The evolutionarily conserved ECHIDNA (ECH) and Ypt Interacting Protein4 (YIP4) proteins form a complex that localizes to the trans-Golgi network (TGN). The ECH-YIP4 complex is required for TGN function and plays a crucial role in the secretion of cell wall polysaccharides in plant cells.
Abstract
The secretion of cell wall polysaccharides through the trans-Golgi network (TGN) is required for plant cell elongation. However, the components mediating the post-Golgi secretion of pectin and hemicellulose, the two major cell wall polysaccharides, are largely unknown. We identified evolutionarily conserved YPT/RAB GTPase Interacting Protein 4a (YIP4a) and YIP4b (formerly YIP2), which form a TGN-localized complex with ECHIDNA (ECH) in Arabidopsis thaliana. The localization of YIP4 and ECH proteins at the TGN is interdependent and influences the localization of VHA-a1 and SYP61, which are key components of the TGN. YIP4a and YIP4b act redundantly, and the yip4a yip4b double mutants have a cell elongation defect. Genetic, biochemical, and cell biological analyses demonstrate that the ECH/YIP4 complex plays a key role in TGN-mediated secretion of pectin and hemicellulose to the cell wall in dark-grown hypocotyls and in secretory cells of the seed coat. In keeping with these observations, Fourier transform infrared microspectroscopy analysis revealed that the ech and yip4a yip4b mutants exhibit changes in their cell wall composition. Overall, our results reveal a TGN subdomain defined by ECH/YIP4 that is required for the secretion of pectin and hemicellulose and distinguishes the role of the TGN in secretion from its roles in endocytic and vacuolar trafficking.
INTRODUCTION
Cell elongation in plants requires the synthesis and remodeling of the rigid cell wall that encloses the plant cells. Primary cell wall consists of ∼20 to 30% cellulose embedded in a matrix of soluble hemicellulosic and pectic polysaccharides with small amounts of structural and enzymatic proteins (reviewed in Sandhu et al., 2009; Carpita, 2011). While cellulose microfibrils are synthesized by plasma membrane (PM)–localized cellulose synthase complexes, pectins and hemicelluloses are synthesized at the Golgi apparatus (reviewed in Driouich et al., 2012). Polysaccharide-specific antibodies, mutants in polysaccharide biosynthesis, and protein glycosylation have revealed the sequence of cell wall polysaccharide biosynthesis on the distinct regions of the cis-, medial-, and trans-cisternae of the Golgi (Lynch and Staehelin, 1992; Zhang and Staehelin, 1992; Vicré et al., 1998; Chevalier et al., 2010). By contrast, the components involved in post-Golgi polysaccharide delivery to the cell wall remain poorly understood. Nevertheless, secretory vesicles emerging from the trans-Golgi network (TGN) contain cell wall components (Toyooka et al., 2009; Kang et al., 2011) and the vesicles number varies depending on secretion activity (Young et al., 2008). Furthermore, pharmacological inhibition of V-ATPase (VHA) activity at the TGN by Concanamycin A leads to secretory defects and related growth phenotypes (Dettmer et al., 2006; Brüx et al., 2008; Viotti et al., 2010). Those data strongly suggest that the TGN plays a central role in polysaccharide secretion. However, a lack of mutants defective in secretion of polysaccharide has limited our understanding of this process, and the molecular mechanisms mediating secretion to the cell wall remain largely unknown.
The TGN is a polymorphic tubular-vesicular network that matures from the trans-most cisternae of the Golgi stack (reviewed in Staehelin and Kang, 2008; Kang, 2011). Originally considered as an extension of the Golgi on the trans-side, recent data indicate that the TGN is actually a highly mobile organelle that can exist independently or in association with a Golgi or another TGN (Viotti et al., 2010). The primary function of the TGN is to correctly pack and transport newly synthesized proteins and carbohydrates en route to vacuoles and the PM (reviewed in Richter et al., 2009). In animal cells, endocytosed material is received by the TGN from early endosome and recycling endosome (reviewed in De Matteis and Luini, 2008). By contrast, the plant TGN functions as the early endosome and directly receives material endocytosed from the PM (Dettmer et al., 2006; Viotti et al., 2010), increasing the complexity of the sorting required at the plant TGN. Moreover, while constantly receiving and secreting vesicles, the TGN must also regulate and maintain the proteins belonging to the intrinsic TGN machinery.
In recent years, knowledge of TGN morphogenesis and function in plants has increased dramatically, and several TGN components have been identified (Dettmer et al., 2006; Chow et al., 2008; Robert et al., 2008; Kang et al., 2011). Nevertheless, little is known about how the material destined for the endocytic, vacuolar, and secretory pathways is sorted at the TGN. In particular, the identity of the TGN components mediating secretion of cell wall material remains largely unknown (reviewed in Worden et al., 2012). We recently identified ECHIDNA (ECH), a protein required for TGN integrity and involved in secretion of unidentified cargo (Gendre et al., 2011). Here, we identified conserved proteins of the YIP (for YPT/RAB GTPase Interacting Protein) family in Arabidopsis thaliana that form a TGN-localized complex with ECH. We showed that the ECH/YIP complex is necessary for cell elongation and is required for the TGN-mediated secretion of cell wall polysaccharides, such as pectins. Importantly, the ECH/YIP complex does not play a role in vacuolar targeting and endocytosis. Thus, we identified the components of a post-Golgi trafficking mechanism that plays an important role in secretion, independently of the other TGN functions, such as endocytosis.
RESULTS
YIP4a and YIP4b Interact with ECH
To better understand the role of ECH (Gendre et al., 2011), we performed a yeast two-hybrid (Y2H) screen using ECH as bait against an Arabidopsis cDNA library (Hybrigenics). This screen identified two proteins from the YIP family encoded by the loci At2g18840 and At4g30260. These proteins were named YIP4a and YIP4b (previously referred to as YIP2; Drakakaki et al., 2011) based on their closest yeast homolog, YIP4p (YGL198W) (see Supplemental Figure 1A and Supplemental References 1 online). In Arabidopsis, seven proteins share the YIP domain. YIP4a and YIP4b are small proteins (30 kD) that exhibit 85% identity (see Supplemental Figure 1B online) and have five predicted transmembrane domains (see Supplemental Figure 1C online). YIP homologs in yeast and mammals interact with RAB GTPases and are involved in vesicle trafficking, but their functions are still poorly understood (Heidtman et al., 2003; Chen and Collins, 2005b, 2005a; Yoshida et al., 2008).
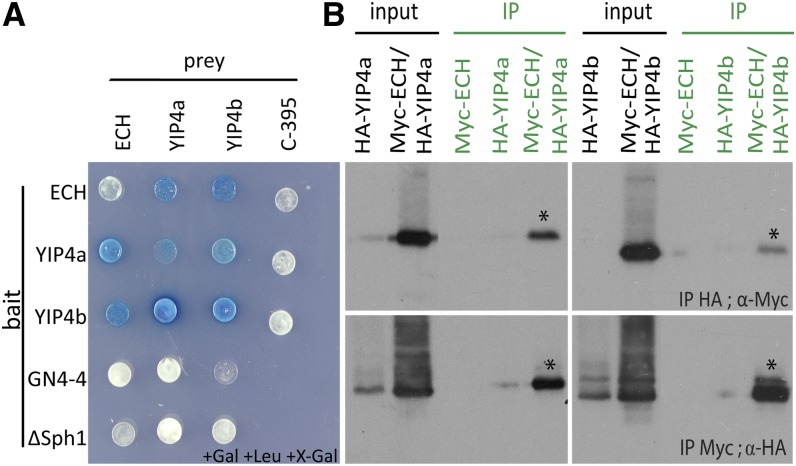
A two-by-two Y2H experiment confirmed that ECH and YIP4a/b interact (Figure 1A) and that YIP4a and YIP4b can hetero- and homodimerize. Importantly, the ECH and YIP4a/b interactions were validated in planta by coimmunoprecipitation (co-IP) assays (Figure 1B). These results clearly establish that ECH interacts with YIP4a and YIP4b either separately or with potential YIP4a/YIP4b dimers.
Figure 1.
YIP4a and YIP4b Interact with ECH.
(A) Y2H assay between ECH, YIP4a, and YIP4b with C-395 and GN4-4 as negative interaction controls for prey and bait, respectively. To detect autoactivation of the prey constructs, a pEG202 derivate with a deleted lexA binding domain sequence (ΔSphI; Grebe et al., 2000) was used as bait.
(B) Co-IP assay of Myc-ECH and HA-YIP4a or HA-YIP4b transiently expressed in Arabidopsis cell culture protoplasts. The top plots show the HA immunoprecipitation (IP), and the protein gel blot was performed using an anti-Myc antibody (α-Myc). The bottom plots show the results of the reciprocal experiment. Asterisks indicate interaction.
[See online article for color version of this figure.]
The yip4a yip4b Double Mutant Displays a Cell Elongation Defect
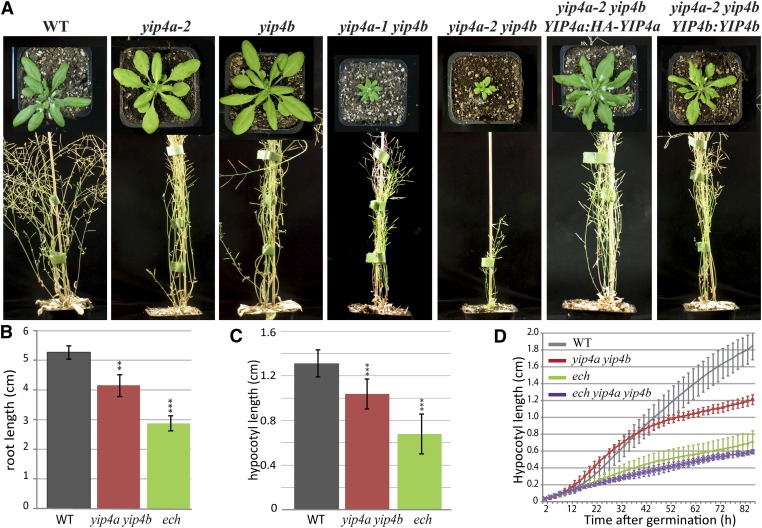
To understand the function of YIP4a and YIP4b, one T-DNA insertion line in the YIP4b gene (SALK_129888, yip4b) and two insertion lines in YIP4a (SALK_066428 and SALK_021897, yip4a-1 and yip4a-2, respectively) were characterized. The insertion in YIP4b occurred in the second intron and generates a transcriptional knockout (see Supplemental Figure 2A online). The insertion in yip4a-1 occurred at the beginning of the first intron of YIP4a and generates a truncated transcript, while the yip4a-2 insertion occurs at the beginning of the 3′ untranslated region (UTR) and does not affect the transcription of YIP4a (see Supplemental Figure 2A online). The phenotype of single yip4 mutants was indistinguishable from the wild type (Columbia-0 [Col-0]) (Figure 2A). By contrast, yip4a-1 yip4b and yip4a-2 yip4b double mutants displayed clear growth defects (Figure 2A), suggesting that YIP4a and YIP4b act redundantly and are involved in growth regulation. The stronger phenotype for yip4a-2 yip4b over yip4a-1 yip4b was surprising because yip4a-2 was not a transcriptional knockout. However, many studies have demonstrated the importance of UTRs for the stability and/or regulation of transcripts (Ortega et al., 2006 and reviewed in Gutièrres et al., 1999), so the T-DNA insertion in the 3′UTR end of YIP4a may cause a drastic reduction in YIP4a protein level. The expression of either YIP4a or YIP4b driven by their respective endogenous promoters fully complemented yip4a-2 yip4b double mutants (Figure 2A), confirming that the yip4a-2 yip4b growth defect was due to the loss of function of the corresponding genes. Subsequent experiments were performed using the yip4a-2 yip4b double mutant, which is henceforth referred to as yip4a yip4b.
