Abstract
AIM: To examine if fulminant Clostridium difficile infections (CDI) resulting in colectomy was associated with a prior appendectomy and whether any association affected the severity of the disease.
METHODS: A retrospective chart review was performed on patients who underwent colectomy for CDI between 2001 and 2011. The appendectomy rate was calculated based on the absence of an appendix on the surgical pathology report. This was compared to an established lifetime risk of appendectomy in the general population. A chart review was performed for mortality and traditional markers of CDI disease severity. Fisher’s exact test was used to calculate the likelihood of association between prior appendectomy, mortality, and clinical markers of severity of infection.
RESULTS: Fifty-five specimens were identified with pseudomembranous colitis consistent with CDI. All patients had a clinical history consistent with CDI and 45 of 55 (81.8%) specimens also had microbiological confirmation of CDI. Appendectomy was observed in 24 of 55 specimens (0.436, 99%CI: 0.280-0.606). This was compared to the lifetime incidence of appendectomy of 17.6%. The rate of appendectomy in our sample was significantly higher than would be expected in the general population (43.6% vs 17.6%, P < 0.01). Disease severity did not differ based on presence or absence of an appendix and no association was detected between prior appendectomy and mortality (OR = 0.588, 95%CI: 0.174-1.970).
CONCLUSION: The rate of appendectomy in the patients whose CDI led to colectomy, was significantly higher than the calculated lifetime risk, suggesting an association of appendectomy and severe CDI resulting in colectomy. Larger prospective studies are needed to assess any potential causal relationships affecting fulminant CDI.
Keywords: Appendectomy, Fulminant colitis, Clostridium difficile
Core tip: We demonstrated a significant relationship between fulminant Clostridium difficile infections and previous appendectomy. Early surgical management of at risk patients might improve outcomes and further studies can hopefully explore the role of appendectomy on chronic colonic colonization and future infection risks.
INTRODUCTION
The appendix has long been considered a vestigial organ. However, comparative analysis of primate anatomy shows that the appendix may have developed independently among different species and has been maintained over time[1]. The exact nature of any function or clinical implications of removal of the appendix is still relatively unknown.
Examination of biofilms in the colons of deceased organ donors demonstrates that the appendix has the highest density of microbes and this density progressively decreases in the bowel distally toward the rectum[2]. For this reason it is thought that the appendix may serve as a “safe house” for flora of the gut. Specifically, in infections of the large bowel, the appendix may potentially serve to re-inoculate the colon with normal flora as a defense mechanism against infection.
A similar mechanism may be seen in exposure and recovery from Clostridium difficile infection (CDI). The normal flora of the bowel is altered secondary to antibiotics allowing an opportunity for Clostridium difficile to overgrow in the colon[3,4]. Therefore, CDI represents a disruption in colonic flora without resolution. Fulminant CDI represents a refractory disruption of the colonic microbiology and physiology and can require surgical intervention.
CDI is clinically important as an increasing phenomenon in the US and other industrialized nations[5,6]. Infection is associated with increased hospital length of stay, total charges, and mortality rate among hospitalized patients[7,8]. The clinical presentation of infected patients is highly variable, ranging from mild diarrhea to fulminant colitis[9]. Clinical severity scores have been created in order to determine severity with mixed results[10]. Additionally, nosocomial spread of CDI can also occur, but is often asymptomatic[11].
CDI involving the appendix is exceedingly rare[12,13]. Additionally, the presence of an appendix has been preliminarily demonstrated to be inversely associated with CDI recurrence[14]. However, additional studies have shown no consequence or even a harm from an intact appendix when concerning development or relapse of CDI[15,16]. It is our belief that an intact appendix may allow for quicker recovery of colonic flora after antibiotic administration and potentially “protect against” the more severe disease. Based on these recent findings, our intent was to assess our own experience for a potential association between prior appendectomy and CDI requiring colectomy.
