Abstract
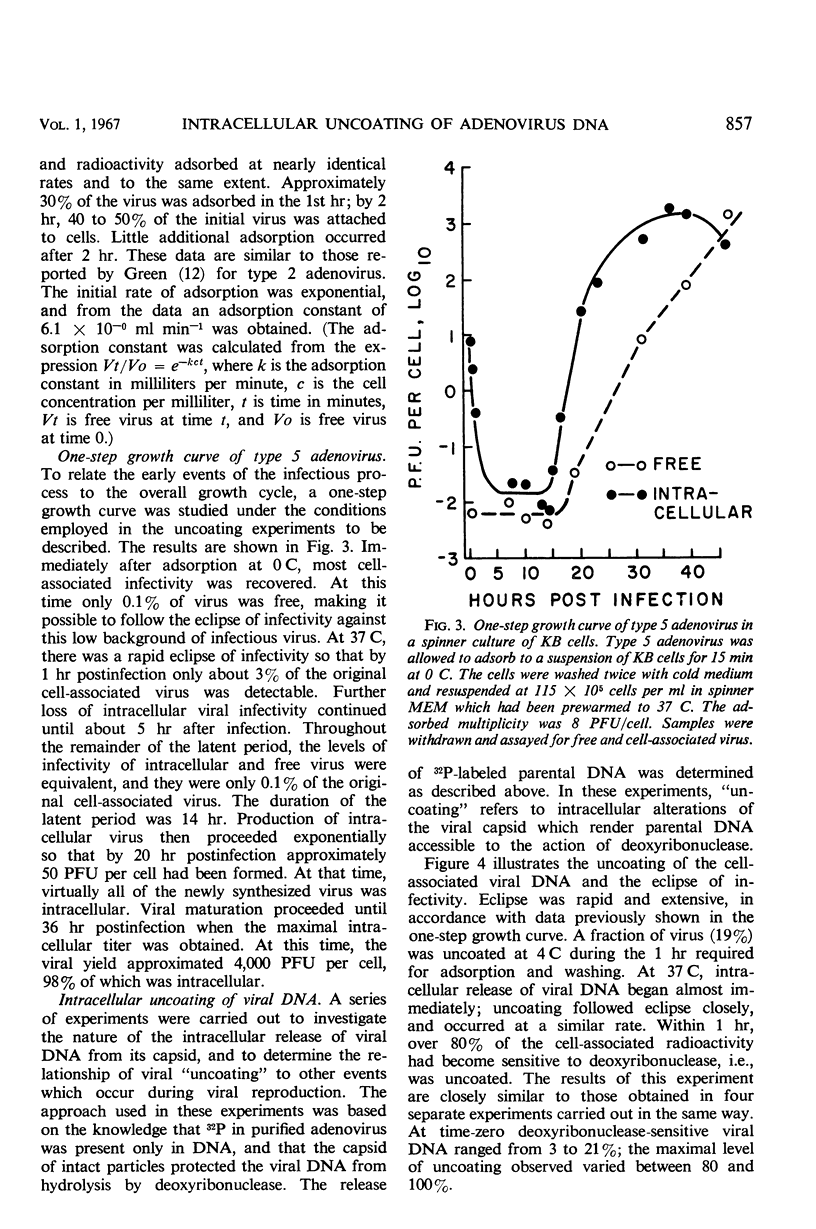
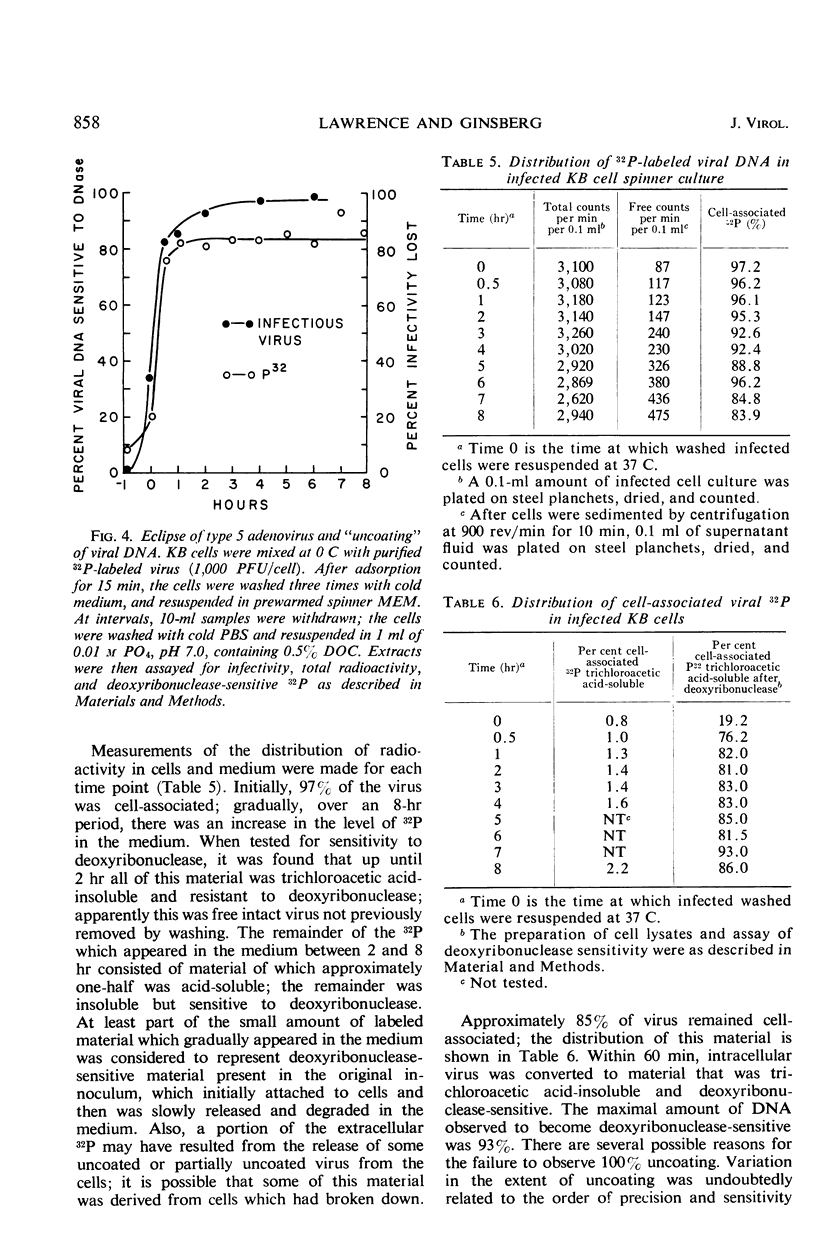
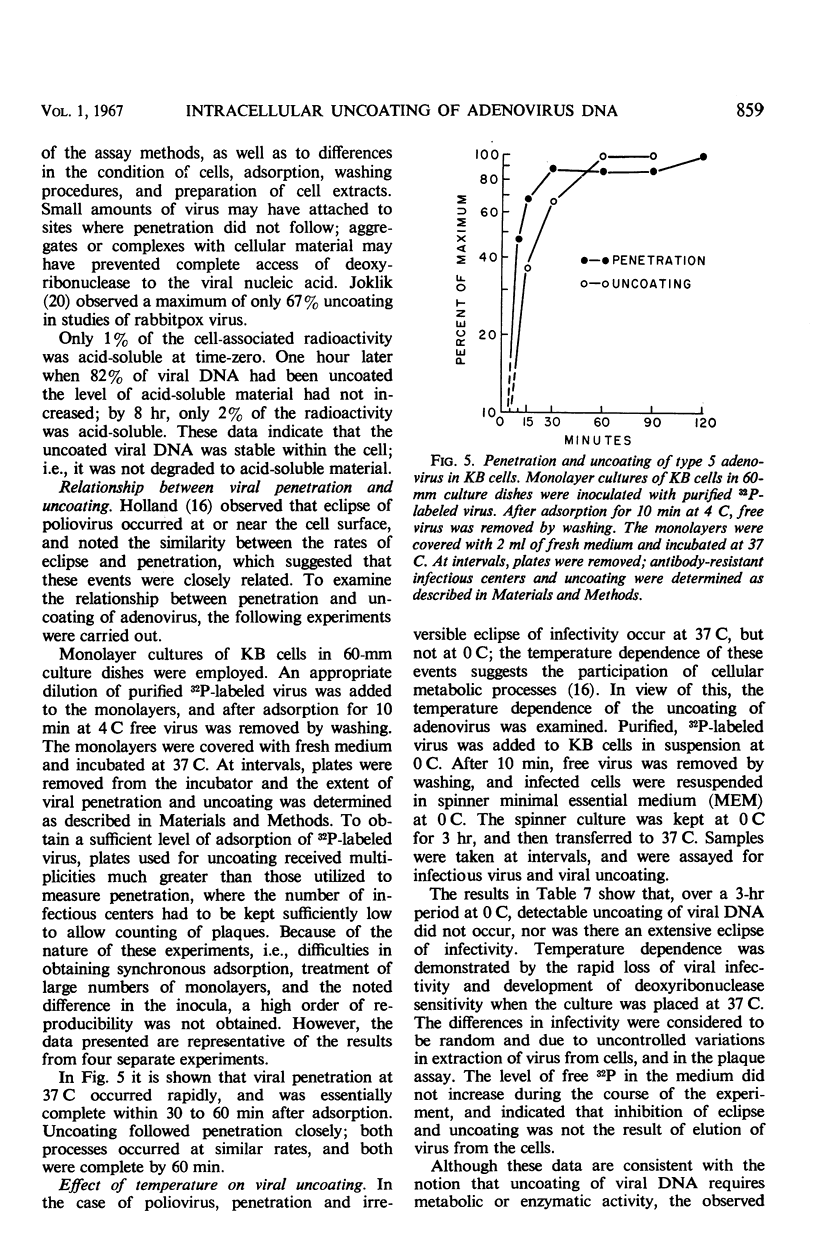
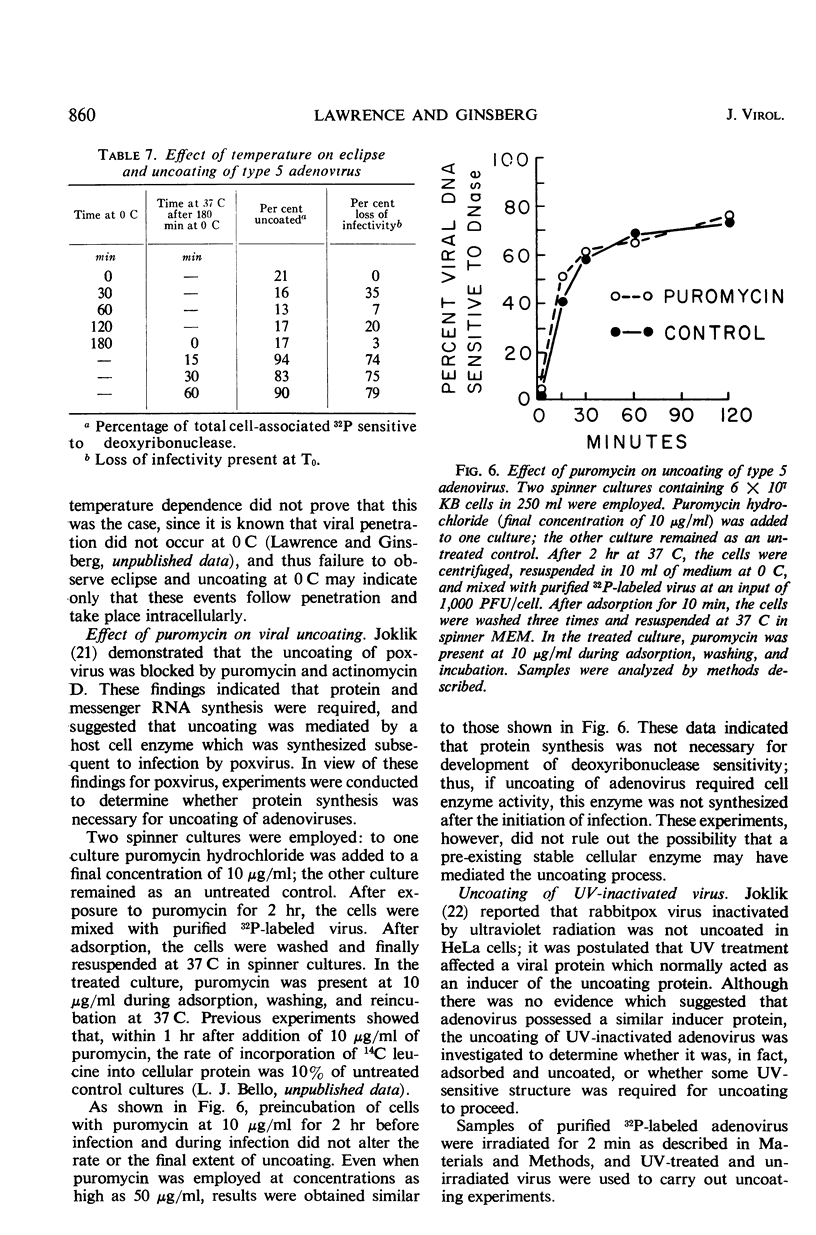
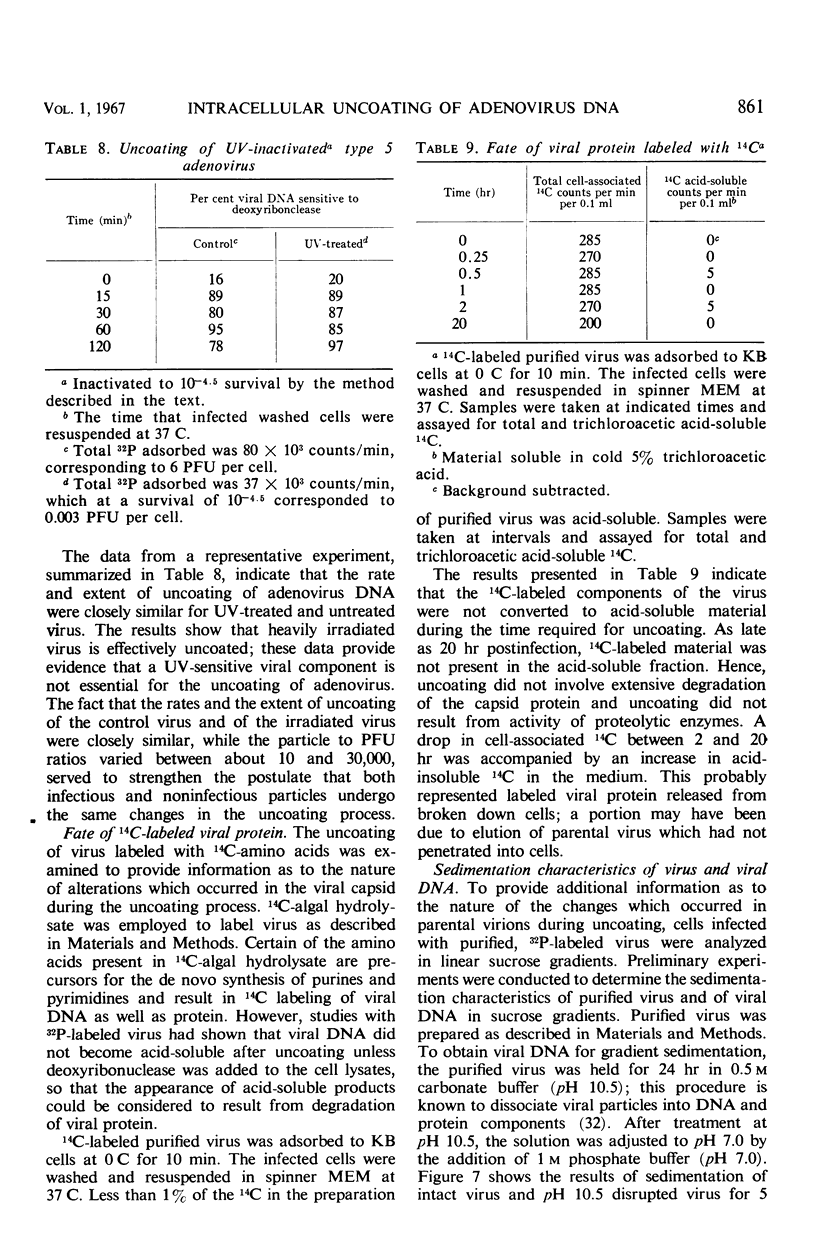
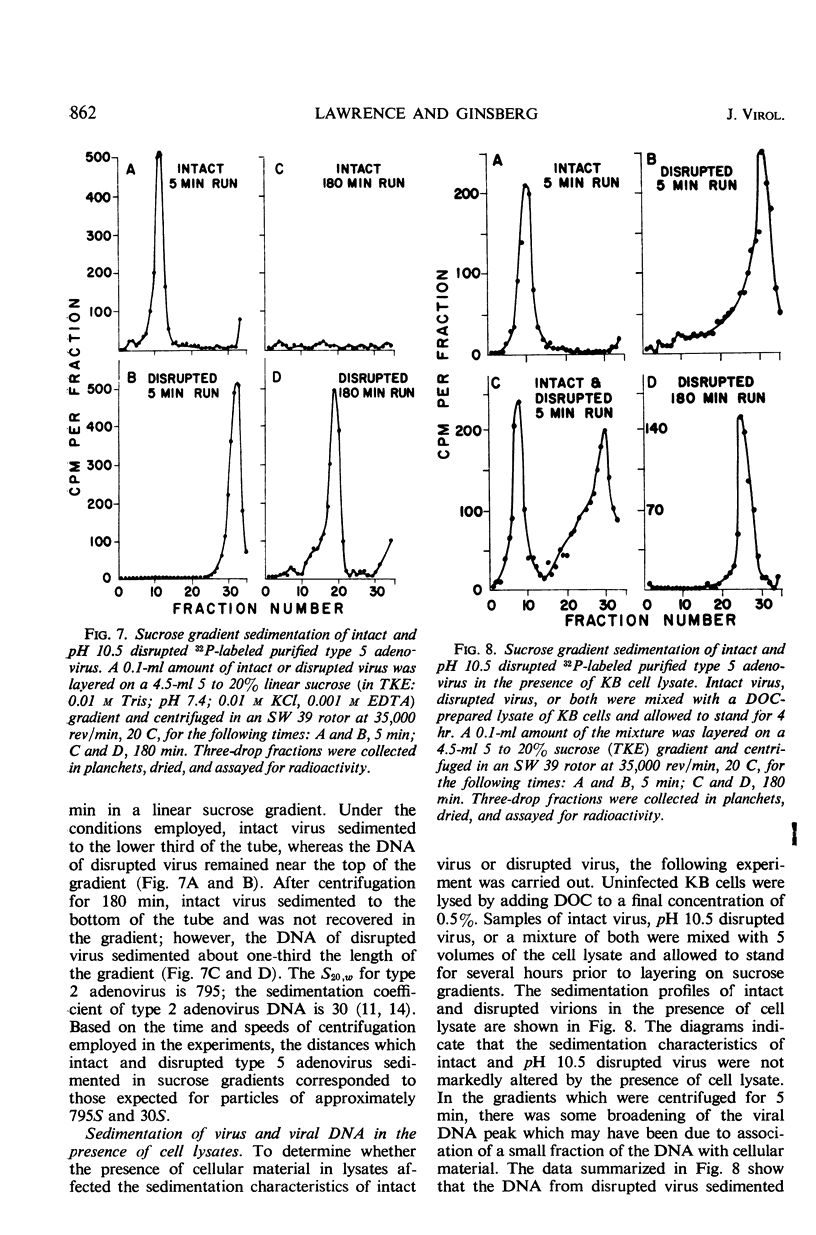
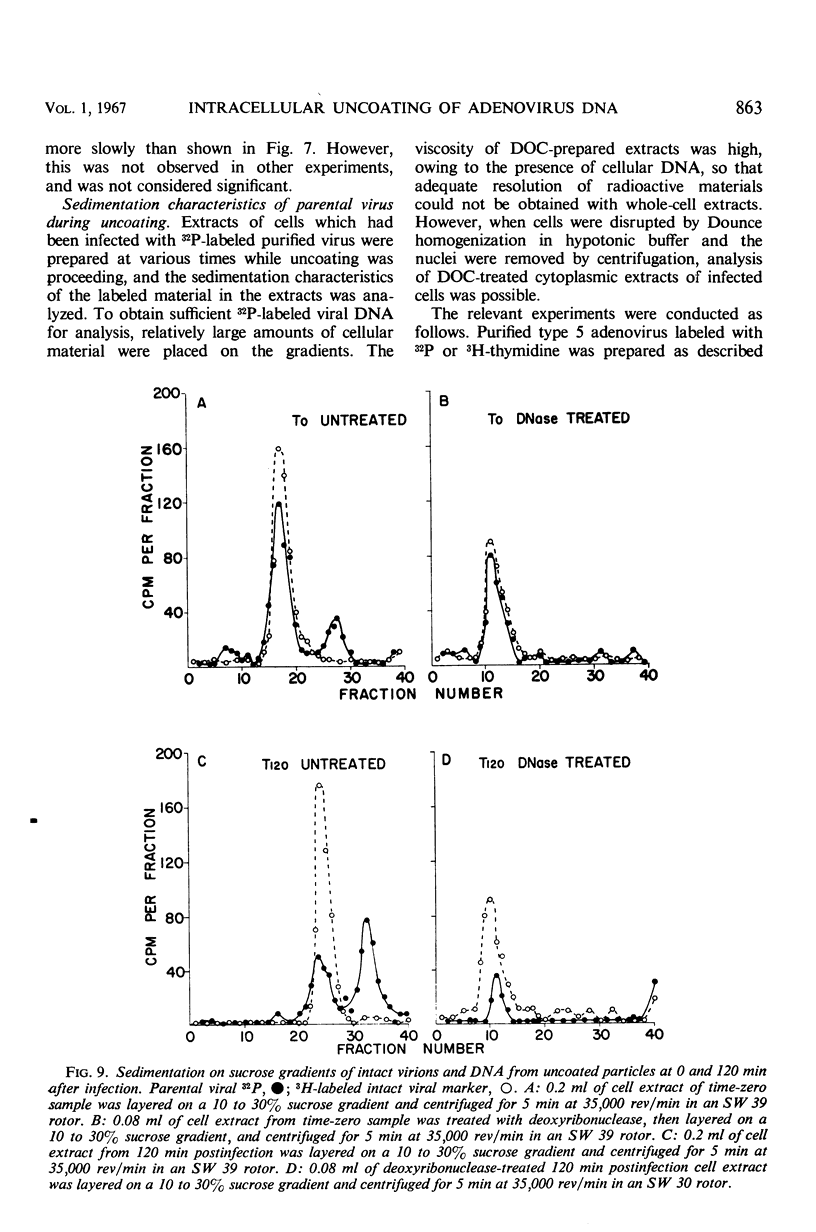
