Abstract
During passage through the female reproductive tract, mammalian sperm undergo a maturation process termed capacitation that renders sperm competent to produce fertilization. Capacitation involves a sequence of changes in biochemical and electrical properties, the onset of a hyperactivated swimming behavior, and development of the ability to undergo successful fusion and penetration with an egg. In mouse sperm, the development of hyperactivated motility is dependent on cytosolic alkalization that then results in an increase in cytosolic Ca2+. The elevation of Ca2+ is thought to be primarily driven by the concerted interplay of two alkalization-activated currents, a K+ current (KSPER) composed of pore-forming subunits encoded by the Kcnu1 gene (also termed Slo3) and a Ca2+ current arising from a family of CATSPER subunits. After deletion of any of four CATSPER subunit genes (CATSPER1–4), the major remaining current in mouse sperm is alkalization-activated KSPER current. After genetic deletion of the Slo3 gene, KSPER current is abolished, but there remains a small voltage-activated K+ current hypothesized to reflect monovalent flux through CATSPER. Here, we address two questions. First, does the residual outward K+ current present in the Slo3 −/− sperm arise from CATSPER? Second, can any additional membrane K+ currents be detected in mouse sperm by patch-clamp methods other than CATSPER and KSPER? Here, using mice bred to lack both SLO3 and CATSPER1 subunits, we show conclusively that the voltage-activated outward current present in Slo3 −/− sperm is abolished when CATSPER is also deleted. Any leak currents that may play a role in setting the resting membrane potential in noncapacitated sperm are likely smaller than the pipette leak current and thus cannot be resolved within the limitation of the patch-clamp technique. Together, KSPER and CATSPER appear to be the sole ion channels present in mouse sperm that regulate membrane potential and Ca2+ influx in response to alkalization.
INTRODUCTION
After ejaculation, mammalian sperm must undergo a maturational process termed capacitation to acquire competence to fertilize an egg (Darszon et al., 2007). Capacitation entails an extensive spectrum of fundamental changes in sperm properties, including biochemical, electrical, and motile (Visconti and Kopf, 1998; Visconti et al., 1998; Darszon et al., 2011). One important and visually obvious step in sperm maturation is the acquisition of a hyperactivated motility, which is thought both to facilitate movement of sperm through the female reproductive tract and, subsequently, to enable successful penetration by sperm of protective layers surrounding an egg (Suarez, 2008). Although the full set of endogenous signals that lead to capacitation and hyperactivation is not entirely understood (Visconti et al., 2002; Fraser et al., 2006), an important step in the process by which mouse sperm acquire hyperactivated motility is cytosolic alkalization (Ho and Suarez, 2001; Suarez, 2008). Alkalization gradually arises, in part, from relocation of sperm from the acidic environment of the epididymis to a more alkaline environment of the female reproductive tract (Kirichok and Lishko, 2011), with proton reequilibration, at least in humans, perhaps being mediated by proton channels (Lishko et al., 2010). Importantly, alkalization is associated with an increase in cytosolic Ca2+ (Wennemuth et al., 2003; Darszon et al., 2005). Together, the rise in pH and [Ca2+]i are the cytosolic signals essential for initiation of hyperactivation (Suarez, 2008).
An extensive body of early work has supported the view that activation of a combination of voltage-dependent Ca2+ channels and K+ channels played a role in controlling sperm membrane potential and [Ca2+]i (Darszon et al., 1999). However, despite an extensive list of proposed sperm channel candidates (Darszon et al., 1999, 2006), the molecular identity of specific channels that might mediate these effects remained elusive until the last 10 years. With the advent of direct patch-clamp recording from sperm and specific genetic knockout (KO) of ion channel subunits (Kirichok and Lishko, 2011; Lishko et al., 2013), it has been demonstrated from work on mouse sperm that the linkage of cytosolic alkalization and Ca2+ elevation involves at least two sperm-specific ion channels: the Ca2+-permeable CATSPER current (Ren et al., 2001; Kirichok et al., 2006; Navarro et al., 2008; Kirichok and Lishko, 2011) and the K+-permeable KSPER K+ current (Navarro et al., 2007; Zeng et al., 2011). KSPER and CATSPER are thought to work in concert during alkalization, with KSPER activation helping to maintain a negative sperm membrane potential (Vm) sufficient to promote influx of Ca2+ through CATSPER channels (Navarro et al., 2007). KSPER activation is the primary or only determinant of Vm of mouse spermatozoa during alkalization (Navarro et al., 2007; Zeng et al., 2011), serving to drive sperm Vm more negative than −40 mV. Simultaneous with the KSPER-mediated hyperpolarization, activation of CATSPER during alkalization (Ren et al., 2001; Kirichok et al., 2006) mediates influx of Ca2+ necessary for activation of Ca2+-dependent processes required for sperm hyperactivation. CATSPER is thought to be assembled from a set of four distinct CATSPER subunits (CATSPER1–4; Carlson et al., 2003; Quill et al., 2003; Jin et al., 2005) and at least three accessory subunits (β, γ, and δ; Liu et al., 2007; Wang et al., 2009; Chung et al., 2011). Genetic deletion of any of the four CATSPER subunits (Qi et al., 2007) or the δ subunit (Chung et al., 2011) abolishes sperm hyperactivated motility and results in male infertility. In fact, the initial demonstration of KSPER current in mouse sperm benefited from the use of CATSPER1-null sperm (Navarro et al., 2007). KSPER activation increases over the range of pH 6.0–8.0, allowing it to play a major hyperpolarizing role during alkalization. The identity of the critical pore-forming subunit of KSPER was recently established, with the demonstration that genetic KO in mice of the pH-regulated SLO3 K+ channel (Santi et al., 2010; Zeng et al., 2011) reduces or abolishes KSPER and results in infertile male mice. Supporting the idea that KSPER encoded by the Slo3 gene plays a predominant role in defining sperm Vm during capacitation, a SLO3-dependent progressive increase in hyperpolarization measured by voltage-sensitive dyes during exposure to capacitating conditions has been observed in mouse sperm (Chávez et al., 2013).
Together, CATSPER and KSPER appear to be the primary participants in regulation of sperm Vm and Ca2+ influx during alkalization (Kirichok et al., 2006; Navarro et al., 2007; Zeng et al., 2011). However, it is possible that other unidentified currents may be present either in resting conditions or after alkalization. In fact, after genetic deletion of SLO3, although most outward K+ current was abolished, voltage steps above ∼100 mV result in a slowly activating increase in outward current (Zeng et al., 2011). This current was proposed to arise from monovalent cation efflux through CATSPER channels, but this proposal requires confirmation. Here, by generating animals in which both Slo3 and CatSper1 genes have been disrupted, we explicitly tested whether the residual K+ current in Slo3 −/− sperm cells can be explained by CATSPER and whether additional currents can be identified in mouse sperm cells. In the absence of both CATSPER and KSPER, we were unable to detect any residual K+ current, either under resting conditions at pH 6.0 or during alkalization at pH 8.0. Although other factors or ligands may result in activation of ion channels not observed here, it is remarkable the extent to which only two ion channels may account for all of the changes in Vm and Ca2+ elevation that are associated with the hyperactivation process.
