Abstract
Inflammasome is a multiprotein complex consisting of Nod-like receptor protein 3 (NALP 3), apoptosis-associated speck-like protein (ASC), and caspase-1 or 5, which functions to switch on the inflammatory process. The present study hypothesized that the formation and activation of NALP3 inflammasomes turn on podocyte injury leading to glomerulosclerosis during hyperhomocysteinemia (hHcys). RT-PCR and Western blot analysis demonstrated that murine podocytes expressed three essential components of NALP3 inflammasome complex, namely, NALP3, ASC and caspase-1. Treatment of podocytes with L-homocysteine (L-Hcys) induced the formation of NALP3 inflammasome complex, increase in caspase-1 activity, podocyte cytoskeleton rearrangement and decreased production of vascular endothelial growth factor (VEGF) from podocytes, which were all blocked by silencing the ASC gene or inhibiting caspase-1 activity. In mice with hHcys induced by feeding them a folate-free (FF) diet, NALP3 inflammasome formation and activation in glomerular podocytes were detected at an early stage, as shown by confocal microscopy, size exclusion chromatography of the assembled inflammasome complex and increased interleukin-1β (IL-1β) production in glomeruli. Locally silencing the ASC gene in the kidney significantly reduced NALP3 inflammasome formation and IL-1β production in glomeruli of mice with hHcys. Pathologically, hHcys-associated albuminuria, foot process effacement of podocytes, loss of podocyte slit diaphragm molecules, and glomerulosclerosis at the late stage were significantly improved by local ASC gene silencing or by caspase-1 inhibition. In conclusion, NALP3 inflammasome formation and activation upon stimulation of Hcys is an important molecular mechanism triggering podocyte injury and ultimately resulting in glomerulosclerosis in hHcys.
Keywords: Homocysteine, inflammatory machinery, podocytes, end-stage renal disease
INTRODUCTION
The inflammasome is recently identified as the cellular machinery responsible for activation of inflammatory processes 1. Among different types of inflammasomes, the NALP3 inflammasome is well characterized in a variety of mammalian cells, which is characteristic of a proteolytic complex mainly composed of the Nod-like receptor protein NALP3 (or nucleotide leukin rich polypeptide 3, NLRP3), the adaptor protein apoptosis-associated speck-like protein (ASC), and caspase-1 2. Caspase-1 is vital for the production of mature interleukin 1β (IL-1β) and IL-18 in response to a variety of agonists or stimuli. It has been reported that IL-1β is an important cytokine with a broad range of biological activities 3 involved in kidney injury and repair 4 and that in glomeruli it is mainly produced by podocytes 5. The active mature IL-1β is formed by cleavage of the inactive pro IL-1β precursor by caspase-1, which is activated in a large multiprotein complex, namely, the inflammasome. The NALP3 inflammasome has been reported to be activated by bacterial toxins 1 or pathogen-associated molecular patterns, such as muramyldipeptide and other stimuli. NALP3 can also detect endogenous stress-associated danger signals, such as ATP 1, monosodium urate crystals (MSU) 6 or β-amyloid, which may be a major mechanism producing local sterile inflammation. With respect to the functional relevance, the NALP3 inflammasome has been implicated in the pathogenesis of various metabolic diseases including diabetes, gout, silicosis, acute myocardial infarction and liver toxicity 2–3, 6. Given the fact that hyperhomocysteinemia (hHcys) is also regarded as a metabolic disorder due to the failure of the clearance of homocysteine (Hcys), the inflammasome may be an attractive candidate as an initiating molecular switch to turn on the inflammatory response observed in hHcys.
Hyperhomocysteinemia is an important pathogenic factor both in the progression of end-stage renal disease (ESRD) and in the development of cardiovascular complications related to ESRD 7. Previously, we have demonstrated that chronic elevations of plasma Hcys levels importantly contribute to the development of glomerular disease independent of hypertension in hHcys mice and rat models 8. Studies from our laboratory 8–9 and by others 10 have demonstrated that Hcys induces podocyte injury, extracellular matrix accumulation and inhibits their degradation in glomeruli, which ultimately leads to glomerulosclerosis and loss of renal function 8. Although these studies increased our understanding of hHcys-associated glomerular injury and sclerosis, the precise mechanism mediating podocyte injury and activating the local inflammatory response in glomeruli has not been fully clarified.
In this regard, earlier reports have shown that hHcys increased levels of inflammatory cytokines and play a crucial role in hHcys-induced endothelial damage and atherosclerosis 11–12. It was also reported that hHcys enhanced the production of MCP-1 in glomerular mesangial cells and tubular epithelial cells, and blockade of these inflammatory processes completely protected the kidney from hHcys-associated damage, supporting the view that hHcys-induced renal injury is associated with its ability to induce inflammation 13. These studies raised the possibility that Hcys may activate an inflammatory response resulting in glomerulosclerosis and end-stage organ damage during hHcys. However, it remains unknown how Hcys activates or initiates the local inflammatory response in renal glomerular residential cells and whether Hcys-induced activation of inflammasomes serves as an early mechanism mediating glomerular injury and sclerosis. The present study tested the hypothesis that hHcys may induce NALP3 inflammasome formation and activation in podocytes and thereby lead to hHcys-associated podocyte dysfunction or injury and consequent glomerular sclerosis.
