Abstract
The aim of this study was to explore the association between outbursts of anger and acute myocardial infarction (AMI) risk. Outbursts of anger are associated with an abrupt increase in cardiovascular events, but it remains unknown whether higher levels of anger intensity are associated with higher levels of AMI risk or whether potentially modifiable factors mitigate the short-term risk of AMI. We conducted a case-crossover analysis of 3886 participants from the multicenter Determinants of Myocardial Infarction Onset Study interviewed during index hospitalization for an AMI between 1989 and 1996. We compared the observed number and intensity of anger outbursts in the 2 hours preceding AMI symptom onset with its expected frequency based on each patient’s control information, defined as the number of anger outbursts in the past year. Among the 3886 participants in the Determinants of Myocardial Infarction Onset Study, 1484 (38%) reported outbursts of anger in the past year. The incidence rate of AMI onset was elevated 2.43-fold (95% confidence interval, 2.01–2.90) within 2 hours of an outburst of anger. The association was consistently stronger with increasing intensities of anger (p-trend <0.001). In conclusion, the risk of having an AMI is >2-fold higher following outbursts of anger compared to other times, and higher intensities of anger were associated with higher relative risks. Compared to non-users, regular beta-blocker users had a lower susceptibility to heart attacks triggered by anger, suggesting that some drugs may lower the risk from each episode of anger.
Keywords: epidemiology, acute myocardial infarction
Introduction
Outbursts of anger have been shown to be associated with a transiently higher risk of acute myocardial infarction (AMI)1,2, acute coronary syndrome (ACS)3,4, arrhythmia5,6, ischemia7 and ischemic8 and hemorrhagic stroke9. However, it remains unknown whether all levels of moderate and high anger carry a similar risk of AMI triggered by anger or whether there is dose-response relationship, with higher AMI risk for each increment in anger intensity. In addition, prior studies have had limited statistical power to assess whether patient characteristics influence susceptibility to cardiovascular events triggered by anger. In this study, we hypothesized that the risk of an AMI triggered by anger is higher for each increment of anger intensity, and that the short-term risk is mitigated by potentially modifiable factors such as physical activity and regular use of aspirin and beta-blockers.
Methods
The Determinants of Myocardial Infarction Onset Study (MIOS) is a multicenter cohort study of patients with confirmed AMI. In the first phase of the Onset Study (August 1989-September 1994), 1937 patients were enrolled in 45 community hospitals and tertiary care medical centers in the United States. In the second phase (October 1994-September 1996), the study was expanded to 64 medical centers across the United States, and an additional 1949 patients were enrolled. Altogether, 3886 patients (2627 men and 1259 women, mean age 61.4 years) were interviewed a median of 4 days (range 0 to 30) after sustaining an AMI. For inclusion in the study, patients were required to meet all of the following criteria: English-speaking, at least 1 creatine kinase level above the upper limit of normal for the clinical laboratory performing the test, positive MB isoenzymes, an identifiable onset of pain or other symptoms typical of infarction, and the ability to complete a structured interview. The protocol was approved by the institutional review board at each participating center and informed consent was obtained from each patient.
As previously described10, detailed chart reviews and patient interviews were conducted by trained research personnel. Data were collected on standard demographic variables and risk factors for coronary artery disease. The interview identified the time, place and quality of AMI pain and other symptoms. If patients reported that they had experienced any chest pain in the week preceding acute onset, they were classified as cases with premonitory symptoms. Subjects were also asked to report the timing of their last exposure to several potential triggers and estimated usual frequency of these factors in the year preceding AMI onset. We used the Onset Anger Scale1 to measure outbursts of anger. Patients were shown a chart of 7 levels of anger and reported the timing of their last episode of anger at each level and their usual frequency of anger outbursts at each level in the past year. They were also asked about their anger levels during each of the 26 hours before AMI onset. Patients were considered exposed if they reported an anger level of 5 or greater during the time window of interest. In another study11, 25 individuals completed the Onset Anger Scale at the time of admission for ischemic stroke and they were re-interviewed up to 6 days later. The intraclass correlation for the usual frequency of anger outbursts was found to be excellent (0.93).
