Abstract
Muscle and bone anabolism and catabolism are tightly coupled during growth, development, and aging, yet the cellular and molecular mechanisms linking these two tissues are not well understood. Here we show that FGF-2 and IGF-1, two growth factors known to play a major role in regulating bone formation, are localized to muscle fibers along the muscle-bone interface of the mouse forelimb. Likewise, receptors for these growth factors are also abundant in periosteum adjacent to fleshy muscle attachments along the diaphysis of long bones. Growth factor levels were quantified from homogenized mouse forelimb muscles and IGF-1 was found to be the most abundant factor with FGF-2 also detected. Growth factor levels were also analyzed in conditioned medium from cultured myotubes, and IGF-1 and FGF-2 were again detected at significant levels. Mechanically wounding C2C12 myotubes increased the release of FGF-2 into conditioned medium, whereas IGF-1 was secreted at lower concentrations than FGF-2 following injury. Together these findings suggest that muscle is an important, local source of growth factors for bone tissue. Hence, the integrated growth and development of bone and muscle is likely to be regulated in part by paracrine mechanisms at the muscle-bone interface involving growth factor signaling.
Keywords: IGF-1, FGF-2, periosteum, muscle injury, C2C12 myotubes
A primary function of the vertebrate skeleton is to provide a set of bony levers to which muscles can attach in order to move and support the body. A close functional association between muscle and bone is underscored by the tight coupling of muscle and bone anabolism and catabolism during growth, development and aging. For example, peak rates of bone mineral acquisition in the skeleton are preceded by peak rates of increase in muscle mass1, and bone loss in later life is accompanied by significant loss of muscle mass2. Likewise, loss of muscle tissue with focal paralysis induces a rapid and significant loss of cortical bone3. Muscle is not only a regulator of pediatric bone gain, and perhaps a determinant of bone loss with aging, but muscle also plays a major role in bone repair4. As noted by Stein et al.5, p. 1382 “Muscle is perhaps the most crucial factor in the physiological process of fracture healing”. Consistent with an important role for muscle in driving bone formation and bone repair, fractures that are covered with relatively intact muscle heal more rapidly than fractures associated with severe muscle damage6. Although there is a clear connection between muscle and bone repair, the underlying mechanisms linking these two tissues are not well understood.
New bone can form nearby, and even within, a traumatized muscle. This phenomena, termed myositis ossificans, has been repeatedly documented both in the clinic and in animal experiments7. Though the molecular basis of muscle injury-induced bone formation remains unknown, important principles were revealed by experiments in which local bone growth in mice was measured after the removal of whole leg muscles8. New bone growth was only observed when the muscle removed was replaced with minced (e.g. injured) muscle. Liver could not substitute as a bone anabolic stimulus. Thus, the presence of muscle, and therefore of some signal derived from it, was essential for new bone formation. The authors8 actually considered the possibility that fibroblast growth factor, known then to be present in skeletal and cardiac muscle, might be the responsible signal, but, to our knowledge, did not further investigate this hypothesis. In this paper we describe morphology of the muscle-bone interface along the diaphysis of the radius as a model for understanding tissue interactions in the growing skeleton. Specifically, we focus on the localization of growth factors in this region in order to highlight the potential of muscle-derived growth factors for regulating the proliferation of cells in the periosteum. We also quantify levels of these growth factors from muscle ex vivo, and in conditioned medium from myotubes cultured in vitro, in order to identify the growth factors that are secreted at the highest concentrations from muscle cells. It is now known that muscle provides an important vascular supply for bone, as well as stem cells that can play a key role in bone repair.9 Our results demonstrate that muscle is an important, local source of growth factors for bone, and that paracrine signaling between muscle and bone tissue is also likely to play a key role in muscle-bone interactions.
MATERIALS AND METHODS
Sample, Histology & Immunohistochemistry
Histological and immunohistochemical studies of the muscle-bone interface were performed using forelimb specimens from a sample of six male and six female CD-1 mice, 8 weeks of age, obtained from Charles River Laboratories. Mice of this age were selected for analysis because they are still growing and have not reached their adult body weight or skeletal dimensions. Mice were euthanized following IACUC approved procedures and the right forelimbs removed by severing the humerus above the elbow joint. Forelimbs were fixed for 24h in paraformaldehyde and then decalcified in EDTA, dehydrated, embedded in paraffin, and sectioned at 4-6 μm. Alternate sections were stained with hematoxylin & eosin or Van Gieson and Celestine blue to visualize detailed morphology of the muscle-bone interface. Alternate sections were also stained with antibodies to FGF-2, IGF-1, and their receptors. These factors were studied because they are well-known osteogenic factors10-12 that are also expressed during muscle proliferation and regeneration13. For immunohistochemistry, sections were blocked in 2% goat serum and incubated with rabbit polyclonal antibodies to IGF-1 (SC-9013, Santa Cruz Biotechnology), IGF-1Rα (SC-712, Santa Cruz Biotechnology), FGF-2 (SC-79, Santa Cruz Biotechnology), and FGF-R2 (ab1068-50, AbCam). Staining was performed using a biotinylated goat anti-rabbit secondary antibody, Vector ABC staining kit, and either Cy3-labeled anti-HRP, DAB or VectorRed. In a separate set of experiments, skeletal muscle from mdx, dystrophin-deficient mice were also prepared from the sample of Montgomery et al.14 and stained as described above.