MATERIALS AND METHODS
Generation of double KO (dKO) mice
Both Slo3 −/− and CatSper1 −/− male mice are infertile, whereas females are reproductively normal. Generation of the dKO mice required, first, generation of Slo3 +/−CatSper1 +/− males, followed by breeding of the heterozygous males with Slo3 −/−CatSper1 −/− females. Such a mating is expected to result in full dKOs in ∼25% of pups. Genotypes of all animals were confirmed by PCR. All animal husbandry and experimental procedures were approved by and performed in accordance with guidelines of the Animal Studies Committee of the Washington University in St. Louis School of Medicine.
Electrophysiology
Before whole-cell access, for the dKO cells studied here, about half had pipette seal resistances between 5 and 10 GΩ, with the others >10 GΩ. Thus, pipette leak conductance (GL) is typically ≤0.2 nS, but on average ∼0.1 nS. In all whole-cell recordings, cytosolic access was gained through the sperm cytosolic droplet (Kirichok et al., 2006; Navarro et al., 2007; Qi et al., 2007). HEPES-buffered saline (HS) was used for sperm swim-out from corpus epididymis and recordings: 135 mM NaCl, 5 mM KCl, 1 mM MgSO4, 2 mM CaCl2, 20 mM HEPES, 5 mM glucose, 10 mM lactic acid, and 1 mM Na pyruvate, pH 7.4 with NaOH. For most voltage-clamp experiments, cells were bathed in a high K+ HS saline: 160 mM KOH, 10 mM HEPES, 150 mM methanesulfonic acid (MES), and 2 mM Ca(MES)2, adjusted to pH 7.4 with MES. The usual high K+ pipette solution contained 155 mM KOH, 5 mM KCl, 10 mM BAPTA, 20 mM HEPES, and 115 mM MES with pH adjusted to 6.0, 7.0, or 8.0 with KOH or MES. For current clamp recordings in which NH4Cl was used to alter cytosolic pH, the following pipette solution was used: 144 mM KOH, 5 mM KCl, 5 mM NaCl, 3 mM MgATP, 0.5 mM Na2GTP, 1 mM BAPTA, 5 mM HEPES, and 140 mM MES with pH adjusted to either 6.0 or 8.0 with MES or KOH. For testing for the presence of Ca2+-activated K+ solutions, a K-MES cytosolic solution with 10 µM Ca2+ contained 140 mM K-MES, 20 mM KOH, 10 mM HEPES (titrated to pH 7.0), and 5 mM HEDTA titrated with Ca-MES to obtain 10 µM Ca2+ as defined by a Ca2+-sensitive electrode calibrated with a commercial set of Ca2+ standards (World Precision Instruments). Solutions were applied directly via a local perfusion system allowing switching between different test solutions. Solution exchange time with this system is typically <1 s. Current waveforms were analyzed with Clampfit (Molecular Devices). Points and error bars on figures correspond to mean ± SEM. All experiments were conducted at room temperature (∼22–25°C). Chemicals were obtained from Sigma-Aldrich.
RESULTS
Simultaneous deletion of Slo3 and CatSper1 abolishes all voltage- and alkalization-activated current
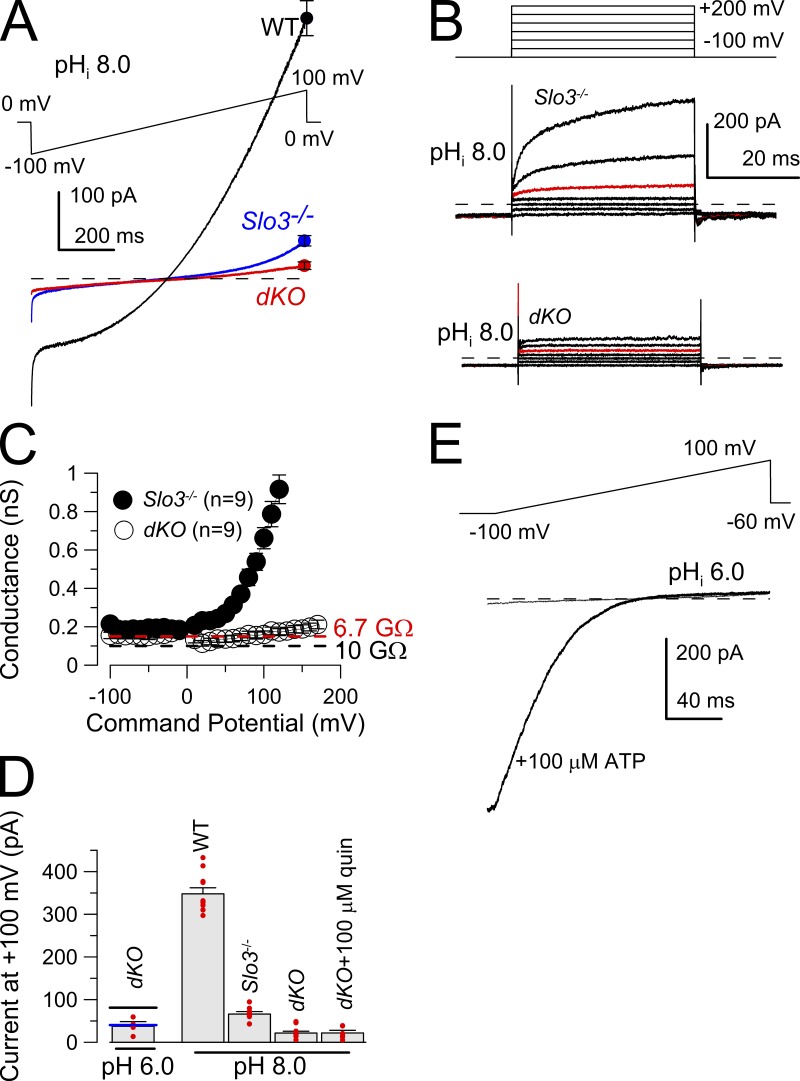
Whole-cell recordings from the cytoplasmic droplet were used to compare K+ currents in WT, Slo3 −/−, and Slo3 −/−CatSper1 −/− (dKO) mouse spermatozoa. With symmetrical K+ solutions and a pipette solution of pH 8.0, WT sperm exhibit a pronounced voltage-dependent K+ conductance (Fig. 1 A), consistent with the previously described KSPER current (Navarro et al., 2007). In Slo3 −/− sperm, total K+ current was markedly reduced (Fig. 1 A), with some small residual voltage-dependent K+ current that becomes particularly apparent at potentials >100 mV (Fig. 1 B; Zeng et al., 2011). It has been previously proposed that the residual K+ current in Slo3 −/− sperm arises from monovalent cation efflux through CATSPER channels (Zeng et al., 2011). Consistent with this suggestion, voltage ramp protocols (Fig. 1 A) and steps (Fig. 1 B) applied to dKO sperm failed to activate any voltage-dependent K+ current despite a pipette pH of 8.0. For a set of Slo3 −/− sperm and dKO sperm studied with the voltage step protocol, the K+ conductance at potentials negative to 0 mV was <0.2 nS for both Slo3 −/− and dKO sperm (Fig. 1 C). For the dKO sperm, this conductance corresponds to a mean resistance of 6.7 GΩ (Fig. 1 C, dashed red line). In contrast, at positive potentials, net conductance markedly differed between the two. We are unable to exclude the possibility that there may be a very small increase in conductance in the dKO sperm at potentials >100 mV. However, command steps to such potentials may increase conductance from compromised recording stability. Given an ∼0.1–0.2-nS conductance (GL) of a 5–10-GΩ pipette seal, we were unable to detect any residual K+ conductance over the range of voltages from −100 to 100 mV in the dKO sperm that was separable from that expected from GL. For the three genotypes, net current at 100 mV was compared (Fig. 1 D). The results indicate that the alkalization-activated current, still present at very positive potentials in the Slo3 −/− sperm, is absent in the dKO sperm. Thus, the alkalization-activated current in the Slo3 −/− sperm arises from monovalent cation flux through CATSPER channels. Furthermore, after deletion of both SLO3 and CATSPER subunits, no remaining alkalization-activated K+ current was detected. Despite the absence of alkalization-activated K+ current in the dKO sperm, application of 100 µM of extracellular ATP to dKO sperm elicited a desensitizing, reversible, rectifying conductance (Fig. 1 E) in the dKO sperm, consistent with the previous observation of a P2X2 current in mouse sperm (Navarro et al., 2011).