MATERIALS AND METHODS
Animals
Twelve week old male C57BL/6J mice (The Jackson Laboratory, Bar Harbor, ME, USA) were used in the present study. All protocols were approved by the Institutional Animal Care and Use Committee of the Virginia Commonwealth University. To speed up the damaging effects of hHcys on glomeruli, all mice were uninephrectomized as described in previous studies 9, 14. After a 1-week recovery period from the uninephrectomy, mice were fed a normal diet or a folate-free (FF) diet (Dyets Inc, Bethlehem, PA, USA) for 1, 2, or 4 weeks to induce hHcys. In another series of experiments, ASC shRNA or a scrambled shRNA (Origene, Rockville, MD, USA) plasmid with a luciferase expression vector was co-transfected into the kidneys of mice via intrarenal artery injection with help of the ultrasound microbubble gene delivery system as we described previously 9. After delivery of plasmids into the kidney, these uninephrectomized mice were maintained on a normal or a FF diet for 4 weeks. In additional experimental groups, mice were injected with Z-WEHD-FMK (WEHD, R&D system, Minneapolis, MN, USA), a caspase-1 inhibitor (1 mg/kg/day, i.p.) during the FF diet treatment. One day before sacrificing these mice, 24-hour urine samples were collected using mouse metabolic cages. After blood samples were collected, the mice were sacrificed and renal tissues were harvested for biochemical and molecular analysis as well as morphological examinations as we described previously 8.
Size-exclusion chromatography (SEC)
SEC was performed in podocytes or isolated mouse glomeruli as described previously 15. For a detailed method, please see the online Data supplement.
All other methods are described in the online Data Supplement at http://hyper.ahajournals.org.
RESULTS
Activation of NALP3 inflammasomes by Hcys in cultured podocytes
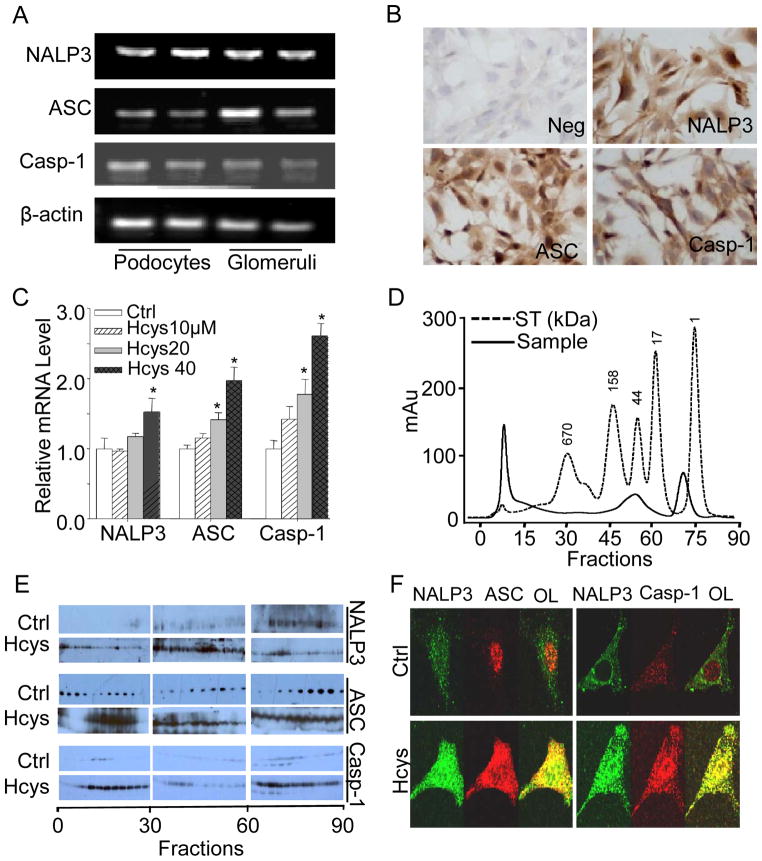
Using cultured murine podocytes, we first characterized the formation and activation of NALP3 inflammasomes. RT-PCR analysis demonstrated that NALP3, ASC, and caspase-1 mRNAs were detectable in these cultured podocytes as well as in the normal mouse renal glomeruli (Figure 1A). By immunocytochemical analyses, the protein expression of these inflammasome components was further confirmed in podocytes (Figure 1B). Upon stimulation of L-Hcys (the active form of Hcys), mRNA levels of NALP3, ASC, and caspase-1 significantly increased in a concentration-dependent manner as detected by real-time RT-PCR (Figure 1C).
Figure 1. Hcys-induced activation of NALP3 inflammasomes in cultured podocytes.
