Abstract
The dairy industry is a multi-billion dollar industry catering the nutritional needs of all age groups globally through the supply of milk. Clinical mastitis has a severe impact on udder tissue and is also an animal welfare issue. Moreover, it significantly reduces animal value and milk production. Mammary tissue damage reduces the number and activity of epithelial cells and consequently contributes to decreased milk production. The high incidence, low cure rate of this highly economic and sometimes deadly disease is an alarming for dairy sector as well as policy makers. Bovine mammary epithelial cells (MECs) and their stem cells are very important in milk production and bioengineering. The adult mammary epithelium consists of two main cell types; an inner layer of luminal epithelial cells, which produce the milk during lactation, and an outer layer of myoepithelial cells resting on a basement membrane, which are responsible for pushing the milk through the ductal network to the teat cistern. Inner layer of columner/luminal cells of bovine MECs, is characterized by cytokeratin18, 19 (CK18, CK19) and outer layer such as myoepithelial cells which are characterized by CK14, α-smooth muscle actin (α-SMA) and p63. Much work has been done in mouse and human, on mammary gland stem cell research, particularly in cancer therapy, but stem cell research in bovine is still in its infancy. Such stem/progenitor cell discoveries in human and mouse mammary gland bring some hope for application in bovines. These progenitors may be therapeutically adopted to correct the structural/cytological defects in the bovine udder due to mastitis. In the present review we focused on various kinds of stem/progenitor cells which can have therapeutic utility and their possibilities to use as a potential stem cell therapy in the management of bovine post-mastitis damage in orders to restore milk production. The possibilities of bovine mammary stem cell therapy offers significant potential for regeneration of tissues that can potentially replace/repair diseased and damaged tissue through differentiation into epithelial, myoepithelial and/or cuboidal/columnar cells in the udder with minimal risk of rejection and side effects.
Keywords: Bovine mastitis, Bovine mammary epithelial cell, Stem cell therapy.
Introduction
The dairy industry is a multi-billion dollar industry catering to the nutritional needs of all age groups globally as well as providing an income to farmers including medium, marginal and landless farmers in developing countries. The bovine mammary gland is an extraordinary organ which is able to produce >3,000 kg of milk in a complete lactation cycle. Mastitis causes a drastic decrease in milk production particularly in acute/peracute mastitis and directly affects the farmer's income by decreased milk production, reduced milk quality, reduced animal value, costs of drugs, risk of culling of animals and sometimes death of animals 1-3. The economic damage for US dairy industry is approximately $2 billion dollars annually only in the US dairy industry and it has a similar impact in Europe 4. Bovine mastitis is also carries public health significance apart of its economic importance 5.
Bovine mastitis, defined as inflammation of the mammary gland, causes physical, chemical and usually bacteriological changes in milk and pathological changes in glandular tissues of the udder which affect the quality and quantity of milk 6. Clinical mastitis is diagnosed by prominent clinical manifestation such as red, hot and swollen mammary gland and presence of blood or flakes or clots in the milk 7, while “subclinical” infections does not show any visible clinical manifestations in the mammary gland and in milk. Bovine mastitis is more prevalent in cows (94.54%) 8 than buffaloes (68.60%) 9-10 particularly in Asian context. A recent review 11 has described the prevalence of bovine mastitis in most of Asian countries. It is a multi-etiological disease which causes severe damage and affects milk producing tissue and later affected tissue become which might permanently fibrosed 12,13. The rate of cure for treatment of mastitis caused by S. aureus with antibiotics is often less than 15% 14, whereas S. aureus is prevalent in more than 50% cases of mastitis 15. The dramatic increase in economic losses, due to high prevalence and low cure rate of this disease is alarmist the dairy sector, which attracts the attention of veterinarians, researchers, policy makers and dairy farmers. Therefore, there is an increasing necessity to treat and prevent the high prevalence of mastitis in dairy cows by using the most effective methodology. Although since last 7 decades several pharmacological and animal husbandry based approaches are being adopted to control the incidence of mastitis in dairy herds, but very often these approaches are unsuccessful and in most cases are associated with severe production losses 2, 16-18. Unfortunately, presently no single therapeutic strategy is available to improve or revert more than 50% of the post-mastitis structural damage of the mammary gland. One of the technologies, which may be of utility in improving the structural defects associated with mastitis, is the use of adult stem/progenitor cells.
Stem cells have been a focus of intense research and publicity for the last decade. They are changing our understanding of development, physiology and pathophysiology of diseases 19-20. Stem cells are commonly defined as “cells capable of self-renewal through replication and differentiating into specific lineages”. The progenitor cells are defined by their ability to self-renew, to generate differentiated progenies, to express specific molecular marker/s and clonal assay. Beside this, stem cells have important property that they also serve as a sort of internal repair system, dividing essentially without limit to replenish other cells as long as the person or animal is alive.
A large number of researchers are working on adult stem cells and trying to discover better ways to grow huge quantities of adult stem cells in laboratories and to manipulate them to generate specific cell types (as per need), and subsequently these specific stem cells can be used to treat specific diseases or repair tissue injury, such as post mastitis mammary tissue damage. An adult stem cell is thought to be an undifferentiated cell, found among differentiated cells of a tissue or an organ that can renew itself and can differentiate to yield some or all of the major specialized cell types of the tissue or an organ. The primary role of adult stem cells in a living organism is to maintain and repair the tissue in which they are found. There are extensive data available on mouse and human mammary gland stem/progenitor cells from normal biological to cancer studies 21-24. In contrast, limited information is available on stem cells and their progeny in the mammary glands of other species. Mammary gland epithelial cells are likely to be important effectors in the defense against intramammary infection 25. The research on bovine stem cells in general and bovine mammary stem cell in particular is very meager. Due to paucity of data on bovine stem cell research, the present paper is prepared with the objective of future possibilities for application of stem cells therapy to repair post-mastitis mammary tissue damage in dairy cows. In this review we have described various aspects including basics of bovine mammary gland anatomy, pathophysiology and udder immune responses, basics of bovine mammary gland stem/progenitor cells, and different kinds of stem cells and their possible applications in the management of mastitis. Some recent papers are available on bovine mammary stem cells 26-28, but no such work on the application of bovine mammary stem cells in the management of bovine mastitis is available. Before understanding the pathophysiology of the mammary stem cell, we should know the basics about mammary gland structure and its functional anatomy.
Structural and Functional Anatomy of the Bovine Mammary Gland
To understand the etiology, pathogenesis and treatment, of mastitis including stem cell therapy, it is very important to know the anatomy of the mammary gland. The bovine mammary gland is an excellent experimental model for studying tissue remodeling events, owing to the fact that much of the growth occurs post-natally rather than during embryonic or fetal stages of development.
Intriguingly, it is likely that the entire cellular repertoire of the mammary gland is formed from a single antecedent cell. Moreover, in order to produce a progeny of varied lineages (e.g., luminal and myoepithelial cells), signals from the local tissue microenvironment influence the fate of mammary stem/progenitor cells 29. Developmentally, the blood vessels and connective tissue of mammary gland are derived from the mesoderm, while alveolar, epithelial and myoepithelial cells are derived from ectoderm 30. It has been suggested that the multipotent mammary stem cells (MaSCs) give rise to epithelial precursor cells, the progeny of which develop into either ductal or alveolar cells 31 (Figure 1).
Figure 1.
Mammary gland epithelium cell lineages. Myoepithelial cells and luminal cells are formed from ductal precursors (DP) as the ducts grow out postnatally, particularly during puberty. On initiation of pregnancy, alveolar precursor cells (AP) give rise to myoepithelial and luminal cells, the latter of which synthesize and secrete milk. After lactation, the alveolar cells are subject to programmed cell death during the process of involution. A simple ductal system containing multipotent (yellow) and committed ductal (green) and luminal (orange) precursor cells persists that will develop into a fully functional epithelium in subsequent pregnancies. (Adopted from Hennighausen and Robinson, 31, with permission from corresponding author).
