Abstract
Osteoarthritis is the most common form of arthritis and a leading cause of disability worldwide, largely due to pain, the primary symptom of the disease. The pain experience in knee osteoarthritis in particular is well-recognized as typically transitioning from intermittent weight-bearing pain to a more persistent, chronic pain. Methods to validly assess pain in osteoarthritis studies have been developed to address the complex nature of the pain experience. The etiology of pain in osteoarthritis is recognized to be multifactorial, with both intra-articular and extra-articular risk factors. Nonetheless, greater insights are needed into pain mechanisms in osteoarthritis to enable rational mechanism-based management of pain. Consequences of pain related to osteoarthritis contribute to a substantial socioeconomic burden.
Keywords: osteoarthritis, pain, risk factors
Introduction
The hallmark symptom of osteoarthritis (OA), the most common form of arthritis, is pain. This is the symptom that drives individuals to seek medical attention, and contributes to functional limitations and reduced quality of life.1–4 Largely because of pain, lower extremity OA is well-recognized as the leading cause of mobility impairment in older adults in the US.5,6
The scope of the problem
Approximately 27 million US adults and 8.5 million UK adults are estimated to have clinical OA defined on the basis of symptoms and physical findings.7,8 Prevalence of OA increases with age; 13.9% of adults age 25 and older have clinical OA of at least one joint, while 33.6% of adults age 65 and older have OA.9
In large epidemiologic studies, OA is often defined on the basis of standard radiographic assessments, such as the Kellgren and Lawrence grade. Symptomatic OA indicates the presence of both radiographic OA and symptoms (i.e., pain, aching, stiffness) in the same joint attributable to OA; as such, its prevalence is generally lower than that of radiographic OA (i.e., regardless of symptoms). For example, the prevalence of radiographic knee OA was 19% and 28% among adults age ≥45 years in the Framingham study and Johnston County Osteoarthritis Project, respectively, while the prevalence of symptomatic knee OA was 7% in Framingham and 17% in the Johnston County Osteoarthritis Project.10,11 The prevalence of symptomatic knee OA in two UK studies ranged from 11–19%, and estimates of 5–15% were noted in surveys undertaken in other countries.12
Symptomatic hip OA has been reported to be 9% in the Johnston County Osteoarthritis Project, with lower prevalence estimates of 0.7–4.4% in the UK.13,14 The prevalence of symptomatic hand OA is higher, with the age-standardized prevalence of symptomatic hand OA being 14.4% and 6.9% in women and men, respectively, in younger Framingham cohorts,15 increasing to 26.2% and 13.4%, respectively, among those age ≥71 in an older Framingham cohort.16 Another study reported an estimate of 8% among adults age 60 and older.17 Incidence of symptomatic hand OA were reported to be 9.7% for women and 4% for men over a 9-year period.15
The lifetime risk of developing symptomatic knee OA is estimated to be ~45% (40% in men and 47% in women) based upon Johnston County Osteoarthritis Project data, with risks increasing to 60.5% among persons who are obese, which is approximately double the risk of those who are of normal weight or are underweight.18 With aging of the population and increasing obesity, the prevalence of OA is expected to rise. Indeed, an increase in prevalence of symptomatic knee OA over the past 20 years has been noted in the Framingham cohort, rising by 4.1% and 6% among women and men, respectively, intriguingly without a concomitant parallel rise in prevalence of radiographic OA.19 Based upon National Health Interview Survey (NHIS) data, the estimated number of US adults with doctor-diagnosed arthritis, the majority of which is related to OA and likely symptomatic if it has had medical attention, is projected to increase to nearly 67 million by 2030.20
Clearly a substantial proportion of adults experience pain related to OA during their lifetime. Further, individuals with OA in one joint will often have OA in another joint(s), with resulting greater symptomatic burden of the disease.
The pain experience in OA
The International Association for the Study of Pain defines pain as “an unpleasant sensory and emotional experience associated with actual or potential tissue damage, or described in terms of such damage.”21 It is a complex subjective phenomenon, with each individual having a unique perception of it, influenced by biological, psychological and social factors.22 Under normal circumstances, pain is a warning that something is wrong: pain from touching a hot stove, having injured a joint, or chest pain due to ischemia, for example. In these instances, pain plays a protective role, signaling to the individual to withdraw from the threat, rest to allow tissue healing, or seek help, etc. However, once its warning role is over, persistence or continued pain, i.e., chronic pain, is considered maladaptive.
Unlike many other pain conditions in which the underlying injury typically heals or resolves, OA is a disease that does not resolve. Thus, OA is typically accompanied by chronic pain. Whether, and to what degree, this ongoing chronic pain i) plays an important nociceptive role, ii) represents maladaptive pain, or iii) reflects other aspects of the pain experience is not clear.
The pain experience among persons with OA has been evaluated through a number of qualitative research efforts. In the first qualitative study to focus explicitly on pain and related distress as well as changes in pain over time by Hawker et al., individuals with hip and knee OA identified two distinct types of OA pain: one that was intermittent but generally severe or intense, and another that was a persistent background pain or aching.23 Stages of OA-related pain could be discerned, with early stages characterized by activity-related pain, becoming more constant over time and punctuated by intermittent intense pain. A decrease in participation subsequently occurs in an attempt to avoid triggering such episodes. The more intense but less frequent pain that comes and goes (i.e., intermittent), particularly when unpredictable, had greater impact on quality of life than the ‘background’ (i.e., constant) pain. The pain had negative effects on mood, participation in social and recreational activities, and sleep. Similar findings were noted in another study of individuals who had a recent diagnosis of knee OA or were symptomatic but undiagnosed (i.e., “prediagnostic knee OA”).24 The significance of intermittent knee symptoms was not clear for several years before participants became aware of development of chronic knee symptoms. They then altered activities to avoid more symptoms, until symptoms affected participation, at which time they sought medical care.
In addition to the concepts of “intermittent” and “constant” pain, the intensity of daily pain varies widely,25 although the underlying reasons for such variation are not well-understood. The quality of pain in OA also varies, with approximately one-third of individuals with knee OA using descriptors such as burning, tingling, numbness, and pins and needles to characterize their knee symptoms.26 Such descriptors suggest that neuropathic pain may contribute to the OA pain experience, although specific nerve lesions have not been identified in OA.
Pain assessment in OA
Given the variation in pain intensity, frequency, pattern, and quality in OA, a single, simple question about pain is unlikely to adequately capture the full pain experience. Some of the variation in reported prevalence of symptomatic OA is related to differences in study design and populations examined, but importantly, it is also due to the way in which questions about knee pain were formulated. Differences in descriptors used to assess pain (e.g., “pain” vs. “pain, aching, or stiffness”) may elicit different responses. Duration over which pain is being assessed (e.g., “pain on most days of a month in the past year” vs. “pain on most days of the past month”) can be prone to recall bias. Ideally, uniform, standardized, and valid questionnaires should be used to evaluate pain, particularly to enable more precise pain phenotyping and facilitate cross-study comparisons, genetic association studies, and drug trial protocol development.
