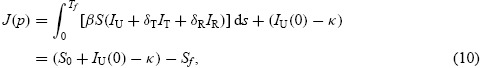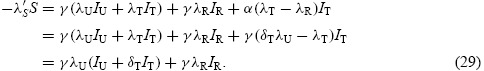Abstract
The emergence and spread of drug resistance is one of the most challenging public health issues in the treatment of some infectious diseases. The objective of this work is to investigate whether the effect of resistance can be contained through a time-dependent treatment strategy during the epidemic subject to an isoperimetric constraint. We apply control theory to a population dynamical model of influenza infection with drug-sensitive and drug-resistant strains, and solve the associated control problem to find the optimal treatment profile that minimizes the cumulative number of infections (i.e. the epidemic final size). We consider the problem under the assumption of limited drug stockpile and show that as the size of stockpile increases, a longer delay in start of treatment is required to minimize the total number of infections. Our findings show that the amount of drugs used to minimize the total number of infections depends on the rate of de novo resistance regardless of the initial size of drug stockpile. We demonstrate that both the rate of resistance emergence and the relative transmissibility of the resistant strain play important roles in determining the optimal timing and level of treatment profile.
AMS Subject Classification: 34, 49, 92
Keywords: epidemic modelling, influenza, limited antiviral stockpiles, drug resistance, control theory
1. Introduction
Optimizing intervention strategies is an important policy issue for the management of infectious diseases, in particular when facing an emerging pathogen. In case of influenza, antiviral treatment is a key pharmaceutical measure that reduces illness and duration of infection, and consequently lowers the transmissibility of disease in the population. However, treatment exerts a significant selection pressure that favours resistance and encourages its wide spread in the population [3,8,26,28,31]. The transmissibility of resistance is generally lower than its competitor (sensitive strain) due to a fitness cost associated with de novo resistance emergence. However, since treatment suppresses the growth and spread of the sensitive strain, the relative transmissibility of resistance can be improved through further replication in multiple hosts [3,5,9,26–29]. The effectiveness of antiviral drugs may therefore be trivialized once resistance has developed and spread between individuals. This poses an important question for optimizing treatment strategies in order to reduce the impact of disease on the population, but also minimize the selective pressure of drugs on the evolutionary-epidemiological consequences of resistance emergence.
In the present paper, we extend our previous work [14] on optimal treatment profile during an influenza epidemic by taking into consideration isoperimetric constraints which are imposed on the resources (i.e. the amount of drug stockpile available for treatment). With a limited drug stockpile in the presence of resistance, identifying optimal treatment strategies is a control problem and therefore requires the application of control theory for minimizing the total number of infections during an epidemic episode. To address this problem, we employ an established population dynamical model of influenza infection and investigate analytic solutions for the time-dependent control components. Our objective is to find the optimal treatment profile that minimizes the epidemic final size, resulted from disease transmission through a bilinear mass action incidence. A time-dependent treatment profile was initially introduced by Moghadas et al. [28], proposing a switch in the level of treatment from a low value (at the early stages of disease outset) to a higher value (at a later stage during the epidemic). However, the optimality of this strategy depends on the initial level of treatment and the timing for the switch to a higher level, both of which are important factors for preventing wide-scale resistance spread and reducing the final size.
Using a control theory approach, we analyse different scenarios of optimal treatment profile and investigate how the rate of de novo resistance and its relative transmissibility affect the timing and outcomes of such optimal solutions. We describe the model and its assumptions and constraints, and define the control problem in Section 2. The effect of changes in parameters associated with resistance and the size of drug stockpile will be discussed in Section 3, and simulation results of particular scenarios will be presented. Finally, we close the paper with some concluding remarks and questions to be addressed in future work.
2. The model and control problem
We consider a deterministic model of influenza epidemic [28] that describes the transmission dynamics of drug-sensitive and drug-resistant strains in a host population. The compartmental model divides a randomly mixed population into classes of individuals who are susceptible to infection (S); infected with the sensitive strain (IU); treated (IT); and infected with the drug-resistant strain (IR). Since treatment is effective only against drug-sensitive infection, we combined treated and untreated classes of individuals infected with the drug-resistant strain. The transitions between these classes are represented in Figure 1, and mathematically formulated by the following system of differential equations:
Figure 1.
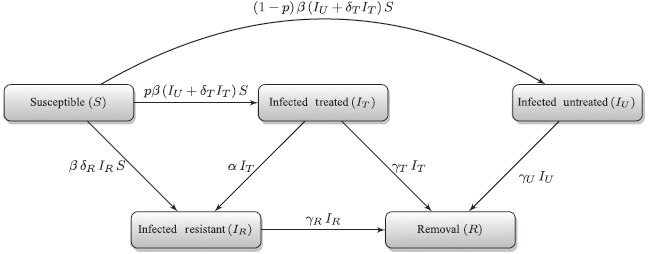
Model diagram for the transitions between sub-populations with emergence of resistance during treatment.