Figure 2.
The yip4a yip4b Double Mutant Displays an Elongation Deficit.
(A) Five-week-old wild type (WT; Col-0), yip4a-2 and yip4b single mutant, yip4a-1 yip4b and yip4a-2 yip4b double mutant, and yip4a-2 yip4b rescued by expression of YIP4a (HA N-terminal fusion) and YIP4b under their own promoter.
(B) Root length measurements of 10-d-old wild-type, yip4a yip4b, and ech seedlings grown in vitro (three replicates, each with 15 seedlings; average ± sd).
(C) Etiolated hypocotyl length measurements of 5-d-old wild-type, yip4a yip4b, and ech seedlings grown in vitro in darkness (three replicates, each with 15 seedlings; average ± sd).
(D) Etiolated hypocotyl growth kinematic of the wild type, yip4a yip4b, ech, and ech yip4a yip4b triple mutant (n = 10, average ±sd). Significant differences are indicated as *P < 0.05, **P < 0.01, and ***P < 0.001 (t test).
[See online article for color version of this figure.]
We then investigated hypocotyl and root elongation to better understand the growth defects in yip4a yip4b. The lengths of both 10-d-old seedling roots (Figures 2B; see Supplemental Figure 2B online) and 5-d-old dark-grown hypocotyls (Figures 2C; see Supplemental Figure 2C online) were 20% lower in yip4a yip4b than in the wild type. Since hypocotyl growth in darkness is due to cell elongation and not cell division (Gendreau et al., 1997), the growth reduction in yip4a yip4b suggests that YIP4a and YIP4b are required for cell elongation. While the exact reason for the less severe elongation phenotype of yip4a yip4b compared with ech is not known, this phenotype could potentially be due to other YIP4 related proteins substituting for the lack of YIP4, such as YIP5b, which also localizes to the TGN (Drakakaki et al., 2011).
The triple ech yip4a yip4b mutant displays an identical phenotype to ech with 50% length reduction, relative to the wild type, for both root (see Supplemental Figure 2D online) and etiolated hypocotyl elongation (Figure 2D; see Supplemental Figure 2C online). In conjunction with the interaction data, the lack of additive effects in the triple mutant indicates that ECH and YIP4a/b act together to mediate a common step in cell elongation.
YIP4a and YIP4b Localize to the TGN
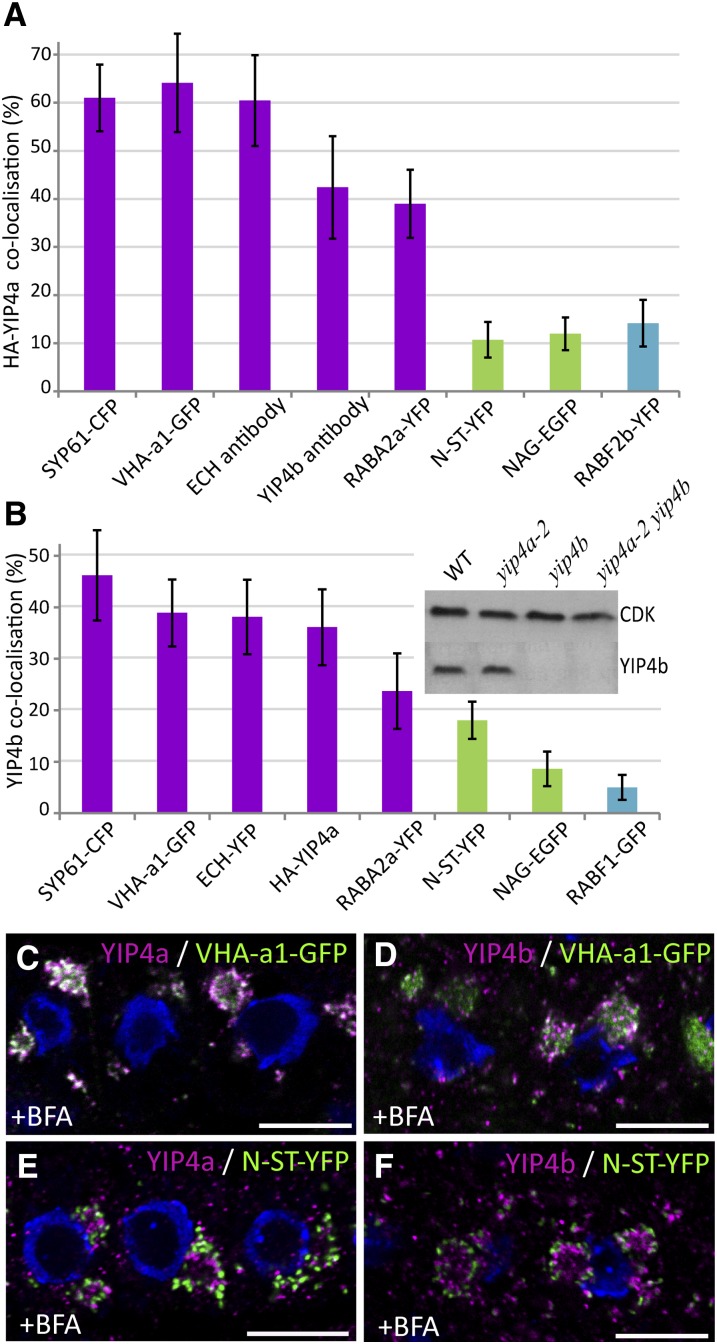
To better understand the function of the YIP4 proteins, the subcellular localization of YIP4a and YIP4b was investigated using N-terminal hemagglutinin (HA)-tagged YIP4a expressed under its native promoter and a specific YIP4b antibody (Figure 3B, inset). The HA-YIP4a fusion is functional, as shown by the successful rescue of the yip4a yip4b mutant (Figure 2A). YIP4a and YIP4b subcellular localisation were analyzed in root epidermal cells by colocalising anti-HA and anti-YIP4b signals with green, yellow or cyan fluorescent proteins (GFP, YFP, CFP) targeted to different organelles. G/Y/CFP fused to the Syntaxin of plant 61 (SYP61), V-ATPase subunit a1 (VHA-a1) and Rab GTPase A2a (RABA2a) are indicative of TGN localisation. While the fluorescent proteins fused with N-acetylglucosaminyl transferase I (NAG1) and N-α-2,6-sialyltransferase (N-ST-YFP) are indicative of the Golgi. Both YIP4a (Figure 3A) and YIP4b (Figure 3B) signals strongly colocalized with TGN markers (Figures 3A and 3B; see Supplemental Figures 3A and 4A online), the highest colocalization is observed with SYP61-CFP (61% ± 6.9% and 46.2% ± 8.7%, respectively; Robert et al., 2008) and VHA-a1-GFP (64% ± 10% and 38.9% ± 6.5%; Dettmer et al., 2006) and to a lesser extent with fused to RABA2a-YFP (39% ± 7% and 23.7% ± 7.3%; Chow et al., 2008). By contrast, YIP4a and YIP4b signals displayed only minor colocalization with Golgi-localized proteins, such as NAG-EGFP (12% ± 3.4% and 8.6% ± 3.3%, respectively; see Supplemental Figures 3A and 3B online) and N-ST-YFP (10.7% ± 3.7% and 18% ± 3.6%; see Supplemental Figures 3A and 4F online), or with multivesicular body (MVB) markers, such as RABF2b-YFP (14% ± 4.8% for YIP4a; see Supplemental Figure 3A online) and RABF1-GFP (5% ± 2.4% for YIP4b; see Supplemental Figure 4C online). Importantly, a strong colocalization is observed between YIP4a and ECH antibody (60.4% ± 9.4%; see Supplemental Figure 3A online), as well as YIP4b and ECH-YFP (38% ± 7%; see Supplemental Figure 3B (38% ± 7%; see Supplemental Figure 3B online). Moreover, YIP4a and YIP4b colocalized with each other as well (42.4% ± 10.6% and 36% ± 7% for the reverse; see Supplemental Figure 3A online). This confirms that the three proteins colocalize largely on the same TGN compartment (Figures 3A and 3B), in agreement with the protein–protein interaction between ECH and YIP4a/b.
Figure 3.
YIP4a and YIP4b Are TGN Localized.
(A) and (B) Quantitative measurement of colocalization of YIP4a (A) or YIP4b (B) with various markers. Markers of the TGN (magenta), Golgi apparatus (green), and MVB compartments (blue) are indicated by color coding. Quantification was achieved using the centroids method (Boutté et al., 2006) (n = 10 roots with four cells each; average ± sd). The inset of the graph in (B) shows the specificity of the YIP4b antibody, which detected a single 30-kD band on protein gel blots only with protein extracts from the wild type (WT) and yip4a-2. CDK (cyclin-dependant kinase A) was used as a loading control.
(C) to (F) Colocalization between anti-HA (HA-YIP4a; [C] and [E]) or anti-YIP4b ([D] and [F]) labeling (magenta) and VHA-a1-GFP ([C] and [D]) or N-ST-YFP ([E] and [F]) after 1 h treatment with 50 µM BFA. The 4′,6-diamidino-2-phenylindole–stained nuclei are blue. Bars = 10 µm.