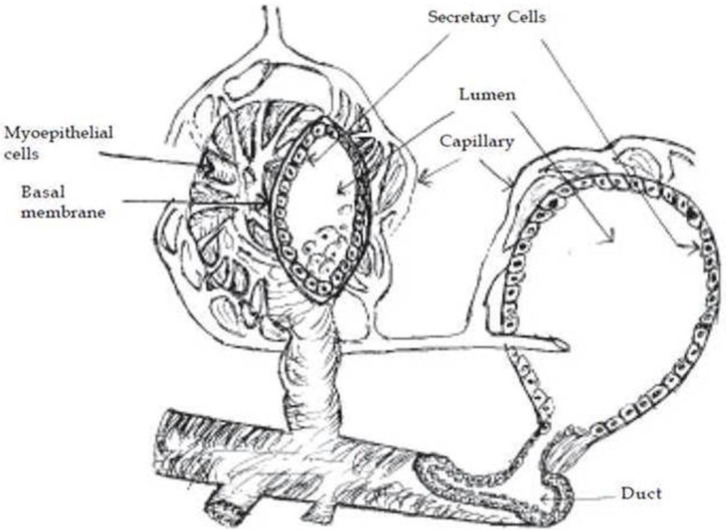
The parenchyma is the portion of the mammary gland (udder) that is considered to be the functional aspect of the gland as this region contains the mammary epithelial cells (MECs) 32. MECs, responsible for producing milk, are the focus of the majority of research pertaining to mammary gland in cattle. The mammary gland consists of milk producing unit i.e. “alveolus”, a duct system, a gland cisterns and a teat with a teat cistern. The alveolus, which is a central unit in the milk production process, is made-up of secretory epithelial cells and surrounded by myoepithelial cells. The milk is synthesized in the secretory cells, which are arranged as a single layer on a basal memberane in a spherical structure called “alveoli”. The alveoli are microscopic, spherical structures 50 to 250 mm in diameter, depending upon the volume of milk accumulated. Bovine mammary glands typically consist of 5 trillion secretory cells in the epithelium of the alveolar tissue 33. Smooth muscle-like cells called “myoepithelial cells” cover the epithelial cells of the alveoli, ducts and cisterns of the entire gland. Outside the myoepithelial cells, the alveolus is surrounded by a basement membrane of connective tissue. Each alveolus is supplied with tiny capillaries, which lie in the stroma (Figure 2). An alveolus is the sac like structure, where milk is synthesized and secreted, and therefore considered the milk producing structural unit of the udder (Figure 2). The mammary gland tissue is highly organized branched ductal network consisting bilayered system: basal (myoepithelial) and luminal (secretory epithelial cells) 34. Myoepithelial cells generally express the cytokeratin 14 (CK14, 50 kDa), CK5 (58 kDa) and CK17 (46 kDa), alpha-smooth muscle actin (α-SMA), whereas the luminal/secretory epithelial cells mainly express the CK18, CK19, CK7 and CK8 35-37. A recent study of Martignani et al 37 on bovine mammary epithelial stem cells, reported that the inner layer of cuboidal cells are positive to CK18 and myoepithelial cells of an outer layer consisted of more elongated cells with flattened nuclei were strongly positive for CK14, α-smooth muscle actin (α-SMA) and p63. The luminal cells in the alveoli can produce milk, while the myoepithelial cells by virtue of their contractile function, which is regulated by oxytocin and mechanical stimulus, facilitate the milk ejection process. The duct system of mammary gland is lined by epithelial cells varies from cuboidal/collumnar cells, while the cisterns are surrounded by smooth muscle cells 38-40. The cells lining the interlobular ducts and cisterns are positive for CK5, CK6, CK8, CK18 and CK14 35, 36, 40-42.
Figure 2.
Structure of alveolus.
Mastitis causing tissue damage and cellular rearrangement
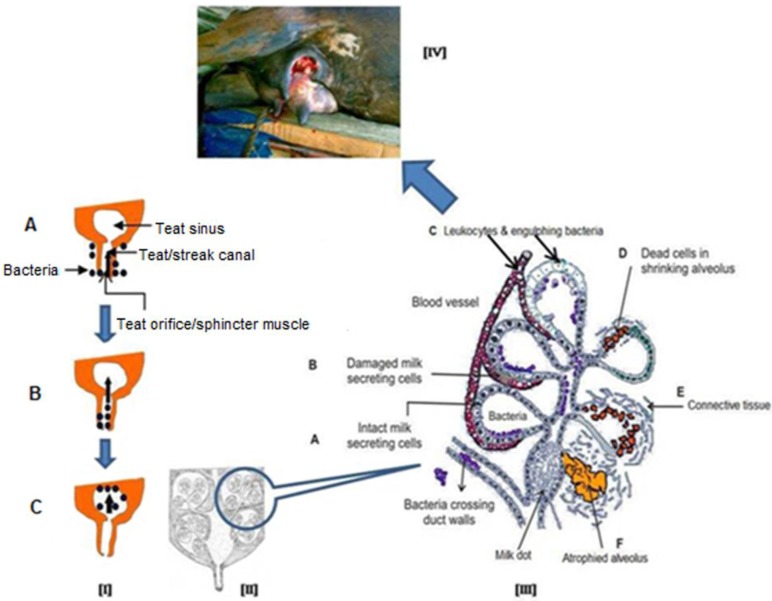
Mastitis always causes a certain irreversible destruction of milk producing tissue which leads to a decrease in milk production. Intramammary infection results once bacteria breach the teat sphincter and pass through the teat duct of a mammary quarter, multiply in the teat and gland cisterns, and progress dorsally to the milk-producing tissues. Bacteria produce toxins and immunological reactions, resulting in the complete loss of milk synthetic tissue and complete loss of milk production due to fibrosis (Figure 3) particularly in acute/peracute clinical mastitis cases. Some recent reviews on bovine mastitis and its impact on structure and function in the ruminant mammary gland covers the most aspects of direct or indirect damage caused to mammary tissue by mastitis 43-44.
Figure 3.
Schematic representation of process of intramammary infection and subsequently damage to mammary gland. [I] Bacteria present on the surface of mammary gland (udder, A) and enter into the teat canal (B) after getting opportunity and finally setup the infection in mammary gland (C), [II] A longitudinal diagram of normal mammary gland, [III] After getting bacterial infection, cellular defence mechanism become active and phagocytic cells (from blood) effort to engulf and kill the bacteria, phagocytosis by-products and release of bacterial toxins damage to the secretory mammary epithelial cells and finally cause fibrosis of mammary gland (A to F), [IV] Final outcome of acute and/or chronic mastitis.
Mastitis is a multi-etiological disease 45 and the S. aureus is considered to be an important root cause of acute mastitis 7, 46. This bacterium produces a toxin that destroys MECs and permanently damages milk-producing tissue (vesicle), resulting in complete loss of milk production due to fibrosis (Figure 3).
In severe and chronic intramammary infections the secretory tissue changed into non-secretory or fibrous tissue 33. Hence, during and immediately post mastitis, the infected udder has less of alveolar epithelium, adipose tissue, luminal areas and it has more inter-alveolar connective tissue. Ensuingly, there is reduction in milk secretory activity, resulting in drastically reduced milk production 18. It is a well-established fact that milk production in dairy cattle is a function of number and activity of MEC and these factors can be influenced by diverse environmental and management practices 47.
Leitner et al 48 have demonstrated the detail of distribution of cellular arrangement in the healthy and infected bovine mammary tissue and found that CD18+ leukocytes are the most prevalent cells (>95%) in infected mammary tissue. During and post mastitis, there is strong evidence for the severely compromised cytology in udders of dairy cows and buffaloes both.
Mammary gland stem/Progenitor cells
Mammary cell proliferation, turnover and tissue regeneration are functions of mammary stem cells. The existence of adult mammary stem cells was established several decades ago when DeOme et al 49 observed that mammary epithelium was able to generate normal mammary outgrowths containing all structures, like ductal, alveolar, and myoepithelial cells of the mammary gland.
The mammary glands contain stem/progenitor functional hierarchies that are maintained through the entire life span of the animal 22. The evidence for the existence of stem cells in the bovine mammary gland has accumulated over the last decades and it is now widely accepted that a variety of different cell subpopulations exist, ranging from undifferentiated stem cells to terminally differentiated luminal epithelial and myoepithelial cells 28, 30, 42, 50-53. Pioneering studies have distinguished bovine MECs according to their morphology and DNA label retention 50, 54, 55. A pioneered study has identified a “pale staining” cell population present in bovine mammary glands that may include bovine MaSCs 51. They carried out an analysis of mammary epithelial cell proliferation in prepubertal bromodeoxyuridine (BrdU)-injected Holstein heifers to investigate this hypothesis and found three different cell types viz. light, dark, and intermediate staining cells in histologic sections. Light cells comprised 10% of the total epithelial cell population but accounted for 50% of the cell proliferation. Intermediate cells comprised 60% of the cell population and 43% of proliferating cells. Dark cells comprised 30% of the parenchymal cell population but only 7% of proliferating cells.
Adult/somatic stem cells have capability to self renewal and differentiation to generate all cell types of the tissue or organ in which they reside 55. Delineation of mammary epithelial cell hierarchy, as perceived today, consists of MaSCs giving rise to uni-potent luminal-restricted progenitors that, in turn, differentiate into alveolar myoepithelial or secretory cells. According to recent studies, some in vitro and in vivo methods are currently being utilized to isolate, characterize and study stemness and progenitor activity of bovine putative mammary stem/progenitor cells, including morphology, histopathology 42, 56-59, bromodeoxyuridine retention 50, Hoechst dye-effluxing to identify side population (SP) properties and surface markers by flowcytometer or immunochemistry 23, 42.