where ‘′’ is used to show the derivative of the compartments with respect to time (t) and p(t) (as function of time) is the fraction of infected individuals who receive treatment at time t. In this model, β denotes the baseline transmission rate of the drug-sensitive strain; δT (≤ 1) represents the relative infectiousness of treated individuals infected with the drug-sensitive strain (which is reduced compared to untreated infection); δR (≤ 1) is the relative transmissibility of the resistant infection (and is reduced compared to the sensitive infection due to the fitness cost of resistance emergence); α represents the rate of resistance emergence during treatment; and γU, γT, and γR are the recovery rates of untreated, treated, and resistant infections, respectively. The removal compartment consists of recovered individuals (R), and its dynamics are described by the equation
We also assume that recovery upon infection provides protection against re-infection. For a single course of an influenza epidemic, natural birth and death rates are neglected. For a limited drug stockpile, the usage can be described by the following equation:
with Δ(0) = K, where K represents the initial size of drug stockpile. The function Δ(t) represents the size of drug stockpile available for treatment at time t during the epidemic without replenishment [2,12,22,30]. For simplicity, we rewrite the model in the following form:
where X = [S, IU, IT, IR, Δ]T denotes the state of the control system, and G = [S′, I′U, IT, IR, Δ′]T, with S(0) = S0 > 0, IU(0) = I0 > 0, IR(0) = IT(0) = R(0) = 0.
2.1. The control problem
To show the existence of an optimal solution related to the time-dependent treatment level, we define the control problem with the objective of minimizing the total number of infections (epidemic final size). To do so, we express the control problem as
where the cost functional is defined by
and f(t, X, p) = (γUIU + γTIT + γRIR). For consistency with the published literature [12,13], the upper bound of integration Tf denotes the end of epidemic, where Tf = inf{t | (IU + I T + IR)(t) = κ ≪ 1}. We also assume that the initial size of drug stockpile is limited and may be exhausted before the end of epidemic (run-out scenario). This corresponds to the situation in which there exists some tK < Tf, where (IU + IT + IR)(tK) ≥ 1, but Δ(t) = 0 for t ≥ tK.
Remark
Let the pair (p∗(t),X∗(t)) be an optimal control for the corresponding solution of the system (1)–(5). Then, there is a t1 < Tf such that the optimal control value is given by p(t) = 0 for t ≥ t1.
We consider the effect of treatment as a reduction (by a factor of δT < 1) in transmissibility of the disease [28] and assume that recovery rates are the same for all infectious classes (i.e. γU = γT = γR), denoted by γ. In this case, the cost functional reduces to
A direct application of Filippov's theorem [1] shows that the pair (p∗(t),X∗(t)) of admissible optimal control exists for our objective functional. Therefore, in what follows, the existence of an optimal control will be assumed. Now, we discuss the necessary conditions of optimality by introducing the Pontryagin minimum principle (PMP) [11,12,22]. Readers may also consult published literature for further applications of the PMP theorem [10,15,–19,21,25]. The main technique in the PMP is to determine an admissible set of necessary conditions for an optimal control problem. This admissible set decreases the cost functional given by Equation (8) provided that the adjoint variable and the corresponding state variables satisfy certain conditions presented by PMP Theorem 2.1 [11,20,22].
Let ℘ be a bounded subset of ℝ as the collection of all admissible controls, p, with values in ℝ where admissible controls p: [0, T] → ℘ are measurable functions. We introduce
as the set of all bounded measurable functions with values in ℝ for arbitrary positive time T, where T is the time of completion for the control program.
Theorem 2.1
Let the pair (p∗(t),X∗(t)) be an admissible control for the corresponding solution of system (1)–(4). Then, there exists an absolutely continuous function
such that Λ(t) ≠ 0 for t ∊ [0, Tf] with λ0 ∊ {0,1} and
for all admissible controls p at time t, where the Hamiltonian function is defined by
in which the map «.,.» represents the standard inner product and the adjoint variables are obtained from
Furthermore, Λ(Tf) is orthogonal to ker(DΨ(X(Tf))), where DΨ denotes the Jacobian of the set of possible values for the final state values.
The following lemma provides a different way of considering the objective functional and its proof is given in [14].
Lemma 2.2
Minimizing the cost functional given by Equation (9) is equivalent to reducing the influx rates from susceptible class to infection classes, that is, the cost functional can be written as
where Sf = S(Tf) and S0 = S(0).
It is worth noting that minimizing the cumulative number of infections (final size of the epidemic) given by Equation (8) is equivalent to maximizing the final number of susceptibles Sf since S0 + IU(0) − κ is fixed. From now on, we consider the new cost functional for minimizing –Sf resulted from Equation (10).
The function of Hamiltonian for the optimal control theory obtained by the PMP can be expressed by
where λ0 ≡ {0,1} and f ≡ 0. As a result of the PMP, the adjoint equations corresponding to system (1)–(4) are obtained from
where the transversality conditions are given by
with q ≥ 0. The Hamiltonian function can then be represented by multiple identities which will be used to study the dynamical model of influenza infection associated with the following system:
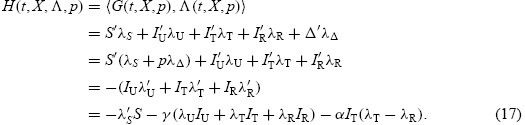 |
We consider the maximization of an objective functional over a variable time interval, where the end time Tf is not fixed, and thus based on the PMP, H(t, X, Λ,p) = 0 for the entire course of epidemic (t ∊ [0, Tf]). We note that λS(Tf) ≠ 0 (or q ≠ 0); otherwise, using the Hamiltonian at Tf gives q = 0 (i.e. λS(Tf) = 0) which contradicts the fact that Λ(t) ≠ 0 for all t ∊ [0, Tf] in PMP Theorem 2.1.