Figure 1.
Alkalization-activated K+ current is absent in sperm from CatSper1 −/−Slo3 −/− (dKO) mice. (A) The indicated 1-s voltage ramp was used to activate current from WT, Slo3 −/−, and dKO spermatozoa. Each trace is the mean of currents (point corresponds to mean ± SEM at 100 mV) activated by the ramp protocol in six sperm of each genotype. Pipette pH was 8.0; intracellular and extracellular salines each contained 160 mM K-MES, with 2 mM extracellular Ca2+. (B) The indicated voltage step protocol was applied to a Slo3 −/− sperm and a dKO sperm, with the red traces being the step to 100 mV. (C) Currents activated with the protocol shown in B were converted to conductances for nine Slo3 −/− sperm and nine dKO sperm. The red dashed line corresponds to the mean of conductances in the dKO sperm over the range of −100 to 100 mV and equates to a resistance of 6.7 GΩ. The black dashed line indicates a 0.1-nS conductance, typical for the seal conductance for a 10-GΩ pipette seal. Pipette saline was pH 8.0. Error bars are SEM. (D) Mean current (error bars are SEM) measured at 100 mV is compared for the three genotypes, along with individual estimates (red circles). Values for Slo3 −/− sperm differ significantly (P < 0.003) from those of dKO sperm. The horizontal lines associated with the pH 6.0 estimates for the dKO sperm correspond to earlier mean current measurements for WT (black; 81.8 ± 7.9 pA [Zeng et al., 2011]) and Slo3 −/− (blue; 40.3 ± 2.9 pA [Zeng et al., 2011]) sperm. (E) The indicated 200-ms voltage ramp was used to activate current from a dKO spermatozoa, before and during application of 100 µM ATP. Pipette solution: pH 6.0. Extracellular: HS solution. (A, B, and E) The dashed lines represent 0 current level.
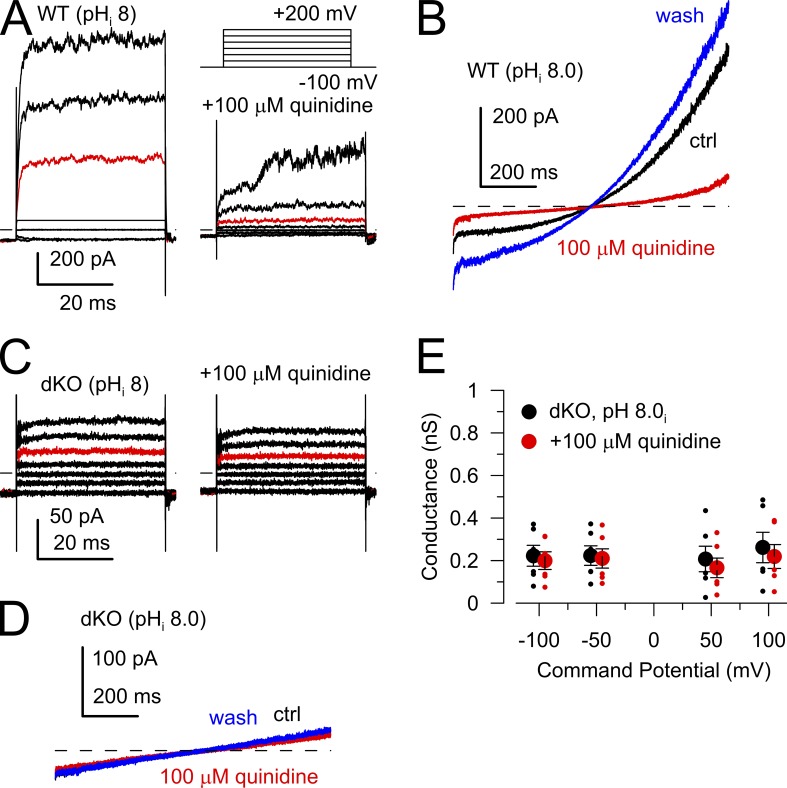
The small residual conductance in the dKO sperm is certainly dominated by GL but might also include some small contribution of membrane ion channels, which might be important in intact sperm when KSPER and CATSPER are not activated. As an additional test of the nature of any conductance that might still be present in dKO sperm with pipette pH 8.0, we used quinidine, a rather general ion channel blocker which has been shown to block SLO3 currents (>90% inhibition at 100 µM quinidine at >100 mV; Tang et al., 2010; Zeng et al., 2011) as well as CATSPER (Zeng et al., 2011). In addition, quinidine also inhibits two pore leak channels (e.g., TASK2: 65% inhibition at 100 µM [Reyes et al., 1998]; and TASK3: 37% block by 100 µM [Kim et al., 2000]) that have also been proposed as possible sperm ion channels (Barfield et al., 2005), in addition to Kv channels (Fedida, 1997; Wang et al., 2003). Thus, because of the general effectiveness of quinidine on a host of cation channels, any sensitivity of the residual currents in the dKO sperm to quinidine might suggest the presence of additional types of channels. In WT sperm, 100 µM quinidine substantially inhibits both outward and inward currents recorded in symmetric K+ solutions with pipette pH of 8.0 (Fig. 2, A and B). This reflects inhibition of both KSPER and CATSPER currents (Zeng et al., 2011). Note that the residual conductance in WT sperm at negative potentials after application of 100 µM quinidine approaches that of the dKO sperm (Fig. 2, B and D). When 100 µM quinidine was applied to dKO sperm at pHi 8.0 (Figs. 1 D and 2, C and D), the residual current was little affected. The effects of 100 µM quinidine on conductances measured from −100 to 100 mV were determined for six dKO sperm (Fig. 2 E). Individual sperm in some cases exhibited small decreases in current during application of quinidine (e.g., Fig. 2 C). Although the differences measured over the set of cells was not significant, the mean conductance in the presence of quinidine (Fig. 2 C) corresponds to a decrease of 12.7 ± 4.9% compared with control levels. We conclude that quinidine-sensitive ion channels make minor contributions, if any, to the residual conductance of dKO sperm.