A. RT-PCR gel document showing the expression of NALP3, ASC, and caspase-1 in cultured podocytes. B. Immunocytochemical staining of NALP3, ASC, and caspase-1 in podocytes (original magnification, ×100). C. mRNA expression of NALP3, ASC, and caspase-1 in podocytes with or without stimulation of L-Hcys (n=6). D. Elution profile of proteins (optical density at 280 nm) from podocytes by SEC. The molecular mass scale was determined using a wide range of molecular mass standard. ST=standard. E. SDS-PAGE of proteins fractions via SEC from control and Hcys-treated podocytes that were probed with anti-NALP3, ASC, or casapse-1 antibodies, respectively. F. Representative confocal microscopic images showing colocalization of NALP3 (green) with ASC (red) or NALP3 (green) with caspase-1 (red, Casp-1) in podocytes (original magnification, ×400). OL=overlay. * P<0.05 vs. control.
We next analyzed the assembly of NAPL3 inflammasome proteins as a complex in podocytes by SEC. As shown in Figure 1D, total proteins from podocytes were eluted through a Sepharose 6 SEC column, and all proteins were separated into different fractions according to their size and detected by Western blot analysis. It was observed that the specific band for NALP3, ASC, and caspase-1 were located in the low-molecular weight fractions under control conditions. However, upon stimulation of L-Hcys for 24 h, these bands specific to inflammasome components migrated into high-molecular weight factions, which were termed inflammasome fractions (Figure 1E). Furthermore, confocal microscopic analysis demonstrated that colocalization of NALP3 with ASC or caspase-1 was increased in podocytes upon L-Hcys stimulation compared to control podocytes (Figure 1F), indicating the aggregation or assembly of these inflammasome molecules, namely, the formation of NALP3 inflammasome complex in podocytes.
Effects of ASC gene silencing and caspase-1 inhibition on podocyte inflammasome activation and functional changes induced by L-Hcys
As shown in Figure 2A, ASC siRNA transfection in podocytes markedly inhibited the L-Hcys-induced colocalization of NALP3 with ASC or caspase-1. Consistent with these findings, ASC gene silencing or blockade of caspase-1 activity dramatically blocked the Hcys-induced caspase-1 activity (Figure 2B) and IL-1β production (Figure 2C) in podocytes. VEGF-A secretion was dramatically reduced in podocytes treated with L-Hcys, and this Hcys-induced decrease in VEGF-A secretion was substantially blocked by ASC gene silencing or inhibition of caspase-1 activity (Figure 2D).
Figure 2. Effects of ASC gene silencing and caspase-1 inhibition on inflammasome activation and functional changes in podocytes.
A. Representative confocal microscopic images showing colocalization of NALP3 (green) with ASC (red) or caspase-1 (red, Casp-1) in podocytes (original magnification, ×400), OL=overlay. B. Caspase-1 activity. C. IL-1β production. D. VEGF-A secretion in podocytes with or without treatment of L-Hcys and/or ASC shRNA and caspase-1 inhibitor. Ctrl: control; Veh: vehicle; Scra: scrambled siRNA; Casp-1: Caspase-1; WEHD: Z-WEHD-FMK, n=5. * P<0.05 vs. control; # P<0.05 vs. Hcys.
Activation of podocyte NALP3 inflammasomes in mice with hHcys
In mice on the FF diet, the plasma total Hcys levels were gradually increased, starting from the 1st week on the FF diet (Control: 5.8 ± 1; 1st week: 9.9 ± 1; 2nd week: 14.9 ± 2; 4th week: 18 ± 1 μM). As shown in Figure 3A, under normal condition NALP3, ASC and caspase-1 were expressed at a low level within glomeruli, and very little colocalization of these molecules could be detected by confocal microscopy. The FF diet increased the colocalization of NALP3 with ASC or caspase-1 in a time dependent manner as shown by large yellow spots or patches in glomeruli of mice. The Pearson correlation coefficient (PCC) of NALP3 with ASC or caspase-1 was summarized in Figure 3B. Such colocalization of NALP3 molecules suggests the formation of inflammasomes in glomeruli. Furthermore SEC demonstrated that inflammasome components (NALP3 and ASC) markedly shifted to large molecular fractions in glomeruli of hHcys mice (Figure S2A and S2B). Although podocin staining as a podocyte marker was found decreased in podocytes even in the 1st week of the FF diet feeding, we could still detect the colocalization of podocin with NALP3 or caspase-1 which was more remarkable in the 2nd and 4th week (Figure 3C and 3D). Consistent with the aggregation of these inflammasome molecules in the glomeruli, the caspase-1 activity and IL-1β production were increased even in the 1st week of FF diet treatment (Figure 3E, 3F, S2C and S2D), suggesting the activation of NALP3 inflammasomes. In addition, the creatinine clearance was gradually decreased in FF diet fed mice starting from the 2nd week (Control: 3.4 ± 0.6; 1st week: 3.3 ± 0.8; 2nd week: 2.6 ± 0.6; 4th week: 1.7 ± 0.2 μl/min/g BW).
Figure 3. Formation and activation of podocyte NALP3 inflammasomes during hHcys in mice.