2.2. Analysis of treatment profiles
The main challenge in implementing Pontryagin fundamental theory appears in Hamiltonian depending on the linear control p, that is, the function H is generated from the form
where the function ψ explicitly consists of only state and adjoint variables, the control component p is constrained to upper and lower bounds 0 ≤ p(t) ≤ 1, and Ψ is the remaining nonlinear terms which depend only on the state and adjoint variables. To reduce the value of Hamiltonian H (t, X,Λ,p) to zero, we need to make the control function p as large or small as possible depending on the sign of ψ(X, Λ,t). The optimality condition represented by the switching time function, ψ,
takes positive or negative values at some times and is non-zero with the possible exception of at most a finite number of times t, and the solution to the control problem is easily obtained from Equation (19). In this case, the optimal control is referred to as bang–bang control switches from 0 to 1 at finite number of times corresponding to sign changes in ψ(t) at each switch. However, the case of singularity for control problem arises when ψ(t) continues to be zero on a time interval t1 ≤ t ≤ t2. In this case, the minimization of the Hamiltonian function with respect to the control function p does not provide any informative solution within that time interval. The common technique to explicitly characterize the control function is to recurrently take the derivative of ϱH/ϱp with respect to time, which guarantees to generate the explicit solution [6]. Setting the expression for ψ(t) to zero, the control p is determined by the requirement that the singularity condition continues to hold. This characterizes the optimal control as follows:
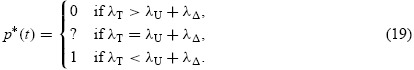 |
Lemma 2.3
If δR = 0 or α = 0, then a shorter delay in switching the treatment level (from p = 0 to p = 1) corresponds to a lower epidemic final size regardless of the size of drug stockpile. The minimum cumulative number of infection is achieved when the switch occurs at the onset of epidemic (i.e. t = 0). Also, we have ψ ≤ 0 on [0, Tf] when λΔ = 0.
Proof
Case 1: Assume that λΔ = 0. If α = 0, then IR = 0 on [0, Tf]. We first show that the optimal treatment strategy is non-singular. Suppose there exists some interval [t0,t1] with t0 ≤ t1 such that ψ(t) = 0 for all t ∊ [t0,t1]. Thus, we have λT(t) = λU(t) + λΔ and λ′U(t) = λ′T(t) for all t ∊ [t0,t1]. Using Equations (13) and (14), it follows that λU(t) = λS(t) = λT(t) − λΔ. Considering Equation (12), when λΔ = 0, we have λ′U (t) = λ′T(t) = λ′S(t) = 0, and consequently λS(t) = λU(t) = λT(t) = 0 which is a contradiction with Λ(t) = 0. Thus, the optimal control is a bang-bang problem.
Suppose ψ(t0) = 0 and ψ(t) > 0 on [0, t0). Hence, λ′U(t0) = 0 and we have
Solving the above ordinary differential equation gives
Since λT(0) > λU, (λT(0) − δTλU) > (λU − δTλU); however, evaluating this at t = 0 gives (λT(0) − δTλU) eγt0 = (λU − δT λU), which is a contradiction since eγt0 > 1.
Now we show that ψ < 0 for the entire course of epidemic. For a contrary proposition, assuming that there is a switch before Tf, from the above results, it follows that there exist at least two switches occurring in (0, Tf). Let T0 be the last switch where T0 < Tf. Since λU(T0) = λT(T0) < λS (T0), there exists some t∗ ∊ (T0, Tf) such that λT(t∗) = λS(t∗) and λ′S(t∗) = 0 < λT(t∗). Therefore, t∗ is an inflection point at which λS″(t∗) = 0, and from Equation (12) it follows that λT(t∗) = 0, which is a contradiction.
Case 2: Suppose λΔ ≠ 0 and that there exists some interval [t0, t1] ⊂ [0, Tf] on which ψ(t) < 0, and therefore λU(t) + λΔ = λT(t) for t ∊ [t0, t1]. Using Equations (13) and (14), it follows that λU(t0) = λT(t0) − λΔ < λS (t0) and λU(t1) = λT(t1) − λΔ > λS(t1), implying that there exists some t∗ ∊ (t0, t1) such that λT(t∗) − λΔ = λS(t∗) and λ′S(t∗) = 0 < λ′T(t∗). This shows that t∗ is an inflection point at which λS″(t∗) = 0, and from Equation (12) it follows that λ′T(t∗) = 0, again leading to a contradiction.
Theorem 2.4
Let (p∗(t),X∗(t)) be an optimal control for the corresponding solution of the system (1)–(5). Then, the adjoint variable corresponding to the isoperimetric constraint (5) is always non-positive.
Proof
Without loss of generality, we assume that the initial size of drug stockpile, K, is smaller than the total number of infections in the absence of any treatment. Otherwise, the adjoint equation λΔ = 0. With limited drug stockpile over the time period of [0, Tf], there exists tK < Tf such that the switching time function ψ must be non-negative over [tK, Tf]. Thus, it follows from Equation (5) that λΔ remains either positive or negative constant for the entire epidemic course. Furthermore, taking into account the transversality conditions at Tf, it follows that there exists some t1 < Tf such that
for t ≥ t1. However, since ψ(t) ≥ 0 on [tK, Tf], we have
If λΔ > 0, then we have λT ≥ λU + λΔ > λU on [tK, Tf], which contradicts the inequality (21). Consequently, the adjoint variable corresponding to the isoperimetric constraint (5) is always non-positive.