In OA cohort studies and trials, a number of approaches are typically used to assess pain. For evaluation of knee OA pain, the most common are a visual analog scale (VAS) or numerical rating scale (NRS) assessment of pain intensity; a single question about presence of “pain, aching of stiffness in or around the knee” over a specified period of time; and/or the pain subscale of the Western Ontario and McMaster Universities Arthritis Index (WOMAC)27 or the Knee injury and Osteoarthritis Outcome Score (KOOS).28 The pain subscales of these latter two instruments assess pain experienced with specific activities. As a result, the pain and function subscale scores are highly correlated. Nonetheless, these validated instruments are responsive and are used in assessing efficacy of interventions. A number of additional validated generic pain instruments are available that are also appropriate for use in OA.29 A meta-analysis concluded that different patient-reported outcome measures of pain severity have generally comparable responsiveness to treatment, with the single-item pain assessments with the VAS or NRS resulting in effect estimates comparable to the WOMAC pain subscale, although their mean standardized effect sizes were lower.30 To enable meaningful interpretation of response to therapy rather than relying on mean group responses, the OMERACT-OARSI set of responder criteria were developed and validated for use in clinical trials.31 To be considered a responder, at least a minimum threshold of relative and absolute improvement in pain or a lesser degree of absolute and relative improvement in at least 2 out of 3 domains (pain, function, patient global assessment) is required. Many of these same questions and instruments (e.g., WOMAC) can be used for hip OA; the Hip disability and Osteoarthritis Outcome Score (HOOS) is specific for hip OA.32 To assess pain, stiffness and physical functioning in hand OA, the Australian/Canadian Osteoarthritis Hand Index (AUSCAN) is commonly used.33
Despite widespread use of these pain assessments, the complex pain experience of those living with OA is not adequately captured by existing measures. To address this issue, a multicenter international OARSI/OMERACT initiative led to development of a new measure informed by qualitative research findings that was subsequently validated. This new instrument, ICOAP (Intermittent and Constant OA Pain), assesses various facets regarding both intermittent and constant pain for the knee and hip separately, including frequency (for intermittent pain), intensity, effects on sleep and quality of life, degree of frustration or annoyance and upset or worried feelings associated with the pain, as well as whether the intermittent pain occurs without warning or after a trigger.34 The ICOAP has recently been demonstrated to be responsive to change in intervention studies.35
In keeping with the acknowledgement of the multidimensional nature of pain, the Initiative on Methods, Measurement, and Pain Assessment in Clinical Trials (IMMPACT) has recommended six core domains and associated measures that should be considered when studying any type of chronic pain in clinical trials: pain (intensity and use of rescue medications), physical functioning (with a focus on pain interference), emotional functioning, participant ratings of improvement and satisfaction with treatment, symptoms and adverse events, and participant disposition.36,37 Other domains related to pain in OA include fatigue, sleep, and cognition. With the increasing importance of patient-reported outcomes, the NIH-funded Patient Reported Outcomes Measurement Information System (PROMIS) provides an opportunity to collect a variety of validated patient-reported health outcomes related to physical, mental, and social well-being, in addition to pain.
Risk factors for pain in OA
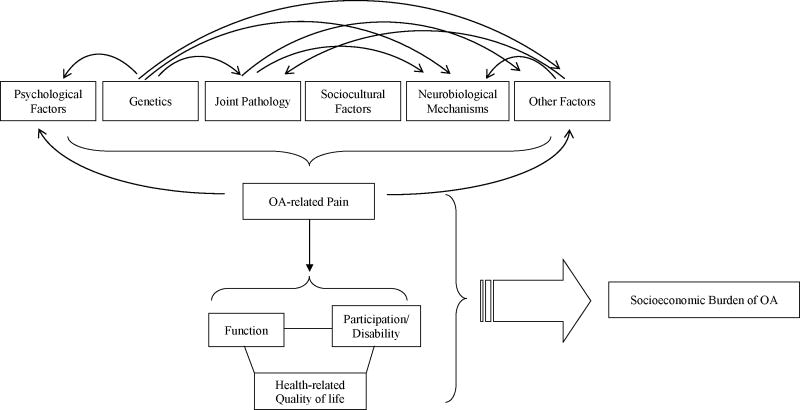
In view of the complex, multidimensional nature of the pain experience in OA, it is perhaps not surprising that the underlying etiology of pain is multifactorial, most often considered in a biopsychosocial framework (Figure 1). A few such risk factors are discussed below.
Figure 1.
Schematic illustrating the multifactorial nature of pain in OA, with complex inter-relationships between various risk factors, and the potential wide-ranging effects of OA pain.
The extent to which structural pathology in OA contributes to the pain experience has been controversial. A structure-symptom discordance in OA has been widely noted, based upon observations of weak correlations between radiographic severity of OA and pain presence or severity, although the discordance is less with more severe stages of radiographic disease.10,38–43 In a systematic review, 15–76% of those with knee pain had radiographic OA, and 15–81% of those with radiographic OA had knee pain.44 The extent of additional x-ray views obtained, the definition of pain symptoms, and the nature of the study sample (e.g., age, race) affected the prevalence of these findings, and therefore interpretation of the degree of concordance. For example, in studies evaluating both the tibiofemoral and patellofemoral joints that also obtained WOMAC pain assessments, a more consistent association was noted between pain severity and radiographic OA.45,46 Supporting such findings, a randomized trial demonstrated intraarticular lidocaine to effectively decrease knee pain in comparison with placebo,47 lending further support to the notion that structural pathology within the knee must be contributing to pain.
Beyond measurement issues, there are additional reasons that contribute to an apparent discordance. As discussed above, pain is a subjective experience, influenced by a number of factors, including genetic predisposition,48,49 prior experience,50,51 expectations about analgesic treatment,52,53 current mood,54 coping strategies and catastrophizing,55 and sociocultural environment,56–58 as some examples. Without taking into account such factors that can contribute to between-person differences, assessment of the relation of structure to symptoms will be confounded. Unfortunately most such factors that contribute to individual variation in pain cannot be feasibly measured or collected in most studies. By adequately controlling for between-person differences using a within-person knee-matched approach, a strong dose-response relationship can be demonstrated between radiographic severity and pain presence, severity, and incidence (i.e., new onset).59,60
While such studies provide confirmation that structural pathology of OA does indeed contribute to the pain experience, radiographs do not provide insight into what particular structural pathologies may contribute to such pain. A review elsewhere in this issue examines the structural correlates of pain in greater detail.(REF) In brief, based upon MRI studies, bone marrow lesions, synovitis, and effusions appear to have the greatest evidence supporting their relation to pain in OA to date.61
Although such studies have highlighted the importance of structural pathology to pain in OA, attempts at structure modification have been largely unsuccessful to date with regards to pain. Some recent exceptions include promising pain results from trials evaluating zoledronic acid targeting bone marrow lesions, with possible additional bone and cartilage effects, and strontium ranelate which may have both bone and cartilage effects.62,63
Other risk factors for pain in OA may be more amenable to modification. Psychological factors are well-recognized as being correlated with pain in OA, and the role of cognitive behavioural therapy is outlined elsewhere in this issue.39,64 (+REF) Specifically, some traits, such as catastrophizing, coping, and self-efficacy may be amenable to intervention.65–67 While depression, anxiety, and negative affect, among others, have been associated with OA pain,42,68 the causal direction of such relationships is difficult to discern. Fluctuation in pain has been linked to fluctuation in psychological factors, but whether the pain influences the mood or vice versa is difficult to disentangle.69 Although psychological factors can certainly contribute to a heightened pain experience, it is also possible that pain itself can contribute to poor mood. Such relationships can only be discerned from longitudinal studies, of which there are relatively few to date. For example, pain from OA contributed to functional limitations and fatigue, which in turn contributed to depressed mood and worse pain and function in one study evaluating these complex inter-relationships.70 Functional brain imaging studies of OA also demonstrate an important role of affective and motivational aspects of pain71,72 that should be addressed to improve effective management of OA-related pain. This is particularly important in light of the prevalence and impact of comorbid mood disorders on health outcomes.