ELISA Assays & Cell Culture
For ELISA assays using skeletal muscle homogenates, triceps brachii muscles of the mice described above were rinsed once with 1X PBS, placed in 50 ml tubes with 5 ml 1X PBS, and homogenized on ice. Homogenates were frozen at −20C overnight, thawed, and centrifuged for 5 min at 5000xG following the recommended manufacturer procedure. Supernatant was removed, aliquoted, and stored at −20C. ELISA assays were performed using kits for FGF2 (DFB50, R&D Systems) and IGF-I (MG100, R&D Systems) following manufacturer specifications. C2C12 cells were obtained from the American Type Culture Collection (ATCC Number CRl-1772). Cells were cultured in differentiating medium (DMEM + 10% horse serum) for 10 days. Conditioned medium was removed at 4 and 10 days for ELISA assays using the kits for FGF2 and IGF-1 noted above.
For analysis of muscle injury in cultured mouse C2C12 myotubes, C2C12 cells were obtained from the American Type Culture Collection (ATCC Number CRl-1772). Cells were plated in 12 well dishes at a density of 20,000 per well in DMEM+10%FBS. The next day the cells were at 30% confluency, and were treated with differentiation media (DMEM+10% horse serum) and left for 4 days until myotubules began to form. A sterile rubber policeman was used to injure the C2C12 cells in half of the wells, and conditioned media was collected 3 hours after injury. Cells in the other half of the wells were not injured. All media was centrifuged for 5 min at 1000rpm to remove cell debris from both injured and non-injured cells. ELISA assays for FGF-2 (DFB50, R&D Systems) and IGF-1 (MG100, R&D Systems) were run on conditioned medium from both injured and non-injured cells according to manufacturer specifications.
Statistical analysis
Growth factor concentrations from skeletal muscle and from cultured myotubes were compared using single-factor ANOVA with growth factor type as the factor.
RESULTS
Localization of growth factors and their receptors at the muscle-bone interface
Sections along the mid-diaphysis of the mouse radius and ulna show that the forelimb muscles make fleshy attachments along virtually the entire periosteal surface of both the radius and ulna (Fig. 1A). High magnification micrographs demonstrate that as muscle fibers reach the periosteum there is a slight increase in collagen staining (pink, asterisks; Fig 1B, C) where the plasma membrane of myocytes is adjacent to the fibrous periosteum, which is characterized by a collagen rich matrix and abundant fibroblast nuclei. It is clear that while the periosteum is relatively thick and fibrous in certain locations where it merges with muscle fibers (Fig. 1B), it is only a single cell-layer thick in other locations (Fig. 1C), where muscle fibers appear to attach directly onto the bone surface. Immunohistochemistry shows that the growth factor IGF-1 can be detected along the surface of myocytes, and extracellularly, along the fleshy muscle-bone interface (Fig. 2A). Likewise, the IGF-1 receptor is localized to periosteum (Fig. 2B). FGF-2 is also detected in myofibers along their junction with periosteum (Fig. 2C), and receptors for FGF-2 are abundant in periosteum (Fig. 2D).
Fig. 1.
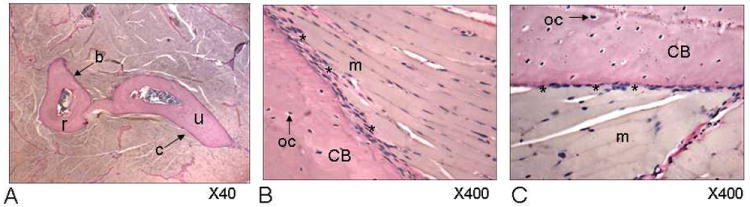
Van Gieson-stained sections of the mouse forelimb demonstrating microanatomy of the muscle-bone interface along the mid-diaphysis of the radius (r) and ulna (u). Arrow a indicates region of image shown in 1B and arrow b indicates region of image shown in 1C. B. Asterisks indicate relatively thick and fibrous peristeum where it merges with muscle fibers, whereas in other locations (C) the periosteum is only a single cell-layer thick and muscle fibers appear to attach directly onto the bone surface. m=skeletal muscle, oc=osteocytes, CB=cortical bone.
