Abstract
Cholangiocarcinoma (CCA) carries a severe prognosis because of its strong invasiveness and early metastasization. In several patients, otherwise eligible for surgical resection, micrometastasis are already present at the time of surgery. The mechanisms responsible for CCA invasiveness are unclear. S100A4, a member of the S100 family of small Ca2+-binding proteins, is expressed in mesenchymal cells, regulates cell motility in several cell types, and is expressed in some epithelial cancers. Thus, we aimed to study the role of S100A4 in CCA invasiveness and metastasization. The expression of S100A4 was studied by immunohistochemistry in 93 human liver samples of CCA patients undergoing surgical resection and correlated with metastases development (67 cases) and patient survival following surgery using log rank tests and multivariate analysis. S100A4 expression was studied in EGI-1 and TFK-1, human CCA cell lines with and without nuclear S100A4 expression, respectively. Metastatic properties of CCA cells were assessed by xenotransplantation in severe combined immunodeficiency (SCID) mice after transduction with lentiviral vectors encoding firefly luciferase gene. Proliferation, motility (wound healing), invasiveness (Boyden chamber), and metalloproteinases (MMPs) secretion were studied in CCA cells, with or without lentiviral silencing of S100A4. Nuclear expression of S100A4 by neoplastic ducts was a strong predictor of metastasization and reduced survival after resection (P < 0.01). EGI-1 CCA cells showed stronger metastatic properties than TFK-1 when xenotransplanted in SCID mice. S100A4-silenced EGI-1 cells showed significantly reduced motility, invasiveness, and MMP-9 secretion in vitro, without changes in cell proliferation.
Conclusion
Nuclear S100A4 identifies a subset of CCA patients with a poor prognosis after surgical resection. Nuclear expression of S100A4 increases CCA cells invasiveness and metastasization, indicating that S100A4 may also represent a potential therapeutic target.
Cholangiocarcinoma (CCA) is the second most common primary malignancy of the liver; the incidence of intrahepatic CCA in Western countries has been steadily growing in the last two decades.1 In spite of the rising incidence, treatment options for CCA remain unsatisfactory,1,2 particularly because of the strong and early invasiveness of the tumor. In many patients, lymphnodal or distant metastasis or micrometastasis are present already at the time of the diagnosis, limiting and worsening the prognosis in patients otherwise eligible for surgical resection. However, a subset of patients with less aggressive CCA may even undergo liver transplantation after neoadjuvant radiochemotherapy and have excellent survival. Biomarkers able to predict tumor invasiveness and prognosis would be an important decision-making tool. Unfortunately, mechanisms that determine CCA invasiveness are largely unknown.
Cancer invasiveness and metastasization requires tightly adherent epithelial cells to convert to a more motile phenotype expressing several mesenchymal features. 3 During this process, some molecular programs typical of the mesenchymal phenotype are activated, as shown by the expression of specific cell surface proteins, cytoskeletal proteins, extracellular matrix-degrading enzymes, and transcription factors.4 One such proteins is S100A4, a member of the S100 family of small calcium-binding proteins, expressed by mesenchymal cells, macrophages,5 and by epithelial cells in mesenchymal transition (EMT). Expression of S100A4 was shown to be a predictor of metastasization in colon cancer.6,7
The mechanisms of action of A100A4 depends on the cellular localization of the protein. In the cytoplasm S100A4 interacts with a number of partner proteins in cytoskeleton and in the plasma membrane (such as myosin IIa or liprin-β1). When localized in the nucleus, S100A4 may exert transcriptional functions that affect several genes, including matrix metalloproteinase (MMP)-98 and E-cadherin.9 However, the mechanism of action of S100A4 remains largely unknown, as it remains unclear whether S100A4 is just a biomarker of cancer cell aggressiveness or actually represents a functional target amenable of therapeutic intervention.
While examining the expression of EMT markers in CCA specimens, we noticed that a subgroup of CCAs expressed S100A4 in the nucleus. In this study we addressed: (1) the prognostic significance of S100A4 nuclear expression in a large series of patients undergoing surgical resection, and (2) the functional relevance of S100A4 expression on the metastatic potential, motility, and invasiveness of CCA cell lines in vivo and in vitro. Our results show that nuclear expression of S100A4 by neoplastic bile ducts significantly correlated with increased metastasization and reduced survival after surgery, that human CCA cells with nuclear expression of S100A4 have a much stronger metastatic ability when xenotransplanted into severe combined immunodeficiency (SCID) mice, and that silencing S100A4 in CCA cells that originally overexpressed S100A4 significantly reduced motility and invasive capabilities in vitro.
Patients and Methods
Patients and Tissues Samples
A total of 93 samples of CCA, obtained from subjects undergoing surgical resection between 1989 and 2009, in three different medical centers in northern Italy (Bergamo n = 23, Padova n = 49, Treviso n = 21) were considered for the immunohistochemical study; among them, matched peritumoral liver samples were available in 23 cases. To adjust for possible effects on survival related to surgical complications, rather than tumor prognosis, we excluded subjects who died during the first 30 days after surgery; thus, the follow-up period started from 30 days after resection. Taking this approach, 86 subjects out of the original 93 subjects were considered for statistical analysis. The follow-up period ranged from 0.13 to 195.67 months (median: 13.37 months). Clinical, epidemiological, anatomical (hepatic localization), and histopathological (staging, grade, margin involvement) data of CCA patients according to S100A4 expression are reported in Table 1. In a subset of 67 patients (78%), metastatic data were available. Informed consent and local regional Ethical Committee approval were obtained before tissue collection.
Table 1.