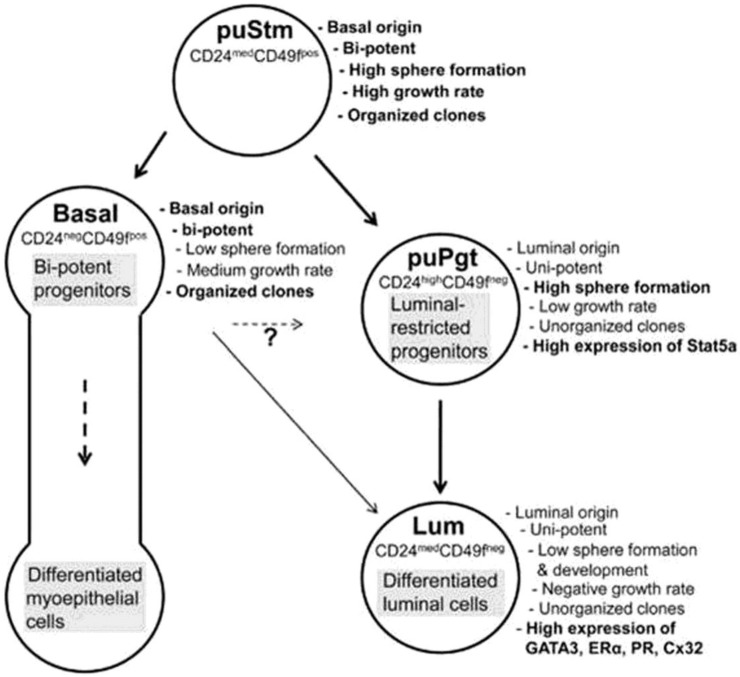
Progenitor cells are different than stem cells because progenitor cells have a finite proliferation capacity and restricted differentiation capacity. Transplantation studies have suggested that the mammary epithelium is maintained by the presence of multipotent mammary stem cells 23. The mammary gland contains stem/progenitor functional hierarchies that are maintained through the entire life span of the animal 22. A recent study of Rauner and Barash 42 has described the bovine mammary stem cell/progenitor hierarchy with their specific markers (Figure 4).
Figure 4.
Schematic representation of proposed bovine mammary epithelial cell hierarchy. (Adopted from Rauner and Barash 42, with permission from publisher).
MaSCs are essential for mammary tissue regeneration with each cycle of lactation. Therefore, isolation and characterization of bovine MaSCs and their progenitors is of primary interest, not only to extend our knowledge regarding the diverse regulation of MaSCs among mammals, but also for the dairy industry, since their activity may directly affect lactation persistency 42. MaSCs required for net growth, renewal and turnover of mammary epithelial cells and are, therefore, potential targets for strategies to increase production efficiency 55, 60. Appropriate regulation of MaSC can potentially benefit milk yield, persistency, dry period management and tissue repair 26. For the next sections we will describe the individual stem/progenitor cell types of the bovine mammary gland and provide possibilities of their application in the correction of post-mastitis damage.
Epithelial stem/Progenitor cells
The isolation of epithelial stem cells from both mouse 35, 36 and human 61 mammary glands, and the assessment of their ability to regenerate mammary tissue in vivo, provide some hope for the application of stem/progenitor cells in the repair of post-mastitis damage in the mammary glands of dairy animals. Tissue damage and/or immune stimuli can activate progenitor cells through growth factors or cytokines, leading to increased cell division, which are important for the repair process.
It has even been postulated that the mammary gland itself may be an extension of the innate immune system 62. Until recently, we thought that only blood cells that are neutrophils and monocytes migrate at the site of infections in response to invading mastitis causing pathogens and worked as innate immunity in the udder. However a study of Gray et al. 63 has reported that mammary epithelial cells may also play an important role in the innate immune response through secretion of antimicrobial peptides and then attraction of circulating immune effector cells. Stelwagen et al. 64 have been reviewed the interaction and function of mammary epithelial cells against bacteria. Mammary epithelial cells express a range of pathogen recognition receptors, most notably the Toll-like receptors (TLR), which recognize specific molecular motifs (pathogen-associated molecular patterns, or PAMPs) on the surface of pathogens. A key element in the initiation of an innate immune response is the early detection of potentially harmful microbes by recognizing components commonly found on these foreign pathogens, referred to as PAMPs 65. Activation of these receptors initiates a signaling cascade in which nuclear factor-κB plays a pivotal role in coordinating multiple signals and directing expression of effector response genes 65-67.
The discovery of toll-like receptors as evolutionary conservative molecules and their role in innate defence has opened a new area of interest. A number of research groups have reported on the importance of TLR as the first line of defence against invading pathogens through initiation of the innate immune response 66, 68. The identification of CD14, TLR-2 and TLR-4 on milk fat globule membranes suggests a direct role for the mammary gland parenchyma in pathogen detection 69. TLR2 is a receptor for S. aureus lipoteichoic acid (LTA) and TLR4 is a receptor for E. coli lipopolysaccharide (LPS).
Shackleton et al 35 and Stingl et al 36 have worked extensively on mouse mammary stem cells. They characterized that a single stem/progenitor cell e.g. CD29hi/CD49fhi/ CD24+/mod/Sca-1- can give rise to all epithelial cell types required to produce a functional mammary gland and possesses self-renewal properties. It is believed that this mammary epithelial stem cell gives rise to a common progenitor that splits into two lineages, which includes the “myoepithelial progenitors” that produce myoepithelial cells and the “luminal progenitors” that generate both ductal luminal epithelial cells and alveolar luminal epithelial cells 70, 71. A recent study reported that bovine mammary gland epithelial stem cells share basic characteristics with the human breast cells, hence the identification of its cell composition may broaden our understanding of the diversity in cell hierarchy among mammals 42. They have characterized the bovine mammary epithelial stem/progenitor cells as Linneg epithelial cells and divided these cell populations into four groups according to the expression of CD24 and CD49f surface markers- 1) putative stem cells (CD24medCD49fpos), 2) basal cells (CD24negCD49fpos), 3) putative progenitors (CD24highCD49fneg) and 4) luminal cells (CD24medCD49fneg). These findings of bovine MaSCs confirmed the reports on mouse mammary gland stem cells 35, 36 that there is a close relationship between bovine MaSCs and MaSCs in other mammals. However, a recent publication had claimed that neither CD24, CD29 nor CD49f are exclusive markers of bovine mammary epithelial progenitor cells 33. This is counter facted by the Rauner and Barash's 42 who proved that CD24, CD29 and CD49f are also markers of bovine mammary epithelial progenitor cells similar to mouse mammary epithelial progenitor cells. In contrast, Machado et al 72 have reported that these markers (CD24+CD29HCD49fHSca1-) enrich for cell subpopulations that harbor MaSCs, they do not identify regenerative stem cells uniquely. They found that cells >10 μm in size with a higher forward scatter cells (FSC) had increased outgrowth potential as compared with lineage-negative (LIN-) control cells.
Rauner and Barash 42 have also reported that these bovine MaSCs maintained differential gene expression of lineage markers and markers of stem cells and luminal progenitors as high expression of Stat5a in the putative progenitors, and of Notch1, Delta1, Jagged1 and Hey1 in the putative stem cells and basal populations. Choudhary et al 27 have demonstrated three novel candidate markers for bovine MaSC: nuclear receptor subfamily 5 group A member 2 (NR5A2), nucleoporin 153 (NUP153) and fibronectin type III domain containing 3B (FNDC3B). They found that NR5A2 and NUP153-positive nuclei were more abundant in prepubertal than lactating mammary glands and their distributions were consistent with expectations for a MaSC marker. The study on expression of these markers in bovine MaSCs would also support further identification and characterization of specific bovine mammary stem/progenitor cells. The isolation and characterization of bovine mammary epithelial stem/progenitor cells could open a new avenue for the management and correction of post-mastitis damage and increase milk production after genetic manipulations and subsequent transplantation in damaged udder. Therefore, mammary epithelial progenitors could possibly be used as cells of high therapeutic interest for the correction of mastitis damage.
