Abstract
Leukemia inhibitory factor (LIF), a downstream target of estrogen, is essential for implantation in mice. LIF function is thought to be mediated by its binding to LIF receptor (LIFR) and recruitment of coreceptor GP130 (glycoprotein 130), and this receptor complex then activates signal transducer and activator of transcription (STAT)1/3. However, the importance of LIFR and GP130 acting via STAT3 in implantation remains uncertain, because constitutive inactivation of Lifr, Gp130, or Stat3 shows embryonic lethality in mice. To address this issue, we generated mice with conditional deletion of uterine Gp130 or Stat3 and show that both GP130 and STAT3 are critical for uterine receptivity and implantation. Implantation failure in these deleted mice is associated with higher uterine estrogenic responses prior to the time of implantation. These heightened estrogenic responses are not due to changes in ovarian hormone levels or expression of their nuclear receptors. In the deleted mice, estrogen-responsive gene, Lactoferrin (Ltf), and Mucin 1 protein, were up-regulated in the uterus. In addition, progesterone-responsive genes, Hoxa10 and Indian hedgehog (Ihh), were markedly down-regulated in STAT3-inactivated uteri. These changes in uteri of deleted mice were reflected by the failure of differentiation of the luminal epithelium, which is essential for blastocyst attachment.
Implantation failure is a major contributor to female infertility. Successful implantation requires an intimate cross talk between the blastocyst and the receptive uterus (1). Although the molecular basis of the initiation of uterine receptivity is not fully understood, leukemia inhibitory factor (LIF) is one of few known critical cytokines in implantation (2, 3).
LIF is a member of the IL-6 cytokine family which also includes IL-6, IL-11, oncostatin M (OSM), ciliary neurotropic factor, and cardiotrophin-1. This family of cytokines has pleiotropic biological functions, including cell growth and differentiation of various types of target cells, bone metabolism, neural development, embryogenesis, and inflammation (4). Cytokines in the LIF/IL-6 family execute their biological function by activating their own receptors followed by the recruitment of glycoprotein 130 (GP130/IL6ST). These cytokines are clustered into 2 classes due to their different signaling initiation mechanisms. After IL-6 and IL-11 bind to their ligand-specific receptors, a homodimer of GP130 is recruited to transduce signaling. In contrast, LIF, OSM, ciliary neurotropic factor, and cardiotrophin-1 signaling is initiated by the formation of heterodimers consisting of GP130/LIF receptor (LIFR) or GP130/OSM complex (4, 5). In addition, LIF is capable of signaling through the homodimers of the LIFR cytoplasmic domain to block differentiation of embryonic stem cells (6). Although the homodimer of LIFR is considered less efficient than the LIFR/GP130 heterodimer in transducing LIF signaling, in certain cases LIFR homodimers can generate equivalent signals for growth arrest and differentiation as observed in a mouse myeloid leukemia cell line (7). The activation of LIFR and GP130 leads to the initiation of 3 main signaling pathways: cytoplasmic Jak kinases (8), Src homology 2 domain-containing protein tyrosine phosphatase-2, which activates MAPK (9), and signal transducer and activator of transcription 1/3 (STAT1/3) (10). Several YXXQ motifs on the C terminus of the GP130 cytoplasmic tail mediate the binding and activation of STAT1/3 (11).
LIF is indispensable for implantation in mice. Lif mutant (Lif−/−) females are viable and Lif−/−embryos develop to the blastocyst stage. Mutant females are infertile due to implantation failure, and administration of recombinant LIF can rescue implantation in Lif−/− females (2, 12). Reciprocal embryo transfer experiments between wild-type (WT) and Lif−/− females have shown that maternal LIF, but not the embryonic LIF, is essential for implantation. In contrast, Lifr−/− offspring show perinatal lethality due to placental, skeletal, neural, and metabolic defects (13). Gp130−/− embryos, which are generated by heterozygous crossing, also die between day 13 and term due to abnormal cardiac development (14). Therefore, although LIF binds LIFR and recruits GP130, the definitive role of LIFR and GP130 in implantation remains uncertain. To study the downstream signaling pathways of GP130, different motifs or regions in the cytoplasmic portion of GP130 were genetically manipulated. In Gp130YXXQ/YXXQ mice, all tyrosine (Y) residues in 4 YXXQ motifs that recruit STAT3 were replaced by phenylalanine. This resulted in perinatal lethality similar to Gp130−/− mice (11). However, when a C-terminal region of the Gp130 gene containing all STAT-binding sites (YXXQ motifs) were deleted, the mutant mice (Gp130ΔSTAT/ΔSTAT) were viable, but showed implantation failure (15). This phenotype suggests that GP130 plays an important role in implantation, but questions still remain regarding the role of uterine GP130 in implantation because Gp130ΔSTAT/ΔSTAT mice represent global inactivation of GP130. In addition, the differences in viability of Gp130ΔSTAT/ΔSTAT mice and Gp130YXXQ/YXXQ/Gp130−/− mice suggest that subtle manipulations of different functional domains in Gp130 cause disparate phenotypes.
The disparate expression pattern of Lifr and Gp130 suggests a GP130-independent function of LIFR in mouse uteri. For example, on the morning of day 4 of pregnancy, Lifr mRNA is expressed primarily in luminal epithelial cells whereas Gp130 expression is observed in glandular epithelial cells (16). Given that both LIFR and GP130 are able to function separately (4, 6, 7), further studies are warranted to reveal their specific function. Conditional deletion of Gp130 or Stat3 has helped to study their specific function in adulthood, because both Gp130−/− and Stat3−/− mice exhibit embryonic lethality (14, 17). In this study, we generated 2 mouse lines with uterine-specific deletion of Gp130 (Gp130d/d) and Stat3 (Stat3d/d) to study the role of Gp130 and Stat3 in implantation. We established these mouse lines using floxed Gp130 (Gp130f/f) (18) or Stat3 (Stat3f/f) (19) mice crossed with mice carrying a Cre gene driven by the progesterone receptor promoter (Pgrcre/+). We found that mice with uterine inactivation of Gp130 or Stat3 are infertile due to implantation failure and that this phenotype is associated with higher uterine estrogen responses, especially in the Stat3d/d females in the context of uterine gene expression and luminal epithelial differentiation.
Materials and Methods
Animals and treatments
To generate mice with uterine deletion of Stat3 and Gp130, Stat3loxP/loxP (19) and Gp130loxP/loxP (18) mice were crossed with PgrCre/+ mice (20). All mice were housed in the animal care facility of Cincinnati Children's Hospital Medical Center according to NIH and institutional guidelines for laboratory animals. All protocols of the present study were approved by Cincinnati Children's Research Foundation Institutional Animal Care and Use Committee.
Female mice were mated with fertile males to induce pregnancy (vaginal plug = day 1 of pregnancy). Embryos at the blastocyst stage were recovered by flushing uteri on day 4. To examine receptivity and implantation, pregnant dams were humanely destroyed on day 4 (8:00 am to 10:00 am) and day 5 (9:00 am), respectively. Implantation sites were visualized by an iv injection of 0.1 mL of 1% Chicago blue dye solution in saline 3–5 minutes before killing, and the number of implantation sites, demarcated by distinct blue bands, was recorded.
In situ hybridization
In situ hybridization was performed as previously described (21). In brief, frozen sections (12 μm) were mounted onto poly-l-lysine-coated slides and fixed in cold 4% (wt/vol) paraformaldehyde in PBS. The sections were prehybridized and hybridized at 45°C for 4 hours in 50% (vol/vol) formamide hybridization buffer containing the 35S-labeled antisense or sense cRNA probes. RNase A-resistant hybrids were detected by autoradiography. Sections were poststained with hematoxylin and eosin. Sections hybridized with the sense probes did not exhibit any positive signals and served as negative controls.