Weight is a potential modifiable factor contributing not only to OA risk, but also to pain. The effect of obesity on pain may be two-fold. For the lower extremities, the effect of excess weights on symptoms may be due to mechanical loading. Increased relative fat mass in obesity may potentially contribute to pain symptoms related to elaboration of adipokines, although studies are conflicting in this regard.73,74 While the mechanism by which obesity contributes to pain may not be clear, effects of altering weight on OA-related pain have been studied. Observational cohort data was used to demonstrate a lower risk of developing symptomatic knee OA among women who lost ≥5kg.75 Subsequent randomized trials have noted reductions in pain with ~10% weight loss,76–78 with more substantial effects on pain reduction with greater weight loss.79 Importantly, weight gain significantly increases pain, highlighting the dose-response relationship of change in weight with change in pain.80
While not directly modifiable, there may be a genetic predisposition to development of chronic pain or experiencing greater pain severity that may provide insight into novel therapeutic targets. The availability of large cohort studies with standardized pain and x-ray data has facilitated genetic association studies to address such hypotheses. A functional polymorphism (Val158Met) in the COMT gene, which has been associated with pain sensitivity in other clinical conditions, was associated with hip OA-related pain in one cohort study,81 but has not yet been replicated in other cohorts. TRPV1 and the PACE4 gene PCSK6 were associated with pain in knee OA in two separate meta-analyses,82,83 while an association with a SCN9 SNP could not be replicated.84 A missense variant in P2RX7, a target identified through a genome-wide screen in mice with assessment of mechanical allodynia, has been associated with OA-related pain in one cohort.85 Greater details of genetic determinants of pain can be found elsewhere in this issue.(REF)
Another area that may provide potential therapeutic targets is related to risk factors that contribute to the transition from acute to chronic pain in OA, which at present is not well-understood. As noted in the qualitative work described above, there is a general progression of symptoms from the early stages of OA with activity-related (e.g., weight-bearing) symptoms that appear to be nociceptive in nature, to a more persistent constant pain that likely reflects other additional processes, such as neurobiological mechanisms. Tissue injury and/or inflammation, as may be seen in OA, leads to a decrease in the excitation threshold and an increase in responsiveness to suprathreshold stimuli of peripheral nociceptors, i.e., peripheral sensitization.86–88 Noxious mechanical stimuli can then evoke exaggerated responses (primary hyperalgesia), and normally innocuous stimuli, such as movement of the joint through its normal range of motion, may evoke a pain response (allodynia). As a result of nociceptor activity after tissue injury or inflammation, a number of changes occur in the central nervous system. These include changes to dorsal horn transmission neuron receptors, leading the transmission neurons to become increasingly responsive to peripheral input (central sensitization), with reduction in the threshold for mechanically induced pain and an expansion of the receptive field of dorsal horn neurons (spatial summation).89 Radiating pain in OA likely reflects this latter phenomenon. Once established, central sensitization is maintained by low-level noxious and even non-nociceptive input from the periphery.90 Such changes in the central nervous system are mainly responsible for the enhanced sensitivity to mechanical stimuli that develops outside the area of the injury (secondary hyperalgesia).91–93
Beyond the clinical observations of hyperalgesia, allodynia, and radiating pain that suggest a role for sensitization in OA-related pain, there are some experimental neurophysiologic findings that also support the presence of sensitization in OA. Persons with knee OA experience a greater intensity, duration, and area of hyperalgesia after intramuscular injection of hypertonic saline compared with controls.94 Lidocaine injected into a painful OA knee resulted in pain reduction in both the injected knee and the untreated contralateral knee, supporting central pain modulation in OA.47 Persons with knee OA have higher pain intensities compared with controls to the same level of pressure stimuli, as well as lower pressure pain thresholds.95 Other studies have also documented lower pain thresholds in persons with OA compared with controls.96–98 Temporal summation, a progressive increase in discharges of dorsal horn neurons in response to repetitive afferent stimulation thought to reflect central sensitization, is increased in persons with painful knee OA compared with age-matched healthy controls, and the degree of sensitization correlated with pain.99 What pathologies of OA may contribute to peripheral and/or central sensitization, other risk factors for sensitization, and identification of the transition from appropriate nociceptive input to sensitization are important research questions that need to be addressed for improved understanding of pain mechanisms in OA. In addition, further development and validation of tools to assess sensitization will be necessary to support such research efforts.100
Thus, there appears to be substantial opportunities to gain further insights into causes and contributors to pain in OA. Such insights in turn will provide opportunities for rational mechanism-based targeting of pain for more efficacious therapeutic management of OA patients.
Impact of OA-related pain
Because effective treatment for OA and its related pain is not available to date, and the disease can be present for decades, the public health impact of OA is substantial on an individual and societal level (Figure 1). With the high prevalence of knee OA globally,101 not only is OA a leading cause of disability among older adults in the US,5,6 but it is among the top 10 causes of disability worldwide.101,102 In recent estimates of global years lived in disability, musculoskeletal-related conditions ranked second, with low back pain, neck pain, and knee OA being the three most common such conditions, and knee OA itself ranked within the top 10 noncommunicable diseases for global disability-adjusted life years (i.e., years of life lost and years lived with disability).102