The State-Trait Personality Inventory (STPI)12is a well-validated measure with strong psychometric properties; itconsists of 6 10-item self-report scales for measuring state and trait anxiety, anger and curiosity. Subjects reported how often they experienced a particular feeling by rating themselves on a 4-pointfrequencyscale: (1) Almost never; (2) Sometimes; (3) Often; (4) Almost always. The 10-item scale measuring anger over the 24 hours prior to AMI was used as an indicator of a tendency toward anger feelings. Although cut-off values that relate to clinical diagnostic categories have not been established, a score >11 points on the anger scaleand 21 points on the anxiety scale correspond to the 75th percentile of the scores in our sample.
The MIOS Study used a case-crossover design, a variation of a case-control design that is appropriate when a transient exposure (outbursts of anger) is associated with an acute change in the risk of an acute outcome (nonfatal AMI)13,14. Because control information for each subject is based on his or her own past exposure experience, self-matching eliminates confounding by risk factors that are constant within individuals over the sampling period but often differ between study subjects. Since we have previously shown1 that the transiently increased AMI risk following episodes of anger extends for up to 2 hours, we compared a subject’s report of anger episodes in the 2 hours prior to AMI onset (the hazard period) with the same subject’s report of anger in the past year (the control period). In a sensitivity analysis, we compared scores from the Onset Anger Scale in the 2 hours prior to AMI onset with anger in the 2 hours at the same time on the day before the AMI (pair-matched intervals) and a similar analysis for the STPI scores of anger adjusted for anxiety above the 75th percentile. Anger episodes are assumed to be rare enough that hazard periods do not overlap.
Each subject in a case-crossover study forms his or her own stratum and thus is his or her own control13,14. For the primary analysis, the ratio of the observed exposure frequency in the hazard period to the expected frequency based on control information about outbursts of anger in the previous year was used to calculate estimates of the rate ratio as a measure of relative risk (RR). In order to calculate the expected frequency of exposure in the case period, we multiplied the usual annual frequency of anger outbursts by the hypothesized window of its physiologic effect (2 hours in the primary analysis) to estimate the amount of person-time exposed to anger, and the unexposed person-time was calculated by subtracting this value from the number of hours in a year. The data were analyzed using methods for cohort studies with sparse data in each stratum15. As a sensitivity analysis, we used conditional logistic regression to compare each person’s exposure during the hazard period to their exposure at the same time on the day before AMI onset14.
We examined whether the triggering effect was greater for higher levels of anger in the 2 hours prior to AMI onset compared to the risk for lower anger levels by estimating the RRs for levels 4, 5, 6, and 7 on the Onset Anger Scale and testing for linear component of trend using a χ2 test for linear trend15, and we calculated the incremental change in risk for each unit increase in anger16. We stratified by demographic, clinical, pharmacological, psychological and behavioral factors and compared the RRs by means of a Wald test for homogeneity15. Potential modifiers included sex, age (<50, 50–69, ≥70), educational attainment (<high school, completed high school, some college), prior coronary artery disease (CAD) defined as history of AMI or angina pectoris, diabetes, hypertension (yes/no), aspirin use, beta-blocker use (yes/no), trait anger score below versus above 75th percentile, weekly physical activity (<3 vs. ≥3 times per week) and coffee intake (0, 1–4, ≥5 per week). We conducted several sensitivity analyses; in the first sensitivity analysis, we restricted the analysis to participants reporting no other potential triggers (physical activity, caffeine, alcohol, tobacco) in the 2 hours prior to AMI onset. It is possible that premonitory symptoms evoke feelings of anger rather than the anger eliciting AMI onset, resulting in reverse causation bias. Therefore, we conducted a sensitivity analysisrestricted to participants that reported no premonitory symptoms. In the final sensitivity analysis, we conducted separate analyses for each phase of the study. All reported p values are 2-sided.
Results
The characteristics of the population are summarized in Table 1. Among the 3886 study participants, 1484 (38%) reported outbursts of anger in the past year. Among the 1484 reporting episodes of anger in the past year, 617 (42%) reported that the outbursts occurred < once per month, 271 (18%) reported outbursts > once per month, 340 (23%) reported outbursts > once per week and 256 (17%) reported outbursts at least daily. Among the 3886 participants, 110 (2.8%) reported episodes of anger in the 2 hours prior to AMI onset. The most frequent causes of anger outbursts were family (38%), conflicts at work (25%) and commuting (6%).