Figure 2.
Residual current in dKO sperm is insensitive to 100 µM quinidine. (A) Currents were activated in a WT sperm with the indicated voltage protocol with symmetrical K+ gradients and pHi of 8.0. (left) Control saline. (right) 100 µM quinidine. Red traces correspond to 100-mV step. (B) In another WT sperm, the ±100-mV voltage ramp was used to activate currents before, during, and after application of 100 µM quinidine (red), highlighting effects of quinidine at more negative voltages. (C) Currents were activated in a dKO sperm as in A without (left) and with (right) 100 µM quinidine. Red traces correspond to 100 mV. (D) Ramp-activated currents in a dKO spermatozoa were monitored before, during (red), and after 100 µM quinidine. (A–D) The dashed lines represent 0 current level. (E) Mean conductances were calculated at the indicated voltages for six dKO sperm without (black) and with (red) 100 µM quinidine. There were no significant differences between groups. Error bars indicate SEM.
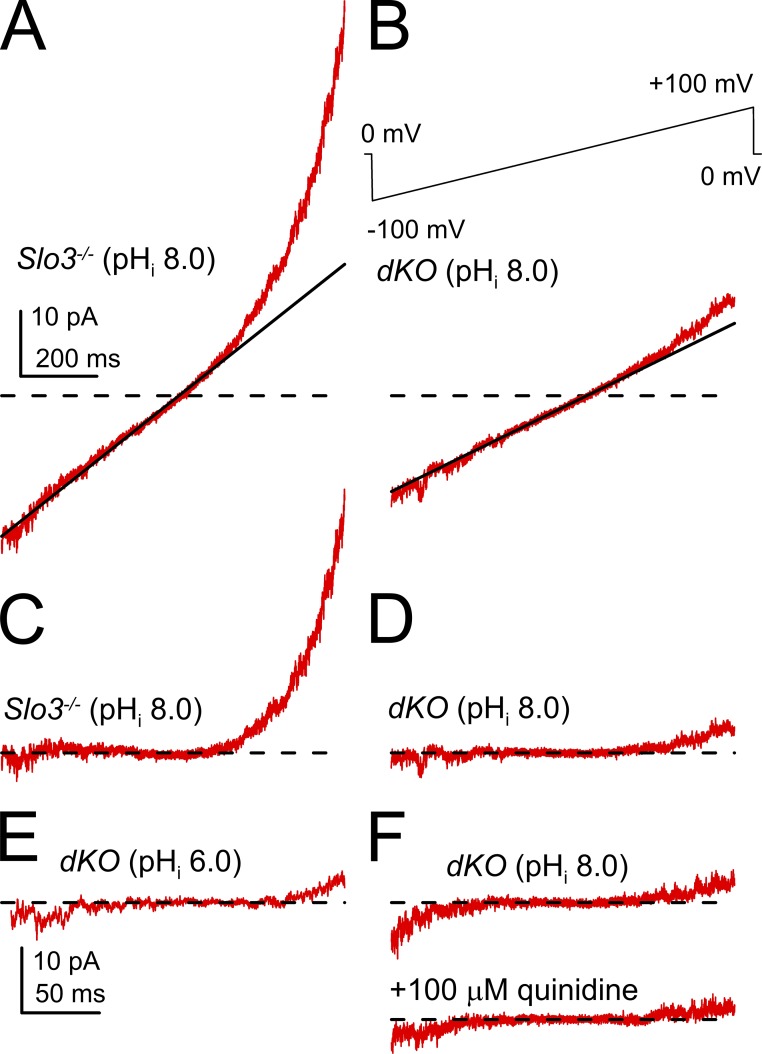
As a final evaluation of this issue, we examined ramp-activated currents over the range of −90 to 90 mV at high gain (Fig. 3). Such traces were filtered at 2 kHz, and a linear leak current (Fig. 3, A and B) defined by the slope of the currents over the range of −30 to 30 mV was subtracted from the records. According to this procedure, asymmetries around the 0 current level would be potentially indicative of additional voltage-dependent conductances. Close inspection of raw traces suggested that in the dKO sperm, some sperm exhibited a small nonlinear increase in current at both the most negative and most positive voltages (Fig. 3, A and B). After filtering and subtraction, currents from Slo3 −/− sperm at pHi 8.0 (Fig. 3 C) show the upward curvature associated with monovalent flux through CATPSER channels, while also showing a small asymmetric increase in current variance at the most negative potentials. For dKO sperm at pHi 8.0 (Fig. 3 D), there remained some small asymmetric current at both positive and negative potentials, with the currents at negative potentials indistinguishable from Slo3 −/− sperm and from those in dKO sperm at pHi 6.0 (Fig. 3 E). Whatever the origins of this asymmetric increase in current, it is not only insensitive to pH but also to 100 µM quinidine (Fig. 3 F). We suspect that the small asymmetric currents at very negative and positive potentials may reflect recording instabilities (i.e., unstable seal leak conductance).
Figure 3.
Examples of ramp-activated currents recorded at high gain. (A) Currents activated over the range of −90 to 90 mV are shown at high gain for one sperm. The line corresponds to a linear fit to current over the range of −30 to 30 mV. (B) Similar currents and the linear fit are shown for a dKO sperm. The full ramp protocol is shown on the top. Currents were filtered at 2 kHz. (C) The sweep shows currents from the Slo3 −/− sperm with pHi 8.0 in A, but after subtraction of the linear component. The time base in A also applies to B–D and F. (D) Subtracted currents from the dKO sperm in B with pHi 8.0. (E) Filtered subtracted currents from one dKO sperm with pHi 6.0. (F) Currents from a dKO sperm at pHi 8.0 before and during application of 100 µM quinidine. The dashed lines represent 0 current level.
