Abstract
The incorporation of 3H-thymidine (3H-dT) into deoxyribonucleic acid (DNA) has been studied in uninfected confluent monolayer cultures of monkey kidney and mouse kidney cells, simian virus 40 (SV40)-infected cells, and in SV40-transformed mouse kidney cells. Radioautographic measurements revealed that during the period from 28 to 51 hr after productive SV40 infection of monkey kidney cultures about 80% of the cells synthesized DNA, compared to about 16% in uninfected cultures. At 28 to 43 hr after abortive SV40 infection of mouse kidney cultures, 24 to 37% of the cells synthesized DNA, compared to about 6 to 8% in uninfected cultures. The infected monkey kidney and mouse kidney cultures, respectively, incorporated about 5 to 10 times and 3 to 5 times as much 3H-dT into DNA as did uninfected cultures. Moreover, the net DNA synthesized by SV40-infected monkey kidney cultures, estimated by colorimetric methods, substantially exceeded that of uninfected cultures.
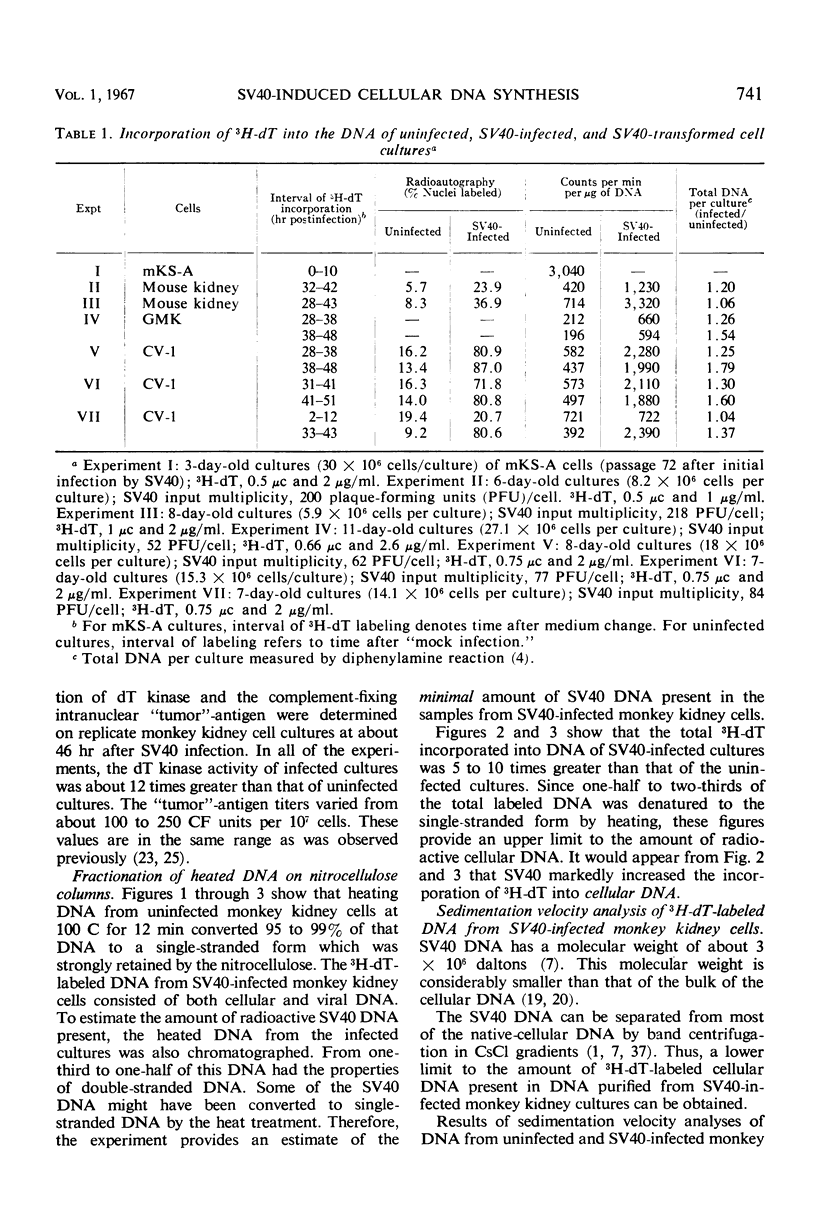
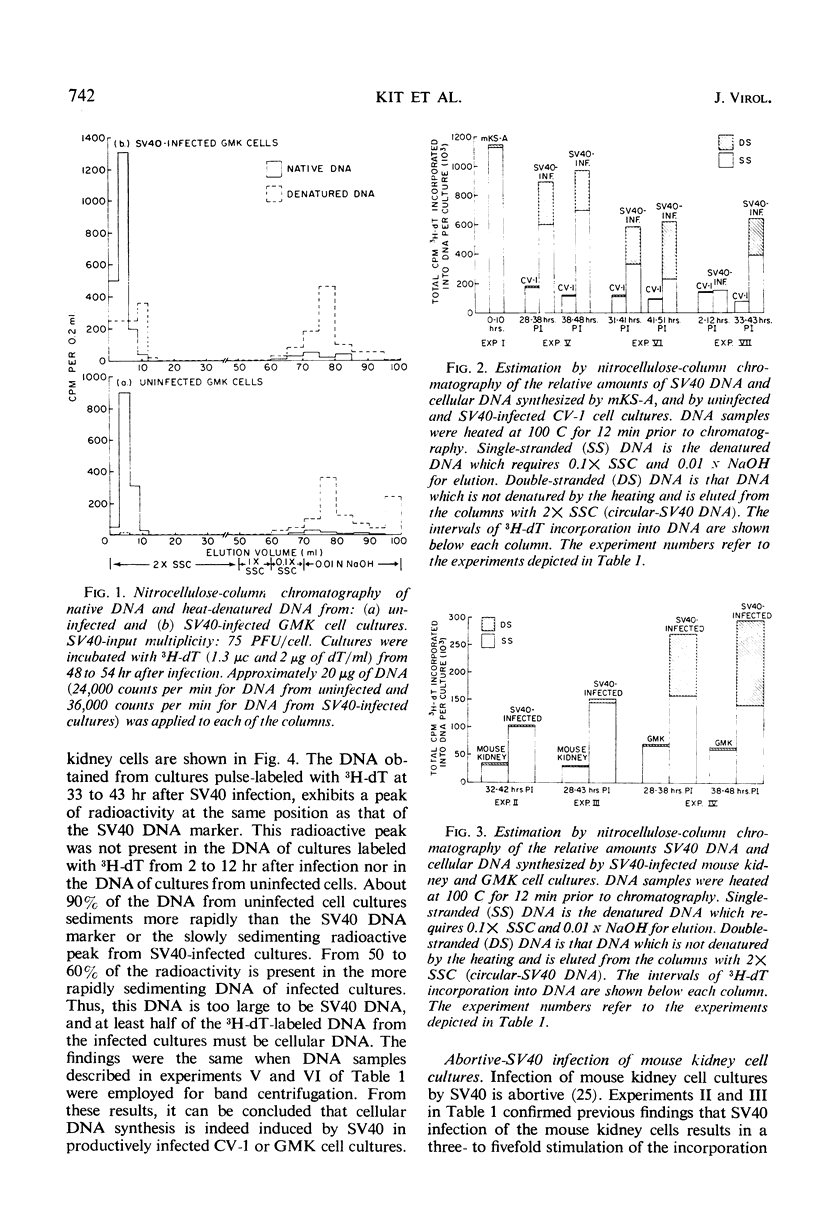
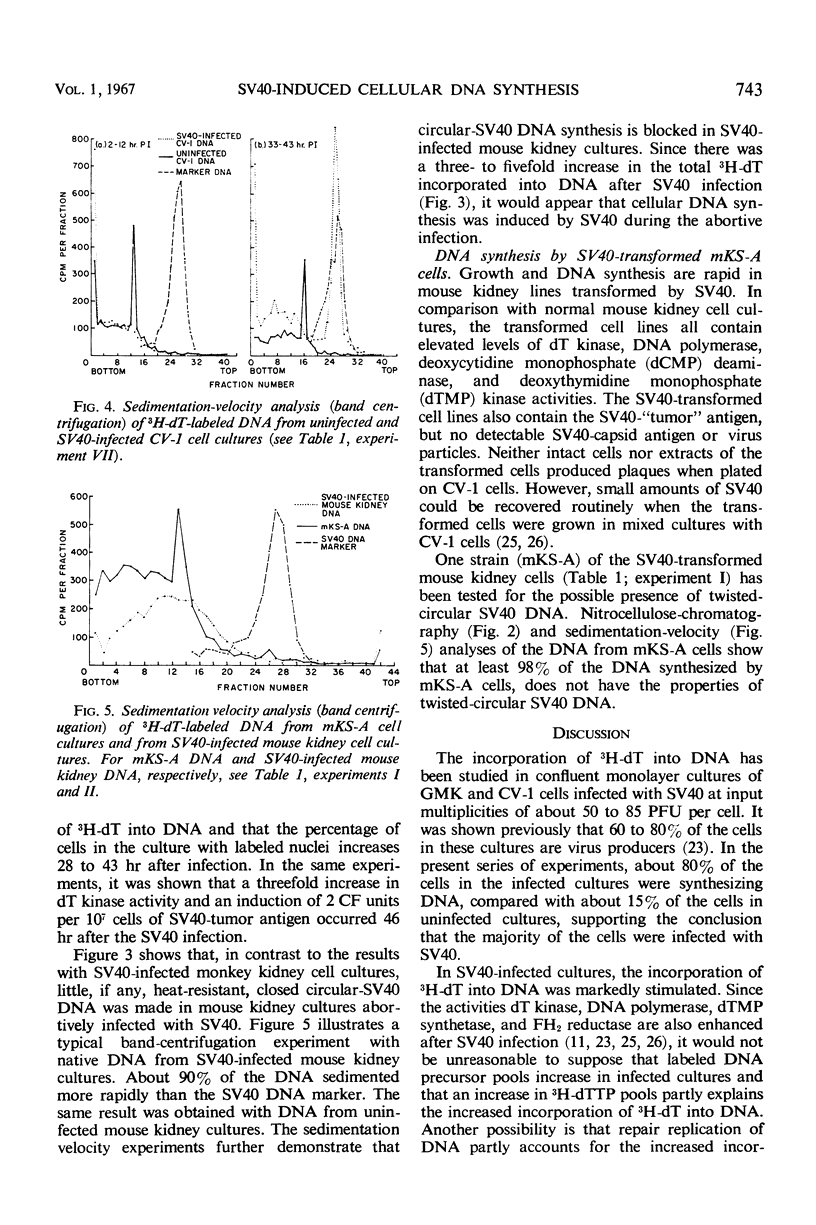
Nitrocellulose chromatography and band centrifugation experiments were performed to elucidate the kinds of DNA synthesized in the cultures. In uninfected monkey kidney cultures and at 2 to 12 hr after SV40 infection, almost all of the 3H-dT labeled DNA sedimented more rapidly than SV40 DNA, and the radioactive DNA was denatured by heating for 12 min at 100 C (cellular DNA). Almost all of the labeled DNA obtained from abortively infected mouse kidney cultures and from SV40-transformed cells also had the properties of cellular DNA. However, approximately one-third to one-half of the labeled DNA obtained from monkey kidney cultures 28 to 51 hr after infection sedimented more slowly than cellular DNA and was not denatured by the heating (SV40 DNA). It is concluded that cellular DNA synthesis was induced during either the productive or abortive SV40 infections.