Table 1.
Clinical Characteristics of the Myocardial Infarction Onset Study Population. Mean ± standard deviation or n (%)
| Anger Outbursts in the Past Year | |||
|---|---|---|---|
| Variable | Yes | No | |
| n=(1484) | n=(2402) | ||
| Age (years) | 56 ± 12 | 64 ± 12 | |
| Female | 438 (30%) | 821 (34%) | |
| White | 2130 (89%) | 1290 (87%) | |
| Married | 1488 (62%) | 1009 (68%) | |
| Income, $ | 39147 ± 17174 | 39119 ± 17081 | |
| Education | |||
| < high school | 285 (19%) | 639 (27%) | |
| Completed high school | 534 (36%) | 859 (36%) | |
| Some college | 665 (45%) | 904 (38%) | |
| Body mass index (kg/m2) | 28 ± 5 | 27 ± 5 | |
| Smoking status | |||
| Never | 312 (21%) | 705 (29%) | |
| Former | 546 (37%) | 1012 (42%) | |
| Current | 616 (42%) | 662 (28%) | |
| Hypertension (by history) | 604 (41%) | 1094 (46%) | |
| Diabetes mellitus | 246 (17%) | 512 (21%) | |
| Angina pectoris | 344 (23%) | 611 (25%) | |
| Acute myocardial infarction | 351 (24%) | 654 (27%) | |
| 141 (10%) | 376 (16%) | ||
| Regular use of | |||
| ACE inhibitors | 173 (12%) | 342 (14%) | |
| Aspirin | 580 (39%) | 969 (40) | |
| Beta blockers | 275 (19%) | 559 (23%) | |
| Calcium channel blockers | 322 (22%) | 606 (25%) | |
| Digoxin | 68 (5%) | 190 (8%) | |
| Index Hospitalization | |||
| Thrombolytic use | 703 (47%) | 846 (35%) | |
| Congestive heart failure | 28 (10%) | 252 (15%) | |
| Ventricular tachycardia | 166 (11%) | 215 (9%) | |
| Peak creatine kinase level (units/liter) | 1662 ± 2008 | 1419 ± 1681 | |
| Physical Activity (times/week) | |||
| <3 | 1141 (77%) | 2123 (88%) | |
| ≥3 | 343 (23%) | 279 (12%) | |
The risk of AMI was 2.43 (95%CI 2.01–2.90; p<0.001) times higher in the 2 hours following self-reported outbursts of moderate or extreme anger compared to other times. Rather than a similar risk at any level of self-reported moderate or high anger, Figure 1 shows that there was a dose-response relationship, with higher AMI risk for each increment in blockersthan nonusers. The RR was lower among people with CAD historythan people with no CAD history. Even among people that were anger intensity, (p-trend <0.001). Compared to other times, the risk of AMI was greater in the 2 hours after feeling “moderately angry, so hassled it shows in your voice” (RR=1.68, 95%CI 1.39–2.03); the association was even higher after feeling, “very angry, body tense, clenching fists or teeth” (RR=2.27, 95%CI 1.83–2.82), feeling “furious, almost out of control, very angry, pound table, slam door” (RR=2.91, 95%CI 1.97–4.30) and the risk was strongest after feeling “enraged! lost control, throwing objects, hurtingyourself or others” (RR=4.50, 95%CI 2.77–7.30).
Figure 1.
