Abstract
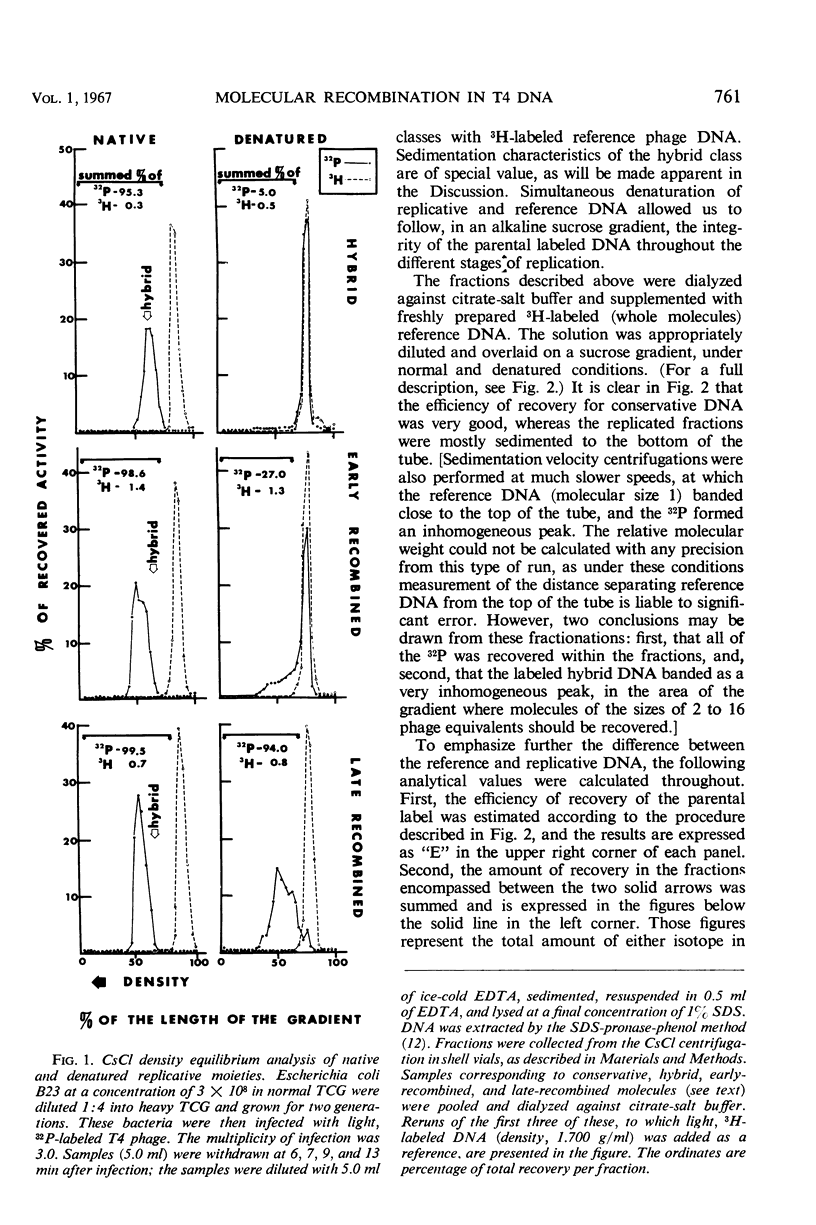
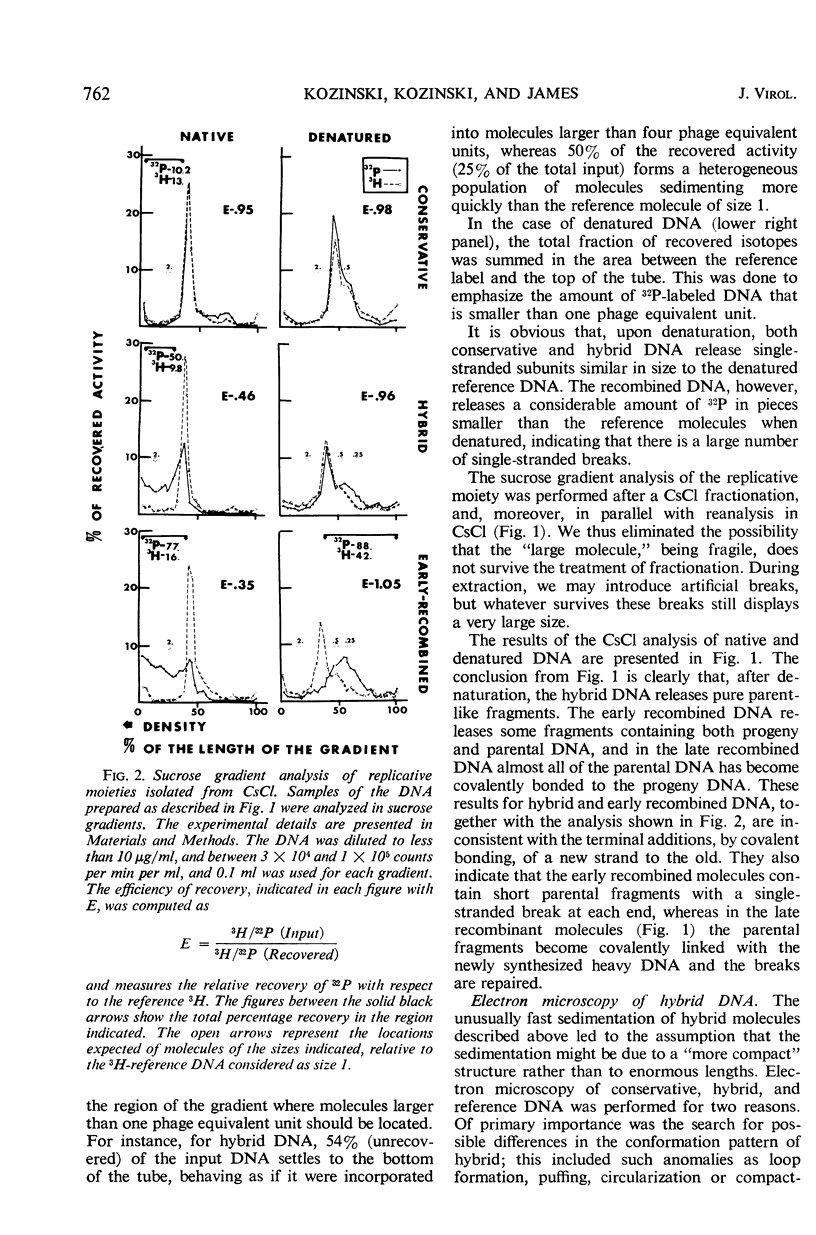
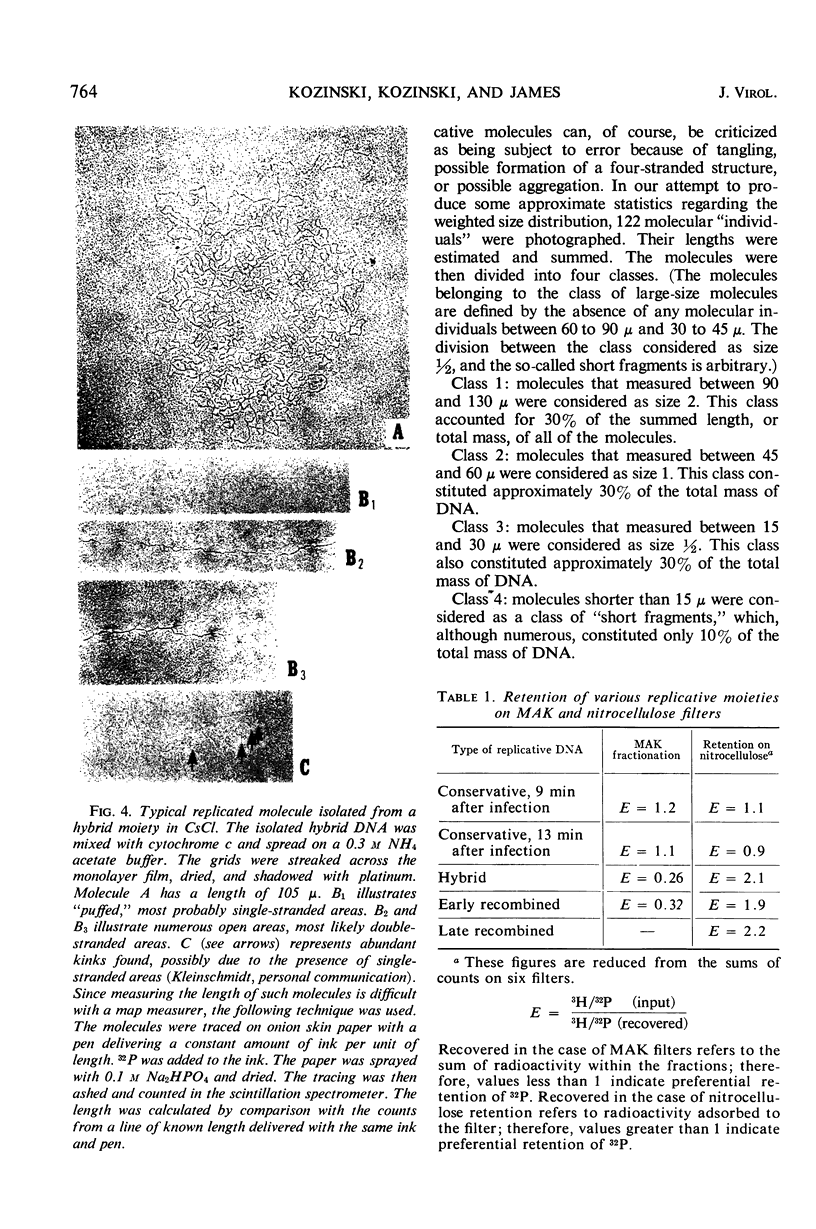
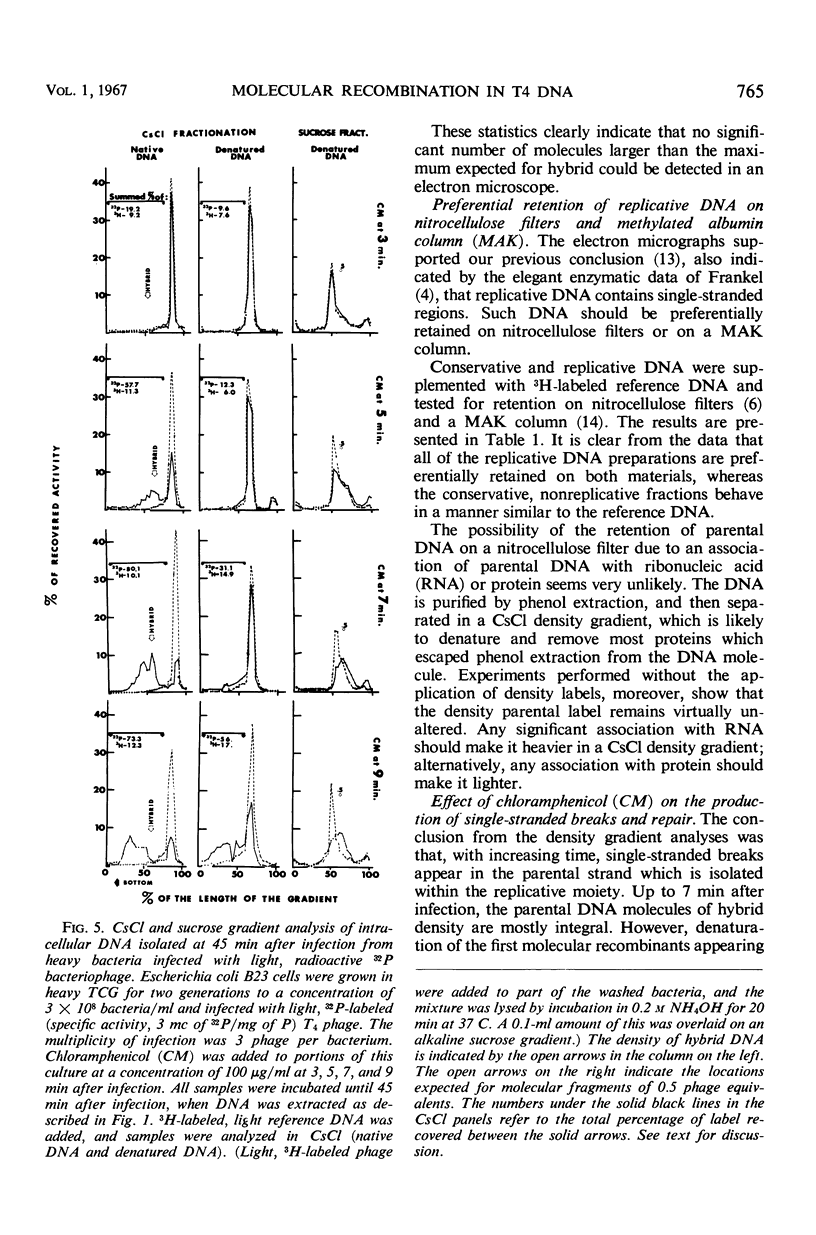
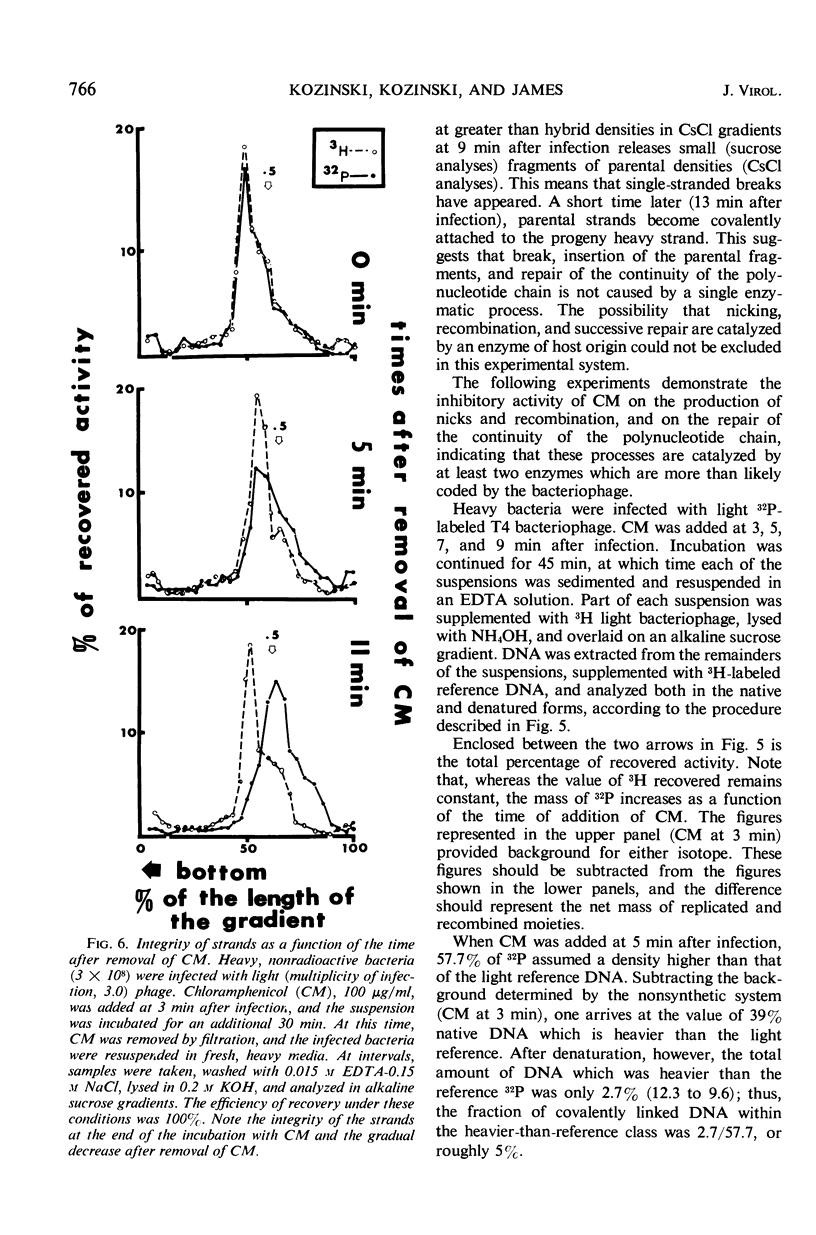
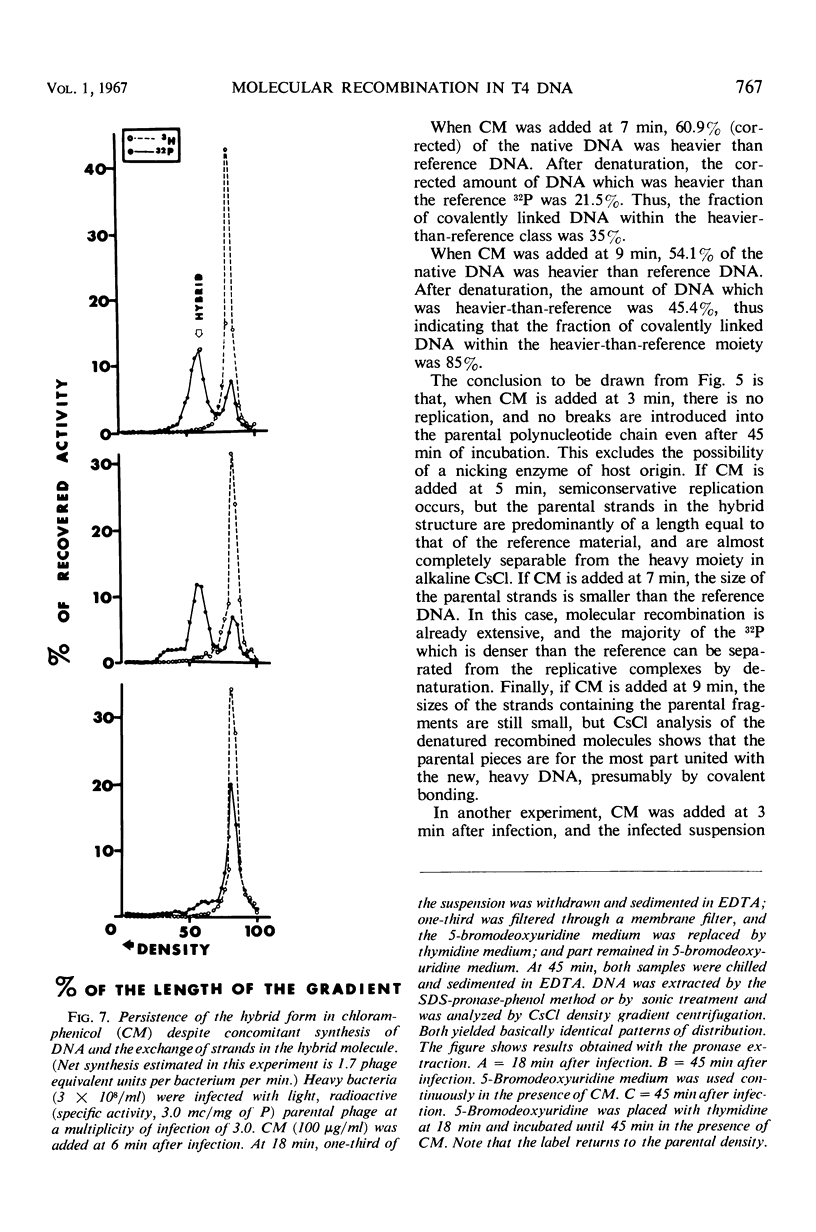
A replicative hybrid resulting from the infection of heavy (substituted with 5-bromodeoxyuridine) bacteria with light (not substituted with 5-bromodeoxyuridine) radioactive bacteriophage was isolated from a CsCl density gradient. Sedimentation studies indicate that 60% of the deoxyribonucleic acid (DNA) behaves as if it were in units more than four times as large as an intact reference molecule. Under the electron microscope, hybrid molecules appeared tangled, showed puffs and loops, occupied a small area, and often had a total length twice that of mature phage. This indicates that sucrose gradient sedimentation is not applicable as a method for estimating the relative molecular size of