Abstract
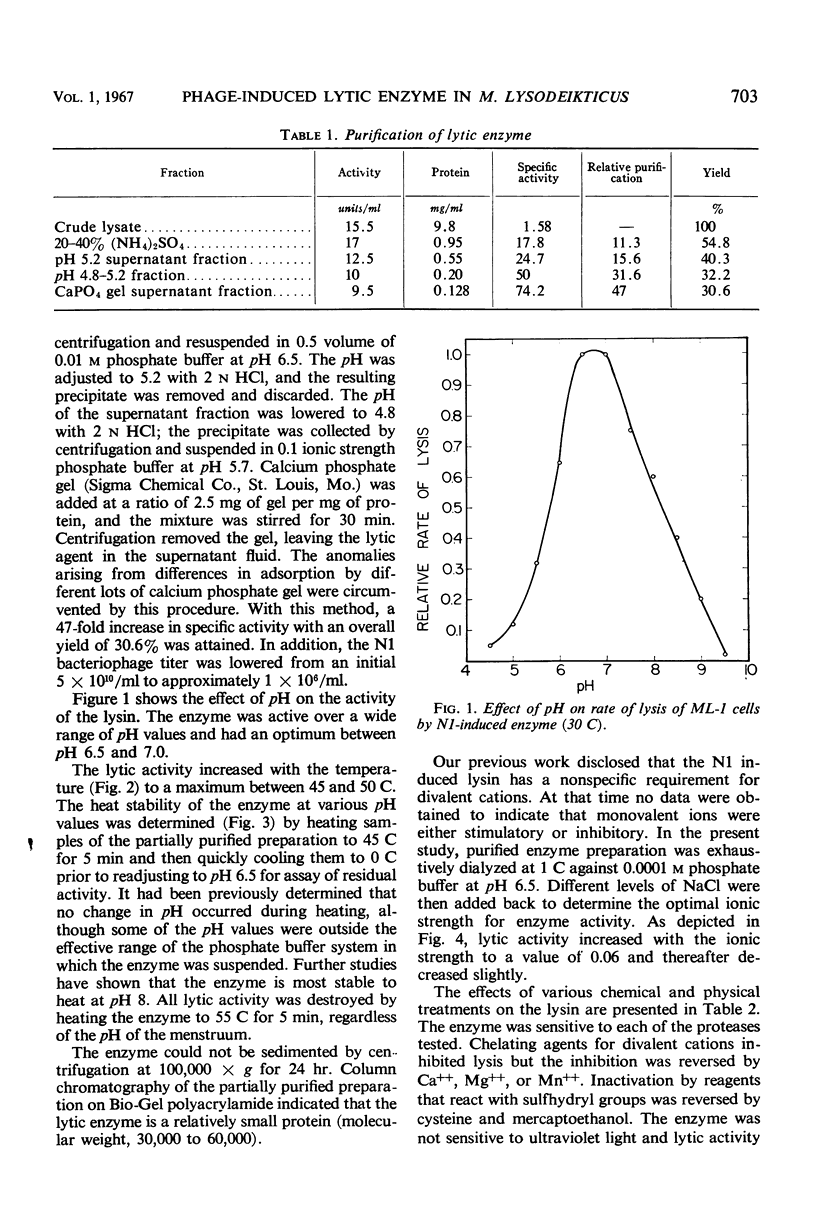
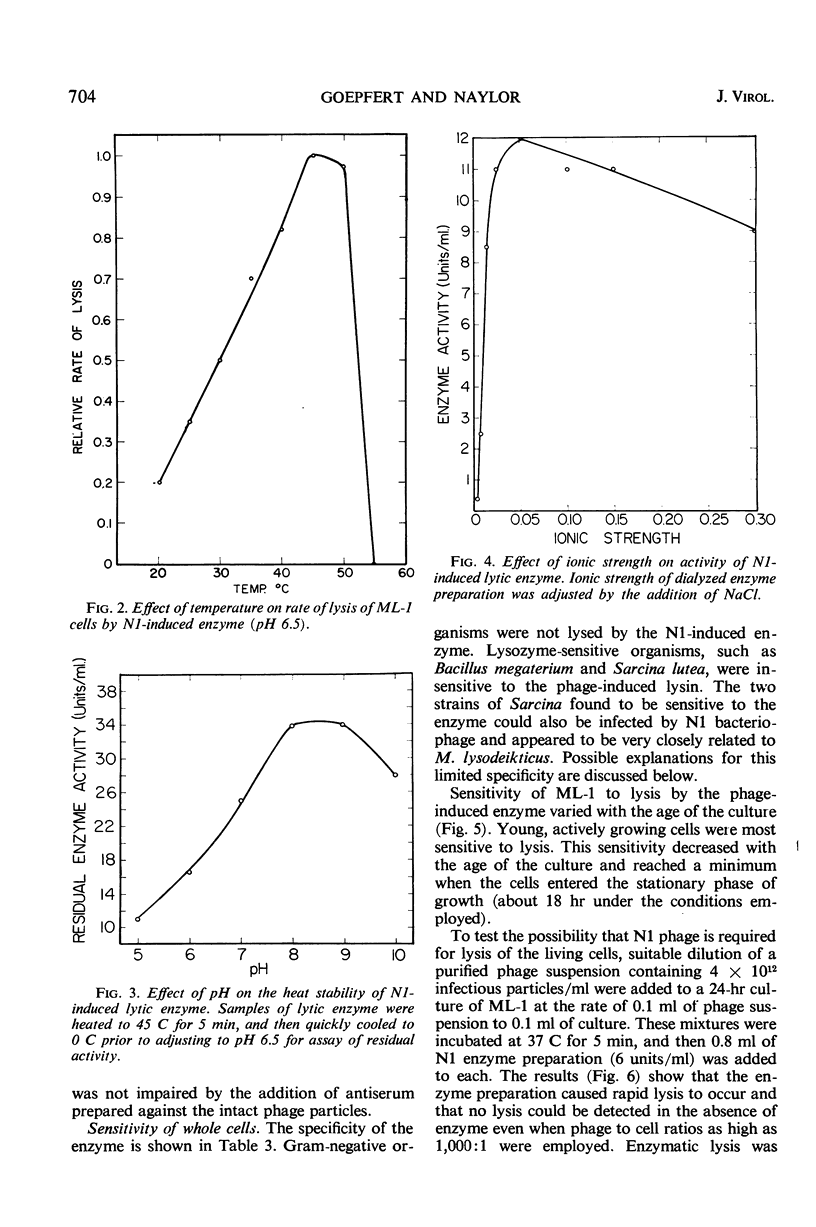
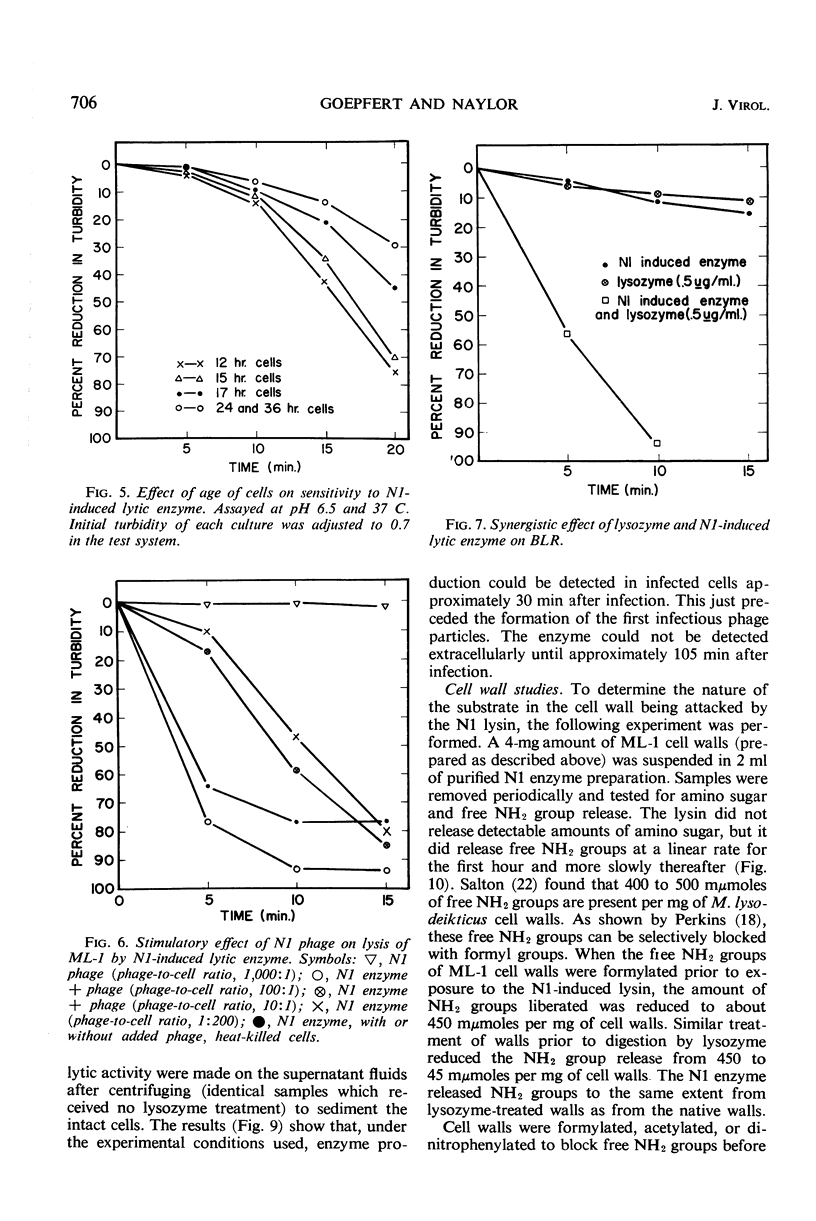
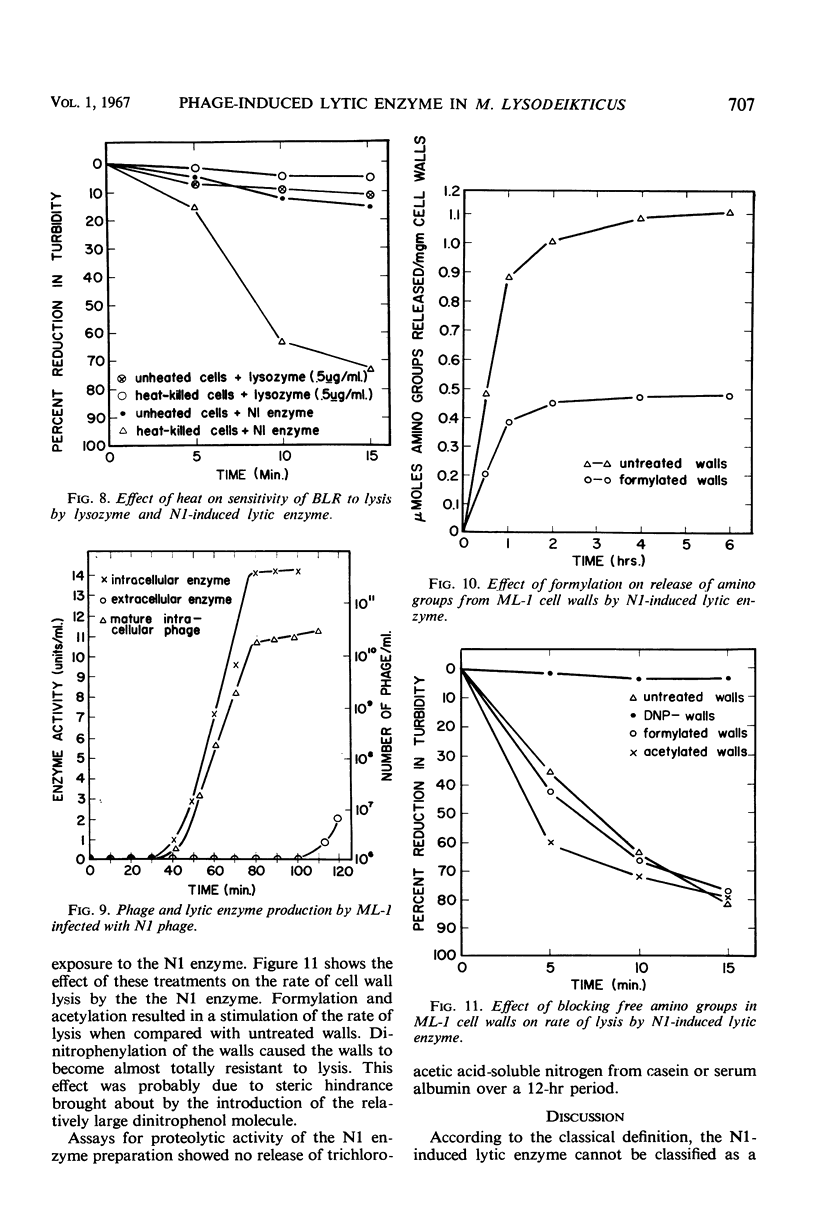
A lytic enzyme induced in Micrococcus lysodeikticus strain 1 by infection with N1 bacteriophage was purified 45- to 50-fold by ammonium sulfate precipitation, acid precipitation, and selective adsorption of contaminating proteins with calcium phosphate gel. The optimal pH for activity of the enzyme was 6.5 to 7.0. Maximal activity occurred at 45 to 50 C and at an ionic strength of 0.06. The enzyme had a limited specificity and lysed cell walls of M. lysodeikticus with the release of dinitrofluorobenzene reactive groups. Living cells were lysed in the absence of phage; however, the rate of lysis increased when phage was present in excess of 10 particles per bacterial cell. Young cells were most sensitive, and the sensitivity decreased to a minimum with stationary-phase cells. Acting synergistically, lysozyme and the N1-induced lysin caused lysis of cells which were resistant to either enzyme acting independently. The N1 lysin did not exhibit proteolytic activity.