Suppose ψ(t) = 0 for some interval [t0, t1], where 0 ≤ t0 ≤ t1 < Tf. From the previous results, we have λΔ < 0 and (IU + δTIT − λΔδRIR)S > 0. Therefore, λT = λU + λΔ and λ′T = λ′U on [t0, t1] (singularity condition). Substituting this into Equations (13) and (14), we obtain
From Equations (23) and (24), we see that
Theorem 2.5 [14]
Suppose βS0 > α and p(0) = 0, and let (p∗(t),X∗(t)) be an optimal control for the corresponding solution of the system (1)–(5). Then, a switch in the level of treatment (from p = 0 to p > 0) must occur before Tf for minimizing the epidemic final size and the expression for the final size relation is given by
where N0 = S0 + IU(0), with equality occurring for the second inequality ‘ ≤ ’only if p(t) = 0 for all t.
Remark
We denote the ratio βS0/γ by R0, the so-called basic reproduction number. In the epidemiological context, R0 is defined as the number of secondary infectious cases generated by a single infected case introduced into an entirely susceptible population N0 ⋍ S0 (assuming IU(0) is small compared to S0) [7]. If R0 > 1, then the outbreak will occur, whereas if R0 < 1, then the outbreak is expected to die out.
A switch in the treatment level corresponds to a change of sign in ψ. Now, we show that no singularity can occur at the first switching time and the optimal control is a bang-bang problem.
Theorem 2.6
Suppose δTδR < 1. Let (p∗(t),X∗(t)) be an optimal control for the corresponding solution of the system (1)–(4). If the switch function ψ remains zero on some interval [t0, t1], then
- (1)
λ′U = λ′T ≠ 0 on the same interval, that is, the adjoint variables λU = λT + λΔ must be either increasing or decreasing on [t0, t1];
- (2)
λ′R ≠ 0 with opposite sign of λU = λ′T, that is, λ′Uλ′R = λ′Tλ′R < 0 on [t0, t1].
Furthermore, no singularity occurs at the first switching time.
Proof
Suppose the switching time function ψ is zero on some interval [t0,t1] (0 ≤ t0 < t1 ≤ Tf). Thus, λT(t) = λU(t) + λΔ on [t0,t1]. If λ′T(t) = λ′U(t) = 0 for some t ∊ [t0,t1], then from the Hamiltonian function H(t, X, Λ, p) = −(λ′U IU + λ′TIT + λ′RIR) = 0 on [0, Tf], it follows that λ′U = λ′T = λ′R = 0 (since IR ≠ 0). Taking into account Equations (22)–(25), it can be seen that
From the first and second equalities, we have
which is equivalent to
Furthermore, using the equation for the Hamiltonian function, we obtain
However from Equation (26) or (28), it follows that
Comparing Equation (29) with Equation (30), we have
Equality (31) holds only if λR = 0 (since otherwise Λ = 0). Using Equation (28), it then follows that (1 − δT)γ = α(δR − 1), which holds only if δT = δR = 1. In this case, the system (1)−(4) reduces to the basic SIR epidemic model, which essentially eliminates the effect of control problem. If, however, δT δR < 1, we have a contradiction, and therefore when ψ(t) is zero on [t0, t1], we get λ′U = λ′T = 0 with opposite sign of λ′R = 0.
Now suppose δT δR < 1 and p∗(t) = 0 for t ∊ [0,t0), where t0 is the first time at which ψ(t0) = 0. Using Equation (23), it is easy to see that λ′U(t0−) = λ′U(t0+) = 0. At t0, we must then have λ′U(t0) = 0. If λ′T(t0) < 0, then clearly the control is non-singular at the first switch. If λ′T(t0) = 0, then there are two cases: either ψ(t) ≠ 0 or ψ(t) = 0 on (t0, t∗] for some t∗ > t0. The first case shows that the first switch is non-singular. The second case may generate a singular control. If ψ(t) = 0 on (t0, t∗], then it follows that λ′U(t) = λ′T(t) = 0 on (t0, t∗]. Also λU(t0) = 0, since otherwise λS(t0) = λU (t0) = λR(t0) = 0 > λT(t0) = λΔ which is a contradiction based on the previous result. In addition, there exists some t∗∗, where t0 < t∗∗ ≤ t∗ such that (λS − λU)βS < γλU(t) on (t0,t∗∗], and therefore λ′U(t) = λ′T(t) < 0. This implies that t0 is an inflection point for λU and λT at which λ″U(t0) = λ″T(t0) = 0. Using Equations (23) and (24), it follows that
which yields λ′R(t0) = 0. This implies that λ′U(t0) = λ′T(t0) = λ′R(t0) = 0, which contradicts the above result for λ′R (t0) ≠ 0, and therefore, the optimal control is a bang-bang problem at t0.
The following theorem shows the optimal treatment profile to minimize the overall impact of the disease and the total number of drug-resistant infections.
Theorem 2.7
Let (p∗(t), X∗(t)) be an optimal control for the corresponding solution of the system (1)–(5). Then, there exists some positive interval [0, t0] (t0 < Tf) such that ψ(t) = 0 on [0, t0].