Myoepithelial and Luminal Stem/Progenitor Cells
The first and most important questions that come in mind are how myoepithelial cells arise in the mammary gland. This has gained increased interest in the light of accumulating findings that they play a pivotal role in the overall development and repair of mammary tissue. Myoepithelial restricted progenitors generate colonies that are composed solely of basal like epithelial cells 73. Functionally, myoepithelial cells have characteristics of both smooth muscle (“myo”) and epithelial cells 74. Myoepithelial cells are characteristically elongated in shape. The myoepithelial cell cytoplasm is filled with actin and myosin, which are responsible for the contractile phenotype mediated by oxytocin during milking 75. Myoepithelial cells surround the epithelial cells of each alveolus. Milk ejection in response to oxytocin is an important function of myoepithelial cells. Therefore, myoepithelial stem/progenitor cells are important for the milk producing unit of the mammary gland and could support the repair process of mammary tissue after injury and/or mastitis.
Zavizion et al 76 isolated and characterized the bovine mammary myoepithelial cells on the basis of alpha-smooth muscle actin (α-SMA), α-actinin and vimentin markers. A recent study reported that bovine mammary luminal and myoepithelial stem/progenitor cells could be isolated and characterized by their aldehyde dehydrogenase (ALDH) activity as ALDHhigh and ALDHlow, respectively 37.
The bovine mammary myoepithelial cells are characterized by expression of α-SMA 42, 56, 57, 76, vimentin 76, CK6, CK14, CK18, connexin-43 (Cx43) 42, 57, p63 and CD49f 42. However, Hellmen and Isaksson 56 reported that CK14 is a less specific marker for bovine myoepithelial cells than α-SMA, while a recent study claimed a strong expression of CK14 in bovine myoepithelial cells 57. CK18 is a potent lineage marker of bovine mammary duct compartment 42, 56, 57. The expression of cytokeratin and smooth muscle actin markers on myoepithelial cells indicate the close similarity with smooth muscle cells 57, 77. Bovine myoepithelial cells express the common acute lymphoblastic leukemia antigen (CALLA), also known as CD10 and neutral endopeptidase 58.
A recent study in human breast cells found that bipotent K5+/K19- stem/progenitor cells differentiated into stable clonal populations (under specific culture conditions) of K5-/K19- and exhibit self-renewal and unipotent myoepithelial differentiation potential in contrast to the parental K5+/K19- cells which are bipotent 78. These K5-/K19- cells function as myoepithelial progenitor cells and constitutively express markers of an epithelial to mesenchymal transition (EMT) and show high invasive and migratory abilities. This indicates that K5-/K19- myoepithelial progenitors may open the avenue for application of unipotent progenitor cells for specific therapy in post-mastitis damage. Active myoepithelial cell division could be maintained at least 3 months, and cells could be serially subcultured at least seven times 76. Still we need to know more about molecular work on bovine mammary myoepithelial cells for a better understanding of their origin and biology. The lineage development of myoepithelial cells in the bovine mammary gland along with their subsequent application in the therapeutic management of mammary gland and cause quantity increase in the milk production are the areas which still need understanding.
Stem cells from other origin
Hematopoietic Stem/Progenitor Cells
Hematopoietic stem cells (HSCs) are the multipotent stem cells, which are able to give rise to all types of blood cells including myeloid (monocytes, macrophages, neutrophils, basophils, eosinophils, erythrocytes, megakaryocytes/platelets, and dendritic cells) and lymphoid lineages (T cells, B cells, NK cells) 79. Hematopoietic stem cells have direct antibacterial function through phagocytosis and ultimately improve the immune system Aquired immunity, mediated by B and T lymphocytes, detects and responds to non-self molecules through the recognition of specific peptide antigens by affinity antigen receptors expressed on the surface of B and T-cells and subsequent production of antibodies and antigen specific T-cells 67. However, the establishment of a primary adaptive immune response is not rapid enough to eradicate invading microorganisms as it involves cell proliferation, gene activation and protein synthesis 80.
Our laboratory is standardizing the techniques for isolation and characterization of bovine hematopoietic stem cells 81 and their further possible application for management of bovine mastitis. We are collecting bovine peripheral blood and separating the peripheral blood mononuclear cells by using Ficolpaque Plus, for isolation of bovine hematopoietic stem cells as CD34+ cells. The final objective of our study is differentiation of hematopoietic stem cells into immune cells to enhance the animal immunity. A CD34 marker is commonly used to identify hematopoietic precursor cells. The discovery of myeloid progenitor cells has offered the exciting prospect of merging classical concepts of myeloid cell biology in neovascularization with evolving notions of myeloid cell plasticity and endothelial/smooth muscle cell differentiation. The dendritic cells can differentiate into endothelial like cells when stimulated with angiogenic factors 82-84. Thus, dendritic cells might exert an important impact on the neovascularization process in different physiopathological conditions 84.
The hematopoietic system is also an important compartment of the mammary gland and may play an important role in the repair of tissue damage. It is found that side population of hematopoietic stem cells promotes wound healing in diabetic mice, proving that hematopoietic stem/progenitor cells can repair damaged tissue 85. A transcriptional study by Siegel and Muller 71 reported that the differentiation potential of mammary epithelial cells within hematopoietic compartment is controlled by the action of some specific transcription factors. For instance, ETS related genes (ERG), a member of the ETS family (E26 family of transcription factors) have been shown to play a significant role in hematopoietic and endothelial development 86.
The most important thing is that the origin of both, mammary gland (particularly vascular and connective tissue) and HSCs is the same; that is mesoderm. Hence, they have some common markers like the peripheral blood mononuclear cells and the bovine mammary epithelial cell line (MAC-T), which express macrophage markers e.g. granulocyte chemotactic protein (GCP)-2 87. This means that peripheral blood mononuclear cells and bone marrow derived mononuclear cells have the potential to give rise to a variety of vascular precursors, which can support in the repair of post-mastitis udder damage. Some workers have reported that hematopoietic (CD34+) cells could differentiate into endothelial cells (ECs) in vitro and in vivo in mouse models. Therefore, these cells contribute to the regeneration of vascular tissue through neoendothelialization and neovascularization 88. The undergoing hematopoietic differentiating cells may reprogram into mammary epithelial cells and promote mammary epithelial morphogenesis 21. These findings provide insight into regeneration of damaged mammary gland and the role of the mammary microenvironment in reprogramming cell fate.
Endothelial progenitor cells (EPCs)
Endothelial progenitor cells are a population of rare cells that circulate in the blood with the ability to differentiate into endothelial cells which make the lining of blood vessels and are capable of generating new blood vessels in areas of ischaemia or infarction 89. Two major cell types, epithelial and myoepithelial stem/progenitor cells are of considerable therapeutic interest in mammary gland tissue. These can support the development of a vascular network (endothelial and smooth muscle cells) within the mammary gland. The vascular degeneration of mammary epithelium is the most prominent sign of Staphylococcal mastitis 45. Landmark studies 90, 91 on EPCs have challenged the traditional notion that endothelial regeneration and angiogenesis occurs exclusively through the proliferation of the pre-existing resident ECs of vessel wall. However, it is a novel concept that EPCs enter the blood stream from the bone marrow and provide a pool of circulatory EPCs in postnatal life 91, which could support the regeneration of vascular damage during mastitis.
Numerous surface markers have been reported as endothelial stem/progenitor cell marker like CD133+ 92, CD34+CD31+ 93, CD34+CD133+ 94, CD34+CD45+ 95, CD34+CD133+VEGFR-2+CD45+ 96, CD34+CD45+CD146+ 97; ALDHbright 98, Sca-1+ 99, Sca-1- Lin-cKit- 100 etc. in humans and mouse, while there is limited work in bovines. Vascular endothelial growth factor (VEGF) is a heparin-binding growth factor specific for vascular endothelial cells and VEGF receptor is used as endothelial stem/progenitor cell marker 57. The severe inflammatory process during mastitis results in extensive damage to the vascular network in the bovine mammary gland 45. The therapeutic approaches with EPCs to improve/restore the compromised vascular network are worth a thought. Therefore, EPCs are proposed as a potential regenerative tool for treating vascular diseases 91, and thus the EPCs may support in the repair of post mastitis damaged vasculature.
Bone marrow (BM) progenitor cells
As we know all living beings including human, animals and plants have a intrinsic healing response to mobilize progenitor cells from the bone marrow to the site of injury. When any injury will start to heal, the progenitor cells that emerge from the bone marrow depend on the type of injury. Haematopoietic progenitor (HP) cells move to new sites in the bone marrow and begin to generate all blood types like EPCs are recruited to sites of injury/ischemia to help in forming new blood vessels; and mesenchymal stem cells (MSCs) help in healing of injuries by differentiating into concerned cell types 101. Stem/progenitor cells from bone marrow and other sources have been seen to repair injured tissues by differentiating into tissue specific phenotypes, by secreting chemokines and in part, by cell fusion 102. Since, multipotent MSCs are easily expandable in culture and differentiate into multiple tissue lineages; there has been much interest in their clinical potential for tissue repair and gene therapy 103. Harris et al 104 study has given the idea regarding the use of bone marrow-derived cells (BMDC) comprising stroma, stem and progenitor cells, may contribute a significant part of regenerating epithelial tissue. They found that a single injection into the wound margin is sufficient to reverse the wounding process and promote normal wound healing.