Immunohistochemistry and immunofluorescence
Immunostaining of estrogen receptor-α (ESR1, sc543, Santa Cruz Biotechnology, Inc; 1:200), progesterone receptor (PGR, 18–0172, Invitrogen; 1:300) and Ki67 (RM-9106-S; Neomarkers; 1:300) was visualized using a Histostain-Plus (diaminobenzidine) kit (2014, Invitrogen). The antibody for mucin 1 (MUC1) was a generous gift from Dan Carson (Rice University, Houston, Texas). Immunofluorescence for MUC1 was performed using a secondary antibody Cy3-conjugated donkey antirabbit (711–165-152; Jackson ImmunoResearch Laboratories). Nuclear staining was performed by Hoechst 33342 (H1399, Molecular Probes; 2 μg/mL). Immunofluorescence was performed on fresh-frozen sections. Sections were fixed in absolute ethanol for 10 minutes and incubated with primary antibody at 4°C overnight, followed by incubation in secondary antibody for 1 hour in PBS. Fluorescent signals were visualized under a confocal microscope (Nikon Eclipse TE2000, Nikon).
Measurement of 17β-estradiol (E2) and progesterone (P4) levels
Mouse blood samples were collected on day 4 of pregnancy. Serum levels of E2 (17β-estradiol) and P4 were measured by EIA kits (Cayman Chemical Co, Ann Arbor, Michigan).
RNA extraction and real-time PCR
RNA was extracted from uterine tissue using TRIzol reagent (Invitrogen). After DNase treatment, cDNA was synthesized using Superscript II. Quantitative RT-PCR for GP130 mRNA was performed using fast SYBR Green master mix (Applied Biosystems) and StepOnePlus real-time PCR system (Applied Biosystems). Primers were 5′-CAACACCAAAGTTCGCTCAA-3′ and 5′-GCCGTCCGAGTACATTTGAT-3′ for GP130.
Western blotting
These experiments were performed using antibodies against GP130 (AF468; R&D Systems) and STAT3 (catalog no. 4904, Cell Signaling Technology) as described previously (16).
Statistical analysis
Comparison of means was performed by using Student's t test. Data are shown as mean ± SEM.
Results
Gp130 and Lifr are disparately expressed in the periimplantation mouse uterus
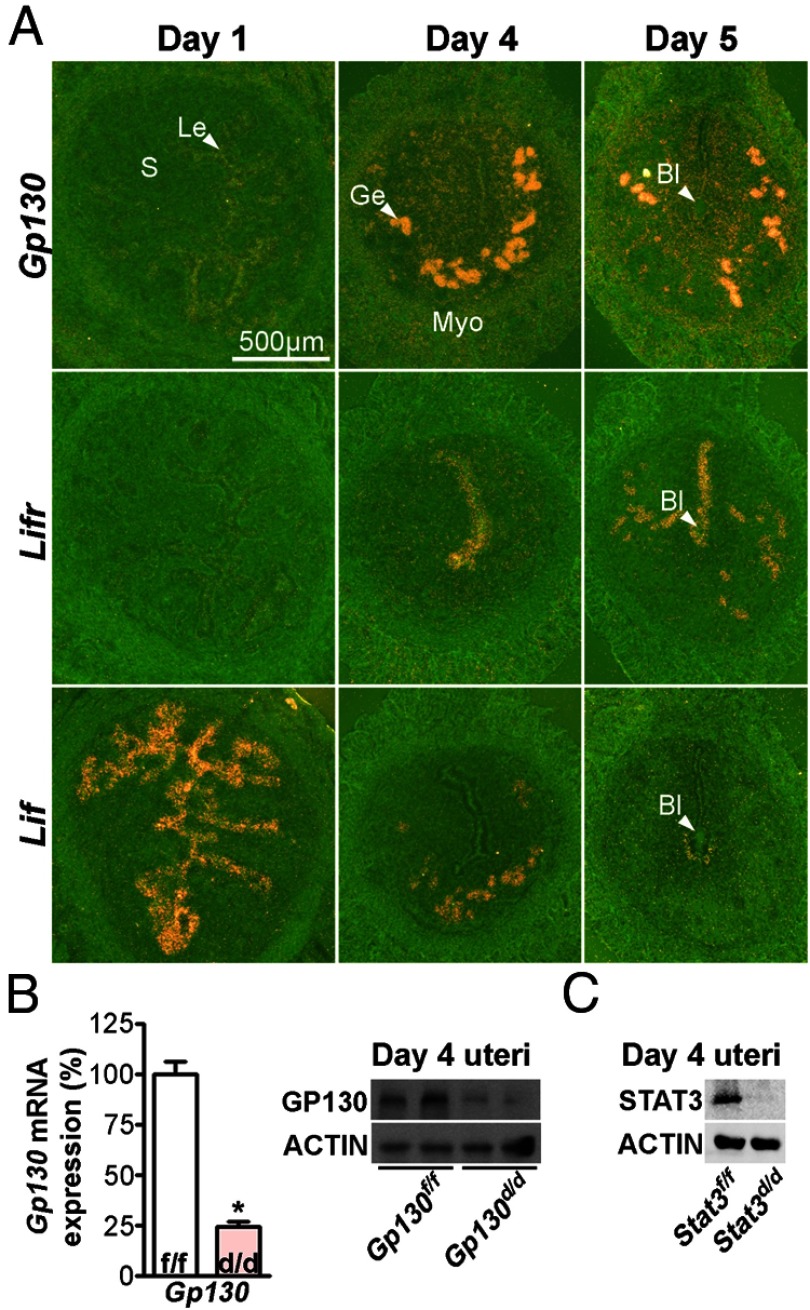
LIF signaling in implantation is considered to be mediated by interacting with the LIFR/GP130 heterodimer. To study the nature and role of Lif signaling, expression of Lif, Gp130, and Lifr was examined in the periimplantation mouse uterus. On day 1 of pregnancy, Gp130 is virtually absent in the uterus, which is under the influence of preovulatory estrogen secretion. On day 4, the uterus is receptive to implantation under the influence of rising progesterone (P4) levels from the newly formed corpora lutea superimposed with a small amount of ovarian estrogen (1). Gp130 is highly expressed in the glandular epithelium with very low levels of expression in the luminal epithelium (Figure 1A). On day 5, stromal cells at the implantation site are undergoing proliferation and beginning to differentiate into decidual cells (22). Gp130 expression is robust in the glandular epithelium and also evident in the luminal epithelium and proliferating stromal cells. In contrast, Lifr, the heterodimer partner of GP130, is primarily expressed in the luminal epithelium on day 4 of pregnancy. On the morning of day 5 when blastocyst implantation is in progress, distinct expression of Lifr persists in the luminal epithelium, including the epithelial cells surrounding the blastocyst. Modest expression of Lifr is also observed in the glandular epithelium (Figure 1A). On the other hand, the expression of Lif, the ligand for LIFR or for the LIF/GP130 complex, is differently expressed compared with its receptors. Lif expression is first observed in the epithelium on day 1 of pregnancy under the influence of preovulatory estrogen secretion. On the morning of day 4 (the day of uterine receptivity), Lif is exclusively expressed in the glandular epithelia. Upon initiation of the blastocyst attachment with the luminal epithelium on midnight of day 4, Lif expression is evident in stromal cells around the blastocyst, and this expression continues through the morning of day 5 of pregnancy. In addition, Lif and Gp130, but not Lifr, are primarily expressed in the uterine glands on this day. These results show that coexpression of Lifr and Gp130 are not fully realized until the attachment reaction occurs. Taken together, the results prompted us to ask whether Gp130 has an independent role, and whether Gp130 is indispensable for the process of implantation.
Figure 1.