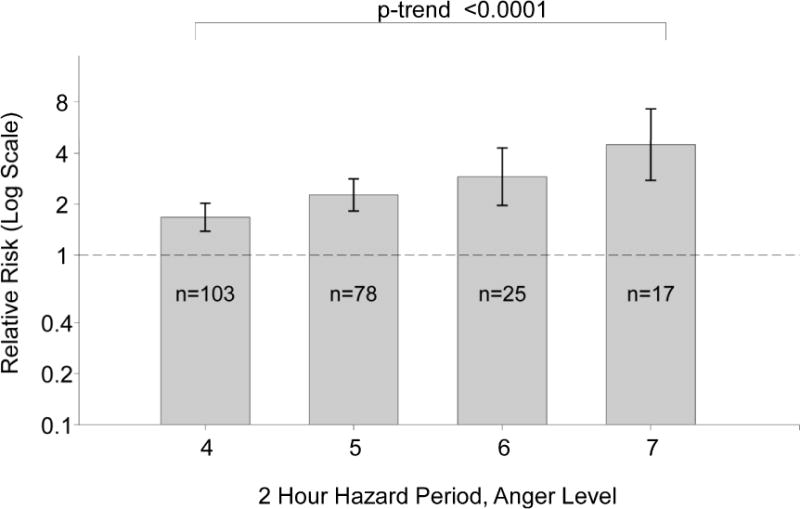
Relative Risk for the Risk of Acute Myocardial Infarction in the 2 Hours after Anger Episodes by Level of Anger according to the Onset Anger Scale. The error bars indicate the 95% confidence limits. The dashed line indicates the baseline risk. The sample size represents the number of participants reporting anger at that level in the 2 hours prior to the index acute myocardial infarction.
Concordant with the original study1, the RR was lower among regular users of beta- not regular beta-blocker users, the RR was lower among people with CAD history (RR=1.83, 95%CI 1.17–2.85) than people with no CAD history (RR=3.08, 95%CI 2.45–3.88;p for homogeneity=0.02). Table 2 shows that there were no other statistically significant differences by any of the other pre-specified potential modifiers. There was not enough data to further stratify these subgroups to determine whether other patient characteristics were responsible for the heterogeneity in the observed RRs. In a sensitivity analysis restricting to participants that did not report exposure to other potential triggers (physical activity, caffeine, alcohol, tobacco) in the 2 hours prior to AMI onset, the association was lower but remained statistically significant (RR=1.88, 95%CI 1.46–2.42; p<0.001); the results were also lower but remained statistically significant in sensitivity analyses restricted to participants with no premonitory symptoms (RR=2.34, 95%CI 1.92–2.85; p<0.001). The results were similar for the first and the second phase of the study.
Table 2.
Relative Risk and 95% Confidence Interval for Acute Myocardial Infarction Within 2 Hours of Anger Outburst According to Patient Characteristics.
| Number Exposed in the Past Year |
Number Exposed in the Past 2 Hours |
Relative Risk (95% Confidence Interval) |
P-Value for χ2 Test for Homogeneity |
|
|---|---|---|---|---|
| All Patients | 3886 | 110 | 2.43 (2.01–2.92) | |
| Sex | ||||
| Male | 2627 | 73 | 2.26 (1.79–2.84) | 0.26 |
| Female | 1259 | 37 | 2.83 (2.04–3.93) | |
| Age (Years) | ||||
| <50 | 766 | 36 | 1.93 (1.36–2.72) | 0.19 |
| 50–69 | 1982 | 62 | 2.85 (2.23–3.64) | |
| ≥70 | 1135 | 12 | 2.35 (1.40–3.96) | |
| Education | ||||
| <High School | 924 | 32 | 3.54 (2.45–5.11) | 0.03 |
| Completed High School | 2161 | 52 | 1.96 (1.51–2.55) | |
| Some College | 801 | 26 | 2.67 (1.79–3.98) | |
| Frequency of Weekly Activity | ||||
| <3 | 3264 | 73 | 2.20 (1.75–2.76) | 0.11 |
| ≥3 | 622 | 37 | 3.05 (2.20–4.25) | |
| Smoking Status | ||||
| Never Smoker | 1017 | 25 | 2.56 (1.74–3.75) | 0.79 |
| Former Smoker | 1558 | 29 | 2.17 (1.49–3.14) | |
| Current Smoker | 1278 | 55 | 2.49 (1.91–3.25) | |
| Body Mass Index >30 | ||||
| Yes | 986 | 36 | 2.37 (1.69–3.32) | 0.89 |
| No | 2859 | 73 | 2.42 (1.93–3.04) | |
| History of Diabetes | ||||
| Yes | 758 | 16 | 1.94 (1.20–3.13) | 0.31 |
| No | 3128 | 94 | 2.54 (2.07–3.11) | |
| History of Hypertension | ||||
| Yes | 1698 | 47 | 2.56 (1.89–3.47) | 0.63 |
| No | 2188 | 63 | 2.33 (1.84–2.96) | |
| History of Acute Myocardial Infarction | ||||
| Yes | 1005 | 19 | 1.71 (1.11–2.64) | 0.08 |
| No | 2808 | 88 | 2.65 (2.14–3.27) | |
| History of Angina Pectoris | ||||
| Yes | 955 | 19 | 1.75 (1.12–2.71) | 0.09 |
| No | 2931 | 91 | 2.65 (2.15–3.25) | |