A. Colocalization of NALP3 (green) with ASC (red) or caspase-1 (red) in mouse glomeruli on the normal diet or folate free diet (FF diet). Casp-1: Caspase-1. B. Summarized data showing the fold changes in PCC for the colocalization of NALP3 with ASC or caspase-1 (n=6). C. Colocalization of podocin (red) with NALP3 (green) or caspase-1 (green) in mouse glomeruli. D. Summarized data showing the fold changes in PCC for the colocalization of podocin with NALP3 or caspase-1 (n=6). E. Caspase-1 activity, F. IL-1β concentrations in mouse glomeruli (n=6) * P<0.05 vs. 0 week.
Blockade of glomerular inflammasome formation and activation by ASC shRNA and caspase-1 inhibition
To further determine the role of NALP3 inflammasome activation in glomerular injury, we transfected ASC shRNA into the kidney via the renal artery to silence the ASC gene locally. As illustrated in Figure 4A, the ASC shRNA transfection substantially suppressed the hHcys-induced colocalization of NALP3 with ASC or caspase-1 in the glomeruli. The summarized data was shown in Figure 4B. Using podocin as a podocyte marker, the inflammasome formation in glomeruli induced by the FF diet were found again to be mainly located in podocytes, as demonstrated by colocalization of podocin with NALP3 or caspase-1, which was substantially blocked by local ASC gene silencing (Figure S4A and S4B). Consistent with decreased aggregation of inflammasome components in the glomeruli, FF diet-enhanced caspase-1 activity and IL-1β production were markedly attenuated in glomeruli of ASC shRNA transfected mice. Similarly, caspase-1 inhibitor, WEHD also reduced FF diet-induced increase in caspase-1 activity and IL-1β production (Figure 4C and 4D).
Figure 4. Effects of ASC shRNA or caspase-1 inhibition on podocyte inflammasome formation and activation during hHcys.
A. Colocalization of NALP3 (green) with ASC (red) or caspase-1 (red) in mouse glomeruli. B. Summarized data showing the fold changes in PCC for the colocalization of NALP3 with ASC or caspase-1 (n=6). C. Caspase-1 activity in different groups of mice (n=6). D. IL-1β production in mouse glomeruli (n=6 per group). N Diet: Normal Diet; Casp-1: Caspase-1; Scra: Scrambled shRNA-transfected; ASC sh: ASC shRNA-transfected. *P<0.05, vs. scrambled shRNA-transfected mice on the N diet; # P<0.05, vs. scrambled shRNA-transfected mice on the FF diet.
Effects of silencing ASC gene or inhibition of caspase-1 activity on hHcys-induced glomerular damage
As shown in Figure 5A, the scrambled shRNA-transfected mice on the FF diet had severe albuminuria. When the ASC gene was silenced in the kidney and caspase-1 inhibited by WEHD, albuminuria in mice on the FF diet was significantly improved (Figure 5A). By PAS staining, morphologic examinations showed typical sclerotic changes in glomeruli of scrambled shRNA-transfected mice on the FF diet such as mesangial expansion, collapse of glomerular capillaries, and hypercellularity in these glomeruli (Figure 5B). Correspondingly, the glomerular damage index increased significantly in FF diet fed mice (Figure 5C). Renal ASC gene silencing or WEHD treatment significantly blocked the glomerular damage induced by the FF diet (Figure 5B and 5C). Under TEM, the intact structures of podocyte foot processes shown in glomeruli from mice on the normal diet were destroyed by hHcys after 4 weeks, as shown by evident foot process effacement in scrambled shRNA-transfected mice on the FF diet. In contrast, podocytes of ASC shRNA-transfected or WEHD-treated mice on the FF diet had relatively normal ultrastructures (Figure 5D). In addition, real-time RT-PCR and immunofluorescence analysis demonstrated that the expression of nephrin, a podocyte functional marker significantly decreased in scrambled shRNA-transfected mice on the FF diet, but this decrease in nephrin expression was not seen in ASC shRNA-transfected or WEHD-treated mice on the same diet. In contrast, the expression of desmin significantly increased in scrambled shRNA-transfected mice on the FF diet, but not in ASC shRNA-transfected or WEHD-treated mice (Figure S4C and S4D). Furthermore, we determined the effect of ASC shRNA transfection on FF diet-induced mean arterial pressure (MAP) and heart rate in mice. It was found that body weight, MAP and heart rate was similar in ASC shRNA transfected mice fed a normal diet or FF diet (Table S1).
Figure 5. Effects of ASC gene silencing and caspase-1 inhibition on hHcys-induced glomerular damage.
A. Urinary albumin excretion in 6 groups of mice as indicated (n=8 per group). B. PAS staining showing glomerular morphological changes (original magnification, ×400). C. Summarized data of glomerular damage index in 6 different groups of mice (n=6 per group). D. ASC gene silencing and caspase-1 inhibition improved podocyte ultrastructure in FF diet-treated mice as shown by TEM examination. Arrow denotes the area of foot process effacement in WT mice on the FF diet (n=3). Original magnification: ×8,000.