Symptoms such as pain, stiffness, and gelling in OA have clear contributions to functional limitations in OA, with well-documented associations of pain severity with degree of functional limitation.103,104 While most of the research focus to date has been on the knee or hip, symptomatic hand OA has important functional limitations, predominantly related to weaker grip strength and activities requiring precise pincer grip or power grip.105 Nonetheless, a particular focus on lower extremity OA is warranted given the high prevalence of associated disability. In a longitudinal panel survey conducted by the US Census bureau, arthritis or rheumatism was the most commonly reported cause of disability, and difficulties related to lower extremity functioning or activities were the most commonly reported limitations among all respondents.106 Specifically, the most common limitation was in walking 3 city blocks, which affected an estimated 22.5 million US adults, and difficulty with climbing stairs, affecting an estimated 21.7 million US adults.106 While not all such individuals have symptomatic knee or hip OA, it is likely that OA accounts for a large proportion of these limitations. Based upon NHANES III data, among persons with OA, about 80% have some degree of movement limitation and 25% cannot perform major activities of daily living; 11% of adults with knee OA require help with personal care, and 14% require help with routine needs.9 Symptomatic knee OA can have less obviously apparent effects on functioning as well. For example, persons with knee OA have slower walking speeds than those without OA.107 Further, those with symptomatic knee OA have a faster decline in gait speed over time than those with either knee OA alone or knee pain alone.108 It is not surprising that knee pain also leads to restrictions in mobility outside of the house, impacting upon participation.109
Symptomatic OA’s economic impact is also substantial. Average direct medical charges related to OA care were estimated to be ~$2600 per year per individual in 1997,110 and the total (i.e., direct and indirect) annual disease costs were estimated to be $5700 per individual (USD, FY 2000).111 Those costs need to be considered in the context of the prevalence of the disease to appreciate the overall societal economic impact. OA as a primary diagnosis accounted for 11.25 million (22.3%) of all arthritis-related ambulatory medical care visits in 2006.112 Further, arthritis-related conditions were the second most common reason for medical visits related to chronic conditions in 2005, second only to hypertension, which is asymptomatic.113 In terms of inpatient costs, OA was the fifth most expensive condition treated in US hospitals in 2008, with a cost of ~$40 billion in total national hospital expenditures, comprising 3.5% of the national hospital bill, and accounting for 70% of all arthritis-related inpatient hospitalizations.112,114 Much of those hospitalizations were related to joint replacement surgery. Pain is clearly among the main reasons for individuals seeking joint replacement. Knee replacement surgeries are one of the most commonly performed orthopedic procedures in the US, with ~50% of all joint arthoplasties performed on the knee, and 97% of those are performed for knee OA.112 In 2004, 478,000 knee replacement surgeries were performed, representing a 3-fold increase since 1991, with total hospitalization charges of $14.26 billion in 2004.112,115,116 This increase exceeds expectations based upon overall population growth and increase in the proportion of the population that is elderly and/or obese. The demand for primary total knee replacement is expected to grow by 673% to 3.48 million procedures by 2030.117 Adding to these costs is the increase in health care utilization in the two years preceding the surgery.118
To appreciate the total economic burden of OA on society, indirect or productivity costs must also be examined. Productivity costs typically reflect costs due to lost productivity while being present at work, costs due to absence from work, and costs for compensation of household work by others.119 Unfortunately, there are significant variations among indirect cost studies in OA regarding methodology, cost estimation, and cost presentation, limiting one’s ability to determine the magnitude of OA’s economic impact.120 For example, in one review, indirect costs of OA per patient per year varied from $831 in Hong Kong to $12,789 in Canada (costs in 2006 USD).120 Considering the prevalence of OA, work-related OA costs have been estimated to range from $3.4 to $13.2 billion per year.121 Estimates from 1999 indicate that adults with knee OA reported more than 13 days of lost work due to health issues.9 Using a more recent large US employer benefits database, those with OA had an average of 63 days of absenteeism compared with 37 days among a matched comparator group, with mean total direct and indirect costs being 2- to 3-fold higher.122 Similar findings were noted in a Swedish population-based cohort, in which those with physician-diagnosed knee OA had a 2-fold increased risk of sick leave and 40–50% increased risk of disability pension compared with the general population.123 Further, ~2% of all sick days in the population were attributable to knee OA. In a systematic literature review regarding work participation, occupational limitations and reduced work capacity or job effectiveness were reported more frequently in those with OA than by controls.124 Aggregate annual absenteeism costs of OA were estimated to be ~$10 billion from the US Medical Expenditure Panel Survey, higher than many other major chronic diseases.125 Taking into account both productivity costs and medical costs among adults with paid employment in a study from the Netherlands, the total economic burden of knee OA was estimated to be €871 per person, per month, with the majority of the costs being related to productivity.119 Regardless of the methodologic differences, issues with cost estimation, and difficulties in comparing costs across studies, it is clear that OA has a tremendous economic impact that will only continue to grow with its rising prevalence.
Summary
OA is highly prevalent worldwide, with a tremendous symptomatic and economic global burden. Although a number of risk factors have been identified for pain in OA, the research focus to date has primarily been on structural targets. Pharmacologic treatment options remain limited and nonpharmacologic options are underutilized. An expansion of the research agenda to more fully explore pain mechanisms operational in OA is urgently needed to enable comprehensive mechanism-based pain management strategies in this prevalent, disabling, and costly disease.
Acknowledgments
NIAMS K23AR055127
NIAMS R01AR062506
NIAMS P60AR47785
Footnotes
Publisher's Disclaimer: This is a PDF file of an unedited manuscript that has been accepted for publication. As a service to our customers we are providing this early version of the manuscript. The manuscript will undergo copyediting, typesetting, and review of the resulting proof before it is published in its final citable form. Please note that during the production process errors may be discovered which could affect the content, and all legal disclaimers that apply to the journal pertain.
References
- 1.Hadler NM. Knee pain is the malady--not osteoarthritis. Ann Intern Med. 1992;116:598–9. doi: 10.7326/0003-4819-116-7-598. [DOI] [PubMed] [Google Scholar]
- 2.Ayis S, Dieppe P. The natural history of disability and its determinants in adults with lower limb musculoskeletal pain. J Rheumatol. 2009;36:583–91. doi: 10.3899/jrheum.080455. [DOI] [PubMed] [Google Scholar]
- 3.Dominick KL, Ahern FM, Gold CH, Heller DA. Health-related quality of life and health service use among older adults with osteoarthritis. Arthritis Rheum. 2004;51:326–31. doi: 10.1002/art.20390. [DOI] [PubMed] [Google Scholar]