Clinical Features of the CCA Series (N=86) According to S100A4 Grouping Considered for Kaplan-Meier Analysis and Cox Model*
| Clinical Features | Total Number of Cases | S100A4 Nuclear Staining | P† | ||
|---|---|---|---|---|---|
|
| |||||
| 0 (N=49) | (0, 30%) (N=19) | ≥30% (N=18) | |||
| Status | 0.009‡ | ||||
| Alive | 42 | 31 (73.8) | 6 (14.3) | 5 (11.9) | |
| Dead | 44 | 18 (40.9) | 13 (29.6) | 13 (29.6) | |
| Age | 86 | 64.4 ±10.1 | 66.7 ±10.4 | 61.0 ±13.0 | 0.27§ |
| Gender | 0.98 | ||||
| Male | 35 | 20 (51.1)‖ | 8 (22.9) | 7 (20.0) | |
| Female | 51 | 29 (56.9) | 11 (21.6) | 11 (21.6) | |
| Localization | 0.96 | ||||
| Intrahepatic | 55 | 31 (56.4) | 12 (21.8) | 12 (21.8) | |
| Extrahepatic | 31 | 18 (58.1) | 7 (22.6) | 6 (19.4) | |
| Size | 0.38‡ | ||||
| T1 | 11 | 5 (45.5) | 5 (45.5) | 1 (9.1) | |
| T2 | 29 | 18 (62.1) | 5 (17.2) | 6 (20.7) | |
| T2 | 35 | 21 (60.0) | 5 (14.3) | 9 (25.7) | |
| T4 | 11 | 5 (45.5) | 4 (36.4) | 2 (18.2) | |
| Node involvement | 0.08‡ | ||||
| N0 | 35 | 24 (68.6) | 6 (17.1) | 5 (14.3) | |
| N1 | 26 | 11 (42.3) | 5 (19.2) | 10 (38.5) | |
| NX | 25 | 14 (56.0) | 8 (32.0) | 3 (12.0) | |
| Histological Grade | 0.93‡ | ||||
| G1 | 8 | 5 (62.5) | 2 (25.0) | 1 (12.5) | |
| G2 | 50 | 28 (56.0) | 12 (24.0) | 10 (20.0) | |
| G3 | 28 | 16 (57.1) | 5 (17.9) | 7 (25.0) | |
| Resection Margin | 0.31 | ||||
| R0 | 64 | 39 (60.9) | 14 (21.9) | 11 (17.2) | |
| R1 | 22 | 10 (45.5) | 5 (22.7) | 7 (31.8) | |
Table values are mean ± SD for continuous variables and n (column %) for categorical variables (percentages may not add to 100% due to rounding).
P-value is for t test (continuous variables)§ or χ2 or Fisher’s Exact test (categorical variables).‡
Immunohistochemical Analysis
Details on the immunohistochemical staining techniques are given in the Supporting Materials.
Survival and Multivariate Analysis
The impact of S100A4 nuclear expression on cumulative patient’s survival after resection was estimated using the Kaplan-Meier method; hazard ratios (HRs) were estimated using the multivariate Cox proportional hazard model (see Supporting Material for details). To examine the association between S100A4 nuclear expression and the development of metastases, we used an alternative approach designed to overcome the limitations related to the interval censored data of metastatization, based on a survival curve using a nonparametric maximum likelihood estimator (NPMLE) and a generalized log-rank test.10 The Weibull model was used to study the impact of S100A4 on the development of metastasis among several other variables, previously considered in the Cox regression analysis (see Supporting Material).
CCA Cell Lines and Detection of S100A4 by Immunofluorescence
On the basis of their expression of S100A4, two different human CCA cell lines were selected, EGI-1 and TFK-111,12 (see Supporting Material).
Western Blot Analysis
Cytoplasmic and nuclear expression of S100A4 was also evaluated by western blot (WB) on cytoplasmic and nuclear cell fractions. Methodological details are given in the Supporting Materials.
Xenotransplantation Studies in SCID Mice
Prior to xenotransplantation, to enable the performance of in vivo imaging EGI-1 and TFK-1 cells were transduced with a lentiviral vector encoding the Luciferase reporter gene13 produced on 293T packaging cells as described.14 After transduction, luciferase-expressing EGI-1 and TFK-1 cells were transplanted through intrasplenic injection into 6 to 8-week-old female SCID mice (Charles River, Wilmington, MA) (n = 6 for each group). (See Supporting Materials for further details.)
Lentiviral Silencing of Nuclear S100A4 in EGI-1 Cells
To silence S100A4 expression, EGI-1 cells were transduced with lentiviral vectors encoding short hairpin RNA (shRNA) targeting human S100A4 (clones TRCN53609-12) or a scrambled shRNA as control (purchased from Sigma-Aldrich, Milan, Italy), together with the gene encoding for the resistance to puromycine, as described.15 (See Supporting Materials for further details.)
CCA Cell Migration, Invasion, Proliferation, Apoptosis, and Secretion of MMP-2 and MMP-9
The functional effects of S100A4 silencing were evaluated by studying the motility, invasion, proliferation, apoptosis, and secretory capabilities of MMP-2 and MMP-9 of EGI-1 cells before and after lentiviral silencing of nuclear S100A4. Effective silencing was evaluated by WB analysis and, following puromycine selection, cells were compared to scrambled shRNA and parental EGI-1 as well as TFK-1 cells. Methods for cell migration (wound healing),16 cell invasion (Boyden chamber),17 cell proliferation assay,18 cleaved caspase-3 expression, and MMP-2 and MMP-9 secretion assay are detailed in the Supporting Materials.
Results
S100A4 Nuclear Expression in Histological Sections of CCAs
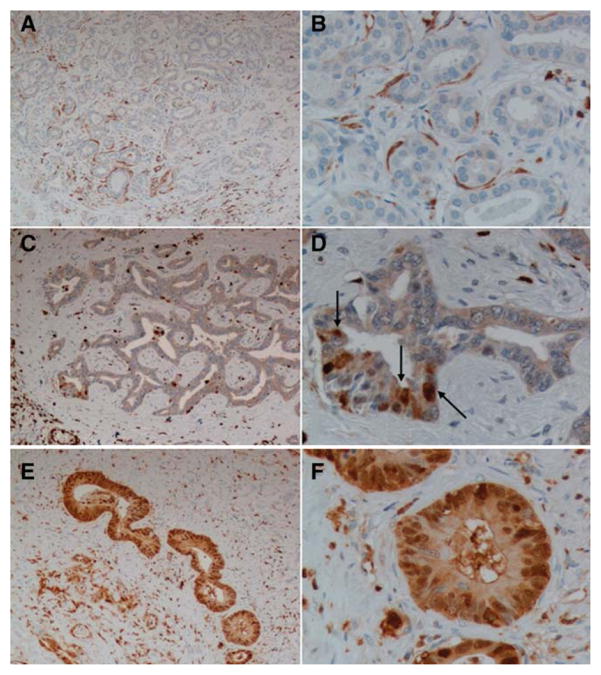
Forty-nine out of 86 patients (57%) considered for the survival analysis were negative for nuclear expression of S100A4 in the neoplastic cells; scattered cytoplasmic expression was found in 70% of these cases (34/49). In the remaining 37 patients the median value of nuclear expression of S100A4 was 30% of the discernible nuclei in the neoplastic ducts. Among the positive samples, 51% (19/37) were in the weakly positive (S100A4 <30% of the nuclei) and 49% (18/37) were in the strongly positive group (S100A4 ≥30% of the nuclei) (Fig. 1A–F). Table 1 shows the distribution of demographic and clinical characteristics of the patients included in the study, stratified by S100A4 grouping. Baseline characteristics were comparable between the three S100A4 groups. None of the 23 tissue sections available from the peritumoral areas showed nuclear and/or cytoplasmic expression of S100A4 on the bile ducts. By Spearman’s rho test we found no correlation between the expression of keratin 19 (K19) and that of nuclear S100A4 in the neoplastic bile ducts.
