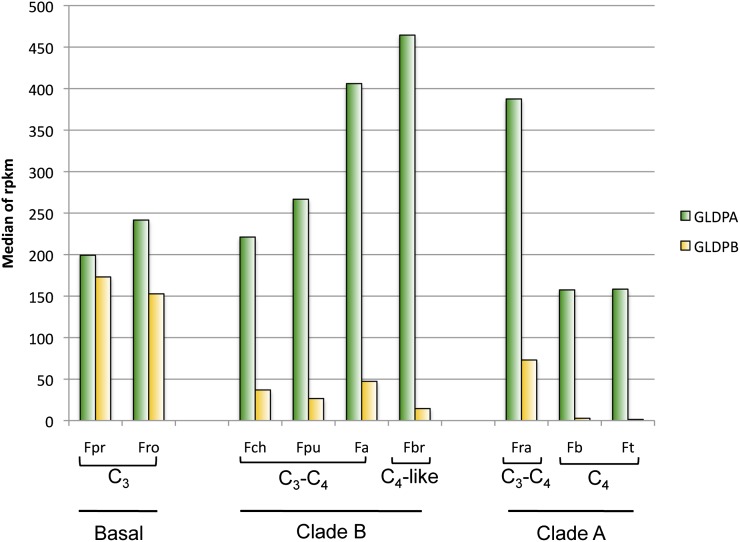
Expression of Group B GLDP Genes in Different Flaveria Species Is Negatively Correlated to the Degree of C4-Ness, While Group A GLDP Genes Show a Maximum of Expression in C3-C4 Intermediate Species
The genus Flaveria offers the unique opportunity to study the steps taken during evolution from C3 to C4 photosynthesis, particularly because of its large number of C3-C4 intermediate species. According to McKown et al. (2005), the genus contains three main phylogenetic groups. The first diverging group includes the three C3 Flaveria species (F. pringlei, Flaveria cronquistii and F. robusta) and the C3-C4 intermediate Flaveria sonorensis. Clade B contains seven C3-C4 intermediate species and the C4-like species Flaveria brownii. All true C4 Flaveria species belong to clade A, which also contains several C4-like species (for instance, Flaveria palmeri) and the C3-C4 intermediate Flaveria ramosissima. It is thought that C3-C4 intermediate (C2) photosynthesis evolved twice in Flaveria, once in the predecessor of the extant basal species F. sonorensis, and once in the line leading to the last common ancestor of clade A and B species (McKown et al., 2005).
To obtain insight into changes in the expression of group A and B GLDP genes, we used RNaseq to compare their RNA amounts in the leaves of different Flaveria species, ranging from C3 (F. pringlei and F. robusta [basal Flaveria species]), via C3-C4 intermediate species with varying degrees of C4-ness (F. chloraefolia, F. pubescens, F. anomala [all belonging to clade B], and F. ramossisima [clade A]), and the C4-like species F. brownii (clade B), to the fully fledged C4 species F. bidentis and F. trinervia (both belonging to clade A).
Figure 5 illustrates that the amounts of group B GLDP transcripts are lower in C3-C4 intermediates than in the C3 reference. Even lower levels of group B GLDP transcripts were found in the C4-like species F. brownii, and no group B GLDP transcripts were detected in the two C4 Flaveria species. By contrast, group A GLDP transcripts increased continuously from C3, to C3-C4 intermediates, to the C4-like species, but dropped to lower levels in the true C4 species, as expected. These findings indicate a progressive increase of C2 cycle activity in both Flaveria clades until the establishment of a fully functional C4 pathway in clade A species.
Figure 5.
Transcript Abundance of Group A and Group B GLDP Genes in C3, C3-C4 Intermediate, and C4 Flaveria Species.
The transcript abundance was calculated as the median of four Illumina RNaseq experiments and is expressed in reads per kilobase per million (rpkm). Transcript abundances of group A GLDP genes are displayed in green, and transcript abundances of group B are displayed in yellow. Fp, F. pringlei; Fro, F. robusta; Fch, F. chloraefolia; Fpu, F. pubescens; Fa, F. anomala; Fra, F. ramossisima; Fbr, F. brownii; Fb, F. bidentis; Ft, F. trinervia.
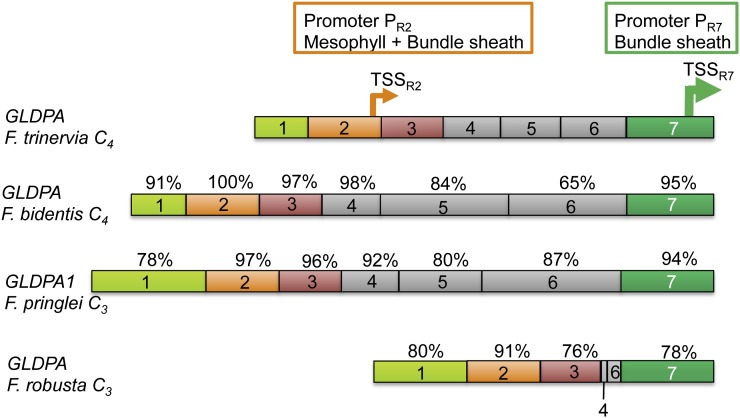
The Tandem Promoter Structure of the GLDPA Gene of F. trinervia (C4) Is Evolutionary Conserved in GLDPA-Type Genes
The transcriptional regulation of the GLDPA gene of F. trinervia (C4) appears to be rather complex, since its 5′ flanking sequence was shown to contain two transcriptional start sites, each of which is preceded by a promoter (Wiludda et al., 2012). The proximal promoter, defined by region 7 (PR7; Figure 6) of the 5′ flanking sequence, is responsible for the expression in the bundle sheath and the vasculature, while the distal promoter, defined by region 2 (PR2; Figure 6), is active in all green leaf tissues (Wiludda et al., 2012). The most reasonable explanation for this dual promoter structure is the need for small amounts of GLDP in the mesophyll cells of C4 plants for maintaining C1 metabolism, after the complete switch off of the GLDPB genes (Wiludda et al., 2012). We wanted to know whether this promoter organization is specific for C4 Flaveria species or whether it is an ancient feature of GLDPA genes and occurred already in C3 Flaveria species.
Figure 6.
Schematic Comparison of the 5′ Flanking Sequences of Group A GLDP Genes from C4 and C3 Flaveria Species.
The 5′ flanking sequence of the GLDPA gene of F. trinervia was divided into seven functionally characterized regions (Engelmann et al., 2008). Regions 2 (orange) and 7 (dark green) contain the two subpromoters PR2 and PR7. Region 1 (light green) enhances the activities of both subpromoters. Region 3 (red) is required to suppress the mesophyll activity of the subpromoter PR2, but only in Arabidopsis. Regions 4 to 6 (gray) are not required for promoter activity. The 5′ flanking sequences of the GLDPA genes from F. bidentis (C4), F. pringlei (C3), and F. robusta (C3) are color-labeled according to their homologous regions in the GLDPA 5′ flanking region of F. trinervia. Similarities are given as percentage of identical nucleotide positions relative to the corresponding regions of GLDPA-Ft.