Next we used treatment with Brefeldin A (BFA) and Wortmannin (Wm), two well-studied drugs affecting different steps in vesicle trafficking, to confirm the TGN localization of YIP4a and YIP4b. BFA affects trafficking mediated by some ARF GTPases (Jackson and Casanova, 2000) and results in a heterogeneous aggregation of TGN membrane encircled by Golgi apparatus in Arabidopsis root cells (Geldner et al., 2003; Lam et al., 2009; reviewed in Satiat-Jeunemaitre et al., 1996). Wm targets phosphatidylinositol kinases (Matsuoka et al., 1995) and causes fusion, swelling, and vacuolization of MVB in Arabidopsis root cells (Jaillais et al., 2008; Niemes et al., 2010). Thus, TGN and Golgi membranes would respond to a BFA treatment, while MVB membranes react to Wm. In accordance with the colocalization data, the majority of YIP4a and YIP4b signals accumulated in BFA bodies together with VHA-a1-GFP (Figures 3C and 3D, respectively) and was excluded from the Golgi (here labeled with N-ST-YFP; Figures 3E and 3F, respectively). Moreover, YIP4a and YIP4b compartments were mostly excluded from the characteristic ring-shaped bodies formed upon Wm treatment (here labeled with RABF1 or RabF2b) (see Supplemental Figure 3C online). Taken together, the colocalization data strongly support the localization of YIP4 proteins at the TGN with ECH.
The Localization of ECH and YIP4 at the TGN Is Interdependent
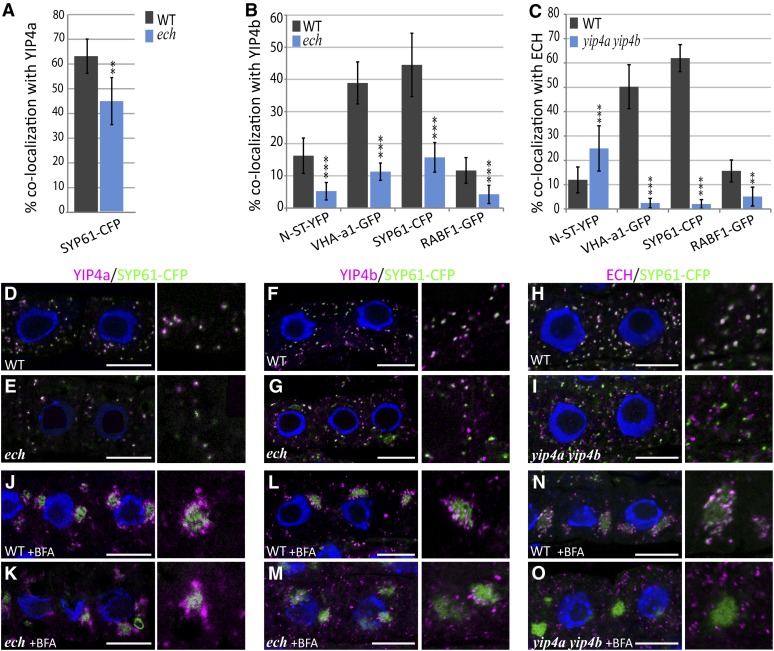
The colocalization of ECH and YIP4 and the interactions between these proteins prompted us to investigate whether the YIP4 proteins influence ECH localization at the SYP61/VHAa1 subdomain of the TGN and vice versa. To address this, we investigated the colocalization of ECH and YIP4 proteins with the TGN markers SYP61 and VHA-a1 in yip4a yip4b and ech mutant backgrounds. In contrast with the wild type (Figures 4D to 4F), colocalizations of YIP4a or YIP4b with SYP61-CFP are significantly reduced in the ech mutant background by 30 and 65%, respectively (Figures 4A, 4B, and 4E to 4G). Strikingly, ECH colocalization with SYP61-CFP is drastically reduced by 96% in yip4a yip4b (Figures 4C and 4I) compared with the wild type (Figure 4H). Additionally, colocalizations of ECH or YIP4 with VHA-a1-GFP are also significantly reduced in the yip4a yip4b and the ech mutant background, respectively, compared with the wild type (Figures 4A to 4C; see Supplemental Figures 4A and 4B online). BFA treatment indicates that while SYP61-CFP still labeled BFA bodies in both the yip4a yip4b and ech mutant, YIP4a/b signal was reduced in BFA bodies in the ech mutant (Figures 4K to 4M). Similarly, ECH signals failed to localize to BFA bodies in the yip4a yip4b mutant (Figure 4O). Identical results were obtained with VHA-a1-GFP (see Supplemental Figures 4G and 4H online). These results further confirm that when YIP4 proteins are not present, the TGN localization of ECH is disrupted and vice versa.
Figure 4.
ECH, YIP4a, and YIP4b Are Dependent on Each Other for TGN Localization.
(A) to (C) Quantitative measurement of colocalization of YIP4a (A), YIP4b (B), or ECH (C) with various markers in wild-type (WT; gray bars) or mutant (blue bars) root epidermal cells (n = 10 roots with four cells, average ± sd). Significant differences are indicated as *P < 0.05, **P < 0.01, and ***P < 0.001 (t test).
(D) to (O) Representative images are displayed in Supplemental Figure 4 online and in (D) to (O) for colocalization with SYP61-CFP. Colocalization of anti-HA (YIP4a; [D], [E], [J], and [K]) anti-YIP4b ([F], [G], [L], and [M]) or anti-ECH ([H], [I], [N], and [O]) labeling (magenta) with SYP61-CFP (green) without treatment ([D] to [I]) or after 1 h treatment with 50 µM BFA ([J] to [O]) in the wild type ([D] to [H] and [J] to [N]) or mutant ([E] to [I] and [K] to [O]). The 4′,6-diamidino-2-phenylindole–stained nuclei are blue. Bars = 10 µm.
As ECH and YIP4 are depleted from SYP61/VHA-a1–positive TGN membranes, we then investigated which compartments they are mislocalized to in yip4a yip4b and ech. The absence of colocalization with RABF1b (Figures 4A and 4B; see Supplemental Figures 4C and 4D online) demonstrates that ECH and YIP4b are not mislocalized to MVBs in the yip4a yip4b and ech backgrounds, respectively. Interestingly, the ECH signal at the trans-Golgi cisternae (labeled by N-ST-YFP) is substantially increased in yip4a yip4b (Figures 4C; see Supplemental Figure 4F online), implying that some of the ECH signal could be retained in the trans-most Golgi cisternae in the absence of YIP4 proteins. Conversely, no such Golgi enrichment of YIP4b signal is found in ech (Figures 4B; see Supplemental Figure 4 online). Thus, our data show that the TGN localization of ECH and YIP4 proteins is interdependent.
TGN Markers Are Partially Mislocalized in yip4a yip4b
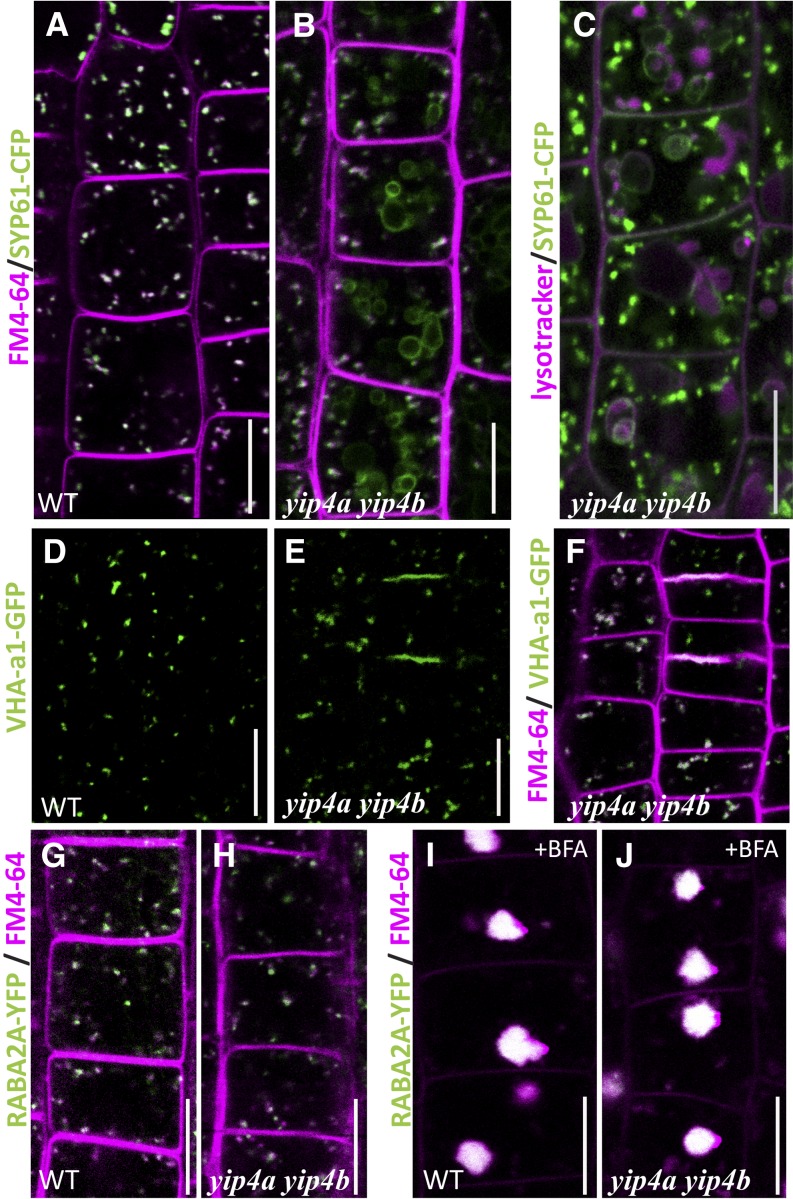
We then investigated whether the TGN was altered by the loss of YIP4 proteins and made use of the known TGN resident proteins. In the yip4a yip4b mutants, SYP61-CFP was partially mislocalized to large ring-shaped compartments (Figure 5B) in addition to the small punctate compartments observed in the wild type (Figure 5A). Lysotracker, which probes acidic organelles, indicated that those ring-shaped compartments are of lytic nature and therefore may be vacuoles (Figure 5C). Similarly, VHA-a1-GFP signal was more diffuse in yip4a yip4b cells than in the wild type (Figure 5D) and was also partly mislocalized to the cell plates (Figure 5E). Interestingly, RabA2a-YFP, a TGN protein residing on a domain partly distinct from SYP61/VHAa1 (Chow et al., 2008), is not mislocalized in yip4a yip4b (Figures 5G and 5H).
Figure 5.
TGN-Localized Proteins Are Partially Mislocalized in yip4a yip4b.
(A) and (B) Colocalization between SYP61-CFP (green) and FM4-64 (magenta) after 5 min internalization in wild-type (WT) (A) and yip4a yip4b (B) root epidermal cells.
(C) Colocalization between SYP61-CFP and lysotracker red (50 nM, 30 min) in yip4a yip4b.