Fig. 2.
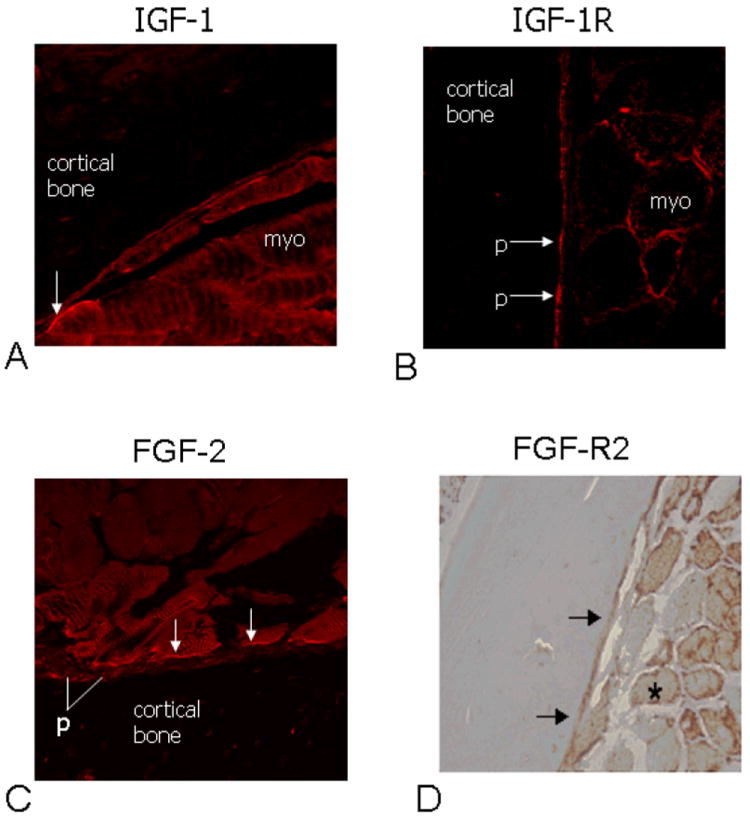
Immunofluorescence using antibodies to IGF-1 (A) and the IGF-1 receptor (IGF-1R; B) imaged with confocal microscopy show (arrows) IGF-1 in myofibers (myo) adjacent to the IGF-1 receptor in periosteum (p). Likewise, antibodies to FGF-2 localize this growth factor in myofibers along the muscle-bone interphase (C), and the FGF-2 receptor (FGF-R2) is abundant in muscle fibers (asterisk) and in periosteum (arrows) (D).
Growth factor levels from muscle ex vivo and myotubes in vitro
ELISA assays run on mouse muscle homogenates revealed significantly (P<0.01) higher levels of IGF-1 (0.32 ng/ml) in skeletal muscle compared to FGF-2 (0.15 ng/ml), suggesting that under normal conditions IGF-1 protein is more abundant in skeletal muscle than FGF-2 (Fig. 3A). Conditioned medium from mouse C2C12 myotubes was analyzed after 4 days of culture in differentiating medium using ELISA to measure levels of IGF-1 and FGF-2 secreted by muscle cells in vitro. FGF-2 was detected at relatively low concentrations (0.08 ng/ml) whereas IGF-1 was detected at significantly (P<0.01) higher (0.77 ng/ml) levels in C2C12-conditioned medium (Fig. 3B). In the second set of experiments we tested the hypothesis that muscle injury stimulates FGF-2 release. Immunostaining for FGF-2 in skeletal muscles of dystrophin-deficient, mdx mice, which show elevated levels of myfiber wounding, reveal abundant FGF-2 in injured myofibers (Fig. 3C). We also tested the hypothesis that wounded myofibers release FGF-2 in vitro using C2C12 myotubes. Results indicate that muscle cell injury produces a significant increase in FGF-2 protein secreted into the culture medium, whereas injury actually decreases production of IGF-1, another factor produced by myocytes that can also stimulate bone formation (Fig. 3D).
Fig. 3.