The comparison of the 5′ flanking sequences of the GLDPA1 and GLDPA genes from the C3 species F. pringlei and F. robusta with those from the C4 species F. trinervia and F. bidentis (accession number KC545946; Figure 6) revealed a high degree of sequence conservation in regions 2 and 7, which define the distal and proximal promoters, respectively. High levels of sequence similarity were also observed for regions 1 and 3, while the regions in between (i.e., regions 4 to 6) are much less conserved and may even be drastically shortened as in F. robusta (Figure 6).
To test experimentally whether regions 2 and 7 of the 5′ flanking regions of the GLDPA1 and GLDPA genes of F. pringlei and F. robusta, respectively, function as promoters, they were fused to the GUS reporter gene, and the constructs were transformed into F. bidentis (GLDPA1 of F. pringlei) and Arabidopsis (GLDPA1 of F. pringlei and GLDPA of F. robusta).
Figure 7 illustrates that region 7 of the GLDPA1 5′ flanking region of F. pringlei directs bundle sheath expression in F. bidentis, while region 2 shows promoter activity in all green leaf tissues. Similar expression profiles were observed when the two regions from the GLDPA1 gene of F. pringlei or from the GLDPA gene of F. robusta were analyzed in transgenic Arabidopsis (Figure 7; see Supplemental Figure 4 online). It follows that the GLDPA1 and GLDPA genes of F. pringlei and F. robusta, respectively, possess the same dual promoter structure as found in the 5′ flanking sequence of the GLDPA gene of the C4 species F. trinervia.
Figure 7.
Functional Analysis of the Subpromoters PR7 and PR2 of the GLDPA1 Gene of F. pringlei.
Regions 1 and 2 (GLDPA-Fp1-2; 641 bp) and 7 (GLDPA-Fp7; 318 bp) of the 5′ flanking region of the GLDPA gene of F. pringlei (C3) were fused to the GUS reporter gene and analyzed in transgenic F. bidentis (C4) and transgenic Arabidopsis (C3).
(A) Histochemical localization of GUS activity in leaf sections of transgenic plants.
(B) GUS activities in leaves of transgenic plants. Each dot indicates an independent transgenic line. The black line represents the median. MU, 4-methylumbelliferone.
The Amounts of Transcripts Derived from the Distal Subpromoter of the GLDPA Gene of C3 Flaveria Species Are Negligible
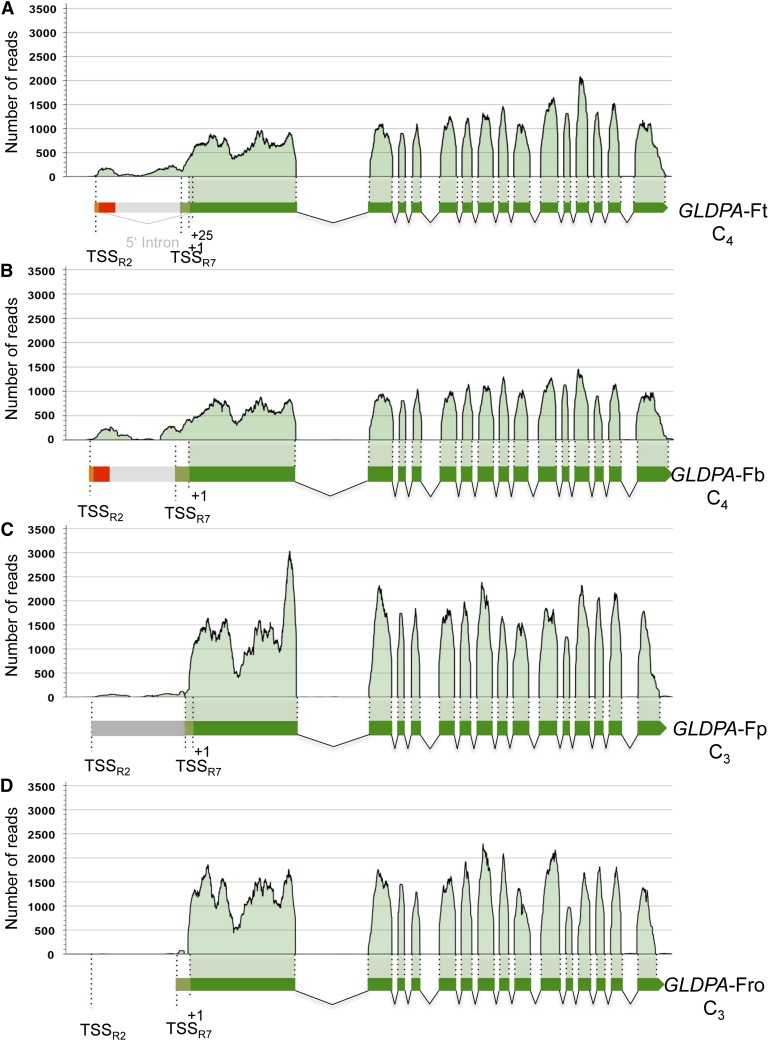
When assayed by promoter-reporter gene fusions, the distal GLDPA subpromoter of F. trinervia (defined by region 2) turned out to be much stronger than the proximal subpromoter (defined by region 7; Wiludda et al., 2012). By contrast, when the two promoters are in their natural configuration as in the 5′ flanking region of the F. trinervia GLDPA gene, their RNA output was opposite. Only traces of transcripts originating from the distal transcriptional start site were detectable, while RNAs starting at the proximal site made up the vast majority of all GLDPA transcripts (Wiludda et al., 2012). To test if the same was true for the GLDPA promoters of C3 and C3-C4 intermediate Flaveria species, we used the RNaseq data available for C3, C3-C4, and C4 Flaveria species and mapped the reads against the genomic sequence of GLDPA from F. trinervia.
We found that the vast majority of all GLDPA transcripts of F. trinervia (C4), but also of F. bidentis (C4), originated from the proximal transcriptional start site (TSSR7) and that only small but clearly detectable amounts arose from the distal site (TSSR2; Figures 8A and 8B). By contrast, transcripts derived from the distal transcriptional start site were drastically reduced (F. pringlei) or even absent (F. robusta) in the GLDPA transcript populations of the C3 Flaveria species (Figures 8C and 8D). In C3-C4 intermediate species, the amounts of transcripts derived from the distal transcription start site were in between those of the C4 and C3 Flaveria species (see Supplemental Figures 5A to 5E online). Taken together, the data indicate that RNA output from the distal promoter of the GLDPA 5′ flanking region is negligible in the C3 species but that it rose to small but clearly detectable amounts during C4 evolution. Moreover, it has to be inferred that the promoter structures of the GLDPA 5′ flanking regions were functionally conserved during evolution within clades A and B of the genus Flaveria.
Figure 8.
Transcript Coverage of the GLDPA Genes of F. trinervia, F. bidentis, F. pringlei, and F. robusta Leaf RNaseq Experiments.