- 4.McAlindon TE, Cooper C, Kirwan JR, Dieppe PA. Determinants of disability in osteoarthritis of the knee. Ann Rheum Dis. 1993;52:258–62. doi: 10.1136/ard.52.4.258. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 5.Centers for Diseaes Control and Prevention. Prevalence of disabilities and associated health conditions among adults: United States, 1999. MMWR Morb Mortal Wkly Rep. 2001:120–5. [PubMed]
- 6.Guccione AA, Felson DT, Anderson JJ, et al. The effects of specific medical conditions on the functional limitations of elders in the Framingham Study. Am J Public Health. 1994;84:351–8. doi: 10.2105/ajph.84.3.351. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 7.Lawrence RC, Felson DT, Helmick CG, et al. Estimates of the prevalence of arthritis and other rheumatic conditions in the United States. Part II. Arthritis Rheum. 2008;58:26–35. doi: 10.1002/art.23176. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 8.National Collaborating Centre for Chronic Conditions. Osteoarthritis: national clinical guideline for care and management in adults. London: Royal College of Physicians; 2008. [PubMed] [Google Scholar]
- 9.Centers for Disease Control and Prevention. [Accessed January 4, 2013];Osteoarthritis. at http://www.cdc.gov/arthritis/basics/osteoarthritis.htm.)
- 10.Felson DT, Naimark A, Anderson J, Kazis L, Castelli W, Meenan RF. The prevalence of knee osteoarthritis in the elderly. The Framingham Osteoarthritis Study. Arthritis Rheum. 1987;30:914–8. doi: 10.1002/art.1780300811. [DOI] [PubMed] [Google Scholar]
- 11.Jordan JM, Helmick CG, Renner JB, et al. Prevalence of knee symptoms and radiographic and symptomatic knee osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2007;34:172–80. [PubMed] [Google Scholar]
- 12.Peat G, McCarney R, Croft P. Knee pain and osteoarthritis in older adults: a review of community burden and current use of primary health care. Ann Rheum Dis. 2001;60:91–7. doi: 10.1136/ard.60.2.91. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 13.Jordan JM, Helmick CG, Renner JB, et al. Prevalence of hip symptoms and radiographic and symptomatic hip osteoarthritis in African Americans and Caucasians: the Johnston County Osteoarthritis Project. J Rheumatol. 2009;36:809–15. doi: 10.3899/jrheum.080677. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 14.Arden N, Nevitt MC. Osteoarthritis: epidemiology. Best practice & research. Clinical rheumatology. 2006;20:3–25. doi: 10.1016/j.berh.2005.09.007. [DOI] [PubMed] [Google Scholar]
- 15.Haugen IK, Englund M, Aliabadi P, et al. Prevalence, incidence and progression of hand osteoarthritis in the general population: the Framingham Osteoarthritis Study. Ann Rheum Dis. 2011;70:1581–6. doi: 10.1136/ard.2011.150078. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 16.Zhang Y, Niu J, Kelly-Hayes M, Chaisson CE, Aliabadi P, Felson DT. Prevalence of symptomatic hand osteoarthritis and its impact on functional status among the elderly: The Framingham Study. Am J Epidemiol. 2002;156:1021–7. doi: 10.1093/aje/kwf141. [DOI] [PubMed] [Google Scholar]
- 17.Dillon CF, Hirsch R, Rasch EK, Gu Q. Symptomatic hand osteoarthritis in the United States: prevalence and functional impairment estimates from the third U.S. National Health and Nutrition Examination Survey, 1991–1994. Am J Phys Med Rehabil. 2007;86:12–21. doi: 10.1097/phm.0b013e31802ba28e. [DOI] [PubMed] [Google Scholar]
- 18.Murphy L, Schwartz TA, Helmick CG, et al. Lifetime risk of symptomatic knee osteoarthritis. Arthritis Rheum. 2008;59:1207–13. doi: 10.1002/art.24021. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 19.Nguyen US, Zhang Y, Zhu Y, Niu J, Zhang B, Felson DT. Increasing prevalence of knee pain and symptomatic knee osteoarthritis: survey and cohort data. Ann Intern Med. 2011;155:725–32. doi: 10.1059/0003-4819-155-11-201112060-00004. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 20.Hootman JM, Helmick CG. Projections of US prevalence of arthritis and associated activity limitations. Arthritis Rheum. 2006;54:226–9. doi: 10.1002/art.21562. [DOI] [PubMed] [Google Scholar]
- 21.IASP Task Force on Taxonomy. Classification of Chronic Pain. 2. Seattle: IASP Press; 1994. [Google Scholar]
- 22.Institute of Medicine. Relieving pain in America: A blueprint for transforming prevention, cure, education and researech. Washington, DC: The National Academies Press; 2011. [PubMed] [Google Scholar]
- 23.Hawker GA, Stewart L, French MR, et al. Understanding the pain experience in hip and knee osteoarthritis--an OARSI/OMERACT initiative. Osteoarthritis Cartilage. 2008;16:415–22. doi: 10.1016/j.joca.2007.12.017. [DOI] [PubMed] [Google Scholar]
- 24.Maly MR, Cott CA. Being careful: a grounded theory of emergent chronic knee problems. Arthritis Rheum. 2009;61:937–43. doi: 10.1002/art.24611. [DOI] [PubMed] [Google Scholar]
- 25.Allen KD, Coffman CJ, Golightly YM, Stechuchak KM, Keefe FJ. Daily pain variations among patients with hand, hip, and knee osteoarthritis. Osteoarthritis Cartilage. 2009;17:1275–82. doi: 10.1016/j.joca.2009.03.021. [DOI] [PubMed] [Google Scholar]
- 26.Hochman JR, French MR, Bermingham SL, Hawker GA. The nerve of osteoarthritis pain. Arthritis Care Res (Hoboken) 2010;62:1019–23. doi: 10.1002/acr.20142. [DOI] [PubMed] [Google Scholar]
- 27.Bellamy N, Buchanan WW, Goldsmith CH, Campbell J, Stitt LW. Validation study of WOMAC: a health status instrument for measuring clinically important patient relevant outcomes to antirheumatic drug therapy in patients with osteoarthritis of the hip or knee. J Rheumatol. 1988;15:1833–40. [PubMed] [Google Scholar]
- 28.Roos EM, Lohmander LS. The Knee injury and Osteoarthritis Outcome Score (KOOS): from joint injury to osteoarthritis. Health and quality of life outcomes. 2003;1:64. doi: 10.1186/1477-7525-1-64. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 29.Hawker GA, Mian S, Kendzerska T, French M. Measures of adult pain: Visual Analog Scale for Pain (VAS Pain), Numeric Rating Scale for Pain (NRS Pain), McGill Pain Questionnaire (MPQ), Short-Form McGill Pain Questionnaire (SF-MPQ), Chronic Pain Grade Scale (CPGS), Short Form-36 Bodily Pain Scale (SF-36 BPS), and Measure of Intermittent and Constant Osteoarthritis Pain (ICOAP) Arthritis Care Res (Hoboken) 2011;63 (Suppl 11):S240–52. doi: 10.1002/acr.20543. [DOI] [PubMed] [Google Scholar]
- 30.Dworkin RH, Peirce-Sandner S, Turk DC, et al. Outcome measures in placebo-controlled trials of osteoarthritis: responsiveness to treatment effects in the REPORT database. Osteoarthritis Cartilage. 2011;19:483–92. doi: 10.1016/j.joca.2011.02.020. [DOI] [PubMed] [Google Scholar]
- 31.Pham T, van der Heijde D, Altman RD, et al. OMERACT-OARSI initiative: Osteoarthritis Research Society International set of responder criteria for osteoarthritis clinical trials revisited. Osteoarthritis Cartilage. 2004;12:389–99. doi: 10.1016/j.joca.2004.02.001. [DOI] [PubMed] [Google Scholar]
- 32.Klassbo M, Larsson E, Mannevik E. Hip disability and osteoarthritis outcome score. An extension of the Western Ontario and McMaster Universities Osteoarthritis Index. Scand J Rheumatol. 2003;32:46–51. doi: 10.1080/03009740310000409. [DOI] [PubMed] [Google Scholar]