(D) and (E) VHA-a1-GFP labeling in wild-type (D) and yip4a yip4b (E) epidermal cells.
(F) Colocalization, in yip4a yip4b, of VHA-a1-GFP signal with FM4-64 after 5 min internalization.
(G) to (J) Colocalization of RABA2a-YFP with FM4-64 in untreated ([G] and [H]) or BFA-treated ([I] and [J]) wild-type ([G] and [I]) and yip4a yip4b seedlings ([H] and [J]). Bars = 10 µm.
Although SYP61-CFP and VHA-a1-GFP are partially mislocalized in yip4a yip4b, the residual punctate signal is found in the core of BFA bodies following BFA treatment (Figure 4O; see Supplemental Figure 4H online), as described for the wild type (Figures 3C, 3D, and 4N; Geldner et al., 2003; Dettmer et al., 2006; Lam et al., 2009; Viotti et al., 2010). Furthermore, SYP61-CFP and VHA-a1 puncta colocalizes with FM4-64 within 5 min in the yip4a yip4b mutant (Figures 5B and 5F), revealing that those compartments still act as early endosome. These results indicate that a functional SYP61/VHA-a1 TGN subdomain still exists in the yip4a yip4b mutant.
TGN-Golgi Association Is Affected by the Lack of YIP4 Proteins
As the TGN originates from the trans-most cisternae of the Golgi bodies (Staehelin and Kang, 2008), we next investigated the TGN-Golgi association in the yip4a yip4b mutant. Confocal images indicate that neither Golgi bodies nor MVBs are affected, as the N-ST-YFP and RABF1-GFP signals remain unchanged in the yip4a yip4b double mutant (see Supplemental Figures 4D and 4F online). Transmission electron microscopy of the Golgi confirmed that the mean cisternal length is not affected in yip4a yip4b mutants compared with the wild type (see Supplemental Figure 5A online). The main difference observed between wild-type and yip4a yip4b cells was that fewer Golgi stacks have an associated TGN in yip4a yip4b compared with the wild type (see Supplemental Figure 5B online), as has been observed in the ech mutant (Gendre et al., 2011).
The YIP4a/YIP4b/ECH Complex Does Not Play a Role in Endocytosis or Vacuolar Transport
The mislocalization of TGN-resident proteins in yip4a yip4b and the dramatic growth phenotypes of these plants prompted us to investigate whether endocytosis, vacuolar trafficking, and/or secretion were perturbed. We investigated the potential effects on the endocytic pathway using FM4-64, a lipophilic dye that initially labels the PM and then every compartment traversed by the endocytic cargo before it reaches the tonoplast (Bolte et al., 2004). FM4-64 internalization followed the same time course in yip4a yip4b cells as in wild-type cells, staining the first endosomes within 5 min and reaching the tonoplast within 3 to 4 h (see Supplemental Figure 6A online). This suggests that the endocytic machinery is not impaired in yip4a yip4b mutants.
Furthermore, as both VHA-a3-GFP (Dettmer et al., 2006) and γ-TIP-GFP (Boursiac et al., 2005) labeled the tonoplast with equal intensity in the wild type and yip4a yip4b (see Supplemental Figure 6B online), vacuolar trafficking of membrane proteins is unaffected by the loss of the YIP4 proteins. However, yip4a yip4b did exhibit some defects in vacuolar morphology similar to those described for ech (Gendre et al., 2011). Nevertheless, the soluble vacuolar cargo aleurain-GFP, which is known to use a vacuolar transport pathway distinct from γ-TIP-GFP (Gomez and Chrispeels, 1993), is correctly targeted to the main lytic vacuole in yip4a yip4b (see Supplemental Figure 6C online). In conclusion, the loss of YIP4 proteins does not affect endocytosis or transport to vacuolar cargo from the TGN.
The YIP4a/YIP4b/ECH Complex Is Required for Cell Wall Polysaccharide Secretion
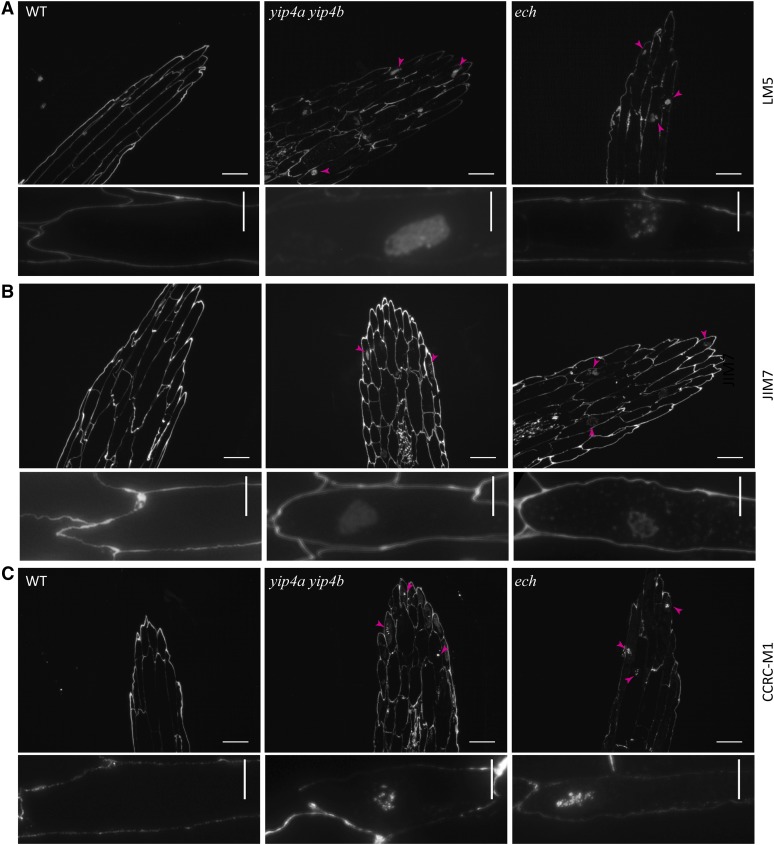
In plants, cell elongation requires the biosynthesis of cell wall components, including pectins and hemicellulose, which are proposed to be delivered to the cell wall via TGN (reviewed in Carpita, 2011; Driouich et al., 2012). The cell elongation defects in the ech and yip4a yip4b mutants, along with the TGN localization of ECH and YIP4, prompted us to investigate whether the complex could be involved in secretion of soluble cell wall polysaccharides (pectin and hemicellulose) via TGN. The pectic matrix is structurally complex, featuring homogalacturonan, rhamnogalacturonan I (RGI), and rhamnogalacturonan II. Antibodies, including anti-RGI (LM5; Figure 6A), antimethylesterified homogalacturonan (JIM7; Figure 6B), and antixyloglucan (CCRC-M1; Figure 6C), labeled the cell wall of the wild-type, ech, and yip4a yip4b dark-grown hypocotyls. However, labeled pectin and xyloglucan also accumulated inside the ech and yip4a yip4b mutant cells (Figures 6A to 6C), which is never found in the wild type (Figure 6A, left panel). The most drastic intracellular polysaccharide accumulations were observed for LM5 (Figure 6A) and CCRC-M1 (Figure 6C), suggesting that the secretion of xyloglucan and RGI pectins are equally affected in ech and yip4a yip4b.
Figure 6.
Intracellular Accumulation of Pectin and Xyloglucan in Dark-Grown Hypocotyls of ech and yip4a yip4b.
Wild-type (WT), yip4a yip4b, and ech etiolated hypocotyls immunolabeled with LM5 (A), JIM7 (B), or CCRC-M1 (C). Arrowheads indicate intracellular accumulation. Bars = 100 µm; bars = 10 µm in close-up images in bottom rows.
[See online article for color version of this figure.]
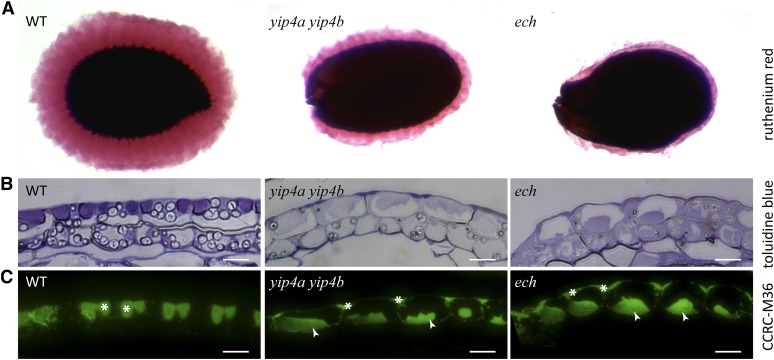
We further confirmed the defects in polysaccharide secretion by investigating the seed coat epidermis, in which the TGN is highly specialized for pectic mucilage secretion (Young et al., 2008). Ruthenium red staining of mucilage released from mature seeds was greatly reduced in both yip4a yip4b and ech seeds, relative to the wild type (Figure 7A). Sections through the seed coat that were stained with Toluidine Blue (Figure 7B) or labeled with the antimucilage antibody CCRC-M36 (Figure 7C; Young et al., 2008) revealed that yip4a yip4b and ech mucilage secretory cells display aberrant pectin localization. In the wild type, Toluidine Blue and CCRC-M36 strongly label the mucilage pockets situated at distinct apical domains of the secretory cells. In both the yip4a yip4b and ech mutants, Toluidine and M36 signal mainly labeled the center of the cell (Figures 7B and 7C), probably in the vacuolar lumen, with some signal also detected at the apoplastic mucilage pockets. Taken together, these data confirm that cell wall matrix polysaccharides, including both hemicelluloses and pectins, are not properly secreted and instead accumulate within ech and yip4a yip4b mutant seed coat cells and hypocotyls.
Figure 7.
Intracellular Accumulation of Pectin Mucilage in ech and yip4a yip4b Seed Coats.
(A) Ruthenium red–stained seed coat mucilage after imbibition of wild-type (WT), yip4a yip4b, and ech seeds.
(B) and (C) Wild-type, yip4a yip4b, and ech seed coat sections stained with Toluidine Blue (B) or immunolabeled with CCRC-M36 (C). Arrowheads indicate intracellular accumulation, and asterisks indicate mucilage pockets. Bars = 10 µm.
[See online article for color version of this figure.]
