Abstract
Objective
Evidence suggests that chronic low-grade inflammation and oxidative stress are related to cardiovascular disease (CVD) and mortality.
Approach and Results
We examined 11 established and novel biomarkers representing inflammation and oxidative stress (C-reactive protein [CRP], fibrinogen, interleukin-6, intercellular adhesion molecule-1 [ICAM-1], lipoprotein-associated phospholipase A2 (mass and activity), monocyte chemoattractant protein-1, myeloperoxidase, CD40 ligand, P-selectin, tumor necrosis factor receptor II [TNFRII]) in relation to incident major CVD and mortality in the community. We studied 3035 participants (mean age 61±9 years, 53% women). During follow-up (median 8.9 years), 253 participants experienced a CVD event and 343 died. CRP (hazard ratios [HR] reported per standard deviation ln-transformed biomarker, 1.18, 95% confidence interval [CI] 1.02-1.35; nominal P=0.02) and TNFRII (HR 1.15, 95% CI; 1.01-1.32; nominal P=0.04) were retained in multivariable-adjusted models for major CVD, but were not significant after adjustment for multiple testing. The biomarkers related to mortality were TNFRII (HR 1.33, 95% CI: 1.19-1.49; P<0.0001); ICAM-1 (HR 1.24, 95% CI: 1.12-1.37; P<0.0001), and interleukin-6 (HR 1.25, 95% CI: 1.12-1.39; P<0.0001). The addition of these markers to the model including traditional risk factors increased discrimination and reclassification for risk of death (P<0.0001), but not for CVD.
Conclusions
Of 11 biomarkers, TNFRII was associated nominally with incident major CVD, and significantly with all-cause mortality, which renders it an interesting target for future research. The combination of TNFRII with CRP in relation to CVD and with interleukin-6 to mortality increased the predictive ability in addition to CVD risk factors for total mortality but not for incident CVD.
Keywords: mortality, cardiovascular disease, inflammation, epidemiology, cohort
Cardiovascular diseases (CVD), diabetes, chronic lower respiratory disease, arthritis, and cancer are the major causes of associated morbidity and mortality in aging populations such as the United States (http://www.cdc.gov/nchs/nhis.htm). Each of these conditions is associated with a pro-inflammatory state.1-3 On the contrary, avoidance of CVD risk factors and the absence of low-grade chronic inflammation delay or prevent onset of disease and are strongly related to survival and successful aging.4,5
Circulating fibrinogen, C-reactive protein (CRP) and white blood count were the first inflammatory biomarkers investigated in large-scale studies of initially healthy individuals, mostly in relation to cardiovascular events.6-9 All three biomarkers are relatively nonspecific and represent the common final path at the end of the inflammatory cascade. More recently other biomarkers representing different stages of the inflammatory pathway and oxidative stress have become available. CD40 ligand, intercellular adhesion molecule-1 (ICAM-1) and P-selectin initiate cell adhesion and transmigration; cytokines (e.g. tumor necrosis factor receptor II (TNFRII)) and chemokines (monocyte chemoattractant protein-1) induce leukocyte recruitment and the acute-phase response.10 Pro-inflammatory actions and oxidative stress are reflected by lipoprotein-associated phospholipase A2 activity and mass, whereas myeloperoxidase originates from the oxidative burst that is part of anti-microbial defense.
To further elucidate the role of inflammatory biomarkers representing distinct pathophysiological pathways, we examined the association of diverse inflammatory blood biomarkers together and individually with risk of CVD and mortality in the community-based Framingham Heart Study.
MATHERIALS AND METHODS
Details on materials and methods are provided in the online Supplement.
RESULTS
Participant Characteristics
The baseline characteristics of our sample are shown in Table 1. The cohort consisted of middle-aged to older adults (mean age 61±9 years) and 53% women. At baseline, 7% of the study sample had major CVD. During a median follow-up of 8.9 years (maximum 11.3 years), 253 participants experienced incident major CVD events and 343 died (CVD death=80, cancer death n=142, other or unknown n=121). Baseline characteristics of individuals excluded from analysis were similar to participants who entered analyses (Supplementary Table I).
Table 1.
Baseline Characteristics of the Study Sample
| Overall cohort (n=3035) |
|
|---|---|
| Age, years | 61±9 |
| Women, % | 1623 (53) |
| Cigarette smoking, % | 398 (13) |
| Body mass index, kg/m2 | 28.1±5.3 |
| Systolic blood pressure, mm Hg | 127±19 |
| Diabetes, % | 400 (13) |
| Total/high density lipoprotein cholesterol | 4.1±1.3 |
| Hypertension treatment, % | 1028 (34) |
| Lipid treatment, % | 622 (20) |
| Aspirin ≥3 per week, % | 963 (32) |
| Hormone replacement therapy, % | 499 (16) |
| Prevalent cardiovascular disease, % | 208 (7) |
Data are presented as mean±SD for continuous variables and n (percentage) for dichotomous variables.
Prediction of CVD
Supplementary Table II presents the multivariable-adjusted association of the separate inflammatory biomarkers with major CVD incidence among the 2827 participants free of major CVD at the 7th examination cycle. TNFRII and CRP were individually associated with major incident CVD. With stepwise selection CRP (hazard ratio [HR] per ln biomarker standard deviation 1.18, 95% confidence interval [CI]: 1.02-1.35; nominal P=0.02) and TNFRII (HR, 1.15, 95% CI: 1.01-1.32; nominal P=0.04) were retained as predictors of major CVD (Table 2). After Bonferroni correction on the number of principal components, none of the biomarkers retained statistical significance.
Table 2.
Final Model from Stepwise Selection Analysis for Inflammatory Biomarkers in Relation to CVD
| Variable | Hazard Ratio | 95% Confidence | Interval | Nominal P-Value |
|---|---|---|---|---|
| C-reactive protein | 1.18 | 1.02 | 1.35 | 0.02 |
| Tumor necrosis factor receptor II | 1.15 | 1.01 | 1.32 | 0.04 |
The covariates included age, sex, current smoking, body mass index, systolic blood pressure, total/high density lipoprotein cholesterol, diabetes, and hypertension treatment. Prevalent major CVD were excluded from this analysis.
Hazard ratios are per one standard deviation increase in ln-biomarker concentration.