Proof
Suppose that there exists some positive interval [0, t0] on which ψ(t) = 0 for the control problem corresponding to solutions of system (1)–(4). Thus, λT(t) = λU(t) + λΔ on the same interval [0, t0]. Using Equation (12), it follows that
From the Hamiltonian condition which shows that H(0) = 0, we have
Equating the above two equations, we obtain
Subtracting the second adjoint equation (23) from the third one in Equation (24) and eliminating the expression for λS(0) give
Now, by taking the derivative of each expression and then solving for λR(t), we have
Using the fact that the function of Hamiltonian obtained from the Pontryagin fundamental theory must be zero at any time, that is, H(t, X, Λ, p) = −(λ′U IU + λ′TIT + λ′RIR) = 0 for t ∊ [0, T], in particular, at the start point of time 0, it follows that
Applying the same argument as in Theorem 2.6, the above expression is a contradiction, and consequently, we have λT(t) ≠ λU(t) on [0, t0] before the first switch occurs.
3. Numerical experiments
In this section, we numerically illustrate the optimal scenarios of the treatment profile, when the drug stockpile is limited, and compare the results for variations in the rate of resistance emergence (α) and relative transmissibility of the resistant strain (δR). We show that, under some conditions, the system (1)–(4) has an infinite number of solutions generated by the optimal control (19). In all the simulations presented here, we fixed the basic reproduction number of the sensitive strain to be R0 = 1.8.
3.1. Run-out scenarios
For a limited drug stockpile sufficient to treat 10% of the population, we simulated the model with the optimal treatment profile when α and δR vary in their respective ranges given in Table 1. Figure 2(a) shows the epicurves of untreated, treated, and resistant infections with α = 10−3 and δR = 0.9. Since, according to the optimal control, treatment starts with a delay following the onset of epidemic, resistance will be absent in the early stages of the epidemic. Initiation of treatment leads to the rapid suppress of the outbreak caused by the sensitive infection, but leads to the emergence and spread of the resistant outbreak. However, a second wave of infection with the sensitive strain occurs when the drug stockpile is exhausted. When the stockpile is increased (i.e. sufficient to treat 20% of the population), then the optimal control suggests similar delay in the start of treatment in the population; however, the size of drug stockpile is adequate to prevent the second wave of infection by the sensitive strain (Figure 2(b)).
Table 1.
Values of the model parameters obtained from the published literature.
Figure 2.
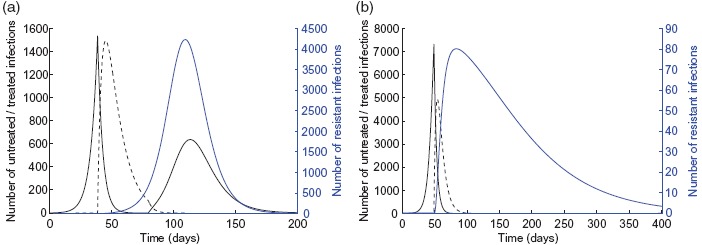
Epidemic profiles with α = 10−3 and δR = 0.9 for (a) K = 10% and (b) K = 20%. Untreated, treated, and resistant infections are illustrated by the solid black, dashed black, and blue curves, respectively. Other parameter values are given in Table 1.
To explore the effect of the size of drug stockpile on the optimal treatment profile, we simulated the model for K = 5%, 10%, 15%, 20%. Figure 3 shows the epidemic final size for different amounts of drug stockpile. Clearly, as the size of stockpile increases, a longer delay in start of treatment is required to minimize the total number of infections, which corresponds to a smaller region for optimal profile. It is, however, important to note that the amount of drugs used to minimize the total number of infections (corresponding to the optimal scenario) depends on the rate of resistance emergence regardless of the initial size of drug stockpile. Figure 4 shows the final size as a function of the drug stockpile for different values of α. As α increases, a larger drug stockpile is required to minimize the total number of infections.
Figure 3.
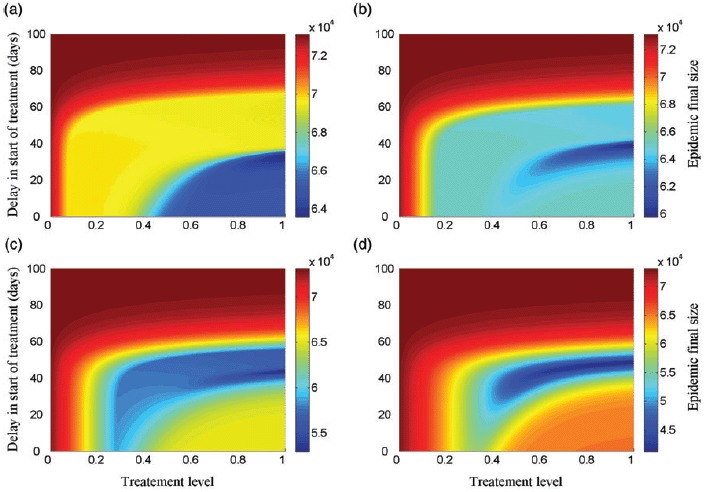
Epidemic final size as a function of the treatment level and delay in start of treatment, with α = 10−3 and δR = 0.9, for (a) K = 5%; (b) K = 10%; (c) K = 15%; and (d) K = 20%. Other parameter values are given Table 1.
Figure 4.