Illumina reads obtained from sequencing the leaf transcriptomes of F. trinervia (C4) (A), F. bidentis (C4) (B), F. pringlei (C3) (C), and F. robusta (C3) (D) were mapped on the sequence of the GLDPA gene of F. trinervia including its 5′ flanking sequence. Region 2 (orange); region 3 (red); regions 4, 5, and 6 (gray); and region 7 (light green) are indicated in the 5′ flanking region, and exon parts of the coding region are indicated in dark green. The numbers of reads covering each position of the gene sequence were counted.
Splicing of Transcripts Derived from the Distal Transcriptional Start Site Changed during C4 Evolution
Transcripts originating from the distal transcriptional start site of the GLDPA gene of F. trinervia contain a large intron of ∼1000 nucleotides that has to be spliced in order to generate functional GLDPA mRNAs (Wiludda et al., 2012). These spliced mRNAs can be detected in F. trinervia, although only in small amounts. The large GLDPA 5′ intron is spliced out much less efficiently than the gene-internal introns (Wiludda et al., 2012). Unspliced GLDPA transcripts of F. trinervia appear to be unstable, possibly due to the presence of many open reading frames within the 5′ intron, which could be involved in activating the nonsense-mediated mRNA decay pathway (Wiludda et al., 2012). Since RNA output from the distal promoter was barely detectable in the C3 species (Figures 8C and 8D) but detectable in small amounts in the C3-C4 intermediates (see Supplemental Figures 5A to 5E online), we wanted to know when during C4 evolution the 5′ intron and its splicing were established.
Inspection of the RNaseq data did not reveal any correctly spliced GLDPA RNAs derived from the distal transcriptional start site in the two C3 species or the C3-C4 intermediates. By contrast, in the C4-like species F. brownii, small amounts of spliced GLDPA RNA accumulate (see Supplemental Figure 6 online). As expected, splicing of the 5′ intron occurs also in the C4 species F. bidentis (see Supplemental Figure 6 online). A functional spliced 5′ intron was therefore not only observed in the C4 Flaveria species F. bidentis and F. trinervia, but also in the C4-like species F. brownii. This indicates that its presence is a typical feature of C4 and C4-like Flaveria species.
Interestingly, the splice acceptor sites differ in the two C4 species (see Supplemental Figure 6 online). While the splice acceptor site of the GLDPA gene of F. trinervia is located ∼16 nucleotides downstream of the first ATG codon of the GLDPA open reading frame, the acceptor sites found in F. bidentis and also in F. brownii are positioned ∼15 nucleotides upstream (see Supplemental Figure 6 online). We conclude that the splicing of the 5′ intron was established at least two times independently during C4 speciation in clade A of Flaveria.
DISCUSSION
The photorespiratory CO2 pump is considered to be an essential and early step in the evolutionary trajectory toward C4 photosynthesis (Sage, 2004; Bauwe, 2011; Sage et al., 2012). The establishment of this pump required that the Gly-decarboxylating step of the photorespiratory pathway, which is performed by GDC, became restricted to the bundle sheath cells. Earlier studies with C3-C4 intermediate species indicated that modifications to the gene encoding the P-protein, the actual GDC, were responsible for this reallocation of GDC activity from the mesophyll to the bundle sheath and that this event occurred rather early during C4 evolution (Monson et al., 1984; Morgan et al., 1993; Sage, 2004; Bauwe, 2011; Sage et al., 2012). Two questions immediately arose from this evolutionary scenario: By which gene regulatory mechanism did GLDP become restricted to the bundle sheath cells, and how was this compartmentation achieved in time? Work presented here, using the genus Flaveria as an evolutionary model, provides conclusive answers to both questions.
C3 Flaveria Species Contain a Bundle Sheath Cell–Specific GLDP Gene
Two scenarios can be imagined how a bundle sheath–specific expression of GLDP evolved during the transition from C3 to C4. First, a ubiquitously expressed GLDP gene changed its expression behavior to become bundle sheath specific. Alternatively, a GLDP gene with the requested bundle sheath specificity of expression was already present in C3 species and an additional, ubiquitously expressed GLDP gene became inactivated during C4 evolution. The first scenario is best exemplified by the evolution of mesophyll expression specificity in the ppcA phosphoenolpyruvate carboxylase gene of the genus Flaveria (Stockhaus et al., 1997; Gowik et al., 2004; Akyildiz et al., 2007).
By contrast, the evolution of bundle sheath specificity of GLDP expression in Flaveria followed the second scenario. The GLDPA genes of the C3 Flaveria species F. pringlei and F. robusta already contained promoter sequences driving bundle sheath–specific gene expression in both C4 and C3 plants (Figure 2; see Supplemental Figure 4 online). The other leaf-expressed GLDP genes (i.e., those of group B) were expressed in all photosynthetically active tissues of C3 Flaveria species, but turned into pseudogenes in the C4 species (Cossu and Bauwe, 1998; Figure 5; see Supplemental Figure 3 online).
The occurrence of bundle sheath–specific GLDP genes is not a universal feature of C3 plants. The C3 species Arabidopsis, for instance, contains two GLDP genes both of which are expressed similarly in all leaf chlorenchyma cells as concluded from their promoter activities (Figure 4). The presence of a GLDP gene with bundle sheath specificity of expression in C3 Flaveria species should therefore be viewed as part of a preconditioning syndrome that distinguishes C3 taxa that evolved C4 photosynthesis from others that did not (Sage, 2004; Sage et al., 2011, 2012). Such a preconditioning phase has been proposed as an inherent, most likely necessary step in C4 evolution. One can envision that the presence of a bundle sheath–specific GLDP gene, in addition to a ubiquitously expressed GLDP gene, in a C3 species facilitated the evolution of C3-C4 intermediate photosynthesis. This is because a knockout or the drastic downregulation of the ubiquitously expressed GLDP gene would suffice to initiate the establishment of a photorespiratory CO2 pump as a precondition to evolve the C4 pathway (Sage, 2004; Bauwe, 2011; Sage et al., 2012). As deduced from the phylogeny (McKown et al., 2005), a photorespiratory CO2 pump must have evolved twice in Flaveria, once in the lineage leading to the C3-C4 intermediate F. sonorensis, and once in the lineage leading to the C3-C4 intermediate and C4 species of clades A and B.
To support the notion that the presence of bundle sheath–specific GLDP genes in C3 species could be a general enhancer of C4 evolution, one could analyze GLDP genes from C3 species closely related to clades with several C4 origins, such as the Amaranthaceae or the PACMAD clade of grasses. The grasses are probably the oldest angiosperm lineage in which C4 species evolved (Edwards et al., 2010) and therefore illustrate how fully optimized C4 species finally look with respect to metabolic organization and the underlying transcriptional regulation. While the genome of the C3 grass rice (Oryza sativa) contains two GLDP genes (Goff et al., 2002; LOC_Os01g51410 and LOC_OS06g40940), the genomes of the C4 grasses maize (Zea mays; Schnable et al., 2009) (GRMZM2G104310), sorghum (Sorghum bicolor; Paterson et al., 2009) (Sb08g003440), and Setaria italica (Bennetzen et al., 2012) (Si000068m) harbor only one GLDP copy. If the C4 grasses represent the terminal stage of C4 evolution and if they pursued a similar evolutionary path as Flaveria, one may speculate that their ubiquitously expressed GLDP gene(s) have been lost from the genomes after pseudogenization.