Fig. 1.
Immunohistochemical expression of S100A4 in human liver samples. Different patterns of S100A4 expression in surgical samples obtained from liver resection for CCA. (A,B) Negative group. S100A4 is negative or faintly expressed in the cytoplasm of tumoral cholangiocytes, whereas it is expressed by some fibroblasts adjacent to tumoral ducts, some inflammatory cells. (C,D) Weakly positive group. S100A4 is expressed in the nucleus in less than 30% of tumoral duct cells (arrows). (E,F) Strongly positive group. S100A4 is expressed in the nucleus in more than 30% of tumoral duct cells. Original magnification: A,C,E: 100×; B,D,F: 400×.
Nuclear Expression of S100A4 Is a Risk Factor Indicating Worse Prognosis Following Surgical Resection in CCA Patients
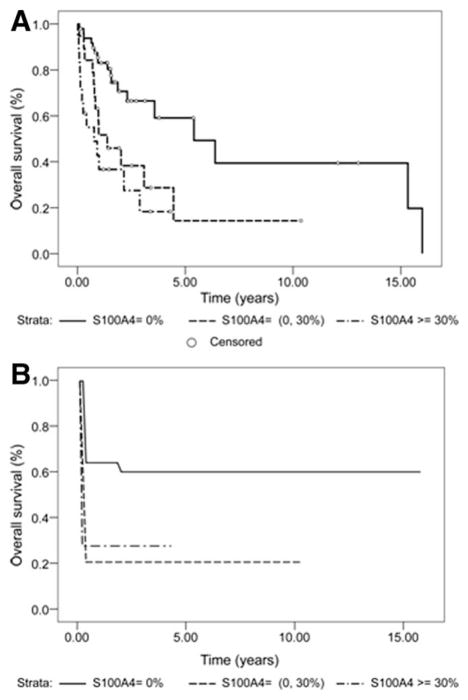
Kaplan-Meier and Cox proportional hazard models were performed in the 86 patients surviving more than 1 month after surgery. The median survival time based on 86 subjects was 2.89 years (95% confidence interval [CI] = 1.57,5.40). As shown in Table 2, Supporting Table S1, and Fig. 2A, for subjects with no nuclear expression of S100A4 (n = 49), the median survival time was 5.40 years (95% CI = 2.31,16.00). In sharp contrast, for subjects with weakly positive nuclear expression of S1004A (<30% of nuclei, n = 19) the median survival time was 1.38 years (95% CI = 0.76,4.45), whereas for subjects with strongly positive nuclear expression of S100A4 (≥30% of nuclei, n = 18) the median survival time was further reduced to 0.77 years (95% CI = 0.14,2.89). These data indicate that nuclear S100A4 is associated with a significant reduction in survival, even when weakly expressed.
Table 2.
Summary of Kaplan-Meier Analysis According to S100A4 Grouping
| S100A4 Nuclear Expression | Median Survival (95% CI) | Strata Comparison* | Strata Comparison* | Log-Rank Trend Test |
|---|---|---|---|---|
| Negative (0%) | 5.40 (2.31, 16.00) | Control | 0.006 | 0.003 |
| Weakly positive (0–30%) | 1.38 (0.76, 4.45) | 0.006 | Control | |
| Strongly positive (≥ 30%) | 0.77 (0.14, 2.89) | <0.001 | 0.77 |
Bonferroni method was used for adjustment of multiple comparison between strata.
Fig. 2.
Nuclear expression of S100A4 strongly correlates with poor prognosis and development of metastases following surgical resection in CCA patients. Kaplan-Meier (A) and NPMLE (B) survival curves estimates for patients after surgical resection with negative nuclear S100A4 expression (0%), weakly positive nuclear S100A4 expression (<30%), or strongly positive S100A4 nuclear expression (≥30%). The maximum timepoint was set up to 16 years.
Consistent with the interpretation that S100A4 is associated with a significant reduction in survival, the univariate analysis showed that S100A4 nuclear expression levels were strongly predictive of survival (P = 0.003, log-rank test for trend) and that higher levels of nuclear expression of S100A4 were associated with shorter survival times for patients.
Furthermore, the Cox proportional hazards regression analysis showed that S100A4 is an independent predictor of survival whether it was treated as a continuous (HR = 1.02, P = 0.007) (Table 3) or categorical variable (0–30% versus 0: HR = 2.58, P = 0.03 and ≥30% versus 0: HR = 3.02, P = 0.01), and even after controlling for other covariates. The HR of 1.02 when S100A4 is treated as a continuous variable indicates that a 10% increase in S100A4 expression levels is associated with a 22% increase in a patient’s hazard rate. Notably, a 30% increase in S100A4 expression levels is associated with a 82% increase in a patient’s hazard rate. When S100A4 is treated as a categorical variable in multivariate Cox proportional hazard model, the HR of 2.59 (0%–30% group) and 3.02 (≥30% group) indicate that the hazard rate is close to three times greater for people in these groups compared to those with 0% expression of nuclear S100A4. Besides S100A4, the only other covariates that were significant independent predictors of survival were the involvement of resection margins and of regional lymph nodes, with an HR similar to S100A4 ≥30% (2.62 for resection margin involvement, 3.56 for lymph node involvement).
Table 3.