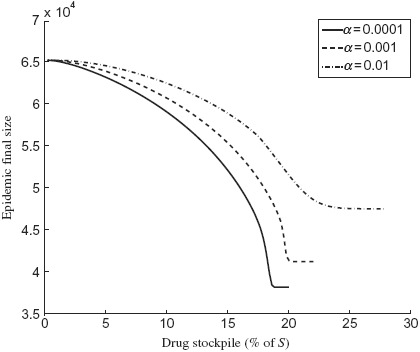
Epidemic final size as a function of drug stockpile (K) with δR = 0.9 for (a) α = 0.0001 (solid curve); (b) α = 0.001 (dashed curve); and (c) α = 0.01 (dot-dashed curve). Other parameter values are given in Table 1.
3.2. Effect of relative transmissibility and resistance development
The effect of relative transmissibility of the resistant strain (δR) on the optimal treatment level has been investigated in previous work [12–14,23,26,28,31]. However, as shown in our simulations, the rate of resistance emergence (α) also plays a critical role in identifying the optimal treatment profile for a limited drug stockpile. To illustrate the combination effect of α and δR, we simulated the model to determine the optimal treatment rate (i.e. time dependent with a switch) for different sizes of drug stockpile (Figure 5(a)–(c)). These simulations indicate that when δR is relatively low (i.e. remains below 0.6 in these scenarios), the optimal control suggests that the minimum final size corresponds to the start of treatment at the onset of epidemic without any delay. However, as δR increases above a certain threshold, then the resistant strain gains a competitive advantage, and the size of stockpile becomes important in determining the delay in start of treatment. For some values of δR (0.6 < δR < 0.8 in these scenarios), as the size of drug stockpile increases, a longer delay is required to minimize the total number of infections (Figure 5(b)–(c)). This delay is shorter for higher values of α. For relatively high values of δR (δR > 0.8 in these scenarios), the rate of resistance emergence (α) has very little impact in determining the optimal timing for switch (regions to the right side of δR = 0.8 in Figure 5(a)–(c)).
Figure 5.
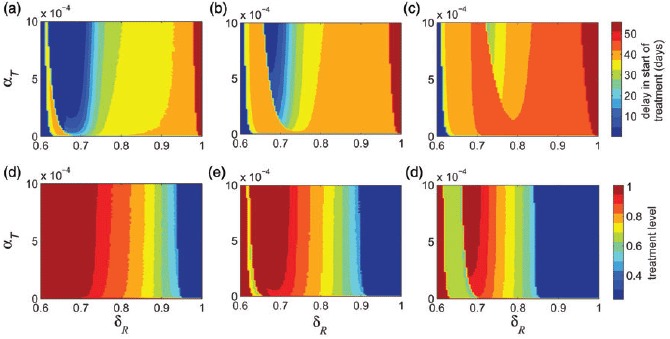
Delay in start of treatment in the time-dependent profile as a function of δR and α to minimize the epidemic final size is shown for (a) K = 8%; (b) K = 12%; and (c) K = 16%. Optimal level in the constant treatment profile as a function of δR and α to minimize the epidemic final size is shown for (d) K = 8%; (e) K = 12%; and (f) K = 16%. Other parameter values are given in Table 1.
We obtained similar results when treatment profile remains constant throughout the epidemic without any switch (Figure 5(d)–(f)). Figure 5(d) shows that for low values of δR (below 0.6), the minimum final size corresponds to the highest treatment level. As δR increases, the size of drug stockpile determines the optimal treatment level, and this level decreases as K increases. Similar to the case of time-dependent treatment profile, for some values of δ R (0.6 < δR < 0.8 in these scenarios), the rate of resistance emergence (α) plays an important role in determining the optimal treatment level. However, for relatively high values of δR (above approximately 0.8 in these scenarios), the rate of resistance emergence has very little impact on changing the optimal treatment level, regardless of the size of drug stockpile (Figure 5(d)–(f)).
3.3. Comparison between time-dependent and constant treatment profiles
To compare the outcomes of optimal scenarios in the two treatment profiles simulated in Figure 5, we defined Fop and Foc to be the final sizes of the epidemic in the time-dependent and constant treatment strategies, respectively. We simulated the model to determine the minimum number of infections as a function of δR and α in each strategy. Simulations in Figure 6(a) and 6(b) show contour plots for the ratio Fop/Foc with two different ranges of α. Simulation results for this ratio indicate that as the transmissibility of the resistant strain increases above a certain threshold, the ratio Fop/Foc decreases below 1, implying that the optimal time-dependent treatment profile outperforms the optimal constant strategy in reducing the final size. However, the corresponding reduction in the final size depends significantly on the rate of resistance emergence. We also observe that, as δR increases, the change in the ratio Fop/Foc is less pronounced for variation of α in the range 10−6 − 10−3 (Figure 6(b)) compared to the range 10−3 − 10−1 (Figure 6(a)).
Figure 6.