The Photorespiratory CO2 Pump in Flaveria Is Established Gradually
It was proposed that the photorespiratory CO2 pump was established by an abrupt loss of GLDP in the mesophyll cells (Sage, 2004; Sage et al., 2012). This hypothesis does not comply with our RNA profiling studies, which included a representative set of species from all different phylogenetic groups of Flaveria ranging from those with C3 through C3-C4 intermediate and C4-like to C4 photosynthesis. Our investigations demonstrated that GDC disappeared from the mesophyll cells not abruptly but gradually. We showed that the ubiquitously expressed group B GLDP genes are downregulated in the C3-C4 intermediate Flaveria species belonging to clade A as well as to clade B compared with the C3 species. However, they are not completely switched off (Figure 5). Clade A and B Flaveria species derived from a common ancestor that was most likely a C3-C4 intermediate (McKown et al., 2005). The reduction in the expression of group B GLDP genes has therefore been initiated during the establishment of the photorespiratory CO2 pump in this last common ancestor of clade A and B Flaveria species. After the separation of clade A and B species, expression of these genes was progressively further reduced in clade B species (i.e., the C4-like species F. brownii) or even completely switched off in the C4 species of clade A.
These results are in line with a recent study that modeled the evolution of the C4 pathway based on combining a biochemical model with a population genetic framework (Heckmann et al., 2013). The model shows that all C3-C4 intermediates, including those from clade B, can be treated as true evolutionary intermediates with the potential to develop a fully functional C4 cycle. The model predicts that the photorespiratory CO2 concentrating cycle must have originated early during the evolution of C4 photosynthesis and that the cycle was established gradually.
Is it plausible that the photorespiratory CO2 pump was not established abruptly (Sage, 2004), but step by step? One can imagine that the capacities to decarboxylate large amounts of Gly efficiently and recapture the correspondingly large amounts of photorespiratory CO2 were not ab initio present in the bundle sheaths of C3 ancestors of contemporary C4 plants. Indeed, bundle sheath cells of present C3 species with “Proto-Kranz” anatomy are still relatively poor in chloroplasts and mitochondria (Muhaidat et al., 2011; Sage et al., 2012). If such a C3 species were to abruptly lose all its Gly decarboxylation activity in the mesophyll, it would most probably not be viable anymore. A gradual reduction of Gly decarboxylation in the mesophyll cells could initiate a series of steps organized in a positive feedback loop (Bauwe, 2011; Muhaidat et al., 2011; Sage et al., 2012). Gly had to diffuse to the bundle sheath for decarboxylation, thereby creating a higher CO2 concentration around Rubisco in the bundle sheath. The Rubisco in the bundle sheath would become more engaged in CO2 fixation than the mesophyll enzyme, thus creating a selection pressure to enhance the number of bundle sheath chloroplasts and the amount of Rubisco in these cells. Even more Gly decarboxylation activity could then be shifted to the bundle sheath cells; concomitantly, the number of mitochondria would increase. This would lead to further CO2 enrichment in the bundle sheath and allow an increase in the amount of Rubisco in this compartment, in which the enzyme would operate under the favorable condition of an elevated CO2 concentration. Due to the surrounding mesophyll, a second beneficial outcome of this positive feedback loop would have been a higher CO2 refixation capacity than before. Other evolutionary adaptations would have occurred in parallel, for instance, the optimization of organelle positioning in the bundle sheath cells (i.e., centripetal mitochondria and centrifugal chloroplasts), the upregulation of inter- and intracellular Gly and Ser transport, and/or changes in overall leaf anatomy.
The Tandem Promoter Structure of GLDPA Is Conserved in Both C3 and C4 Flaveria Species, but the RNA Output Is Not
The 5′ flanking sequences of the GLDPA genes of C3 Flaveria species are very similar to those of their counterparts in the C4 species. The tandem promoter structure is highly conserved (Figure 6) and both promoters of the C3 species direct the same expression specificities in transgenic Arabidopsis and F. bidentis as the corresponding regions from the C4 species F. trinervia (compared with Wiludda et al., 2012; Figure 7; see Supplemental Figure 4 online). By contrast, the GLDPA transcript profiles differ between the C3 and C4 Flaveria species. While the RNA output from the distal GLDPA promoter is negligible in C3 Flaveria species, RNAs derived from this promoter accumulate to small amounts in C4 Flaveria species (Figure 8).
These findings could suggest that the distal GLDPA promoter is silent in the context of the authentic 5′ flanking region in the C3 Flaveria species, whereas its counterpart in the C4 Flaveria species is active. This would imply that the distal promoter is cryptic in the C3 species and became activated only in the course of C4 evolution. The promoter activation could be brought about by changes in the rates of transcriptional initiation, pausing, or elongation (reviewed in Shearwin et al., 2005; Levine, 2011; Palmer et al., 2011). Alternatively, the different RNA output from the distal promoter in C3 versus C4 Flaveria species may not be regulated transcriptionally but posttranscriptionally at the level of transcript stability. Indeed, tentative evidence indicates that the 5′ intron is involved in regulating the accumulation of stable transcripts from the distal promoter of the GLDPA gene of F. trinervia (C4) (Wiludda et al., 2012), possibly via nonsense-mediated mRNA decay (Kertesz et al., 2006; Hori and Watanabe, 2007; Brogna and Wen, 2009). How much each of these regulatory levels contributes to the differences in RNA output from these two types of orthologous promoters remains to be investigated.
Why do the distal promoters of C3 and C4 Flaveria species differ in their RNA output? We proposed recently (Wiludda et al., 2012) that expression from the distal GLDPA promoter must be leaky in C4 Flaveria species because each plant cell must be capable of synthesizing C1 compounds regardless of whether or not it photorespires. A complete shutdown of GDC in the mesophyll cells of C4 plants would thus be fatal (Bauwe, 2011). The promoter could be silent in C3 and C3-C4 intermediate species because these plants possess a group B GLDP gene that is active in all leaf chlorenchyma cells (Figure 5); consequently, no selective pressure would favor leakiness in expression, as is observed in C4 species.