Results of Cox Proportional Hazards Model (Multivariate Analysis) with Outcome as Death, and of Weibull Model with Outcome as Development of Metastases, Including Gender, Age, Intensity of S100A4 Immunostaining (Continuous Variable), Histological Grading, Hepatic Localization, Marginal and Lymphnodal Involvement, and Tumor Size
| Parameter | Cox Model (n=86) | Weibull Model (n=67) | ||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
|
|
|
|||||||||||
| Estimate | SE | P | HR | 95% HR Confidence Limits | Estimate | SE | P | HR | 95% HR Confidence Limits | |||
| Age | 0.014 | 0.015 | 0.35 | 1.01 | 0.98 | 0.01 | −0.012 | 0.035 | 0.724 | 1.01 | 0.97 | 1.04 |
| Sex (female vs. male) | −0.419 | 0.373 | 0.26 | 0.66 | 0.32 | −0.42 | 0.555 | 0.837 | 0.51 | 0.75 | 0.31 | 1.77 |
| S100A4 (% of positive nuclear staining) | 0.020 | 0.007 | 0.007 | 1.02 | 1.00 | 0.02 | −0.042 | 0.019 | 0.027 | 1.02 | 1.00 | 1.04 |
| Differentiation (G2 vs. G1) | 1.316 | 0.842 | 0.12 | 3.73 | 0.72 | 1.32 | −5.405 | 2.313 | 0.02 | 17.58 | 1.60 | 192.73 |
| Differentiation (G3 vs. G1) | 1.553 | 0.841 | 0.06 | 4.73 | 0.91 | 1.55 | −5.243 | 2.358 | 0.03 | 16.14 | 1.37 | 190.62 |
| Extra– vs. intrahepatic | −0.372 | 0.378 | 0.33 | 0.69 | 0.33 | −0.37 | 1.305 | 0.924 | 0.16 | 0.50 | 0.19 | 1.31 |
| Margins (involvement vs. no involvement) | 0.947 | 0.414 | 0.02 | 2.58 | 1.14 | 0.95 | −1.747 | 1.008 | 0.08 | 2.53 | 0.84 | 7.56 |
| Node involvement (N1 vs. N0) | 1.176 | 0.515 | 0.02 | 3.24 | 1.18 | 1.18 | −1.843 | 0.962 | 0.05 | 2.66 | 0.96 | 7.32 |
| Node involvement (Nx vs. N0) | 1.001 | 0.503 | 0.05 | 2.72 | 1.01 | 1.00 | −1.311 | 0.963 | 0.17 | 2.00 | 0.72 | 5.58 |
| Size (T2 vs. T1) | 0.757 | 0.665 | 0.25 | 2.13 | 0.58 | 0.76 | −1.861 | 1.550 | 0.23 | 2.68 | 0.56 | 12.86 |
| Size (T3 vs. T1) | 0.546 | 0.719 | 0.45 | 1.73 | 0.42 | 0.55 | −1.263 | 1.584 | 0.42 | 1.95 | 0.38 | 10.04 |
| Size (T4 vs. T1) | 0.939 | 0.836 | 0.26 | 2.56 | 0.50 | 0.94 | −3.42 | 1.786 | 0.06 | 6.14 | 1.02 | 36.88 |
| Scale | 1.885 | 0.377 | ||||||||||
| Weibull shape | 0.530 | 0.106 | ||||||||||
| Linear Hypotheses Testing Results | ||||||||||||
| S100A4 (0–30 % ) vs. S100A4 ( ≥30%) | Wald chi-square | DF | Pr > chi sq | |||||||||
| 0.094 | 1 | 0.760 | ||||||||||
Nuclear Expression of S100A4 Is a Risk Factor Associated with Increased Development of Metastasis Following Surgical Resection in CCA Patients
To study whether nuclear S100A4 expression was associated with increased development of metastasis we analyzed a subset of 67 subjects (78%) for which metastatic data were available. This subgroup, as shown in Supporting Table S2, was well representative of the complete series as expression of nuclear S100A4, clinical features, and outcome. The survival curve (Fig. 2B) showed a significant difference in time to metastasis between patients with negative S100A4 and those with weak/strong positive S100A4 (P = 0.0052). Using the Weibull model, we also analyzed the impact of S100A4 nuclear positivity on death and on the development of metastasis in relation to the same variables, studied with the Cox model. The analysis showed that the effect of S100A4 on death and metastasis was very similar and confirm that nuclear S100A4 has a strong predictive power on the development of metastasis when considered both as a continuous (HR = 1.022, P = 0.0274) and as a categorical variable (HR = 5.894, P = 0.0012) (Table 3). As a further proof of the reliability of this approach, the results with Weibull model for death were very similar to those obtained with the Cox model (Tables 3, S3). Noteworthy, by comparing the estimated hazard function for death and metastasis with the Weibull model we found that, whereas for death the hazard over time increased, the rate decreased for metastasis, the hazard was very high at the beginning, and it dropped very rapidly over time (see Fig. S1).