Highly purified, 32P-labeled type 5 adenovirus was employed to study “uncoating” of viral deoxyribonucleic acid (DNA)—defined as the development of sensitivity to deoxyribonuclease. Viral infectivity and radioactivity adsorbed to KB cells at the same rate, and significant amounts of 32P did not elute from cells throughout the eclipse period. Kinetic studies of viral penetration, eclipse of infectivity, and uncoating of viral DNA indicated that the three events were closely related temporally, that the rates of each were similar, and that they were completed within 60 to 90 min after infection. Viral penetration, eclipse, and uncoating proceeded normally under conditions which blocked protein synthesis, but they did not occur at 0 to 4 C. Neither viral DNA nor viral protein was degraded to acid-soluble material during the eclipse period. The nature of adenovirus DNA was studied after it was converted intracellularly from deoxyribonuclease-resistant to deoxyribonuclease-susceptible. Intact virions centrifuged in sucrose gradients had a sedimentation coefficient of approximately 800, and viral DNA sedimented as a particle of about 30S. Infection of KB cells with purified 32P-labeled virus yielded deoxyribonuclease-susceptible viral nucleic acid which was in particles with sedimentation coefficients of 350 to 450S, i.e., greater than 10 times faster than DNA obtained from purified virions which had been disrupted by exposure to pH 10.5. When the DNA from disrupted virions was mixed with cell lysates, its sedimentation characteristics were essentially unchanged by the presence of cellular material.