The Expression of Gp130 and Lifr Is Temporally and Spatially Regulated in the Periimplantation Mouse Uterus. A, In situ hybridization of Gp130, Lifr, and Lif in WT pregnant uteri. Le, luminal epithelium; Ge, glandular epithelium; S, Stroma; Bl, blastocyst; Myo, myometrium. B, Relative Gp130 mRNA levels and protein levels in Gp130f/f and Gp130d/d uteri on day 4 of pregnancy assessed by real-time PCR and Western blotting. Unpaired t test, *, P < .05. C, Western blotting of STAT3 levels using protein extracts from Stat3f/f and Stat3d/d uteri on day 4 of pregnancy.
To study the importance of different components in the LIF signaling pathway, we conditionally deleted Gp130 or Stat3 in the mouse uterus. Notably, GP130 facilitates the binding of LIF to LIFR and activates STAT3. We crossed Gp130flox/flox (Gp130f/f) or Stat3flox/flox (Stat3f/f) mice with Pgrcre/+ mice to generate mice with uterine deletion of Gp130 (Gp130d/d) and Stat3 (Stat3d/d), respectively. To confirm uterine deletion of Gp130, levels of Gp130 transcripts were examined by quantitative RT-PCR (qPCR) and Western blotting in Gp130f/f and Gp130d/d uteri on day 4. The results show that levels of Gp130 in Gp130d/d uteri at the mRNA and protein levels are robustly diminished as compared with the levels in Gp130f/f uteri (Figure 1B). The deletion of Stat3 was assessed by Western blotting using uterine protein extracts collected from Stat3f/f and Stat3d/d females on day 4. Positive signals of STAT3 protein were not detected in Stat3d/d uterine extracts, suggesting that Stat3 is also efficiently deleted in Stat3d/d uteri (Figure 1C).
Mice with uterine deletion of Gp130 are infertile due to implantation failure
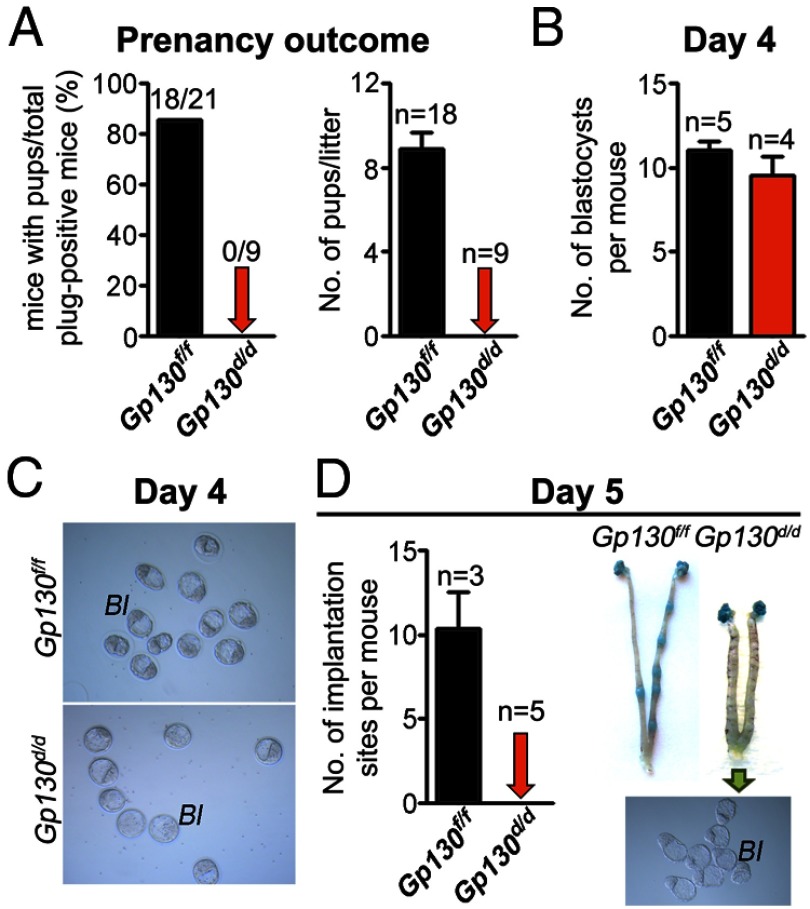
To examine the effects of uterine inactivation of Gp130 on pregnancy outcome, Gp130f/f and Gp130d/d females were mated with WT fertile males. Although the rate of vaginal plugs was comparable between Gp130f/f and Gp130d/d females, all Gp130d/d females examined failed to produce any pups as opposed to Gp130f/f females that generated a full complement of offspring (Figure 2A). To further determine the stage-specific failure of pregnancy in Gp130d/d females, females of both genotypes were killed at different times during the early stages of pregnancy. Fertilized eggs through successive stages of development form blastocysts on day 4; blastocysts subsequently attach to the uterine luminal epithelium on the evening of day 4 of pregnancy (1). To examine preimplantation embryo development, pregnant females were killed on the morning of day 4. A comparable number of blastocysts were recovered from day 4 uteri from both Gp130f/f and Gp130d/d mice with blastocyst morphology appearing normal (Figure 2, B and C). This suggests that Gp130d/d females have normal ovulation and Gp130d/d oviducts and uteri are competent to support normal fertilization and preimplantation embryo development, respectively. To further examine whether blastocysts can successfully implant into Gp130d/d uteri, Gp130d/d and Gp130f/f mice were examined on day 5. An increase in localized endometrial vascular permeability at the site of blastocyst attachment is an early distinct feature of the implantation process that can be monitored by an iv injection of a macromolecular blue dye solution, demarcating the implantation sites as discrete blue bands along the uterus (23). Whereas Gp130f/f females showed a normal number of distinct blue bands, Gp130d/d mice failed to show such blue bands; unattached blastocysts were recovered after flushing the uterine lumen with saline (Figure 2D). These results show that Gp130d/d uteri fail to support implantation, although normal ovulation, fertilization, and preimplantation embryo development occur in these mice.
Figure 2.
Uterine Deletion of Gp130 Confers Female Infertility. A, Pregnancy outcomes in Gp130f/f and Gp130d/d mice. Gp130d/d females are completely infertile. B and C, Blastocysts recovered from Gp130f/f and Gp130d/d uteri on day 4. D, Number of implantation sites and representative uteri from Gp130f/f and Gp130d/d mice on day 5. Photographs of implantation sites were taken after blue dye injection and embryos were recovered from Gp130d/d uteri. Bl, blastocyst.