The defects in the secretion of cell wall components in ech and yip4 mutants should result in alterations in the cell walls in these mutants. Therefore, we analyzed cell wall composition in the hypocotyls of etiolated ech and yip4a yip4b seedlings using Fourier transform-infrared (FT-IR) microspectroscopy, a powerful method for identifying changes in the cell wall composition (Mouille et al., 2003). FT-IR analysis demonstrated that the cell walls of ech, yip4a-1 yip4b, and yip4a-2 yip4b are similar to one another, indicating similar cell wall defects in these mutants and that they are distinct from the wild type (see Supplemental Figure 7 online). Moreover, ech and yip4a yip4b generally cluster closer to mutants defective in soluble polysaccharides synthesis. Thus, these results indicate that the ECH/YIP4 complex is important for the TGN-mediated secretion of cell wall components such as pectins and xyloglucan, which ultimately affect cell wall composition. Interestingly, the secretion defect observed with polysaccharides is not a general secretion defect, as the PM proteins PIN2- and PIN3-GFP did not accumulate inside yip4a yip4b cells and are found at similar intensity levels at the PM of yip4a yip4b compared with the wild type (see Supplemental Figure 8 online).
DISCUSSION
Here, we show that the evolutionarily conserved ECH/YIP4 complex acts at the TGN to mediate the secretion of cell wall polysaccharides without affecting either endocytic or vacuolar trafficking in Arabidopsis. Secretion via the TGN plays a key role in cell elongation, as revealed by the impairment of cell elongation when TGN function is altered in the ech and yip4a yip4b mutants
ECH/YIP4a/YIP4b Is a Conserved Eukaryotic Interaction
We identified two members of the YIP domain family, YIP4a (At2g18840) and YIP4b (At4g30260), as interactors of ECH at the TGN. The similarities between ech and yip4a yip4b mutant phenotypes and the genetic, biochemical, and cell biological data indicate that ECH and YIP4a/YIP4b form a TGN-localized complex. Furthermore, YIP4 proteins are required for the maintenance of ECH on SYP61-/VHA-a1–positive TGN compartments, and YIP4a and 4b rely at least partly on ECH for localization to this specific subdomain. Proteins of the YIP family are found in all eukaryotic organisms but have been studied most extensively in yeast and, more recently, in mammals. YIPs play a crucial role in vesicle trafficking (Yang et al., 1998; Matern et al., 2000; Barrowman et al., 2003; Heidtman et al., 2003; Chen et al., 2004; Chen and Collins, 2005b, 2005a; Yoshida et al., 2008; Kano et al., 2009; Tanimoto et al., 2011), which may involve their ability to bind RAB GTPases (RABs; hence their name Ypt/Rab Interacting Protein) (Yang et al., 1998), making them attractive candidates for the recruitment of RABs onto target membranes (Yang et al., 1998; Barrowman et al., 2003; Calero et al., 2003; Heidtman et al., 2003; Chen et al., 2004). YIP1p, the best characterized member of the yeast YIP family, acts in the secretory pathway at an early stage (Yang et al., 1998; Matern et al., 2000; Calero et al., 2003), but it remains unclear whether YIP1p regulates vesicle fusion at the Golgi apparatus (Barrowman et al., 2003) or vesicle budding from the endoplasmic reticulum (ER) (Heidtman et al., 2003). While YIP1p acts at the ER-Golgi interface, the other yeast YIPs, YIP4p and YIP5p, may function later in the secretory pathway (Inadome et al., 2007). Arabidopsis YIP4a and YIP4b display the YIP domain topology (Shakoori et al., 2003) and are most similar to yeast YIP4p. Like Arabidopsis YIP4 proteins, yeast YIP4p interacts with TVP23p, the yeast ortholog of ECH (Gendre et al., 2011), as demonstrated by Y2H (Uetz et al., 2000; Ito et al., 2001) and co-IP (Inadome et al., 2007). Moreover, ECH and YIP4 localizations are also conserved across species, as both TVP23p and YIP4p localize to the late Golgi/early endosome (Inadome et al., 2007), which corresponds to the plant TGN. Thus, ECH and YIP4a/b localizations and interaction have been conserved through evolution between the organisms as distant as yeast and plants, indicating their functional importance for vesicle trafficking.
The ECH/YIP4a/YIP4b Interaction Is Important for TGN Maintenance and Function
Although the ECH (TVP23)/YIP4 interaction is evolutionarily conserved from yeast to plants, little was previously known about its role and function at the TGN in any organism partly due to the lack of easily detectable phenotypes for the corresponding mutants. For example loss of yeast TVP23 or YIP4 has no measurable effect on growth (Calero et al., 2002; Stein et al., 2009). Nevertheless, when investigated, YIP proteins have been shown to be important for the maintenance and function of the compartments to which they localize. For example, in HeLa cells, YIPF3, YIPF4 (Tanimoto et al., 2011), and YIPF5 (Yoshida et al., 2008) are cis-Golgi located, and their loss leads to a partial fragmentation of the Golgi apparatus. We did not detect such defects in ech or yip4a yip4b mutants; instead, less TGN was associated with the Golgi in these mutants.
All YIP family members described so far in yeast and mammalian systems are Golgi located and are involved in ER-to-Golgi (Barrowman et al., 2003; Heidtman et al., 2003; Jin et al., 2005; Tanimoto et al., 2011) or Golgi-to-ER (Kano et al., 2009) transport. In Arabidopsis, the partial mislocalization of SYP61-CFP and VHA-a1-GFP in the absence of ECH (Gendre et al., 2011) or YIP4 proteins (this work) indicates that the ECH/YIP4 complex plays a key role in the maintenance of the TGN. Interestingly, in contrast with ECH, the loss of YIP4 does not lead to the mislocalization of RABA2a-YFP, which defines a subdomain of TGN that only partially overlaps the SYP61/VHAa1 domain (Chow et al., 2008). This suggests that ECH/YIP4 complex function could be restricted more specifically to the VHAa1/SYP61 subdomain.
We previously proposed that the TGN defects in ech (i.e., VHA-a1 and SYP61 partial mislocalization) could explain the growth/secretion defects (Gendre et al., 2011), as inhibition of V-ATPase activity with Concanamycin A mimics the ech and yip4a yip4b phenotypes. In yip4 double mutants, VHA-a1-GFP localization is less affected than in ech, which correlates with the reduced severity of the yip4a yip4b double mutation on cell elongation and mucilage secretion compared with ech. The localization of SYP61-CFP is also greatly affected by the absence of the ECH/YIP4a/YIP4b complex. Low levels of SYP61-CFP are often observed on the PM; recently, proteomic analysis of SYP61 compartments revealed that they may have a role in secretion (Drakakaki et al., 2011). The mislocalization of SYP61-CFP could thus be another factor that contributes to the phenotypes of ech and yip4a yip4b. However, the cell biological phenotypes of syp61 loss-of-function mutants have not been described; therefore, the extent to which loss of SYP61 from TGN contributes to the ech and yip4a yip4b phenotypes remains to be seen.
The ECH/YIP4a/YIP4b Complex Defines a TGN Subdomain Mediating Polysaccharide Secretion
The ECH/YIP4 complex plays a key role in TGN maintenance since its absence leads to mislocalization of key components, such as VHA-a1, from the TGN. Since the TGN is a hub for several trafficking pathways, the defects in TGN could perturb any of the functions of the TGN, including vacuolar trafficking, endocytosis, and/or secretion. However, neither the endocytic nor the vacuolar route is affected in ech and yip4a yip4b. Instead, the secretion of cell wall polysaccharides is perturbed, as demonstrated by the intracellular accumulation of pectin and xyloglucan within ech and yip4a yip4b etiolated hypocotyl cells. This result explains the altered cell wall composition of the mutants revealed by FT-IR analysis and may underlie the cell elongation defects observed in both ech and yip4a yip4b. Furthermore, the seed coat phenotype of the yip4a yip4b and ech provides a rare opportunity to observe the perturbation of pectin traffic during a developmental period of abundant pectin secretion. The loss of ECH or YIP4a/b leads to ineffective pectin transport to the seed coat mucilage pockets, resulting in almost no mucilage release from seeds upon hydration. Thus, the data from the seed coat analysis, together with that from hypocotyls, strongly support the role of ECH/YIP4 complex in secretion of pectin and xyloglucan via TGN.
Pectin and xyloglucan are important for cell elongation and play a key role in determining the mechanical properties of the cell walls. Changes in pectin and xyloglucan are associated with cell elongation and morphogenesis (Derbyshire et al., 2007; Peaucelle et al., 2011; reviewed in Hayashi and Kaida, 2011). Additionally, increased growth in pollen tubes is associated with increased exocytosis of pectins (McKenna et al., 2009), and deficiency in xyloglucans affects various aspects of growth (Cavalier et al., 2008), indicating that the delivery of these components plays an important role in the cell elongation process. Nevertheless, how these cell wall polysaccharides are delivered to the cell wall and which components mediate their secretion are largely unknown. Therefore, our identification of the role of the ECH/YIP4a/YIP4b complex in TGN-mediated secretion of pectin and hemicellulose provides a foundation for dissecting the molecular mechanisms that underpin polysaccharide secretion to the plant cell wall. Importantly, several hormones (e.g., indole-3-acetic acid, ethylene, and brassinosteroids) are thought to mediate their effects on cell elongation via alteration of cell walls (reviewed in Wolf et al., 2012). Thus, identification of components such as ECH and YIP4 in mediating the secretion of cell wall polysaccharides provides an additional control point for the regulation of cell elongation by hormonal and developmental signals.
METHODS
Plant Material
For growth conditions and genotyping of ech, see Gendre et al. (2011). The Arabidopsis thaliana (Col-0) T-DNA insertion lines yip4a-1 (SALK_066428), yip4a-2 (SALK_021897), and yip4b (SALK_129888) were obtained from the SALK Institute. Molecular genotyping was performed using the primers listed in Supplemental Table 1 online.
Transcript levels of the 5′UTR, 3′UTR, and the full-length transcripts of YIP4a and YIP4b were evaluated using EF1α transcription level as control. PCR reactions were performed on cDNA obtained from leaves (see Supplemental Table 1 online for primers).
The following transgenic fluorescent protein marker lines were used in the Col-0 background: pRAB-A2a:YFP-RABA2a (Chow et al., 2008), pSYP61:SYP61-CFP (Robert et al., 2008), pRABF1:RABF1-GFP (Goh et al., 2007), pRABF2b:GFP-RABF2b (Goh et al., 2007), pVHA-a3:VHA-a3-GFP (Dettmer et al., 2006), pVHA-a1:VHA-a1-GFP (Dettmer et al., 2006), p35S:N-ST-YFP and p35S:NAG1-EGFP (Grebe et al., 2003), p35S:γ-TIP-GFP (Boursiac et al., 2005), and p35S:Aleu-GFP (Di Sansebastiano et al., 2001) in the Wassilewskija background. pECH:ECH-YFP (Gendre et al., 2011) is in the ech background.