The two selected biomarkers did not significantly improve the discrimination ability for CVD of the model comprising traditional risk factors (c-statistic of 0.769, 95% CI: 0.743-0.796 before, and 0.773, 95% CI: 0.746-0.80 after adding the biomarkers). The estimated increment of c-statistic was 0.0038 (95% CI: −0.0023-0.010, P=0.11). The Hosmer-Lemeshow statistic showed adequate calibration between observed and predicted mortality risk of the final model (X2=13.2, df=10, P=0.15).
We also performed reclassification analyses for CVD risk categories over 10 years (Table 3). Net-reclassification improvement was not statistically significant (-1.1%, P=0.62).The relative integrated discrimination improvement was 7.9%; P=0.004, the integrated discrimination improvement, 0.9%; P=0.0039. Supplementary Figure I shows sex-specific adjusted survival curves by tertile of the biomarker score incorporating the top two inflammatory markers. Decreased survival was observed across tertiles.
Table 3.
Reclassification Matrix for CVD During 10 Years of Follow-up
| Without Biomarkers | With TNFRII, CRP | |||
|---|---|---|---|---|
| <6% | 6-20% | >20% | Total | |
| Participants without CVD events | ||||
| <6% | 1142 (94.5) | 67 (5.5) | 0 (0.0) | 1209 |
| 6-20% | 73 (7.1) | 904 (88.5) | 45 (4.4) | 1022 |
| >20% | 0 (0.0) | 41 (12.0) | 302 (88.1) | 343 |
| Total | 1215 | 1012 | 347 | 2574 |
|
|
||||
| Participants with CVD events | ||||
| <6% | 27 (90.0) | 3 (10.0) | 0 (0.0) | 30 |
| 6-20% | 7 (6.5) | 91 (84.3) | 10 (9.3) | 108 |
| >20% | 0 (0.0) | 9 (7.8) | 106 (92.2) | 115 |
| Total | 34 | 103 | 116 | 253 |
Analyses were censored at 10-year follow-up. Provided are the number of individuals and row percent. Net-reclassification improvement was −1.1%, P=0.62. Participants with prevalent major CVD were excluded from analyses of incident major CVD resulting in N=253 individuals for analysis. Green shaded cells represent favorable reclassification, red shaded cells unfavorable reclassification.
Prediction of Mortality
Supplementary Table III provides the HRs per standard deviation of natural log-transformed biomarkers for multivariable-adjusted single biomarker associations with mortality. Four of the 11 markers were individually associated with mortality: CRP, ICAM-1, interleukin-6, and TNFRII (all P<0.0001). In the stepwise biomarker model the three markers selected were ICAM-1, interleukin-6, and TNFRII (all P<0.0001) (Table 4). TNFRII revealed the highest HR (HR 1.33, 95% CI: 1.19-1.49).
Table 4.
Final Model from Stepwise Selection Analysis for Inflammatory Biomarkers in Relation to Overall Mortality
| Variable | Hazard Ratio |
95% Confidence Interval |
Nominal P-Value |
|
|---|---|---|---|---|
| Multivariable model | ||||
| Tumor necrosis factor receptor II | 1.33 | 1.19 | 1.49 | <0.0001 |
| Intercellular adhesion molecule-1 | 1.24 | 1.12 | 1.37 | <0.0001 |
| Interleukin-6 | 1.25 | 1.12 | 1.39 | <0.0001 |
|
| ||||
| Multivariable Model + Interim CVD | ||||
| Tumor necrosis factor receptor II | 1.29 | 1.15 | 1.44 | <0.0001 |
| Intercellular adhesion molecule-1 | 1.23 | 1.11 | 1.36 | <0.0001 |
| Interleukin-6 | 1.23 | 1.10 | 1.38 | 0.0002 |
The covariates included age, sex, smoking, systolic blood pressure, body mass index, total/HDL cholesterol, hypertension treatment, diabetes and interim CVD (time-dependent variable).
Hazard ratios are per one standard deviation increase in log-biomarker concentration.
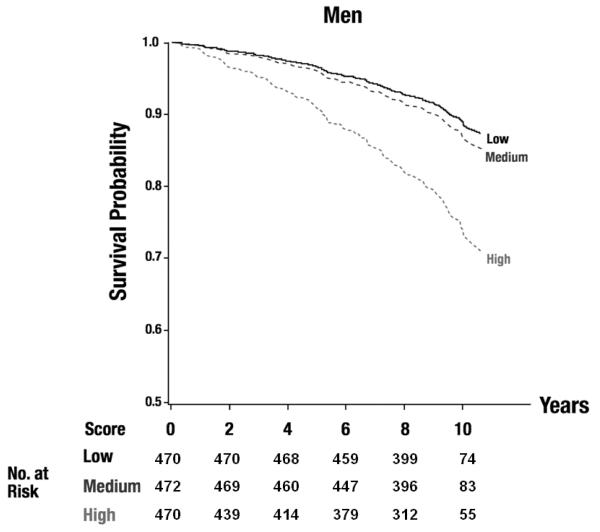
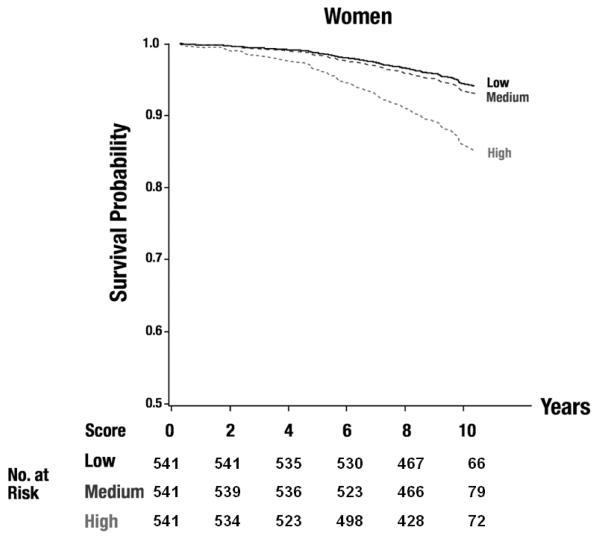
The additional adjustment for interim CVD marginally decreased the estimated HRs, but the same set of biomarkers was selected in the final model. Figure 1 shows sex-specific adjusted survival curves by tertile of the biomarker score incorporating the top three inflammatory markers. Decreased survival was observed across tertiles. Whereas the two lower tertiles differed little, the event rate clearly was highest in the upper tertile.
Figure 1.
Adjusted survival curves for tertiles of the biomarker score (including TNFRII, interleukin-6, ICAM-1) for men (A) and women (B), Plog rank<0.0001 for both. The numbers of participants at risk are displayed in the figure.