MATERIALS AND METHODS
After obtaining Institutional Review Board approval, a review of the Department of Pathology’s database of pathological colon resection specimens was performed. A search of all the surgical pathology specimens from January 1, 2000 to January 1, 2012 at Summa Akron City Hospital in Akron, Ohio was conducted using SoftPath v 4.3. All pathological specimens of colectomies during the time period that demonstrated pseudomembranes were initially included. Seventy-three cases were suitable for chart review based on examination of final diagnosis and gross description. The pathology reports of the selected cases were reviewed for the presence or absence of an appendix, ischemia, and perforation.
A total of 73 specimens were initially identified with the presence of pseudomembranes. All of the specimens were colectomy specimens that included the cecum (total, subtotal, or partial colectomy). The choice as well as timing of surgery was made by the surgeon based on each patient’s individual clinical picture. The pathology reports were reviewed and the presence or absence of an appendix in each specimen was recorded. Seven cases were excluded because they did not specifically mention either the presence or absence of an appendix in the specimen.
A retrospective chart review of the included cases was performed in order to identify and record patient characteristics and traditional markers of severity of infection. Patient characteristics included age and sex only. Severity markers included white blood cell count, lactic acid level, tachycardia (defined as heart rate greater than 100), hypotension (systolic blood pressure less than 80) fever (maximum temperature > 101.5 oF), abdominal pain, diarrhea, or the need for vasopressor support. Thirty-day mortality was also recorded for each patient by electronic chart review.
The charts were then reviewed for a microbiological confirmation of the diagnosis of CDI. The early confirmatory test of choice was a stool enzyme immuno-assay (EIA) for Clostridium difficile toxin A and B. Late in the study period, in 2011, the test at the study hospital was changed to a more sensitive molecular polymerase chain reaction test.
After the chart review, four cases were excluded because the diagnosis was confirmed to be something other than CDI. Seven additional cases were excluded because they had a negative microbiological test for CDI, all by EIA testing. Ten cases were retained in the sample population because although clinical history was consistent with CDI, due to patient’s rapidly progressive course no testing of CDI was performed before surgery. A clinical history of CDI included both the presence of diarrhea or abdominal pain and a recent history (within the last 30 d) of antibiotic use. Fifty-five cases with pseudomembranes present on pathology plus a clinical history of CDI were included in the initial analysis. Of these 55, 45 patients had preoperative microbiologic confirmation of CDI.
As a cohort, the study sample was compared to the appendectomy rate in the general population. This rate was obtained using the National Hospital Discharge Survey (NHDS). This dataset is maintained by the Centers for Disease Control and Prevention (CDC) for the evaluation of national trends of disease processes and procedures. Greater than 200000 charts from hundreds of hospitals are reviewed to generate a nationally representative sample of hospital discharges. The NHDS is the longest continuously running survey of hospital utilization, collecting data since 1965. The rate of appendectomy as published in the Journal of Epidemiology in 1990 was initially used[17]. Current NHDS data from 2007-2010 was reviewed to determine any changes in the rate of appendectomy in the population over time.
For analysis, 95% and 99% confidence intervals for the proportion of appendectomy in the sample population were calculated using a modified Wald method. This was then compared to the calculated lifetime risk of appendectomy from the NHDS data, both as a group and divided individually by gender. Fisher’s exact test and Student’s t-test were also used to determine whether there were independent associations between history of appendectomy and all markers of severity including thirty-day mortality. Fisher’s exact test was used for discrete variables while a Student’s t-test was used for continuous variables.
RESULTS
Fifty-five pathological specimens were identified with pseudomembranous colitis consistent with CDI. All patients had a clinical history consistent with CDI and 82% (45/55) had microbiological confirmation of CDI. In the sample population, 44% (24/55) of patients had a surgically absent appendix noted on pathology (95%CI: 0.314-0.567, 99%CI: 0.280-0.606). There were 27 male specimens and 28 female specimens. Divided by gender, 41% of the observed male specimens had a documented prior appendectomy (95%CI: 0.245-0.593, 99%CI: 0.206-0.645), while 46% of the female specimens had a documented prior appendectomy (95%CI: 0.295-0.642, 99%CI: 0.253-0.690) (Figure 1).