Plasmid Construction and Plant Transformation
In total, 1.8 kb of YIP4a and 1.3 kb of YIP4b promoters were amplified, as well as 2 kb of the YIP4a open reading frame and YIP4b coding sequence (see Supplemental Table 1 online for all primers). After digestion, promoters were cloned into the BamHI- and AscI-digested vector pSL34 (Ikeda et al., 2009), and the resulting plasmid was SpeI and NotI digested to ligate either YIP4a or b. The promoter:gene construct was isolated by BamHI-AscI digestion and transferred into pGreenII 0229 (Basta resistance). Additionally, the insertion of 3*HA in frame at the N-terminal side of YIP4a open reading frame was used by annealing HA forward/HA reverse primers (heating to 96°C in ligase buffer followed by cooling to room temperature). The Agrobacterium tumefaciens strain C58C1 was transformed with pGreen YIP4b:YIP4b and YIP4a:HA-YIP4a and used to transform the Arabidopsis yip4a yip4b double mutant by the floral dip method (Clough and Bent, 1998).
Length Measurement
Root and hypocotyl lengths were measured with Image J (Abramoff et al., 2004) on pictures taken at full resolution with a Canon 350D camera.
Colocalization Quantification
Colocalization was performed by assessing the proximity of the geometric centers (centroids) of objects in two different channels within the objective resolution (Boutté et al., 2006). The centroid coordinates were obtained using 3D objects counter in Image J (Bolte and Cordelières, 2006). Four to six cells from each of 10 individual roots were analyzed for quantification.
YIP4b Antibody Production
The first 330 bp of YIP4b cDNA was amplified, cloned into pET28a, and overexpressed with a C-terminal HIS tag in Escherichia coli (strain Rosetta). Four hours after induction with 1 mM isopropyl β-d-1-thiogalactopyranoside, the expressed 15-kD protein was extracted, purified twice under native conditions using nickel-nitrilotriacetic acid beads, and dialyzed overnight. The correct band was excised from the gel and used for rabbit inoculation. Antibody production and ELISA testing were performed by Agrisera (Sweden). The YIP4b polyclonal antibody from the final bleed serum was affinity purified using the expressed 330-bp fragment of YIP4b. This antibody detected a single 30-kD band on protein gel blots (anti-YIP4b 1:250 followed by anti-rabbit-HRP Bio-Rad 1:10,000) with protein extracts from the wild type, yip4a-1, and yip4a-2, but no band corresponding to YIP4b was observed with the yip4b, yip4a-1 yip4b, and yip4a-2 yip4b protein extracts (Figure 3B, inset), indicating its specificity for YIP4b.
Confocal Laser Scanning Microscopy and Immunolabeling
Fluorescence signals were viewed under an Axioplan inverted microscope with a Zeiss LSM 780 spectral confocal laser scanning microscope. GFP was detected using a 488-nm laser and 493- to 598-nm emission filter; CFP was detected with a 405-nm laser and 454- to 581-nm emission filter. Arabidopsis root whole-mount immunolabeling and drug treatments were performed using the same protocol as previously described (Gendre et al., 2011) using anti-YIP4b (1:150) and anti-rabbit-CY5 (1:300; Jackson Immunoresearch) or anti-HA (1:700; Covance) followed by anti-mouse TRITC (1:250; Jackson Immunoresearch). Cy5 was detected using a 633-nm laser and 638- to 759-nm emission filter followed by ab anti-mouse antibody conjugated with tetramethylrhodamine isothiocyanate with a 561-nm laser and 569- to 638-nm emission filter. For acidic organelle labeling, seedlings were incubated for 30 min in 50 nM lysotracker red (Invitrogen) and detected using a 561-nm laser and 560- to 700-nm emission filter. FM4-64 (5 µM) and pretreatment with BFA (50 µM, 1 h) and Wm (33 µM, 1 h) were executed as previously described (Gendre et al., 2011).
For hypocotyl and seed coat immunolabeling, samples were high-pressure frozen, freeze-substituted, and embedded in LR White resin as previously described (McFarlane et al., 2008). Thick sections (250 nm) were prepared using a Leica Ultracut UCT Ultramicrotome and placed on Teflon-coated glass slides (Electron Microscopy Sciences). The morphology of each sample was surveyed by staining with aqueous 1% Toluidine Blue in 1% sodium borate using a Leica DMR microscope. Unstained sections were processed for immunofluorescence as described (Young et al., 2008) using 1:10 of mouse CCRC-M36 or CCRC-M1 from the Complex Carbohydrate Research Center (University of Georgia; Pattathil et al., 2010) or 1:10 rat LM5 (Jones et al., 1997) or JIM7 (Knox et al., 1990) from Leeds University. Sections were incubated in 1:100 goat-anti-mouse or goat-anti-rat antibody conjugated to Alexa488 (Molecular Probes) and viewed under a Leica DMR microscope equipped with an ebq100 mercury fluorescent source with a 450- to 490-nm band-pass excitation filter, no emission filter, and a QICAM digital camera (QImaging).
Transmission Electron Microscopy
High-pressure freezing of 7-d-old root tips was done using a Leica EM HPM100. See Gendre et al. (2011) for more details.
Ruthenium Red Staining
Mature seeds were incubated in 0.05 M EDTA for 1 h at room temperature with gentle shaking, followed by 1 h incubation in 0.01% (w/v) aqueous solution of Ruthenium red. Seeds were mounted in water and viewed using a Leica MZ9.5 stereomicroscope coupled to a Leica DC300 camera.
Y2H
Coding sequences of ECH, YIP4a, and YIP4b were amplified from Arabidopsis root cDNA (see Supplemental Table 1 online for primers), cloned into pGEM-T, and subcloned in the EcoRI- and XhoI-digested pEG202 and pJG4-5. Y2H was performed according to the manufacturer’s instructions (DupLEX-A; OriGene) with pEG202 ΔSphI, a truncated version of GNOM (GN4-4), and pJG4-5 CYCLOPHILIN5 (C-395) as controls (Grebe et al., 2000).
Co-IP
The cloning procedure was identical to cloning for the Y2H assay. Fragments were introduced into pRT104 (N-terminal fusion with 3*Myc or 3*HA). For the co-IP assay, 5 µg of ECH fused to a Myc-tag and YIP4a or YIP4b fused to a HA tag were transiently expressed in Arabidopsis cell culture protoplasts (Fülöp et al., 2005). Immunocomplexes were captured on Protein G sepharose (GE Healthcare) as described by Cruz-Ramírez et al. (2012). Immunodetection of tagged proteins was performed using monoclonal anti-HA-peroxidase conjugate (clone 3F10; Roche), monoclonal anti-c-Myc (clone 9E10; Covance), and chicken anti-c-Myc (Molecular Probes) antibodies.
FT-IR
Four-day-old seedlings were squashed between two BaF2 windows and abundantly rinsed in distilled water for 2 min. The samples were then dried on the window at 37°C for 20 min. An area of 50 µm × 50 µm halfway up the hypocotyl, on the side of the central cylinder, was selected for spectra collection. Spectra were collected using a ThermoNicolet Nexus spectrometer with a Continuum microscope accessory. Five hypocotyls were measured for each of the four biological replicate giving a total of 20 spectra collected (see Supplemental Data Set 1 online for normalized spectral data). See Mouille et al. (2003) for further info and Robin et al. (2003) for more info on statistical analysis.
Bioinformatic and Phylogenetic Analysis
Multiple protein sequence alignments for YIP4a and YIP4b were generated with T-coffee (Poirot et al., 2003), using Boxshade for shading. Related proteins from yeast and Arabidopsis were aligned using MUSCLE (Edgar, 2004), and a phylogenetic tree was constructed using PhyML (www.phylogeny.fr) (Dereeper et al., 2008, 2010) based on the full protein sequence (for details of the procedure; see Supplemental Data Set 2 online).
Accession Numbers
Sequence data from this article can be found in GenBank/EMBL data libraries under the following accession numbers: ECH, At1g09330; YIP4a, At2g18840; YIP4b At4g30260; YIP1a, At2g36300; YIP1b, At3g52760; YIP5a, At2g39805; YIP5b, At3g05280; YIP5c, At5g27490; Sc-YIP1, YGR172C; Sc-YIP2, YPR028W; Sc-YIP3, YNL044W; Sc-YIP4, YGL198W; Sc-YIP5, YGL161C; VHA-a1, At2g28520; and SYP61, At1g28490.
Supplemental Data
The following materials are available in the online version of this article.
Supplemental Figure 1. YIP4a and YIP4b Belong to a Multigenic Family Encoding Transmembrane Proteins.
Supplemental Figure 2. Characterization of yip4 Single and Double Mutants.
Supplemental Figure 3. YIP4a and YIP4b Are TGN Localized.
Supplemental Figure 4. ECH and YIP4b Are Partially Mislocalized in yip4a yip4b and ech, Respectively.
Supplemental Figure 5. TGN-Golgi Association Is Affected by the Lack of YIP4 Proteins.
Supplemental Figure 6. Endocytic and Vacuolar Routes Are Not Affected in yip4a yip4b.
Supplemental Figure 7. Cell Wall Composition Altered in Etiolated Hypocotyls of ech and yip4a yip4b.
Supplemental Figure 8. PIN2- and PIN3-GFP Intensity at the Plasma Membrane of the Wild Type and yip4a yip4b.
Supplemental Table 1. Sequences of Primers.
Supplemental Data Set 1. FT-IR Normalized Data Used to Create the Dendrogram of Supplemental Figure 7.
Supplemental Data Set 2. Procedure Used for the Phylogenetic Analysis of Supplemental Figure 1.
Supplemental References 1. References for Supplemental Figures.
Acknowledgments
We thank Natasha Raikhel (SYP61-CFP), Karin Schumacher (VHA-a1 and VHA-a3), Christophe Maurel (γ-TIP), Markus Grebe (C-395, GN4-4, N-ST-YFP, and NAG-EGFP), and Tomohiro Uemura (RabF1-GFP and GFP-RabF2b) for providing published material, the University of British Columbia Bioimaging Facility for technical assistance, and Ingela Sandström for constant help in the lab. We also thank Stephanie Robert and Sebastian Bednarek for helpful comments. This research was funded by an Natural Sciences and Engineering Research Council Discovery Grant to L.S. and by grants from Sveriges lantbruksuniversitet (Excellence), Formas (Funcfiber), and Knut och Alice Wallenbergs Stiftelse Foundations to R.P.B.
AUTHOR CONTRIBUTIONS
D.G., H.E.M., L.S., and R.P.B. designed the research. D.G., H.E.M., G.M., G.L.-T., Y.W., E.J., and J.O. performed research. A.S. contributed new computational tools. D.G., H.E.M., L.S., and R.P.B. analyzed data. D.G., H.E.M., L.S., and R.P.B. wrote the article.