DISCUSSION
The major goal of the present study was to determine whether NALP3 inflammasomes are activated in glomerular podocytes and thereby lead to podocyte dysfunction and subsequent glomerular injury during hHcys. Our results demonstrated that hHcys induces NALP3 inflammasome formation and activation in podocytes even at a very early stage of Hcys stimulation (24 hours in vitro and 1 week in vivo). This inflammasome activation served as an intracellular molecular machinery to initiate the inflammatory response and to directly damage podocytes in glomeruli, ultimately leading to glomerular sclerosis. Silencing of the ASC gene and inhibition of caspase-1 activity almost completely blocked podocyte injury and late glomerular dysfunction and sclerosis. These results indicate that activation of NALP3 inflammasomes in podocytes may be an early mechanism turning on podocyte injury and consequent glomerulosclerosis during hHcys.
It has been reported that non-microbial or sterile inflammation is an important pathological process in many kidney diseases including hHcys-associated glomerulosclerosis 16. In particular, the residential renal cells have been found to play an important role in initiating renal inflammation and kidney damage. In this regard, a delicate study by Niemir et al revealed that podocytes are the major source of glomerular IL-1β, which participates in the progression of many non-proliferative forms of human glomerulonephritis by induction of a local inflammatory process 5. Many other studies from humans and animals such as rats and mice have also demonstrated that podocytes are one of the major sources of glomerular IL-1β under various pathological conditions and that activation of inflammasomes may contribute to IL-1β production 5, 17–19. Since activation of the NALP3 inflammasomes has been known to cause caspase-1 activation and cleavage of pro–IL-1β and pro–IL-18 into their active and mature form 20, namely, IL-1β or IL-18, we first characterized the expression and activity of this inflammasome complex in murine podocytes and determined whether it is indeed involved in Hcys-induced podocyte injury. By various approaches such as RT-PCR, Western blot analysis, immunohistocytochemistry and SEC, it was found that the main NALP3 inflammasome molecules such as NALP3, ASC and caspase-1 were expressed in murine podocytes. Importantly, L-Hcys stimulation induced the formation of NALP3 inflammasome complex in podocytes, as shown by colocalization of NALP3 with ASC or caspase-1 using confocal microscopy, by increase in NALP3-specific large molecular fractions detected by SEC, and by biochemical analysis of caspase-1 activity and production of IL-1β. These results clearly suggest that the NALP3 inflammasomes are functioning in podocytes and that Hcys stimulation can lead to its activation. Although this type of inflammasome was firstly characterized in immune cells, recent studies have demonstrated that it can be detected in various non-immune cells including intrinsic glomerular cells 17, 21–22 and other residential cells in the brain, heart, and vessels 23–24. Although there are some reports that NALP3 and other inflammasomes can be activated in glomeruli during different pathological conditions 17, the results from the present study provide the first experimental evidence that L-Hcys activates NALP3 inflammasomes in podocytes, which may be an important pathogenic mechanism responsible for glomerular injury during hHcys.
Another interesting finding of the present study is that Hcys directly induced podocyte dysfunction in vitro, as shown by a decrease in podocin expression, the disruption of actin cytoskeleton and the decrease in VEGF production in these cells. This Hcys-induced podocyte dysfunction was almost completely blocked by caspase-1 inhibition or ASC gene silencing. These results imply that podocytes are not only a glomerular cell type with intracellular inflammatory machinery featured by the formation and activation of NALP3 inflammasomes, but also a cell type that is a target of inflammatory factors derived from activated NALP3 inflammasomes. The present study did not attempt to define the mechanism mediating the effect of NALP3 inflammasome activation to induce podocyte dysfunction or injury. However, two main mechanisms may contribute to such effect of NALP3 inflammasome activation on podocytes. First, the production of inflammatory factors such as IL-1β due to activation of NALP3 inflammasomes may act in an autocrine fashion to change podocyte function. Indeed, there were some reports that various inflammatory factors including IL-1β can induce podocyte injury by reduction of nephrin production 21, 25. Another mechanism may be related to the intrinsic functional changes in podocytes during the formation and activation of NALP3 inflammasomes, which may lead to intracellular signaling alterations and thereby result in podocyte dysfunction. Some ongoing studies in our laboratory will further clarify these mechanisms.
In animal experiments, we produced experimental hHcys by feeding mice with the FF diet. It was found that the FF diet gradually increased the plasma total Hcys levels starting from the 1st week. Our confocal microscopy and SEC experiments indeed detected the formation and activation of NALP3 inflammasomes in the glomeruli of mice on the FF diet, which mainly occurred in podocytes given the colocalization of increased NALP3 molecules with podocin. Correspondingly, glomerular caspase-1 activity and IL-1β production were dramatically elevated during hHcys, suggesting the activation of NALP3 inflammasomes in glomeruli. In particular, most of these changes related to the formation and activation of NALP3 inflammasome could be seen at a very early stage of hHcys (1st week on the FF diet), when functional or structural changes in glomeruli could not be seen. These data may afford a reasonable explanation for previous results reported by others that podocytes are a major source of IL-1β seen in different kinds of glomerular injuries or sclerosis 5, 17, 19. Consistent with our findings, some recent reports demonstrated that NALP3 mRNA expression increased and this inflammasome can be activated in different glomerular diseases. It is assumed that NALP3 serves as a sensor of danger factors and consequent activated inflammasomes may integrate several triggering signals leading to the secretion of IL-1β, ultimately resulting in glomerular inflammatory injury 26. However, a recent study has shown that NALP3 inflammasomes may not be activated in glomeruli or podocytes in anti-GBM glomerulonephritis or by LPS 27. It is possible that under some pathological conditions such as anti-GBM glomerulonephritis or upon stimulation of LPS, activation of NALP3 inflammasomes is dominant in immune cells such as dendritic cells. However, in other pathological conditions that were caused by some autoinflammatory stimuli or factors such as hHcys, hyperglycemia, increased plasma uric acid and cholesterol, activation of NALP3 inflammasomes may occur in glomerular residential cells at the early stage of such pathological processes.