replicative forms of DNA. After denaturation, the separated strands of hybrid were of the same size as those of reference DNA. CsCl density gradient analysis revealed no terminal covalent addition of new material to the old parental strand. The possibility of a continuous growth of the DNA molecule, either on a single-stranded level or as a double helical structure, is disproved. When chloramphenicol (CM) was added at critical times after infection, DNA synthesis continued at a constant rate. The parental label soon assumed and retained a hybrid density, despite concomitant synthesis of DNA, throughout the rest of the period of incubation in CM. The hybrid moiety, however, actively participated in replication and exchanged its partner strand for a new one; this was demonstrated by changing the density label during incubation in CM. A new enzyme synthesized shortly after infection introduced single-stranded “nicks” into the parental DNA. Since nicking can be inhibited by chloramphenicol, the responsible enzyme is not of host origin. The time of the appearance of this enzyme coincided with the onset of molecular recombination. Another enzyme, which mediates the repair of the continuity of the polynucleotide chain after recombination, appeared after recombination. If selectively inhibited by chloramphenicol, recombinant molecules remained unrepaired, and, upon denaturation, the parental fragment was liberated in pure form.
Full text
PDF






Images in this article
Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- Anraku N., Tomizawa J. Molecular mechanisms of genetic recombination of bacteriophage. V. Two kinds of joining of parental DNA molecules. J Mol Biol. 1965 Jul;12(3):805–815. doi: 10.1016/s0022-2836(65)80329-1. [DOI] [PubMed] [Google Scholar]
- BERNS K. I., THOMAS C. A., Jr ISOLATION OF HIGH MOLECULAR WEIGHT DNA FROM HEMOPHILUS INFLUENZAE. J Mol Biol. 1965 Mar;11:476–490. doi: 10.1016/s0022-2836(65)80004-3. [DOI] [PubMed] [Google Scholar]