Due to the differentiating capability of BM mesenchymal progenitor cells into osteocytes, chondrocytes, adipocytes, smooth muscle cells, endothelial cells etc. these cells may be of noteworthy utility in restoring the mammary gland cytological architecture after mastitis damage. Osteogenic differentiation potential of these cells is assumed due to high levels of osteopontin (OPN). Interestingly a significant association of the microsatellite markers in the region of OPN is observed with the synthesis of milk proteins, milk yield and other desirable milk traits 105. Hence mesenchymal progenitor cells are among the most promising vascular progenitors, which can be adopted for therapy of post mastitis cytological defects. The adipocytes are a component of the bovine mammary gland and an inflammatory reaction significantly impairs the adipocyte function through production of free radicals. Therefore, mesenchymal progenitor cells can be specifically used to improve the adipocyte content in the udder. As we have also discussed above that mammary gland is composed of epithelial cells, mesenchymal cells, adipocytes, fibroblasts, blood vessels and immune cells.
Induced pluripotent stem cells (iPSCs)
Various kinds of stem and/or progenitor cells have pluripotency and can differentiate into any type of body cell. However, still a major impediment is the lack of availability of bounteous number of these cells for clinical/therapeutic use. Pluripotent stem cells from mouse adult fibroblasts were produced, by introducing four factors, Oct3/4, Sox2, c-Myc, and Klf4, known as “induced pluripotent stem cells” 106. This work has been awarded with the Nobel prize in 2012. This breakthrough discovery has created a powerful new way which can address the lack of availabile stem/progenitor cells. By using this novel technology, any stromal cell can be transformed into a desired pluripotent progenitor cell. Some studies have reported that only two transcription factors (Oct4/Sox2 or Oct4/Klf4) are enough to generate iPS cell lines 107. These cells also have the potential to differentiate into endothelial (CD31+) and smooth muscle cells, indicating their multi-lineage potential. It would be fascinating to study if iPSC technology can be useful to differentiate stromal cells into epithelial, myoepithelial and other (columnar and cuboidal) cells of the mammary gland, which would be a breakthrough in the management of post-mastitis damage. Induced pluripotent stem cells have been successfully generated from domestic animals including bovine 108-111. At the movement there is no report of an application of iPSCs in the repair of bovine mammary gland damage.
Conclusion and future research needs
Mastitis in dairy animals is a multietiological in nature and causing heavy economic losses worldwide. Unfortunately, the current therapeutic strategy can not able to improve or revert the post-mastitis structural damage more than 50% in the mammary gland. Although from last few decades, the stem cell techniques are being used as a therapeutic tool for regenerative medicine in human but it is still lacking in the treatment/corrections of various challenging ailments in livestock such as the mastitis. Present review is providing the insight into the possible application of various stem/progenitor cells including mammary stem cells and other origin adult stem cells in the repair of post-mastitis structural defects in the dairy animals. Due to the self-renewal ability and the subsequent generations with variable degrees of differentiation capacities has given the impact of these cells in therapeutic research and applications. Therefore, the significant potential of these progenitors could be used for generation of tissues that can potentially replace or repair diseased and also the damaged tissue like epithelial, myoepithelial and or cuboidal/columnar cells in the udder. Two major cell types, epithelial and myoepithelial stem/progenitor cells are of considerable therapeutic interest in mammary gland tissue. These can support the development of a vascular network (endothelial and smooth muscle cells) within the mammary gland. In this direction, a study of Capuco et al 55 have been tried to establish in-vivo expansion of bovine mammary stem cells using an intramammary infusion of xanthosine to improve the growth of mammary epithelial cells. Moreover, a more precise study has need to identify bovine mammary gland stem/progenitor cells markers for isolation of specific cell populations for further application in udder repair. Simultaneously easy and accurate techniques for isolation of bovine mammary gland epithelial stem/progenitor cells and its long term culture methods are needed to develop. The isolation and characterization of mammary stem cells is an important step towards elucidating the hierarchy of epithelial cell development in the mammary gland and identifying the pluripotent cells in the udder along with their further application in the correction of damage. If possible then it can consider the most important research aspect of the reprogramming of adult somatic/stromal cells to differentiate into mammary epithelial, myoepithelial and cuboidal/columnar cells by using specific factors. Myoepithelial stem/progenitor cells are important for the milk producing unit of the mammary gland and could support the repair process of mammary tissue after injury and/or mastitis and mesenchymal progenitor cells can be specifically used to improve the adipocyte content in the udder. Therefore, adult stem cells have the novel boulevard for bovine mastitis management and further reduction in heavy economic losses.
Acknowledgments
Stem cell research is a broad topic and huge numbers of researchers are contributing to this field. Hence we apologize to those, whose work could not be cited due to space constraints. This research was supported by a grant from the Next-Generation BioGreen 21 Program (No. PJ009032022012), Rural Development Administration, Republic of Korea, hence the authors are thankful to this organization. Authors are equally thankful to the Indian Council of Agricultural Research, New Delhi, India for providing international ICAR fellowship to first author. We are also thanks to Dr. Henk Hogeveen, Bovine mastitis research group, Wageningen University, The Netherland for thorough reviewing of manuscript as a native English speaker.
References
- 1.Seegers H, Fourichon C, Beaudeau F. Production effects related to mastitis and mastitis economics in dairy cattle herds. Vet Res. 2003;34:475–491. doi: 10.1051/vetres:2003027. [DOI] [PubMed] [Google Scholar]
- 2.Halasa T, Huijps K, Osteras O. et al. Economic effects of bovine mastitis and mastitis management: a review. The Vet Quart. 2007;29:18–31. doi: 10.1080/01652176.2007.9695224. [DOI] [PubMed] [Google Scholar]
- 3.Nielsen C. Economic impact of mastitis in dairy cows. Dissertation, Swedish University of Agricultural Sciences. Uppasala, Sweden. 2009.
- 4.Donovan DV, Kerr DE, Wall RJ. Engineering disease resistant cattle. Transgenic Res. 2005;14:563–567. doi: 10.1007/s11248-005-0670-8. [DOI] [PubMed] [Google Scholar]
- 5.Sharma N, Maiti SK, Roy S. Role of vitamin E in the control of mastitis in dairy cows. Vet Practitioner. 2003;4(2):140–143. [Google Scholar]
- 6.Sharma N, Singh NK, Bhadwal MS. Relationship of somatic cell count and mastitis: An overview. Asian-Australasian J Anim Sci. 2011;24(3):429–438. [Google Scholar]
- 7.Sharma N, Gupta SK, Sharma U. et al. Treatment of clinical mastitis in buffalo-A case report. Buffalo Bull. 2007;26(2):56–58. [Google Scholar]
- 8.Sharma N, Maiti SK, Sharma KK. Prevalence, etiology and antibiogram of microorganisms associated with Sub-clinical mastitis in buffaloes in Durg, Chhattisgarh State (India) Int J Dairy Sci. 2007;2(2):145–151. [Google Scholar]
- 9.Sharma N, Maiti SK, Koley KM. Studies on the incidence of sub clinical mastitis in buffaloes of Rajnandgaon district of Chhattisgarh state. Vet Practitioner. 2004;5(2):123–124. [Google Scholar]
- 10.Beheshti R, Eshratkhah B, Shayegh J. et al. Prevalence and etiology of subclinical mastitis in Buffalo of the Tabriz region. Iran J Am Sci. 2011;7(5):642–645. [Google Scholar]
- 11.Sharma N, Rho GJ, Hong YH. et al. Bovine mastitis: An Asian perspective. Asian J Anim Vet Adv. 2012;7(6):454–476. [Google Scholar]
- 12.Sharma N, Gautam A, Upadhyay SR. et al. Role of antioxidants in udder health: a review. Indian J Field Vet. 2006;2(1):73–76. [Google Scholar]
- 13.Sharma N. SMVS Dairy Year Book-2010. Gurgoan, India: Sarva Manav Vikash Samiti; 2010. Economically important production diseases of dairy animals; pp. 47–65. [Google Scholar]
- 14.Kerr DE, Wellnitz O. Mammary expression of new genes to combat mastitis. J Anim Sci. 2003;819(Suppl 3):38–47. doi: 10.2527/2003.81suppl_338x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Sharma N. Epidemiological investigation on subclinical mastitis in dairy animals: Role of vitamin E and selenium supplementation on its control. Dissertation, I.G.K.V.V, Raipur (C.G.) India. 2003.