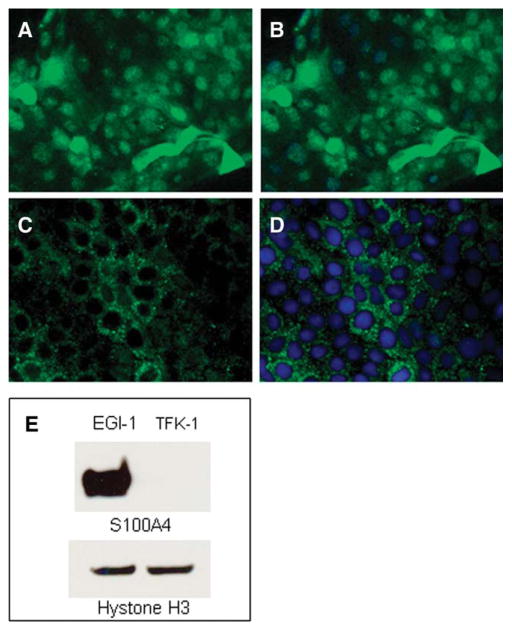
Nuclear S100A4-Expressing Human CCA Cell Lines Show an Increased Metastasization in SCID Mice
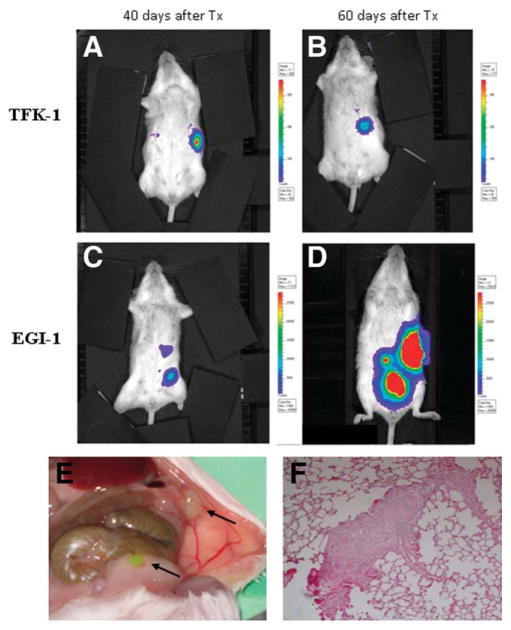
Because of its strong association with survival, we hypothesized that nuclear expression of S100A4 was functionally involved in determining the invasiveness of the tumor. TFK-1 and EGI-1 are human CCA cell lines that differ in terms of S100A4 expression. In contrast to TFK-1 cells, which showed a weak immunoreactivity for S100A4 strictly confined to the cytoplasm, in EGI-1 cells staining for S100A4 showed a strong nuclear positivity, at immunohistochemistry as well as at WB (Fig. 3A–E). In fact, WB analysis of nuclear extracts confirmed that an intense, specific band at 12 kDa was present in the nuclear protein fraction in EGI-1, but not in TFK-1 cells (Fig. 3E). We used EGI-1 and TFK-1 cells to compare, by in vivo bioluminescence as well as at autopsy, their metastatic behavior, following xenotransplantation into SCID mice by intrasplenic injection. By in vivo bioluminescence detection, EGI-1 cells showed a metastatic spread to different abdominal locations within 60 days from injection in five out of six transplanted mice. TFK-1 cells, on the other hand, presented no metastasis up to 120 days from transplant in 6/6 animals, even though a persistent signal could still be detected in the site of injection (spleen) (Fig. 4A–D). Consistent with the findings derived from imaging studies, and in sharp contrast with mice transplanted with TFK-1 cells, which did not present new masses at distance from the spleen (not shown), at necropsy mice transplanted with EGI-1 cells showed multiple masses in different abdominal organs (Fig. 4E). In all five mice sacrificed after transplantation with EGI-1, histological examination by hematoxylin and eosin (H&E) of serial sections derived from different organs revealed the presence of micrometastases, particularly in the liver and in the lung (Fig. 4F). Immunohistochemistry for MMPs in tissue sections obtained from liver samples revealed that metastasizing EGI-1 cells were strongly decorated by MMP-9 but not MMP-2 antibodies (Fig. S4A,B).
Fig. 3.
Different pattern of expression of S100A4 in two human CCA cell lines, EGI-1 and TFK-1 cells. Immunofluorescence for S100A4 showed a strong immunoreactivity in both nucleus and cytoplasm in EGI-1 cells (A, Alexa Fluor-488; B, merged), in contrast to TFK-1 cells that were decorated only in the cytoplasm (C, Alexa Fluor-488; D, merged). Original magnification: 200×. This difference was confirmed by WB analysis performed on nuclear cell fraction using Hystone H3 as reference proteins for nuclear extracts (E).
Fig. 4.
Different metastatic capabilities of EGI-1 and TFK-1 cells in SCID mice. Following xenotransplantation into SCID mice by intrasplenic injection, in sharp contrast with TFK-1 cells, which were free of metastasis beyond 60 days from transplant (A,B), EGI-1 cells showed a diffuse metastatic dissemination to different abdominal sites within 60 days (C,D). Autoptic examination performed in the same animal as picture (C,D) confirmed the development of multiple abdominal masses in peritoneum and bowel, in accordance with bioluminescence findings (E, arrows). Histological examination of serial sections by H&E revealed the presence of micrometastases in distant organs, including the lung (F, original magnification: 200×).
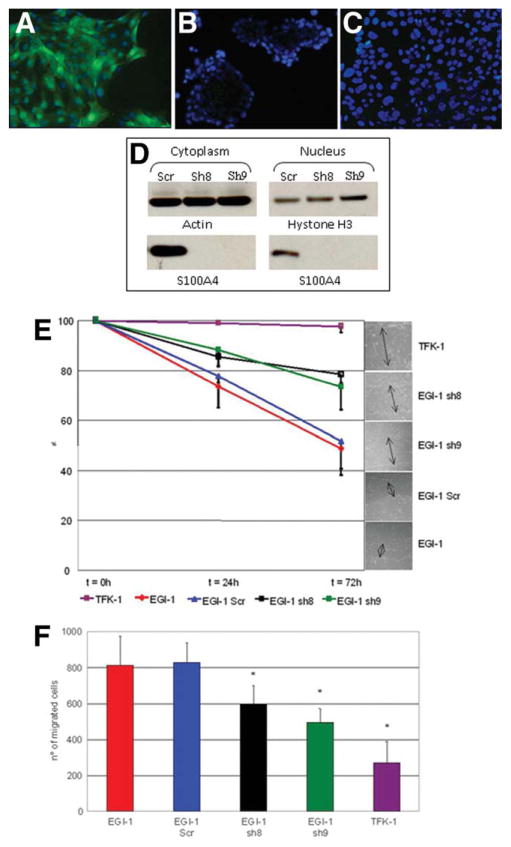
Down-Regulation of S100A4 Expression in EGI-1 Cells Results in a Significant Reduction in Cell Motility, Invasion, and MMP-9 Secretion, Without Affecting Cell Proliferation and Apoptosis
Lentiviral silencing of S100A4 expression in EGI-1 cells, with S100A4-specific shRNA, generated two cultures (sh8 and sh9) that presented a strong inhibition of cytoplasmic and nuclear expression of the S100A4 protein as compared to scramble shRNA (Fig. 5A–D). Phenotypically, silencing of S100A4 did not result in changes in K19 expression in EGI-1 cells (Fig. S2). Using these clones, we investigated the effects of S100A4 silencing on cell motility, invasion, proliferation, apoptosis, and secretion of MMP-2 and MMP-9. Data were compared to scramble EGI-1 and to TFK-1 cells. Data shown below indicate that down-regulation of nuclear S100A4 inhibits the capability of EGI-1 cells to migrate, secrete MMP-9, and invade the extracellular matrix, without affecting the proliferative and apoptotic activities.
Fig. 5.