From Evolutionary Analysis to Synthetic Experimental Evolution
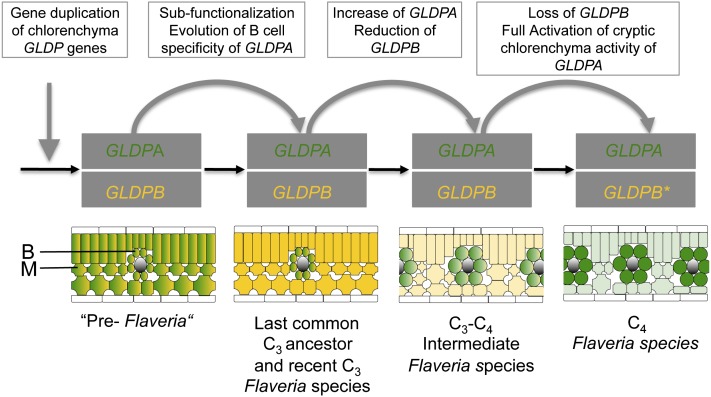
Flaveria is the youngest genus with respect to C4 evolution (Christin et al., 2011a), and the large number of C3-C4 intermediate species (Edwards and Ku, 1987; McKown et al., 2005) suggests that C4 evolution is still going on in Flaveria. We used Flaveria to analyze the evolutionary trajectory toward the establishment of a photorespiratory CO2 pump and its further integration into the C4 pathway. The model derived from these studies shows that the photorespiratory CO2 pump evolved step by step and that this gradual evolution was eased by the presence of duplicated GLDP genes differing in expression specificity (Figure 9).
Figure 9.
Model for the Evolution of Bundle Sheath–Specific GLDP Expression in the Genus Flaveria.
The duplication of a photorespiratory GLDP gene in early, ancestral C3 Flaveria species led to two ubiquitously expressed GLDP genes with identical expression patterns in all chlorenchyma tissues (M, mesophyll; B, bundle sheath) of the leaf. Subfunctionalization remodeled the expression of the group A GLDP genes to become bundle sheath specific and led to an ancestral C3 species with the same spatial GLDP expression pattern as today’s C3 species. During transition to C3-C4 intermediate photosynthesis, the expression level of group B GLDP genes was reduced and the distal GLDPA subpromoter became activated. Finally, group B GLDP genes were inactivated by pseudogenization. Green, group A GLDP spatial expression; yellow, group B GLDP spatial expression.
We do not know whether this evolutionary scenario is unique for Flaveria or whether it represents a general model for C3-to-C4 transitions. It would be worthwhile, therefore, to study phylogenetically diverse genera that contain both C3 and C4 species, and ideally also C3-C4 intermediates, as for instance Mollugo (Christin et al., 2011b), Cleome (Marshall et al., 2007), or Heliotropium (Muhaidat et al., 2011). An alternative approach could pursue synthetic experimental evolution (Morange, 2009) using C3 model plants such as Arabidopsis that can easily be manipulated by genetic engineering and are suitable for multiple rounds of mutation and selection due to their fast life cycles. The optimization of photosynthesis by placing a C4 pathway into current C3 species is on the agenda of crop biologists (Hibberd et al., 2008; von Caemmerer et al., 2012). Setting up a photorespiratory CO2 pump by synthetic experimental evolution is an important and necessary component of this endeavor.
METHODS
Sequence Alignments and Phylogenetic Analyses
Sequences used to construct the phylogenetic tree of the GLDP genes of the Flaveria species were obtained from either known sequences (GLDPA-Ft [Cossu and Bauwe, 1998], GLDPB*-Ft [Cossu and Bauwe, 1998], GLDPA1-Fp [Bauwe et al., 1995], GLDPA2-Fp [Bauwe et al., 1995], GLDPB1-Fp [Chu, 1996], GLDPB2-Fp [Chu, 1996], and GLDPC-Fp [Chu, 1996]) or from contigs assembled from either 454 (Gowik et al., 2011) or Illumina (J. Mallmann, U. Gowik, and P. Westhoff, unpublished data) sequencing. Alignments of sequences for phylogenetic analyses were performed with ClustalX 2.0.8 (Higgins and Sharp, 1988, 1989; Thompson et al., 1997; Larkin et al., 2007). A full alignment of the sequences used is available as Supplemental Data Set 1 online. Phylogenetic analyses were done with the program MEGA 5 (Tamura et al., 2011) using the maximum likelihood method with the Tamura 3 parameter model. Bootstrapping was performed 1000 times. Sequence comparisons for similarity studies between the promoters were done with the Genomatix DiAlign Web interface (Morgenstern et al., 1996; Morgenstern et al., 1998; Morgenstern, 1999).
Mapping and Quantification of Reads
Illumina reads from sequencing the leaf transcriptomes of Flaveria pringlei, Flaveria robusta, Flaveria chloraefolia, Flaveria pubescens, Flaveria anomala, Flaveria ramossisima, Flaveria brownii, Flaveria bidentis, and Flaveria trinervia were obtained in four independent experiments. All plant species were grown next to each other in the greenhouse, RNA was isolated as described (Westhoff et al., 1991), and construction of sequence libraries and sequencing followed Illumina protocols. Between 30 and 58 million reads could be obtained per species and experiment (J. Mallmann, U. Gowik, and P. Westhoff, unpublished data). The reads were mapped against the sequences of the GLDPA gene of F. trinervia and the GLDPB* gene of F. trinervia to obtain an overall distribution of reads along the 5′ flanking and coding regions of the genes. Mapping was performed with the CLC Genomics server version 3.2.1 by CLC Bio with the “map reads against reference” tool for high-throughput sequencing.
To determine the abundance of GLDPA, GLDPB, and GLDPC transcripts in roots, stems, and leaves of F. bidentis and F. robusta, the coding sequences of GLDPA-Ft, GLDPB-Fro, and GLDPC-Fro (the latter two obtained from full-length contigs from assembled Illumina reads) were used as references and Illumina reads from sequencing the root, stem, and leaf transcriptomes of F. bidentis and F. robusta were mapped on these. Leaf, stem, and root transcriptomes of F. robusta and F. bidentis were sequenced in a single experiment as described above. Between 37 and 42 million reads could be obtained per species and tissue. Plants were grown next to each other in the greenhouse. The sequences for GLDPB-Fro and GLDPC-Fro are available as Supplemental Data Set 3 online.
Isolation of GLDP 5′ Flanking Regions by Vectorette PCR
The 5′ flanking regions of GLDP genes from F. robusta and F. bidentis were isolated by vectorette PCR (Siebert et al., 1995) as implemented in the Genome Walking method. Libraries of genomic DNA were prepared as described in the GenomeWalker Universal Kit manual from Clontech. DNA for library construction was isolated from leaves of the respective Flaveria species with the Qiagen DNeasy plant mini kit.
Cloning of Promoter-Reporter Gene Constructs
The 5′ regions of GLDPA-Fp and GLDPB-Fp genes were fused to the GUS (uidA) gene (Novel and Novel, 1973) and cloned into the pBin19 plant transformation vector (Bevan, 1984). All other constructs were constructed using restriction sites added with PCR to the respective sequences. Following sequencing for confirmation of the sequence, these fragments were inserted in pBI121 (Jefferson et al., 1987; Chen et al., 2003). The sequences of the oligonucleotides used can be found in Supplemental Table 1 online.
Transformation of Arabidopsis thaliana and F. bidentis
Transformation of Arabidopsis was performed following the floral dip protocol (Clough and Bent, 1998) as modified by Logemann et al. (2006). Strain GV3101 of Agrobacterium tumefaciens (Holsters et al., 1980; Koncz and Schell, 1986) provided the helper plasmid for the transformations. F. bidentis was transformed as described by Chitty et al. (1994) using Agrobacterium strain AGL1 (Hood et al., 1986; Lazo et al., 1991).