Mice with uterine inactivation of Stat3 show implantation failure
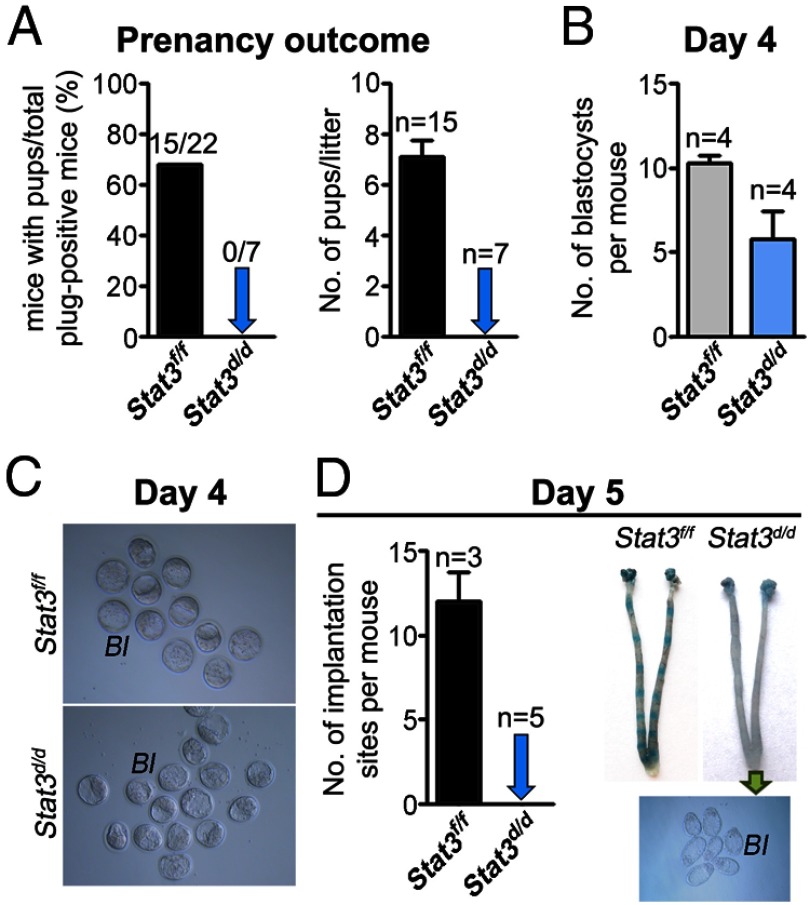
To see whether uterine deletion of Stat3 gives rise to similar phenotypes as seen in Gp130d/d females, Stat3 was similarly inactivated in the uterus. Stat3d/d females mated with WT fertile males failed to produce any pups and remained infertile (Figure 3A). Well-developed blastocysts were recovered from Stat3d/d uteri on day 4 of pregnancy (Figure 3, B and C), but they failed to implant as examined on day 5 by the blue dye method (Figure 3D) compared with Stat3f/f females, which showed a full complement of implantation. However, the number of blastocysts recovered from Stat3d/d uteri on day 4 of pregnancy was relatively smaller compared with that recovered from the Stat3f/f uteri (Figure 3B). This finding may suggest that ovulation, fertilization, and/or preimplantation development are somewhat compromised in Stat3d/d females. Furthermore, 11 of 18 plug-positive Stat3d/d females (61%) examined failed to yield any blastocyst. It is possible that the reproductive tract environment in Stat3d/d females is not conducive to fully support fertilization and preimplantation embryo development. This may also account for the relatively small number of blastocysts recovered from uteri of Stat3d/d females (Figure 3B). These findings are somewhat different from a recent study published prior to the submission of our present work (24). This report showed that although implantation fails to occur in Stat3d/d females, ovulation, fertilization, and preimplantation development were normal in these deleted mice. Nonetheless, the results of these 2 studies suggest that Stat3d/d females are infertile due to implantation failure.
Figure 3.
Uterine Deletion of Stat3 Confers Female Infertility. A, Pregnancy outcomes in Stat3f/f and Stat3d/d mice. Stat3d/d females are completely infertile. B and C, Blastocysts recovered from Stat3f/f and Stat3d/d uteri on day 4. D, Number of implantation sites and representative uteri from Stat3f/f and Stat3d/d mice on day 5. Photographs of implantation sites were taken after blue dye injection and embryos were recovered from Stat3d/d uteri. Bl, blastocysts.
Uterine inactivation of Gp130 or Stat3 confers increased estrogenic responses
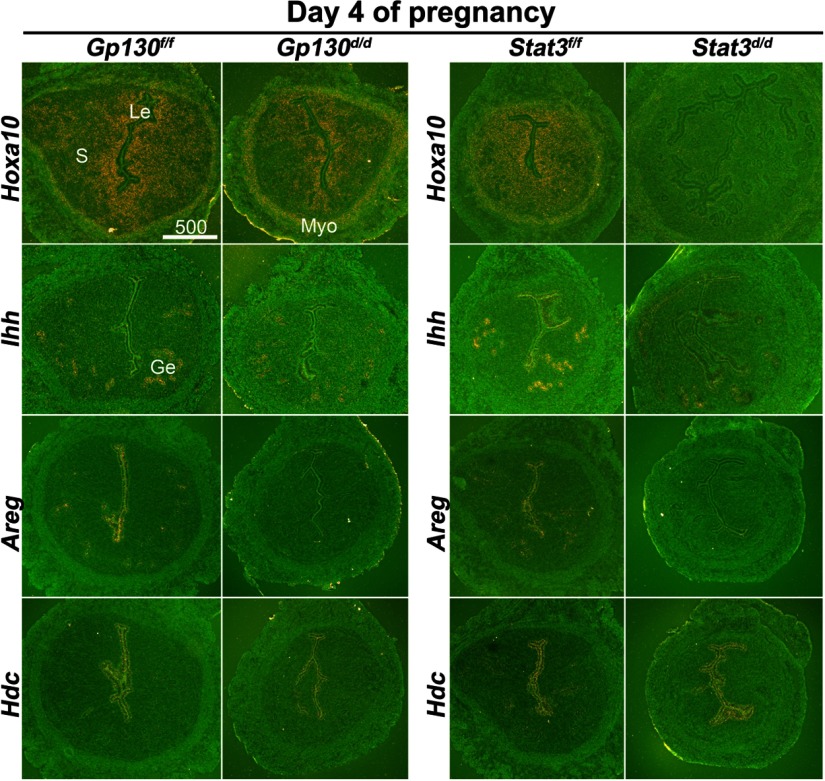
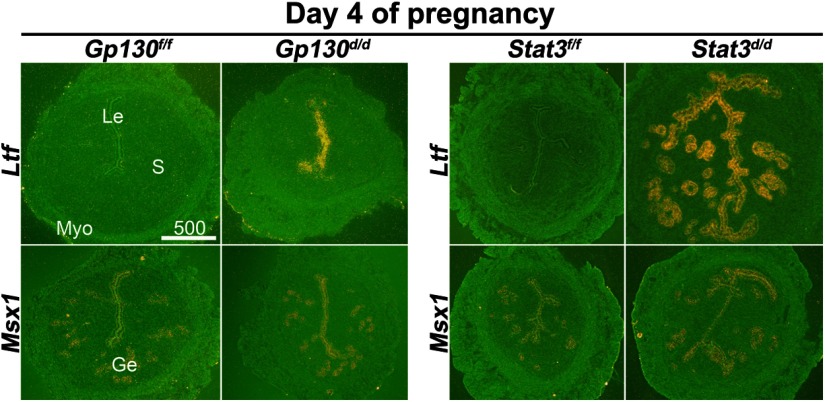
To further study the consequences of uterine inactivation of Gp130 or Stat3, we examined the expression of genes known to be critical for uterine receptivity. We examined P4 -responsive genes Indian hedgehog (Ihh) and Hoxa10, and an estrogen-responsive gene Lactoferrin (Ltf) (3) in uteri of floxed and deleted mice. We also examined Msx1, a recently identified gene crucial to uterine receptivity and implantation (25), in Gp130d/d and Stat3d/d uteri. Cell-specific expression of these genes in day 4 uteri was examined by in situ hybridization (Figures 4 and 5). The expression pattern of Ihh in the epithelium was similar in Gp130d/d, and control uteri on day 4, but its expression levels were lower in Stat3d/d uteri. As previously observed (26), Hoxa10 expression was observed in the stroma and circular muscle layer of Gp130f/f and Stat3f/f uteri. Its expression was lower in Gp130d/d uteri and became almost undetectable in Stat3d/d uteri (Figure 4). In contrast, the expression of estrogen-responsive Ltf was undetectable in floxed uteri, but showed its distinct presence in the luminal epithelium of Gp130d/d females and significantly higher levels of expression in the luminal and glandular epithelia in Stat3d/d uteri on day 4 of pregnancy (Figure 5). However, the expression of Msx1, which is not directly influenced by ovarian hormones (25), showed no obvious differences in Gp130d/d and Stat3d/d uteri as compared with their floxed counterparts. These results show that the balance between the estrogen and P4 responsiveness in the uterus is impaired, manifesting reduced P4 responsiveness, but higher estrogenic response. To further explore whether the reduced P4 responsiveness was associated with other P4-responsive genes, we examined uterine expression of 2 additional P4 responsive genes amphiregulin (Areg) and histidine decarboxylase (Hdc) employing in situ hybridization (27, 28). As expected, Areg were expressed in the luminal and glandular epithelia in floxed uteri, but the expression was very low to undetectable in Gp130d/d or Stat3d/d uteri on day 4 of pregnancy (Figure 4). In contrast, the expression of Hdc was comparable in all groups examined. This differential regulation of P4-responsive genes in the uterus suggests that the inactivation of Gp130 or Stat3 affects specific instead of all P4-responsive genes.