The most statistically significant biomarker, TNFRII alone in the model for death incorporating clinical risk factors had higher model discrimination (c-statistic of 0.789 (95% CI, 0.765 to 0.813) rose to 0.799 (95% CI, 0.776 to 0.823). The three selected biomarkers combined only marginally improved the discrimination ability of the model for death beyond traditional risk factors, with c-statistic 0.811 (95% CI, 0.788 to 0.834) after adding biomarkers. The estimated increment of c-statistic was 0.022 (95% CI, 0.011 to 0.033, P<0.0001). The Hosmer-Lemeshow statistic showed adequate calibration between observed and predicted mortality risk in the final model (X2=12.5, df=10, P=0.19). The relative integrated discrimination improvement index was 25.7%; P<0.0001; integrated discrimination improvement 5.1%, p<0.0001. For the top biomarker, TNFRII, alone, the relative integrated discrimination improvement index was 17.9%, P<0.0001, integrated discrimination improvement 3.6%, P<0.0001.
We tested reclassification for three categories of risk of death over 10 years <5%, 5-10% and >10% (Table 5). The net-reclassification improvement was 9.4%, P<0.0001. The NRI table shows that there is substantial downward reclassification in those who survived (net downward 212/2697=8.0%), but little reclassification in those who died (net upward 5/338=1.5%). We have shaded cells representing favorable reclassification green and unfavorable reclassification red to further emphasize the utility of reclassification metrics. Thus, net reclassification was mainly driven by a downward reclassification of those who survived, whereas there was little upward reclassification of those who died.
Table 5.
Reclassification matrix for 10-year mortality
| Without Biomarkers | With TNFRII, ICAM-1, interleukin-6 | |||
|---|---|---|---|---|
| <5% | 5-10% | >10% | Total | |
| Participants surviving | ||||
| <5% | 989 (92.3) | 73 (6.8) | 9 (0.8) | 1071 |
| 5-10% | 188 (31.1) | 335 (55.4) | 82 (13.6) | 605 |
| >10% | 9 (0.9) | 179 (17.5) | 833 (81.6) | 1021 |
| Total | 1186 | 587 | 924 | 2697 |
|
| ||||
| Participants who died | ||||
| <5% | 19 (73.1) | 4 (15.4) | 3 (11.5) | 26 |
| 5-10% | 5(13.2) | 19 (50.0) | 14 (36.8) | 38 |
| >10% | 1 (0.4) | 10(3.6) | 263 (96.0) | 274 |
| Total | 25 | 33 | 280 | 338 |
Analyses were censored at 10-year follow-up. Provided are the number of individuals and row percent. Relative Net-reclassification improvement was 9.4%, P<0.0001.
Green shaded cells represent favorable reclassification, red shaded cells unfavorable reclassification.
Secondary Analyses
We did not observe significant interactions by age or sex for the two biomarkers in relation to incident CVD and the three biomarkers that were associated with mortality. Accounting for estimated glomerular filtration rate, hormone replacement, aspirin or lipid treatment did not change the associations of the three markers with mortality markedly (data not shown).
DISCUSSION
Principal Findings
In our large contemporary community-based cohort, circulating TNFRII was associated with both CVD events and mortality over a follow-up period of about 10 years. CRP and TNFRII were nominally associated with major CVD, but not after adjustment for multiple testing. Furthermore, they did not substantively improve the risk prediction model based on classical CVD risk factors. TNFRII, ICAM-1 and interleukin-6 were selected into the final model for death using a stepwise procedure. We showed significant discrimination and reclassification increments for mortality when TNFRII was assessed in addition to classical risk factors. The combined incorporation of the top three biomarkers only modestly improved the c-statistic and reclassification metrics. The relation between the selected markers and mortality was similar in models accounting for interim CVD, suggesting that they provide risk information beyond CVD. Overall, our data on a large panel of biomarkers representing varying aspects of inflammation extend the knowledge on the association of inflammation with incident CVD and death in the community.9,11-13
The mechanisms relating TNFRII to CVD and mortality are uncertain. Tumor necrosis factor-alpha cytokine is central to the inflammatory cascade that promotes the acute-phase response.14 The more stable TNFRII may be a good indicator of the biological effects of the tumor necrosis factor-alpha system. In the cardiovascular system, tumor necrosis factor-alpha signaling via TNFRII is protective in post-ischemic recovery in adult myocardium.15 In mice, endothelial TNFRII plays a critical role in arteriogenesis and ischemia-mediated adaptive angiogenesis.16 TNFRII blood concentrations have been related to autoimmune diseases, their prognosis and the response to anti-tumor necrosis factor-alpha therapy.17-20 Higher concentrations of circulating TNFRII have been reported with advancing age21 and in overweight individuals.21 We and others found an association of TNFRII with mean arterial pressure, hypertension treatment, arterial stiffness, endothelial function, ankle-brachial index, and kidney disease, all of which are strong predictors of overall mortality.22-27 More specifically, moderately increased TNFRII concentrations are observed in atherosclerosis28 and coronary artery disease,29 and TNFRII has been related to the risk of CVD in women with diabetes and in smaller study samples.30,31 However, the relation with CVD is not completely consistent; no significant relation was observed in a cross-sectional study of older adults.32 Our findings extend the epidemiological evidence relating TNFRII to CVD and death in an unselected community-based sample.
The glycoprotein ICAM-1 is expressed in different cell types. The membrane-bound form on endothelial cells is involved in early stages of inflammatory processes by facilitating the transmigration of leukocytes through the interaction with lymphocyte function-associated antigen.33 A strong relation has been demonstrated with viral infections,34 graft rejection after transplantation,35 endothelial function,36 CVD,37 and cancer.38 Elevated ICAM-1 concentrations have been reported to be associated with increased all-cause mortality in a smaller sample of individuals aged 65 years or older, which may be explained by the relation to CVD.39 With our current findings, we can extend these observations to middle-age to older community-dwelling adults in the context of other inflammatory biomarkers.
An association of the correlated biomarkers CRP and interleukin-6 with overall mortality has been reported.40-42 CRP was not selected into the final model for the outcome of death, likely due to its correlation with the already selected markers, especially interleukin-6 (r=0.47 in our sample). Most inflammatory processes end in the downstream activation of the interleukin-6, CRP pathways. Thus, they are relatively nonspecific acute-phase markers induced by infections, cancer, tissue damage, and inflammation.43 Whereas some studies found CRP improved risk prediction for CVD,9,44 some studies reported otherwise after accounting for standard risk factors.7,45 Our well-characterized sample provides supportive evidence for the role of the moderately correlated CRP and interleukin-6 as risk predictors of mortality and CVD. Our results do not indicate a clear superiority of either of the two biomarkers.