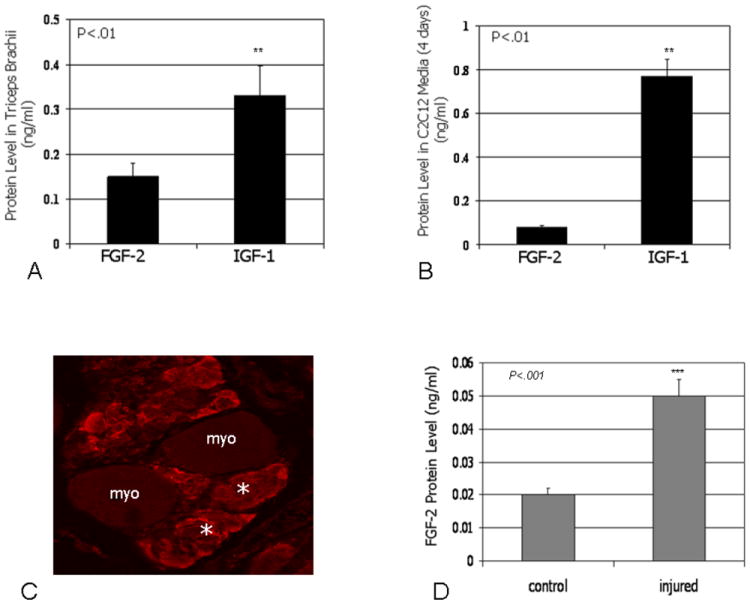
Protein levels of IGF-1 are significantly higher than FGF-2 levels in homogenates from forelimb muscles of normal mice mice (A). Protein levels of FGF-2 and IGF-1 detected using ELISA assays in conditioned medium from C2C12 cells (B). FGF-2 is abundant in injured myofibers (myo) of dystrophin-deficient mdx mice in vivo (asterisks) (C), and injury of mouse C2C12 myotubes increases the concentration of FGF-2 secreted into culture medium (D).
DISCUSSION
In his classic On Growth and Form D’Arcy Thompson15, p.263 wrote “Muscle and bone are inseparably associated and connected; they are moulded one with another; they come into being together…”. Indeed, muscle paralysis or the congenital absence of muscle development in utero decreases bone mineralization, bone cross-sectional diameter, and the development of long bone curvatures16. Mechanical loading is thought to be the primary mechanism by which muscle mass influences bone modeling, and a substantial body of literature supports this biomechanical relationship underlying the muscle-bone unit17. Apart from its well-known role in providing a mechanical stimulus to bone tissue, our results suggest that muscle may also influence bone metabolism by secreting local growth factors that can stimulate osteogenesis (Fig. 4). This hypothesis was given additional support recently by the study of Kaufman et al.18, who demonstrated that when nitrocellulose membranes were interposed between muscle and injured bone, bone healing was improved if pore sizes were large enough for the diffusion of growth factors. Indeed, Kaufman et al.18, p. 755 concluded that “osteogenesis and bone remodeling activities induced by muscle cells are, in fact, paracrinic activities of muscle-derived metabolites acting on the nearby bone”. We believe that this paracrine signaling mechanism explains a number of situations in which muscle has been observed to induce bone formation independent of mechanical loading. For example, fractures that are covered with relatively intact muscle heal more rapidly than fractures associated with more severe muscle damage6, muscle flaps applied to autogenous bone grafts improve healing but coverage with skin does not19, and minced muscle applied directly to intact bone tissue can stimulate bone formation but minced liver does not 8. Likewise, muscle coverage of open tibial fractures accelerates bone healing, but coverage with fasciocutaneous tissue does not.20 Muscle injury also stimulates proliferation of osteoprogenitor cells in the periosteum at the muscle-bone interface21. It was previously suggested that in these cases muscle might function as a local source of trophic factors for bone8, 14, but the trophic factors emanating from muscle tissue were never specified.
Fig. 4.
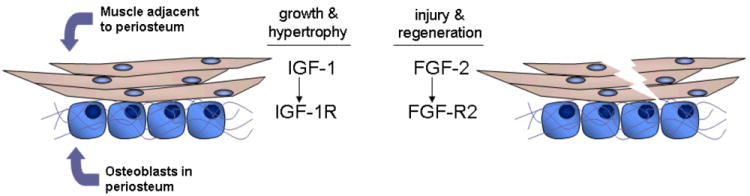
Generalized model showing paracrine interactions at the muscle-bone interface. Muscle growth, development, and hypertrophy lead to the secretion of IGF-1, which stimulates bone formation by osteoprogenitor cells in the periosteum that express IGF-1R. Muscle injury during strenuous exercise or traumatic extremity injury promotes the release of FGF-2, which induces bone formation and stimulates fracture healing by periosteal osteoprogenitor cells expressing FGF-R2.