Full text
PDF






Selected References
These references are in PubMed. This may not be the complete list of references from this article.
- BURTON K. A study of the conditions and mechanism of the diphenylamine reaction for the colorimetric estimation of deoxyribonucleic acid. Biochem J. 1956 Feb;62(2):315–323. doi: 10.1042/bj0620315. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DALES S. An electron microscope study of the early association between two mammalian viruses and their hosts. J Cell Biol. 1962 May;13:303–322. doi: 10.1083/jcb.13.2.303. [DOI] [PMC free article] [PubMed] [Google Scholar]
- DISCHE Z. Qualitative and quantitative colorimetric determination of heptoses. J Biol Chem. 1953 Oct;204(2):983–997. [PubMed] [Google Scholar]
- EAGLE H. Amino acid metabolism in mammalian cell cultures. Science. 1959 Aug 21;130(3373):432–437. doi: 10.1126/science.130.3373.432. [DOI] [PubMed] [Google Scholar]
- EAGLE H. Propagation in a fluid medium of a human epidermoid carcinoma, strain KB. Proc Soc Exp Biol Med. 1955 Jul;89(3):362–364. doi: 10.3181/00379727-89-21811. [DOI] [PubMed] [Google Scholar]
- FLANAGAN J. F., GINSBERG H. S. Synthesis of virus-specific polymers in adenovirus-infected cells; effect of 5-fluorodeoxyuridine. J Exp Med. 1962 Aug 1;116:141–157. doi: 10.1084/jem.116.2.141. [DOI] [PMC free article] [PubMed] [Google Scholar]
- Flanagan J. F., Ginsberg H. S. Role of ribonucleic acid biosynthesis in multiplication of type 5 adenovirus. J Bacteriol. 1964 May;87(5):977–987. doi: 10.1128/jb.87.5.977-987.1964. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBERG H. S., BADGER G. F., DINGLE J. H., JORDAN W. S., Jr, KATZ S. Etiologic relationship of the RI-67 agent to acute respiratory disease (ARD). J Clin Invest. 1955 Jun;34(6):820–831. doi: 10.1172/JCI103137. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GINSBERG H. S., DIXON M. K. Nucleuc acid synthesis in types 4 and 5 adenovirus-infected HeLa cells. J Exp Med. 1961 Feb 1;113:283–299. doi: 10.1084/jem.113.2.283. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M., DAESCH G. E. Biochemical studies on adenovirus multiplication. II. Kinetics of nucleic acid and protein synthesis in suspension cultures. Virology. 1961 Feb;13:169–176. doi: 10.1016/0042-6822(61)90051-4. [DOI] [PubMed] [Google Scholar]
- GREEN M., PINA M. BIOCHEMICAL STUDIES ON ADENOVIRUS MULTIPLICATION, VI. PROPERTIES OF HIGHLY PURIFIED TUMORIGENIC HUMAN ADENOVIRUSES AND THEIR DNA. Proc Natl Acad Sci U S A. 1964 Jun;51:1251–1259. doi: 10.1073/pnas.51.6.1251. [DOI] [PMC free article] [PubMed] [Google Scholar]
- GREEN M., PINA M. Biochemical studies on adenovirus multiplication. IV. Isolation, purification, and chemical analysis of adenovirus. Virology. 1963 May;20:199–207. doi: 10.1016/0042-6822(63)90157-0. [DOI] [PubMed] [Google Scholar]
- GREEN M. Studies on the biosynthesis of viral DNA. Cold Spring Harb Symp Quant Biol. 1962;27:219–235. doi: 10.1101/sqb.1962.027.001.022. [DOI] [PubMed] [Google Scholar]
- HERSHEY A. D., CHASE M. Independent functions of viral protein and nucleic acid in growth of bacteriophage. J Gen Physiol. 1952 May;36(1):39–56. doi: 10.1085/jgp.36.1.39. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HOLLAND J. J. Irreversible eclipse of poliovirus by HeLa cells. Virology. 1962 Feb;16:163–176. doi: 10.1016/0042-6822(62)90292-1. [DOI] [PubMed] [Google Scholar]