Overall, several easily measurable inflammatory biomarkers were related to all-cause mortality. The exact mechanisms through which TNFRII, ICAM-1 and interleukin-6 are associated with increased risk of death are not well-established. Further research is needed to unravel the pathophysiology of inflammation in healthy aging and disease. A better characterization on the community level is needed to identify causal pathways and define interventional strategies.
Strengths and Limitations
Strengths of the study are the well-characterized community-based sample with routine ascertainment of potential clinical confounders, near complete follow-up, and a comprehensive and standardized adjudication process for outcomes. A broad range of biomarkers representing different phases and pathways of inflammation measured with strict quality control also were assets. We were able to demonstrate significant improvement in model discrimination and reclassification by incorporating the three markers in addition to traditional risk factors of mortality. Since widely applied risk categories for mortality are not available from the literature,46 we defined them to ensure that there were adequate numbers of events in each category (without reference to the inflammatory biomarkers). We concede that the risk categories for mortality lack external validation, proof of clinical relevance, and might vary in other datasets. Due to a moderate number of outcomes we did not have the power to perform subgroup analyses for cause-specific mortality (e.g., cardiovascular mortality, cancer mortality). Due to limited numbers of individuals with cardiovascular medication such as statins, aspirin and hormone replacement therapy and a variety of biases (e.g. indication) in a community-based sample, our analyses adjusting for cardiac medications remain exploratory and need refinement in better suited samples. Furthermore, despite long-term follow-up with carefully adjudicated outcomes, the sample size does not permit us to identify smaller associations. Lack of statistical power may be one of the reasons of non-replication of findings from large consortia as seen for Lp-PLA2.47 Apart from overall small effect sizes, non-replication of published associations of inflammatory biomarkers such as CD40 ligand, monocyte chemoattractant protein-1, myeloperoxidase, and P selectin in multivariable models may be due to our rigorous cardiovascular risk factor adjustment, the sample structure of community-based individuals compared to often smaller clinical samples, presenting with acute disease states.
We acknowledge that the clinical relevance of our data is uncertain because it may not be cost-effective to measure three biomarkers as long as a specific, targeted intervention to reduce mortality based on this knowledge is not available. In addition, our findings will need to be replicated in external cohorts. Finally, the pathophysiological background for our findings and potential causal mechanisms need to be examined, which will require further investigation in the experimental setting.
In conclusion, we report estimates of the associations of eleven distinct circulating inflammatory biomarkers in a community-based cohort with CVD and all-cause mortality during nearly a decade of follow-up. Multivariable-selection analyses revealed TNFRII was nominally predictive of CVD and significantly predictive of mortality. However, the magnitude and strength of association were modest. Even the combination of information on TNFRII and CRP for incident CVD and TNFRII and interleukin-6 for mortality increased the predictive ability of a model consisting of classical CVD risk factors only modestly, if at all. Embedded in the ongoing discussion of the clinical utility of the measurement of circulating inflammatory biomarkers48 in addition to classical CVD risk factors for risk prediction in the community, our results thus speak against a direct clinical application for risk determination. Rather, our data may be of interest to clinicians and scientists concerned about “inflammaging”, i.e. how alterations of the inflammatory system contribute to immunosenescence, frailty and increased risk for death.49 Our findings may stimulate future research at the epidemiologic as well as basic science level, to establish the pathophysiological role of the inflammatory marker TNFRII as a risk marker for CVD events and mortality in the middle-aged to older individuals.
MATERIALS AND METHODS
Study Sample
Individuals (n=5124) were recruited to the Framingham Offspring cohort, in the early 1970s and were followed by examinations every four to eight years.(1) The seventh examination cycle was attended by 3539 participants (1998 2001); inflammatory biomarkers were measured in 3325 attendees. For the present investigation, we excluded individuals missing any inflammatory biomarker measurements (n=286), incomplete or missing follow up (n=2), and missing covariate data (n=2), leaving 3035 participants (85.8% of all examination 7 attendees) for analysis. The study protocols were reviewed and approved by the Boston University Medical Center Institutional Review Board. Participants provided informed consent at every examination cycle. All authors have read and agreed to the manuscript as written.
Clinical Evaluations & Follow-up
Health history updates were performed biennially by Framingham staff. At the Framingham clinic questionnaires, physician administered interviews and physical examinations, and laboratory assessments provided information on CVD risk factors. Assessment of current smoking, alcohol consumption, and medications relied on self report. Clinic physicians performed blood pressure measurements with seated participants; two systolic blood pressure values were averaged. Diabetes was defined as an elevated fasting blood glucose ≥126 mg/dL or the use of diabetes drugs.
Follow up was cutoff at December 31, 2009. Routine collection of participants’ physician office visit and hospitalization records was performed by Framingham Heart Study staff. For death reviews official death certificates and medical records were acquired. If medical records were unavailable, next of kin were contacted. Major CVD events, defined as myocardial infarction, coronary insufficiency (prolonged angina and electrocardiographic alterations), heart failure, or stroke, including deaths resulting from these diseases, were regularly adjudicated by a committee of three investigators using standardized written criteria.(2)
Biomarker determination
Venous blood samples were obtained from fasting participants and immediately processed and stored at −80°C. Eleven inflammatory markers were measured: plasma CD40 ligand, CRP, fibrinogen, lipoprotein associated phospholipase A2 activity and mass, P selectin, and TNFRII, and serum ICAM 1, interleukin 6, monocyte chemoattractant protein 1, and myeloperoxidase. Characteristics of measurement kits, test properties and blood specimens have been published.(3) Mean inter assay coefficients of variation were less than 10% for all markers. Correlations among biomarkers, clinical correlates, heritability and genetic variation in inflammatory SNPs in relation to the biomarker panel have been reported previously.(4)
Statistical Analyses
We applied natural logarithmical (ln) transformation to biomarker values to normalize their skewed distributions and we carried out sex pooled standardization (mean 0, standard deviation [SD] 1) prior to analyses. We used multivariable adjusted Cox proportional hazards regression models to relate inflammatory biomarkers to occurrence of CVD events and death after confirming that the assumption of proportionality of hazards was met.(5) We forced clinical covariates for the multivariable prospective analyses of incident CVD and mortality into the model. We based the covariate selection on prior publications.(6) We included age, sex, current smoking, body mass index, systolic blood pressure, total/high density lipoprotein cholesterol, diabetes, hypertension treatment, as an indicator of elevated blood pressure, and prevalent major CVD. Since we were interested in the prediction of first onset of CVD, participants with prevalent major CVD were excluded from analyses of incident major CVD. We further investigated whether the association of inflammatory markers with mortality was due to occurrence of interim major cardiovascular events. We performed the final inflammatory marker stepwise selection model by incorporating CVD as a time dependent variable. Regression coefficients were calculated for one SD increase in ln transformed biomarker concentrations.