In Situ Detection of GUS and Fluorimetric Activity Measurements
Fluorimetric measurements of GUS activity were performed according to Jefferson et al. (1987) and Kosugi et al. (1990). In the case of F. bidentis, the fifth leaf of a 40- to 50-cm tall T0 plant was harvested for the analysis, and in the case of Arabidopsis, three rosette leafs were harvested from T1 plants that were around 4 weeks old. For both species, leaves were harvested before the onset of flowering. Histochemical GUS staining and light microscopy were performed as described by Engelmann et al. (2008).
Accession Numbers
Sequence data from this article are available in the GenBank/EMBL data libraries under the following accession numbers: Z36879 (F. pringlei GLDPA2; formerly gdcsPA), Z54239 (F. pringlei GLDPA1; formerly gdcsPB), KC545949 (F. pringlei GLDPC; formerly gdcsPC), KC545950 (F. pringlei GLDPB2; formerly gdcsPD), KC545951 (F. pringlei GLDPB1; formerly gdcsPE), Z99767 (F. trinervia GLDPA; formerly gdcsPA), Z99768 (F. trinervia GLDPB*; formerly gdcsPB pseudogene), KC545946 (5′ flanking sequence of F. bidentis GLDPA), and KC545947 (5′ flanking sequence of F. robusta GLDPA). The Arabidopsis genes GLDP1 (AT4G33010) and GLDP2 (AT2G26080) can be found at The Arabidopsis Information Resource (www.Arabidopsis.org).
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. Molecular Phylogenetic Analysis of GLDP Genes.
Supplemental Figure 2. Functional Analysis of the 5′ Flanking Sequence of the GLDPA2 Gene of F. pringlei.
Supplemental Figure 3. Functional Analysis of the 5′ Flanking Sequence of the GLDPB* Gene of F. trinervia.
Supplemental Figure 4. Functional Analysis of the Proximal (PR7) and Distal (PR2) Subpromoters of the GLDPA Gene of the C3 Species F. robusta.
Supplemental Figure 5. Transcript Coverage of the GLDPA Genes of the C3-C4 Intermediates F. chloraefolia, F. pubescens, F. anomala, F. ramosissima, and F. brownii.
Supplemental Figure 6. Splice Variants of GLDPA Transcripts Derived from the Distal Transcriptional Start Site.
Supplemental Table 1. List of Oligonucleotides Used to Amplify Promoter Sequences for GUS Constructs and the 5′ Splice Site of Transcripts Derived from Distal Transcriptional Start Site of F. bidentis.
Supplemental Data Set 1. ClustalX Alignment of the GLDP Sequences Used for the Phylogenetic Analysis Shown in Figure 1A.
Supplemental Data Set 2. ClustalX Alignment of the GLDP Sequences Used for the Phylogenetic Analysis in Supplemental Figure 1 online.
Supplemental Data Set 3. Sequences of the GLDPB and GLDPG Genes of F. robusta.
Acknowledgments
We thank Borjana Arsova for carefully reading this article and giving useful comments. This work was funded by the Deutsche Forschungsgemeinschaft through the Sonderforschungsbereich SFB 590, the Forschergruppe FOR1186 (Promics), and the Excellence Cluster EXC 1028.
AUTHOR CONTRIBUTIONS
S.S., H.B., U.G., and P.W. designed the research. S.S., J.M., J.B., M.K., and M.S. performed the research. S.S. and U.G. analyzed the data. S.S., H.B., U.G., and P.W. wrote the article.
Glossary
- Rubisco
ribulose-1,5-bis-phosphate carboxylase/oxygenase
- GDC
Gly decarboxylase
- GUS
β-glucuronidase
References
- Akyildiz M., Gowik U., Engelmann S., Koczor M., Streubel M., Westhoff P. (2007). Evolution and function of a cis-regulatory module for mesophyll-specific gene expression in the C4 dicot Flaveria trinervia. Plant Cell 19: 3391–3402 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bauwe, H. (2011). Photorespiration: The bridge to C4 photosynthesis. In C4 Photosynthesis and Related CO2 Concentrating Mechanisms, A.S. Raghavendra and R.F. Sage, eds (Dordrecht, The Netherlands: Springer), pp. 81–108. [Google Scholar]
- Bauwe H., Chu C.C., Kopriva S., Nan Q. (1995). Structure and expression analysis of the gdcsPA and gdcsPB genes encoding two P-isoproteins of the glycine-cleavage system from Flaveria pringlei. Eur. J. Biochem. 234: 116–124 [DOI] [PubMed] [Google Scholar]
- Bauwe H., Kopriva S. (1995). The gdcsPA gene from Flaveria pringlei (Asteraceae). Plant Physiol. 107: 655. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Bennetzen J.L., et al. (2012). Reference genome sequence of the model plant Setaria. Nat. Biotechnol. 30: 555–561 [DOI] [PubMed] [Google Scholar]
- Bevan M. (1984). Binary Agrobacterium vectors for plant transformation. Nucleic Acids Res. 12: 8711–8721 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Brogna S., Wen J. (2009). Nonsense-mediated mRNA decay (NMD) mechanisms. Nat. Struct. Mol. Biol. 16: 107–113 [DOI] [PubMed] [Google Scholar]
- Cameron R.G., Bassett C.L., Bouton J.H., Brown R.H. (1989). Transfer of C4 photosynthetic characters through hybridization of Flaveria species. Plant Physiol. 90: 1538–1545 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen P.Y., Wang C.K., Soong S.C., To K.Y. (2003). Complete sequence of the binary vector pBI121 and its application in cloning T-DNA insertion from transgenic plants. Mol. Breed. 11: 287–293 [Google Scholar]
- Chitty J.A., Furbank R.T., Marshall J.S., Chen Z., Taylor W.C. (1994). Genetic transformation of the C4 plant, Flaveria bidentis. Plant J. 6: 949–956 [Google Scholar]
- Christin P.A., Osborne C.P., Sage R.F., Arakaki M., Edwards E.J. (2011a). C4 eudicots are not younger than C4 monocots. J. Exp. Bot. 62: 3171–3181 [DOI] [PubMed] [Google Scholar]
- Christin P.A., Sage T.L., Edwards E.J., Ogburn R.M., Khoshravesh R., Sage R.F. (2011b). Complex evolutionary transitions and the significance of C3-C4 intermediate forms of photosynthesis in Molluginaceae. Evolution 65: 643–660 [DOI] [PubMed] [Google Scholar]
- Chu, C. (1996). Molecular Structure and Expression Patterns of Glycine Decarboxylase Genes from Flaveria pringlei C3 and Flaveria anomala C3-C4 PhD dissertation (Halle-Wittenberg, Germany: Martin-Luther University). [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cossu R., Bauwe H. (1998). The electronic Plant Gene Register. Plant Physiol. 116: 445–446 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dengler, N.G., and Nelson, T. (1999). Leaf structure and development in C4 plants. In C4 Plant Biology, R.F. Sage and R.K. Monson, eds (San Diego, CA: Academic Press), pp. 133–172. [Google Scholar]