Functional effects of S100A4 silencing in EGI-1 cells as compared to TFK-1 cells on cell motility and invasion. Effective silencing of S100A4 in EGI-1 cells by lentiviral vectors was tested by immunofluorescence and WB. ShRNA (scrambled) EGI-1 cells regularly maintained the immunoreactivity for S100A4 (green) in both nucleus and cytoplasm (A), whereas sh8- and sh9-EGI-1 cells showed no immunostaining for S100A4 (nuclei counterstained by 4′,6-diamidino-2-phenylindole [DAPI] in blue, B,C). Original magnification: 200×. Absence of S100A4 protein expression in sh8 and sh9 was confirmed by WB on nuclear cell fractions; actin was used as reference for cytoplasmic proteins and Hystone H3 for nuclear proteins (D). Cell motility, assessed by wound-healing assay, showed that EGI-1 and scrambled shRNA EGI-1 cells significantly reduce the distance between the edges of the scratch at 72 hours; this property was potently impaired in both silenced clones (P < 0.001 n = 6) (E). Cell invasion, assessed by Boyden chambers on Matrigel, showed that, with respect to EGI-1 or scrambled shRNA EGI-1 cells, the number of cells crossing through the filter after 48 hours was significantly reduced in sh8 and in sh9 EGI-1 cells (P = 0.01) and was significantly different from TFK-1 cells (P < 0.001) (F).
Cell Motility
In cell monolayers, cells were scraped and the distance between the two edges of the epithelial wound was measured over time. Contrary to TFK-1 cells, EGI-1 cells rapidly reduced the distance between the wound edges (Fig. 5E). In wildtype EGI-1, 73.60% ± 8.49% of the distance remained 24 hours after the scraping, whereas at 72 hours 48.88% ± 8,08% of the distance remained. Results with shRNA EGI-1 were similar (77.89% ± 2.84% at 24 hours, and 51.89% ± 13.61% at 72 hours). On the contrary, the ability of clones sh8 and sh9 to migrate was significantly impaired (78.50% ± 3.38% and 73.41% ± 9.18% remained to be covered at 72 hours for sh8 and sh9, respectively).
Cell Invasion
CCA cells were seeded on the top of transwells coated with Matrigel and the number of cells that migrated on the other side of the counted after 48 hours. Taking this approach, we found that invasiveness of parental EGI-1 and scrambled shRNA EGI-1 cells (812.83 ± 163.72 and 828.33 ± 110.41 cells after 48 hours, respectively, P not significant) was significantly higher compared with sh8 and sh9 bulk cultures (597.33 ± 102.79 cells in sh8-EGI-1, P = 0,02 with respect to EGI-1 and shRNA; 496.50 ± 76.01 cells in sh9-EGI-1, P = 0.001) (Fig. 5F).
Cell Proliferation and Cell Apoptosis
We found no difference in proliferation rates and caspase-3 expression among the CCA cell lines and the S100A4 silenced clones. The results are shown in the Supporting Materials (text and Fig. S3).
Cell Secretion of MMP-2 and MMP-9
By enzyme-linked immunosorbent assay (ELISA), we found that EGI-1 cells were able to secrete MMP-9 but not MMP-2, in accordance with immunohistochemistry performed in liver metastases of SCID mice. This secretory property was significantly reduced by lentiviral silencing of S100A4 in sh8 and sh9 clones (4141 ± 520 pg/mL in EGI-1, 1649 ± 128 in sh9, 146 ± 59 in sh8, P < 0.001 versus parental EGI-1 cells). TFK-1 ability to secrete MMP-9 is conversely negligible (74 ± 110) (Fig. S4C).
Discussion
Cholangiocarcinoma is characterized by a poor prognosis and strong invasiveness. The availability of biomarkers of early metastatic behavior would help to allocate CCA patients to their best treatment, which may even include transplant. Unfortunately, little is known about the mechanisms that favor invasiveness in CCA.
Among the molecular signatures of cancer invasiveness, S100A4, a member of the S100 family of small calcium-binding proteins, normally expressed by mesenchymal cells, is of particular interest because it was shown to correlate with metastatic potential in breast and colon cancers.7,19 However, it is currently unknown whether S100A4 merely represents a surrogate marker of cancer invasiveness or actually plays a key role in the development of a metastatic phenotype, thereby potentially representing a functional target amenable to specific therapeutic interference.
To understand the role of S100A4 as a predictor of CCA invasiveness, we studied the expression of S100A4 in a large series of resected human CCA samples and correlated it to the clinical outcomes, considered either as death or as development of metastases. We found that nuclear expression of S100A4 by neoplastic bile ducts significantly correlated with increased metastasization and reduced survival after surgery. The association of S100A4 expression with worse patient outcome has been reported earlier in colonic and breast cancers.7,19 These studies did not distinguish between its nuclear or cytoplasmic expression, although prior data from Flatmark et al.20 indicated that nuclear localization of S100A4 was more closely correlated with advanced tumor stage at diagnosis in colorectal cancer. This is important, because expression of S100A4 in the cytoplasm of biliary epithelial cells can be seen also in nonneoplastic diseases, as a part of the epithelial reaction to damage.21 Therefore, in our study on CCA we focused on the nuclear expression of S100A4, a feature not seen in nonneoplastic biliary diseases.
It is important to underline that, because only a minority of patients with CCA (about 30%) can be treated by surgical resection, the population of our study represents a subgroup of CCA with more clinically localized disease at the time of diagnosis. In our series of CCAs, nuclear expression of S100A4 identified a subgroup of patients (43%) with a markedly reduced survival after surgical resection, but without significant differences in their clinical features at presentation (Table 1). The median survival following resection was between 0.77 years and 1.38 years in subjects with nuclear expression of S100A4, whereas patients with no nuclear expression of S100A4 showed a median survival of 5.4 years. Taking this approach, we demonstrated that nuclear expression of S100A4 by cancer cells is a strong and independent predictor of survival even when expressed by a minority of cancer cells, with a dose-response effect, as shown by log rank test and Cox proportional hazards regression analysis. In fact, an increase in S100A4 expression levels from 10% to 30% is associated with an increase in a subject’s hazard rate from 22% to 82%. Notably, by considering the percentage of S100A4-positive nuclei as a continuous variable, at Cox analysis the prognostic power yielded by S100A4 was much more significant than that of the other covariates, including resection margin and lymph node involvement (P = 0.007 for S100A4 versus P = 0.022 for margin involvement and P = 0.023 for lymph node involvement; see Table 3). Furthermore, nuclear S100A4 was strongly associated with an enhanced metastatic behavior. Analysis of the relationship between the estimated hazard function for death and metastasis with the Weibull model over time showed that the peak of hazard of metastasis preceded that of the hazard of death (Fig. S1 in the Supporting Material), a finding consistent with a direct effect of metastasis on death, as expected for cancers with strong aggressiveness.