We calculated the c statistics before and after adding the significant biomarkers to examine model discrimination with respect to risk of CVD and death over 10 years. We further assessed net reclassification improvement for risk categories <6%, 6 20%, >20% risk for CVD,(7) and categories of <5%, 5 10%, >10% risk for mortality(8) during follow up. Mortality risk categories were chosen to ensure that there were adequate numbers of events in each category independent of the relation to the biomarkers. We calculated integrated discrimination improvement using a recently published method.(7)
We used a stepwise procedure to select biomarker(s) associated with outcomes with two sided P<0.05 for retention. A biomarker score was created for each participant as a sum of (biomarker*coefficient) for biomarkers retained in the final models for CVD and mortality. Adjusted survival curves were calculated using corrected group prognosis method(9) implemented in a SAS macro by Zhang et al.(10) Event free survival curves were generated by tertile of the biomarker score for each sex.
Secondary Analyses
The biomarkers retained in the final models were tested for their interactions with age and sex. In addition, we examined whether adjustment for estimated glomerular filtration rate calculated according to the Modification of Diet in Renal Disease Study formula,(11) hormone replacement therapy, aspirin intake or lipid treatment substantively changed our results.
The 11 biomarkers were correlated. In a covariate adjusted principal component analysis, we found that among them there were only 4 principal components with eigenvalues greater than one. To account for multiple testing, we used Bonferroni correction approach on the number of principal components. A P value <0.0125 (0.05/4) was the significance criterion for both endpoints – CVD and mortality. Analyses were performed using SAS version 9.2 (Cary, North Carolina, http://www.sas.com/presscenter/guidelines.html) statistical software.
Supplementary Material
Supplementary Table I Baseline Characteristics of the Study Sample by exclusion
Supplementary Table II. Multivariable-Adjusted Cox Proportional Hazards Regression Models Examining Each Circulating Inflammatory Biomarker Separately in Relation to CVD
Supplementary Table III Multivariable-Adjusted Cox Proportional Hazards Regression Models Examining Each Circulating Inflammatory Biomarker Separately in Relation to Overall Mortality
Supplementary Figure I. Sex-specific adjusted survival curves by tertile of the biomarker score incorporating the top two inflammatory markers (CRP and TNFRII) for men (A) and women (B). The numbers of participants at risk are displayed below the figure.
SIGNIFICANCE.
There is an ongoing discussion on the clinical utility of the measurement of circulating inflammatory biomarkers for cardiovascular risk prediction. In our middle-aged to older community-based cohort we report the associations of 11 distinct circulating inflammatory biomarkers with first major cardiovascular disease [CVD] events and all-cause mortality during long-term follow-up. In multivariable-adjusted analyses tumor necrosis factor receptor II [TNFRII] was nominally related to CVD and significantly predictive of mortality. The magnitude and strength of association were modest. Even the combination of the two strongest biomarkers, TNFRII and C-reactive protein for incident CVD and TNFRII and interleukin-6 for mortality increased the predictive ability of a model consisting of classical CVD risk factors only modestly. Our findings may stimulate future research to understand the pathophysiological role of TNFRII and other inflammatory biomarkers in the process of aging, CVD risk and mortality.
Acknowledgments
Sources of Funding Supported by NIH/NHLBI contract N01-HC-25195, 6R01-NS17950, and NIH grants HL064753. HL076784, AG028321 (EJB), 1 RO1HL71039 (RSV); NIH Research career award K24 HL04334 (RSV); Deutsche Forschungsgemeinschaft (German Research Foundation) Research Fellowship SCHN 1149/1-1 and Emmy Noether Program SCHN 1149/3-1 (RS); 1K23HL083102, Doris Duke Charitable Foundation Clinical Scientist Development Award (SK).
Lipoprotein-associated phospholipase A2 activity and mass measurements were provided to the Heart Study by GlaxoSmithKline at no cost.
Acknowledgments None.
Abbreviations
- CVD
cardiovascular disease
- CI
confidence Interval
- CRP
C-reactive protein
- HR
hazard ratio
- ICAM-1
intercellular adhesion molecule-1
- SD
standard deviation
- TNFRII
tumor necrosis factor receptor II
Footnotes
Disclosure Lipoprotein-associated phospholipase-A2 activity measurements were provided by GlaxoSmithKline and mass measurements by diaDexus at no cost to the FHS.