Figure 4.
P4-Responsive Genes Are Differentially Regulated in Stat3d/d and Gp130d/d Uteri on Day 4 of Pregnancy. In situ hybridization of Hoxa10, Ihh, Areg, and Hdc in uteri on day 4 of pregnancy. Le, luminal epithelium; Ge, glandular epithelium; S, stroma; Myo, myometrium.
Figure 5.
Genes Critical for Implantation Are Dysregulated in Stat3d/d and Gp130d/d Uteri on Day 4 of Pregnancy. In situ hybridization of Ltf and Msx1 in uteri on day 4 of pregnancy. Le, luminal epithelium; Ge, glandular epithelium; S, stroma; Myo, myometrium.
Expression pattern of Lif is variable in Stat3d/d uteri
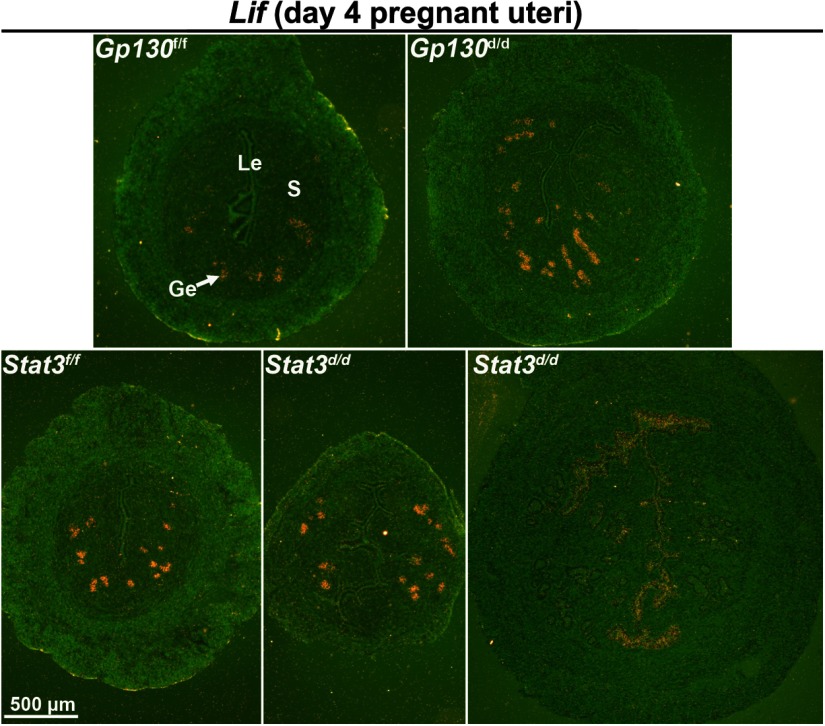
Activation of GP130 and STAT3 depends on the presence of LIF, and Lif is considered as an estrogen-responsive gene. It is possible that uterine expression of Lif is altered due to deletion of the downstream signaling components or the aberrant hyperestrogenic responses in Gp130d/d and Stat3d/d uteri. Thus, we examined the uterine expression of Lif on day 4 of pregnancy in the absence of Gp130 or Stat3 by in situ hybridization. The expression of Lif in the glandular epithelium appeared normal in Gp130d/d uteri (Figure 6). However, the expression of Lif varied among Stat3d/d uteri. The morphology and histology of some Stat3d/d uteri were normal looking as in floxed uteri on day 4 in which the pattern of Lif was normal, whereas some Stat3d/d uteri were more edematous and larger in size similar to WT day 1 uteri in which Lif expression was observed in the luminal epithelium as is seen in WT day 1 uteri under the influence of preovulatory estrogen. These results suggest that Lif expression is normal in Gp130d/d uteri but becomes variable in Stat3d/d uteri depending on the degree of estrogenic responses.
Figure 6.
Lif Expression Is Variable in Stat3d/d Uteri on Day 4 of Pregnancy. In situ hybridization of Lif in uterine sections on day 4. Positive signals of Lif were observed in Stat3d/d luminal epithelial cells. Le, luminal epithelium; Ge, glandular epithelium; S, stroma.
Luminal epithelia in Gp130d/d and Stat3d/d females are less differentiated under heightened uterine estrogenic responses without altered ovarian hormone levels
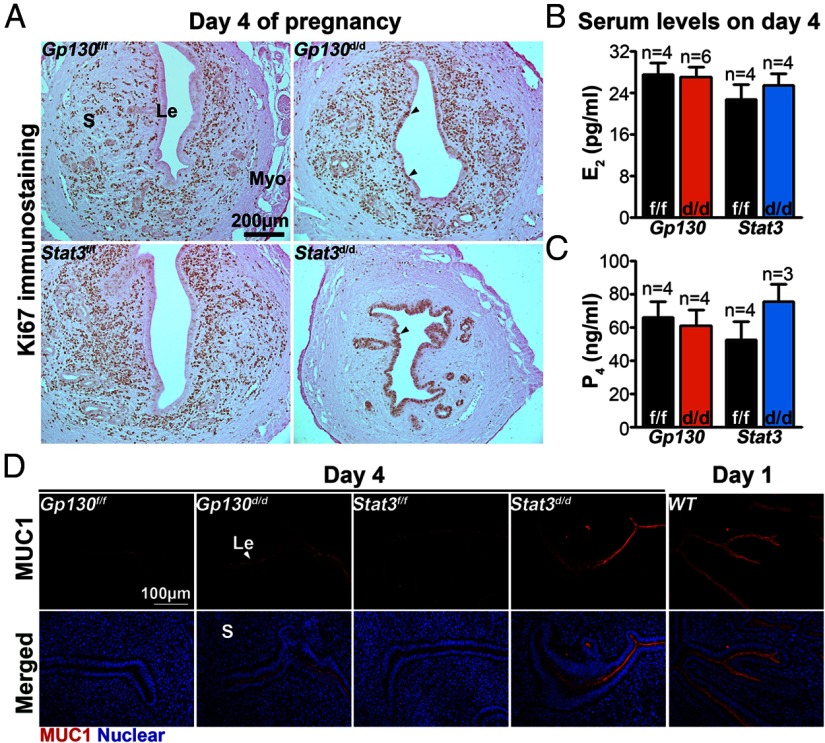
Successful implantation requires the uterus to attain the receptive phase. In mice, differentiation of the luminal epithelium after they have been primed with P4 for 2 days prepares the uterus to accommodate blastocysts for implantation if exposed to a small amount of estrogen (1). One distinct characteristic of the luminal epithelial cells on day 4 of pregnancy is that they cease proliferating and undergo differentiation. We examined the status of uterine cell proliferation by Ki67 immunostaining on day 4. In Gp130d/d uteri, the number of Ki67-positive epithelial cells was significantly more than that of Gp130f/f epithelial cells (Figure 7A). In Stat3d/d uteri, most, if not all, epithelial cells were Ki67 positive, whereas Ki67-positive cells were sparse in the stroma, indicating impaired epithelial cell differentiation and stromal cell proliferation in these deleted females (Figure 7A). These results provide evidence that Gp130 or Stat3 deficiency compromises uterine receptivity perhaps due to aberrant epithelial-stromal interactions.
Figure 7.
The Less Differentiated Luminal Epithelia in Gp130d/d and Stat3d/d Females Are Associated with Heightened Uterine Estrogenic Responses without Altered Ovarian Hormone Levels. A, Immunostaining of Ki67 in implantation sites on day 4 of pregnancy. Arrowheads indicate Ki67-positive luminal epithelial cells. B and C, Serum levels of E2 and P4 on day 4. D, Immunostaining of MUC1 (red) at the day 4 implantation sites. Nuclei are stained with Hoechst dye (blue). Le, luminal epithelium; Myo, myometrium; S, stroma.