Figure 1.
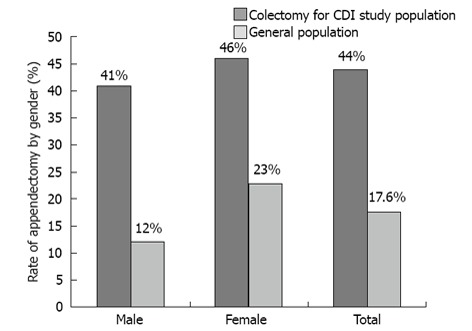
Rate of appendectomy by gender. CDI: Clostridium difficile infection.
All patients had undergone total or subtotal colectomies except for two patients who had partial right colectomies performed. The description of pseudomembranes ranged from focal and patchy to extensive and diffuse. Ischemia was present in 51% (28/55) of specimens. Perforation was noted in only one pathology specimen, with a prior appendectomy.
The lifetime risk of appendectomy from the NHDS is 17.6% overall (12.0% for males and 23.1% for females)[17]. A standard deviation was determined to be 0.003625583% due to the large sample size of the data.
The 95% and 99% confidence intervals of appendectomy rate for the CDI colectomy group were compared to the confidence interval for the rate in the general population. There was a statistically significant difference in the rate of appendectomy between the sample group and the overall population (P < 0.01). This finding was consistent when the sample group was divided by gender (P < 0.01).
A separate analysis was performed excluding all patients who did not have a confirmatory microbiological test for CDI (leaving 45 total patients). Evaluating only the patients with pseudomembranes on pathology, a clinical history of CDI, and a positive microbiological test for CDI yielded similar results. Forty-nine percent (22/45 cases) had a prior appendectomy (95%CI: 0.350-0.630, 99%CI: 0.311-0.670). Using the above method, this also yielded a statistically significant difference (P < 0.01).
There was a mortality rate of 49% (27/55) in our sample population. There was no association between mortality and prior appendectomy (OR = 0.588, 95%CI: 0.174-1.970). Presence of ischemia had no correlation with prior appendectomy (OR = 0.666, P = 0.7245). Furthermore, there were no statistically significant associations for any markers of clinical severity and appendectomy (Table 1).
Table 1.
Characteristics of and severity of illness within the Clostridium difficile infection colectomy group
| Appendix absent | Appendix present | P-value | |
| (n = 24) | (n =31) | ||
| Age (yr), mean ± SD, (range) | 78.4 ± 6.6 | 72.3 ± 13.9 | 0.051 |
| (60-96) | (36-90) | ||
| Sex (male) | 46.00% | 52.00% | 0.788 |
| Mortality | 42.00% | 55.00% | 0.418 |
| Vasopressor use | 37.50% | 42.00% | 0.787 |
| WBC (thou/cm2) (pre-op) | 34.70% | 37.70% | 0.647 |
| Lactate (mmol/L) (72 h max) | 2.6 | 3.3 | 0.192 |
| Diarrhea | 58% | 65% | 0.781 |
| Abdominal pain | 83% | 68% | 0.226 |
CDI: Clostridium difficile infection; WBC: White blood cells.
DISCUSSION
Our study demonstrated a significant difference in the rate of appendectomy in patients with fulminant CDI resulting in colectomy compared to the general population. The national incidence of appendectomy was far less than our sample’s appendectomy rate associated with fulminant CDI. This remained true when our sample group was examined by gender as well. While a statistically significant association between incidence of appendectomy and CDI was seen, appendectomy did not appear to influence either mortality or severity of CDI.