| History of Coronary Artery Disease (Acute Myocardial Infarction or Angina Pectoris) | ||||
| Yes | 1443 | 28 | 1.63 (1.14–2.33) | 0.006 |
| No | 2443 | 82 | 2.92 (2.34–3.65) | |
| STPI Score | ||||
| Ag24 <75th | 1486 | 28 | 2.19 (1.53–3.14) | 0.51 |
| Ag24 ≥75th | 469 | 34 | 2.58 (1.83–3.64) | |
| Aspirin | ||||
| Yes | 1549 | 43 | 2.57 (1.91–3.45) | 0.64 |
| No | 2337 | 67 | 2.34 (1.84–2.98) | |
| Beta-Blocker | ||||
| Yes | 834 | 14 | 1.42 (0.87–2.32) | 0.02 |
| No | 3052 | 96 | 2.71 (2.21–3.32) | |
In the pair-matched analysis using scores from the Onset Anger Scale, 97 cases reported exposure only during the 2-hour hazard period, 41reported exposed during the same 2-hour period on the prior day (the control period) and 13reported exposure during both periods. The analysis yielded results similar to that from the usual frequency approach (RR=2.37, 95%CI 1.64–3.41; p<0.001). In the pair-matched analysis using the STPI subscales, there was a higher risk of AMIfrom anger even after adjusting for anxiety (RR=1.50, 95%CI 1.19–1.91; p<0.001). In a sensitivity analysis including information on co-exposure to other potential triggers, the results from the pair-matched analysis were not materially different when we further adjusted for physical activity, coffee consumption and alcohol consumption.
Discussion
Similar to our prior findings based on a smaller study population1, we found that the risk of having an AMI is >2-fold higher following outbursts of anger compared to other times. A novel finding in this study is that there was a striking monotonic increase in AMI risk with higher levels of self-reported anger intensityrather than a similar magnitude of risk for all higher anger levels. Compared to non-users, beta-blocker users had a lower susceptibility to heart attacks triggered by anger. The RR was lower among people with a history of coronary artery disease than people with no CAD history, even among people that were not beta-blocker users. This may be due to other medications or health characteristics that lower the risk of an anger-triggered AMI among people with a CAD history. Unlike the prior study, there was no difference in risk by aspirin use1 or by level of educational attainment17.
Our results are consistent with prior research on the short-term risk of cardiovascular events following episodes of anger1,2. In a study of the first 1623 patients enrolled in the MIOS Study1, 39 (2.4%) patients reported an episode of anger in the 2 hours prior to AMI onset, resulting in a 2.3-fold (95%CI 1.7 to 3.2) increased risk of AMI. Similarly, anger is associated with an abruptly higher risk of acute coronary syndrome3,4, ventricular arrhythmia5,6, myocardial ischemia7 and ischemic8 and hemorrhagic stroke9.
Acute anger6,18 and anxiety19have been shown to increase heart rate, blood pressure and vascular resistance. These hemodynamic changes may cause transient myocardial ischemia and/or disruption of a vulnerable coronary plaque, especially among susceptible patients. Furthermore, it may stimulate an inflammatory and pro-thrombotic response, including increased platelet aggregation and plasma viscosity. These changes may cause plaque disruption and thrombotic occlusion, resulting in an ischemic event20,21.