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- ADAMS M. H., PARK B. H. An enzyme produced by a phage-host cell system. II. The properties of the polysaccharide depolymerase. Virology. 1956 Dec;2(6):719–736. doi: 10.1016/0042-6822(56)90054-x. [DOI] [PubMed] [Google Scholar]
- BRUMFITT W. Studies on the mechanism of adsorption and penetration by bacteriophage. J Pathol Bacteriol. 1960 Jan;79:1–9. [PubMed] [Google Scholar]
- BRUMFITT W., WARDLAW A. C., PARK J. T. Development of lysozyme-resistance in Micrococcus lysodiekticus and its association with an increased O-acetyl content of the cell wall. Nature. 1958 Jun 28;181(4626):1783–1784. doi: 10.1038/1811783a0. [DOI] [PubMed] [Google Scholar]
- BURGI E., NAYLOR H. B. Observations on abortive infection of Micrococcus lysodeikticus with bacteriophage. Virology. 1956 Oct;2(5):577–593. doi: 10.1016/0042-6822(56)90039-3. [DOI] [PubMed] [Google Scholar]
- CHAMPE S. P. BACTERIOPHAGE REPRODUCTION. Annu Rev Microbiol. 1963;17:87–114. doi: 10.1146/annurev.mi.17.100163.000511. [DOI] [PubMed] [Google Scholar]
- CUMMINS C. S., HARRIS H. The chemical composition of the cell wall in some gram-positive bacteria and its possible value as a taxonomic character. J Gen Microbiol. 1956 Jul;14(3):583–600. doi: 10.1099/00221287-14-3-583. [DOI] [PubMed] [Google Scholar]
- DOUGHTY C. C., HAYASHI J. A. Enzymatic properties of a phage-induced lysin affecting group A streptococci. J Bacteriol. 1962 May;83:1058–1068. doi: 10.1128/jb.83.5.1058-1068.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Eklund C., Wyss O. ENZYME ASSOCIATED WITH BACTERIOPHAGE INFECTION. J Bacteriol. 1962 Dec;84(6):1209–1215. doi: 10.1128/jb.84.6.1209-1215.1962. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ensign J. C., Wolfe R. S. Characterization of a small proteolytic enzyme which lyses bacterial cell walls. J Bacteriol. 1966 Feb;91(2):524–534. doi: 10.1128/jb.91.2.524-534.