Abstract
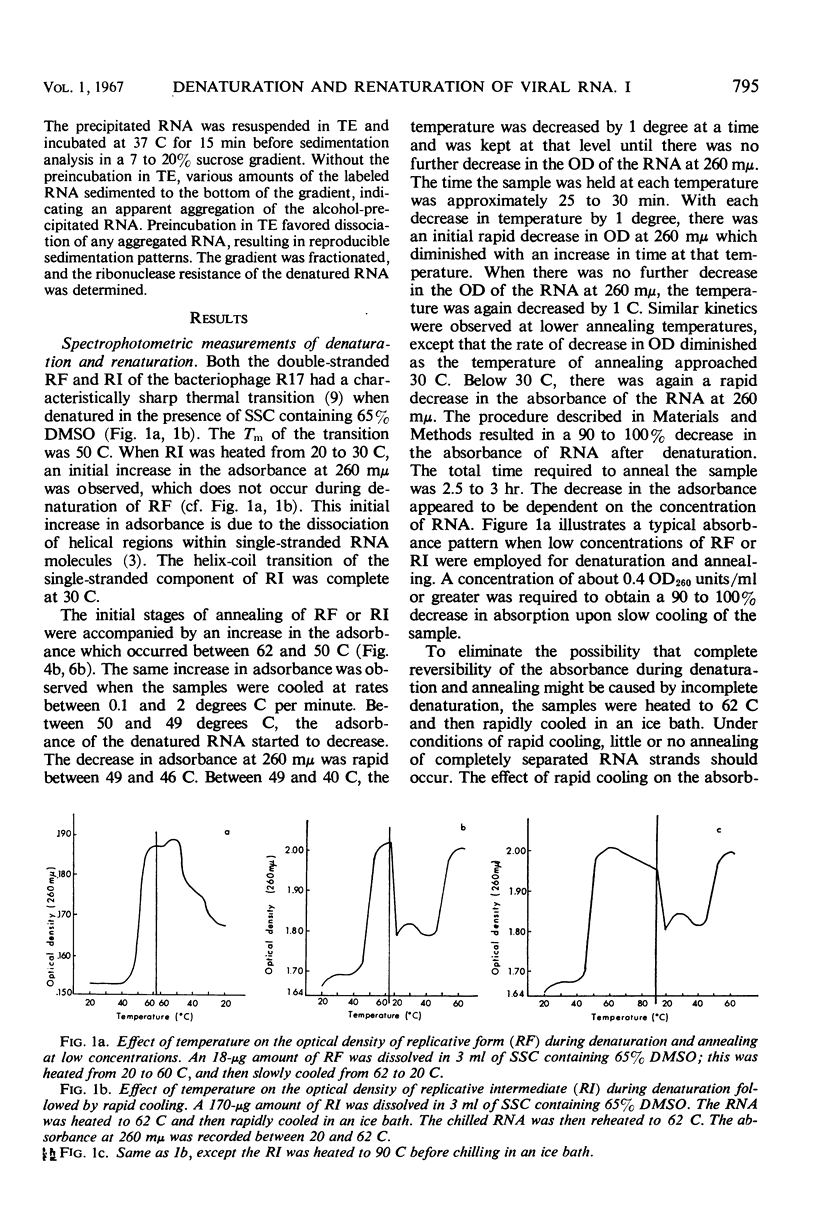
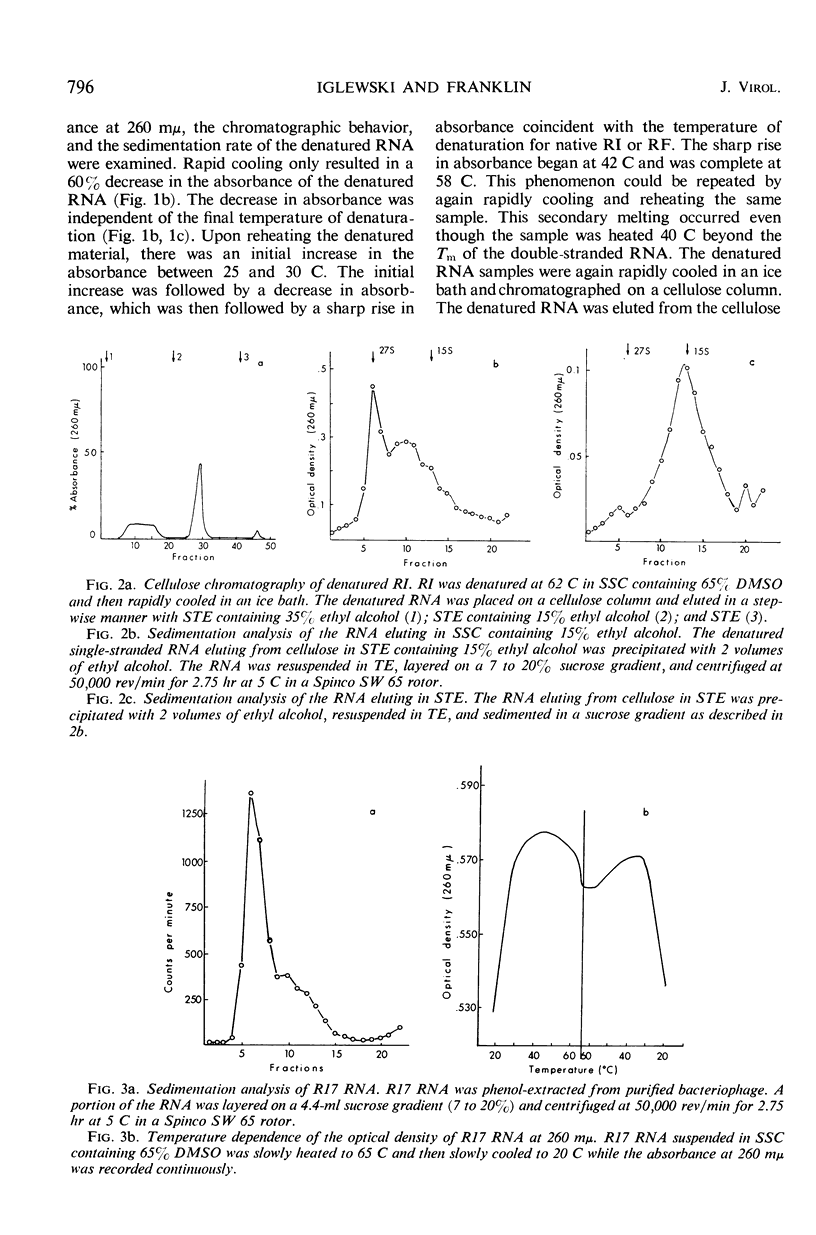
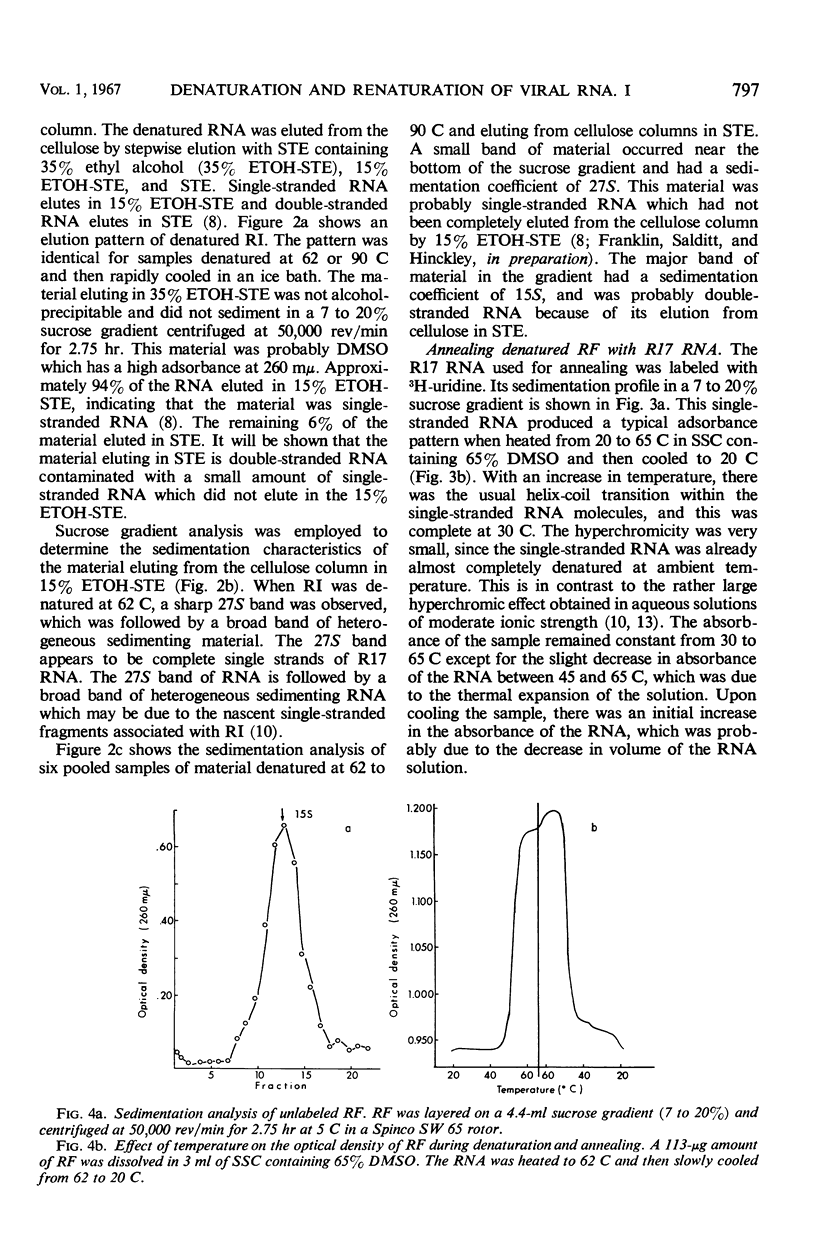
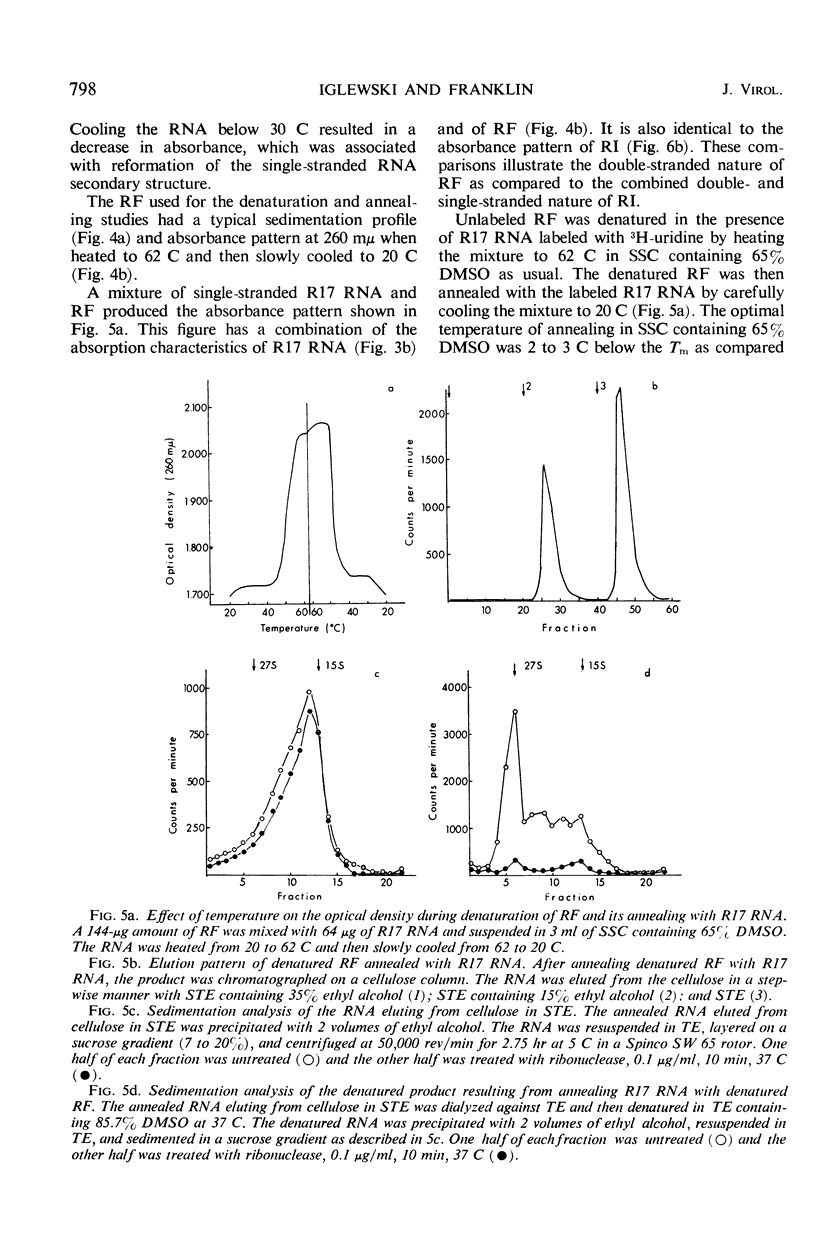
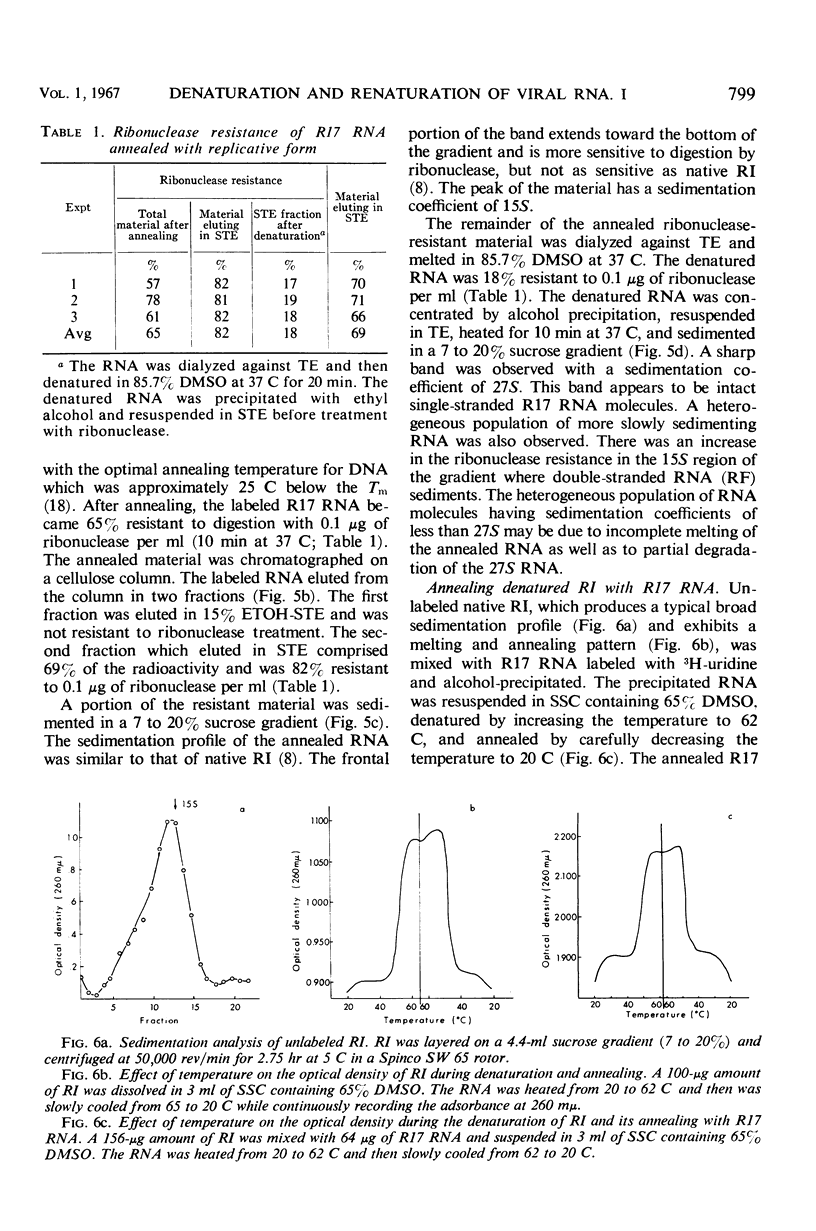
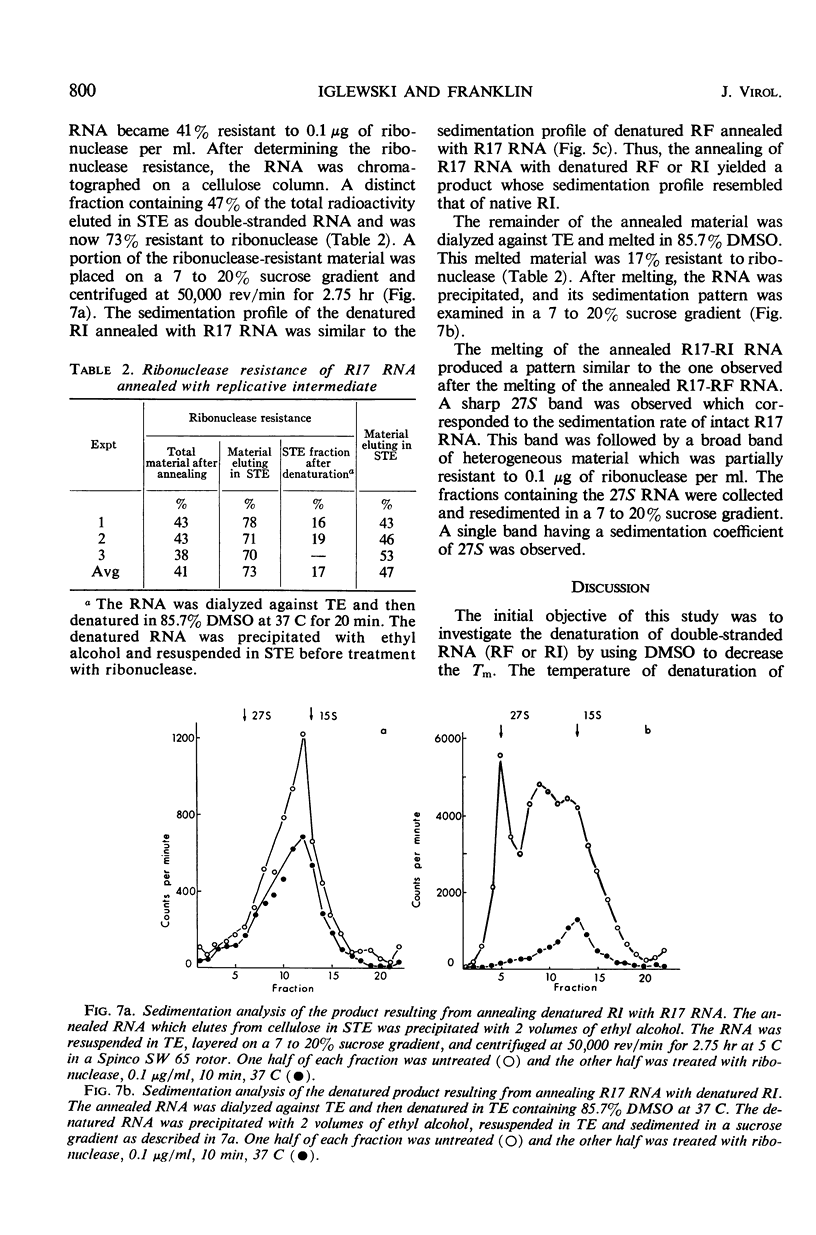
Purified replicative form (RF) and replicative intermediate (RI) prepared from Escherichia coli infected with R17 were denatured in 0.15 m NaCl, 0.015 m sodium citrate containing 65% dimethylsulfoxide. Denaturation of RF or RI was demonstrated spectrophotometrically, chromatographically, and by sedimentation analysis. Denatured RF or RI was annealed by carefully decreasing the temperature from 62 to 20 C. Annealing was accompanied by a decreased absorbance at 260 mμ. The decrease in absorbance during annealing appeared to be dependent upon the rate of cooling and the concentration of ribonucleic acid (RNA). Denatured RF or RI was annealed with R17 RNA which was labeled with 3H-uridine. The annealed product was 73 to 82% resistant to 0.1 μg/ml of ribonuclease. Annealing R17 RNA with either denatured RF or RI resulted in the formation of a ribonuclease-resistant product with a sedimentation profile resembling that of native RI. Melting the annealed products in 85.7% dimethyl sulfoxide produced 27S single-stranded R17 RNA and a heterogeneous population of more slowly sedimenting RNA.