It is well established that exogenous delivery of factors such as IGF-1 and FGF-2 will stimulate bone formation12, 22. The endogenous source of these proteins for bone is thought to be both local, as osteoblasts and chondrocytes express IGF-1 and FGF-2, and systemic, as all three factors can be detected in serum. Our data show that myofibers secrete these osteogenic factors in vitro, and that these same factors are localized to the muscle-bone interface in vivo. Furthermore, receptors for these factors are abundant in both periosteum and skeletal muscle. These results are consistent with previous studies showing that IGF-1 is abundant in wound exudates of skeletal muscle flaps23, both IGF-1 and FGF-2 are present in extracts from crushed muscle24-26, and levels of extracellular FGF-2 and FGF-2 in serum are elevated with muscle injury.27,28 Local increases of FGF-2 at the muscle-bone interface during muscle regeneration would also explain why mdx mice, which have large but very weak muscles along with increased myofiber injury and regeneration, also show greater bone diameters than normal mice14. Skeletal muscle regeneration is also associated with increased expression of IGF-113, 29 and IGF-1 secretion from myofibers may couple muscle growth, development, and repair with bone formation. It is certainly possible that the secretion of these factors may be altered with muscle contraction and resistance exercise, perhaps increasing local delivery of muscle-derived growth factors to bone (Fig. 4). This hypothesis would be consistent with the observation that overexpression of IGF-1 in muscle increases bone size and bone mass30, and that ectopic expression of IGF-1 in muscle can prevent bone loss with disuse31. Mechanical and biological stimuli may therefore function synergistically with one another, where growth factor secretion by muscle is one of several possible ways that mechanical signals are transduced biologically.
It is likely that other factors secreted by muscle can also significantly increase bone formation. A recent study found that proteins such as matrix metalloproteinase-2 (MMP-2), secreted protein rich in cysteine protein (SPARC, or osteonectin), and insulin-like growth factor binding protein-5 (IGFBP5) are secreted by C2C12 myotubes in vitro32. Each of these factors is known to influence bone metabolism. Mice lacking MMP-2 show decreased bone mineral density in the limbs33, mice lacking SPARC develop osteopenia due to significantly decreased bone formation by osteoblasts34, and IGFBP5 can stimulate bone formation in vivo and in vitro35. Muscle is also a primary source of leptin36, which is known to regulate both osteoblast and osteoclast differentiation37, and serum leptin levels increase with increased muscle mass38. Finally, muscle cells are known to secrete transforming growth factor beta-1 (TGFβ1),39 and tgf beta is a potent mitogen for osteoblasts that is generally thought to have anti-resorptive effects on bone.40 TGF β1 does, however, contribute to the accumulation of fibrotic tissue in muscle, and TGF β1 stimulates expression of myostatin, which suppresses muscle growth and hypertrophy. 39 Thus, it is likely that muscle growth and hypertrophy in response to loading involve downregulation of TGF β1, whereas muscle growth and exercise-induced loading involve increased expression of muscle IGF-1 and decreased expression of myostatin.
A role for muscle-derived growth factors in bone metabolism raises several questions about how muscle-bone interactions may change with growth, development, and aging, and what specific regions of bone (e.g., cortical vs. trabecular) and cell populations (osteoblasts or osteoclasts) might be affected. We suggest that periosteal surfaces are most likely to be influenced by the secretion of growth factors from muscle because of the fact that periosteal tissue is closely apposed to muscle at many skeletal sites. Allen and colleagues41 have proposed that osteoprogenitor cells in periosteum differ markedly from marrow-derived osteoprogenitors in their threshold sensitivity to physical and hormonal stimuli. A future area of research is to determine how periosteal osteoprogenitors differ from marrow- and endosteal-derived stem cells in their response to muscle-derived growth factors. A second area of research is to determine how the interactions between muscle-derived growth factors and osteoprogenitor cells, particularly periosteal cells, are altered with age. It is known that muscle mass as well as periosteal cellularity and thickness all decline with age. One attractive hypothesis is that prevention of age-associated muscle wasting (sarcopenia) with treatments such as androgen receptor modulators or myostatin inhibitors might also attenuate age-related bone fragility by enhancing periosteal apposition via increased secretion of local muscle-derived growth factors. Finally, the periosteal surface is also a site of bone resorption42. It remains to be determined how muscle tissue might be involved in the paracrine regulation of osteoclast activity, but it is certainly possible that declines in muscle-derived factors with age or disuse could stimulate periosteal bone resorption.