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GHUYSEN J. M., STROMINGER J. L. STRUCTURE OF THE CELL WALL OF STAPHYLOCOCCUS AUREUS, STRAIN COPENHAGEN. I. PREPARATION OF FRAGMENTS BY ENZYMATIC HYDROLYSIS. Biochemistry. 1963 Sep-Oct;2:1110–1119. doi: 10.1021/bi00905a035. [DOI] [PubMed] [Google Scholar]
- HEYMANN H., MANNIELLO J. M., BARKULIS S. S. STRUCTURE OF STREPTOCOCCAL CELL WALLS. 3. CHARACTERIZATION OF AN ALANINE-CONTAINING GLUCOSAMINYLMURAMIC ACID DERIVATIVE LIBERATED BY LYSOZYME FROM STREPTOCOCCAL GLYCOPEPTIDE. J Biol Chem. 1964 Sep;239:2981–2985. [PubMed] [Google Scholar]
- Humphries J. C. Enzymic Activity of Bacteriophage-Culture Lysates: I. A Capsule Lysin Active against Klebsiella pneumoniae Type A. J Bacteriol. 1948 Nov;56(5):683–693. [PMC free article] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MURPHY J. S. A phage-associated enzyme of Bacillus megaterium which destroys the bacterial cell wall. Virology. 1957 Dec;4(3):563–581. doi: 10.1016/0042-6822(57)90086-7. [DOI] [PubMed] [Google Scholar]