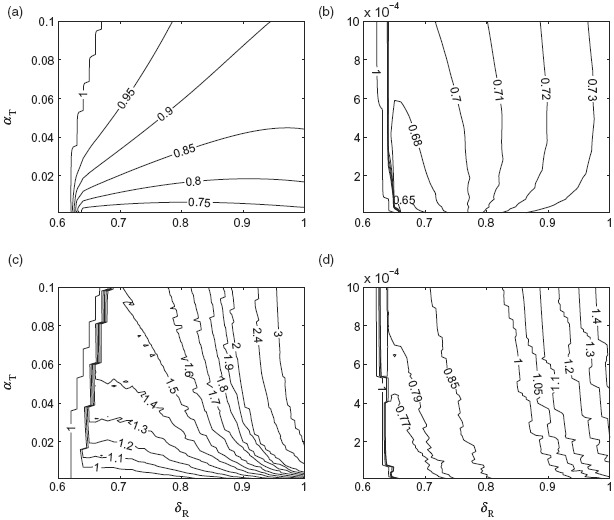
Contour plots for the ratio Fop/Foc for α in the range (a) 10−3 − 10−1 and (b) 10−6 − 10−3. Contour plots for the ratio Kop/Koc for α in the range (c) 10−3 − 10−1 and (d) 10−6 − 10−3. Other parameter values are given in Table 1.
For further comparison, we simulated the model to find the corresponding ratio of Kop/Koc, where Kop and Koc are the minimum sizes of drug stockpile to achieve the optimal scenarios in the time-dependent and constant treatment strategies, respectively. Figures 6(c) and 6(d) show contour plots for Kop/Koc as a function of δR and α, corresponding to simulations presented in Figure 6(a) and 6(b). As evident, when Fop/Foc < 1 for α in the range 10−3 − 10−1, a significantly larger drug stockpile may be required in the time-dependent treatment profile (Kop/Koc > 1) as δ increases. However, for low values of α in the range 10−6 − 10−3 and δ below 0.8, the optimal time-dependent treatment profile outperforms the optimal constant treatment level (Figure 6(b)), with considerably smaller size of the drug stockpile (Figure 6(d)). As δ increases above 0.8, while Fop/Foc < 1, a larger drug stockpile is required in the time-dependent treatment profile compared to the constant treatment strategy. These simulations clearly illustrate the trade-off between minimizing the epidemic final size and the size of drug stockpile in the two scenarios discussed here, and highlight the importance of relative transmissibility and the rate of resistance emergence.
4. Concluding remarks
Summarizing our findings with simulation results, we observed that a time-dependent treatment profile can provide the optimal scenario for minimizing the epidemic final size in the presence of limited drug stockpile. We have shown the effect of two key parameters (i.e. the relative transmissibility of resistance and the rate of resistance emergence) in the optimal control problem. In some scenarios, we found that while the optimal time-dependent treatment strategy outperforms the optimal constant treatment policy, it requires significantly larger drug stockpile, and therefore a cost-benefit analysis would be desirable to inform an antiviral policy with regard to effectiveness and cost-effectiveness of treatment strategies. Combining findings of this study with those of our previous work with no constraint on the size of drug stockpile, Jaberi Douraki et al. [14] provide the grounding for further investigation on the optimal solution of the control problem. Based on the theoretical findings and numerical simulations, we conjecture that for any pair of optimal control (p∗(t),X∗(t)) corresponding to the solution of the control system (1)–(5), there exists a unique t0 ∊ [0, tK) such that the switching function changes its sign at t0 from positive to negative.
Acknowledgements
The authors would like to thank the reviewers for their insightful comments that have improved the paper. This work was supported in part by Mprime and the Natural Sciences and Engineering Research Council of Canada (NSERC).
References
- [1].Agrachev A.A., Sachkov Y.L. Encyclopedia of Mathematical Sciences. Vol. 87. New York: Springer-Verlag; 2004. Control Theory from the Geometric Viewpoint. [Google Scholar]
- [2].Arinaminpathy N., McLean A.R. Soc J. R., editor. Antiviral treatment for the control of pandemic influenza: Some logistical constraints. Interface. 2008;5(22):545–553. doi: 10.1098/rsif.2007.1152. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [3].Arino J., Bowman C.S., Moghadas S.M. Antiviral resistance during pandemic influenza: Implications for stockpiling and drug use. BMC Infect. Dis. 2009;9:8. doi: 10.1186/1471-2334-9-8. doi:10.1186/1471-2334-9-8. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [4].Arino J., Brauer F., van den Driessche P., Watmough J., Wu J. A model for influenza with vaccination and antiviral treatment. J. Theor. Biol. 2008;253(1):118–130. doi: 10.1016/j.jtbi.2008.02.026. [DOI] [PubMed] [Google Scholar]
- [5].Brauer F., van den Driessche P., Wu J. Mathematical Epidemiology. Berlin, Heidelberg: Springer-Verlag; 2008. [Google Scholar]
- [6].Bryson A.E., Jr., Ho Y.-C. Estimation and Control. New York: Taylor & Francis; 1975. Applied Optimal Control: Optimization; p. 246. [Google Scholar]
- [7].Diekmann O., Heesterbeek J.A.P. Mathematical Epidemiology of Infectious Diseases: Model Building. Analysis and Interpretation. New York: John Wiley and Sons; 2000. [Google Scholar]
- [8].Ferguson N.M., Mallett S., Jackson H., Roberts N., Ward P. A population-dynamic model for evaluating the potential spread of drug-resistant influenza virus infections during community-based use of antivirals. J.Antimicrob. Chemother. 2003;51:977–990. doi: 10.1093/jac/dkg136. doi:10.1093/jac/dkg136. [DOI] [PubMed] [Google Scholar]