The resting potential of dKO sperm measured by patch-clamp is insensitive to changes in cytosolic pH
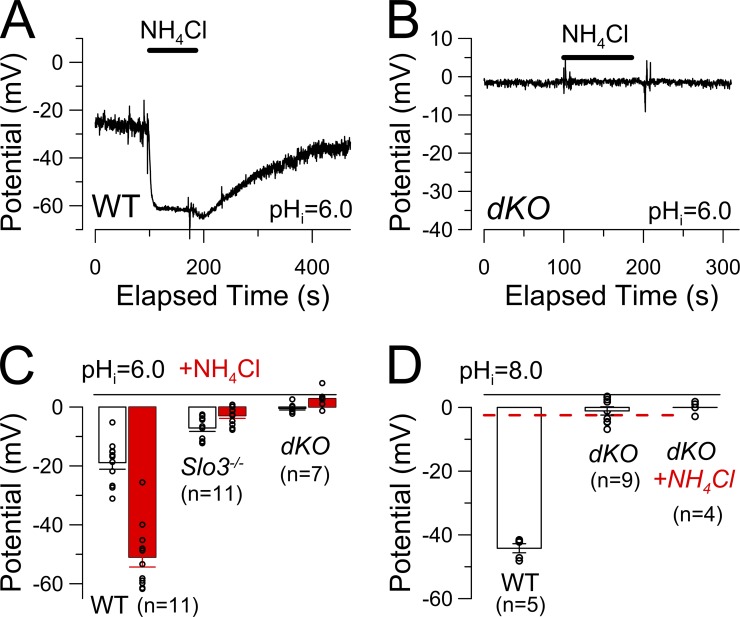
Activation of KSPER is sufficient to drive Vm changes during cytosolic alkalization in mouse spermatozoa (Navarro et al., 2007; Zeng et al., 2011). In WT sperm with physiological cation gradients, NH4Cl-mediated alkalization typically results in a pronounced hyperpolarization (Fig. 4 A), whereas in Slo3 −/− sperm, alkalization produces a small depolarization presumably because of CATSPER activation (Zeng et al., 2011). In current clamp experiments with physiologically normal Na+o/K+i cation gradients, we measured membrane potentials in dKO sperm during NH4Cl application (Fig. 4 B). With a pipette pH of 6.0, Vm in dKO sperm was indistinguishable from 0 mV (Fig. 4 C) and NH4Cl induced no obvious changes in potential. Likewise, with an intracellular (pipette) solution of pH 8.0, the dKO sperm Vm was indistinguishable from 0 mV (Fig. 4 D), similar to earlier results for the Slo3 −/− KO sperm (Zeng et al., 2011). These results further support the idea that there are no alkalization-activated K+ conductances other than KSPER available to influence mouse sperm membrane potential during sperm alkalization. This conclusion must be tempered by the fact that any residual conductance, in order to influence Vm under our experimental conditions, would have to be an appreciable fraction of GL.
Figure 4.
Membrane potential in dKO sperm is close to 0 mV and unregulated by alkalization. (A) Membrane potential of WT mouse spermatozoa was recorded with physiological Na+/K+ gradients and a pipette pH of 6.0. Application of 10 mM NH4Cl resulted in a pronounced reversible hyperpolarization. (B) Application of 10 mM NH4Cl induced no change in membrane potential in a dKO spermatozoon. (C) Mean sperm membrane potentials with pipette pH 6.0 are plotted for WT and dKO genotypes before and during (red bars) application of 10 mM NH4Cl. (D) Mean sperm membrane potential with pipette pH 8.0 is plotted for WT and dKO sperm with the red dashed line showing values for Slo3 −/− sperm (−2.44 ± 1.2 mV) from Zeng et al. [2011]. (C and D) Error bars indicate SEM.
Elevated cytosolic Ca2+ does not activate additional conductances in dKO mouse sperm
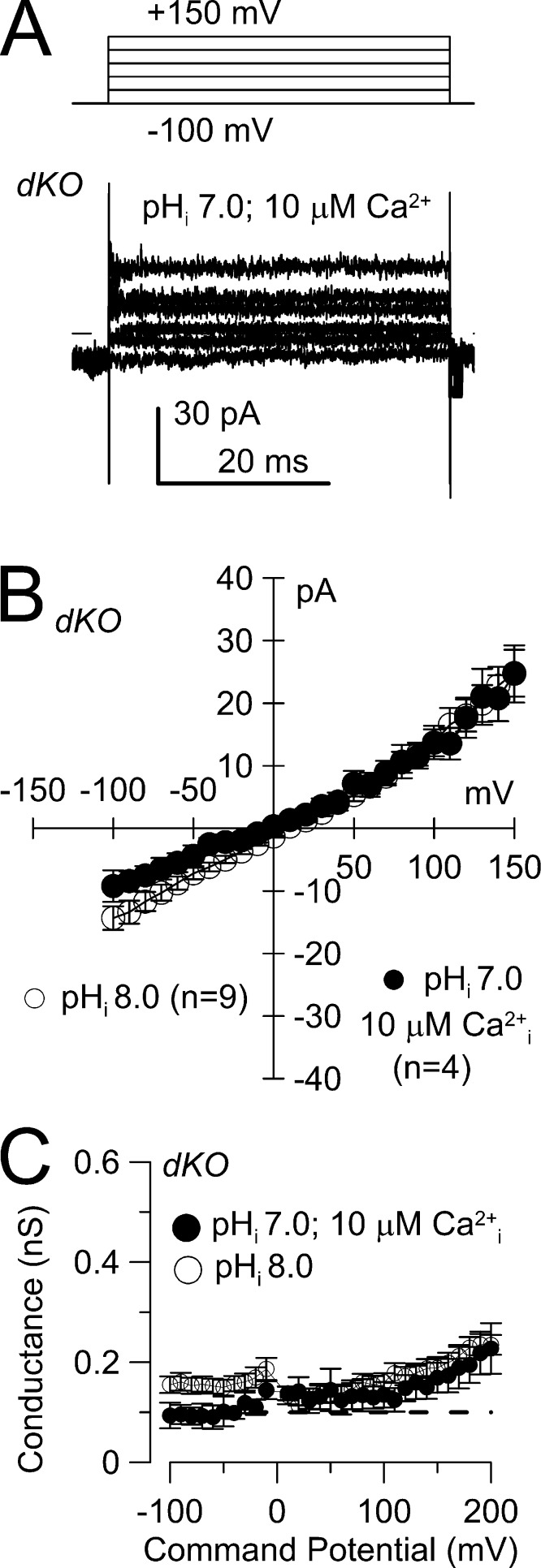
The absence of KSPER and CATSPER currents in the dKO sperm provide a useful background environment to test for the presence of other conductances. Given the elevations in cytosolic Ca2+ that occur during CATSPER activation (Carlson et al., 2003; Wennemuth et al., 2003), we tested for the presence of known Ca2+-activated K+ currents. We used a 10 µM Ca2+ pipette solution with symmetrical K+ to assess whether there might be any contributions of Ca2+-activated K+ conductances (Fig. 5, A and B). Such ionic conditions would be expected to be sufficient to result in activation of all known forms of Ca2+-activated K+ currents (Vergara et al., 1998), including the Ca2+- and voltage-dependent BK-type K+ channels, the voltage-independent small conductance SK-type type Ca2+-dependent channels, and intermediate conductance IK-type K+ channels. For four dKO sperm, the measured currents over the range of −100 to 100 mV were indistinguishable from currents recorded from dKO sperm with low cytosolic Ca2+ and pH 8.0 (Fig. 5 B). Based on the inability of 10 µM Ca2+ and positive voltages to increase K+ current in the dKO sperm, it is unlikely that any known Ca2+-dependent K+ currents are expressed in mouse sperm.
Figure 5.