Previous studies reporting the value of S100A4 as a risk factor for tumor progression did not address its mechanism. To obtain experimental proof that nuclear expression of S100A4 was associated with an invasive phenotype in CCA, we studied the metastatic behavior of two human CCA cell lines characterized by the presence or absence of nuclear expression of S100A4. EGI-1 and TFK-1 cells were xenotransplanted by intrasplenic injection into SCID mice and the metastatic behavior was followed by bioluminescence imaging and then autopsy and histological examination. Although no significant metastasis was found with TFK-1 cells (nuclear expression negative) in the examined time-frame, diffuse spreading was found in all mice transplanted with EGI-1 cells (nuclear expression positive).
The ability to translocate to the nucleus in human cancer has been reported for proteins belonging to the S100 family (such as S100A11 in glioblastoma cells).22 However, little is known about the function of S100A4 proteins in the nucleus. S100A4, a small 12 kD molecule, does not have intrinsic enzymatic activity, and its effects require interactions with different binding partners. The number of intracellular binding partners targeted by S100A4 includes proteins involved in cytoskeletal rearrangement (F-actin, nonmuscle myosin IIA and IIB, tropomyosin), proteins promoting extracellular matrix remodeling (Annexin II), proteins regulating angiogenesis (thrombospondin), and proteins regulating the balance between cell proliferation and apoptosis (p53, liprinβ1, methionine aminopeptidase 2). Recent evidence indicates that, similar to other S100 proteins, i.e., S100A1 and S100B,23,24 S100A4 may also have transcriptional activity, either by direct DNA binding or by interacting with other DNA-binding proteins. For example, S100A4 regulates the transcriptional activation of MMP-9 in human prostate cancer.8 S100A4 also negatively regulates expression of E-cadherin, an important prerequisite for cancer cell motility.9
To better understand the functional effects of S100A4 in CCA, we then studied if silencing S100A4 expression in the EGI-1 cell line interfered with cell motility, invasion, cell proliferation, and apoptosis. Our results demonstrate a significant reduction in both cell migration and invasiveness as measured by transwell migration through Matrigel in Boyden chambers in the absence of chemotactic stimuli. Contrary to motility and invasion, we observed no effects on cell proliferation and apoptosis in S100A4-silenced EGI-1 cells. Thus, S100A4 appears to be a key determinant of CCA invasiveness, given its involvement in the regulation of cellular motility and invasion, without affecting the local growth of the tumor that depends on the balance between cell proliferation and apoptosis. Noteworthy, expression of MMP-9 was significantly reduced in EGI-1 clones with silenced S100A4, indicating that S100A4 modulates MMP-9, an important mediator of cancer invasiveness.8
In summary, our study demonstrates that nuclear expression of S100A4 identifies a subtype of CCA with poor response to surgical resection. Furthermore, the functional data generated in this work strongly suggest that S100A4 is a mechanistic determinant of CCA invasiveness. In fact, nuclear expression of S100A4 was associated with enhanced metastatic potential of CCA cell lines xenotransplanted into SCID mice, and silencing of S100A4 with reduced motility, invasiveness, and expression of MMP9 in vitro. If validated prospectively, nuclear expression of S100A4 may eventually become a tool in clinical decision making to allocate patients with CCA that are candidates for potentially curative therapies, including liver transplantation. On the other hand, down-regulation of S100A4 may become an attractive strategy to reduce CCA progression. This could eventually be achieved also using small molecules.25
Supplementary Material
Acknowledgments
Supported by Telethon (grant GGP09189) and Federazione Amici Di Epatologia (FADE) to L.F. Supported by Progetto di Ricerca Ateneo 2008 (grant CPDA083217 to L.F., M.C., and L.O.). Associazione Scientifica Gastroenterologica di Treviso (ASGET, “associazione di promozione sociale senza scopo di lucro” to L.F. and L.O.). Yale University Liver Center (NIH DK34989 to M.S.). Fondazione S. Martino, Bergamo.
The authors thank Dr. Luigi Dall’Olmo (Department of Surgical and Gastroenterological Sciences, University of Padova) for surgical assistance in experiments with SCID mice, and Dr. Enrico Gringeri (Department of Surgical and Gastroentrological Sciences, University of Padova) for collecting data on metastases.
Abbreviations
- CCA
cholangiocarcinoma
- DAPI
4′,6-diamidino-2-phenylindole
- EMT
epithelial-mesenchymal transition
- H&E
hematoxylin and eosin
- HR
hazard ratios
- K19
keratin 19
- SCID
severe combined immunodeficiency
- Sh
short hairpin
- WB
western blot
Footnotes
Potential conflict of interest: Nothing to report.
Additional Supporting Information may be found in the online version of this article.