Glossary
- PM
plasma membrane
- TGN
trans-Golgi network
- Y2H
yeast two-hybrid
- co-IP
coimmunoprecipitation
- UTR
untranslated region
- Col-0
Columbia-0
- MVB
multivesicular body
- BFA
Brefeldin A
- Wm
Wortmannin
- RGI
rhamnogalacturonan I
- FT-IR
Fourier transform-infrared
- ER
endoplasmic reticulum
- HA
hemagglutinin
References
- Abramoff M.D., Magelhaes P.J., Ram S.J. (2004). Image Processing with ImageJ. Biophotonics International 11: 36–42 [Google Scholar]
- Barrowman J., Wang W., Zhang Y., Ferro-Novick S. (2003). The Yip1p.Yif1p complex is required for the fusion competence of endoplasmic reticulum-derived vesicles. J. Biol. Chem. 278: 19878–19884 [DOI] [PubMed] [Google Scholar]
- Bolte S., Cordelières F.P. (2006). A guided tour into subcellular colocalization analysis in light microscopy. J. Microsc. 224: 213–232 [DOI] [PubMed] [Google Scholar]
- Bolte S., Talbot C., Boutte Y., Catrice O., Read N.D., Satiat-Jeunemaitre B. (2004). FM-dyes as experimental probes for dissecting vesicle trafficking in living plant cells. J. Microsc. 214: 159–173 [DOI] [PubMed] [Google Scholar]
- Boursiac Y., Chen S., Luu D.T., Sorieul M., van den Dries N., Maurel C. (2005). Early effects of salinity on water transport in Arabidopsis roots. Molecular and cellular features of aquaporin expression. Plant Physiol. 139: 790–805 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Boutté Y., Crosnier M.T., Carraro N., Traas J., Satiat-Jeunemaitre B. (2006). The plasma membrane recycling pathway and cell polarity in plants: Studies on PIN proteins. J. Cell Sci. 119: 1255–1265 [DOI] [PubMed] [Google Scholar]
- Brüx A., Liu T.Y., Krebs M., Stierhof Y.D., Lohmann J.U., Miersch O., Wasternack C., Schumacher K. (2008). Reduced V-ATPase activity in the trans-Golgi network causes oxylipin-dependent hypocotyl growth Inhibition in Arabidopsis. Plant Cell 20: 1088–1100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calero M., Chen C.Z., Zhu W., Winand N., Havas K.A., Gilbert P.M., Burd C.G., Collins R.N. (2003). Dual prenylation is required for Rab protein localization and function. Mol. Biol. Cell 14: 1852–1867 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Calero M., Winand N.J., Collins R.N. (2002). Identification of the novel proteins Yip4p and Yip5p as Rab GTPase interacting factors. FEBS Lett. 515: 89–98 [DOI] [PubMed] [Google Scholar]
- Carpita N.C. (2011). Update on mechanisms of plant cell wall biosynthesis: How plants make cellulose and other (1->4)-β-D-glycans. Plant Physiol. 155: 171–184 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Cavalier D.M., Lerouxel O., Neumetzler L., Yamauchi K., Reinecke A., Freshour G., Zabotina O.A., Hahn M.G., Burgert I., Pauly M., Raikhel N.V., Keegstra K. (2008). Disrupting two Arabidopsis thaliana xylosyltransferase genes results in plants deficient in xyloglucan, a major primary cell wall component. Plant Cell 20: 1519–1537 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.Z., Calero M., DeRegis C.J., Heidtman M., Barlowe C., Collins R.N. (2004). Genetic analysis of yeast Yip1p function reveals a requirement for Golgi-localized rab proteins and rab-Guanine nucleotide dissociation inhibitor. Genetics 168: 1827–1841 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chen C.Z., Collins R.N. (2005a). Insights into biological functions across species: Examining the role of Rab proteins in YIP1 family function. Biochem. Soc. Trans. 33: 614–618 [DOI] [PubMed] [Google Scholar]
- Chen C.Z., Collins R.N. (2005b). Analysis and properties of the yeast YIP1 family of Ypt-interacting proteins. Methods Enzymol. 403: 333–339 [DOI] [PubMed] [Google Scholar]
- Chevalier L., Bernard S., Ramdani Y., Lamour R., Bardor M., Lerouge P., Follet-Gueye M.L., Driouich A. (2010). Subcompartment localization of the side chain xyloglucan-synthesizing enzymes within Golgi stacks of tobacco suspension-cultured cells. Plant J. 64: 977–989 [DOI] [PubMed] [Google Scholar]
- Chow C.M., Neto H., Foucart C., Moore I. (2008). Rab-A2 and Rab-A3 GTPases define a trans-Golgi endosomal membrane domain in Arabidopsis that contributes substantially to the cell plate. Plant Cell 20: 101–123 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Clough S.J., Bent A.F. (1998). Floral dip: A simplified method for Agrobacterium-mediated transformation of Arabidopsis thaliana. Plant J. 16: 735–743 [DOI] [PubMed] [Google Scholar]
- Cruz-Ramírez A., et al. (2012). A bistable circuit involving SCARECROW-RETINOBLASTOMA integrates cues to inform asymmetric stem cell division. Cell 150: 1002–1015 [DOI] [PMC free article] [PubMed] [Google Scholar]
- De Matteis M.A., Luini A. (2008). Exiting the Golgi complex. Nat. Rev. Mol. Cell Biol. 9: 273–284 [DOI] [PubMed] [Google Scholar]
- Derbyshire P., Findlay K., McCann M.C., Roberts K. (2007). Cell elongation in Arabidopsis hypocotyls involves dynamic changes in cell wall thickness. J. Exp. Bot. 58: 2079–2089 [DOI] [PubMed] [Google Scholar]
- Dereeper A., Audic S., Claverie J.M., Blanc G. (2010). BLAST-EXPLORER helps you building datasets for phylogenetic analysis. BMC Evol. Biol. 10: 8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dereeper A., Guignon V., Blanc G., Audic S., Buffet S., Chevenet F., Dufayard J.F., Guindon S., Lefort V., Lescot M., Claverie J.M., Gascuel O. (2008). Phylogeny.fr: robust phylogenetic analysis for the non-specialist. Nucleic Acids Res. 36 (Web Server issue): W465–W469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Dettmer J., Hong-Hermesdorf A., Stierhof Y.D., Schumacher K. (2006). Vacuolar H+-ATPase activity is required for endocytic and secretory trafficking in Arabidopsis. Plant Cell 18: 715–730 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Di Sansebastiano G.P., Paris N., Marc-Martin S., Neuhaus J.M. (2001). Regeneration of a lytic central vacuole and of neutral peripheral vacuoles can be visualized by green fluorescent proteins targeted to either type of vacuoles. Plant Physiol. 126: 78–86 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Drakakaki G., van de Ven W., Pan S., Miao Y., Wang J., Keinath N.K., Weatherly B., Jiang L., Schumacher K., Hicks G., Raikhel N. (2011). Isolation and proteomic analysis of the SYP61 compartment reveal its role in exocytic trafficking in Arabidopsis. Cell Res. 22: 413–424. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Driouich A., Follet-Gueye M.L., Bernard S., Kousar S., Chevalier L., Vicré-Gibouin M., Lerouxel O. (2012). Golgi-mediated synthesis and secretion of matrix polysaccharides of the primary cell wall of higher plants. Front. Plant Sci. 3: 79. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Edgar R.C. (2004). MUSCLE: Multiple sequence alignment with high accuracy and high throughput. Nucleic Acids Res. 32: 1792–1797 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fülöp K., Pettkó-Szandtner A., Magyar Z., Miskolczi P., Kondorosi E., Dudits D., Bakó L. (2005). The Medicago CDKC;1-CYCLINT;1 kinase complex phosphorylates the carboxy-terminal domain of RNA polymerase II and promotes transcription. Plant J. 42: 810–820 [DOI] [PubMed] [Google Scholar]
- Geldner N., Anders N., Wolters H., Keicher J., Kornberger W., Muller P., Delbarre A., Ueda T., Nakano A., Jürgens G. (2003). The Arabidopsis GNOM ARF-GEF mediates endosomal recycling, auxin transport, and auxin-dependent plant growth. Cell 112: 219–230 [DOI] [PubMed] [Google Scholar]
- Gendre D., Oh J., Boutté Y., Best J.G., Samuels L., Nilsson R., Uemura T., Marchant A., Bennett M.J., Grebe M., Bhalerao R.P. (2011). Conserved Arabidopsis ECHIDNA protein mediates trans-Golgi-network trafficking and cell elongation. Proc. Natl. Acad. Sci. USA 108: 8048–8053 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gendreau E., Traas J., Desnos T., Grandjean O., Caboche M., Höfte H. (1997). Cellular basis of hypocotyl growth in Arabidopsis thaliana. Plant Physiol. 114: 295–305 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Goh T., Uchida W., Arakawa S., Ito E., Dainobu T., Ebine K., Takeuchi M., Sato K., Ueda T., Nakano A. (2007). VPS9a, the common activator for two distinct types of Rab5 GTPases, is essential for the development of Arabidopsis thaliana. Plant Cell 19: 3504–3515 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gomez L., Chrispeels M.J. (1993). Tonoplast and soluble vacuolar proteins are targeted by different mechanisms. Plant Cell 5: 1113–1124 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebe M., Gadea J., Steinmann T., Kientz M., Rahfeld J.U., Salchert K., Koncz C., Jürgens G. (2000). A conserved domain of the Arabidopsis GNOM protein mediates subunit interaction and cyclophilin 5 binding. Plant Cell 12: 343–356 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Grebe M., Xu J., Möbius W., Ueda T., Nakano A., Geuze H.J., Rook M.B., Scheres B. (2003). Arabidopsis sterol endocytosis involves actin-mediated trafficking via ARA6-positive early endosomes. Curr. Biol. 13: 1378–1387 [DOI] [PubMed] [Google Scholar]
- Gutiérrez R.A., MacIntosh G.C., Green P.J. (1999). Current perspectives on mRNA stability in plants: Multiple levels and mechanisms of control. Trends Plant Sci. 4: 429–438 [DOI] [PubMed] [Google Scholar]
- Hayashi T., Kaida R. (2011). Functions of xyloglucan in plant cells. Mol. Plant 4: 17–24 [DOI] [PubMed] [Google Scholar]
- Heidtman M., Chen C.Z., Collins R.N., Barlowe C. (2003). A role for Yip1p in COPII vesicle biogenesis. J. Cell Biol. 163: 57–69 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ikeda Y., Men S., Fischer U., Stepanova A.N., Alonso J.M., Ljung K., Grebe M. (2009). Local auxin biosynthesis modulates gradient-directed planar polarity in Arabidopsis. Nat. Cell Biol. 11: 731–738 [DOI] [PubMed] [Google Scholar]