Ca2+-dependent cation currents are not detected in mouse spermatozoa. (A) Currents were activated by the indicated voltage protocol in a dKO spermatozoon, with 10 µM pipette Ca2+, at pH 7.0, with symmetrical K+. The dashed line represents 0 current level. (B) Mean current (±SEM) activated as in A is plotted as a function of command voltage for four dKO sperm with 10 µM pipette Ca2+ at pHi 7.0. For comparison, currents measured in nine dKO sperm with the standard pipette solution (Fig. 1 C, dKO) buffered with 10 mM BAPTA at pHi 8.0 are also shown. (C) Assuming a 0-mV reversal potential, conductance was determined for the set of cells shown in B, for dKO cells at pHi 7.0 with 10 µM cytosolic Ca2+ and for dKO cells with pHi 8.0. The dashed line corresponds to a residual conductance of 0.1 nS.
DISCUSSION
Previous work on mouse sperm established that the alkalization-activated KSPER current accounts for most of the K+ current in CATSPER-deficient corpus epididymal sperm (Navarro et al., 2007). More recently, alkalization-activated K+ current in testicular sperm was shown to be reduced after Slo3 KO (Santi et al., 2010), and, independently, it was shown the Slo3 KO abolished all KSPER current in mouse corpus epididymal sperm (Zeng et al., 2011). Yet, at very positive potentials, a pharmacologically distinct, slowly activated outward current was present in the Slo3 KO spermatozoa (Zeng et al., 2011). Although we attributed this current to monovalent K+ efflux through CATSPER that persists despite the presence of 2 mM extracellular Ca2+, this result appeared to differ from an earlier result showing that, in cells with 140 mM Cs+ pipette solution, 2 mM extracellular Ca2+ largely prevented any outward current at positive potentials through CATSPER (Kirichok et al., 2006). Our present results now show unambiguously that the residual outward current flux present in Slo3 −/− sperm can only reflect K+ efflux through CATSPER channels. We would suggest that the differences in the effectiveness of 2 mM Ca2+ in reducing monovalent cation efflux probably arise from the differential relative permeabilities of K+ versus Cs+ and their ability to compete with Ca2+ that occupies CATPSER pore sites. This would be generally consistent with differences in relative permeabilities of K+ and Cs+ in classical studies of selectivity of Ca2+ selective channels (Hess et al., 1986). Despite the ability of K+ to permeate CATSPER channels in the presence of extracellular Ca2+ as shown by the present experiments, it should be noted that at potentials negative to 0 mV with physiological ionic gradients, K+ efflux will most likely be negligible. However, rigorous estimates of the selectivity of CATSPER channels to different ions will be required to fully address this topic.
Here we now assess the implications of these results for two situations, during alkalization and then under conditions of low cytosolic pH. The present results further support the view that, during alkalization, KSPER and CATSPER are the primary and perhaps the only two ionic currents present in the mouse corpus epididymal sperm principal piece. After deletion of both CATSPER and KSPER, the sperm membrane potential is indistinguishable from 0 mV at pH 8.0. The patch-clamp method as used here is unable to identify any other current other than KSPER and CATSPER that is activated by voltage and alkalization, and any residual current at pH 8.0 in the dKO sperm cannot be distinguished within the background of pipette leak conductance. Any minor K+ currents hidden in the pipette leak conductance would essentially be irrelevant to sperm function and physiology as long as CATSPER and KSPER remain activated by alkalization.
The situation under so-called resting conditions of lower pH is less clear. Although we have made measurements at pH 6.0, what resting conditions actually are when sperm first enter the female reproductive tract are not entirely clear. As with the results at pH 8.0, our recordings both in voltage-clamp and current-clamp are dominated by the pipette leak conductance. However, for the pH 6.0 condition, the possibility that there may be currents buried in the background pipette leak conductance is potentially more critical to sperm physiology. In a cell of high input impedance, even a mean net conductance corresponding to the amplitude of a single K+ channel might help define resting potential in an intact cell. Such an effect would not be detected in the present work. If we assume a nonselective GL of 0.1 nS (corresponding to a 10-GΩ pipette seal), an additional cell-specific K+ conductance that was, say, 20% of GL would be expected to produce a membrane potential of approximately −4 mV; for GK = 0.5 GL, the predicted Vm would be approximately −10 mV. Given that the present measurements report a Vm under physiological conditions in the dKO sperm indistinguishable from 0 mV, these considerations limit any residual GK in the dKO sperm to probably <0.02 nS (20 pS). Yet, in the absence of CATSPER or KSPER current and an added pipette leak conductance, a current of such a small size might still influence Vm.
The definition of sperm Vm under resting conditions is clearly problematic. Direct measurements with patch-clamp suffer from the limitation that pipette GL will dominate membrane potential measurements. Furthermore, the use of pH 6.0 is probably not an accurate surrogate for basal conditions. Indirect measures of Vm using membrane potential–sensitive dyes (Chávez et al., 2013), although of value in assessing relative changes in intact cells, have uncertainties in regards to absolute membrane potential levels and dye-partitioning issues. At pH 6.0, direct patch-clamp recordings from WT sperm have reported Vm of about −7 mV (Navarro et al., 2007) and −13 mV (Zeng et al., 2011). Here, for a set of WT sperm monitored under identical conditions, Vm was about −18 mV (Fig. 4 C), with notable variability in individual measurements. The variability in estimates is probably not surprising because both differences in GL among cells and variation in the SLO3 current density among cells might be expected to contribute to such differences. Both in previous work (Zeng et al., 2011) and here, Vm at pH 6.0 was somewhat depolarized in Slo3 −/− sperm relative to WT sperm. This supports the view that some weak activation of KSPER at pH 6.0 can influence Vm. However, recent results estimating Vm with potential-sensitive dyes in WT and Slo3 −/− sperm suggest both that SLO3 contributes little to Vm in mouse sperm under noncapacitated conditions and that such sperm have a measureable negative membrane potential arising from other, as yet unidentified K+ channels (Chávez et al., 2013). The present work suggests that if such currents are present in mouse sperm, they are invisible to the patch-clamp technique. To confirm the existence of such currents, molecular deletion of the relevant subunits will probably be required.
Despite the abundance of channels that have been proposed to be present in sperm (Barfield et al., 2005; Darszon et al., 2007), very few transporter/channel proteins have met the test of being indispensable to sperm function. To date, of ion transport proteins that have been proposed to be expressed in sperm, only genetic deletion of CATSPER-related subunits (Quill et al., 2003; Carlson et al., 2005; Jin et al., 2007; Qi et al., 2007; Chung et al., 2011), the SLO3 subunit (Santi et al., 2010; Zeng et al., 2011), a Ca-ATPase (PMCA4; Okunade et al., 2004; Schuh et al., 2004), a sperm-specific Na,K-ATPase α4 isoform (Jimenez et al., 2011), and a sperm-specific Na+-H+ exchanger (NHE5; Wang et al., 2003) have resulted in clear loss of mouse fertility in a fashion related to sperm function and not as the result of a developmental defect. It is notable that, of these five examples, four involve sperm-specific proteins, whereas PMCA4 has a broader tissue distribution. Given the pace of genetic deletions of various categories of ion channels, the absence of additional cases of loss of male fertility seems telling. Of course, if a channel/transporter important to sperm function was also more broadly expressed, the genetic deletion approach might result in deleterious consequences that preclude tests of sperm function and fertility.