To further confirm the role of podocyte NALP3 inflammasome activation in the development of glomerular injury during hHcys, a well-established in vivo gene silencing strategy was used by delivery of ASC shRNA into the kidney 9. Using this method, ASC shRNA was introduced into renal cortical tissue including glomeruli, and renal local ASC expression could be dynamically monitored in vivo and thereby guided functional studies. At the end of experiments, real-time RT-PCR was conducted routinely to confirm the inhibitory efficiency of local gene silencing in the glomeruli. We demonstrated that ASC gene silencing substantially attenuated the NALP3 inflammasome formation and activation, caspase-1 activity and IL-1β production as well as glomerular damage in hHcys mice. In contrast, ASC shRNA transfection did not alter the mean arterial pressure and heart rate in C57BL/6J WT mice fed a normal diet or FF diet. Similarly, inhibition of caspase-1 in the kidney also ameliorated production of IL-1 β and glomerular injury during hHcys. These data further support the view that podocyte and glomerular NALP3 inflammasome formation and activation occur during hHcys and that this NALP3 inflammasome activation importantly contributes to the initiation or development of glomerulosclerosis independent of hypertension. The present study did not aim to elucidate the exact mechanism of hHcys-induced NALP3 inflammasome activation in podocytes. However, NADPH oxidase derived O2·− production may be an important mechanism mediating hHcys-induced inflammasome activation in podocytes (unpublished observations). Targeting this inflammasome at the stage of its assembling or activation may be a novel strategy to prevent the development of glomerular injury or sclerosis in hHcys. In this regard, although a recent study reported that ASC knockout mice did not protect the kidney from ischemia-reperfusion injury 28, NALP3 knockout mice had less interstitial fibrosis as compared with their wide-type littermates 26. These observations, together with our findings in the hHcys mouse model, point to the idea that renal NALP3 inflammasome formation and activation may be a crucial mechanism mediating a renal inflammatory response that may directly cause podocyte dysfunction or indirectly induce accumulation of inflammatory cells such as T-cells or macrophages in glomeruli, ultimately leading to glomerular injury and sclerosis. It is plausible that NALP3 inflammasome activation is an important early mechanism triggering or promoting chronic renal tissue damage leading to fibrosis and sclerosis.
Our further experiments indeed confirmed that podocytes can be damaged by activation of NALP3 inflammasomes in mice with hHcys. It was found that ASC gene silencing or caspase-1 inhibition by WEHD protected the podocytes from hHcys-induced injury. TEM examinations showed that foot process effacement induced by hHcys was dramatically alleviated in mice transfected with ASC shRNA or injected with WEHD, suggesting that podocyte ultrastructure was improved in these mice. Moreover, the expression of the most important slit diaphragm molecule, nephrin, was almost completely recovered in mice treated with ASC shRNA or WEHD. In contrast, increase of a classic podocyte marker, desmin, was found to be attenuated in ASC-transfected hHcys mice, further indicating that hHcys induced podocyte injury were significantly attenuated due to reduced NALP3 inflammasome activation in these cells. Such protection of podocyte structure and function from hHcys-induced injury further confirms the important role of NALP3 inflammasome activation in the development of glomerular injury.
Supplementary Material
PERSPECTIVES.
The present study revealed a new triggering mechanism of hHcys-induced glomerular injury that is characterized by the formation and activation of an NALP3 inflammasome complex in podocytes. This NALP3 inflammasome activation may represent a novel early event leading to podocyte dysfunction and injury, initiating glomerulosclerosis during hHcys. Based on these findings, the development of strategies that target the activation of podocyte NALP3 inflammasomes may be a promising therapeutic intervention to prevent hHcys-associated glomerular damage.
NOVELTY AND SIGNIFICANCE.
What is New
The present study clarified a novel intracellular mechanism, namely, the formation and activation of inflammasomes, which turns on podocyte injury and glomerular inflammation leading to glomerulosclerosis during hHcys.
What is Relevant
Podocyte and glomerular NALP3 inflammasome formation and activation occurs during hHcys and NALP3 inflammasome activation importantly contributes to the initiation and development of glomerulosclerosis independent of hypertension.
Summary
NALP3 inflammasome formation is an early pathogenic event of podocyte injury and glomerular sclerosis and therefore it may be an important, novel therapeutic target for treatment of hHcys-associated glomerular disease or other chronic degenerative diseases.