- Inadome H., Noda Y., Kamimura Y., Adachi H., Yoda K. (2007). Tvp38, Tvp23, Tvp18 and Tvp15: Novel membrane proteins in the Tlg2-containing Golgi/endosome compartments of Saccharomyces cerevisiae. Exp. Cell Res. 313: 688–697 [DOI] [PubMed] [Google Scholar]
- Ito T., Chiba T., Ozawa R., Yoshida M., Hattori M., Sakaki Y. (2001). A comprehensive two-hybrid analysis to explore the yeast protein interactome. Proc. Natl. Acad. Sci. USA 98: 4569–4574 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jackson C.L., Casanova J.E. (2000). Turning on ARF: The Sec7 family of guanine-nucleotide-exchange factors. Trends Cell Biol. 10: 60–67 [DOI] [PubMed] [Google Scholar]
- Jaillais Y., Fobis-Loisy I., Miège C., Gaude T. (2008). Evidence for a sorting endosome in Arabidopsis root cells. Plant J. 53: 237–247 [DOI] [PubMed] [Google Scholar]
- Jin C., Zhang Y., Zhu H., Ahmed K., Fu C., Yao X. (2005). Human Yip1A specifies the localization of Yif1 to the Golgi apparatus. Biochem. Biophys. Res. Commun. 334: 16–22 [DOI] [PubMed] [Google Scholar]
- Jones L., Seymour G.B., Knox J.P. (1997). Localization of pectic galactan in tomato cell walls using a monoclonal antibody specific to (1[->]4)-[beta]-D-galactan. Plant Physiol. 113: 1405–1412 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B.H. (2011). Shrinkage and fragmentation of the trans-Golgi network in non-meristematic plant cells. Plant Signal. Behav. 6: 884–886 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kang B.H., Nielsen E., Preuss M.L., Mastronarde D., Staehelin L.A. (2011). Electron tomography of RabA4b- and PI-4Kβ1-labeled trans Golgi network compartments in Arabidopsis. Traffic 12: 313–329 [DOI] [PubMed] [Google Scholar]
- Kano F., Yamauchi S., Yoshida Y., Watanabe-Takahashi M., Nishikawa K., Nakamura N., Murata M. (2009). Yip1A regulates the COPI-independent retrograde transport from the Golgi complex to the ER. J. Cell Sci. 122: 2218–2227 [DOI] [PubMed] [Google Scholar]
- Knox J.P., Linstead P.J., King J., Cooper C., Roberts K. (1990). Pectin esterification is spatially regulated both within cell walls and between developing tissues of root apices. Planta 181: 512–521 [DOI] [PubMed] [Google Scholar]
- Lam S.K., Cai Y., Tse Y.C., Wang J., Law A.H., Pimpl P., Chan H.Y., Xia J., Jiang L. (2009). BFA-induced compartments from the Golgi apparatus and trans-Golgi network/early endosome are distinct in plant cells. Plant J. 60: 865–881 [DOI] [PubMed] [Google Scholar]
- Lynch M.A., Staehelin L.A. (1992). Domain-specific and cell type-specific localization of two types of cell wall matrix polysaccharides in the clover root tip. J. Cell Biol. 118: 467–479 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matern H., Yang X., Andrulis E., Sternglanz R., Trepte H.H., Gallwitz D. (2000). A novel Golgi membrane protein is part of a GTPase-binding protein complex involved in vesicle targeting. EMBO J. 19: 4485–4492 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Matsuoka K., Bassham D.C., Raikhel N.V., Nakamura K. (1995). Different sensitivity to wortmannin of two vacuolar sorting signals indicates the presence of distinct sorting machineries in tobacco cells. J. Cell Biol. 130: 1307–1318 [DOI] [PMC free article] [PubMed] [Google Scholar]
- McFarlane H.E., Young R.E., Wasteneys G.O., Samuels A.L. (2008). Cortical microtubules mark the mucilage secretion domain of the plasma membrane in Arabidopsis seed coat cells. Planta 227: 1363–1375 [DOI] [PubMed] [Google Scholar]
- McKenna S.T., Kunkel J.G., Bosch M., Rounds C.M., Vidali L., Winship L.J., Hepler P.K. (2009). Exocytosis precedes and predicts the increase in growth in oscillating pollen tubes. Plant Cell 21: 3026–3040 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Mouille G., Robin S., Lecomte M., Pagant S., Höfte H. (2003). Classification and identification of Arabidopsis cell wall mutants using Fourier-transform infrared (FT-IR) microspectroscopy. Plant J. 35: 393–404 [DOI] [PubMed] [Google Scholar]
- Niemes S., Langhans M., Viotti C., Scheuring D., San Wan Yan M., Jiang L., Hillmer S., Robinson D.G., Pimpl P. (2010). Retromer recycles vacuolar sorting receptors from the trans-Golgi network. Plant J. 61: 107–121 [DOI] [PubMed] [Google Scholar]
- Ortega J.L., Moguel-Esponda S., Potenza C., Conklin C.F., Quintana A., Sengupta-Gopalan C. (2006). The 3′ untranslated region of a soybean cytosolic glutamine synthetase (GS1) affects transcript stability and protein accumulation in transgenic alfalfa. Plant J. 45: 832–846 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Pattathil S., et al. (2010). A comprehensive toolkit of plant cell wall glycan-directed monoclonal antibodies. Plant Physiol. 153: 514–525 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Peaucelle A., Braybrook S.A., Le Guillou L., Bron E., Kuhlemeier C., Höfte H. (2011). Pectin-induced changes in cell wall mechanics underlie organ initiation in Arabidopsis. Curr. Biol. 21: 1720–1726 [DOI] [PubMed] [Google Scholar]
- Poirot O., O’Toole E., Notredame C. (2003). Tcoffee@igs: A web server for computing, evaluating and combining multiple sequence alignments. Nucleic Acids Res. 31: 3503–3506 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Richter S., Voss U., Jürgens G. (2009). Post-Golgi traffic in plants. Traffic 10: 819–828 [DOI] [PubMed] [Google Scholar]
- Robert S., Chary S.N., Drakakaki G., Li S., Yang Z., Raikhel N.V., Hicks G.R. (2008). Endosidin1 defines a compartment involved in endocytosis of the brassinosteroid receptor BRI1 and the auxin transporters PIN2 and AUX1. Proc. Natl. Acad. Sci. USA 105: 8464–8469 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Robin S., Lecomte M., Hofte H., Mouille G. (2003). A procedure for the clustering of cell wall mutants in the model plant Arabidopsis based on Fourier-transform infrared (FT-IR) spectroscopy. J. Appl. Stat. 30: 669–681 [Google Scholar]
- Sandhu A.P., Randhawa G.S., Dhugga K.S. (2009). Plant cell wall matrix polysaccharide biosynthesis. Mol. Plant 2: 840–850 [DOI] [PubMed] [Google Scholar]
- Satiat-Jeunemaitre B., Cole L., Bourett T., Howard R., Hawes C. (1996). Brefeldin A effects in plant and fungal cells: Something new about vesicle trafficking? J. Microsc. 181: 162–177 [DOI] [PubMed] [Google Scholar]
- Shakoori A., Fujii G., Yoshimura S., Kitamura M., Nakayama K., Ito T., Ohno H., Nakamura N. (2003). Identification of a five-pass transmembrane protein family localizing in the Golgi apparatus and the ER. Biochem. Biophys. Res. Commun. 312: 850–857 [DOI] [PubMed] [Google Scholar]
- Staehelin L.A., Kang B.H. (2008). Nanoscale architecture of endoplasmic reticulum export sites and of Golgi membranes as determined by electron tomography. Plant Physiol. 147: 1454–1468 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Stein I.S., Gottfried A., Zimmermann J., Fischer von Mollard G. (2009). TVP23 interacts genetically with the yeast SNARE VTI1 and functions in retrograde transport from the early endosome to the late Golgi. Biochem. J. 419: 229–236 [DOI] [PubMed] [Google Scholar]
- Tanimoto K., et al. (2011). Characterization of YIPF3 and YIPF4, cis-Golgi Localizing Yip domain family proteins. Cell Struct. Funct. 36: 171–185 [DOI] [PubMed] [Google Scholar]
- Toyooka K., Goto Y., Asatsuma S., Koizumi M., Mitsui T., Matsuoka K. (2009). A mobile secretory vesicle cluster involved in mass transport from the Golgi to the plant cell exterior. Plant Cell 21: 1212–1229 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Uetz P., et al. (2000). A comprehensive analysis of protein-protein interactions in Saccharomyces cerevisiae. Nature 403: 623–627 [DOI] [PubMed] [Google Scholar]
- Vicré M., Jauneau A., Knox J.P., Driouich A. (1998). Immunolocalization of beta (1-4) and beta-(1-6)-D-galactan epitopes in the cell wall and Golgi stacks of developing flax root tissues. Protoplasma 203: 26–34 [Google Scholar]
- Viotti C., et al. (2010). Endocytic and secretory traffic in Arabidopsis merge in the trans-Golgi network/early endosome, an independent and highly dynamic organelle. Plant Cell 22: 1344–1357 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wolf S., Hématy K., Höfte H. (2012). Growth control and cell wall signaling in plants. Annu. Rev. Plant Biol. 63: 381–407 [DOI] [PubMed] [Google Scholar]
- Worden N., Park E., Drakakaki G. (2012). Trans-Golgi network: An intersection of trafficking cell wall components. J. Integr. Plant Biol. 54: 875–886 [DOI] [PubMed] [Google Scholar]
- Yang X., Matern H.T., Gallwitz D. (1998). Specific binding to a novel and essential Golgi membrane protein (Yip1p) functionally links the transport GTPases Ypt1p and Ypt31p. EMBO J. 17: 4954–4963 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Yoshida Y., et al. (2008). YIPF5 and YIF1A recycle between the ER and the Golgi apparatus and are involved in the maintenance of the Golgi structure. Exp. Cell Res. 314: 3427–3443 [DOI] [PubMed] [Google Scholar]
- Young R.E., McFarlane H.E., Hahn M.G., Western T.L., Haughn G.W., Samuels A.L. (2008). Analysis of the Golgi apparatus in Arabidopsis seed coat cells during polarized secretion of pectin-rich mucilage. Plant Cell 20: 1623–1638 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Zhang G.F., Staehelin L.A. (1992). Functional compartmentation of the Golgi apparatus of plant cells: Immunocytochemical analysis of high-pressure frozen- and freeze-substituted sycamore maple suspension culture cells. Plant Physiol. 99: 1070–1083 [DOI] [PMC free article] [PubMed] [Google Scholar]