References
- 1.Blechacz B, Gores GJ. Cholangiocarcinoma: advances in pathogenesis, diagnosis, and treatment. Hepatology. 2008;48:308–321. doi: 10.1002/hep.22310. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 2.Valle J, Wasan H, Palmer DH, Cunningham D, Anthoney A, Maraveyas A, et al. Cisplatin plus gemcitabine versus gemcitabine for biliary tract cancer. N Engl J Med. 2010;362:1273–1281. doi: 10.1056/NEJMoa0908721. [DOI] [PubMed] [Google Scholar]
- 3.Thiery JP. Epithelial-mesenchymal transitions in tumour progression. Nat Rev Cancer. 2002;2:442–454. doi: 10.1038/nrc822. [DOI] [PubMed] [Google Scholar]
- 4.Zeisberg M, Neilson EG. Biomarkers for epithelial-mesenchymal transitions. J Clin Invest. 2009;119:1429–1437. doi: 10.1172/JCI36183. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Österreicher CH, Penz-Österreicher M, Grivennikov SI, Guma M, Koltsova EK, Datz C, et al. Fibroblast-specific protein 1 identifies an inflammatory subpopulation of macrophages in the liver. Proc Natl Acad Sci U S A. 2011;108:308–313. doi: 10.1073/pnas.1017547108. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 6.Boye K, Maelandsmo GM. S100A4 and metastasis: a small actor playing many roles. Am J Pathol. 2010;176:528–535. doi: 10.2353/ajpath.2010.090526. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Gongoll S, Peters G, Mengel M, Piso P, Klempnauer J, Kreipe H, et al. Prognostic significance of calcium-binding protein S100A4 in colorectal cancer. Gastroenterology. 2002;123:1478–1484. doi: 10.1053/gast.2002.36606. [DOI] [PubMed] [Google Scholar]
- 8.Saleem M, Kweon MH, Johnson JJ, Adhami VM, Elcheva I, Khan N, et al. S100A4 accelerates tumorigenesis and invasion of human prostate cancer through the transcriptional regulation of matrix metalloproteinase 9. Proc Natl Acad Sci U S A. 2006;103:14825–14830. doi: 10.1073/pnas.0606747103. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 9.Moriyama-Kita M, Endo Y, Yonemura Y, Heizmann CW, Miyamori H, Sato H, et al. S100A4 regulates E-cadherin expression in oral squamous cell carcinoma. Cancer Lett. 2005;230:211–218. doi: 10.1016/j.canlet.2004.12.046. [DOI] [PubMed] [Google Scholar]
- 10.Sun J, Zhao Q, Zhao X. Generalized log-rank tests for intervalcensored failure time data. Scand J Stat. 2005;32:49–57. [Google Scholar]
- 11.Kamenz T, Caca K, Blüthner T, Tannapfel A, Mössner J, Wiedmann M. Expression of c-kit receptor in human cholangiocarcinoma and in vivo treatment with imatinib mesilate in chimeric mice. World J Gastroenterol. 2006;12:1583–1590. doi: 10.3748/wjg.v12.i10.1583. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 12.Sato Y, Harada K, Itatsu K, Ikeda H, Kakuda Y, Shimomura S, et al. Epithelial-mesenchymal transition induced by transforming growth factor-{beta}1/snail activation aggravates invasive growth of cholangiocarcinoma. Am J Pathol. 2010;177:141–152. doi: 10.2353/ajpath.2010.090747. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Keyaerts M, Verschueren J, Bos TJ, Tchouate-Gainkam LO, Peleman C, Breckpot K, et al. Dynamic bioluminescence imaging for quantitative tumour burden assessment using IV or IP administration of D-luciferin: effect on intensity, time kinetics and repeatability of photon emission. Eur J Nucl Med Mol Imaging. 2008;35:999–1007. doi: 10.1007/s00259-007-0664-2. [DOI] [PubMed] [Google Scholar]
- 14.Indraccolo S, Tisato V, Tosello V, Habeler W, Esposito G, Moserle L, et al. Interferon-alpha gene therapy by lentiviral vectors contrasts ovarian cancer growth through angiogenesis inhibition. Hum Gene Ther. 2005;16:957–970. doi: 10.1089/hum.2005.16.957. [DOI] [PubMed] [Google Scholar]
- 15.Indraccolo S, Habeler W, Tisato V, Stievano L, Piovan E, Tosello V, et al. Gene transfer in ovarian cancer cells: a comparison between retroviral and lentiviral vectors. Cancer Res. 2002;62:6099–6107. [PubMed] [Google Scholar]
- 16.Liang CC, Park AY, Guan JL. In vitro scratch assay: a convenient and inexpensive method for analysis of cell migration in vitro. Nat Protoc. 2007;2:329–333. doi: 10.1038/nprot.2007.30. [DOI] [PubMed] [Google Scholar]
- 17.Cannito S, Novo E, Compagnone A, Valfrè di Bonzo L, Busletta C, Zamara E, et al. Redox mechanisms switch on hypoxia-dependent epithelial-mesenchymal transition in cancer cells. Carcinogenesis. 2008;29:2267–2278. doi: 10.1093/carcin/bgn216. [DOI] [PubMed] [Google Scholar]
- 18.Fabris L, Cadamuro M, Fiorotto R, Roskams T, Spirlì C, Melero S, et al. Effects of angiogenic factor overexpression by human and rodent cholangiocytes in polycystic liver diseases. Hepatology. 2006;43:1001–1012. doi: 10.1002/hep.21143. [DOI] [PubMed] [Google Scholar]
- 19.Rudland PS, Platt-Higgins A, Renshaw C, West CR, Winstanley JH, Robertson L, et al. Prognostic significance of the metastasis-inducing protein S100A4 (p9Ka) in human breast cancer. Cancer Res. 2000;60:1595–1603. [PubMed] [Google Scholar]
- 20.Flatmark K, Pedersen KB, Nesland JM, Rasmussen H, Aamodt G, Mikalsen SO, et al. Nuclear localization of the metastasis-related protein S100A4 correlates with tumour stage in colorectal cancer. J Pathol. 2003;200:589–595. doi: 10.1002/path.1381. [DOI] [PubMed] [Google Scholar]
- 21.Svegliati-Baroni G, Faraci G, Fabris L, Saccomanno S, Cadamuro M, Pierantonelli I, et al. Insulin resistance and necroinflammation drives ductular reaction and epithelial-mesenchymal transition in chronic hepatitis C. Gut. 2010;60:108–115. doi: 10.1136/gut.2010.219741. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 22.Davey GE, Murmann P, Hoechli M, Tanaka T, Heizmann CW. Calcium-dependent translocation of S100A11 requires tubulin filaments. Biochim Biophys Acta. 2000;1498:220–232. doi: 10.1016/s0167-4889(00)00098-7. [DOI] [PubMed] [Google Scholar]
- 23.Baudier J, Bergeret E, Bertacchi N, Weintraub H, Gagnon J, Garin J. Interactions ofmyogenic bHLHtranscription factors with calcium-binding calmodulin and S100a (alpha alpha) proteins. Biochemistry. 1995;34:7834–7846. doi: 10.1021/bi00024a007. [DOI] [PubMed] [Google Scholar]
- 24.Onions J, Hermann S, Grundström T. Basic helix-loop-helix protein sequences determining differential inhibition by calmodulin and S-100 proteins. J Biol Chem. 1997;272:23930–23937. doi: 10.1074/jbc.272.38.23930. [DOI] [PubMed] [Google Scholar]
- 25.Parker C, Piura B, Sherbet GV. Taxol, a promoter of microtubule polymerisation, down regulates metastasis associated mts1 gene in the B16 murine melanoma. Proc Am Assoc Cancer Res. 1993;34:62. [Google Scholar]
Associated Data
This section collects any data citations, data availability statements, or supplementary materials included in this article.