- 33.Bellamy N, Campbell J, Haraoui B, et al. Clinimetric properties of the AUSCAN Osteoarthritis Hand Index: an evaluation of reliability, validity and responsiveness. Osteoarthritis Cartilage. 2002;10:863–9. doi: 10.1053/joca.2002.0838. [DOI] [PubMed] [Google Scholar]
- 34.Hawker GA, Davis AM, French MR, et al. Development and preliminary psychometric testing of a new OA pain measure--an OARSI/OMERACT initiative. Osteoarthritis Cartilage. 2008;16:409–14. doi: 10.1016/j.joca.2007.12.015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 35.Bond M, Davis A, Lohmander S, Hawker G. Responsiveness of the OARSI-OMERACT osteoarthritis pain and function measures. Osteoarthritis Cartilage. 2012;20:541–7. doi: 10.1016/j.joca.2012.03.001. [DOI] [PubMed] [Google Scholar]
- 36.Dworkin RH, Turk DC, Farrar JT, et al. Core outcome measures for chronic pain clinical trials: IMMPACT recommendations. Pain. 2005;113:9–19. doi: 10.1016/j.pain.2004.09.012. [DOI] [PubMed] [Google Scholar]
- 37.Turk DC, Dworkin RH, Allen RR, et al. Core outcome domains for chronic pain clinical trials: IMMPACT recommendations. Pain. 2003;106:337–45. doi: 10.1016/j.pain.2003.08.001. [DOI] [PubMed] [Google Scholar]
- 38.Dieppe PA. Relationship between symptoms and structural change in osteoarthritis. what are the important targets for osteoarthritis therapy? J Rheumatol Suppl. 2004;70:50–3. [PubMed] [Google Scholar]
- 39.Davis MA, Ettinger WH, Neuhaus JM, Barclay JD, Segal MR. Correlates of knee pain among US adults with and without radiographic knee osteoarthritis. J Rheumatol. 1992;19:1943–9. [PubMed] [Google Scholar]
- 40.Hannan MT, Felson DT, Pincus T. Analysis of the discordance between radiographic changes and knee pain in osteoarthritis of the knee. J Rheumatol. 2000;27:1513–7. [PubMed] [Google Scholar]
- 41.Hochberg MC, Lawrence RC, Everett DF, Cornoni-Huntley J. Epidemiologic associations of pain in osteoarthritis of the knee: data from the National Health and Nutrition Examination Survey and the National Health and Nutrition Examination-I Epidemiologic Follow-up Survey. Semin Arthritis Rheum. 1989;18:4–9. doi: 10.1016/0049-0172(89)90008-5. [DOI] [PubMed] [Google Scholar]
- 42.Creamer P, Lethbridge-Cejku M, Hochberg MC. Determinants of pain severity in knee osteoarthritis: effect of demographic and psychosocial variables using 3 pain measures. J Rheumatol. 1999;26:1785–92. [PubMed] [Google Scholar]
- 43.Lethbridge-Cejku M, Scott WW, Jr, Reichle R, et al. Association of radiographic features of osteoarthritis of the knee with knee pain: data from the Baltimore Longitudinal Study of Aging. Arthritis Care Res. 1995;8:182–8. doi: 10.1002/art.1790080311. [DOI] [PubMed] [Google Scholar]
- 44.Bedson J, Croft PR. The discordance between clinical and radiographic knee osteoarthritis: a systematic search and summary of the literature. BMC Musculoskelet Disord. 2008;9:116. doi: 10.1186/1471-2474-9-116. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 45.Duncan R, Peat G, Thomas E, Hay E, McCall I, Croft P. Symptoms and radiographic osteoarthritis: not as discordant as they are made out to be? Ann Rheum Dis. 2007;66:86–91. doi: 10.1136/ard.2006.052548. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 46.Szebenyi B, Hollander AP, Dieppe P, et al. Associations between pain, function, and radiographic features in osteoarthritis of the knee. Arthritis Rheum. 2006;54:230–5. doi: 10.1002/art.21534. [DOI] [PubMed] [Google Scholar]
- 47.Creamer P, Hunt M, Dieppe P. Pain mechanisms in osteoarthritis of the knee: effect of intraarticular anesthetic. J Rheumatol. 1996;23:1031–6. [PubMed] [Google Scholar]
- 48.Zubieta JK, Smith YR, Bueller JA, et al. Regional mu opioid receptor regulation of sensory and affective dimensions of pain. Science. 2001;293:311–5. doi: 10.1126/science.1060952. [DOI] [PubMed] [Google Scholar]
- 49.Mogil JS. The genetic mediation of individual differences in sensitivity to pain and its inhibition. Proc Natl Acad Sci U S A. 1999;96:7744–51. doi: 10.1073/pnas.96.14.7744. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 50.Vase L, Riley JL, 3rd, Price DD. A comparison of placebo effects in clinical analgesic trials versus studies of placebo analgesia. Pain. 2002;99:443–52. doi: 10.1016/S0304-3959(02)00205-1. [DOI] [PubMed] [Google Scholar]
- 51.Colloca L, Benedetti F. How prior experience shapes placebo analgesia. Pain. 2006;124:126–33. doi: 10.1016/j.pain.2006.04.005. [DOI] [PubMed] [Google Scholar]
- 52.Fields HL. Pain modulation: expectation, opioid analgesia and virtual pain. Prog Brain Res. 2000;122:245–53. doi: 10.1016/s0079-6123(08)62143-3. [DOI] [PubMed] [Google Scholar]
- 53.Wager TD. Expectations and anxiety as mediators of placebo effects in pain. Pain. 2005;115:225–6. doi: 10.1016/j.pain.2005.03.018. [DOI] [PubMed] [Google Scholar]
- 54.Villemure C, Slotnick BM, Bushnell MC. Effects of odors on pain perception: deciphering the roles of emotion and attention. Pain. 2003;106:101–8. doi: 10.1016/s0304-3959(03)00297-5. [DOI] [PubMed] [Google Scholar]
- 55.Bradley LA. Recent approaches to understanding osteoarthritis pain. J Rheumatol Suppl. 2004;70:54–60. [PubMed] [Google Scholar]
- 56.Giardino ND, Jensen MP, Turner JA, Ehde DM, Cardenas DD. Social environment moderates the association between catastrophizing and pain among persons with a spinal cord injury. Pain. 2003;106:19–25. doi: 10.1016/s0304-3959(03)00226-4. [DOI] [PubMed] [Google Scholar]
- 57.Gamsa A. Is emotional disturbance a precipitator or a consequence of chronic pain? Pain. 1990;42:183–95. doi: 10.1016/0304-3959(90)91161-B. [DOI] [PubMed] [Google Scholar]
- 58.Deshields TL, Tait RC, Gfeller JD, Chibnall JT. Relationship between social desirability and self-report in chronic pain patients. Clin J Pain. 1995;11:189–93. doi: 10.1097/00002508-199509000-00005. [DOI] [PubMed] [Google Scholar]
- 59.Neogi T, Felson D, Niu J, et al. Association between radiographic features of knee osteoarthritis and pain: results from two cohort studies. BMJ. 2009;339:b2844. doi: 10.1136/bmj.b2844. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 60.Niu J, Felson D, Neogi T, Zhang Y. Radiographic osteoarthritis severity is associated with an increased risk of developing knee pain: Findings from the Osteoarthritis Initiative. Arthritis Rheum. 2012;64:S1115. [Google Scholar]
- 61.Yusuf E, Kortekaas MC, Watt I, Huizinga TW, Kloppenburg M. Do knee abnormalities visualised on MRI explain knee pain in knee osteoarthritis? A systematic review. Ann Rheum Dis. 2011;70:60–7. doi: 10.1136/ard.2010.131904. [DOI] [PubMed] [Google Scholar]
- 62.Laslett LL, Dore DA, Quinn SJ, et al. Zoledronic acid reduces knee pain and bone marrow lesions over 1 year: a randomised controlled trial. Ann Rheum Dis. 2012;71:1322–8. doi: 10.1136/annrheumdis-2011-200970. [DOI] [PubMed] [Google Scholar]
- 63.Reginster JY, Badurski J, Bellamy N, et al. Efficacy and safety of strontium ranelate in the treatment of knee osteoarthritis: results of a double-blind, randomised placebo-controlled trial. Ann Rheum Dis. 2013;72:179–86. doi: 10.1136/annrheumdis-2012-202231. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 64.Dieppe PA, Lohmander LS. Pathogenesis and management of pain in osteoarthritis. Lancet. 2005;365:965–73. doi: 10.1016/S0140-6736(05)71086-2. [DOI] [PubMed] [Google Scholar]