In sum, under the conditions of the present experiments, we were unable to observe any conductance above the background of GL other than the cation fluxes arising from KSPER and CATSPER, either at pH 6.0 or pH 8.0. Furthermore, our tests with elevated pipette Ca2+ suggest that any Ca2+ elevation that would be expected to occur as a consequence of CATSPER activation would be unlikely to activate secondary K+ conductances, unless such conductances also require additional regulatory cofactors. Based on the observations presented here, once alkalization is initiated in mouse sperm, the critical ionic currents that control sperm Vm and the elevation of Ca2+ can be accounted for by only two channels, KSPER, arising from the expression of SLO3 subunits, and CATSPER. Because CATSPER is essentially the only current component present after genetic KO of the SLO3 subunit, the Slo3 −/− sperm may serve as a useful tool for investigation of a relatively uncontaminated CATSPER current, at least until successful heterologous expression of CATSPER channels is accomplished.
Acknowledgments
We thank the Department of Anesthesiology, Washington University in St. Louis School of Medicine, for their support of this project. We thank Drs. Chengtao Yang and Vivian Gonzalez-Perez for comments on the manuscript.
This work was also supported in part by National Institutes of Health (NIH) grant GM081748 to C.J. Lingle, Natural Science Foundation of China grants 31070767 and 31171116 to X.-H. Zeng, and NIH grant U01 HD045857 to D.E. Clapham.
Angus C. Nairn served as editor.
Footnotes
Abbreviations used in this paper:
- dKO
- double KO
- KO
- knockout
- MES
- methanesulfonic acid
References
- Barfield J.P., Yeung C.H., Cooper T.G. 2005. Characterization of potassium channels involved in volume regulation of human spermatozoa. Mol. Hum. Reprod. 11:891–897 10.1093/molehr/gah208 [DOI] [PubMed] [Google Scholar]
- Carlson A.E., Westenbroek R.E., Quill T., Ren D., Clapham D.E., Hille B., Garbers D.L., Babcock D.F. 2003. CatSper1 required for evoked Ca2+ entry and control of flagellar function in sperm. Proc. Natl. Acad. Sci. USA. 100:14864–14868 10.1073/pnas.2536658100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Carlson A.E., Quill T.A., Westenbroek R.E., Schuh S.M., Hille B., Babcock D.F. 2005. Identical phenotypes of CatSper1 and CatSper2 null sperm. J. Biol. Chem. 280:32238–32244 10.1074/jbc.M501430200 [DOI] [PubMed] [Google Scholar]
- Chávez J.C., de la Vega-Beltrán J.L., Escoffier J., Visconti P.E., Treviño C.L., Darszon A., Salkoff L., Santi C.M. 2013. Ion permeabilities in mouse sperm reveal an external trigger for SLO3-dependent hyperpolarization. PLoS ONE. 8:e60578 10.1371/journal.pone.0060578 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Chung J.J., Navarro B., Krapivinsky G., Krapivinsky L., Clapham D.E. 2011. A novel gene required for male fertility and functional CATSPER channel formation in spermatozoa. Nat Commun. 2:153 10.1038/ncomms1153 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Darszon A., Labarca P., Nishigaki T., Espinosa F. 1999. Ion channels in sperm physiology. Physiol. Rev. 79:481–510 [DOI] [PubMed] [Google Scholar]
- Darszon A., Nishigaki T., Wood C., Treviño C.L., Felix R., Beltrán C. 2005. Calcium channels and Ca2+ fluctuations in sperm physiology. Int. Rev. Cytol. 243:79–172 10.1016/S0074-7696(05)43002-8 [DOI] [PubMed] [Google Scholar]
- Darszon A., Acevedo J.J., Galindo B.E., Hernández-González E.O., Nishigaki T., Treviño C.L., Wood C., Beltrán C. 2006. Sperm channel diversity and functional multiplicity. Reproduction. 131:977–988 10.1530/rep.1.00612 [DOI] [PubMed] [Google Scholar]
- Darszon A., Treviño C.L., Wood C., Galindo B., Rodríguez-Miranda E., Acevedo J.J., Hernandez-González E.O., Beltrán C., Martínez-López P., Nishigaki T. 2007. Ion channels in sperm motility and capacitation. Soc. Reprod. Fertil. Suppl. 65:229–244 [PubMed] [Google Scholar]
- Darszon A., Nishigaki T., Beltran C., Treviño C.L. 2011. Calcium channels in the development, maturation, and function of spermatozoa. Physiol. Rev. 91:1305–1355 10.1152/physrev.00028.2010 [DOI] [PubMed] [Google Scholar]
- Fedida D. 1997. Gating charge and ionic currents associated with quinidine block of human Kv1.5 delayed rectifier channels. J. Physiol. 499:661–675 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Fraser L.R., Adeoya-Osiguwa S.A., Baxendale R.W., Gibbons R. 2006. Regulation of mammalian sperm capacitation by endogenous molecules. Front. Biosci. 11:1636–1645 10.2741/1910 [DOI] [PubMed] [Google Scholar]
- Hess P., Lansman J.B., Tsien R.W. 1986. Calcium channel selectivity for divalent and monovalent cations. Voltage and concentration dependence of single channel current in ventricular heart cells. J. Gen. Physiol. 88:293–319 10.1085/jgp.88.3.293 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ho H.C., Suarez S.S. 2001. Hyperactivation of mammalian spermatozoa: function and regulation. Reproduction. 122:519–526 10.1530/rep.0.1220519 [DOI] [PubMed] [Google Scholar]
- Jimenez T., McDermott J.P., Sánchez G., Blanco G. 2011. Na,K-ATPase alpha4 isoform is essential for sperm fertility. Proc. Natl. Acad. Sci. USA. 108:644–649 10.1073/pnas.1016902108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Jin J.L., O’Doherty A.M., Wang S., Zheng H., Sanders K.M., Yan W. 2005. Catsper3 and catsper4 encode two cation channel-like proteins exclusively expressed in the testis. Biol. Reprod. 73:1235–1242 10.1095/biolreprod.105.045468 [DOI] [PubMed] [Google Scholar]
- Jin J., Jin N., Zheng H., Ro S., Tafolla D., Sanders K.M., Yan W. 2007. Catsper3 and Catsper4 are essential for sperm hyperactivated motility and male fertility in the mouse. Biol. Reprod. 77:37–44 10.1095/biolreprod.107.060186 [DOI] [PubMed] [Google Scholar]
- Kim Y., Bang H., Kim D. 2000. TASK-3, a new member of the tandem pore K(+) channel family. J. Biol. Chem. 275:9340–9347 10.1074/jbc.275.13.9340 [DOI] [PubMed] [Google Scholar]