- MURPHY J. S. An agent derived from B. megaterium phage G which dissolves the bacterial cell wall. Virology. 1960 Jun;11:510–513. doi: 10.1016/0042-6822(60)90094-5. [DOI] [PubMed] [Google Scholar]
- PERKINS H. R. THE ACTION OF HOT FORMAMIDE ON BACTERIAL CELL WALLS. Biochem J. 1965 Jun;95:876–882. doi: 10.1042/bj0950876. [DOI] [PMC free article] [PubMed] [Google Scholar]
- RALSTON D. J., BAER B. S., LIEBERMAN M., KRUEGER A. P. Virolysin: a virus-induced lysin from staphylococcal phage lysates. Proc Soc Exp Biol Med. 1955 Aug;89(4):502–507. doi: 10.3181/00379727-89-21859. [DOI] [PubMed] [Google Scholar]
- RALSTON D. J., BAER B., LIEBERMAN M., KRUEGER A. P. Virolysin, a virus-induced lysin: its appearance and function in phage-infected staphylococci. J Gen Microbiol. 1961 Mar;24:313–325. doi: 10.1099/00221287-24-3-313. [DOI] [PubMed] [Google Scholar]
- RALSTON D. J. STAPHYLOCOCCAL SENSITIZATION: SPECIFIC BIOLOGICAL EFFECTS OF PHAGE K ON THE BACTERIAL CELL WALL IN LYSIS-FROM-WITHOUT. J Bacteriol. 1963 Jun;85:1185–1193. doi: 10.1128/jb.85.6.1185-1193.1963. [DOI] [PMC free article] [PubMed] [Google Scholar]