- 65.Somers TJ, Blumenthal JA, Guilak F, et al. Pain coping skills training and lifestyle behavioral weight management in patients with knee osteoarthritis: a randomized controlled study. Pain. 2012;153:1199–209. doi: 10.1016/j.pain.2012.02.023. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 66.Riddle DL, Keefe FJ, Nay WT, McKee D, Attarian DE, Jensen MP. Pain coping skills training for patients with elevated pain catastrophizing who are scheduled for knee arthroplasty: a quasi-experimental study. Arch Phys Med Rehabil. 2011;92:859–65. doi: 10.1016/j.apmr.2011.01.003. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 67.Allen KD, Oddone EZ, Coffman CJ, Keefe FJ, Lindquist JH, Bosworth HB. Racial differences in osteoarthritis pain and function: potential explanatory factors. Osteoarthritis Cartilage. 2010;18:160–7. doi: 10.1016/j.joca.2009.09.010. [DOI] [PubMed] [Google Scholar]
- 68.Dekker J, Tola P, Aufdemkampe G, Winckers M. Negative affect, pain and disability in osteoarthritis patients: the mediating role of muscle weakness. Behav Res Ther. 1993;31:203–6. doi: 10.1016/0005-7967(93)90073-4. [DOI] [PubMed] [Google Scholar]
- 69.Wise BL, Niu J, Zhang Y, et al. Psychological factors and their relation to osteoarthritis pain. Osteoarthritis Cartilage. 2010;18:883–7. doi: 10.1016/j.joca.2009.11.016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 70.Hawker GA, Gignac MA, Badley E, et al. A longitudinal study to explain the pain-depression link in older adults with osteoarthritis. Arthritis Care Res (Hoboken) 2011;63:1382–90. doi: 10.1002/acr.20298. [DOI] [PubMed] [Google Scholar]
- 71.Gwilym SE, Keltner JR, Warnaby CE, et al. Psychophysical and functional imaging evidence supporting the presence of central sensitization in a cohort of osteoarthritis patients. Arthritis Rheum. 2009;61:1226–34. doi: 10.1002/art.24837. [DOI] [PubMed] [Google Scholar]
- 72.Kulkarni B, Bentley DE, Elliott R, et al. Arthritic pain is processed in brain areas concerned with emotions and fear. Arthritis Rheum. 2007;56:1345–54. doi: 10.1002/art.22460. [DOI] [PubMed] [Google Scholar]
- 73.Sowers MR, Karvonen-Gutierrez CA. The evolving role of obesity in knee osteoarthritis. Curr Opin Rheumatol. 2010;22:533–7. doi: 10.1097/BOR.0b013e32833b4682. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 74.Yusuf E. Metabolic factors in osteoarthritis: obese people do not walk on their hands. Arthritis Res Ther. 2012;14:123. doi: 10.1186/ar3894. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 75.Felson DT, Zhang Y, Anthony JM, Naimark A, Anderson JJ. Weight loss reduces the risk for symptomatic knee osteoarthritis in women. The Framingham Study. Ann Intern Med. 1992;116:535–9. doi: 10.7326/0003-4819-116-7-535. [DOI] [PubMed] [Google Scholar]
- 76.Bliddal H, Leeds AR, Stigsgaard L, Astrup A, Christensen R. Weight loss as treatment for knee osteoarthritis symptoms in obese patients: 1-year results from a randomised controlled trial. Ann Rheum Dis. 2011;70:1798–803. doi: 10.1136/ard.2010.142018. [DOI] [PubMed] [Google Scholar]
- 77.Messier SP, Loeser RF, Miller GD, et al. Exercise and dietary weight loss in overweight and obese older adults with knee osteoarthritis: the Arthritis, Diet, and Activity Promotion Trial. Arthritis Rheum. 2004;50:1501–10. doi: 10.1002/art.20256. [DOI] [PubMed] [Google Scholar]
- 78.Messier SP, Nicklas BJ, Legault C, et al. The Intenstive Diet and Exercise for Arthritis Trial: 18-month clinical outcomes. Arthritis Rheum. 2011;63:S281. [Google Scholar]
- 79.Richette P, Poitou C, Garnero P, et al. Benefits of massive weight loss on symptoms, systemic inflammation and cartilage turnover in obese patients with knee osteoarthritis. Ann Rheum Dis. 2011;70:139–44. doi: 10.1136/ard.2010.134015. [DOI] [PubMed] [Google Scholar]
- 80.Riddle DL, Stratford PW. Body weight changes and corresponding changes in pain and function in persons with symptomatic knee osteoarthritis: A cohort study. Arthritis Care Res (Hoboken) 2013;65:15–22. doi: 10.1002/acr.21692. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 81.van Meurs JB, Uitterlinden AG, Stolk L, et al. A functional polymorphism in the catechol-O-methyltransferase gene is associated with osteoarthritis-related pain. Arthritis Rheum. 2009;60:628–9. doi: 10.1002/art.24175. [DOI] [PubMed] [Google Scholar]
- 82.Valdes AM, De Wilde G, Doherty SA, et al. The Ile585Val TRPV1 variant is involved in risk of painful knee osteoarthritis. Ann Rheum Dis. 2011;70:1556–61. doi: 10.1136/ard.2010.148122. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 83.Malfait AM, Seymour AB, Gao F, et al. A role for PACE4 in osteoarthritis pain: evidence from human genetic association and null mutant phenotype. Ann Rheum Dis. 2012;71:1042–8. doi: 10.1136/annrheumdis-2011-200300. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 84.Valdes AM, Arden NK, Vaughn FL, et al. Role of the Nav1. 7 R1150W amino acid change in susceptibility to symptomatic knee osteoarthritis and multiple regional pain. Arthritis Care Res (Hoboken) 2011;63:440–4. doi: 10.1002/acr.20375. [DOI] [PubMed] [Google Scholar]
- 85.Sorge RE, Trang T, Dorfman R, et al. Genetically determined P2X7 receptor pore formation regulates variability in chronic pain sensitivity. Nat Med. 2012;18:595–9. doi: 10.1038/nm.2710. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 86.Gold MS, Flake NM. Inflammation-mediated hyperexcitability of sensory neurons. Neurosignals. 2005;14:147–57. doi: 10.1159/000087653. [DOI] [PubMed] [Google Scholar]
- 87.Hucho T, Levine JD. Signaling pathways in sensitization: toward a nociceptor cell biology. Neuron. 2007;55:365–76. doi: 10.1016/j.neuron.2007.07.008. [DOI] [PubMed] [Google Scholar]
- 88.Woolf CJ, Ma Q. Nociceptors-noxious stimulus detectors. Neuron. 2007;55:353–64. doi: 10.1016/j.neuron.2007.07.016. [DOI] [PubMed] [Google Scholar]
- 89.Woolf CJ, Salter MW. Neuronal plasticity: increasing the gain in pain. Science. 2000;288:1765–9. doi: 10.1126/science.288.5472.1765. [DOI] [PubMed] [Google Scholar]
- 90.Woolf CJ. Evidence for a central component of post-injury pain hypersensitivity. Nature. 1983;306:686–8. doi: 10.1038/306686a0. [DOI] [PubMed] [Google Scholar]
- 91.Kilo S, Schmelz M, Koltzenburg M, Handwerker HO. Different patterns of hyperalgesia induced by experimental inflammation in human skin. Brain. 1994;117 (Pt 2):385–96. doi: 10.1093/brain/117.2.385. [DOI] [PubMed] [Google Scholar]
- 92.Koltzenburg M. Neural mechanisms of cutaneous nociceptive pain. Clin J Pain. 2000;16:S131–8. doi: 10.1097/00002508-200009001-00004. [DOI] [PubMed] [Google Scholar]
- 93.Schaible HG, Ebersberger A, Von Banchet GS. Mechanisms of pain in arthritis. Ann N Y Acad Sci. 2002;966:343–54. doi: 10.1111/j.1749-6632.2002.tb04234.x. [DOI] [PubMed] [Google Scholar]
- 94.Bajaj P, Bajaj P, Graven-Nielsen T, Arendt-Nielsen L. Osteoarthritis and its association with muscle hyperalgesia: an experimental controlled study. Pain. 2001;93:107–14. doi: 10.1016/S0304-3959(01)00300-1. [DOI] [PubMed] [Google Scholar]
- 95.Bradley LA, Kersh BC, DeBerry JJ, Deutsch G, Alarcon GA, McLain DA. Lessons from fibromyalgia: abnormal pain sensitivity in knee osteoarthritis. Novartis Found Symp. 2004;260:258–70. discussion 70–9. [PubMed] [Google Scholar]