This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
Reference List
- 1.Perls T, Kunkel LM, Puca AA. The genetics of exceptional human longevity. J Am Geriatr Soc. 2002;50:359–368. doi: 10.1046/j.1532-5415.2002.49283.x. [DOI] [PubMed] [Google Scholar]
- 2.Ferrucci L, Corsi A, Lauretani F, Bandinelli S, Bartali B, Taub DD, Guralnik JM, Longo DL. The origins of age-related proinflammatory state. Blood. 2005;105:2294–2299. doi: 10.1182/blood-2004-07-2599. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 3.Koenig W, Khuseyinova N, Baumert J, Meisinger C. Prospective study of high-sensitivity C-reactive protein as a determinant of mortality: results from the MONICA/KORA Augsburg Cohort Study, 1984-1998. Clin Chem. 2008;54:335–342. doi: 10.1373/clinchem.2007.100271. [DOI] [PubMed] [Google Scholar]
- 4.Terry DF, Pencina MJ, Vasan RS, Murabito JM, Wolf PA, Hayes MK, Levy D, D’Agostino RB, Benjamin EJ. Cardiovascular risk factors predictive for survival and morbidity-free survival in the oldest-old Framingham Heart Study participants. J Am Geriatr Soc. 2005;53:1944–1950. doi: 10.1111/j.1532-5415.2005.00465.x. [DOI] [PubMed] [Google Scholar]
- 5.Evert J, Lawler E, Bogan H, Perls T. Morbidity profiles of centenarians: survivors, delayers, and escapers. J Gerontol A Biol Sci Med Sci. 2003;58:232–237. doi: 10.1093/gerona/58.3.m232. [DOI] [PubMed] [Google Scholar]
- 6.Danesh J, Collins R, Appleby P, Peto R. Association of fibrinogen, C-reactive protein, albumin, or leukocyte count with coronary heart disease: meta-analyses of prospective studies. JAMA. 1998;279:1477–1482. doi: 10.1001/jama.279.18.1477. [DOI] [PubMed] [Google Scholar]
- 7.Danesh J, Wheeler JG, Hirschfield GM, Eda S, Eiriksdottir G, Rumley A, Lowe GD, Pepys MB, Gudnason V. C-reactive protein and other circulating markers of inflammation in the prediction of coronary heart disease. N Engl J Med. 2004;350:1387–1397. doi: 10.1056/NEJMoa032804. [DOI] [PubMed] [Google Scholar]
- 8.Engstrom G, Hedblad B, Stavenow L, Tyden P, Lind P, Janzon L, Lindgarde F. Fatality of future coronary events is related to inflammation-sensitive plasma proteins: a population-based prospective cohort study. Circulation. 2004;110:27–31. doi: 10.1161/01.CIR.0000133277.88655.00. [DOI] [PubMed] [Google Scholar]
- 9.Ridker PM, Hennekens CH, Buring JE, Rifai N. C-reactive protein and other markers of inflammation in the prediction of cardiovascular disease in women. N Engl J Med. 2000;342:836–843. doi: 10.1056/NEJM200003233421202. [DOI] [PubMed] [Google Scholar]
- 10.Gabay C, Kushner I. Acute-phase proteins and other systemic responses to inflammation. N Engl J Med. 1999;340:448–454. doi: 10.1056/NEJM199902113400607. [DOI] [PubMed] [Google Scholar]
- 11.Zethelius B, Berglund L, Sundstrom J, Ingelsson E, Basu S, Larsson A, Venge P, Arnlov J. Use of multiple biomarkers to improve the prediction of death from cardiovascular causes. N Engl J Med. 2008;358:2107–2116. doi: 10.1056/NEJMoa0707064. [DOI] [PubMed] [Google Scholar]
- 12.Kaptoge S, Di AE, Lowe G, Pepys MB, Thompson SG, Collins R, Danesh J. C-reactive protein concentration and risk of coronary heart disease, stroke, and mortality: an individual participant meta-analysis. Lancet. 2010;375:132–140. doi: 10.1016/S0140-6736(09)61717-7. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Woodward M, Lowe GD, Rumley A, Tunstall-Pedoe H. Fibrinogen as a risk factor for coronary heart disease and mortality in middle-aged men and women. The Scottish Heart Health Study. Eur Heart J. 1998;19:55–62. doi: 10.1053/euhj.1997.0573. [DOI] [PubMed] [Google Scholar]
- 14.Fattori E, Cappelletti M, Costa P, Sellitto C, Cantoni L, Carelli M, Faggioni R, Fantuzzi G, Ghezzi P, Poli V. Defective inflammatory response in interleukin 6-deficient mice. J Exp Med. 1994;180:1243–1250. doi: 10.1084/jem.180.4.1243. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 15.Kishore R, Tkebuchava T, Sasi SP, Silver M, Gilbert HY, Yoon YS, Park HY, Thorne T, Losordo DW, Goukassian DA. Tumor necrosis factor-alpha signaling via TNFR1/p55 is deleterious whereas TNFR2/p75 signaling is protective in adult infarct myocardium. Adv Exp Med Biol. 2011;691:433–448. doi: 10.1007/978-1-4419-6612-4_45. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Luo Y, Xu Z, Wan T, He Y, Jones D, Zhang H, Min W. Endothelial-specific transgenesis of TNFR2 promotes adaptive arteriogenesis and angiogenesis. Arterioscler Thromb Vasc Biol. 2010;30:1307–1314. doi: 10.1161/ATVBAHA.110.204222. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Holtmann MH, Schuchmann M, Zeller G, Galle PR, Neurath MF. The emerging distinct role of TNF-receptor 2 (p80) signaling in chronic inflammatory disorders. Arch Immunol Ther Exp (Warsz ) 2002;50:279–288. [PubMed] [Google Scholar]
- 18.Goeb V, Dieude P, Vittecoq O, Mejjad O, Menard JF, Thomas M, Gilbert D, Boumier P, Pouplin S, Daragon A, Fardellone P, Tron F, Cornelis F, Le L, X Association between the TNFRII 196R allele and diagnosis of rheumatoid arthritis. Arthritis Res Ther. 2005;7:R1056–R1062. doi: 10.1186/ar1777. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Mattey DL, Glossop JR, Nixon NB, Dawes PT. Circulating levels of tumor necrosis factor receptors are highly predictive of mortality in patients with rheumatoid arthritis. Arthritis Rheum. 2007;56:3940–3948. doi: 10.1002/art.23075. [DOI] [PubMed] [Google Scholar]
- 20.Ongaro A, De MM, Pellati A, Caruso A, Ferretti S, Masieri FF, Fotinidi M, Farina I, Trotta F, Padovan M. Can tumor necrosis factor receptor II gene 676T>G polymorphism predict the response grading to anti-TNFalpha therapy in rheumatoid arthritis? Rheumatol Int. 2008;28:901–908. doi: 10.1007/s00296-008-0552-5. [DOI] [PubMed] [Google Scholar]