- [9].Ferguson N.M., Cummings D.A.T., Cauchemez S., Fraser C., Riley S., Meeyai A., Iamsirithaworn S., Burke D.S. Strategies for containing an emerging influenza pandemic in Southeast Asia. Nature. 2005;437:209–214. doi: 10.1038/nature04017. doi:10.1038/nature04017. [DOI] [PubMed] [Google Scholar]
- [10].Fister K.R., Panetta J.C. Optimal control applied to cell-cycle-specific cancer chemotherapy. SIAM J. Appl. Math. 2000;60:1059–1072. [Google Scholar]
- [11].Fleming W.H., Rishel R.W. Deterministic and Stochastic Optimal Control. New York: Springer-Verlag; 1975. [Google Scholar]
- [12].Hansen E., Day T. Optimal control of epidemics with limited resources. J. Math. Biol. 2011;62(3):423–451. doi: 10.1007/s00285-010-0341-0. doi 10.1007/s00285-010-0341-0. [DOI] [PubMed] [Google Scholar]
- [13].Hansen E., Day T. Optimal antiviral treatment strategies and the effects of resistance. Proc. R. Soc. B. 2011;278(1708):1082–1089. doi: 10.1098/rspb.2010.1469. doi 10.1098/rspb.2010.1469. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [14].Jaberi-Douraki M., Hefffernan J., Wu J., Moghadas S.M. Optimal treatment profile during an influenza epidemic. Differ. Equ. Dyn. Syst. 2013;21(3):237–252. doi: 10.1007/s12591-012-0149-z. [Google Scholar]
- [15].Joshi H.R. Optimal control of an HIV immunology model. Optim. Control Appl. Methods. 2002;23:199–213. [Google Scholar]
- [16].Joshi H.R., Lenhart S., Gaff H. Optimal harvesting in an integro-difference population model. Optim. Control Appl. Methods. 2006;27:61–75. [Google Scholar]
- [17].Joshi H.R., Lenhart S., Lou H., Gaff H. Harvesting control in an integro-difference population model with concave growth term. Nonlinear Anal. Hybrid Syst. 2007;1:417–429. [Google Scholar]
- [18].Jung E., Lenhart S., Feng Z. Optimal control of treatments in a two-strain tuberculosis model. Discrete Contin. Dyn. Syst. B. 2002;2:473–482. [Google Scholar]
- [19].Jung E., Takeuchi Y., Jo T.C. Optimal control strategy for prevention of avian influenza pandemic. J. Theoret. Biol. 2009;260:220–229. doi: 10.1016/j.jtbi.2009.05.031. [DOI] [PubMed] [Google Scholar]
- [20].Kirk D.E. Optimal Control Theory: An Introduction. Mineola, New York: Dover Publications, Inc.; 2004. [Google Scholar]
- [21].Kyriakidis E.G., Pavitsos A. Optimal intervention policies for a multidimensional simple epidemic process. Math. Comput. Model. 2009;50:1318–1324. [Google Scholar]
- [22].Lenhart S., Workman J.T. Optimal Control Applied to Biological Models. Boca Raton, FL: Chapman & Hall, CRC Press; 2007. [Google Scholar]
- [23].Lipsitch M., Cohen T., Murray M., Levin B.R. Antiviral resistance and the control of pandemic influenza. PLoS Med. 2007;4(1):e15. doi: 10.1371/journal.pmed.0040015. doi:10.1371/journal.pmed.0040015. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [24].Longini I.M., Jr, Nizam A., Xu S., Ungchusak K., Hanshaoworakul W., Cummings D.A.T., Halloran M.E. Containing pandemic influenza at the source. Science. 2005;309:1083–1087. doi: 10.1126/science.1115717. doi:10.1126/science.1115717. [DOI] [PubMed] [Google Scholar]
- [25].Lukes D.L. Mathematics in Science and Engineering. Vol. 162. New York: Academic Press; 1982. Differential Equations: Classical to Controlled. [Google Scholar]
- [26].Moghadas S.M. Management of drug resistance in the population: Influenza as a case study. Proc. R. Soc. B. 2008;275:1163–1169. doi: 10.1098/rspb.2008.0016. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [27].Moghadas S.M. Dynamics of resistance in influenza with compensatory mutations. Math. Popul. Stud. 2011;18:106–121. [Google Scholar]
- [28].Moghadas S.M., Bowman C.S., Röst G., Wu J. Population-wide emergence of antiviral resistance during pandemic influenza. PLoS ONE. 2008;3(3):e1839. doi: 10.1371/journal.pone.0001839. doi: 10.1371/journal.pone.0001839. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [29].Moghadas S.M., Bowman C.S., Röst G., Fisman D.N., Wu J. Post-exposure prophylaxis during pandemic outbreaks. BMC Med. 2009;7:73. doi: 10.1186/1741-7015-7-73. doi: 10.1186/1741-7015-7-73. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [30].Porco T.C., Lloyd-Smith J.O., Gross K.L., Galvani A.P. The effect of treatment on pathogen virulence. J. Theor. Biol. 2005;233:91–102. doi: 10.1016/j.jtbi.2004.09.009. [DOI] [PMC free article] [PubMed] [Google Scholar]
- [31].Regoes R.R., Bonhoeffer S. Emergence of drug resistance influenza virus: Population dynamical considerations. Science. 2006;312:389–391. doi: 10.1126/science.1122947. doi:10.1126/science.1122947. [DOI] [PubMed] [Google Scholar]