Some preventive agents, such as aspirin, beta-blockers, statins and angiotensin-converting enzyme (ACE) inhibitors may break the link between a trigger and its potential adverse cardiovascular consequences20,22. Consistent with our prior report1, the RR was lower among people who reported regular use of beta-blockers and those with a prior history of AMI. On the other hand, our prior observation of a lower RR among aspirin users was not replicated. Two studies2,4 reported that the risk of AMI triggered by anger did not vary by beta-blocker use, possibly due to the small number of exposed cases. We could not examine whether statins lower the risk from each episode of anger since this study was conducted before they were commonly used as a preventive agent.
In addition to lowering the hemodynamic and thrombotic response toanger, some pharmacologic agents may prevent anger outbursts. Low serotonin levels are associated with impulsive aggressive behavior and poor impulse control and emotion regulation23,24, suggesting that drugs intervening on the autonomic nervous system may lower aggressive behavior. Beta-blockers or selective serotonin re-uptake inhibitor (SSRI) antidepressants may lower the frequency of outbursts and hence lower the cumulative absolute risk over time, and based on our findings of a gradient in risk by anger level, lowering the magnitude of anger outbursts may in turn lower the risk from each episode. In this study, there were not enough patients reporting antidepressant use to evaluate the potentially lower risk of AMI among regular users of SSRI’s compared to non-users.
We hypothesized that a person acclimates to the physiologic response to anger so that more frequent outbursts of anger or an anger-prone personality is associated with lowerrisk from each outburst, but the association was not materially different by levels of the STPI anger scale. This may suggest that trait anger does not impact the short-term risk of AMI. Alternatively, differences in susceptibility by anger frequency or trait anger may be masked by the use of a general anger expression scale; it is possible that, similar to studies of long-term cardiovascular risk25,26, anger suppression is harmful whereas anger expression lowers the short-term risk.
Regular physical activity lowers the baseline risk of AMI as well as the risk from each episode of physical activity10,27. Since physical activity and anger cause similar acute physiologic responses, we hypothesized that people who are regularly active would be less susceptible to having an AMI triggered by anger. However, the short-term risk of AMI did not vary by usual physical activity. This may indicate that regular activity and anger act through different pathways or it may be due to other differences between participants with different levels of activity. Nevertheless, maintaining an active lifestyle is still beneficialsince it lowers baseline risk and thereby lowers the absolute risk of sustaining an AMI21.
There are some limitations that warrant discussion. Patients may attempt to explain their cardiac event by emphasizing emotional stressors immediately prior to symptom onset and underestimate exposure during the control period, resulting in recall bias and an overestimate of the RR. Alternatively, patients may be reluctant to report recent anger outbursts, resulting in an underestimate of the RR. However, we used a standardized structured interview and patients were not informed of the duration of the hypothesized hazard period. Since the case-crossover design uses subjects as their own controls, there can be no confounding by risk factors that are stable over time, but confounding by factors that change over time within individualscan occur if other transient risk factors occur during the case period13. However, when we restricted the analysis to patients who did not report recent exposure to other potential triggers, the results remained statistically significant. Since the study involves self-reported anger, only people that survived the index MI are included. Compared to other AMI cases, patients who had an episode of anger prior to AMI onset may be more likely to survive and participate in our study, resulting in an overestimate of the RR or they may be less likely to survive, resulting in an underestimate of the RR. However, it seems unlikely that AMI survival is different for cases triggered by different mechanisms. Although the self-matching in the case-crossover design accounts for within-person differences, between-person differences may at least partially explain differences between stratified results. In our study, angiographic data is not available, so we cannot rule out the possibility that some of the cases included in our sample had Takotsubo cardiomyopathy28 rather than an acute coronary syndrome.
Acknowledgments
None
Funding/Support: This work was supported by grant T32-HL098048 from the National Institutes of Health, United States.
Role of the Sponsor: No funding organization had any role in the design and conduct of the study; collection; management, analysis and interpretation of the data; and preparation of the manuscript.