- 21.McFarlin BK, Johnston CA, Tyler C, Hutchison AT, Kueht ML, Reeves R, Foreyt JP. Inflammatory markers are elevated in overweight Mexican-American children. Int J Pediatr Obes. 2007;2:235–241. doi: 10.1080/17477160701440455. [DOI] [PubMed] [Google Scholar]
- 22.Schnabel R, Larson MG, Dupuis J, et al. Relations of inflammatory biomarkers and common genetic variants with arterial stiffness and wave reflection. Hypertension. 2008;51:1651–1657. doi: 10.1161/HYPERTENSIONAHA.107.105668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 23.Ohgushi M, Taniguchi A, Fukushima M, Nakai Y, Kuroe A, Ohya M, Seino Y. Soluble tumor necrosis factor receptor 2 is independently associated with brachial-ankle pulse-wave velocity in nonobese Japanese type 2 diabetic patients. Diabetes Care. 2006;29:1459–1460. doi: 10.2337/dc06-0528. [DOI] [PubMed] [Google Scholar]
- 24.Kawasaki Y, Taniguchi A, Fukushima M, Nakai Y, Kuroe A, Ohya M, Nagasaka S, Yamada Y, Inagaki N, Seino Y. Soluble TNF receptors and albuminuria in non-obese Japanese type 2 diabetic patients. Horm Metab Res. 2005;37:617–621. doi: 10.1055/s-2005-870536. [DOI] [PubMed] [Google Scholar]
- 25.Keller CR, Odden MC, Fried LF, Newman AB, Angleman S, Green CA, Cummings SR, Harris TB. Shlipak MG. Kidney function and markers of inflammation in elderly persons without chronic kidney disease: The health, aging, and body composition study. Kidney Int. 2007;71:239–244. doi: 10.1038/sj.ki.5002042. [DOI] [PubMed] [Google Scholar]
- 26.Lin J, Hu FB, Rimm EB, Rifai N, Curhan GC. The association of serum lipids and inflammatory biomarkers with renal function in men with type II diabetes mellitus. Kidney Int. 2006;69:336–342. doi: 10.1038/sj.ki.5000021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 27.Murabito JM, Keyes MJ, Guo CY, Keaney JF, Jr., Vasan RS, D’Agostino RB, Sr., Benjamin EJ. Cross-sectional relations of multiple inflammatory biomarkers to peripheral arterial disease: The Framingham Offspring Study. Atherosclerosis. 2009;203:509–514. doi: 10.1016/j.atherosclerosis.2008.06.031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 28.Blann AD, McCollum CN. Increased levels of soluble tumor necrosis factor receptors in atherosclerosis: no clear relationship with levels of tumor necrosis factor. Inflammation. 1998;22:483–491. doi: 10.1023/a:1022346010304. [DOI] [PubMed] [Google Scholar]
- 29.Pai JK, Pischon T, Ma J, Manson JE, Hankinson SE, Joshipura K, Curhan GC, Rifai N, Cannuscio CC, Stampfer MJ, Rimm EB. Inflammatory markers and the risk of coronary heart disease in men and women. N Engl J Med. 2004;351:2599–2610. doi: 10.1056/NEJMoa040967. [DOI] [PubMed] [Google Scholar]
- 30.Shai I, Schulze MB, Manson JE, Rexrode KM, Stampfer MJ, Mantzoros C, Hu FB. A prospective study of soluble tumor necrosis factor-alpha receptor II (sTNF-RII) and risk of coronary heart disease among women with type 2 diabetes. Diabetes Care. 2005;28:1376–1382. doi: 10.2337/diacare.28.6.1376. [DOI] [PubMed] [Google Scholar]
- 31.Benjafield AV, Wang XL, Morris BJ. Tumor necrosis factor receptor 2 gene (TNFRSF1B) in genetic basis of coronary artery disease. J Mol Med. 2001;79:109–115. doi: 10.1007/s001090000168. [DOI] [PubMed] [Google Scholar]
- 32.Cesari M, Penninx BW, Newman AB, Kritchevsky SB, Nicklas BJ, Sutton-Tyrrell K, Tracy RP, Rubin SM, Harris TB, Pahor M. Inflammatory markers and cardiovascular disease (The Health, Aging and Body Composition [Health ABC] Study) Am J Cardiol. 2003;92:522–528. doi: 10.1016/s0002-9149(03)00718-5. [DOI] [PubMed] [Google Scholar]
- 33.Yasuda M, Shimizu S, Ohhinata K, Naito S, Tokuyama S, Mori Y, Kiuchi Y, Yamamoto T. Differential roles of ICAM-1 and E-selectin in polymorphonuclear leukocyte-induced angiogenesis. Am J Physiol Cell Physiol. 2002;282:C917–C925. doi: 10.1152/ajpcell.00223.2001. [DOI] [PubMed] [Google Scholar]
- 34.Whiteman SC, Bianco A, Knight RA, Spiteri MA. Human rhinovirus selectively modulates membranous and soluble forms of its intercellular adhesion molecule-1 (ICAM-1) receptor to promote epithelial cell infectivity. J Biol Chem. 2003;278:11954–11961. doi: 10.1074/jbc.M205329200. [DOI] [PubMed] [Google Scholar]
- 35.Ballantyne CM, Mainolfi EA, Young JB, Windsor NT, Cocanougher B, Lawrence EC, Pollack MS, Entman ML, Rothlein R. Relationship of increased levels of circulating intercellular adhesion molecule 1 after heart transplantation to rejection: human leukocyte antigen mismatch and survival. J Heart Lung Transplant. 1994;13:597–603. [PubMed] [Google Scholar]
- 36.van Buul JD, Kanters E, Hordijk PL. Endothelial signaling by Ig-like cell adhesion molecules. Arterioscler Thromb Vasc Biol. 2007;27:1870–1876. doi: 10.1161/ATVBAHA.107.145821. [DOI] [PubMed] [Google Scholar]
- 37.Becker A, van H, V, Jager A, Kostense PJ, Dekker JM, Nijpels G, Heine RJ, Bouter LM, Stehouwer CD. Why is soluble intercellular adhesion molecule-1 related to cardiovascular mortality? Eur J Clin Invest. 2002;32:1–8. doi: 10.1046/j.1365-2362.2002.00919.x. [DOI] [PubMed] [Google Scholar]
- 38.Viac J, Vincent C, Palacio S, Schmitt D, Claudy A. Tumour necrosis factor (TNF) soluble receptors in malignant melanoma: correlation with soluble ICAM-1 levels. Eur J Cancer. 1996;32A:447–449. doi: 10.1016/0959-8049(95)00541-2. [DOI] [PubMed] [Google Scholar]
- 39.Jenny NS, Arnold AM, Kuller LH, Sharrett AR, Fried LP, Psaty BM, Tracy RP. Soluble intracellular adhesion molecule-1 is associated with cardiovascular disease risk and mortality in older adults. J Thromb Haemost. 2006;4:107–113. doi: 10.1111/j.1538-7836.2005.01678.x. [DOI] [PubMed] [Google Scholar]
- 40.Mendall MA, Strachan DP, Butland BK, Ballam L, Morris J, Sweetnam PM, Elwood PC. C-reactive protein: relation to total mortality, cardiovascular mortality and cardiovascular risk factors in men. Eur Heart J. 2000;21:1584–1590. doi: 10.1053/euhj.1999.1982. [DOI] [PubMed] [Google Scholar]