- HOMMA M., GRAHAM A. F. INTRACELLULAR FATE OF MENGO VIRUS RIBONUCLEIC ACID. J Bacteriol. 1965 Jan;89:64–73. doi: 10.1128/jb.89.1.64-73.1965. [DOI] [PMC free article] [PubMed] [Google Scholar]
- HUEBNER R. J., ROWE W. P., WARD T. G., PARROTT R. H., BELL J. A. Adenoidal-pharyngeal-conjunctival agents: a newly recognized group of common viruses of the respiratory system. N Engl J Med. 1954 Dec 30;251(27):1077–1086. doi: 10.1056/NEJM195412302512701. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K., DARNELL J. E., Jr The adsorption and early fate of purified poliovirus in HeLa cells. Virology. 1961 Apr;13:439–447. doi: 10.1016/0042-6822(61)90275-6. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. THE INTRACELLULAR FATE OF RABBITPOX VIRUS RENDERED NONINFECTIOUS BY VARIOUS REAGENTS. Virology. 1964 Apr;22:620–633. doi: 10.1016/0042-6822(64)90084-4. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. THE INTRACELLULAR UNCOATING OF POXVIRUS DNA. I. THE FATE OF RADIOACTIVELY-LABELED RABBITPOX VIRUS. J Mol Biol. 1964 Feb;8:263–276. doi: 10.1016/s0022-2836(64)80136-4. [DOI] [PubMed] [Google Scholar]
- JOKLIK W. K. THE INTRACELLULAR UNCOATING OF POXVIRUS DNA. II. THE MOLECULAR BASIS OF THE UNCOATING PROCESS. J Mol Biol. 1964 Feb;8:277–288. doi: 10.1016/s0022-2836(64)80137-6. [DOI] [PubMed] [Google Scholar]
- Kates J. R., McAuslan B. R. Messenger RNA synthesis by a "coated" viral genome. Proc Natl Acad Sci U S A. 1967 Feb;57(2):314–320. doi: 10.1073/pnas.57.2.314. [DOI] [PMC free article] [PubMed] [Google Scholar]
- LOCKART R. Z., Jr, EAGLE H. Requirements for growth of single human cells. Science. 1959 Jan 30;129(3344):252–254. doi: 10.1126/science.129.3344.252. [DOI] [PubMed] [Google Scholar]
- LOWRY O. H., ROSEBROUGH N. J., FARR A. L., RANDALL R. J. Protein measurement with the Folin phenol reagent. J Biol Chem. 1951 Nov;193(1):265–275. [PubMed] [Google Scholar]
- MARTIN R. G., AMES B. N. A method for determining the sedimentation behavior of enzymes: application to protein mixtures. J Biol Chem. 1961 May;236:1372–1379. [PubMed] [Google Scholar]
- Munyon W. H., Kit S. Induction of cytoplasmic ribonucleic acid (RNA) synthesis in vaccinia-infected LM cells during inhibition of protein synthesis. Virology. 1966 Jun;29(2):303–309. doi: 10.1016/0042-6822(66)90037-7. [DOI] [PubMed] [Google Scholar]
- Rose J. A., Reich P. R., Weissman S. M. RNA production in adenovirus-infected KB cells. Virology. 1965 Dec;27(4):571–579. doi: 10.1016/0042-6822(65)90183-2. [DOI] [PubMed] [Google Scholar]
- TRAUTMAN R., BREESE S. S., Jr Isodensity ultracentrifugation of foot-and-mouth disease virus in caesium chloride. J Gen Microbiol. 1962 Feb;27:231–239. doi: 10.1099/00221287-27-2-231. [DOI] [PubMed] [Google Scholar]
- WILCOX W. C., GINSBERG H. S. STRUCTURE OF TYPE 5 ADENOVIRUS. I. ANTIGENIC RELATIONSHIP OF VIRUS-STRUCTURAL PROTEINS TO VIRUS-SPECIFIC SOLUBLE ANTIGENS FROM INFECTED CELLS. J Exp Med. 1963 Aug 1;118:295–306. doi: 10.1084/jem.118.2.295. [DOI] [PMC free article] [PubMed] [Google Scholar]