Full text
PDF




Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- AMMANN J., DELIUS H., HOFSCHNEIDER P. H. ISOLATION AND PROPERTIES OF AN INTACT PHAGE-SPECIFIC REPLICATIVE FORM OF RNA PHAGE M12. J Mol Biol. 1964 Dec;10:557–561. doi: 10.1016/s0022-2836(64)80079-6. [DOI] [PubMed] [Google Scholar]
- Billeter M. A., Libonati M., Viñuela E., Weissmann C. Replication of viral ribonucleic acid. X. Turnover of virus-specific double-stranded ribonucleic acid during replication of phage MS2 in Escherichia coli. J Biol Chem. 1966 Oct 25;241(20):4750–4757. [PubMed] [Google Scholar]
- Doty P., Boedtker H., Fresco J. R., Haselkorn R., Litt M. SECONDARY STRUCTURE IN RIBONUCLEIC ACIDS. Proc Natl Acad Sci U S A. 1959 Apr;45(4):482–499. doi: 10.1073/pnas.45.4.482. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Doty P., Marmur J., Eigner J., Schildkraut C. STRAND SEPARATION AND SPECIFIC RECOMBINATION IN DEOXYRIBONUCLEIC ACIDS: PHYSICAL CHEMICAL STUDIES. Proc Natl Acad Sci U S A. 1960 Apr;46(4):461–476. doi: 10.1073/pnas.46.4.461. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Erikson R. L., Erikson E., Gordon J. A. Structure and function of bacteriophage R17 replicative intermediate RNA. I. Studies on sedimentation and infectivity. J Mol Biol. 1966 Dec 28;22(2):257–268. doi: 10.1016/0022-2836(66)90131-8. [DOI] [PubMed] [Google Scholar]
- Erikson R. L., Franklin R. M. Symposium on replication of viral nucleic acids. I. Formation and properties of a replicative intermediate in the biosynthesis of viral ribonucleic acid. Bacteriol Rev. 1966 Jun;30(2):267–278. doi: 10.1128/br.30.2.267-278.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- FENWICK M. L., ERIKSON R. L., FRANKLIN R. M. REPLICATION OF THE RNA OF BACTERIOPHAGE R17. Science. 1964 Oct 23;146(3643):527–530. doi: 10.1126/science.146.3643.527. [DOI] [PubMed] [Google Scholar]
- FREIFELDER D., DAVISON P. F., GEIDUSCHEK E. P. Damage by visible light to the acridine orange--DNA complex. Biophys J. 1961 May;1:389–400. doi: 10.1016/s0006-3495(61)86897-5. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R. M., Granboulan N. Ultrastructure of Escherichia coli cells infected with bacteriophage R17. J Bacteriol. 1966 Feb;91(2):834–848. doi: 10.1128/jb.91.2.834-848.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R. M. Purification and properties of the replicative intermediate of the RNA bacteriophage R17. Proc Natl Acad Sci U S A. 1966 Jun;55(6):1504–1511. doi: 10.1073/pnas.55.6.1504. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R. M. Replication of bacteriophage ribonucleic acid: some physical properties of single-stranded, double-stranded, and branched viral ribonucleic acid. J Virol. 1967 Feb;1(1):64–75. doi: 10.1128/jvi.1.1.64-75.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Franklin R. M. Replication of bacteriophage ribonucleic acid: some properties of native and denatured replicative intermediate. J Virol. 1967 Jun;1(3):514–522. doi: 10.1128/jvi.1.3.514-522.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GESTELAND R. F., BOEDTKER H. SOME PHYSICAL PROPERTIES OF BACTERIOPHAGE R17 AND ITS RIBONUCLEIC ACID. J Mol Biol. 1964 Apr;8:496–507. doi: 10.1016/s0022-2836(64)80007-3. [DOI] [PubMed] [Google Scholar]