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Conflict of Interest Disclosures: None
References
- 1.Mittleman MA, Maclure M, Sherwood JB, Mulry RP, Tofler GH, Jacobs SC, Friedman R, Benson H, Muller JE. Triggering of acute myocardial infarction onset by episodes of anger. Determinants of Myocardial Infarction Onset Study Investigators. Circulation. 1995;92:1720–1725. doi: 10.1161/01.cir.92.7.1720. [DOI] [PubMed] [Google Scholar]
- 2.Möller J, Hallqvist J, Diderichsen F, Theorell T, Reuterwall C, Ahlbom A. Do episodes of anger trigger myocardial infarction? A case-crossover analysis in the Stockholm Heart Epidemiology Program (SHEEP) Psychosom Med. 1999;61:842–849. doi: 10.1097/00006842-199911000-00019. [DOI] [PubMed] [Google Scholar]
- 3.Strike PC, Perkins-Porras L, Whitehead DL, McEwan J, Steptoe A. Triggering of acute coronary syndromes by physical exertion and anger: clinical and sociodemographic characteristics. Heart. 2006;92:1035–1040. doi: 10.1136/hrt.2005.077362. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Lipovetzky N, Hod H, Roth A, Kishon Y, Sclarovsky S, Green MS. Emotional events and anger at the workplace as triggers for a first event of the acute coronary syndrome: a case-crossover study. Isr Med Assoc J. 2007;9:310–315. [PubMed] [Google Scholar]
- 5.Reich P, DeSilva RA, Lown B, Murawski BJ. Acute psychological disturbances preceding life-threatening ventricular arrhythmias. JAMA. 1981;246:233–235. [PubMed] [Google Scholar]
- 6.Lampert R. Anger and ventricular arrhythmias. Curr Opin Cardiol. 2010;25:46–52. doi: 10.1097/HCO.0b013e32833358e8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gabbay FH, Krantz DS, Kop WJ, Hedges SM, Klein J, Gottdiener JS, Rozanski A. Triggers of myocardial ischemia during daily life in patients with coronary artery disease: physical and mental activities, anger and smoking. J Am Coll Cardiol. 1996;27:585–592. doi: 10.1016/0735-1097(95)00510-2. [DOI] [PubMed] [Google Scholar]
- 8.Koton S, Tanne D, Bornstein NM, Green MS. Triggering risk factors for ischemic stroke: a case-crossover study. Neurology. 2004;63:2006–2010. doi: 10.1212/01.wnl.0000145842.25520.a2. [DOI] [PubMed] [Google Scholar]
- 9.Vlak MH, Rinkel GJ, Greebe P, van der Bom JG, Algra A. Trigger factors and their attributable risk for rupture of intracranial aneurysms: a case-crossover study. Stroke. 2011;42:1878–1882. doi: 10.1161/STROKEAHA.110.606558. [DOI] [PubMed] [Google Scholar]
- 10.Mittleman MA, Maclure M, Tofler GH, Sherwood JB, Goldberg RJ, Muller JE. Triggering of acute myocardial infarction by heavy physical exertion. Protection against triggering by regular exertion. Determinants of Myocardial Infarction Onset Study Investigators. N Engl J Med. 1993;329:1677–1683. doi: 10.1056/NEJM199312023292301. [DOI] [PubMed] [Google Scholar]
- 11.Mostofsky E, Burger MR, Schlaug G, Mukamal KJ, Rosamond WD, Mittleman MA. Alcohol and acute ischemic stroke onset: the stroke onset study. Stroke. 2010;41:1845–1849. doi: 10.1161/STROKEAHA.110.580092. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Spielberger C, Gorsuch R, Lushene R. Manual for the State-Trait Anxiety Inventory (STAI) (self-evaluation questionnaire) Palo Alto: Consulting Psychologists Press; 1979. [Google Scholar]
- 13.Maclure M. The case-crossover design: a method for studying transient effects on the risk of acute events. Am J Epidemiol. 1991;133:144–153. doi: 10.1093/oxfordjournals.aje.a115853. [DOI] [PubMed] [Google Scholar]