Recent evidence has suggested an association between CDI and appendectomy[14,15]. However, these studies have been limited by participant accuracy and recall bias when conducted by personal interviews. They may also be confounded by low response rates. A strength of this study is the accurate and precise determination of prior appendectomy based on pathologic specimens. To maximize accuracy, only pathology specimens in which the appendix was noted as present or absent were used to determine the rate of previous appendectomy in our study population. In addition, there was also a strict definition of CDI in this study, which included both a clinical history of CDI and histopathologic confirmation of pseudomembranes on a pathology report. Although the majority of the patients (45/55) had microbiological confirmation, a sub analysis was performed using even stricter criteria - clinical history, pseudomembranes, and microbiological confirmation - which demonstrated similarly significant results.
Although the pathology specimens were initially examined for pseudomembranous colitis, it alone cannot be considered pathognomonic for CDI. Clostridium difficile toxin B has been isolated from > 95% of cases of pseudomembranous colitis colons themselves[18]. However, pseudomembranous colitis is merely a descriptive diagnosis that can still be easily confused with other forms of infectious or ischemic colitis and ultimately relies upon concurrent clinical and/or microbiological confirmation of CDI, as per our inclusion criteria[19].
Given the nature of our sample population, the ability to have a reliable control group is very limited. Our strict use of pathologic specimens makes a control group especially difficult. We considered different methods such as including a random sample of colectomy specimens as a cohort. However, a random sample of colectomy specimens would be a poor representation of the general population and may have confounding factors given the specific indications for colectomy. Therefore, we desired to compare the rate of appendectomy in the study population of patients requiring colectomy for fulminant CDI to the appendectomy rate found in the general public. With this small sample size, strikingly different rates of appendectomy led to significant results in our cohort.
The rate of appendectomy in the general population was obtained using the NHDS. The use of this data for analysis of national trends and comparative groups has been well substantiated in the literature[20-23]. This data provided an appropriate control group comparable to other commonly reviewed large sources of national disease and procedure incidence[24].
We initially reviewed the published data on appendectomy rates from 1990 as well as current NHDS data available considering widespread use of CT scanning and laparoscopy that may have affected the appendectomy rate over time. A decrease in the national number of appendectomies per year was noted from 1990 to 2010, despite an increasing population[25]. Our analysis used the originally published lifetime appendectomy rate of 17.6% from 1990. Had we used only current data, our results comparing appendectomy rate would be even far more significant.
The possibility that the appendix may play a role in Clostridium difficile infection has important clinical implications; primarily as a CDI risk stratification tool. Patients already at an increased risk for developing CDI (i.e., excessive antibiotic exposure, previous history of CDI, etc.) are often monitored closely for signs and symptoms of CDI. If patients can be better categorized into a “high risk” group based on a history of appendectomy, this may result in overall better patient care. However, this may need to be substantiated with a larger number of patients. The patients in our sample had a mortality rate consistent with other published studies[26]. Patients were chosen for operative intervention primarily based on age, associated comorbidities, vasopressor requirements, and ominous laboratory or radiographic findings, consistent with current clinical practice guidelines[27]. In the future, the initial detection of appropriate risk factors can allow for early intervention in CDI, which may positively impact outcome.
Once a commonplace practice, this data may add a new wrinkle to the indifferent performance of the incidental appendectomy during another unrelated surgical procedure[28]. In fact, according to data from the CDC there were still an estimated 30000 to 44000 incidental appendectomies performed each year from 2007 to 2010[25]. If the appendix is seen as a protective organ, an argument could be made for reducing or eliminating incidental appendectomies altogether. Taken a step further, there may be another reason for preservation of the appendix after appendicitis, as is already being practiced in some parts of Europe[29].