Acknowledgments
SOURCES OF FUNDING
This work was supported by grants DK54927, HL075316, and HL57244 from National Institutes of Health.
Footnotes
DISCLOSURES
None
References
- 1.Mariathasan S, Newton K, Monack DM, Vucic D, French DM, Lee WP, Roose-Girma M, Erickson S, Dixit VM. Differential activation of the inflammasome by caspase-1 adaptors asc and ipaf. Nature. 2004;430:213–218. doi: 10.1038/nature02664. [DOI] [PubMed] [Google Scholar]
- 2.Zhou R, Tardivel A, Thorens B, Choi I, Tschopp J. Thioredoxin-interacting protein links oxidative stress to inflammasome activation. Nat Immunol. 2010;11:136–140. doi: 10.1038/ni.1831. [DOI] [PubMed] [Google Scholar]
- 3.Imaeda AB, Watanabe A, Sohail MA, Mahmood S, Mohamadnejad M, Sutterwala FS, Flavell RA, Mehal WZ. Acetaminophen-induced hepatotoxicity in mice is dependent on tlr9 and the nalp3 inflammasome. J Clin Invest. 2009;119:305–314. doi: 10.1172/JCI35958. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Iyer SS, Pulskens WP, Sadler JJ, Butter LM, Teske GJ, Ulland TK, Eisenbarth SC, Florquin S, Flavell RA, Leemans JC, Sutterwala FS. Necrotic cells trigger a sterile inflammatory response through the nlrp3 inflammasome. Proc Natl Acad Sci U S A. 2009;106:20388–20393. doi: 10.1073/pnas.0908698106. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Niemir ZI, Stein H, Dworacki G, Mundel P, Koehl N, Koch B, Autschbach F, Andrassy K, Ritz E, Waldherr R, Otto HF. Podocytes are the major source of il-1 alpha and il-1 beta in human glomerulonephritides. Kidney Int. 1997;52:393–403. doi: 10.1038/ki.1997.346. [DOI] [PubMed] [Google Scholar]
- 6.Martinon F, Petrilli V, Mayor A, Tardivel A, Tschopp J. Gout-associated uric acid crystals activate the nalp3 inflammasome. Nature. 2006;440:237–241. doi: 10.1038/nature04516. [DOI] [PubMed] [Google Scholar]
- 7.Robinson K, Gupta A, Dennis V, Arheart K, Chaudhary D, Green R, Vigo P, Mayer EL, Selhub J, Kutner M, Jacobsen DW. Hyperhomocysteinemia confers an independent increased risk of atherosclerosis in end-stage renal disease and is closely linked to plasma folate and pyridoxine concentrations. Circulation. 1996;94:2743–2748. doi: 10.1161/01.cir.94.11.2743. [DOI] [PubMed] [Google Scholar]
- 8.Yi F, Zhang AY, Li N, Muh RW, Fillet M, Renert AF, Li PL. Inhibition of ceramide-redox signaling pathway blocks glomerular injury in hyperhomocysteinemic rats. Kidney Int. 2006;70:88–96. doi: 10.1038/sj.ki.5001517. [DOI] [PubMed] [Google Scholar]
- 9.Yi F, Xia M, Li N, Zhang C, Tang L, Li PL. Contribution of guanine nucleotide exchange factor vav2 to hyperhomocysteinemic glomerulosclerosis in rats. Hypertension. 2009;53:90–96. doi: 10.1161/HYPERTENSIONAHA.108.115675. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 10.Ingram AJ, Krepinsky JC, James L, Austin RC, Tang D, Salapatek AM, Thai K, Scholey JW. Activation of mesangial cell mapk in response to homocysteine. Kidney Int. 2004;66:733–745. doi: 10.1111/j.1523-1755.2004.00795.x. [DOI] [PubMed] [Google Scholar]
- 11.Woo CW, Siow YL, OK Homocysteine induces monocyte chemoattractant protein-1 expression in hepatocytes mediated via activator protein-1 activation. J Biol Chem. 2008;283:1282–1292. doi: 10.1074/jbc.M707886200. [DOI] [PubMed] [Google Scholar]
- 12.de la Sierra A, Larrousse M. Endothelial dysfunction is associated with increased levels of biomarkers in essential hypertension. J Hum Hypertens. 2010;24:373–379. doi: 10.1038/jhh.2009.91. [DOI] [PubMed] [Google Scholar]
- 13.Cheung GT, Siow YL, OK Homocysteine stimulates monocyte chemoattractant protein-1 expression in mesangial cells via nf-kappab activation. Can J Physiol Pharmacol. 2008;86:88–96. doi: 10.1139/y08-002. [DOI] [PubMed] [Google Scholar]
- 14.Sen U, Basu P, Abe OA, Givvimani S, Tyagi N, Metreveli N, Shah KS, Passmore JC, Tyagi SC. Hydrogen sulfide ameliorates hyperhomocysteinemia-associated chronic renal failure. Am J Physiol Renal Physiol. 2009;297:F410–419. doi: 10.1152/ajprenal.00145.2009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Abulafia DP, de Rivero Vaccari JP, Lozano JD, Lotocki G, Keane RW, Dietrich WD. Inhibition of the inflammasome complex reduces the inflammatory response after thromboembolic stroke in mice. J Cereb Blood Flow Metab. 2009;29:534–544. doi: 10.1038/jcbfm.2008.143. [DOI] [PubMed] [Google Scholar]