Full text
PDF





Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BEN-PORAT T., KAPLAN A. S. The synthesis and fate of pseudorabies virus DNA in infected mammalian cells in the stationary phase of growth. Virology. 1963 Jun;20:310–317. doi: 10.1016/0042-6822(63)90120-x. [DOI] [PubMed] [Google Scholar]
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Basilico C., Marin G., di Mayorca G. Requirement for the integrity of the viral genome for the induction of host DNA synthesis by polyoma virus. Proc Natl Acad Sci U S A. 1966 Jul;56(1):208–215. doi: 10.1073/pnas.56.1.208. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ben-Porat T., Coto C., Kaplan A. S. Unstable DNA synthesized by polyoma virus-infected cells. Virology. 1966 Sep;30(1):74–81. doi: 10.1016/s0042-6822(66)81011-5. [DOI] [PubMed] [Google Scholar]
- CRAWFORD L. V., BLACK P. H. THE NUCLEIC ACID OF SIMIAN VIRUS 40. Virology. 1964 Nov;24:388–392. doi: 10.1016/0042-6822(64)90176-x. [DOI] [PubMed] [Google Scholar]
- Carp R. I., Gilden R. V. A comparison of the replication cycles of simian virus 40 in human diploid and African green monkey kidney cells. Virology. 1966 Jan;28(1):150–162. doi: 10.1016/0042-6822(66)90316-3. [DOI] [PubMed] [Google Scholar]
- DUBBS D. R., KIT S. THE EFFECT OF TEMPERATURE ON INDUCTION OF DEOXYTHYMIDINE KINASE ACTIVITY BY HERPERS SIMPLEX MUTANTS. Virology. 1965 Feb;25:256–270. doi: 10.1016/0042-6822(65)90204-7. [DOI] [PubMed] [Google Scholar]
- DULBECCO R., HARTWELL L. H., VOGT M. INDUCTION OF CELLULAR DNA SYNTHESIS BY POLYOMA VIRUS. Proc Natl Acad Sci U S A. 1965 Feb;53:403–410. doi: 10.1073/pnas.53.2.403. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DULBECCO R., VOGT M. EVIDENCE FOR A RING STRUCTURE OF POLYOMA VIRUS DNA. Proc Natl Acad Sci U S A. 1963 Aug;50:236–243. doi: 10.1073/pnas.50.2.236. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frearson P. M., Kit S., Dubbs D. R. Induction of dihydrofolate reductase activity by SV40 and polyoma virus. Cancer Res. 1966 Aug;26(8):1653–1660. [PubMed] [Google Scholar]
- GINSBERG H. S., DIXON M. K. Nucleuc acid synthesis in types 4 and 5 adenovirus-infected HeLa cells. J Exp Med. 1961 Feb 1;113:283–299. doi: 10.1084/jem.113.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon D., Hausen P., Sachs L., Winocour E. On the mechanism of polyoma virus-induced synthesis of cellular DNA. Proc Natl Acad Sci U S A. 1965 Dec;54(6):1584–1592. doi: 10.1073/pnas.54.6.1584. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Gershon D., Sachs L., Winocour E. The induction of cellular DNA synthesis by simian virus 40 in contact-inhibited and in x-irradiated cells. Proc Natl Acad Sci U S A. 1966 Sep;56(3):918–925. doi: 10.1073/pnas.56.3.918. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HANAWALT P. C., HAYNES R. H. REPAIR REPLICATION OF DNA IN BACTERIA: IRRELEVANCE OF CHEMICAL NATURE OF BASE DEFECT. Biochem Biophys Res Commun. 1965 May 3;19:462–467. doi: 10.1016/0006-291x(65)90147-6. [DOI] [PubMed] [Google Scholar]
- Hatanaka M., Dulbecco R. Induction of DNA synthesis by SV40. Proc Natl Acad Sci U S A. 1966 Aug;56(2):736–740. doi: 10.1073/pnas.56.2.736. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Henry P., Black P. H., Oxman M. N., Weissman S. M. Stimulation of DNA synthesis in mouse cell line 3T3 by Simian virus 40. Proc Natl Acad Sci U S A. 1966 Oct;56(4):1170–1176. doi: 10.1073/pnas.56.4.1170. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIRBY K. S. A new method for the isolation of deoxyribonucleic acids; evidence on the nature of bonds between deoxyribonucleic acid and protein. Biochem J. 1957 Jul;66(3):495–504. doi: 10.1042/bj0660495. [DOI] [PMC free article] [PubMed] [Google Scholar]