- BURGI E., HERSHEY A. D. Sedimentation rate as a measure of molecular weight of DNA. Biophys J. 1963 Jul;3:309–321. doi: 10.1016/s0006-3495(63)86823-x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FRANKEL F. R. An unusual DNA extracted from bacteria infected with phage T2. Proc Natl Acad Sci U S A. 1963 Mar 15;49:366–372. doi: 10.1073/pnas.49.3.366. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Frankel F. R. Studies on the nature of replicating DNA in T4-infected Escherichia coli. J Mol Biol. 1966 Jun;18(1):127–143. doi: 10.1016/s0022-2836(66)80081-5. [DOI] [PubMed] [Google Scholar]
- Frankel F. R. The absence of mature phage DNA molecules from the replicating pool of T-even-infected Escherichia coli. J Mol Biol. 1966 Jun;18(1):109–126. doi: 10.1016/s0022-2836(66)80080-3. [DOI] [PubMed] [Google Scholar]
- Gillespie D., Spiegelman S. A quantitative assay for DNA-RNA hybrids with DNA immobilized on a membrane. J Mol Biol. 1965 Jul;12(3):829–842. doi: 10.1016/s0022-2836(65)80331-x. [DOI] [PubMed] [Google Scholar]
- KOZINSKI A. W. Fragmentary transfer of P32-labeled parental DNA to progeny phage. Virology. 1961 Jan;13:124–134. doi: 10.1016/0042-6822(61)90039-3. [DOI] [PubMed] [Google Scholar]
- KOZINSKI A. W., KOZINSKI P. B. Fragmentary transfer of P32-labeled parental DNA to progeny phage. II. The average size of the transferred parental fragment. Two-cycletransfer. Repair of the polynucleotide chain after fragmentation. Virology. 1963 Jun;20:213–229. doi: 10.1016/0042-6822(63)90109-0. [DOI] [PubMed] [Google Scholar]
- KOZINSKI A. W., KOZINSKI P. B. REPLICATIVE FRAGMENTATION IN T4 BACTERIOPHAGE DNA. II. BIPARENTAL MOLECULAR RECOMBINATION. Proc Natl Acad Sci U S A. 1964 Aug;52:211–218. doi: 10.1073/pnas.52.2.211. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinski A. W., Kozinski P. B. Early intracellular events in the replication T4 phage DNA. II. Partially replicated DNA. Proc Natl Acad Sci U S A. 1965 Aug;54(2):634–640. doi: 10.1073/pnas.54.2.634. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kozinski A. W., Lin T. H. Early intracellular events in the replication of T4 phage DNA. I. Complex formation of replicative DNA. Proc Natl Acad Sci U S A. 1965 Jul;54(1):273–278. doi: 10.1073/pnas.54.1.273. [DOI] [PMC free article] [PubMed] [Google Scholar]
- MANDELL J. D., HERSHEY A. D. A fractionating column for analysis of nucleic acids. Anal Biochem. 1960 Jun;1:66–77. doi: 10.1016/0003-2697(60)90020-8. [DOI] [PubMed] [Google Scholar]
- MESELSON M. ON THE MECHANISM OF GENETIC RECOMBINATION BETWEEN DNA MOLECULES. J Mol Biol. 1964 Sep;9:734–745. doi: 10.1016/s0022-2836(64)80178-9. [DOI] [PubMed] [Google Scholar]
- Shahn E., Kozinski A. Fragmentary transfer of P32 labeled parental DNA to progeny phage. 3. Incorporation of a single parental fragment to the progeny molecule. Virology. 1966 Nov;30(3):455–470. doi: 10.1016/0042-6822(66)90122-x. [DOI] [PubMed] [Google Scholar]
- TOMIZAWA J., ANRAKU N. MOLECULAR MECHANISMS OF GENETIC RECOMBINATION IN BACTERIOPHAGE. IV. ABSENCE OF POLYNUCLEOTIDE INTERRUPTION IN DNA OF T4 AND LAMBDA PHAGE PARTICLES, WITH SPECIAL REFERENCE TO HETEROZYGOSIS. J Mol Biol. 1965 Mar;11:509–527. doi: 10.1016/s0022-2836(65)80007-9. [DOI] [PubMed] [Google Scholar]
- Thomas C. A., Jr Recombination of DNA molecules. Prog Nucleic Acid Res Mol Biol. 1966;5:315–337. doi: 10.1016/s0079-6603(08)60237-8. [DOI] [PubMed] [Google Scholar]
- Weiss B., Richardson C. C. Enzymatic breakage and joining of deoxyribonucleic acid, I. Repair of single-strand breaks in DNA by an enzyme system from Escherichia coli infected with T4 bacteriophage. Proc Natl Acad Sci U S A. 1967 Apr;57(4):1021–1028. doi: 10.1073/pnas.57.4.1021. [DOI] [PMC free article] [PubMed] [Google Scholar]