- 96.Imamura M, Imamura ST, Kaziyama HH, et al. Impact of nervous system hyperalgesia on pain, disability, and quality of life in patients with knee osteoarthritis: a controlled analysis. Arthritis Rheum. 2008;59:1424–31. doi: 10.1002/art.24120. [DOI] [PubMed] [Google Scholar]
- 97.Lee YC, Lu B, Bathon JM, et al. Pain sensitivity and pain reactivity in osteoarthritis. Arthritis Care Res (Hoboken) 2011 doi: 10.1002/acr.20373. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 98.Wessel J. The reliability and validity of pain threshold measurements in osteoarthritis of the knee. Scand J Rheumatol. 1995;24:238–42. doi: 10.3109/03009749509100881. [DOI] [PubMed] [Google Scholar]
- 99.Arendt-Nielsen L, Nie H, Laursen MB, et al. Sensitization in patients with painful knee osteoarthritis. Pain. 2010;149:573–81. doi: 10.1016/j.pain.2010.04.003. [DOI] [PubMed] [Google Scholar]
- 100.Suokas AK, Walsh DA, McWilliams DF, et al. Quantitative sensory testing in painful osteoarthritis: a systematic review and meta-analysis. Osteoarthritis Cartilage. 2012;20:1075–85. doi: 10.1016/j.joca.2012.06.009. [DOI] [PubMed] [Google Scholar]
- 101.Vos T, Flaxman AD, Naghavi M, et al. Years lived with disability (YLDs) for 1160 sequelae of 289 diseases and injuries 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2163–96. doi: 10.1016/S0140-6736(12)61729-2. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 102.Murray CJ, Vos T, Lozano R, et al. Disability-adjusted life years (DALYs) for 291 diseases and injuries in 21 regions, 1990–2010: a systematic analysis for the Global Burden of Disease Study 2010. Lancet. 2012;380:2197–223. doi: 10.1016/S0140-6736(12)61689-4. [DOI] [PubMed] [Google Scholar]
- 103.Thomas E, Peat G, Mallen C, et al. Predicting the course of functional limitation among older adults with knee pain: do local signs, symptoms and radiographs add anything to general indicators? Ann Rheum Dis. 2008;67:1390–8. doi: 10.1136/ard.2007.080945. [DOI] [PubMed] [Google Scholar]
- 104.Creamer P, Lethbridge-Cejku M, Hochberg MC. Factors associated with functional impairment in symptomatic knee osteoarthritis. Rheumatology (Oxford) 2000;39:490–6. doi: 10.1093/rheumatology/39.5.490. [DOI] [PubMed] [Google Scholar]
- 105.Zhang Y, Niu J, Kelly-Hayes M, Chaisson CE, Aliabadi P, Felson DT. Prevalence of symptomatic hand osteoarthritis and its impact on functional status among the elderly: The Framingham Study. Am J Epidemiol. 2002;156:1021–7. doi: 10.1093/aje/kwf141. [DOI] [PubMed] [Google Scholar]
- 106.Centers for Disease Control and Prevention. Prevalence and Most Common Causes of Disability Among Adults — United States, 2005. MMWR. 2009;58:421–6. [PubMed] [Google Scholar]
- 107.Al-Zahrani KS, Bakheit AM. A study of the gait characteristics of patients with chronic osteoarthritis of the knee. Disabil Rehabil. 2002;24:275–80. doi: 10.1080/09638280110087098. [DOI] [PubMed] [Google Scholar]
- 108.White DK, Niu J, Zhang Y. Is symptomatic knee osteoarthritis a risk factor for a fast decline in gait speed? Results from the Osteoarthritis Initiative. Arthritis Care Res. 2012 doi: 10.1002/acr.21816. Online first 16 Aug 2012. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 109.Wilkie R, Peat G, Thomas E, Croft P. Factors associated with restricted mobility outside the home in community-dwelling adults ages fifty years and older with knee pain: an example of use of the International Classification of Functioning to investigate participation restriction. Arthritis Rheum. 2007;57:1381–9. doi: 10.1002/art.23083. [DOI] [PubMed] [Google Scholar]
- 110.Gabriel SE, Crowson CS, Campion ME, O’Fallon WM. Direct medical costs unique to people with arthritis. J Rheumatol. 1997;24:719–25. [PubMed] [Google Scholar]
- 111.Maetzel A, Li LC, Pencharz J, Tomlinson G, Bombardier C. The economic burden associated with osteoarthritis, rheumatoid arthritis, and hypertension: a comparative study. Ann Rheum Dis. 2004;63:395–401. doi: 10.1136/ard.2003.006031. [DOI] [PMC free article] [PubMed] [Google Scholar]
- 112.United States Bone and Joint Initiative. The Burden of Musculoskeletal Diseases in the United States. 2. Rosemont, IL: American Academy of Orthopaedic Surgeons; 2011. [Google Scholar]
- 113.Cherry DK, Woodwell DA, Rechsteiner EA. National Ambulatory Medical Care Survey: 2005 Summary. Advance data from vital and health statistics. Hyattsville, MD: National Center for Health Statistics; 2007. [PubMed] [Google Scholar]
- 114.Wier LM, Thompson Reuters, Andrews RM., AHRQ . HCUP Statistical Brief #107. Agency for Healthcare Research and Quality; Rockville, MD: 2011. The National Hospital Bill: The Most Expensive Conditions by Payer, 2008. [PubMed] [Google Scholar]
- 115.Kurtz S, Mowat F, Ong K, Chan N, Lau E, Halpern M. Prevalence of primary and revision total hip and knee arthroplasty in the United States from 1990 through 2002. J Bone Joint Surg Am. 2005;87:1487–97. doi: 10.2106/JBJS.D.02441. [DOI] [PubMed] [Google Scholar]
- 116.DeFrances CJ, Podgornik MN. 2004 National Hospital Discharge Survey. Advance data from vital and health statistics; no 371. Hyattsville, MD: National Center for Health Statistics; 2006. [PubMed] [Google Scholar]
- 117.Kurtz S, Ong K, Lau E, Mowat F, Halpern M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J Bone Joint Surg Am. 2007;89:780–5. doi: 10.2106/JBJS.F.00222. [DOI] [PubMed] [Google Scholar]
- 118.Berger A, Bozic K, Stacey B, Edelsberg J, Sadosky A, Oster G. Patterns of pharmacotherapy and health care utilization and costs prior to total hip or total knee replacement in patients with osteoarthritis. Arthritis Rheum. 2011;63:2268–75. doi: 10.1002/art.30417. [DOI] [PubMed] [Google Scholar]
- 119.Hermans J, Koopmanschap MA, Bierma-Zeinstra SM, et al. Productivity costs and medical costs among working patients with knee osteoarthritis. Arthritis Care Res (Hoboken) 2012;64:853–61. doi: 10.1002/acr.21617. [DOI] [PubMed] [Google Scholar]
- 120.Xie F. The need for standardization: a literature review of indirect costs of rheumatoid arthritis and osteoarthritis. Arthritis Rheum. 2008;59:1027–33. doi: 10.1002/art.23825. [DOI] [PubMed] [Google Scholar]
- 121.Buckwalter JA, Saltzman C, Brown T. The impact of osteoarthritis: implications for research. Clin Orthop Relat Res. 2004:S6–15. doi: 10.1097/01.blo.0000143938.30681.9d. [DOI] [PubMed] [Google Scholar]
- 122.Berger A, Hartrick C, Edelsberg J, Sadosky A, Oster G. Direct and indirect economic costs among private-sector employees with osteoarthritis. J Occup Environ Med. 2011;53:1228–35. doi: 10.1097/JOM.0b013e3182337620. [DOI] [PubMed] [Google Scholar]
- 123.Hubertsson J, Petersson IF, Thorstensson CA, Englund M. Risk of sick leave and disability pension in working-age women and men with knee osteoarthritis. Ann Rheum Dis. 2012 doi: 10.1136/annrheumdis-2012-201472. Online first. [DOI] [PubMed] [Google Scholar]
- 124.Bieleman HJ, Bierma-Zeinstra SM, Oosterveld FG, Reneman MF, Verhagen AP, Groothoff JW. The effect of osteoarthritis of the hip or knee on work participation. J Rheumatol. 2011;38:1835–43. doi: 10.3899/jrheum.101210. [DOI] [PubMed] [Google Scholar]
- 125.Kotlarz H, Gunnarsson CL, Fang H, Rizzo JA. Osteoarthritis and absenteeism costs: evidence from US National Survey Data. J Occup Environ Med. 2010;52:263–8. doi: 10.1097/JOM.0b013e3181cf00aa. [DOI] [PubMed] [Google Scholar]