There are several limitations to this study. Differences in severity or mortality may be observed with a larger sample size or when non-surgical CDI patients are also included as part of the study population, i.e., those who may be severely ill but do not require colectomy. The data collected was only from a single center. The patients who undergo colectomy represent a small minority of those diagnosed with CDI. The decision for surgical intervention was made individually by the surgeon based on each patient’s clinical picture. Potential risk factors previously linked to CDI as well as previous treatment prior to colectomy were also not individually addressed in this study, as there was not appropriate power to detect any individual effects. A distinction between initial versus recurrent CDI was not able to be collected due to the study design, although if the appendix has an effect on incidence, it would likely similarly affect relapse in the same way. Additionally, age at appendectomy and potential analysis was impossible to determine due to study design. Also, though there is a known variability in the incidence of appendicitis and appendectomy among gender, there was an approximately even gender distribution in our groups of patients with and without an appendix.
In conclusion, there was a statistically significant association between prior appendectomy and fulminant CDI resulting in colectomy compared to the general population. This association was present among those patients with simply a clinical history of CDI and with microbiological confirmatory testing. In the study population, mortality and traditional markers of severity were not associated with prior appendectomy. Our preliminary data supports that appendectomy may place patients at risk for developing fulminant CDI. However, larger prospective studies are needed to elucidate causal relationships. Future prospective studies could include patients with varying degrees of illness.
ACKNOWLEDGEMENTS
We would like to thank Drs. Richard George, Michael Tan, Karen Gil, Sharon Hull, and Julie Mangino for review and assistance with the manuscript.
COMMENTS
Background
The appendix has long been considered a vestigial organ. However, comparative analysis of primate anatomy shows that the appendix may have developed independently among different species and has been maintained over time. The exact nature of any function or clinical implications of removal of the appendix is still relatively unknown.
Research frontiers
A retrospective chart review was performed on patients who underwent colectomy for Clostridium difficile infections (CDI) between 2001 and 2011. The appendectomy rate was calculated based on the absence of an appendix on the surgical pathology report. This was compared to an established lifetime risk of appendectomy in the general population. A chart review was performed for mortality and traditional markers of CDI disease severity. Fisher’s exact test was used to calculate the likelihood of association between prior appendectomy, mortality, and clinical markers of severity of infection.
Innovations and breakthroughs
Fifty-five specimens were identified with pseudomembranous colitis consistent with CDI. All patients had a clinical history consistent with CDI and 45 of 55 specimens also had microbiological confirmation of CDI. Appendectomy was observed in 24 of 55 specimens. This was compared to the lifetime incidence of appendectomy of 17.6%. The rate of appendectomy in our sample was significantly higher than would be expected in the general population. Disease severity did not differ based on presence or absence of an appendix and no association was detected between prior appendectomy and mortality.
Applications
The authors found that the rate of appendectomy in the patients whose CDI led to colectomy, was significantly higher than the calculated lifetime risk, suggesting an association of appendectomy and severe CDI resulting in colectomy. Larger prospective studies are needed to assess any potential causal relationships affecting fulminant CDI.
Peer review
This is a well written and unique designed paper in a subject which is of interest to all.