- 14.Mittleman MA, Maclure M, Robins JM. Control sampling strategies for case-crossover studies: an assessment of relative efficiency. Am J Epidemiol. 1995;142:91–98. doi: 10.1093/oxfordjournals.aje.a117550. [DOI] [PubMed] [Google Scholar]
- 15.Rothman KJ, Greenland S, Lash TL. Modern Epidemiology. Vol. 2008. Philadelphia: Lippincott Williams & Wilkins; p. 279. [Google Scholar]
- 16.Maclure M, Greenland S. Tests for trend and dose response: misinterpretations and alternatives. Am J Epidemiol. 1992;135:96–104. doi: 10.1093/oxfordjournals.aje.a116206. [DOI] [PubMed] [Google Scholar]
- 17.Mittleman MA, Maclure M, Nachnani M, Sherwood JB, Muller JE, Mittleman MA, Maclure M, Nachnani M, Sherwood JB, Muller JE. Educational attainment, anger, and the risk of triggering myocardial infarction onset. The Determinants of Myocardial Infarction Onset Study Investigators. Arch Intern Med. 1997;157:769–775. [PubMed] [Google Scholar]
- 18.Gottdiener JS, Kop WJ, Hausner E, McCeney MK, Herrington D, Krantz DS. Effects of mental stress on flow-mediated brachial arterial dilation and influence of behavioral factors and hypercholesterolemia in subjects without cardiovascular disease. The American journal of cardiology. 2003;92:687–691. doi: 10.1016/s0002-9149(03)00823-3. [DOI] [PubMed] [Google Scholar]
- 19.Krantz DS, Kop WJ, Santiago HT, Gottdiener JS. Mental stress as a trigger of myocardial ischemia and infarction. Cardiol Clin. 1996(14):271–287. [PubMed] [Google Scholar]
- 20.Steptoe A, Brydon L. Emotional triggering of cardiac events. Neurosci Biobehav Rev. 2009;33:63–70. doi: 10.1016/j.neubiorev.2008.04.010. [DOI] [PubMed] [Google Scholar]
- 21.Mittleman MA, Mostofsky E. Physical, psychological and chemical triggers of acute cardiovascular events: preventive strategies. Circulation. 2011;124:346–354. doi: 10.1161/CIRCULATIONAHA.110.968776. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Tofler GH, Muller JE. Triggering of acute cardiovascular disease and potential preventive strategies. Circulation. 2006;114:1863–1872. doi: 10.1161/CIRCULATIONAHA.105.596189. [DOI] [PubMed] [Google Scholar]
- 23.Yanowitch R, Coccaro EF. The neurochemistry of human aggression. Adv Genet. 2011;75:151–169. doi: 10.1016/B978-0-12-380858-5.00005-8. [DOI] [PubMed] [Google Scholar]
- 24.Comai S, Tau M, Gobbi G. The psychopharmacology of aggressive behavior: a translational approach: part 1: neurobiology. J Clin Psychopharmacol. 2012;32:83–94. doi: 10.1097/JCP.0b013e31823f8770. [DOI] [PubMed] [Google Scholar]
- 25.Davidson KW, Mostofsky E. Anger expression and risk of coronary heart disease: evidence from the Nova Scotia Health Survey. Am Heart J. 2010;159:199–206. doi: 10.1016/j.ahj.2009.11.007. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26.Haukkala A, Konttinen H, Laatikainen T, Kawachi I, Uutela A. Hostility, anger control, and anger expression as predictors of cardiovascular disease. Psychosom Med. 2010;72:556–562. doi: 10.1097/PSY.0b013e3181dbab87. [DOI] [PubMed] [Google Scholar]
- 27.Dahabreh IJ, Paulus JK. Association of episodic physical and sexual activity with triggering of acute cardiac events: systematic review and meta-analysis. JAMA. 2011;305:1225–1233. doi: 10.1001/jama.2011.336. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Eitel I, von Knobelsdorff-Brenkenhoff F, Bernhardt P, Carbone I, Muellerleile K, Aldrovandi A, Francone M, Desch S, Gutberlet M, Strohm O, Schuler G, Schulz-Menger J, Thiele H, Friedrich MG. Clinical characteristics and cardiovascular magnetic resonance findings in stress (takotsubo) cardiomyopathy. JAMA. 2011;306:277–286. doi: 10.1001/jama.2011.992. [DOI] [PubMed] [Google Scholar]