- Edwards E.J., et al. (2010). The origins of C4 grasslands: Integrating evolutionary and ecosystem science. Science 328: 587–591 [DOI] [PubMed] [Google Scholar]
- Edwards, G., and Ku, M. (1987). Biochemistry of C3-C4 intermediates. In The Biochemistry of Plants, M.D. Hatch and N.K. Boardman, eds (New York: Academic Press), pp. 275–325. [Google Scholar]
- Engel N., van den Daele K., Kolukisaoglu U., Morgenthal K., Weckwerth W., Parnik T., Keerberg O., Bauwe H. (2007). Deletion of glycine decarboxylase in Arabidopsis is lethal under nonphotorespiratory conditions. Plant Physiol. 144: 1328–1335 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Engelmann S., Wiludda C., Burscheidt J., Gowik U., Schlue U., Koczor M., Streubel M., Cossu R., Bauwe H., Westhoff P. (2008). The gene for the P-subunit of glycine decarboxylase from the C4 species Flaveria trinervia: Analysis of transcriptional control in transgenic Flaveria bidentis (C4) and Arabidopsis (C3). Plant Physiol. 146: 1773–1785 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Felsenstein J. (1985). Confidence limits on phylogenies: An approach using the bootstrap. Evolution 39: 783–791 [DOI] [PubMed] [Google Scholar]
- Goff S.A., et al. (2002). A draft sequence of the rice genome (Oryza sativa L. ssp. japonica). Science 296: 92–100 [DOI] [PubMed] [Google Scholar]
- Gowik U., Brautigam A., Weber K.L., Weber A.P., Westhoff P. (2011). Evolution of C4 photosynthesis in the genus Flaveria: How many and which genes does it take to make C4? Plant Cell 23: 2087–2105 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gowik U., Burscheidt J., Akyildiz M., Schlue U., Koczor M., Streubel M., Westhoff P. (2004). cis-Regulatory elements for mesophyll-specific gene expression in the C4 plant Flaveria trinervia, the promoter of the C4 phosphoenolpyruvate carboxylase gene. Plant Cell 16: 1077–1090 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hanson A.D., Roje S. (2001). One-carbon metabolism in higher plants. Annu. Rev. Plant Physiol. Plant Mol. Biol. 52: 119–137 [DOI] [PubMed] [Google Scholar]
- Hatch M., Kagawa T., Craig S. (1975). Subdivision of C4-pathway species based on differing C4 acid decarboxylating systems and ultrastructural features. Aust. J. Plant Physiol. 2: 111–128 [Google Scholar]
- Hatch M.D. (1987). C4 photosynthesis: A unique blend of modified biochemistry, anatomy and ultrastructure. Biochim. Biophys. Acta 895: 81–106 [Google Scholar]
- Hattersley P.W. (1984). Characterization of C4 type leaf anatomy in grasses (Poaceae). Mesophyll: Bundle sheath area ratios. Ann. Bot. (Lond.) 53: 163–180 [Google Scholar]
- Heckmann D., Schulze S., Denton A., Gowik U., Westhoff P., Weber, A.P., and Lercher, M.J. (2013). Predicting C4 photosynthesis evolution: Modular, individually adaptive steps on a Mount Fuji fitness landscape. Cell 153: 1579–1588 [DOI] [PubMed] [Google Scholar]
- Hibberd J.M., Sheehy J.E., Langdale J.A. (2008). Using C4 photosynthesis to increase the yield of rice-rationale and feasibility. Curr. Opin. Plant Biol. 11: 228–231 [DOI] [PubMed] [Google Scholar]
- Higgins D.G., Sharp P.M. (1988). CLUSTAL: A package for performing multiple sequence alignment on a microcomputer. Gene 73: 237–244 [DOI] [PubMed] [Google Scholar]
- Higgins D.G., Sharp P.M. (1989). Fast and sensitive multiple sequence alignments on a microcomputer. Comput. Appl. Biosci. 5: 151–153 [DOI] [PubMed] [Google Scholar]
- Holsters M., et al. (1980). The functional organization of the nopaline A. tumefaciens plasmid pTiC58. Plasmid 3: 212–230 [DOI] [PubMed] [Google Scholar]
- Hood E.E., Helmer G.L., Fraley R.T., Chilton M.D. (1986). The hypervirulence of Agrobacterium tumefaciens A281 is encoded in a region of pTiBo542 outside of T-DNA. J. Bacteriol. 168: 1291–1301 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Hori K., Watanabe Y. (2007). Context analysis of termination codons in mRNA that are recognized by plant NMD. Plant Cell Physiol. 48: 1072–1078 [DOI] [PubMed] [Google Scholar]
- Jefferson R.A., Kavanagh T.A., Bevan M.W. (1987). GUS fusions: Beta-glucuronidase as a sensitive and versatile gene fusion marker in higher plants. EMBO J. 6: 3901–3907 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kertesz S., Kerenyi Z., Merai Z., Bartos I., Palfy T., Barta E., Silhavy D. (2006). Both introns and long 3′-UTRs operate as cis-acting elements to trigger nonsense-mediated decay in plants. Nucleic Acids Res. 34: 6147–6157 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Koncz C., Schell J. (1986). The promoter of TL-DNA gene 5 controls the tissue-specific expression of chimaeric genes carried by a novel type of Agrobacterium binary vector. Mol. Gen. Genet. 204: 383–396 [Google Scholar]
- Kopriva S., Bauwe H. (1994). P-protein of glycine decarboxylase from Flaveria pringlei. Plant Physiol. 104: 1077–1078 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kopriva S., Chu C.C., Bauwe H. (1996). Molecular phylogeny of Flaveria as deduced from the analysis of nucleotide sequences encoding the H-protein of the glycine cleavage system. Plant Cell Environ. 19: 1028–1036 [Google Scholar]
- Kosugi S., Ohashi Y., Nakajima K., Arai Y. (1990). An improved assay for β-glucuronidase in transformed cells: Methanol almost completely suppresses a putative endogenous β-glucuronidase activity. Plant Sci. 70: 133–140 [Google Scholar]
- Larkin M.A., et al. (2007). Clustal W and Clustal X version 2.0. Bioinformatics 23: 2947–2948 [DOI] [PubMed] [Google Scholar]
- Lazo G.R., Stein P.A., Ludwig R.A. (1991). A DNA transformation-competent Arabidopsis genomic library in Agrobacterium. Biotechnology (NY) 9: 963–967 [DOI] [PubMed] [Google Scholar]
- Levine M. (2011). Paused RNA polymerase II as a developmental checkpoint. Cell 145: 502–511 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Li P., et al. (2010). The developmental dynamics of the maize leaf transcriptome. Nat. Genet. 42: 1060–1067 [DOI] [PubMed] [Google Scholar]