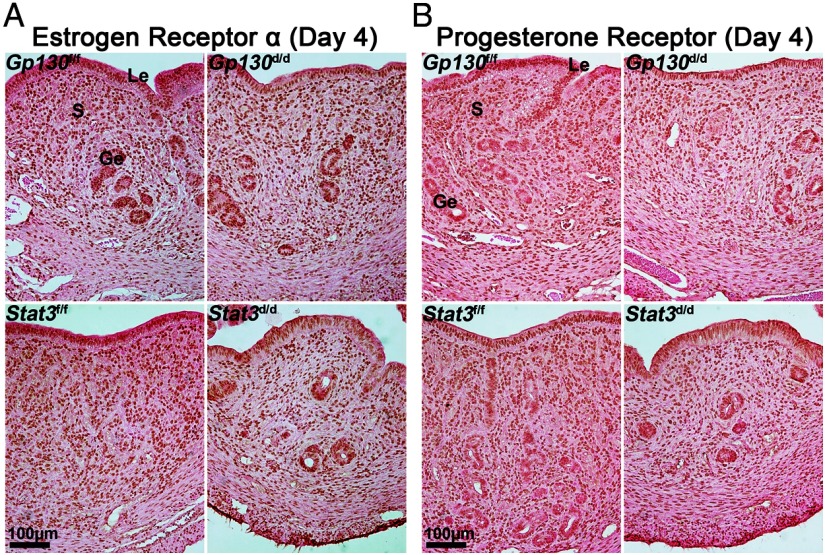
MUC1, which is expressed in the mouse uterine epithelium, is an estrogen-responsive gene (29). Although MUC1 is highly expressed in the uterine epithelium on day 1 of pregnancy, its expression is down-regulated in the epithelium with the attainment of uterine receptivity on day 4 (29). We examined MUC1 localization by immunofluorescence in day 4 uterine sections and found that MUC1 protein highly decorated the apical membrane of the luminal epithelium in both Gp130d/d and Stat3d/d uteri as opposed to Gp130f/f and Stat3f/f uteri (Figure 7D). The up-regulated MUC1 is thought to provide a barrier for blastocyst attachment with the luminal epithelium. Because MUC1 is induced in the uterus under the influence of preovulatory ovarian estrogen on day 1 of pregnancy (30), our observation of increased estrogenic responses in Gp130d/d and Stat3d/d deleted uteri is consistent with the result of MUC1 expression. These results would suggest that either ovarian hormone levels or uterine responsiveness to steroid hormones, or both, are aberrant in pregnant Gp130d/d and Stat3d/d females. Although it is possible that decreased P4 levels and/or increased estrogen levels make Gp130d/d and Stat3d/d uteri more estrogenic, we found serum levels of E2 and P4 in Gp130d/d and Stat3d/d mice to be comparable to those in Gp130f/f and Stat3f/f mice on day 4 (Figure 7, B and C). These results suggest that increased uterine estrogenic responses resulting from uterine deletion of Gp130 or Stat3 are not due to the alteration of ovarian secretion of E2 or P4. The pattern and intensity of expression of estrogen receptor (ESR1) and PGRs in uteri of both deleted and floxed mice on day 4 were also comparable (Figure 8). In conclusion, a functional Gp130-Stat3 signaling pathway is essential for an appropriate uterine estrogen and P4 responsiveness for preparation of the uterus to implantation.
Figure 8.
Gp130d/d and Stat3d/d Mice Have Comparable Expression of ESR1 and PGR on Day 4 Uteri Compared with Those in Gp130f/f and Stat3f/f Females. Le, luminal epithelium; S, stroma; Ge, glandular epithelium.
Discussion
Normally, the uterus differentiates into a receptive state to initiate the attachment of an implantation-competent blastocyst at the antimesometrial side of the uterus in rodents (3). Although several genes including Lif are critical to uterine receptivity and implantation, the precise molecular mechanisms underlying these processes are not fully understood (1). A similar signature of implantation failure in Gp130d/d and Stat3d/d mice, like that in Lif−/− mice, suggests that LIF signaling via Gp130 and Stat3 is indispensable for implantation. As shown here, uterine deletion of Gp130 or Stat3 leads to increased estrogenic responses, precluding uterine differentiation to the receptive state for implantation. Notably, implantation failure in Lif−/− females is not associated with increased uterine estrogenic responses, and LIF injection can rescue implantation in these mutant females (12). Although there is evidence that LIFR is able to function as a homodimer (7), and that blockade of LIFR by a LIFR antagonist (MH35-BD/Q29A+G124R) causes implantation failure (31), our results would suggest that LIF signaling via LIFR alone in the absence of GP130 is not sufficient for inducing implantation in mice.
Lif expression is biphasic in the mouse uterus on day 4 of pregnancy. It is expressed in the glandular epithelium on the morning of day 4 and again in stromal cells at the site of attachment reaction surrounding the blastocyst in the evening of day 4 (16). The overlapping expression of LIFR and GP130 in the luminal epithelia at this time at the site of attachment with Lif expression in the underlying stroma suggests that LIF signaling can be transduced in a paracrine manner by interacting with LIFR-GP130 heterodimers for implantation. The role of Lif in the glands on the morning of day 4 of pregnancy with disparate expression of Lifr and Gp130 is not clearly understood at this time. The discoordinate expression of Gp130 and Lifr in the glandular and luminal epithelial cells on the morning of day 4 would suggest that GP130 has a LIFR-independent role in regulating uterine receptivity. Gp130d/d or Stat3d/d uteri show increased estrogenic responses prior to the anticipated time of implantation on day 4 (the day of uterine receptivity). This could be a factor for observed aberrant expression of Lif in the luminal epithelial cells of Stat3d/d uteri on day 4. These results suggest that the GP130-STAT3 signaling pathway plays a role in preparing the uterus to the receptive state. GP130-STAT3 signaling may also play a role in decidualization, because the binding of IL-11 on IL-11ra further recruits GP130 homodimers, and the deletion of IL11Rα causes decidualization failure (32, 33). However, Gp130d/d or Stat3d/d mouse models, as described here, are not suitable for exploring this role due to the failure of blastocyst attachment prior to the initiation of decidualization.
Stat3d/d uteri are more edematous and they show more branching of the luminal epithelium on day 4, suggesting that the microenvironment in these deleted uteri is more hostile than Gp130d/d uteri as evident from the recovery of fewer healthy blastocysts on day 4 (Figure 3B). Moreover, no blastocysts were recovered from 61% of plug-positive Stat3d/d females (11 of 18 mice examined) on day 4 of pregnancy as opposed to only 2 of 12 Stat3f/f females that failed to yield any blastocysts. It is not clear why some Stat3d/d uteri have histologic characteristics that resemble the phenotype in WT mice treated with estrogen alone (34) or of day 1 WT uteri. Because STAT3 physically interacts with PGR (35) and functions as a coactivator of PGR (36), it is possible that PGR responses are dampened in uteri missing Stat3, thus manifesting heightened estrogenic responses (37). It is also possible that not all P4-responsive genes in the uterus behave in a similar manner in Gp130- and Stat3-deficient mice. Indeed, whereas the expression of Areg was down-regulated, the expression of Hdc was not much altered in Gp130d/d and Stat3d/d uteri as compared with the respective floxed uteri. This interesting finding again reinforces that STAT3 serving as a “signaling hub” confers differential gene regulation in the uterus and directs multiple uterine functions that are not currently fully appreciated. Taken together, these results suggest that certain aspects of P4 effects are mediated by STAT3. Further studies are required to address these issues. Estrogen is critical in determining the duration of the implantation window. The window of uterine receptivity remains open for a longer duration at lower estrogen levels (38), and increased uterine sensitivity to estrogen disrupts implantation (39). Thus, our observation of increased estrogenic responses in Gp130d/d and Stat3d/d uteri may be a cause for implantation failure in these mice.