- 16.Halasa T, Nielen M, De Roos APW. et al. Production loss due to new subclinical mastitis in Dutch dairy cows estimated with a test-day model. J Dairy Sci. 2009;92(2):599–606. doi: 10.3168/jds.2008-1564. [DOI] [PubMed] [Google Scholar]
- 17.McDougall S, Parker KI, Heuer C. et al. A review of prevention and control of heifer mastitis via non-antibiotic strategies. Vet Microbiol. 2009;134:177–185. doi: 10.1016/j.vetmic.2008.09.026. [DOI] [PubMed] [Google Scholar]
- 18.Nickerson SC. Control of heifer mastitis: antimicrobial treatment-an overview. Vet Microbiol. 2009;134:128–35. doi: 10.1016/j.vetmic.2008.09.019. [DOI] [PubMed] [Google Scholar]
- 19.Joseph NM, Morrison SJ. Toward an understanding of the physiological function of mammalian stem cells. Dev Cell. 2005;9:173–183. doi: 10.1016/j.devcel.2005.07.001. [DOI] [PubMed] [Google Scholar]
- 20.Clarke MF, Fuller M. Stem cells and cancer: two faces of eve. Cell. 2006;124(6):1111–1115. doi: 10.1016/j.cell.2006.03.011. [DOI] [PubMed] [Google Scholar]
- 21.Jiang S, Lee BC, Fu Y. et al. Reconstitution of mammary epithelial morphogenesis by murine embryonic stem cells undergoing hematopoietic stem cell differentiation. PLoS ONE. 2010;5(3):e9707. doi: 10.1371/journal.pone.0009707. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Bruno RD, Smith GH. Role of epithelial stem/progenitor cells in mammary cancer. Gene Expr. 2011;15(3):133–140. doi: 10.3727/105221611x13176664479368. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.VanKeymeulen A, Rocha AS, Ousset M. et al. Distinct stem cells contribute to mammary gland development and maintenance. Nature. 2011;479:189–193. doi: 10.1038/nature10573. [DOI] [PubMed] [Google Scholar]
- 24.Pond AC, Bin X, Batts T. et al. Fibroblast growth factor receptor signaling is essential for normal mammary gland development and stem cell function. Stem Cells. 2013;31:178–189. doi: 10.1002/stem.1266. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 25.Wellnitz O, Kerr DE. Cryopreserved bovine mammary cells to model epithelial response to infection. Vet Immunol Immunopathol. 2004;101:191–202. doi: 10.1016/j.vetimm.2004.04.019. [DOI] [PubMed] [Google Scholar]
- 26.Capuco AV, Choudhary RK, Daniels KM. et al. Bovine mammary stem cells: cell biology meets production Agriculture. Animal. 2012;6(3):382–393. doi: 10.1017/S1751731111002369. [DOI] [PubMed] [Google Scholar]
- 27.Choudhary RK, Evock-Clover CM, Capuco AV. Expression of novel, putative stem cell markers in prepubertal and lactating bovine Mammary glands. J Anim Sci 89(E-Suppl. 1)/J Dairy Sci. 2012;94(E-Suppl 1):180–181. [Google Scholar]
- 28.Choudhary RK, Li RW, Evock-Clover CM. et al. Comparison of the transcriptomes of long-term label retaining-cells and control cells microdissected from mammary epithelium: an initial study to characterize potential stem/progenitor cells. Front Oncol. 2013;3:21.. doi: 10.3389/fonc.2013.00021. doi: 10.3389/fonc.2013.00021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Bussard KM, Smith GH. The mammary gland microenvironment directs progenitor cell fate in vivo. Int J Cell Biol. 2011 doi: 10.1155/2011/451676. DOI:10.1155/2011/451676. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 30.Ii M, Takenak H, Asai J. et al. Endothelial progenitor thrombospondin-1 mediates diabetes-induced delay in re-endothelialization following arterial injury. Circ Res. 2006;98:697–704. doi: 10.1161/01.RES.0000209948.50943.ea. [DOI] [PubMed] [Google Scholar]
- 31.Hennighausen L, Robinson GR. Information networks in the Mammary gland. Nat Rev Mol Cell Biol. 2005;6(9):715–725. doi: 10.1038/nrm1714. [DOI] [PubMed] [Google Scholar]
- 32.Thorn SR, Purup S, Vestergaard M. et al. Regulation of mammary parenchymal growth by the fat pad in prepubertal dairy heifers: role of inflammation-related proteins. J Endocrinol. 2008;196:539–546. doi: 10.1677/JOE-07-0501. [DOI] [PubMed] [Google Scholar]
- 33.Kumar AHS, Singh NK. Compromised cytology of bovine udder during mastitis: therapeutic potential of adult stem/progenitor cells; In: Sharma N, Bacic G, Singh NK, eds. Production diseases of dairy animals. Delhi, India: Satish Serial Publishing House; 2011. pp. 391–411. [Google Scholar]
- 34.Muschler J, Streuli CH. Cell -Matrix interactions in mammary gland development and breast cancer. Cold Spring Harb Perspect Biol. 2010;2:a003202. doi: 10.1101/cshperspect.a003202. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Shackleton M, Vaillant F, Simpson KJ. et al. Generation of a functional mammary gland from a single stem cell. Nature. 2006;439:84–88. doi: 10.1038/nature04372. [DOI] [PubMed] [Google Scholar]
- 36.Stingl J, Eirew P, Ricketson I. et al. Purification and unique properties of mammary epithelial stem cells. Nature. 2006;439:993–997. doi: 10.1038/nature04496. [DOI] [PubMed] [Google Scholar]
- 37.Martignani E, Eirew P, Accornero P. et al. Baratta M. Human milk protein production in xenografts of genetically engineered bovine mammary epithelial stem cells. PLoS ONE. 2010;5(10):e13372. doi: 10.1371/journal.pone.0013372. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 38.Harmon RJ. Physiology of mastitis and factors affecting somatic cell counts. J Dairy Sci. 1994;77:2103–2112. doi: 10.3168/jds.S0022-0302(94)77153-8. [DOI] [PubMed] [Google Scholar]
- 39.Sordillo LM, Streicher KL. Mammary gland immunity and mastitis susceptibility. J Mamm Gland Biol Neoplasia. 2002;7:135–146. doi: 10.1023/a:1020347818725. [DOI] [PubMed] [Google Scholar]
- 40.Vangroenweghe F, Lamote I, Burvenich C. Physiology of the periparturient period and its relation to severity of clinical mastitis. Domest Anim Endocrin. 2005;29:283–293. doi: 10.1016/j.domaniend.2005.02.016. [DOI] [PubMed] [Google Scholar]
- 41.Kittrell FS, arletti MZ, Kerbawy S. et al. Prospective isolation and characterization of committed and multipotent progenitors from immortalized mouse mammary epithelial cells with morphogenic potential. Breast Cancer Res. 2011;13:R41. doi: 10.1186/bcr2863. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 42.Rauner G, Barash I. Cell hierarchy and lineage commitment in the bovine mammary gland. PLoS ONE. 2012;7(1):e30113. doi: 10.1371/journal.pone.0030113. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Zhao X, Lacasse P. Mammary tissue damage during bovine mastitis: Causes and control. J Anim Sci. 2008;86:57–65. doi: 10.2527/jas.2007-0302. [DOI] [PubMed] [Google Scholar]
- 44.Akers RM, Nickerson SC. Mastitis and its Impact on Structure and Function in the Ruminant Mammary Gland. J Mammary Gland Biol Neoplasia. 2011;16:275–289. doi: 10.1007/s10911-011-9231-3. [DOI] [PubMed] [Google Scholar]
- 45.El-Metwally AE, Asfour HAE. Mastitis pathogens in relation to histopathological changes in buffalo udder tissues and supramammary lymph nodes. Egypt J Comp Path & Clinic Path. 2008;21(4):190–208. [Google Scholar]
- 46.Sharma N, Maiti SK. Incidence, etiology and antibiogram of sub clinical mastitis in cows in Durg, Chhattisgarh. Indian J Vet Res. 2010;19(2):45–54. [Google Scholar]
- 47.Singh K, Erdman RA, Swanson KM. et al. Epigenetic Regulation of Milk Production in Dairy Cows. J Mammary Gland Biol Neoplasia. 2010;15:101–112. doi: 10.1007/s10911-010-9164-2. [DOI] [PubMed] [Google Scholar]