The data presented here provide new insights into the functional and developmental interactions between muscle and bone at the molecular, cellular and whole-organism levels. It is well-recognized that soft-tissues such as muscle and fat influence bone mass43, and while the role of fat-derived factors such as adiponectin and leptin have received considerable attention with regard to their effects on bonee.g., ref.44 the role of muscle as a source of osteogenic factors has received much less attention. As such, our findings have important implications for both basic and clinical science. First, the results of this study suggest that targeting muscle using novel pharmacological therapies may improve the mass and strength of both muscle and bone tissue through non-mechanical pathways. Perhaps more importantly, our findings underscore the importance of maintaining muscle mass throughout life as a means of maintaining bone density and strength, and as a strategy for preventing falls and debilitating bone fractures.
Acknowledgments
Funding for this research was provided by the National Institutes of Health (NIAMS AR 049717) and the Office of Naval Research (N00014-08-1-0197). We are grateful to Darren Baker and Katsuya Miyake for assistance with confocal imaging.
LITERATURE CITED
- 1.Rauch F, Bailey D, Baxter-Jones A, Mirwald R, Faulkner R. The ‘muscle-bone unit’ during the pubertal growth spurt. Bone. 2004;34:771–775. doi: 10.1016/j.bone.2004.01.022. [DOI] [PubMed] [Google Scholar]
- 2.Greenlund LJ, Nair KS. Sarcopenia--consequences, mechanisms, and potential therapies. Mech Ageing Dev. 2003;124:287–99. doi: 10.1016/s0047-6374(02)00196-3. [DOI] [PubMed] [Google Scholar]
- 3.Warner S, Sanford D, Becker B, Bain S, Srinivasan S, Gross TS. Botox induced muscle paralysis rapidly degrades bone. Bone. 2006;38:257–264. doi: 10.1016/j.bone.2005.08.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 4.Gerstenfeld L, Einhorn T. Developmental aspects of fracture healing and the use of pharmacological agents to alter healing. J Musculoskeletal Neuronal Interact. 2003;3:297–303. [PubMed] [Google Scholar]
- 5.Stein A, Perren S, Cordey J, Kenwright J, Mosheiff R, Francis M. The muscle bed—a crucial factor in fracture healing: a physiological concept. Orthopedics. 2002;25:1379–1383. doi: 10.3928/0147-7447-20021201-16. [DOI] [PubMed] [Google Scholar]
- 6.Utvag S, Grundnes O, Rindal D, Reikeras O. Influence of extensive muscle injury on fracture healing in rat tibia. J Orthop Trauma. 2003;17:430–5. doi: 10.1097/00005131-200307000-00007. [DOI] [PubMed] [Google Scholar]
- 7.Hall BK. Cellular differentiation in skeletal tissues. Biol Rev Camb Philos Soc. 1970;45:455–84. doi: 10.1111/j.1469-185x.1970.tb01174.x. [DOI] [PubMed] [Google Scholar]
- 8.Zacks SI, Sheff MF. Periosteal and metaplastic bone formation in mouse minced muscle regeneration. Lab Invest. 1982;46:405–12. [PubMed] [Google Scholar]
- 9.Schindeler A, Liu R, Little DG. The contribution of different cell lineages to bone repair: exploring a role for muscle stem cells. Differentiation. 2009;77:12–18. doi: 10.1016/j.diff.2008.09.007. [DOI] [PubMed] [Google Scholar]
- 10.Nakamura T, Hanada K, Tamura M, Shibanushi T, Fukomoto S, Matsumoto T. Stimulation of endosteal bone formation by systemic injections of recombinant basic fibroblast growth factor in rats. Endocrinology. 1995;136:1276–84. doi: 10.1210/endo.136.3.7867582. [DOI] [PubMed] [Google Scholar]