- 16.Segelmark M, Hellmark T. Autoimmune kidney diseases. Autoimmun Rev. 2010;9:A366–371. doi: 10.1016/j.autrev.2009.11.007. [DOI] [PubMed] [Google Scholar]
- 17.Anders HJ, Muruve DA. The inflammasomes in kidney disease. J Am Soc Nephrol. 2011;22:1007–1018. doi: 10.1681/ASN.2010080798. [DOI] [PubMed] [Google Scholar]
- 18.Boswell JM, Yui MA, Burt DW, Kelley VE. Increased tumor necrosis factor and il-1 beta gene expression in the kidneys of mice with lupus nephritis. J Immunol. 1988;141:3050–3054. [PubMed] [Google Scholar]
- 19.Tesch GH, Yang N, Yu H, Lan HY, Foti R, Chadban SJ, Atkins RC, Nikolic-Paterson DJ. Intrinsic renal cells are the major source of interleukin-1 beta synthesis in normal and diseased rat kidney. Nephrol Dial Transplant. 1997;12:1109–1115. doi: 10.1093/ndt/12.6.1109. [DOI] [PubMed] [Google Scholar]
- 20.Martinon F, Burns K, Tschopp J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of proil-beta. Mol Cell. 2002;10:417–426. doi: 10.1016/s1097-2765(02)00599-3. [DOI] [PubMed] [Google Scholar]
- 21.Timoshanko JR, Kitching AR, Iwakura Y, Holdsworth SR, Tipping PG. Leukocyte-derived interleukin-1beta interacts with renal interleukin-1 receptor i to promote renal tumor necrosis factor and glomerular injury in murine crescentic glomerulonephritis. Am J Pathol. 2004;164:1967–1977. doi: 10.1016/s0002-9440(10)63757-1. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Timoshanko JR, Kitching AR, Iwakura Y, Holdsworth SR, Tipping PG. Contributions of il-1beta and il-1alpha to crescentic glomerulonephritis in mice. J Am Soc Nephrol. 2004;15:910–918. doi: 10.1097/01.asn.0000115704.86897.f4. [DOI] [PubMed] [Google Scholar]
- 23.Silverman WR, de Rivero Vaccari JP, Locovei S, Qiu F, Carlsson SK, Scemes E, Keane RW, Dahl G. The pannexin 1 channel activates the inflammasome in neurons and astrocytes. J Biol Chem. 2009;284:18143–18151. doi: 10.1074/jbc.M109.004804. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 24.Yamasaki K, Muto J, Taylor KR, Cogen AL, Audish D, Bertin J, Grant EP, Coyle AJ, Misaghi A, Hoffman HM, Gallo RL. Nlrp3/cryopyrin is necessary for interleukin-1beta (il-1beta) release in response to hyaluronan, an endogenous trigger of inflammation in response to injury. J Biol Chem. 2009;284:12762–12771. doi: 10.1074/jbc.M806084200. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Takano Y, Yamauchi K, Hayakawa K, Hiramatsu N, Kasai A, Okamura M, Yokouchi M, Shitamura A, Yao J, Kitamura M. Transcriptional suppression of nephrin in podocytes by macrophages: Roles of inflammatory cytokines and involvement of the pi3k/akt pathway. FEBS Lett. 2007;581:421–426. doi: 10.1016/j.febslet.2006.12.051. [DOI] [PubMed] [Google Scholar]
- 26.Vilaysane A, Chun J, Seamone ME, Wang W, Chin R, Hirota S, Li Y, Clark SA, Tschopp J, Trpkov K, Hemmelgarn BR, Beck PL, Muruve DA. The nlrp3 inflammasome promotes renal inflammation and contributes to ckd. J Am Soc Nephrol. 2010;21:1732–1744. doi: 10.1681/ASN.2010020143. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Lichtnekert J, Kulkarni OP, Mulay SR, Rupanagudi KV, Ryu M, Allam R, Vielhauer V, Muruve D, Lindenmeyer MT, Cohen CD, Anders HJ. Anti-gbm glomerulonephritis involves il-1 but is independent of nlrp3/asc inflammasome-mediated activation of caspase-1. PLoS One. 2011;6:e26778. doi: 10.1371/journal.pone.0026778. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Shigeoka AA, Mueller JL, Kambo A, Mathison JC, King AJ, Hall WF, da Silva Correia J, Ulevitch RJ, Hoffman HM, McKay DB. An inflammasome-independent role for epithelial-expressed nlrp3 in renal ischemia-reperfusion injury. J Immunol. 2010;185:6277–6285. doi: 10.4049/jimmunol.1002330. [DOI] [PMC free article] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.