- Logemann E., Birkenbihl R.P., Ulker B., Somssich I.E. (2006). An improved method for preparing Agrobacterium cells that simplifies the Arabidopsis transformation protocol. Plant Methods 2: 16. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Marshall D.M., Muhaidat R., Brown N.J., Liu Z., Stanley S., Griffiths H., Sage R.F., Hibberd J.M. (2007). Cleome, a genus closely related to Arabidopsis, contains species spanning a developmental progression from C3 to C4 photosynthesis. Plant J. 51: 886–896 [DOI] [PubMed] [Google Scholar]
- McKown A.D., Moncalvo J.M., Dengler N.G. (2005). Phylogeny of Flaveria (Asteraceae) and inference of C4 photosynthesis evolution. Am. J. Bot. 92: 1911–1928 [DOI] [PubMed] [Google Scholar]
- Monson R.K., Edwards G.E., Ku M.S.B. (1984). C3-C4 intermediate photosynthesis in plants. Bioscience 34: 563–574 [Google Scholar]
- Morange M. (2009). Synthetic biology: A bridge between functional and evolutionary biology. Biol. Theory 4: 368–377 [Google Scholar]
- Morgan C.L., Turner S.R., Rawsthorne S. (1993). Coordination of the cell-specific distribution of the four subunits of glycine decarboxylase and of serine hydroxymethyltransferase in leaves of C3-C4 intermediate species from different genera. Planta 190: 468–473 [Google Scholar]
- Morgenstern B. (1999). DIALIGN 2: Improvement of the segment-to-segment approach to multiple sequence alignment. Bioinformatics 15: 211–218 [DOI] [PubMed] [Google Scholar]
- Morgenstern B., Dress A., Werner T. (1996). Multiple DNA and protein sequence alignment based on segment-to-segment comparison. Proc. Natl. Acad. Sci. USA 93: 12098–12103 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Morgenstern B., Frech K., Dress A., Werner T. (1998). DIALIGN: Finding local similarities by multiple sequence alignment. Bioinformatics 14: 290–294 [DOI] [PubMed] [Google Scholar]
- Muhaidat R., Sage T.L., Frohlich M.W., Dengler N.G., Sage R.F. (2011). Characterization of C3-C4 intermediate species in the genus Heliotropium L. (Boraginaceae): Anatomy, ultrastructure and enzyme activity. Plant Cell Environ. 34: 1723–1736 [DOI] [PubMed] [Google Scholar]
- Novel G., Novel M. (1973). Mutants d'Escherichia coli K 12 affectés pour leur croissance sur méthyl-β-D-glucuronide: Localisation du gène de structure de la β-D-glucuronidase (uid A). Mol. Gen. Genet. 120: 319–335 [PubMed] [Google Scholar]
- Ogren W.L. (1984). Photorespiration - Pathways, regulation, and modification. Annu. Rev. Plant Physiol. Plant Mol. Biol. 35: 415–442 [Google Scholar]
- Ogren W.L., Bowes G. (1971). Ribulose diphosphate carboxylase regulates soybean photorespiration. Nat. New Biol. 230: 159–160 [DOI] [PubMed] [Google Scholar]
- Oliver D.J., Raman R. (1995). Glycine decarboxylase: Protein chemistry and molecular biology of the major protein in leaf mitochondria. J. Bioenerg. Biomembr. 27: 407–414 [DOI] [PubMed] [Google Scholar]
- Palmer A.C., Egan J.B., Shearwin K.E. (2011). Transcriptional interference by RNA polymerase pausing and dislodgement of transcription factors. Transcription 2: 9–14 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Paterson A.H., et al. (2009). The Sorghum bicolor genome and the diversification of grasses. Nature 457: 551–556 [DOI] [PubMed] [Google Scholar]
- Powell A. (1978). Systematics of Flaveria (Flaveriinae-Asteraceae). Ann. Mo. Bot. Gard. 65: 590–636 [Google Scholar]
- Rawsthorne S., Hylton C.M., Smith A.M., Woolhouse H.W. (1988). Photorespiratory metabolism and immunogold localization of photorespiratory enzymes in leaves of C3 and C3-C4 intermediate species of Moricandia. Planta 173: 298–308 [DOI] [PubMed] [Google Scholar]
- Sage R.F. (2004). The evolution of C4 photosynthesis. New Phytol. 161: 341–370 [DOI] [PubMed] [Google Scholar]
- Sage R.F., Christin P.A., Edwards E.J. (2011). The C4 plant lineages of planet Earth. J. Exp. Bot. 62: 3155–3169 [DOI] [PubMed] [Google Scholar]
- Sage R.F., Sage T.L., Kocacinar F. (2012). Photorespiration and the evolution of C4 photosynthesis. Annu. Rev. Plant Biol. 63: 19–47 [DOI] [PubMed] [Google Scholar]
- Schnable P.S., et al. (2009). The B73 maize genome: Complexity, diversity, and dynamics. Science 326: 1112–1115 [DOI] [PubMed] [Google Scholar]
- Shearwin K.E., Callen B.P., Egan J.B. (2005). Transcriptional interference–A crash course. Trends Genet. 21: 339–345 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Siebert P.D., Chenchik A., Kellogg D.E., Lukyanov K.A., Lukyanov S.A. (1995). An improved PCR method for walking in uncloned genomic DNA. Nucleic Acids Res. 23: 1087–1088 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stockhaus J., Schlue U., Koczor M., Chitty J.A., Taylor W.C., Westhoff P. (1997). The promoter of the gene encoding the C4 form of phosphoenolpyruvate carboxylase directs mesophyll-specific expression in transgenic C4 Flaveria spp. Plant Cell 9: 479–489 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Tamura K., Peterson D., Peterson N., Stecher G., Nei M., Kumar S. (2011). MEGA5: Molecular evolutionary genetics analysis using maximum likelihood, evolutionary distance, and maximum parsimony methods. Mol. Biol. Evol. 28: 2731–2739 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Thompson J.D., Gibson T.J., Plewniak F., Jeanmougin F., Higgins D.G. (1997). The CLUSTAL_X windows interface: Flexible strategies for multiple sequence alignment aided by quality analysis tools. Nucleic Acids Res. 25: 4876–4882 [DOI] [PMC free article] [PubMed] [Google Scholar]
- von Caemmerer S., Quick W.P., Furbank R.T. (2012). The development of C4 rice: Current progress and future challenges. Science 336: 1671–1672 [DOI] [PubMed] [Google Scholar]
- Westhoff P., Offermann-Steinhard K., Höfer M., Eskins K., Oswald A., Streubel M. (1991). Differential accumulation of plastid transcripts encoding photosystem II components in the mesophyll and bundle-sheath cells of monocotyledonous NADP-malic enzyme-type C4 plants. Planta 184: 377–388 [DOI] [PubMed] [Google Scholar]
- Wiludda C., Schulze S., Gowik U., Engelmann S., Koczor M., Streubel M., Bauwe H., Westhoff P. (2012). Regulation of the photorespiratory GLDPA gene in C4 Flaveria: An intricate interplay of transcriptional and posttranscriptional processes. Plant Cell 24: 137–151 [DOI] [PMC free article] [PubMed] [Google Scholar]