- 48.Leitner G, Eligulashvily R, Krifucks O. et al. Immune cell differentiation in mammary gland tissues and milk of cows chronically infected with Staphylococcus aureus. J Vet Med B. 2003;50:45–52. doi: 10.1046/j.1439-0450.2003.00602.x. [DOI] [PubMed] [Google Scholar]
- 49.DeOme KB, Faulkin LJ Jr. Bern HA, et al. Development of mammary tumors from hyperplastic alveolar nodules transplanted into gland-free mammary fat pads of female C3H mice. Cancer Res. 1959;19:515–520. [PubMed] [Google Scholar]
- 50.Ellis S, Capuco AV. Cell proliferation in bovine mammary epithelium: identification of the primary proliferative cell population. Tissue & Cell. 2002;34:155–163. doi: 10.1016/s0040-8166(02)00025-3. [DOI] [PubMed] [Google Scholar]
- 51.Holland MS, Holland RE. The cellular perspective on mammary gland development: stem/progenitor cells and beyond. J Dairy Sci. 2005;88:E1–8. doi: 10.3168/jds.S0022-0302(05)73132-5. [DOI] [PubMed] [Google Scholar]
- 52.Holland MS, Stasko JA, Holland RE. Influence of extracellular matrix on bovine mammary gland progenitor cell growth and differentiation. Am J Vet Res. 2007;68:476–482. doi: 10.2460/ajvr.68.5.476. [DOI] [PubMed] [Google Scholar]
- 53.Motyl T, Bierła JB, Kozłowski M. et al. Identification, quantification and transcriptional profile of potential stem cells in bovine mammary gland. Livestock Sci. 2001;136:136–149. [Google Scholar]
- 54.Holland MS, Tai MH, Trosko JE. et al. Isolation and differentiation of bovine mammary gland progenitor cell populations. Am J Vet Res. 2003;64:396–403. doi: 10.2460/ajvr.2003.64.396. [DOI] [PubMed] [Google Scholar]
- 55.Capuco AV, Evock-Clover CM, Minuti A. et al. In vivo expansion of the mammary stem/progenitor cell population by xanthosine infusion. Exp Biol Med. 2009;234:475–482. doi: 10.3181/0811-RM-320. [DOI] [PubMed] [Google Scholar]
- 56.Hellmen E, Isaksson A. Immunohistochemical investigation into the distribution pattern of myoepithelial cells in the bovine mammary gland. J Dairy Res. 1997;64:197–205. doi: 10.1017/s0022029997002148. [DOI] [PubMed] [Google Scholar]
- 57.Alkafafy M, Rashed R, Helal A. Immunohistochemical studies on the bovine lactating mammary gland (Bos taurus) Acta Histochemica. 2012;114:87–93. doi: 10.1016/j.acthis.2011.02.012. [DOI] [PubMed] [Google Scholar]
- 58.Safayi S, Korn N, Bertram A. et al. Myoepithelial cell differentiation markers in prepubertal bovine mammary gland: Effect of ovariectomy. J Dairy Sci. 2012;95:2965–2976. doi: 10.3168/jds.2011-4690. [DOI] [PubMed] [Google Scholar]
- 59.Kaushik R, Singh KP, Kumari A. et al. Isolation, characterization, and EGFP expression in the buffalo (Bubalus bubalis) mammary gland epithelial cell line. In Vitro Cell & Dev Biol - Anim. 2013;49(1):1–7. doi: 10.1007/s11626-012-9557-1. [DOI] [PubMed] [Google Scholar]
- 60.Capuco AV, Ellis SE. Comparative aspects of mammary gland development and homeostasis. Annu. Rev Anim Biosci. 2013;1:179–202. doi: 10.1146/annurev-animal-031412-103632. [DOI] [PubMed] [Google Scholar]
- 61.Lim E, Vaillant F, Wu D. et al. Aberrant luminal progenitors as the candidate target population for basal tumor development in BRCA1 mutation carriers. Nat Med. 2009;15:907–913. doi: 10.1038/nm.2000. [DOI] [PubMed] [Google Scholar]
- 62.Vorbach C, Capecchi MR, Penninger JM. Evolution of the mammary gland from the innate immune system. Bioessays. 2006;28:606–616. doi: 10.1002/bies.20423. [DOI] [PubMed] [Google Scholar]
- 63.Gray C, Stranberg Y, Donaldson L. et al. Bovine mammary epithelial cells, initiators of innate immune responses to mastitis. Autralian J Exp Agri. 2005;45:757–761. [Google Scholar]
- 64.Stelwagen K, Carpenter E, Haigh B. et al. Immune components of bovine colostrum and milk. J Anim Sci. 2009;87(Suppl. 1):3–9. doi: 10.2527/jas.2008-1377. [DOI] [PubMed] [Google Scholar]
- 65.Medzhitov R, Janeway Jr. CA. Innate immunity: the virtues of a nonclonal system of recognition. Cell. 1997;91:295–298. doi: 10.1016/s0092-8674(00)80412-2. [DOI] [PubMed] [Google Scholar]
- 66.Akira S, Takeda K. Toll-like receptor signalling. Nat Rev Immunol. 2004;4:499–511. doi: 10.1038/nri1391. [DOI] [PubMed] [Google Scholar]
- 67.Sohn EJ. The interelationship between CD14 and LPS during mastitis: release of soluble CD14 and cytokines by bovine PMN following activation with LPS. PhD dissertation, University of Maryland, Baltimore. 2005.
- 68.Kawai T, Akira S. Pathogen recognition with toll-like receptors. Curr Opin Immunol. 2005;17:338–344. doi: 10.1016/j.coi.2005.02.007. [DOI] [PubMed] [Google Scholar]
- 69.Reinhardt TA, Lippolis JD. Bovine milk fat globule membrane proteome. J Dairy Res. 2006;73:406–416. doi: 10.1017/S0022029906001889. [DOI] [PubMed] [Google Scholar]
- 70.Asselin-Labat ML, Sutherland KD, Barker H. et al. Gata-3 is an essential regulator of mammary-gland morphogenesis and luminal-cell differentiation. Nat Cell Biol. 2007;9:201–209. doi: 10.1038/ncb1530. [DOI] [PubMed] [Google Scholar]
- 71.Siegel PM, Muller WJ. Transcription factor regulatory networks in mammary epithelial development and tumorigenesis. Oncogene. 2010;29:2753–2759. doi: 10.1038/onc.2010.43. [DOI] [PubMed] [Google Scholar]
- 72.Machado HL, Kittrell FS, Edwards D, Separation by cell size enriches for mammary stem cell repopulation activity. Stem Cells Trans Med. 2013. doi:10.5966/sctm.2012-0121. [DOI] [PMC free article] [PubMed]
- 73.Stingl J, Emerman JT, Eaves CJ. Enzymatic dissociation and culture of normal human mammary tissue to detect progenitor activity. Methods Mol Biol. 2005;290:249–63. doi: 10.1385/1-59259-838-2:249. [DOI] [PubMed] [Google Scholar]
- 74.Adriance MC, Inman JL, Petersen OW. et al. Myoepithelial cells: Good fences make good neighbors. Breast Cancer Res. 2005;7:190–197. doi: 10.1186/bcr1286. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Murrell TG. The potential for oxytocin (OT) to prevent breast cancer: A hypothesis. Breast Cancer Res Treat. 1995;35:225–29. doi: 10.1007/BF00668213. [DOI] [PubMed] [Google Scholar]
- 76.Zavizion B, Politis I, Gorewit RC. Bovine mammary myoepithelial cells. 1. Isolation, culture and characterization. J Dairy Sci. 1992;15:3367. doi: 10.3168/jds.S0022-0302(92)78113-2. [DOI] [PubMed] [Google Scholar]
- 77.Deugnier MA, Moiseyeva EP, Thiery JP. et al. Myoepithelial cell differentiation in the developing mammary gland: progressive acquisition of smooth muscle phenotype. Dev Dynamics. 1995;204:107–117. doi: 10.1002/aja.1002040202. [DOI] [PubMed] [Google Scholar]
- 78.Zhao X, Malhotra GK, Band H. et al. Derivation of myoepithelial progenitor cells from bipotent mammary stem/progenitor cells. PLoS ONE. 2012;7(4):e35338. doi: 10.1371/journal.pone.0035338. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 79.Aggarwal R, Lu J, Pompili VJ. et al. Hematopoietic stem cells: Transcriptional regulation, ex-vivo expansion and clinical application. Curr Mol Med. 2012;12(1):34–49. doi: 10.2174/156652412798376125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 80.Werling D, Piercy J, Coffey TJ. Expression of TOLL-like receptors (TLR) by bovine antigen-presenting cells—Potential role in pathogen discrimination. Vet Immunol Immunopathol. 2006;112:2–11. doi: 10.1016/j.vetimm.2006.03.007. [DOI] [PubMed] [Google Scholar]