Footnotes
P- Reviewers Amin AI, Burdette SD, Girotra M S- Editor Gou SX L- Editor A E- Editor Lu YJ
References
- 1.Smith HF, Fisher RE, Everett ML, Thomas AD, Bollinger RR, Parker W. Comparative anatomy and phylogenetic distribution of the mammalian cecal appendix. J Evol Biol. 2009;22:1984–1999. doi: 10.1111/j.1420-9101.2009.01809.x. [DOI] [PubMed] [Google Scholar]
- 2.Randal Bollinger R, Barbas AS, Bush EL, Lin SS, Parker W. Biofilms in the large bowel suggest an apparent function of the human vermiform appendix. J Theor Biol. 2007;249:826–831. doi: 10.1016/j.jtbi.2007.08.032. [DOI] [PubMed] [Google Scholar]
- 3.Borriello SP. The influence of the normal flora on Clostridium difficile colonisation of the gut. Ann Med. 1990;22:61–67. doi: 10.3109/07853899009147244. [DOI] [PubMed] [Google Scholar]
- 4.Tvede M, Rask-Madsen J. Bacteriotherapy for chronic relapsing Clostridium difficile diarrhoea in six patients. Lancet. 1989;1:1156–1160. doi: 10.1016/s0140-6736(89)92749-9. [DOI] [PubMed] [Google Scholar]
- 5.Barbut F, Jones G, Eckert C. Epidemiology and control of Clostridium difficile infections in healthcare settings: an update. Curr Opin Infect Dis. 2011;24:370–376. doi: 10.1097/QCO.0b013e32834748e5. [DOI] [PubMed] [Google Scholar]
- 6.Ricciardi R, Rothenberger DA, Madoff RD, Baxter NN. Increasing prevalence and severity of Clostridium difficile colitis in hospitalized patients in the United States. Arch Surg. 2007;142:624–631; discussion 631. doi: 10.1001/archsurg.142.7.624. [DOI] [PubMed] [Google Scholar]
- 7.Zerey M, Paton BL, Lincourt AE, Gersin KS, Kercher KW, Heniford BT. The burden of Clostridium difficile in surgical patients in the United States. Surg Infect (Larchmt) 2007;8:557–566. doi: 10.1089/sur.2006.062. [DOI] [PubMed] [Google Scholar]
- 8.Lucado J, Gould C, Elixhauser A. Clostridium Difficile Infections (CDI) in Hospital Stays, 2009: Statistical Brief #124. Healthcare Cost and Utilization Project (HCUP) Statistical Briefs [Internet] Rockville (MD): Agency for Health Care Policy and Research (US); 2006-; 2012. p. Jan. [PubMed] [Google Scholar]
- 9.Gonenne J, Pardi DS. Clostridium difficile: an update. Compr Ther. 2004;30:134–140. doi: 10.1007/s12019-004-0009-z. [DOI] [PubMed] [Google Scholar]
- 10.Fujitani S, George WL, Murthy AR. Comparison of clinical severity score indices for Clostridium difficile infection. Infect Control Hosp Epidemiol. 2011;32:220–228. doi: 10.1086/658336. [DOI] [PubMed] [Google Scholar]
- 11.Barbut F, Petit JC. Epidemiology of Clostridium difficile-associated infections. Clin Microbiol Infect. 2001;7:405–410. doi: 10.1046/j.1198-743x.2001.00289.x. [DOI] [PubMed] [Google Scholar]
- 12.Coyne JD, Dervan PA, Haboubi NY. Involvement of the appendix in pseudomembranous colitis. J Clin Pathol. 1997;50:70–71. doi: 10.1136/jcp.50.1.70. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Brown TA, Rajappannair L, Dalton AB, Bandi R, Myers JP, Kefalas CH. Acute appendicitis in the setting of Clostridium difficile colitis: case report and review of the literature. Clin Gastroenterol Hepatol. 2007;5:969–971. doi: 10.1016/j.cgh.2007.04.016. [DOI] [PubMed] [Google Scholar]
- 14.Im GY, Modayil RJ, Lin CT, Geier SJ, Katz DS, Feuerman M, Grendell JH. The appendix may protect against Clostridium difficile recurrence. Clin Gastroenterol Hepatol. 2011;9:1072–1077. doi: 10.1016/j.cgh.2011.06.006. [DOI] [PubMed] [Google Scholar]