- 41.Harris TB, Ferrucci L, Tracy RP, Corti MC, Wacholder S, Ettinger WH, Jr., Heimovitz H, Cohen HJ, Wallace R. Associations of elevated interleukin-6 and C-reactive protein levels with mortality in the elderly. Am J Med. 1999;106:506–512. doi: 10.1016/s0002-9343(99)00066-2. [DOI] [PubMed] [Google Scholar]
- 42.Kizer JR, Arnold AM, Jenny NS, Cushman M, Strotmeyer ES, Ives DG, Ding J, Kritchevsky SB, Chaves PH, Hirsch CH, Newman AB. Longitudinal changes in adiponectin and inflammatory markers and relation to survival in the oldest old: the Cardiovascular Health Study All Stars study. J Gerontol A Biol Sci Med Sci. 2011;66:1100–1107. doi: 10.1093/gerona/glr098. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 43.Pepys MB, Hirschfield GM. C-reactive protein: a critical update. J Clin Invest. 2003;111:1805–1812. doi: 10.1172/JCI18921. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 44.Ridker PM, Glynn RJ, Hennekens CH. C-reactive protein adds to the predictive value of total and HDL cholesterol in determining risk of first myocardial infarction. Circulation. 1998;97:2007–2011. doi: 10.1161/01.cir.97.20.2007. [DOI] [PubMed] [Google Scholar]
- 45.Wilson PW, Nam BH, Pencina M, D’Agostino RB, Sr., Benjamin EJ, O’Donnell CJ. C-reactive protein and risk of cardiovascular disease in men and women from the Framingham Heart Study. Arch Intern Med. 2005;165:2473–2478. doi: 10.1001/archinte.165.21.2473. [DOI] [PubMed] [Google Scholar]
- 46.Cooney MT, Vartiainen E, Laatikainen T, Joulevi A, Dudina A, Graham I. Simplifying cardiovascular risk estimation using resting heart rate. Eur Heart J. 2010;31:2141–2147. doi: 10.1093/eurheartj/ehq164. [DOI] [PubMed] [Google Scholar]
- 47.Thompson A, Gao P, Orfei L, et al. Lipoprotein-associated phospholipase A(2) and risk of coronary disease, stroke, and mortality: collaborative analysis of 32 prospective studies. Lancet. 2010;375:1536–1544. doi: 10.1016/S0140-6736(10)60319-4. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 48.Kaptoge S, Di AE, Pennells L, et al. C-reactive protein, fibrinogen, and cardiovascular disease prediction. N Engl J Med. 2012;367:1310–1320. doi: 10.1056/NEJMoa1107477. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 49.Franceschi C, Capri M, Monti D, Giunta S, Olivieri F, Sevini F, Panourgia MP, Invidia L, Celani L, Scurti M, Cevenini E, Castellani GC, Salvioli S. Inflammaging and anti-inflammaging: a systemic perspective on aging and longevity emerged from studies in humans. Mech Ageing Dev. 2007;128:92–105. doi: 10.1016/j.mad.2006.11.016. [DOI] [PubMed] [Google Scholar]
Reference List
- 1.Quan SF, Howard BV, Iber C, et al. The Sleep Heart Health Study: design, rationale, and methods. Sleep. 1997;20:1077–85. [PubMed] [Google Scholar]
- 2.Kannel WB, Wolf PA, Garrison RJ. The Framingham Study: an epidemiological investigation of cardiovascular disease. National Heart, Lung, and Blood Institute; Bethesda, MD: 1987. [Google Scholar]
- 3.Schnabel R, Larson MG, Dupuis J, et al. Relations of inflammatory biomarkers and common genetic variants with arterial stiffness and wave reflection. Hypertension. 2008;51:1651–7. doi: 10.1161/HYPERTENSIONAHA.107.105668. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Schnabel RB, Lunetta KL, Larson MG, et al. The relation of genetic and environmental factors to systemic inflammatory biomarker concentrations. Circ Cardiovasc Genet. 2009;2:229–37. doi: 10.1161/CIRCGENETICS.108.804245. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Cox DR, Oakes D. Analysis of Survival Data. Chapman and Hall; London, UK: 1984. Analysis of Survival Data; p. 201. [Google Scholar]
- 6.Wang TJ, Gona P, Larson MG, et al. Multiple biomarkers for the prediction of first major cardiovascular events and death. N Engl J Med. 2006;355:2631–9. doi: 10.1056/NEJMoa055373. [DOI] [PubMed] [Google Scholar]
- 7.Pencina MJ, D’Agostino RB, Sr., D’Agostino RB, Jr., Vasan RS. Evaluating the added predictive ability of a new marker: from area under the ROC curve to reclassification and beyond. Stat Med. 2008;27:157–72. doi: 10.1002/sim.2929. [DOI] [PubMed] [Google Scholar]
- 8.Cooney MT, Vartiainen E, Laatikainen T, Joulevi A, Dudina A, Graham I. Simplifying cardiovascular risk estimation using resting heart rate. Eur Heart J. 2010;31:2141–7. doi: 10.1093/eurheartj/ehq164. [DOI] [PubMed] [Google Scholar]
- 9.Chang IM, Gelman R, Pagano M. Corrected group prognostic curves and summary statistics. J Chronic Dis. 1982;35:669–74. doi: 10.1016/0021-9681(82)90019-4. [DOI] [PubMed] [Google Scholar]
- 10.Zhang X, Loberiza FR, Klein JP, Zhang MJ. A SAS macro for estimation of direct adjusted survival curves based on a stratified Cox regression model. Comput Methods Programs Biomed. 2007;88:95–101. doi: 10.1016/j.cmpb.2007.07.010. [DOI] [PubMed] [Google Scholar]
- 11.Levey AS, Bosch JP, Lewis JB, Greene T, Rogers N, Roth D. A more accurate method to estimate glomerular filtration rate from serum creatinine: a new prediction equation. Modification of Diet in Renal Disease Study Group. Ann Intern Med. 1999;130:461–70. doi: 10.7326/0003-4819-130-6-199903160-00002. [DOI] [PubMed] [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.
Supplementary Materials
Supplementary Table I Baseline Characteristics of the Study Sample by exclusion
Supplementary Table II. Multivariable-Adjusted Cox Proportional Hazards Regression Models Examining Each Circulating Inflammatory Biomarker Separately in Relation to CVD
Supplementary Table III Multivariable-Adjusted Cox Proportional Hazards Regression Models Examining Each Circulating Inflammatory Biomarker Separately in Relation to Overall Mortality
Supplementary Figure I. Sex-specific adjusted survival curves by tertile of the biomarker score incorporating the top two inflammatory markers (CRP and TNFRII) for men (A) and women (B). The numbers of participants at risk are displayed below the figure.