- 81.Jeong DK, Sharma N, Kim JN. et al. Optimization of techniques for isolation and identification of bovine hematopoietic stem cells from peripheral blood. Reprod Develop Biol. 2012;36(2):140. [Google Scholar]
- 82.Fernandez N, Renedo M, Garcia-Rodriguez C. et al. Activation of monocytic cells through Fc gamma receptors induces the expression of macrophage-inflammatory protein (MIP)-1 alpha, MIP-1 beta, and RANTES. J Immunol. 2002;169:3321–3328. doi: 10.4049/jimmunol.169.6.3321. [DOI] [PubMed] [Google Scholar]
- 83.Havemann K, Pujol BF, Adamkiewicz J. In vitro transformation of monocytes and dendritic cells into endothelial like cells. Adv Exp Med Biol. 2003;522:47–57. doi: 10.1007/978-1-4615-0169-5_6. [DOI] [PubMed] [Google Scholar]
- 84.Sozzani S, Rusnati M, Riboldi E. et al. Dendritic cell-endothelial cell cross-talk in angiogenesis. Trends Immunol. 2007;28(9):385–392. doi: 10.1016/j.it.2007.07.006. [DOI] [PubMed] [Google Scholar]
- 85.Chan RK, Garfein E, Gigante PR. et al. Side population hematopoietic stem cells promote wound healing in diabetic mice. Plast Reconstr Surg. 2007;120(2):407–411. doi: 10.1097/01.prs.0000267696.98789.66. [DOI] [PubMed] [Google Scholar]
- 86.McLaughlin F, Ludbrook VJ, Cox J. et al. Combined genomic and antisense analysis reveals that the transcription factor Erg is implicated in endothelial cell differentiation. Blood. 2001;98:3332–3339. doi: 10.1182/blood.v98.12.3332. [DOI] [PubMed] [Google Scholar]
- 87.Yu C, Shi ZR, Chu CY. et al. Expression of bovine granulocyte hemotactic protein-2 (GCP-2) in neutrophils and a mammary epithelial cell line (MAC-T) in response to various bacterial cell wall components. Vet J. 2010;186:89–95. doi: 10.1016/j.tvjl.2009.07.012. [DOI] [PubMed] [Google Scholar]
- 88.Carmeliet P. Mechanisms of angiogenesis and arteriogenesis. Nat Med. 2000;6:389–395. doi: 10.1038/74651. [DOI] [PubMed] [Google Scholar]
- 89.Devanesan AJ, Laughlan KA, Girn HRS. et al. Endothelial progenitor cells as a therapeutic option in peripheral arterial disease. Eur J Vasc Endovasc Surg. 2009;38:475–481. doi: 10.1016/j.ejvs.2009.05.019. [DOI] [PubMed] [Google Scholar]
- 90.Li JX, Zhang Y, Ma LB. et al. Isolation and culture of bovine mammary epithelial stem cells. J Vet Med Sci/Japanese Soc Vet Sci. 2009;71:15–19. doi: 10.1292/jvms.71.15. [DOI] [PubMed] [Google Scholar]
- 91.Timmermans F, Plum J, Yoder MC. et al. Endothelial progenitor cells: identity defined. J Cell Mol Med. 2009;13(1):87–102. doi: 10.1111/j.1582-4934.2008.00598.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 92.Werner N, Kosiol S, Schiegl T. et al. Circulating endothelial progenitor cells and cardiovascular outcomes. N Engl J Med. 2005;353:999–1007. doi: 10.1056/NEJMoa043814. [DOI] [PubMed] [Google Scholar]
- 93.Yip HK, Chang LT, Chang WN. et al. Level and value of circulating endothelial progenitor cells in patients after acute ischemic stroke. Stroke. 2007;39:69–74. doi: 10.1161/STROKEAHA.107.489401. [DOI] [PubMed] [Google Scholar]
- 94.Allanore Y, Batteux F, Avouac J. et al. Levels of circulating endothelial progenitor cells in systemic sclerosis. Clin Exp Rheumatol. 2007;25:60–66. [PubMed] [Google Scholar]
- 95.Cogle CR, Wainman DA, Jorgensen ML. et al. Adult human hematopoietic cells provide functional hemangioblast activity. Blood. 2004;103:133–135. doi: 10.1182/blood-2003-06-2101. [DOI] [PubMed] [Google Scholar]
- 96.Kondo T, Hayashi M, Takeshita K. et al. Smoking cessation rapidly increases circulating progenitor cells in peripheral blood in chronic smokers. Arterioscler Thromb Vasc Biol. 2004;24:1442–1447. doi: 10.1161/01.ATV.0000135655.52088.c5. [DOI] [PubMed] [Google Scholar]
- 97.Delorme BA, Gentile C, Sabatier F. et al. Presence of endothelial progenitor cells, distinct from mature endothelial cells, within human CD146+ blood cells. Thromb Haemost. 2005;94:1270–1279. doi: 10.1160/TH05-07-0499. [DOI] [PubMed] [Google Scholar]
- 98.Povsic TJ, Zavodn KL, Kelly FL. et al. Circulating progenitor cells can be reliably identified on the basis of aldehyde dehydrogenase activity. J Am Coll Cardiol. 2007;50:2243–2248. doi: 10.1016/j.jacc.2007.08.033. [DOI] [PubMed] [Google Scholar]
- 99.Takahashi T, Kalka C, Masuda H. et al. Ischemia- and cytokineinduced mobilization of bone marrowderived endothelial progenitor cells for neovascularization. Nat Med. 1999;5:434–438. doi: 10.1038/7434. [DOI] [PubMed] [Google Scholar]
- 100.Zhu S, Liu X, Li Y. et al. Aging in the atherosclerosis milieu may accelerate the consumption of bone marrow endothelial progenitor cells. Arterioscler Thromb Vasc Biol. 2007;27:113–139. doi: 10.1161/01.ATV.0000252035.12881.d0. [DOI] [PubMed] [Google Scholar]
- 101.Chung A. How different progenitor cells are called from the bone marrow. Nature Reports Stem Cell. 2009 DOI:10.1038/stemcells.2009.20. [Google Scholar]
- 102.Munoz JR, Stoutenger BR, Robinson AP. et al. Human stem progenitor cells from bone marrow promote neurogenesis of endogenous neural stem cells in the hippocampus of mice. PNAS. 2005;102(50):18171–18176. doi: 10.1073/pnas.0508945102. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 103.Rawadi G, Vayssiere B, Dunn F. et al. BMP2 controls alkaline phosphatase expression and osteoblast mineralization by a Wnt autocrine loop. J Bone Miner Res. 2003;18:1842–1853. doi: 10.1359/jbmr.2003.18.10.1842. [DOI] [PubMed] [Google Scholar]
- 104.Harris DT, Hilgaertner J, Simonson C. et al. Cell-based therapy for epithelial wounds. Cytotherapy. 2012;14(7):802–810. doi: 10.3109/14653249.2012.671520. [DOI] [PubMed] [Google Scholar]
- 105.Leonard S, Khatib H, Schutzkus V. et al. Effects of the osteopontin gene variants on milk production traits in dairy cattle. J Dairy Sci. 2005;88:4083–4086. doi: 10.3168/jds.S0022-0302(05)73092-7. [DOI] [PubMed] [Google Scholar]
- 106.Takahashi K, Yamanaka S. Induction of pluripotent stem cells from mouse embryonic and adult fibroblast cultures by defined factors. Cell. 2006;126:663–676. doi: 10.1016/j.cell.2006.07.024. [DOI] [PubMed] [Google Scholar]
- 107.Zhang D, Jiang W, Liu M. et al. Highly efficient differentiation of human ES cells and iPS cells into mature pancreatic insulin-producing cells. Cell Res. 2009;19:429–438. doi: 10.1038/cr.2009.28. [DOI] [PubMed] [Google Scholar]
- 108.Han X, Han J, Ding F. et al. Generation of induced pluripotent stem cells from bovine embryonic fibroblast cells. Cell Res. 2011;21:1509–1512. doi: 10.1038/cr.2011.125. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Huang B, Li T, Alonso-Gonzalez L. et al. A virus-free poly-promoter vector induces pluripotency in quiescent bovine cells under chemically defined conditions of dual kinase inhibition. PLoS ONE. 2011;6:e24501. doi: 10.1371/journal.pone.0024501. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 110.Sumer H, Liu J, Malaver-Ortega LF. et al. NANOG is a key factor for induction of pluripotency in bovine adult fibroblasts. J Anim Sci. 2011;89:2708–2716. doi: 10.2527/jas.2010-3666. [DOI] [PubMed] [Google Scholar]
- 111.Cao H, Yang P, Pu Y. et al. Characterization of bovine induced pluripotent stem cells by lentiviral transduction of reprogramming factor fusion proteins. Int J Biol Sci. 2012;8(4):498–511. doi: 10.7150/ijbs.3723. [DOI] [PMC free article] [PubMed] [Google Scholar]