- 15.Merchant R, Mower WR, Ourian A, Abrahamian FM, Moran GJ, Krishnadasan A, Talan DA. Association Between Appendectomy and Clostridium difficile Infection. J Clin Med Res. 2012;4:17–19. doi: 10.4021/jocmr770w. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Khanna S, Baddour LM, Dibaise JK, Pardi DS. Appendectomy is not associated with adverse outcomes in clostridium difficile infection: a population-based study. Am J Gastroenterol. 2013;108:626–627. doi: 10.1038/ajg.2012.475. [DOI] [PubMed] [Google Scholar]
- 17.Addiss DG, Shaffer N, Fowler BS, Tauxe RV. The epidemiology of appendicitis and appendectomy in the United States. Am J Epidemiol. 1990;132:910–925. doi: 10.1093/oxfordjournals.aje.a115734. [DOI] [PubMed] [Google Scholar]
- 18.Bartlett JG, Taylor NS, Chang T, Dzink J. Clinical and laboratory observations in Clostridium difficile colitis. Am J Clin Nutr. 1980;33:2521–2526. doi: 10.1093/ajcn/33.11.2521. [DOI] [PubMed] [Google Scholar]
- 19.Odze RD, Goldblum JR. Surgical pathology of the GI Tract, Liver, Biliary Tract, and Pancreas. 2nd ed. Philadelphia: Saunders; 2009. [Google Scholar]
- 20.Ford E, Cooper R, Castaner A, Simmons B, Mar M. Coronary arteriography and coronary bypass survey among whites and other racial groups relative to hospital-based incidence rates for coronary artery disease: findings from NHDS. Am J Public Health. 1989;79:437–440. doi: 10.2105/ajph.79.4.437. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 21.Fryzek JP, Martone WJ, Groothuis JR. Trends in chronologic age and infant respiratory syncytial virus hospitalization: an 8-year cohort study. Adv Ther. 2011;28:195–201. doi: 10.1007/s12325-010-0106-6. [DOI] [PubMed] [Google Scholar]
- 22.Akinbami LJ, Moorman JE, Garbe PL, Sondik EJ. Status of childhood asthma in the United States, 1980-2007. Pediatrics. 2009;123 Suppl 3:S131–S145. doi: 10.1542/peds.2008-2233C. [DOI] [PubMed] [Google Scholar]
- 23.DeFrances CJ, Hall MJ. 2005 National Hospital Discharge Survey. Advance data from vital and health statistics; no 385. Hyattsville, MD: National Center for Health Statistics; 2007. [PubMed] [Google Scholar]
- 24.Gorina Y, Owings M, Elgaddal N, Weeks J. Comparability between the rates for all-listed inpatient procedures using National Hospital Discharge Survey and Medicare claims, 1999 and 2007. National health statistics reports; no 57. Hyattsville, MD: National Center for Health Statistics; 2012. [PubMed] [Google Scholar]
- 25. Available from: http:// www.cdc.gov.
- 26.Bhangu A, Nepogodiev D, Gupta A, Torrance A, Singh P. Systematic review and meta-analysis of outcomes following emergency surgery for Clostridium difficile colitis. Br J Surg. 2012;99:1501–1513. doi: 10.1002/bjs.8868. [DOI] [PubMed] [Google Scholar]
- 27.Kasper AM, Nyazee HA, Yokoe DS, Mayer J, Mangino JE, Khan YM, Hota B, Fraser VJ, Dubberke ER. A multicenter study of Clostridium difficile infection-related colectomy, 2000-2006. Infect Control Hosp Epidemiol. 2012;33:470–476. doi: 10.1086/665318. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Salom EM, Schey D, Peñalver M, Gómez-Marín O, Lambrou N, Almeida Z, Mendez L. The safety of incidental appendectomy at the time of abdominal hysterectomy. Am J Obstet Gynecol. 2003;189:1563–1567; discussion 1563-1567. doi: 10.1016/s0002-9378(03)00936-0. [DOI] [PubMed] [Google Scholar]
- 29.Hansson J, Körner U, Khorram-Manesh A, Solberg A, Lundholm K. Randomized clinical trial of antibiotic therapy versus appendicectomy as primary treatment of acute appendicitis in unselected patients. Br J Surg. 2009;96:473–481. doi: 10.1002/bjs.6482. [DOI] [PubMed] [Google Scholar]