- Kirichok Y., Lishko P.V. 2011. Rediscovering sperm ion channels with the patch-clamp technique. Mol. Hum. Reprod. 17:478–499 10.1093/molehr/gar044 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Kirichok Y., Navarro B., Clapham D.E. 2006. Whole-cell patch-clamp measurements of spermatozoa reveal an alkaline-activated Ca2+ channel. Nature. 439:737–740 10.1038/nature04417 [DOI] [PubMed] [Google Scholar]
- Lishko P.V., Botchkina I.L., Fedorenko A., Kirichok Y. 2010. Acid extrusion from human spermatozoa is mediated by flagellar voltage-gated proton channel. Cell. 140:327–337 10.1016/j.cell.2009.12.053 [DOI] [PubMed] [Google Scholar]
- Lishko P., Clapham D.E., Navarro B., Kirichok Y. 2013. Sperm patch-clamp. Methods Enzymol. 525:59–83 10.1016/B978-0-12-397944-5.00004-3 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Liu J., Xia J., Cho K.H., Clapham D.E., Ren D. 2007. CatSperbeta, a novel transmembrane protein in the CatSper channel complex. J. Biol. Chem. 282:18945–18952 10.1074/jbc.M701083200 [DOI] [PubMed] [Google Scholar]
- Navarro B., Kirichok Y., Clapham D.E. 2007. KSper, a pH-sensitive K+ current that controls sperm membrane potential. Proc. Natl. Acad. Sci. USA. 104:7688–7692 10.1073/pnas.0702018104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro B., Kirichok Y., Chung J.J., Clapham D.E. 2008. Ion channels that control fertility in mammalian spermatozoa. Int. J. Dev. Biol. 52:607–613 10.1387/ijdb.072554bn [DOI] [PMC free article] [PubMed] [Google Scholar]
- Navarro B., Miki K., Clapham D.E. 2011. ATP-activated P2X2 current in mouse spermatozoa. Proc. Natl. Acad. Sci. USA. 108:14342–14347 10.1073/pnas.1111695108 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Okunade G.W., Miller M.L., Pyne G.J., Sutliff R.L., O’Connor K.T., Neumann J.C., Andringa A., Miller D.A., Prasad V., Doetschman T., et al. 2004. Targeted ablation of plasma membrane Ca2+-ATPase (PMCA) 1 and 4 indicates a major housekeeping function for PMCA1 and a critical role in hyperactivated sperm motility and male fertility for PMCA4. J. Biol. Chem. 279:33742–33750 10.1074/jbc.M404628200 [DOI] [PubMed] [Google Scholar]
- Qi H., Moran M.M., Navarro B., Chong J.A., Krapivinsky G., Krapivinsky L., Kirichok Y., Ramsey I.S., Quill T.A., Clapham D.E. 2007. All four CatSper ion channel proteins are required for male fertility and sperm cell hyperactivated motility. Proc. Natl. Acad. Sci. USA. 104:1219–1223 10.1073/pnas.0610286104 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Quill T.A., Sugden S.A., Rossi K.L., Doolittle L.K., Hammer R.E., Garbers D.L. 2003. Hyperactivated sperm motility driven by CatSper2 is required for fertilization. Proc. Natl. Acad. Sci. USA. 100:14869–14874 10.1073/pnas.2136654100 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Ren D., Navarro B., Perez G., Jackson A.C., Hsu S., Shi Q., Tilly J.L., Clapham D.E. 2001. A sperm ion channel required for sperm motility and male fertility. Nature. 413:603–609 10.1038/35098027 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Reyes R., Duprat F., Lesage F., Fink M., Salinas M., Farman N., Lazdunski M. 1998. Cloning and expression of a novel pH-sensitive two pore domain K+ channel from human kidney. J. Biol. Chem. 273:30863–30869 10.1074/jbc.273.47.30863 [DOI] [PubMed] [Google Scholar]
- Santi C.M., Martínez-López P., de la Vega-Beltrán J.L., Butler A., Alisio A., Darszon A., Salkoff L. 2010. The SLO3 sperm-specific potassium channel plays a vital role in male fertility. FEBS Lett. 584:1041–1046 10.1016/j.febslet.2010.02.005 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Schuh K., Cartwright E.J., Jankevics E., Bundschu K., Liebermann J., Williams J.C., Armesilla A.L., Emerson M., Oceandy D., Knobeloch K.P., Neyses L. 2004. Plasma membrane Ca2+ ATPase 4 is required for sperm motility and male fertility. J. Biol. Chem. 279:28220–28226 10.1074/jbc.M312599200 [DOI] [PubMed] [Google Scholar]
- Suarez S.S. 2008. Control of hyperactivation in sperm. Hum. Reprod. Update. 14:647–657 10.1093/humupd/dmn029 [DOI] [PubMed] [Google Scholar]
- Tang Q.Y., Zhang Z., Xia X.M., Lingle C.J. 2010. Block of mouse Slo1 and Slo3 K+ channels by CTX, IbTX, TEA, 4-AP and quinidine. Channels (Austin). 4:22–41 10.4161/chan.4.1.10481 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Vergara C., Latorre R., Marrion N.V., Adelman J.P. 1998. Calcium-activated potassium channels. Curr. Opin. Neurobiol. 8:321–329 10.1016/S0959-4388(98)80056-1 [DOI] [PubMed] [Google Scholar]
- Visconti P.E., Kopf G.S. 1998. Regulation of protein phosphorylation during sperm capacitation. Biol. Reprod. 59:1–6 10.1095/biolreprod59.1.1 [DOI] [PubMed] [Google Scholar]
- Visconti P.E., Galantino-Homer H., Moore G.D., Bailey J.L., Ning X., Fornes M., Kopf G.S. 1998. The molecular basis of sperm capacitation. J. Androl. 19:242–248 [PubMed] [Google Scholar]
- Visconti P.E., Westbrook V.A., Chertihin O., Demarco I., Sleight S., Diekman A.B. 2002. Novel signaling pathways involved in sperm acquisition of fertilizing capacity. J. Reprod. Immunol. 53:133–150 10.1016/S0165-0378(01)00103-6 [DOI] [PubMed] [Google Scholar]
- Wang H., Liu J., Cho K.H., Ren D. 2009. A novel, single, transmembrane protein CATSPERG is associated with CATSPER1 channel protein. Biol. Reprod. 81:539–544 10.1095/biolreprod.109.077107 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wang S., Morales M.J., Qu Y.J., Bett G.C., Strauss H.C., Rasmusson R.L. 2003. Kv1.4 channel block by quinidine: evidence for a drug-induced allosteric effect. J. Physiol. 546:387–401 10.1113/jphysiol.2002.029512 [DOI] [PMC free article] [PubMed] [Google Scholar]
- Wennemuth G., Carlson A.E., Harper A.J., Babcock D.F. 2003. Bicarbonate actions on flagellar and Ca2+ -channel responses: initial events in sperm activation. Development. 130:1317–1326 10.1242/dev.00353 [DOI] [PubMed] [Google Scholar]
- Zeng X.H., Yang C., Kim S.T., Lingle C.J., Xia X.M. 2011. Deletion of the Slo3 gene abolishes alkalization-activated K+ current in mouse spermatozoa. Proc. Natl. Acad. Sci. USA. 108:5879–5884 10.1073/pnas.1100240108 [DOI] [PMC free article] [PubMed] [Google Scholar]