As mentioned earlier, a very recent report also shows implantation failure in mice with uterine inactivation of Stat3. This study found that implantation failure is due to reduced P4 signaling in Stat3d/d uteri, resulting from the reduction in PGR-positive stromal cells (24). However, we found that whereas estrogen-responsive genes are up-regulated in Stat3d/d uterine epithelial cells, not all P4-responsive genes were down-regulated in the Stat3d/d uteri. Although both studies suggest that an appropriate balance required for epithelial-stromal interactions with respect to steroid hormone action in the uterus is dysregulated in the absence of Gp130 and Stat3, our results show that the reduced P4 responsiveness is not due to the changes in P4 or PGR levels. The discrepancy between the 2 studies could be due to the use of different diets and effects of seasonal variations depending on the geographic locations, impacting early pregnancy effects (40). Collectively, the findings indicate that the GP130-STAT3 signaling pathway regulating ESR1 and PGR is more complex than presumed.
We cannot preclude the possibility of STAT3 mediating other signaling pathways in addition to the Gp130 pathway, because STAT3 serves as a signaling hub for many molecules engaged in diverse cell-signaling pathways (41). ChIP-Seq experiments using STAT3 antibodies suggested STAT3 interacts with many proteins critical for implantation and decidualization (42, 43), including Sgk1 (44), Msx1 (25), Adm (45), and Bmp2/Bmpr1a (46). Therefore, the inactivation of STAT3 in the uterus is likely to adversely impact female fertility via multiple pathways.
The presence of LIF, LIFR, and GP130 in the human endometrium during the secretory phase suggests the importance of LIF-LIFR-GP130 signaling in human pregnancy. Maximal LIF levels are detected in the secretory endometria when implantation normally occurs in humans (47, 48) with coexpression of LIFR and GP130 in the luminal epithelium during the same period (47). Therefore, this study may have high clinical relevance. In addition, persistent and excessive uterine fluid, as found in many Stat3d/d uteri, is detrimental to human implantation. The pregnancy rate is much lower in women with uterine fluid accumulation compared with women with no fluid accumulation following in vitro fertilization (49). Our study presents another signaling pathway of interest to be considered for treating excessive uterine fluid accumulation in human infertility.
Acknowledgments
We thank Serenity Curtis for editing the manuscript, and John Lydon and Francesco DeMayo (Baylor College of Medicine, Houston, Texas) for initially providing us with PgrCre/+ mice. We also thank Takiko Daikoku and Jeeyeon Cha for their help in the mouse breeding strategy, and Poornima Chandran for her help during her short-term graduate training (Cincinnati Children's Hospital Medical Center, Cincinnati, Ohio).
This work was supported by grants from National Institutes of Health (HD12304 and HD068524; to S.K.D.) and the March of Dimes. X.S. is supported by a Lalor Foundation postdoctoral fellowship.
Disclosure Summary: The authors have nothing to disclose.
Footnotes
- E2
- 17β-estradiol
- ESR1
- estrogen receptor-α
- GP130
- glycoprotein 130
- LIF
- leukemia inhibitory factor
- LIFR
- LIF receptor
- MUC1
- mucin 1
- OSM
- oncostatin M
- P4
- progesterone
- PGR
- progesterone receptor
- STAT
- signal transducer and activator of transcription
- WT
- wild type.
References
- 1. Wang H, Dey SK. Roadmap to embryo implantation: clues from mouse models. Nat RevGenet. 2006;7:185–199 [DOI] [PubMed] [Google Scholar]
- 2. Stewart CL, Kaspar P, Brunet LJ, et al. Blastocyst implantation depends on maternal expression of leukaemia inhibitory factor. Nature. 1992;359:76–79 [DOI] [PubMed] [Google Scholar]
- 3. Cha J, Sun X, Dey SK. Mechanisms of implantation: strategies for successful pregnancy. Nat Med. 2012;18:1754–1767 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4. Taga T, Kishimoto T. Gp130 and the interleukin-6 family of cytokines. Annu Rev Immunol. 1997;15:797–819 [DOI] [PubMed] [Google Scholar]
- 5. Kishimoto T, Taga T, Akira S. Cytokine signal transduction. Cell. 1994;76:253–262 [DOI] [PubMed] [Google Scholar]
- 6. Starr R, Novak U, Willson TA, et al. Distinct roles for leukemia inhibitory factor receptor alpha-chain and gp130 in cell type-specific signal transduction. J Biol Chem. 1997;272:19982–19986 [DOI] [PubMed] [Google Scholar]
- 7. Tomida M, Heike T, Yokota T. Cytoplasmic domains of the leukemia inhibitory factor receptor required for STAT3 activation, differentiation, and growth arrest of myeloid leukemic cells. Blood. 1999;93:1934–1941 [PubMed] [Google Scholar]
- 8. Heinrich PC, Behrmann I, Muller-Newen G, Schaper F, Graeve L. Interleukin-6-type cytokine signalling through the gp130/Jak/STAT pathway. Biochem J. 1998;334 (Pt 2):297–314 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9. Fukada T, Hibi M, Yamanaka Y, et al. Two signals are necessary for cell proliferation induced by a cytokine receptor gp130: involvement of STAT3 in anti-apoptosis. Immunity. 1996;5:449–460 [DOI] [PubMed] [Google Scholar]
- 10. Stahl N, Farruggella TJ, Boulton TG, Zhong Z, Darnell JE, Jr., Yancopoulos GD. Choice of STATs and other substrates specified by modular tyrosine-based motifs in cytokine receptors. Science. 1995;267:1349–1353 [DOI] [PubMed] [Google Scholar]
- 11. Ohtani T, Ishihara K, Atsumi T, et al. Dissection of signaling cascades through gp130 in vivo: reciprocal roles for STAT3- and SHP2-mediated signals in immune responses. Immunity. 2000;12:95–105 [DOI] [PubMed] [Google Scholar]
- 12. Song H, Lim H, Das SK, Paria BC, Dey SK. Dysregulation of EGF family of growth factors and COX-2 in the uterus during the preattachment and attachment reactions of the blastocyst with the luminal epithelium correlates with implantation failure in LIF-deficient mice. Mol Endocrinol. 2000;14:1147–1161 [DOI] [PubMed] [Google Scholar]
- 13. Ware CB, Horowitz MC, Renshaw BR, et al. Targeted disruption of the low-affinity leukemia inhibitory factor receptor gene causes placental, skeletal, neural and metabolic defects and results in perinatal death. Development. 1995;121:1283–1299 [DOI] [PubMed] [Google Scholar]
- 14. Yoshida K, Taga T, Saito M, et al. Targeted disruption of gp130, a common signal transducer for the interleukin 6 family of cytokines, leads to myocardial and hematological disorders. Proc Natl Acad Sci USA. 1996;93:407–411 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15. Ernst M, Inglese M, Waring P, et al. Defective gp130-mediated signal transducer and activator of transcription (STAT) signaling results in degenerative joint disease, gastrointestinal ulceration, and failure of uterine implantation. J Exp Med. 2001;194:189–203 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16. Song H, Lim H. Evidence for heterodimeric association of leukemia inhibitory factor (LIF) receptor and gp130 in the mouse uterus for LIF signaling during blastocyst implantation. Reproduction. 2006;131:341–349 [DOI] [PubMed] [Google Scholar]