- 11.Yakar S, Rosen CJ, Beamer WG, Ackert-Bicknell CL, Wu Y, Liu J-L, Ooi G, Setser J, Frystyk J, Boisclair YR, LeRoith D. Circulating levels of IGF-1 directly regulate bone growth and density. J Clin Invest. 2002;110:771–81. doi: 10.1172/JCI15463. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Fowlkes J, Thrailkill K, Liu L, Wahl E, Bunn R, Cockrell G, Perrien D, Aronson J, Lumpkin C. Effects of systemic and local administration of recombinant human IGF-I (rhIGF-I) on de novo bone formation in an aged mouse model. J Bone Miner Res. 2006;21:1359–66. doi: 10.1359/JBMR.060618. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Charge S, Rudnicki M. Cellular and molecular regulation of muscle regeneration. Physiol Rev. 2004;84:209–38. doi: 10.1152/physrev.00019.2003. [DOI] [PubMed] [Google Scholar]
- 14.Montgomery E, Pennington C, Isales CM, Hamrick MW. Muscle-bone interactions in dystrophin-deficient and myostatin-deficient mice. Anat Rec. 2005;286A:814–822. doi: 10.1002/ar.a.20224. [DOI] [PubMed] [Google Scholar]
- 15.Thompson D. On Growth and Form. Cambridge: University Press; 1961. [Google Scholar]
- 16.Gomez C, Valentin D, Peet N, Vico L, Chenu C, Malaval L, Skerry T. Absence of mechanical loading in utero influences bone mass and architecture but not innervation in MyoD-Myf5-deficient mice. J Anat. 2007;210:259–271. doi: 10.1111/j.1469-7580.2007.00698.x. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 17.Rittweger J. Ten years muscle-bone hypothesis: What have we learned so far? J Musculoskelet Neuronal Interact. 2008;8:174–178. [PubMed] [Google Scholar]
- 18.Kaufman H, Reznick A, Stein H, Barak M, Maor G. The biological basis of the bone-muscle inter-relationship in the algorithm of fracture healing. Orthopedics. 2008;31:751. [PubMed] [Google Scholar]
- 19.Richards R, McKee M, Paitich C, Anderson G, Bertoia J. A comparison of the effects of skin coverage and muscle flap coverage on the early strength of union at the site of osteotomy after devascularization of a segment of canine tibia. J Bone Jt Surg. 1991;73:1323–30. [PubMed] [Google Scholar]
- 20.Harry L, Sandison A, Paleolog E, Hansen U, Pearse M, Nanchanal J. Comparison of the healing of open tibial fractures covered with either muscle or fasciocutaneous tissue in a murine model. J Orthop Res. 2008;26:1238–1244. doi: 10.1002/jor.20649. [DOI] [PubMed] [Google Scholar]
- 21.Landry P, Marino A, Sadasivan K, Albright J. Effect of soft-tissue trauma on the early periosteal response of bone to injury. J Trauma. 2000;48:479–483. doi: 10.1097/00005373-200003000-00018. [DOI] [PubMed] [Google Scholar]
- 22.Liang H, Pun S, Wronski TJ. Bone anabolic effects of basic fibroblast growth factor in ovariectomized rats. Endocrinology. 1999;140:5780–8. doi: 10.1210/endo.140.12.7195. [DOI] [PubMed] [Google Scholar]
- 23.Vogt P, Boorboor P, Vaske B, Topsakal E, Schneider M, Muehlberger T. Significant angiogenic potential is present in the microenvironment of muscle flaps in humans. J Reconstructive Microsurgery. 2005;21:517–523. doi: 10.1055/s-2005-922429. [DOI] [PubMed] [Google Scholar]
- 24.Anderson J, Liu L, Kardami E. Distinctive patterns of basic fibroblast growth factor (bFGF) distribution in degenerating and regenerating areas of dystrophic (mdx) striated muscles. Dev Biol. 1991;147:96–109. doi: 10.1016/s0012-1606(05)80010-7. [DOI] [PubMed] [Google Scholar]
- 25.Anderson J, Mitchell C, McGeachie J, Grounds M. The time course of basic fibroblast growth factor expression in crush-injured skeletal muscles of SJL/J and BALB/c mice. Exp Cell Res. 1995;216:325–334. doi: 10.1006/excr.1995.1041. [DOI] [PubMed] [Google Scholar]
- 26.Haugk K, Roeder R, Garber M, Schelling G. Regulation of muscle cell proliferation by extracts from crushed muscle. J Anim Sci. 1995;73:1972–1981. doi: 10.2527/1995.7371972x. [DOI] [PubMed] [Google Scholar]
- 27.DiMario J, Buffinger N, Yamada S, Strohman RC. Fibroblast growth factor in the extracellular matrix of dystrophic (mdx) mouse muscle. Science. 1989;244:688–90. doi: 10.1126/science.2717945. [DOI] [PubMed] [Google Scholar]