- Iglewski W. J., Franklin R. M. Denaturation and renaturation of viral ribonucleic acd. II. Characterization of the products resulting from annealing R17 ribonucleic acid with denatured replicative form or with denatured replicative intermediate. J Virol. 1967 Aug;1(4):804–809. doi: 10.1128/jvi.1.4.804-809.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Iglewski W. J., Franklin R. M. Purification and properties of reovirus ribonucleic acid. J Virol. 1967 Apr;1(2):302–307. doi: 10.1128/jvi.1.2.302-307.1967. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Katz L., Penman S. Association by hydrogen bonding of free nucleosides in non-aqueous solution. J Mol Biol. 1966 Jan;15(1):220–231. doi: 10.1016/s0022-2836(66)80222-x. [DOI] [PubMed] [Google Scholar]
- Katz L., Penman S. The solvent denaturation of double-stranded RNA from poliovirus infected HeLa cells. Biochem Biophys Res Commun. 1966 May 25;23(4):557–560. doi: 10.1016/0006-291x(66)90765-0. [DOI] [PubMed] [Google Scholar]
- MARMUR J., DOTY P. Thermal renaturation of deoxyribonucleic acids. J Mol Biol. 1961 Oct;3:585–594. doi: 10.1016/s0022-2836(61)80023-5. [DOI] [PubMed] [Google Scholar]
- SINHA N. K., FUJIMURA R. K., KAESBERG P. RIBONUCLEASE DIGESTION OF R17 VIRAL RNA. J Mol Biol. 1965 Jan;11:84–89. doi: 10.1016/s0022-2836(65)80173-5. [DOI] [PubMed] [Google Scholar]
- STOLLAR D., GROSSMAN L. The reaction of formaldehyde with denatured DNA: spectrophotometric, immunologic, and enzymic studies. J Mol Biol. 1962 Jan;4:31–38. doi: 10.1016/s0022-2836(62)80114-4. [DOI] [PubMed] [Google Scholar]
- THOMAS C. A., Jr, BERNS K. I. The utility of formaldehyde in stabilizing polynucleotide chains from bacteriophage DNA. J Mol Biol. 1962 Apr;4:309–312. doi: 10.1016/s0022-2836(62)80008-4. [DOI] [PubMed] [Google Scholar]
- Vasquez C., Granboulan N., Franklin R. M. Structure of the ribonucleic acid bacteriophage R17. J Bacteriol. 1966 Dec;92(6):1779–1786. doi: 10.1128/jb.92.6.1779-1786.1966. [DOI] [PMC free article] [PubMed] [Google Scholar]
- WEISSMANN C., BORST P., BURDON R. H., BILLETER M. A., OCHOA S. REPLICATION OF VIRAL RNA, III. DOUBLE-STRANDED REPLICATIVE FORM OF MSW PHAGE RNA. Proc Natl Acad Sci U S A. 1964 Apr;51:682–690. doi: 10.1073/pnas.51.4.682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Weissmann C. Replication of viral RNA, VII. Further studies on the enzymatic replication of MS2 RNA. Proc Natl Acad Sci U S A. 1965 Jul;54(1):202–207. doi: 10.1073/pnas.54.1.202. [DOI] [PMC free article] [PubMed] [Google Scholar]