- KIT S. Compositional heterogeneity of normal and malignant tissue deoxyribonucleic acids (DNA). Biochem Biophys Res Commun. 1960 Oct;3:361–367. doi: 10.1016/0006-291x(60)90045-0. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R. BIOCHEMISTRY OF VACCINIA-INFECTED MOUSE FIBROBLASTS (STRAIN L-M). IV. 3H-THYMIDINE UPTAKE INTO DNA OF CELLS EXPOSED TO COLD SHOCK. Exp Cell Res. 1963 Aug;31:397–406. doi: 10.1016/0014-4827(63)90016-8. [DOI] [PubMed] [Google Scholar]
- KIT S., DUBBS D. R., HSU T. C. Biochemistry of vaccinia-infected mouse fibroblasts (strain L-M). III. Radioautographic and biochemical studies of thymidine-H3 uptake into DNA of L-M cells and rabbit cells in primary culture. Virology. 1963 Jan;19:13–22. doi: 10.1016/0042-6822(63)90019-9. [DOI] [PubMed] [Google Scholar]
- KIT S. Equilibrium sedimentation in density gradients of DNA preparations from animal tissues. J Mol Biol. 1961 Dec;3:711–716. doi: 10.1016/s0022-2836(61)80075-2. [DOI] [PubMed] [Google Scholar]
- Kit S., Dubbs D. R., Frearson P. M. Enzymes of nucleic acid metabolism in cells infected with polyoma virus. Cancer Res. 1966 Apr;26(4):638–646. [PubMed] [Google Scholar]
- Kit S., Dubbs D. R., Frearson P. M., Melnick J. L. Enzyme induction in SV40-infected green monkey kidney cultures. Virology. 1966 May;29(1):69–83. doi: 10.1016/0042-6822(66)90197-8. [DOI] [PubMed] [Google Scholar]
- Kit S., Dubbs D. R., Piekarski L. J., de Torres R. A., Melnick J. L. Acquisition of enzyme function by mouse kidney cells abortively infected with papovavirus SV40. Proc Natl Acad Sci U S A. 1966 Aug;56(2):463–470. doi: 10.1073/pnas.56.2.463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kit S., Piekarski L. J., Dubbs D. R. DNA polymerase induced by Simian virus 40. J Gen Virol. 1967 Apr;1(2):163–173. doi: 10.1099/0022-1317-1-2-163. [DOI] [PubMed] [Google Scholar]
- Klamerth O. Separation of high-molecular deoxyribonucleic acid and ribonucleic acid. Nature. 1965 Dec 25;208(5017):1318–1319. doi: 10.1038/2081318a0. [DOI] [PubMed] [Google Scholar]
- Rapp F., Feldman L. A., Mandel M. Synthesis of virus deoxyribonucleic acid during abortive infection of simian cells by human adenoviruses. J Bacteriol. 1966 Oct;92(4):931–936. doi: 10.1128/jb.92.4.931-936.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Sauer G., Fischer H., Munk K. The effect of SV40 infection on DNA synthesis in cercopithecus kidney cells. Virology. 1966 Apr;28(4):765–767. doi: 10.1016/0042-6822(66)90264-9. [DOI] [PubMed] [Google Scholar]
- Sheinin R. DNA synthesis in rat embryo cells infected with polyoma virus. Virology. 1966 May;29(1):167–170. doi: 10.1016/0042-6822(66)90206-6. [DOI] [PubMed] [Google Scholar]
- Sheinin R. Deoxyribonucleic acid synthesis in cells replicating polyoma virus. Virology. 1966 Apr;28(4):621–632. doi: 10.1016/0042-6822(66)90247-9. [DOI] [PubMed] [Google Scholar]
- VINOGRAD J., BRUNER R., KENT R., WEIGLE J. Band-centrifugation of macromolecules and viruses in self-generating density gradients. Proc Natl Acad Sci U S A. 1963 Jun;49:902–910. doi: 10.1073/pnas.49.6.902. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vinograd J., Lebowitz J., Radloff R., Watson R., Laipis P. The twisted circular form of polyoma viral DNA. Proc Natl Acad Sci U S A. 1965 May;53(5):1104–1111. doi: 10.1073/pnas.53.5.1104. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vogt M., Dulbecco R., Smith B. Induction of cellular DNA synthesis by polyoma virus. 3. Induction in productively infected cells. Proc Natl Acad Sci U S A. 1966 Apr;55(4):956–960. doi: 10.1073/pnas.55.4.956. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weil R., Michel M. R., Ruschmann G. K. Induction of cellular DNA synthesis by polyoma virus. Proc Natl Acad Sci U S A. 1965 Jun;53(6):1468–1475. doi: 10.1073/pnas.53.6.1468. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Winocour E., Kaye A. M., Stollar V. Synthesis and transmethylation of DNA in polyoma-infected cultures. Virology. 1965 Oct;27(2):156–169. doi: 10.1016/0042-6822(65)90155-8. [DOI] [PubMed] [Google Scholar]