- 28.D’Amore PA, Brown R, Ku P, Hoffman E, Watanabe H, Arahata K, Ishihara T. Elevated basic fibroblast growth factor in the serum of patients with Duchenne muscular dystrophy. Ann Neurol. 1994;35:362–5. doi: 10.1002/ana.410350320. [DOI] [PubMed] [Google Scholar]
- 29.Goetsch SC, Hawke TJ, Gallardo TD, Richardson JA, Garry DJ. Transcriptional profiling and regulation of the extracellular matrix during muscle regeneration. Physiol Genomics. 2003;14:261–71. doi: 10.1152/physiolgenomics.00056.2003. [DOI] [PubMed] [Google Scholar]
- 30.Banu J, Wang L, Kalu D. Effects of increased muscle mass on bone in male mice overexpressing IGF-I in skeletal muscles. Calcif Tissue Int. 2003;73:196–201. doi: 10.1007/s00223-002-1072-z. [DOI] [PubMed] [Google Scholar]
- 31.Alzghoul M, Gerrard D, Watkins B, Hannon K. Ectopic expression of IGF-I and Shh by skeletal muscle inhibits disuse-mediated skeletal muscle atrophy and bone osteopenia in vivo. FASEB J. 2004;18:221–223. doi: 10.1096/fj.03-0293fje. [DOI] [PubMed] [Google Scholar]
- 32.Chan X, McDermott J, Siu K. Identification of secreted proteins during skeletal muscle development. J Proteome Res. 2007;6:698–710. doi: 10.1021/pr060448k. [DOI] [PubMed] [Google Scholar]
- 33.Inoue K, Mikuni-Takagaki Y, Oikawa K, Itoh T, Inada M, Noguchi T, Park J, Onodera T, Krane S, Noda M, Itohara S. A crucial role for matrix metalloproteinase 2 in osteocytic canalicular formation and bone metabolism. J Biol Chem. 2006;281:33814–24. doi: 10.1074/jbc.M607290200. [DOI] [PubMed] [Google Scholar]
- 34.Delany A, Kalajzic I, Bradshaw A, Sage E, Canalis E. Osteonectin-null mutation compromises osteoblast formation, maturation, and survival. Endocrinology. 2003;144:2588–96. doi: 10.1210/en.2002-221044. [DOI] [PubMed] [Google Scholar]
- 35.Andress D. IGF-binding protein-5 stimulates osteoblast activity and bone accretion in ovariectomized mice. Am J Physiol Endocrinol Metab. 2001;281:E283–8. doi: 10.1152/ajpendo.2001.281.2.E283. [DOI] [PubMed] [Google Scholar]
- 36.Wang J, Liu R, Hawkins M, Barzilai N, Rossetti L. A nutrient-sensing pathway regulations leptin gene expression in muscle and fat. Nature. 1998;393:684–688. doi: 10.1038/31474. [DOI] [PubMed] [Google Scholar]
- 37.Hamrick MW, Ferrari SL. Leptin and the sympathetic connection of fat to bone. Osteoporosis International. 2008;19:905–912. doi: 10.1007/s00198-007-0487-9. [DOI] [PubMed] [Google Scholar]
- 38.Fernandez-Real H, Vayreda M, Casamitjana R, Gonzalez-Huix Ricart W. The fat-free mass compartment influences serum leptin in men. Eur J Endocrinol. 2000;142:25–29. doi: 10.1530/eje.0.1420025. [DOI] [PubMed] [Google Scholar]
- 39.Zhu J, Li Y, Shen W, Qiao C, Ambrosio F, Lavasani M, Nozaki M, Branca M, Huard J. Relationships between transforming growth factor beta-1, myostatin, and decorin: implications for skeletal muscle fibrosis. J Biol Chem. 2007;282:25852–63. doi: 10.1074/jbc.M704146200. [DOI] [PubMed] [Google Scholar]
- 40.Bonewald L. Transforming growth factor-β. In: Bilezikian J, Raisz L, Rodan G, editors. Principles of Bone Biology. 2. Sand Diego: Academic Press; 2002. pp. 903–918. [Google Scholar]
- 41.Allen MR, Hock JM, Burr DB. Periosteum: biology, regulation, and response to osteoporosis therapies. Bone. 2004;35:1003–1012. doi: 10.1016/j.bone.2004.07.014. [DOI] [PubMed] [Google Scholar]
- 42.Seeman E. The periosteum—a surface for all seasons. Osteopor Intl. 2007;18:123–28. doi: 10.1007/s00198-006-0296-6. [DOI] [PubMed] [Google Scholar]
- 43.Reid IR. Relationships among body mass, its components, and bone. Bone. 2002;31:547–555. doi: 10.1016/s8756-3282(02)00864-5. [DOI] [PubMed] [Google Scholar]
- 44.Reid IR, Cornish J, Baldock P. Nutrition-related peptides and bone homeostasis. J Bone Miner Res. 2006;21:495–500. doi: 10.1359/jbmr.051105. [DOI] [PubMed] [Google Scholar]