- 17. Takeda K, Noguchi K, Shi W, et al. Targeted disruption of the mouse Stat3 gene leads to early embryonic lethality. Proc Natl Acad Sci USA. 1997;94:3801–3804 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 18. Betz UA, Bloch W, van den Broek M, et al. Postnatally induced inactivation of gp130 in mice results in neurological, cardiac, hematopoietic, immunological, hepatic, and pulmonary defects. J Exp Med. 1998;188:1955–1965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19. Takeda K, Kaisho T, Yoshida N, Takeda J, Kishimoto T, Akira S. Stat3 activation is responsible for IL-6-dependent T cell proliferation through preventing apoptosis: generation and characterization of T cell-specific Stat3-deficient mice. J Immunol. 1998;161:4652–4660 [PubMed] [Google Scholar]
- 20. Soyal SM, Mukherjee A, Lee KY, et al. Cre-mediated recombination in cell lineages that express the progesterone receptor. Genesis. 2005;41:58–66 [DOI] [PubMed] [Google Scholar]
- 21. Tan J, Paria BC, Dey SK, Das SK. Differential uterine expression of estrogen and progesterone receptors correlates with uterine preparation for implantation and decidualization in the mouse. Endocrinology. 1999;140:5310–5321 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22. Dey SK, Lim H. Implantation. In: Neill JD, ed. Knobil and Neill's Physiology of Reproduction. 3rd ed New York, NY: Elsevier; 2005:147–188 [Google Scholar]
- 23. Psychoyos A. Endocrine Control of Egg Implantation. Washington, DC: American Physiology Society; 1973 [Google Scholar]
- 24. Lee JH, Kim TH, OH SJ, et al. Signal transducer and activator of transcription-3 (Stat3) plays a critical role in implantation via progesterone receptor in uterus. FASEB J. 2013; [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25. Daikoku T, Cha J, Sun X, et al. Conditional deletion of Msx homeobox genes in the uterus inhibits blastocyst implantation by altering uterine receptivity. Dev cell. 2011;21:1014–1025 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 26. Ma L, Benson GV, Lim H, Dey SK, Maas RL. Abdominal B (AbdB) Hoxa genes: regulation in adult uterus by estrogen and progesterone and repression in müllerian duct by the synthetic estrogen diethylstilbestrol (DES). Dev Biol. 1998;197:141–154 [DOI] [PubMed] [Google Scholar]
- 27. Das SK, Chakraborty I, Paria BC, Wang XN, Plowman G, Dey SK. Amphiregulin is an implantation-specific and progesterone-regulated gene in the mouse uterus. Mol Endocrinol. 1995;9:691–705 [DOI] [PubMed] [Google Scholar]
- 28. Paria BC, Das N, Das SK, Zhao X, Dileepan KN, Dey SK. Histidine decarboxylase gene in the mouse uterus is regulated by progesterone and correlates with uterine differentiation for blastocyst implantation. Endocrinology. 1998;139:3958–3966 [DOI] [PubMed] [Google Scholar]
- 29. DeSouza MM, Surveyor GA, Price RE, et al. MUC1/episialin: a critical barrier in the female reproductive tract. J Reprod Immunol. 1999;45:127–158 [DOI] [PubMed] [Google Scholar]
- 30. DeSouza MM, Mani SK, Julian J, Carson DD. Reduction of mucin-1 expression during the receptive phase in the rat uterus. Biol Reprod. 1998;58:1503–1507 [DOI] [PubMed] [Google Scholar]
- 31. White CA, Zhang JG, Salamonsen LA, et al. Blocking LIF action in the uterus by using a PEGylated antagonist prevents implantation: a nonhormonal contraceptive strategy. Proc Natl Acad Sci USA. 2007;104:19357–19362 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 32. Bilinski P, Roopenian D, Gossler A. Maternal IL-11Ralpha function is required for normal decidua and fetoplacental development in mice. Genes Dev. 1998;12:2234–2243 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 33. Robb L, Li R, Hartley L, Nandurkar HH, Koentgen F, Begley CG. Infertility in female mice lacking the receptor for interleukin 11 is due to a defective uterine response to implantation. Nat Med. 1998;4:303–308 [DOI] [PubMed] [Google Scholar]
- 34. Martin L, Finn CA, Trinder G. Hypertrophy and hyperplasia in the mouse uterus after oestrogen treatment: an autoradiographic study. J Endocrinol. 1973;56:133–144 [DOI] [PubMed] [Google Scholar]
- 35. Liu T, Ogle TF. Signal transducer and activator of transcription 3 is expressed in the decidualized mesometrium of pregnancy and associates with the progesterone receptor through protein-protein interactions. Biol Reprod. 2002;67:114–118 [DOI] [PubMed] [Google Scholar]
- 36. Proietti CJ, Béguelin W, Flaqué MC, et al. Novel role of signal transducer and activator of transcription 3 as a progesterone receptor coactivator in breast cancer. Steroids. 2011;76:381–392 [DOI] [PubMed] [Google Scholar]
- 37. Lydon JP, DeMayo FJ, Funk CR, et al. Mice lacking progesterone receptor exhibit pleiotropic reproductive abnormalities. Genes Dev. 1995;9:2266–2278 [DOI] [PubMed] [Google Scholar]
- 38. Ma WG, Song H, Das SK, Paria BC, Dey SK. Estrogen is a critical determinant that specifies the duration of the window of uterine receptivity for implantation. Proc Natl Acad Sci USA. 2003;100:2963–2968 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 39. Kawagoe J, Li Q, Mussi P, et al. Nuclear receptor coactivator-6 attenuates uterine estrogen sensitivity to permit embryo implantation. Dev Cell. 2012;23:858–865 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 40. Wang H, Tranguch S, Xie H, Hanley G, Das SK, Dey SK. Variation in commercial rodent diets induces disparate molecular and physiological changes in the mouse uterus. Proc Natl Acad Sci USA. 2005;102:9960–9965 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 41. Bromberg J, Darnell JE., Jr The role of STATs in transcriptional control and their impact on cellular function. Oncogene. 2000;19:2468–2473 [DOI] [PubMed] [Google Scholar]
- 42. Hutchins AP, Poulain S, Miranda-Saavedra D. Genome-wide analysis of STAT3 binding in vivo predicts effectors of the anti-inflammatory response in macrophages. Blood. 2012;119:e110–119 [DOI] [PubMed] [Google Scholar]
- 43. Zhang JX, Zhang J, Yan W, et al. Unique genome-wide map of TCF4 and STAT3 targets using ChIP-seq reveals their association with new molecular subtypes of glioblastoma. Neuro Oncol. 2013;15:279–289 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44. Salker MS, Christian M, Steel JH, et al. Deregulation of the serum- and glucocorticoid-inducible kinase SGK1 in the endometrium causes reproductive failure. Nat Med. 2011;17:1509–1513 [DOI] [PubMed] [Google Scholar]
- 45. Li M, Yee D, Magnuson TR, Smithies O, Caron KM. Reduced maternal expression of adrenomedullin disrupts fertility, placentation, and fetal growth in mice. J Clin Invest. 2006;116:2653–2662 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46. Lee KY, Jeong JW, Wang J, et al. Bmp2 is critical for the murine uterine decidual response. Mol Cell Biol. 2007;27:5468–5478 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 47. Cullinan EB, Abbondanzo SJ, Anderson PS, Pollard JW, Lessey BA, Stewart CL. Leukemia inhibitory factor (LIF) and LIF receptor expression in human endometrium suggests a potential autocrine/paracrine function in regulating embryo implantation. Proc Natl Acad Sci USA. 1996;93:3115–3120 [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48. Vogiagis D, Marsh MM, Fry RC, Salamonsen LA. Leukaemia inhibitory factor in human endometrium throughout the menstrual cycle. J Endocrinol. 1996;148:95–102 [DOI] [PubMed] [Google Scholar]
- 49. Chien LW, Au HK, Xiao J, Tzeng CR. Fluid accumulation within the uterine cavity reduces pregnancy rates in women undergoing IVF. Hum Reprod. 2002;17:351–356 [DOI] [PubMed] [Google Scholar]